Abstract
According to the global cancer observatory (GLOBOCAN), there are approximately 18 million new cancer cases per year worldwide. Cancer therapies are largely limited to surgery, radiotherapy, and chemotherapy. In radiotherapy and chemotherapy, the maximum tolerated dose is presently being used to treat cancer patients. The integrated development of innovative nanoparticle (NP) based approaches will be a key to address one of the main issues in both radiotherapy and chemotherapy: normal tissue toxicity. Among other inorganic NP systems, gold nanoparticle (GNP) based systems offer the means to further improve chemotherapy through controlled delivery of chemotherapeutics, while local radiotherapy dose can be enhanced by targeting the GNPs to the tumor. There have been over 20 nanotechnology-based therapeutic products approved for clinical use in the past two decades. Hence, the goal of this review is to understand what we have achieved so far and what else we can do to accelerate clinical use of GNP-based therapeutic platforms to minimize normal tissue toxicity while increasing the efficacy of the treatment. Nanomedicine will revolutionize future cancer treatment options and our ultimate goal should be to develop treatments that have minimum side effects, for improving the quality of life of all cancer patients.
Keywords: gold nanoparticles, radiation, chemotherapy, radiosensitizer, drug delivery system, chemoradiotherapy
1. Introduction
According to American Cancer Society statistics in 2020, there will be an estimated 1.8 million new cancer cases diagnosed and 606,520 cancer deaths in the United States alone. Cancer is an abnormal growth of cells caused by multiple changes in gene expression leading to deregulation of the balance of cell death and proliferation, ultimately leading to an evolving population of cells that can invade tissues and metastasize to other sites [1]. The main types of cancer treatments include surgery, chemotherapy and radiotherapy according to the Canadian Cancer Society [2]. The treatment plan of each cancer patient will vary depending on the type of cancer and the advancement of cancer [2,3]. Radiotherapy is one of the most widely used treatment approaches, being used in approximately 50% of all cancer patients. In radiotherapy, a high dose of ionizing radiation is delivered to the tumor site, which interacts with and excites the atoms inside the cancer cells, causing damage to important structures, ultimately killing the cell [4]. Currently, the clinic mainly employs gamma or X-ray photons, ion-based electrons, or protons as radiation sources in the treatment [5,6]. While radiotherapy is widely used in many different types of cancers, a major issue still present is the normal tissue toxicity [7]. A photon beam will irradiate some of the surrounding healthy tissue no matter how well shaped or conformed the beam is to the dimensions of the tumor, and this dose to normal tissue limits the amount of radiation a patient can receive [8].
Chemotherapy is also used to eradicate micro-metastases and to improve local control of the primary tumor [9]. In chemotherapy, anticancer drugs are administered either orally or intravenously to disrupt the rapid overgrowth of malignant cells [10,11]. Similar to radiotherapy, the side effects caused by anti-cancer drugs remain as one of the important limitations in the advancement of cancer treatment [12,13]. Therefore, we need to improve the bioavailability of the drug in the tumor region, while confining them to this target, to reduce the amount of the drug needed, and thus the number, and severity, of side effects [14]. Some nanoparticle (NP)-based therapeutic systems have already been introduced into the pharmaceutical market. For example, Doxil, a polyethylene glycol (PEG)-liposome containing Doxorubicin, is approved for AIDS-related Kaposi’s sarcoma, ovarian cancer, and multiple myeloma [15,16]. Liposomal drugs and polymer drug conjugates account for most of the FDA (Food and Drug Administration, Tulsa, OK, USA)-approved systems so far [17]. However, in radiotherapy, NP-driven radiosensitization strategies that use inorganic high-Z (atomic number) materials have been pursued to improve the local radiation dose and minimize the damage to surrounding healthy tissue [18]. The interaction of high-Z materials with therapeutic X-ray photons results in an increase in the production of cell damaging species, such as free radicals and low energy electrons [19,20]. Inorganic NP systems such as gold nanoparticles (GNPs), silver NPs, gadolinium-based NPs, lanthanide-based NPs, and titanium oxide nanotubes have been reported as radiosensitizers [21,22,23,24,25,26,27]. Gadolinium-based NPs offer an innovative approach because of their capacity to act as a radiosensitizer as well as a powerful contrast agent in magnetic resonance imaging [26]. The high Z-nature of silver-based NPs along with their antimicrobial properties made them a good candidate in radiotherapy [27]. However, GNPs are the most widely used NP system in radiotherapy due to their ease of production, high Z-nature, advantageous surface chemistry, and biocompatibility [25,28,29,30].
There are different gold-based nanotherapeutic systems available, such as spherical GNPs, gold nanorods, gold nanoshells, gold nanoclusters, and GNP-incorporated liposomal nanoparticles, with many new anisotropic geometries being developed regularly. Spherical GNPs are the most commonly used gold-based nanotherapeutic, as their production is relatively simple and alteration of size and surface chemistry, such as conjugation with polyethylene glycol, is easily achieved [31,32]. Further, GNPs are heavily studied for use in the treatment of cancer through X-ray irradiation and as an anticancer drug carrier [33]. The use of gold nano-rods and gold nanoshells for the treatment of cancer involves the induction of hyperthermia, due to their larger cross-section at near-infrared (NIR) frequencies [34,35]. A comprehensive review of the use of gold-based nanomaterials such as gold nanoshells and gold nanorods in photothermal therapy has been described previously by Vines et al. [36]. It has also recently been shown that gold-based nanotherapeutics can absorb radiofrequency (RF) frequencies and generate heat, opening an avenue to treat more deep-set tumors with the use of gold and hyperthermia-based options [37]. Although more research must be completed, the use of RF waves with gold nanomaterials is very promising. Furthermore, due to the surface plasmon resonance effect present in GNPs, visible light irradiation can also allow for hyperthermia via photothermal therapy, recently shown by Mendes et al. with a green laser light in combination with 14 nm GNPs and doxorubicin [38]. However, the penetration depth of green light is even less than NIR and is thus limited in applicability [39]. Due to their theranostic benefits, such as imaging and biosensing, along with therapeutic properties such as drug delivery, gold nanoclusters have emerged as a useful tool [40,41]. The use of gold nanoclusters can allow for molecular imaging, improving diagnostics and imaging in the future [42]. Ultrasmall gold nanoclusters have also emerged as a useful technology due to their near 100% renal clearance, allowing for the improved probing of disease when utilized as a biosensor [43]. Lipid-based nanoparticles are an avenue that is being explored due to their ability to encapsulate GNPs for radiosensitization purposes and simultaneously act as a drug delivery platform [44]. Utilizing liposomal nanoparticles as a ‘smart’ drug carrier can allow for controlled release of the internalized cargo, such as in response a NIR light source, allowing more control over the treatment process [45].
GNP-based platforms are being researched and have been tested extensively in the field of cancer nanomedicine [46]. For example, a novel nanomedicine that conjugated human tumor necrosis factor alpha (rhTNF) and thiolated PEG onto the surface of colloidal GNPs (named CYT-6091) has been tested in phase 1 clinical trial in cancer patients [47]. The results from the CYT-6091 trial showed that doses up to 600 µg/g of rhTNF were administered without encountering dose-limiting toxicity and was less toxic than a treatment with just rhTNF, as evidenced by a lack of hypertension in patients. Furthermore, the GNPs had gathered in the tumor and mostly avoided healthy tissue. Other phase 1 clinical trials involved the use of PEGylated gold nanoshells around a silica nanoparticle, called AuroLase®, in head and neck, lung, and prostate cancer, with laser irradiation [48,49,50]. Results have, however, not translated to an effective treatment outcome. Another early phase 1 clinical trial involves the use of NU-0129, a platform consisting of nucleic acids attached to the surface of spherical GNPs [51]. The goal of this study is to use the conjugated nucleic acids to bypass the blood-brain barrier and target the BcL2L12 gene present in recurrent glioblastoma. If successful, this platform could supress this gene, which would lead to reduced proliferation and containing the spread of the tumor. However, translation of GNPs to the clinic is still in progress, and further optimization of protocols will have to be elucidated before the majority of research can move out of the preclinical stage, as described in the extensive review by Schuemann et al. [52].
For patients with locally advanced disease, a combination of treatments, such as surgery with chemotherapy and/or radiotherapy is being used. A combination of chemotherapy and radiotherapy (referred to as chemoradiation) is a logical and reasonable approach that has greatly improved the cure rates of solid tumor [8,53]. This combined treatment modality provides local control of the primary tumor mass through radiation while tumor metastasis is suppressed through anticancer drugs [8]. One of the major limitations of chemoradiation as a treatment option is the normal-tissue toxicity, as either radiotherapy or chemotherapy can cause major normal tissue toxicity, as described previously. In order to overcome the normal tissue toxicity in current cancer treatment modalities mentioned previously, NPs are being used to enhance either the local radiation dose or improve delivery of anticancer drugs, or both, as seen in Figure 1. GNPs are one of the materials extensively tested for both radiotherapy and chemotherapy. Therefore, this review article will be focused on prospects of GNP-mediated cancer therapeutics.
Figure 1.
Gold nanoparticle-based cancer therapeutics. Radiotherapy and chemotherapy are the two main modalities, besides surgery, in treating cancer. However, normal tissue toxicity in both methods remains a large issue in limiting the effective dose to the tumor. Thus, gold nanomaterials have been introduced to improve the locally deposited dose into tumors and act as a drug delivery system. The combination of radiotherapy and chemotherapy, called chemoradiotherapy, allows for an optimum platform for eradicating the tumor and improving cancer therapeutics.
Due to the large amount of recent interest in GNPs as a therapeutic agent, there have been many reviews on the topic [33,36,37,52,54,55,56,57,58,59,60,61]. Beik et al. have a recent, extensive review on the use of GNPs in various different modalities, including radiotherapy and chemotherapy, with a larger focus on photothermal therapy and combined treatment options [56]. However, the focus on radiotherapy is limited mainly to kV energy ranges, where GNPs have the largest differential in absorption cross section compared to soft tissue. To be clinically relevant in a larger variety of cancers, the efficacy of GNPs at an MV energy range needs to be explored. As previously mentioned, recent reviews on the use of irradiation in the NIR and RF range with gold nanomaterials for hyperthermia have shown promise [36,37]. Despite continuing research, however, irradiation involving X-rays dominate clinical treatment schemes, occurring in greater than 50% of patients [62]. Of all the gold nano-based therapeutics, spherical GNPs are extensively tested for both radiotherapy and chemotherapy. Therefore, this review article will be focused on prospects of GNP-mediated cancer therapeutics with clinically relevant radiotherapy, chemotherapy, and with a combined modality. This includes information that is necessary in order to improve efficacy, such as an understanding of GNP uptake at a cellular level, and how the size, shape, and functionalization of the GNPs alters effectiveness. In order to better understand the application of GNPs in cancer treatment, an introductory section is presented to understand the behavior of GNPs at a single cell level.
2. Intracellular Fate of Gold Nanoparticles Based on Their Physicochemical Properties
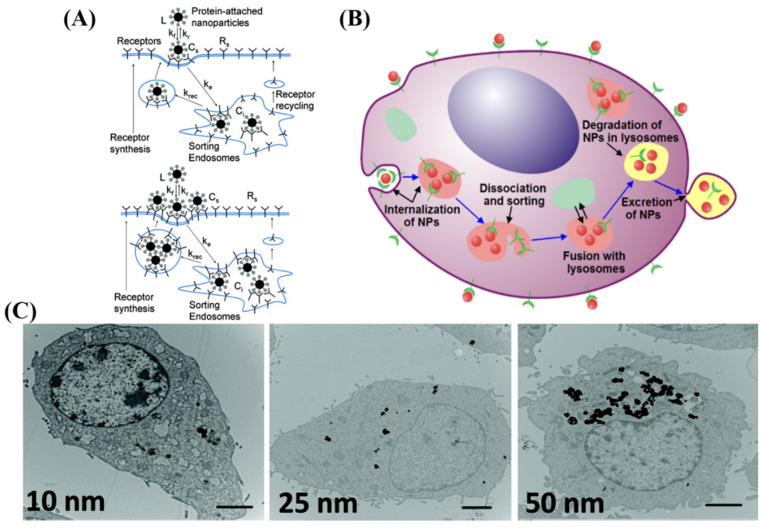
There are different methods of entry into cells for NPs, including clathrin-mediated endocytosis, clathrin-caveolin independent endocytosis, and caveolae-mediated endocytosis [63]. Most NPs, including GNPs, enter the cell mostly via clathrin-mediated, or receptor-mediated, endocytosis (RME) [46,64,65,66,67,68]. The efficiency of the RME process depends on the interaction between molecules on the NP surface (ligands) and the cell membrane receptors. As illustrated in Figure 2A by Jin et al., cell surface receptors bind to molecules on surface of NPs, causing membrane wrapping of the NP with a corresponding increase in elastic energy [64,68]. The receptor-ligand binding immobilizes receptors causing configurational entropy to be reduced. More receptors diffuse to the wrapping site, driven by the local reduction in free energy, allowing the membrane to wrap completely around the particle [69].
Figure 2.
Uptake of GNP by receptor-mediated endocytosis. (A,B) Schematic illustrating pathway of citrate-capped GNP uptake into the cell. (A) Describes the entry and sort mechanism for a single NP and multiple NPs, while (B) describes the entire flow of internalization and excretion. Once GNPs are attached to the receptors on the surface of the cell, membrane invagination occurs followed by budding into the cell, forming a vesicle. The internalized GNPs are sorted inside the vesicle and eventually fuse with lysosomes. GNPs are then excreted out of the cell. (C) Transmission electron microscope images of rat kidney cells treated with three different sizes of GNPs. Scale bar is 2 μm. Reproduced with permission [68,70]. Copyright American Chemical Society, 2009, 2011.
RME is therefore an energy dependent process where the path of the NPs within the cell is explained in Figure 2B. NPs first reach the cell membrane and connect with the cell membrane receptors, which are mobile on the surface. Internalization of NPs occurs via invagination of the membrane, which then get trapped in endosomal vesicles. These internalized NPs are sorted inside the vesicle and eventually fuse with lysosomes, which can be seen within the cell as shown in Figure 2C by Ma et al. [70]. NPs are then excreted out of the cell. This intracellular path of NPs was further confirmed by Liu et al. by using a NP complex tagged with a fluorophore [71]. This group suggested that NPs are eventually transported to lysosomes by observing the co-localization of the fluorescently tagged NPs and lysosomes stained with lysotrackers.
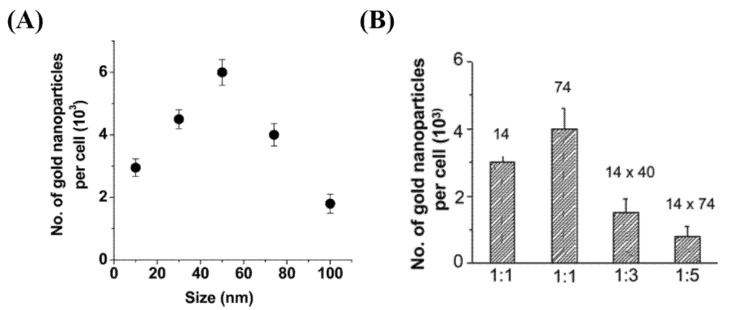
The RME is also dependent on the size, shape, and surface properties of NPs. Chithrani et al. investigated the effect of both size and shape on GNP internalization (see Figure 3A,B) [66]. Among the size range of 10–100 nm, bare GNPs of diameter 50 nm had the highest uptake. They also found that the cellular uptake of rod-shaped NPs was lower than their spherical counter parts. This outcome was explained as a result of balance between energy needed for membrane wrapping of NPs and kinetics of receptor diffusion along the cell membrane [67,68]. They used citrate-capped NPs for the study which were not functionalized, where the RME process of the NPs was facilitated via non-specific binding of serum proteins on the NP surface once they were introduced to the tissue culture media [72]. However, it is important to optimize NPs properly for efficient in vivo delivery to the tumor.
Figure 3.
Effect of size and shape on cellular uptake of gold nanoparticles. (A) Dependence of gold nanoparticle cellular uptake as a funtion of their diameter. (B) Comparison of uptake of rod-shaped nanoparticles (aspect ratios 1:3 and 1:5) and spherical nanoparticles (1:1). Reproduced with permission from [66]. Copyright American Chemical Society, 2006.
There are many factors to consider when optimizing GNPs for use in an in vivo environment. For example, the administration route of the GNPs affects their absorption, toxicity, and tissue distribution [73,74]. Oral and intraperitoneal routes of administration had the largest toxicity, while a tail vein injection had the least, suggesting that an intravenous injection is most promising. Upon administration, the pharmacokinetics of the GNPs is another factor that must be optimized. GNPs exhibit very complex and varying pharmacokinetics, due the vast number of options in size, shape, and functionalization. Avoidance of opsonization and the reticuloendothelial system (e.g., liver and spleen), while also targeting the tumor, are important goals in nanotechnology [75].
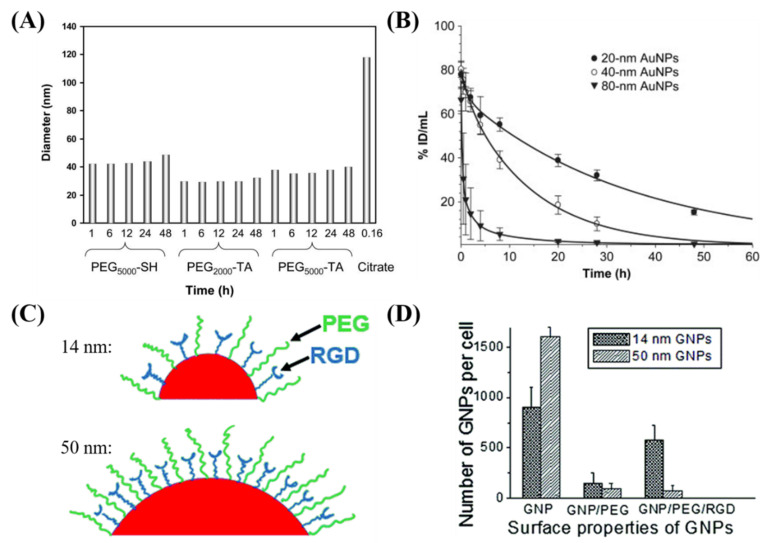
Prolonged in vivo residency time and preferential localization in tumors are key features of an efficient NP system [76]. If not functionalized properly, the opsonin protein in the blood plasma will attach to the NP surface, leading to the removal of the NP from the circulatory system by macrophages [77,78]. Furthermore, the protein corona that can form from interactions of the GNPs with blood, as a result of size, shape, charge, and functionalization, can alter the behavior of the nanoplatform [79]. Therefore, surface modifications of GNPs are performed to protect the particle from the environment and to target the particle to a specific cell or tissue type. This is critical, because the GNPs need to be present long enough for the process of accumulation within a tumor through its leaky vasculature, known as the enhanced permeability and retention (EPR) effect [80]. Previous studies have shown that the addition of PEG molecules to the surface of NPs increases blood circulation time [80,81,82]. The process of PEGylation allows for the ethylene glycol to form associations with water molecules, allowing for the formation of a protective hydrating layer, which in turn hinders protein adsorption and clearance by macrophages [83]. The stability of GNPs functionalized with PEG molecules was done by Zhang et al. in Figure 4A, who showed that PEGylated GNPs maintain stability over time, compared to bare GNPs who aggregate quickly [82]. GNPs functionalized with PEG molecules have also shown the capacity to evade the immune system and remain in the blood undetected by macrophages [76]. Further, Zhang et al. showed that GNPs maintained a large blood concentration over time for 20 nm and 40 nm PEGylated GNPs, as seen in Figure 4B [82]. However, the drawback of PEGylating the NP surface is that RME is very much retarded. To overcome this lower uptake of NPs, researchers have added targeting moieties to overcome the reduced NP uptake. One approach was to add a peptide containing arginine-glycine-aspartic acid (RGD) sequence, as performed by Cruje et al. in Figure 4C [77]. The RGD sequence can recognize the integrin αvβ3 that is highly expressed by several solid tumors and has demonstrably higher uptake than GNPs functionalized with just PEG [76,84]. Depending on the size of the PEG molecule and GNP, the uptake dynamics shown in Figure 4D was changed. For example, it was shown that smaller GNPs had a higher uptake compared to GNPs of diameter 50 nm [76,85]. The peptide and PEG molecules were on the order of 2 kDa and smaller NPs were able to maximize the ligand–receptor interaction of RGD peptide using their higher surface curvature [76,85].
Figure 4.
Effect of functionalization on cellular uptake of gold nanoparticles. (A) Diameter as measured using dynamic light scattering of GNPs functionalized with different PEG moieties, compared to bare GNPs. (B) Pharmacokinetics of different sized PEGylated GNPs expressed as a percentage of injected dose per gram of tissue in mice. (C) GNPs can be functionalized with PEG for stability and a peptide containing integrin binding domain RGD for targeting. (D) The use of the RGD functionalized GNPs allowed for improved uptake into tumors cells compared to GNPs functionalized with solely PEG. Reproduced with permission from [76,82]. Copyright Elsevier, 2009; Copyright Royal Society of Chemistry, 2015.
Various factors can affect the pharmacokinetics of the GNPs. Depending on the size, the GNPs will have a different fate in vivo [86]. Smaller PEGylated GNPs of sizes 4 nm and 13 nm had high blood levels for 24 h and were cleared after 7 days, while larger GNPs (100 nm) were completed cleared after 24 h. Furthermore, the accumulation of smaller GNPs in the liver and spleen was peaked after 7 days, and in the mesenteric lymph node after a month, followed by clearance after 6 months. Larger GNPs were taken up into the liver, spleen, and mesenteric lymph node within 30 min. In general, larger GNPs concentrate in the kidney and spleen, and smaller GNPs are found throughout more organs [87]. Ultrasmall GNPs (<10 nm) have been studied due to their improved capabilities to be cleared from the reticuloendothelial system [88]. Further, Bugno et al. showed that that smaller GNPs (2 nm) have a three-fold increased tumor penetration compared to their larger counterparts (4 nm) [89]. As hypoxic regions far from capillaries tend to be the driver for treatment resistance, the ability to reach these regions with GNPs to increase local damage is a very important goal [90]. However, due to the large surface of curvature, despite surface coating with moieties like PEG, ultrasmall GNPs can have gaps that can be filled with blood proteins such as fibrinogen. As a result, smaller GNPs can contribute to an inflammatory response, due to their interactions with these proteins, highlighting the necessity for proper functionalization [91]. Another factor that impacts biodistribution is the surface charge, which can be controlled by various surface conjugations, such as with PEG [92,93]. The addition of PEG to 20 nm glucose-functionalized GNPs has been shown to increase the half-life period from 1.23 h to 6.17 h [94]. Furthermore, Geng et al. found that the functionalization of the GNPs lead to 20 times higher concentration in tumor tissue compared to normal tissue in the same organ, leading to an increase in damage to tumor following radiation [94]. This highlights the importance of proper functionalization to properly target GNPs to the tumor.
Other functionalization methods have also been tested to effectively target GNPs to tumors. In a variety of different epithelial cancers, epidermal growth factor receptors (EGFRs) can have significantly higher expression on cancer cells compared to normal cells [95]. Cetuximab (C225) is an antibody that allows for EGFR targeting, and has been shown to be effective at improving uptake compared to PEGylated GNPs in-vitro and in-vivo, by Kao et al. [96]. Another method involves the use of aptamer-based targeting. Aptamers are short single-stranded DNA or RNA oligonucleotides that are capable of binding to biological targets [97]. Aptamer-based GNPs can allow for specific targeting as well as aid in diagnostics [98]. Transferrin is a serum glycoprotein that can also be used to target GNPs to tumor cells, as there is an upregulation of receptors on metastatic and drug-resistant malignant cells [99]. The use of transferrin coated GNPs have been shown to improved uptake and allow for specific targeting to improve delivery of therapeutic agents [100]. Folic acid is another targeting molecule that can be employed, as the folate-receptor can be upregulated on human tumors while being minimally expressed on most normal tissue, as evidenced by Zhang et al. [99,101]. While there are many different functionalization modalities that can be employed, it is very important to test the efficiency of functionalized NP systems by varying their size, shape, and surface properties to optimize their internalization within tumor cells to cause the maximum damage. No matter what system that is employed, careful consideration of the functionalized GNPs with the protein corona that can form in vivo can allow for proper targeting and a predictable fate. [102] GNPs have also been associated with anti-inflammatory responses [103]. Thus, the toxicity of GNPs is an important factor that has been explored.
A number of groups studying GNP cytotoxicity concluded that GNP biocompatibility depends on size, surface properties and concentration [104,105]. Many experimental works reported that GNPs are non-toxic. For example, Connor et al. found various sizes (4, 12, 18 nm) and capping agents (citrate, cysteine, glucose, biotin, and cetyltrimethyl ammonium bromide) were nontoxic to K562 human leukemia cell line up to micromolar concentrations based on MTT assays [105]. Steckiewicz et al. found that the shape and concentration of the GNP complexes impact toxicity, with spheroidal GNPs (14 nm) imparting the least toxicity [106]. Sukla et al. observed lysine capped 35 nm GNPs did not show detectable cytotoxicity up to 100 μM concentration in RAW265.7 macrophage cells based on MTT assays [104]. It has been shown that PEGylated 12.1 nm sized GNPs incubated in HeLa cells had an IC50 of 0.477 mM [107]. Despite the many reports on non-toxicity of GNPs, contradictory research results are also present [108,109]. The lack of general consensus on NP toxicity is due to different experimental methods employed, incubation conditions (concentrations and exposure time), variability of sizes and functionalities of GNPs, variability of cell lines, and different measures and assays for toxicity [108,110]. However, most current research platforms are working in conditions that have previously been shown to be non-toxic, and future work should focus on maintaining this important constraint.
3. Gold Nanoparticles as Radiosensitizers
The use of high atomic number (Z) material to enhance radiation dose has been studied for more than 50 years. The interest in using high-Z material stems from the production of secondary electrons, such as photoelectrons, Auger electrons, and Compton electrons. These secondary products are effective at damaging DNA as well as ionizing surrounding water molecules, forming free radicals [110]. While the atomic number of tissue is approximately Z~7.5, materials with a higher atomic number used in the past such as Iodine (Z = 53) and gold (Z = 79) have a larger cross-section for absorption of radiation. For example, it was demonstrated in vitro that incorporating iodine into cellular DNA using iododeoxyuridine enhanced radiosensitivity at keV ranges by a factor of three [111]. The outcome of the in vitro study was also seen in an in vivo study, where an intratumoral injection of iodine and 200 kVp X-ray radiation suppressed the tumor growth by 80% [112]. In addition to having a great difference in mass attenuation between gold and soft tissue, gold has been shown to be biocompatible, simple, and economical to manufacture in many different shapes and sizes [113].
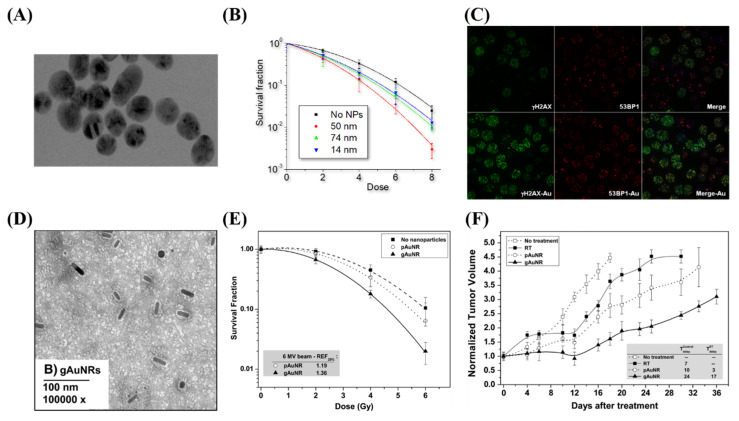
Radiation dose enhancement due to GNPs was first demonstrated using 1.9 nm GNPs in a mouse model, in one of the pioneering studies in GNP-mediated radiation dose enhancement by Hainfeld et al. [29]. A radiation dose of 30 Gy with 250 kVp X-rays to subcutaneous tumors in mice resulted in a significant decrease in tumor volume. However, the concentration of gold in this study was considerably high, at 2.7 g Au/kg body weight, which is not clinically feasible. Furthermore, the use of kV energies, while allowing for prominent photoelectric absorption in gold, is hindered due to the reduced penetration for deep-set tumors. Thus, as previously discussed, optimization of the internalization of the GNPs into the tumor cells, both in-vitro and in-vivo, is required for ideal efficacy. Whenever gold was internalized in vitro, radiosensitization was achievable at MV energy ranges, at concentrations as low as 1 ng/g [25,114,115,116]. This was demonstrated by Chithrani et al. in Figure 5A–C, which found a 17% increase in radiosensitization at 6 MV with 50 nm spherical GNPs [25]. When moving to an in vivo environment, radiosensitization was seen at a delivered dose of 10 μg/g of body weight [117]. This was accomplished by Wolfe et al. using targeted GNRs, as seen in Figure 5D–F, where there was a 36% increase in radiosensitization in vitro in PC3 cells, and a significantly enhanced tumor-growth delay when treated in vivo [117]. The treated dose is a ~ improvement over the original treatment seen in Hainfeld’s pioneering study. The addition of targeting and improvements in the optimization of uptake has allowed significant progress in facilitating the progress of gold nanomaterials to the clinic.
Figure 5.
Radiosensitization due to gold nanoparticles. (A–C) Spheroidal GNPs improve radiosensitization in vitro, with the largest effect occurring with 50 nm GNPs, as they have the optimum uptake. This can be seen both through clonogenic assays as well as through imaging of double strand break foci with confocal microscopy. (D–F) GNRs displayed increased radiosensitization when targeted towards prostate cancer cell lines both in vitro through a clonogenic assay as well as in vivo through tumor volume measurements in a mouse model. Reproduced with permission from [117]. Copyright Elsevier, 2015.
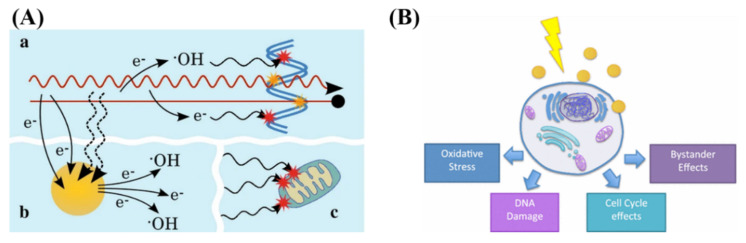
GNPs localized intracellularly increases the probability of ionization events leading to local enhanced deposition of energy causing more damage to tumor cells [25]. The physical mechanism of GNP radiosensitization, seen in Figure 6A, occurs within the first nanoseconds of exposure, and is based on the difference in mass energy absorption coefficients between gold and soft tissue, enabling dose enhancement. The range of electrons released from GNPs is short, only a few micrometers, causing highly localized ionizing events. Thus, to achieve any enhancement from GNPs in radiation therapy, GNPs must be delivered and internalized specifically by tumor cells.
Figure 6.
Radiosensitization and radiobiological effects. (A) Schematic showing chemical mechanism of GNP radiosensitization. While the radiation causes direct and indirect damage (yellow and red stars, respectively), there can be induction of secondary electrons and reactive oxygen species through gold nanoparticles. This can lead to damage to the DNA as well as secondary parts of the cell, such as the mitochondria. (B) GNPs can influence the cell through generation of reactive oxygen species, DNA damage, as well as cell cycle and bystander effects. Reproduced with permission from [110,118]. Copyright Springer Nature, 2016, 2017.
The chemical mechanism of GNP radiosensitization occurs through the radiochemical sensitization of DNA by increasing catalytic surface activity and increasing radical generation from the GNP surface [60]. Despite the prevailing notion that GNPs are chemically inert, there is a growing body of evidence that, due to the electronically active surface of GNPs, they are capable of catalyzing chemical reactions [119]. Catalysis by GNPs occurs mainly through surface interaction with molecular oxygen, generating free radicals [60]. This seems more evident in small GNPs (<5 nm in diameter) where surface to volume ratio is greater [120]. When combined with irradiation, the catalytic effects appear to be enhanced, with smaller GNPs with larger surface areas yielding more ROS [121]. However, it has been shown that at all energy levels, the dose enhancement observed cannot be simply explained by physical or chemical mechanisms [122]. To explain this, a radiobiological effect must be occurring.
The main radiobiological mechanisms involved in the cell’s response to irradiated GNPs results are the production of reactive oxygen species (ROS), oxidative stress, DNA damage induction, potential bystander effects, and cell cycle effects, as explained by Rosa et al. in Figure 6B [118]. Oxidative stress can cause cellular damage to the cell, including the oxidation of lipids, proteins, and DNA, which can result in apoptotic and necrotic cell death [123]. The mitochondria do appear to play a role, and the data indicate loss of function due to high intracellular ROS levels. It has been shown that the use of 1.4-nm triphenyl monosulfonate (TPPMS)-coated GNPs resulted in a loss of mitochondrial potential through elevated oxidative stress, resulting in necrotic cell death [122]. There have also been studies suggesting that GNPs may cause cell cycle disruptions and induce apoptosis. Radiosensitivity varies throughout the cell cycle with S phase being where a cell is most radioresistant and G2/M phase being most sensitive [124]. This could also depend on cell type, expression of cyclin kinases, and NP characteristics such as coating and size. For example, the use of 1.9 nm GNPs in DU-145 and MDA-MB-231 resulted in an increase in sub-G1 population in DU-145 population but not in MDA-MB-231 [125].
The biocompatibility of GNPs has already been tested in a phase I clinical trial. Furthermore, both in vitro and in vivo studies have shown the possibility of using GNPs as a radiosensitizer at clinically feasible concentrations, as discussed previously. Radiotherapy can also be combined with chemotherapy (chemoradiation) in cases where the tumor is not localized anymore, but metastasized as well, or to reduce potential micro-metastases spread. We will discuss the recent research conducted towards adding GNPs to this chemoradiation protocol in the next section.
4. Rationale for Gold Nanoparticles in Chemoradiotherapy
Radiotherapy is mainly used to control the tumor locally as discussed previously. Chemotherapy is used to control the tumor metastasis. Therefore, a combination of chemotherapy and radiotherapy (chemoradiation) is being practiced in the clinic to treat patients with locally advanced disease. Considering the variety of drugs available for cancer treatment, the possible choice of sequencing of combined chemotherapy and radiation therapy is countless, and the treatment plan differs between each patient. The standard treatment sequence refers to chemotherapy regimen before a traditional external beam radiation therapy treatment [53]. Chemotherapy used prior to irradiation is expected to cause maximal tumor regression for locally advanced tumors. The major limitation of combining chemotherapy and radiation therapy is normal tissue toxicity, since either modality can cause major normal tissue toxicity [8]. The main problems currently associated with chemotherapy are the biodistribution of pharmaceuticals, the lack of drug-specific affinity towards the tumor, limited plasma half-life, poor solubility and stability in physiological fluids, and nonspecific toxicity [126]. GNPs, due to their high surface area-to-volume ratio, as well as a large number of surface bio conjugation possibilities, are an ideal platform for delivering pharmaceutics for chemotherapy [46,127,128,129]. The use of GNPs as drug delivery system (DDS) can improve the pharmacokinetics, the pharmacodynamics, and the biodistribution of various drugs, as well as allow for improved targeting to reduce normal tissue toxicity. Beyond being an effective radiosensitizer, GNPs allow for a 100- to 1000-fold increase in ligand density compared to that of liposomal or polymeric DDSs [55]. Thus, the combination of the GNPs with radiotherapy and chemotherapy is part of the natural progression of the exploration of GNPs as a complete treatment modality.
The conjugation of moieties such as chemotherapeutic agents to the surface of the GNPs can be done using various techniques. The most common method is through the use of thiol group-containing biomolecules [130]. The use of thiolated biomolecules allows for functionalization of the GNPs with various agents, such as DNA, peptides, antibodies, and proteins [131,132]. This is a very robust method, as the majority of anticancer drugs can be thiolated, so as to be compatible with GNPs as a DDS [133,134]. Furthermore, capping agents, such as carboxyl terminated PEG molecules, with a thiol bond can allow for further functionalization techniques [131,135]. A general overview of various drug loading techniques using GNPs was explored by Fratoddi et al. [136]. GNPs, due to their favorable surface chemistry, are a suitable drug carrier for use in chemotherapeutics, and may be available for use in a wide range of drug delivery applications.
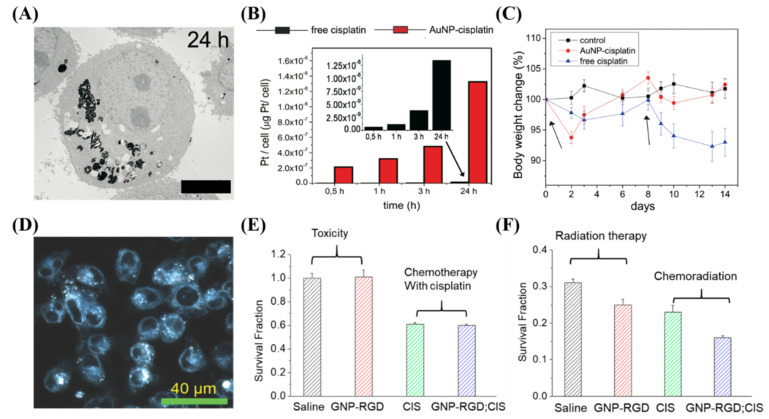
GNPs have been conjugated to a large variety of cytotoxic, anticancer drugs, and combined with radiation for improved efficacy. This includes paclitaxel, methotrexate, gemcitabine, doxorubicin, docetaxel, bleomycin, and platinum-based drugs like cisplatin [133,137,138,139,140,141]. Many different drugs can be used for different purposes, a few of which we will expand on. For instance, the antitumor activity of cisplatin was first discovered by Rosenberg and co-workers in 1960s, when they were examining whether electrical currents affect cellular division [142]. The researchers discovered that the inhibition of cellular division observed in the study was not due to electrical current, but platinum hydrolysis products formed from platinum electrodes. They reported that cis-tetrachlorodiammineplatinum (IV), cis-[PtCl4 (NH3)2], was the potent agent responsible for the inhibition. Cisplatin is now used to treat various types of cancers (i.e., cervical cancer, non-small-cell lung cancer, ovarian cancer, germ cell tumors, osteosarcomas, etc.), with a cure rate as high as 90% in testicular cancer [143]. However, long term cisplatin usage results in drug resistance [144]. To counteract this resistance, very high systemic doses of cisplatin should be administered, which results in severe systemic toxicity and poor patient compliance, limiting its clinical use [144,145,146]. It was shown recently that GNPs can be used to enhance damage caused by platinum-based anticancer drugs [147,148]. Comenge et al. conjugated cisplatin to GNPs and tested the efficacy of this DDS compared to the free drug along, as shown in Figure 7A–C [147]. They found that the use of GNPs led to 300 times more platinum being encapsulated in A549 cells in vitro, and while moving to in vivo, found similar efficacy but largely absent normal tissue toxicity. Yang et al. instead used free cisplatin along with GNPs in a combined chemoradiotherapy modality in vitro, seen in Figure 7D–F [116]. An additive relationship was discovered when treated with GNPs, cisplatin, and radiation in MDA-MB-231 cells. The use of GNPs may be an important avenue to explore when integrating cisplatin into chemoradiation protocols.
Figure 7.
Cisplatin and gold nanoparticles. (A–C) Cisplatin conjugated GNPs lead to an increased deposition of platinum into A549 cells compared to the free drug in vitro, which led to less side effects in vivo with similar efficacy. Scale bar is 4 μm. (D–F) The use of free cisplatin to synergize with GNPs in a chemoradiation modality in vitro lead to a synergistic effect. Scale bar is 40 μm. Reproduced with permission from [116,147]. Copyright Public Library of Science, 2012; Copyright Multidisciplinary Digital Publishing Institute, 2018.
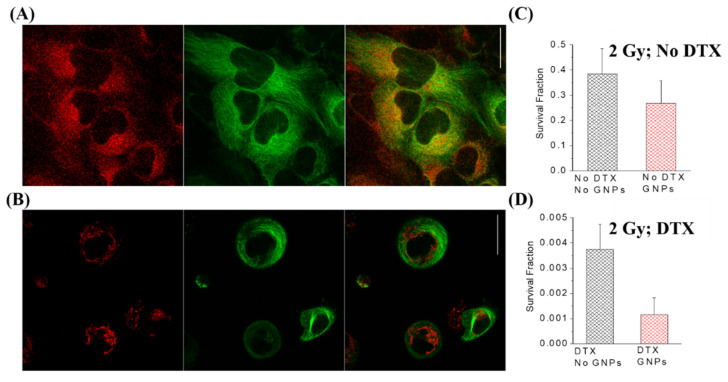
Another chemotherapy agent that is limited due to high normal tissue toxicity is docetaxel (DTX). DTX is a cytotoxic member of the taxanes and is an effective antimicrotubular agent that is effective in the treatment of multiple different types of cancers including head and neck, breast, prostate, and non-small-cell lung cancer [149,150,151,152]. Docetaxel’s mechanism of action is primarily through the ability to enhance microtubule assembly and stabilize free microtubules within the cytoplasm, thus preventing their depolymerization during normal cell division [153]. This has many consequences for the fate of the cell, including inhibition of progression through the cell cycle and inevitably death via mitotic catastrophe, depending on the dose [154]. Francois et al. tested DTX-conjugated GNPs on MCF7 and HCT15 cells and found a 2.5 times more efficient response compared to free DTX [139]. DTX has also been shown to block the cell cycle at the G2/M phase [155]. This is critical because the G2/M phase has shown special sensitivity to the ionizing radiation, causing more cell death [156]. Moreover, by arresting tumor cells in the M phase of the cell cycle, it synergizes the lethal effects of radiotherapy, thereby serving as an ideal radiosensitizer. Both in vitro and in vivo studies have demonstrated the synergistic effects of DTX when combined with radiotherapy [157,158]. Furthermore, it has been shown that the uptake of NPs, including GNPs, is increased when the cell population is synchronized in the G2/M phase [85,159]. This suggests the use of DTX concomitantly with other drugs or radiation, which was tested by Bannister et al. as seen in Figure 8 [160]. DTX used as a synchronizing agent when paired with GNPs lead to higher uptake, a higher localization of the GNPs to the nucleus, and a larger, synergistic response to radiotherapy.
Figure 8.
Docetaxel and gold nanoparticles. (A) Confocal imaging of GNPs (labelled in red) and the microtubule (MT) structure (labelled in green) in HeLa cells. (B) Confocal imaging of GNPs and MTs after treatment with 50 nM of DTX. (C) Radiosensitization of GNPs without DTX. (D) Radiosensitization of GNPs with 50 nM DTX, showing a synergistic effect. Scale bar is 25 μm. Reproduced with permission from [160]. Copyright British Journal of Radiology, 2020.
Normal tissue toxicity is an issue in escalating current dose regimes in many tumors. However, in pancreatic cancer, despite advancements in chemotherapy and radiotherapy, the current 5 year survival rate is only 9% [161]. Gemcitabine is a pyrimidine analog that is a mainstay treatment of adenocarcinoma of the pancreas, but is also used for treatment of bladder and non-small cell lung cancer [162,163,164]. Upon cellular encapsulation, gemcitabine is phosphorylated to its active diphosphate and triphosphate metabolites, which inhibit RR and DNA synthesis, respectively [165]. Despite its prominent use in the clinic, only a small portion of the drug is converted to its active forms. Up to 90% of the injected dose is collected from urine one week after treatment, with 75% of that being in the first 24 h [166]. The use of gemcitabine-conjugated GNPs could be an avenue for both improved uptake of the drug as well as improved efficacy in the very deadly pancreatic disease. It has been shown that 20 nm GNPs by themselves can sensitive pancreatic cell to the effects of gemcitabine by Huai et al. [167]. This is explained by showing that GNPs inhibited epithelial to mesenchymal transition and reduced cancer cell stemness—possible causes of anticancer drug resistance [168]. Furthermore, Pal et al. showed in Figure 9 that gemcitabine-conjugated GNPs that specifically target pancreatic cancer cells with a plectin-1 peptide have improved efficacy in situ compared to the free drug alone [169]. The use of gemcitabine with radiotherapy has also been shown to be more effective than the drug alone through clinical trials [170]. Thus, in the future, the addition of gemcitabine-conjugated GNPs to a radiotherapy protocol may prove highly beneficial.
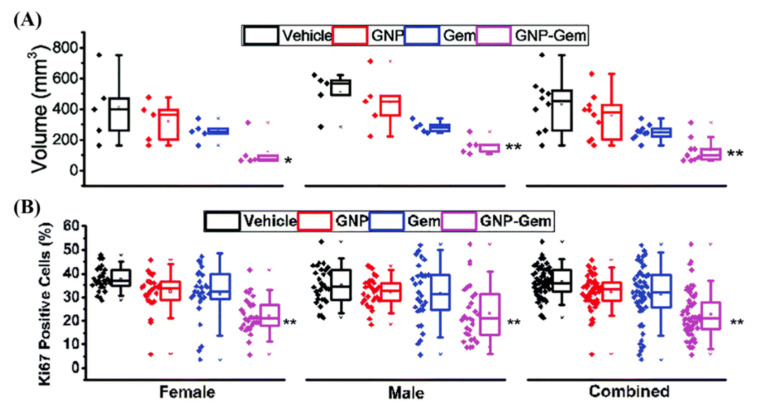
Figure 9.
Gold nanoparticle mediated delivery of Gemcitabine. (A) Gemcitabine-conjugated GNPs showed a significant increase in efficacy when treating mice, as measured through volume. (B) KI67, a marker for proliferation, also showed reduced proliferative cells when treated with the GNP complex compared to the free drug alone, signifying improved efficacy. Black = vehicle, Red = GNP, Blue = Gem and Purple = GNP-Gem. * and ** denote p < 0.05 and p < 0.01 compared to vehicle-treated group respectively. Reproduced with permission from [169]. Copyright Royal Society of Chemistry, 2017.
The use of GNPs as chemotherapeutic DDSs is increasing, and the combination of chemotherapy with radiotherapy remains one of the most effective treatment modalities available in the clinic. A brief summary of recent studies, no older than 2016, involving the use of GNPs with chemotherapy and radiotherapy, can be seen in Table 1. The use of GNPs in combination with anticancer drugs and radiation is still limited in literature; however, the published work thus far shows a trend of improved dose response to the tumor coupled with reduced normal tissue toxicity. Further studies need to be completed, however, before translation to the clinic.
Table 1.
Recent applications of anticancer drugs with gold nanoparticles in drug delivery and combined radiation therapy with clinically relevant energies.
| Nanoparticle Complex | Treatment Parameters | Modality | Experimental Outcomes | Cell Line/Tumor Model | Ref. |
|---|---|---|---|---|---|
| PTX-TNFα-PEG-GNPs | 32.6 nm GNPs; 2.5 mg/kg dose | Chemotherapy | Selective delivery of nanoparticles to tumor and improved efficacy | Ovarian Cancer Cell Line (A2780); B16/F10 tumor-bearing C57BL/6 mice | [133] |
| DOX-PEG-GNPs | 41 nm GNPs; 6 mg/kg dose | Chemotherapy | Dramatically reduced normal tissue toxicity | Ovarian Cancer Cell Line (A2780); CD-1 mice | [138] |
| BLM-DOX-PEG-GNPs | 13 nm GNPs; 10–100 nM dose | Chemotherapy | Cancer cell environment-mediated drug release and improve EC50 | Cervical Cancer Cell Line (HeLa) | [140] |
| CIS-GLC-PEG-GNP | 20 nm GNPs; 10 mg/kg dose; 25 Gy at 6 MV | Chemo-radiotherapy | Similar effect to free cisplatin; dramatically improve result when combined with radiation | Skin Cancer Cell line (A-431); A-431 tumor-bearing mice | [171] |
| DOX@GNPs | 2 nm GNPs; 5 mg/kg dose | Chemotherapy | Efficient renal clearance with effective targeting. Reduced normal tissue toxicity with improved antitumor efficacy | Breast Cancer Cell lines (MCF-7 and MDA-MB-231); Murine Mammary 4T1; CD-1 Mice | [172] |
| PDC-PEG-GNPs | 25–50 nm GNPs; 0–50 µM dose | Chemotherapy | Improved half-life of drug, similar cytotoxicity towards target cells, and active for longer | Murine Lymphoma cells (A20) | [173] |
| Alginate co-loaded with GNPs and CIS | 44 nm NP; 20 µg/mL dose of GNP with 5 µg/mL CIS; 4 Gy at 6 MV | Chemo-radiotherapy with photothermal therapy | ACA and radiotherapy saw improved efficacy over cisplatin and radiation. The addition of photothermal therapy further improved therapeutic results. | Cervical Cancer Cell line (KB) | [174] |
| 5-FU/GSH-GNPs | 9–17 nm GNPs; 0.5–1.5 mg/mL dose | Chemotherapy | Better anticancer effect against the cancer, and reduced drug doses as a result | Colorectal Cancer Cell lines isolated from patients | [175] |
| CS-GNPS-DOX | 21 nm GNPs; 0.05–0.3 mM dose; 0.5, 1, and 3 Gy at 6 MV | Chemo-radiotherapy | Enhanced treatment results including lowered survival fraction, increased apoptosis, and increased DNA damage | Breast Cancer Cell line (MCF-7) | [176] |
| GNP-PEG-RGD; CIS | 10 nm GNPs with 435 nM CIS; 0.3 nM dose; 2 Gy at 6 MV | Chemo-radiotherapy | Improved efficacy of treatment compared to cisplatin and radiation alone | Breast Cancer Cell line (MDA-MB-231) | [116] |
| GNP-PEG-RGD; DTX | 17.2 nm GNPs; 0.2 nM GNPs with 50 nM DTX; 2 Gy at 6 MV | Chemo-radiotherapy | Improved retention of GNPs due to cell synchronicity induced by DTX. Synergistic therapeutic effect found when GNPs and DTX combined | Breast Cancer Cell line (MDA-MB-231) and Cervical Cancer Cell line (HeLa) | [160] |
GNP: Gold Nanoparticle; PAC: Paclitaxel; TNF: Tumor Necrosis Factor; PEG: Polyethylene Glycol; DOX: Doxorubicin; BLM: Bleomycin; CIS: Cisplatin; GLC: Glucose; PDC: Peptide-drug-conjugate containing chlorambucil, melphalan, or bendamustine; 5-FU: 5-Fluorouracil; GSH: Glutathione; CS: Chitosan; RGD: arginyl-glycyl-aspartic acid tripeptide; DTX: Docetaxel
5. Future Considerations
A large issue that is plaguing nanotechnology in general is that a very low (~0.7%) portion of the administered dose is being delivered to the tumor [177]. While Wilhelm et al. describe the issue as improving understanding of the processes present in the body that inhibit the uptake of NPs, and then optimizing those processes, a more personalized approach could be introduced. Personalized medicine involves the analysis of a patient’s genetic code in order to have a better understanding of the potential response to treatment [178]. This is a very important avenue to explore, as it has been estimated that any class of anticancer drug used is ineffective in 75% of patients [179]. This is a result of no two cancers from different patients being the same. Beyond using genomic information to improve each individual’s cancer treatments, the use of an in vitro model that can better mimic the in vivo environment present in a patient may allow for the actual testing of treatment prior to administration. This can be achieved through the use of three-dimensional organoid models [180].
Organoid models have many advantages if implemented into a personalized medicine protocol. The use of a patient’s own cells, as described in Figure 10 by Fan et al., allows for the maintenance of the heterogeneity present [181]. Furthermore, normal spheroids can be engineered to have similar genetic alterations present in a patient, as discovered using their genomic information, through the use of gene-editing [182]. There are many other advantages as well, including low-cost generation, and quick (~4 weeks) results can be obtained. The capability of organoids to enable drug screening in an in vitro environment is being widely explored [180]. Furthermore, the use of organoid models has recently been tested on a chemoradiotherapy treatment of advanced rectal cancer and was able to accurately predict the response [183]. In the future, the use of organoids to screen chemoradiotherapy protocols with GNPs may enable an accurate assessment of response and allow for tailored, personalized medical care.
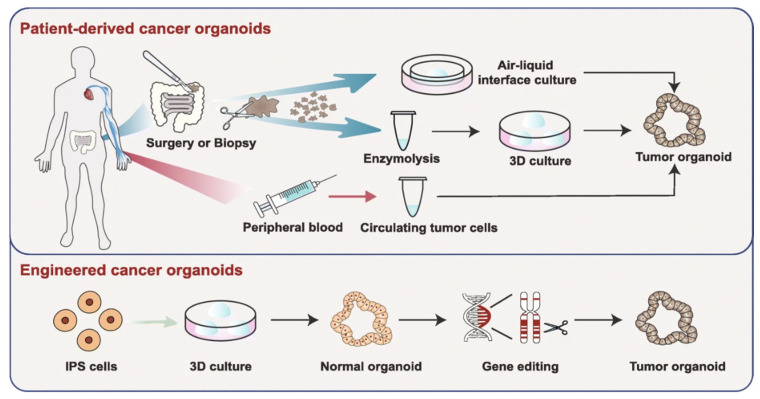
Figure 10.
Organoid models toward personalized medicine. Patient-derived cancer organoids can be derived from surgically resected/biopsied tissues and circulating tumor cells. Furthermore, using gene-editing, normal spheroids can be mutated into tumor organoids. Reproduced with permission from [180]. Copyright Springer Nature, 2019.
Spheroids, and patient derived organoids, should be seen as avascular tumors, with limitations. To move towards personalized medical care with GNPs—using organoids as an assessment tool—certain strategies will have to be employed and obstacles overcome [184]. First, many more preclinical studies will have to be undertaken involving the use of GNPs with spheroids and their ability to accurately predict tumor response. This will have to be a large expanse of research, with many different types of treatment options including chemotherapy and radiotherapy as well as combined modalities. Second, scalability is very important: a high throughput method of testing efficacy of drugs and radiation modalities will have to be elucidated, as well as producing the organoids in a large-scale manner. Work is under way to improve production of spheroids as well as protocols for high throughput drug and radiation testing [185,186,187]. However, translation to the clinic will require more work at the bio–nano interface.
The use of GNPs in this workflow has limited published work, with most research focusing on individual treatment modalities, and not overall high throughput methods for translation to personalized medicine. Towards this, cheap and efficient GNP systems that can be easily functionalized with various moieties such as anticancer drugs or targeting ligands need to be designed for mass-scale production. Furthermore, comparisons of GNP-treated spheroids and organoids with in vivo models must be undertaken for improved confidence for translation to the clinic. Finally, it must be accepted the spheroids are a simplistic model when compared to an in vivo environment and will not be able to predict everything. However, despite this, the use of GNPs with organoid models for personalized medicine may be able to help save lives and improve the quality of lives in the future.
6. Conclusions
In the pursuit of improved cancer therapeutics, the use of GNPs offers the potential of improving on many different facets of the treatment process. Despite progress, the translation of GNPs to clinical practice has been limited due to the lack of coordination between researchers and clinicians. Many advances covered in this review aim to address issues that have arisen in the past, including targeted therapy, and the combination of radiotherapy and chemotherapy paired with GNPs for improved efficacy. However, it is still important to improve upon the current research so that translation to the clinic can be expedited.
Acknowledgments
The authors would like to acknowledge Canada Foundation for Innovation (CFI), the British Columbia government, Natural Sciences and Engineering Research Council of Canada (NSERC), British Columbia Cancer, Vancouver Island (BCC), Centre for Advanced Materials and Related Technologies (CAMTEC), and University of Victoria for their financial support.
Author Contributions
All authors have made substantial contributions in preparation of the manuscript. All authors agreed to be personally accountable for the author’s own contributions and to ensure that the questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution agreement published in the literature. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Natural Sciences and Engineering Research Council of Canada (NSERC), grant number 418453.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Ruddon R.W. Cancer Biology. 4th ed. Oxford University Press; Oxford, UK: 2007. [Google Scholar]
- 2.Treatment-Canadian Cancer Society. [(accessed on 6 August 2020)]; Available online: https://www.cancer.ca/en/cancer-information/diagnosis-and-treatment/treatment/?region=on.
- 3.Types of Cancer Treatment-National Cancer Institute. [(accessed on 6 August 2020)]; Available online: https://www.cancer.gov/about-cancer/treatment/types.
- 4.Joiner M.C., van der Kogel A.J. Basic Clinical Radiobiology. 5th ed. CRC Press; Boca Raton, FL, USA: 2018. [Google Scholar]
- 5.Podgorsak E.B. Radiation Oncology Physics: A Handbook for Teachers and Students. International Atomic Energy Agency; Vienna, Austria: 2003. [Google Scholar]
- 6.Podgoršak E.B. Radiation Physics for Medical Physicists. Springer International Publishing; Cham, Switzerland: 2016. [Google Scholar]
- 7.Delaney G.P., Barton M.B. Evidence-based Estimates of the Demand for Radiotherapy. Clin. Oncol. 2015 doi: 10.1016/j.clon.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Herscher L.L., Cook J.A., Pacelli R., Pass H.I., Russo A., Mitchell J.B. Principles of chemoradiation: Theoretical and practical considerations. Oncology. 1999;13:11–22. [PubMed] [Google Scholar]
- 9.Tannock I.F., Hill R.P., Bristow R.G., Harrington L. Basic Science of Oncology. 5th ed. McGraw-Hill Education; Beijing, China: 2005. [Google Scholar]
- 10.Hanahan D., Weinberg R.A. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Crawford S. Is it time for a new paradigm for systemic cancer treatment? Lessons from a century of cancer chemotherapy. Front. Pharmacol. 2013:68. doi: 10.3389/fphar.2013.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jain R.K. Transport of molecules, particles, and cells in solid tumors. Annu. Rev. Biomed. Eng. 1999;1:241–263. doi: 10.1146/annurev.bioeng.1.1.241. [DOI] [PubMed] [Google Scholar]
- 13.Georgelin T., Bombard S., Siaugue J.-M., Cabuil V. Nanoparticle-Mediated Delivery of Bleomycin. Angew. Chem. Int. Ed. 2010;49:8897–8901. doi: 10.1002/anie.201003316. [DOI] [PubMed] [Google Scholar]
- 14.Strebhardt K., Ullrich A. Paul Ehrlich’s magic bullet concept: 100 Years of progress. Nat. Rev. Cancer. 2008;8:473–480. doi: 10.1038/nrc2394. [DOI] [PubMed] [Google Scholar]
- 15.Davis M.E., Chen Z., Shin D.M. Nanoparticle therapeutics: An emerging treatment modality for cancer. Nat. Rev. Drug Discov. 2008;7:771–782. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 16.Lytton-Jean A.K.R., Kauffman K.J., Kaczmarek J.C., Langer R. Cancer nanotherapeutics in clinical trials. Cancer Treat. Res. 2015;166:293–322. doi: 10.1007/978-3-319-16555-4_13. [DOI] [PubMed] [Google Scholar]
- 17.Zhang L., Gu F., Chan J., Wang A., Langer R., Farokhzad O. Nanoparticles in Medicine: Therapeutic Applications and Developments. Clin. Pharmacol. Ther. 2008;83:761–769. doi: 10.1038/sj.clpt.6100400. [DOI] [PubMed] [Google Scholar]
- 18.Retif P., Pinel S., Toussaint M., Frochot C., Chouikrat R., Bastogne T., Barberi-Heyob M. Nanoparticles for radiation therapy enhancement: The key parameters. Theranostics. 2015;5:1030–1044. doi: 10.7150/thno.11642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Z., Berg A., Levanon H., Fessenden R.W., Meisel D. On the interactions of free radicals with gold nanoparticles. J. Am. Chem. Soc. 2003;125:7959–7963. doi: 10.1021/ja034830z. [DOI] [PubMed] [Google Scholar]
- 20.Zheng Y., Sanche L. Low Energy Electrons in Nanoscale Radiation Physics: Relationship to Radiosensitization and Chemoradiation Therapy. Rev. Nanosci. Nanotechnol. 2013;2:1–28. doi: 10.1166/rnn.2013.1022. [DOI] [Google Scholar]
- 21.Townley H.E., Kim J., Dobson P.J. In vivo demonstration of enhanced radiotherapy using rare earth doped titania nanoparticles. Nanoscale. 2012;4:5043–5050. doi: 10.1039/c2nr30769c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mirjolet C., Papa A.L., Créhange G., Raguin O., Seignez C., Paul C., Truc G., Maingon P., Millot N. The radiosensitization effect of titanate nanotubes as a new tool in radiation therapy for glioblastoma: A proof-of-concept. Radiother. Oncol. 2013;108:136–142. doi: 10.1016/j.radonc.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi J., Misawa M. Analysis of potential radiosensitizing materials for x-ray-induced photodynamic therapy. Nanobiotechnology. 2007;3:116–126. doi: 10.1007/s12030-008-9009-x. [DOI] [Google Scholar]
- 24.Yang W., Read P.W., Mi J., Baisden J.M., Reardon K.A., Larner J.M., Helmke B.P., Sheng K. Semiconductor Nanoparticles as Energy Mediators for Photosensitizer-Enhanced Radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2008;72:633–635. doi: 10.1016/j.ijrobp.2008.06.1916. [DOI] [PubMed] [Google Scholar]
- 25.Chithrani D.B., Jelveh S., Jalali F., Van Prooijen M., Allen C., Bristow R.G., Hill R.P., Jaffray D.A. Gold nanoparticles as radiation sensitizers in cancer therapy. Radiat. Res. 2010 doi: 10.1667/RR1984.1. [DOI] [PubMed] [Google Scholar]
- 26.Le Duc G., Miladi I., Alric C., Mowat P., Bräuer-Krisch E., Bouchet A., Khalil E., Billotey C., Janier M., Lux F., et al. Toward an image-guided microbeam radiation therapy using gadolinium-based nanoparticles. ACS Nano. 2011;5:9566–9574. doi: 10.1021/nn202797h. [DOI] [PubMed] [Google Scholar]
- 27.Liu P., Huang Z., Chen Z., Xu R., Wu H., Zang F., Wang C., Gu N. Silver nanoparticles: A novel radiation sensitizer for glioma? Nanoscale. 2013;5:11829–11836. doi: 10.1039/c3nr01351k. [DOI] [PubMed] [Google Scholar]
- 28.Hainfeld J.F., Dilmanian F.A., Slatkin D.N., Smilowitz H.M. Radiotherapy enhancement with gold nanoparticles. J. Pharm. Pharmacol. 2008;60:977–985. doi: 10.1211/jpp.60.8.0005. [DOI] [PubMed] [Google Scholar]
- 29.Hainfeld J.F., Slatkin D.N., Smilowitz H.M. The use of gold nanoparticles to enhance radiotherapy in mice. Phys. Med. Biol. 2004 doi: 10.1088/0031-9155/49/18/N03. [DOI] [PubMed] [Google Scholar]
- 30.Zheng Y., Sanche L. Gold nanoparticles enhance DNA damage induced by anti-cancer drugs and radiation. Radiat. Res. 2009 doi: 10.1667/RR1689.1. [DOI] [PubMed] [Google Scholar]
- 31.Yeh Y.C., Creran B., Rotello V.M. Gold nanoparticles: Preparation, properties, and applications in bionanotechnology. Nanoscale. 2012;4:1871–1880. doi: 10.1039/C1NR11188D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stiufiuc R., Iacovita C., Nicoara R., Stiufiuc G., Florea A., Achim M., Lucaciu C.M. One-step synthesis of PEGylated gold nanoparticles with tunable surface charge. J. Nanomater. 2013 doi: 10.1155/2013/146031. [DOI] [Google Scholar]
- 33.Sztandera K., Gorzkiewicz M., Klajnert-Maculewicz B. Gold Nanoparticles in Cancer Treatment. Mol. Pharm. 2018;16:1–23. doi: 10.1021/acs.molpharmaceut.8b00810. [DOI] [PubMed] [Google Scholar]
- 34.Huff T.B., Tong L., Zhao Y., Hansen M.N., Cheng J.X., Wei A. Hyperthermic effects of gold nanorods on tumor cells. Nanomedicine. 2007 doi: 10.2217/17435889.2.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rastinehad A.R., Anastos H., Wajswol E., Winoker J.S., Sfakianos J.P., Doppalapudi S.K., Carrick M.R., Knauer C.J., Taouli B., Lewis S.C., et al. Gold nanoshell-localized photothermal ablation of prostate tumors in a clinical pilot device study. Proc. Natl. Acad. Sci. USA. 2019 doi: 10.1073/pnas.1906929116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vines J.B., Yoon J.H., Ryu N.E., Lim D.J., Park H. Gold nanoparticles for photothermal cancer therapy. Front. Chem. 2019;7 doi: 10.3389/fchem.2019.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abadeer N.S., Murphy C.J. Recent Progress in Cancer Thermal Therapy Using Gold Nanoparticles. J. Phys. Chem. C. 2016;120:4691–4716. doi: 10.1021/acs.jpcc.5b11232. [DOI] [Google Scholar]
- 38.Mendes R., Pedrosa P., Lima J.C., Fernandes A.R., Baptista P.V. Photothermal enhancement of chemotherapy in breast cancer by visible irradiation of Gold Nanoparticles. Sci. Rep. 2017 doi: 10.1038/s41598-017-11491-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ash C., Dubec M., Donne K., Bashford T. Effect of wavelength and beam width on penetration in light-tissue interaction using computational methods. Lasers Med. Sci. 2017 doi: 10.1007/s10103-017-2317-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang J., Wang F., Yuan H., Zhang L., Jiang Y., Zhang X., Liu C., Chai L., Li H., Stenzel M. Recent advances in ultra-small fluorescent Au nanoclusters toward oncological research. Nanoscale. 2019;11:17967–17980. doi: 10.1039/C9NR04301B. [DOI] [PubMed] [Google Scholar]
- 41.Porret E., Le Guével X., Coll J.L. Gold nanoclusters for biomedical applications: Toward: In vivo studies. J. Mater. Chem. B. 2020;8:2216–2232. doi: 10.1039/C9TB02767J. [DOI] [PubMed] [Google Scholar]
- 42.Bouché M., Hsu J.C., Dong Y.C., Kim J., Taing K., Cormode D.P. Recent Advances in Molecular Imaging with Gold Nanoparticles. Bioconjug. Chem. 2020;31:303–314. doi: 10.1021/acs.bioconjchem.9b00669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Loynachan C.N., Soleimany A.P., Dudani J.S., Lin Y., Najer A., Bekdemir A., Chen Q., Bhatia S.N., Stevens M.M. Renal clearable catalytic gold nanoclusters for in vivo disease monitoring. Nat. Nanotechnol. 2019 doi: 10.1038/s41565-019-0527-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bromma K., Rieck K., Kulkarni J., O’Sullivan C., Sung W., Cullis P., Schuemann J., Chithrani D.B. Use of a lipid nanoparticle system as a Trojan horse in delivery of gold nanoparticles to human breast cancer cells for improved outcomes in radiation therapy. Cancer Nanotechnol. 2019 doi: 10.1186/s12645-019-0046-z. [DOI] [Google Scholar]
- 45.Mathiyazhakan M., Wiraja C., Xu C. A Concise Review of Gold Nanoparticles-Based Photo-Responsive Liposomes for Controlled Drug Delivery. Nano-Micro Lett. 2018 doi: 10.1007/s40820-017-0166-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chithrani D.B. Optimization of Bio-Nano Interface Using Gold Nanostructures as a Model Nanoparticle System. Insci. J. 2011 doi: 10.5640/insc.0103115. [DOI] [Google Scholar]
- 47.Libutti S.K., Paciotti G.F., Byrnes A.A., Alexander H.R., Gannon W.E., Walker M., Seidel G.D., Yuldasheva N., Tamarkin L. Phase I and pharmacokinetic studies of CYT-6091, a novel PEGylated colloidal gold-rhTNF nanomedicine. Clin. Cancer Res. 2010 doi: 10.1158/1078-0432.CCR-10-0978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clinicaltrials Pilot Study of AuroLase(tm) Therapy in Refractory and/or Recurrent Tumors of the Head and Neck. U.S. National Library of Medicine; Bethesda, MD, USA: Goodyear; Akron, OH, USA: 2010. [Google Scholar]
- 49.Nanospectra Biosciences Inc . Efficacy Study of AuroLase Therapy in Subjects with Primary and/or Metastatic Lung Tumors. U.S. National Library of Medicine; Philadelphia, PA, USA: 2016. [Google Scholar]
- 50.Nanospectra Biosciences Inc . MRI/US Fusion Imaging and Biopsy in Combination With Nanoparticle Directed Focal Therapy for Ablation of Prostate Tissue. U.S. National Library of Medicine; Baltimore, MD, USA: 2016. [Google Scholar]
- 51.Northwesten Universty . NU-0129 in Treating Patients With Recurrent Glioblastoma or Gliosarcoma Undergoing Surgery. U.S. National Library of Medicine; Chicago, IL, USA: 2019. [Google Scholar]
- 52.Schuemann J., Bagley A., Berbeco R., Bromma K., Butterworth K.T., Byrne H., Chithrani D.B., Cho S.H., Cook J.R., Favaudon V., et al. Roadmap for metal nanoparticles in radiation therapy: Current status, translational challenges, and future directions. Phys. Med. Biol. 2020 doi: 10.1088/1361-6560/ab9159. [DOI] [PubMed] [Google Scholar]
- 53.Rubin P., Carter S.K. Combination Radiation Therapy and Chemotherapy: A Logical Basis for Their Clinical Use. CA. Cancer J. Clin. 1976 doi: 10.3322/canjclin.26.5.274. [DOI] [PubMed] [Google Scholar]
- 54.Sharifi M., Attar F., Saboury A.A., Akhtari K., Hooshmand N., Hasan A., El-Sayed M.A., Falahati M. Plasmonic gold nanoparticles: Optical manipulation, imaging, drug delivery and therapy. J. Control. Release. 2019;311:170–189. doi: 10.1016/j.jconrel.2019.08.032. [DOI] [PubMed] [Google Scholar]
- 55.Dykman L.A., Khlebtsov N.G. Gold nanoparticles in chemo-, immuno-, and combined therapy: Review [Invited] Biomed. Opt. Express. 2019 doi: 10.1364/BOE.10.003152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beik J., Khateri M., Khosravi Z., Kamrava S.K., Kooranifar S., Ghaznavi H., Shakeri-Zadeh A. Gold nanoparticles in combinatorial cancer therapy strategies. Coord. Chem. Rev. 2019;387:299–324. doi: 10.1016/j.ccr.2019.02.025. [DOI] [Google Scholar]
- 57.Elahi N., Kamali M., Baghersad M.H. Recent biomedical applications of gold nanoparticles: A review. Talanta. 2018;184:537–556. doi: 10.1016/j.talanta.2018.02.088. [DOI] [PubMed] [Google Scholar]
- 58.Riley R.S., Day E.S. Gold nanoparticle-mediated photothermal therapy: Applications and opportunities for multimodal cancer treatment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017;9:e1449. doi: 10.1002/wnan.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Siddique S., Chow J.C.L. Gold nanoparticles for drug delivery and cancer therapy. Appl. Sci. 2020;10:3824. doi: 10.3390/app10113824. [DOI] [Google Scholar]
- 60.Her S., Jaffray D.A., Allen C. Gold nanoparticles for applications in cancer radiotherapy: Mechanisms and recent advancements. Adv. Drug Deliv. Rev. 2017;109:84–101. doi: 10.1016/j.addr.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 61.Kong F.Y., Zhang J.W., Li R.F., Wang Z.X., Wang W.J., Wang W. Unique roles of gold nanoparticles in drug delivery, targeting and imaging applications. Molecules. 2017;22:1445. doi: 10.3390/molecules22091445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Citrin D.E. Recent developments in radiotherapy. N. Engl. J. Med. 2017;377:1065–1075. doi: 10.1056/NEJMra1608986. [DOI] [PubMed] [Google Scholar]
- 63.Foroozandeh P., Aziz A.A. Insight into Cellular Uptake and Intracellular Trafficking of Nanoparticles. Nanoscale Res. Lett. 2018;13 doi: 10.1186/s11671-018-2728-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chithrani B.D., Stewart J., Allen C., Jaffray D.A. Intracellular uptake, transport, and processing of nanostructures in cancer cells. Nanomed. Nanotechnol. Biol. Med. 2009 doi: 10.1016/j.nano.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 65.Chithrani D.B. Intracellular uptake, transport, and processing of gold nanostructures. Mol. Membr. Biol. 2010;27:299–311. doi: 10.3109/09687688.2010.507787. [DOI] [PubMed] [Google Scholar]
- 66.Chithrani B.D., Ghazani A.A., Chan W.C.W. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006 doi: 10.1021/nl052396o. [DOI] [PubMed] [Google Scholar]
- 67.Gao H., Shi W., Freund L.B. Mechanics of receptor-mediated endocytosis. Proc. Natl. Acad. Sci. USA. 2005 doi: 10.1073/pnas.0503879102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jin H., Heller D.A., Sharma R., Strano M.S. Size-dependent cellular uptake and expulsion of single-walled carbon nanotubes: Single particle tracking and a generic uptake model for nanoparticles. ACS Nano. 2009 doi: 10.1021/nn800532m. [DOI] [PubMed] [Google Scholar]
- 69.Jin H., Heller D.A., Strano M.S. Single-particle tracking of endocytosis and exocytosis of single-walled carbon nanotubes in NIH-3T3 cells. Nano Lett. 2008 doi: 10.1021/nl072969s. [DOI] [PubMed] [Google Scholar]
- 70.Ma X., Wu Y., Jin S., Tian Y., Zhang X., Zhao Y., Yu L., Liang X.J. Gold nanoparticles induce autophagosome accumulation through size-dependent nanoparticle uptake and lysosome impairment. ACS Nano. 2011 doi: 10.1021/nn202155y. [DOI] [PubMed] [Google Scholar]
- 71.Liu M., Li Q., Liang L., Li J., Wang K., Li J., Lv M., Chen N., Song H., Lee J., et al. Real-Time visualization of clustering and intracellular transport of gold nanoparticles by correlative imaging. Nat. Commun. 2017 doi: 10.1038/ncomms15646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chithrani B.D., Chan W.C.W. Elucidating the mechanism of cellular uptake and removal of protein-coated gold nanoparticles of different sizes and shapes. Nano Lett. 2007 doi: 10.1021/nl070363y. [DOI] [PubMed] [Google Scholar]
- 73.Bednarski M., Dudek M., Knutelska J., Nowiński L., Sapa J., Zygmunt M., Nowak G., Luty-Błocho M., Wojnicki M., Fitzner K., et al. The influence of the route of administration of gold nanoparticles on their tissue distribution and basic biochemical parameters: In vivo studies. Pharmacol. Rep. 2015 doi: 10.1016/j.pharep.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 74.Zhang X.D., Wu H.Y., Wu D., Wang Y.Y., Chang J.H., Zhai Z.B., Meng A.M., Liu P.X., Zhang L.A., Fan F.Y. Toxicologic effects of gold nanoparticles in vivo by different administration routes. Int. J. Nanomed. 2010 doi: 10.2147/IJN.S8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nie S. Editorial: Understanding and overcoming major barriers in cancer nanomedicine. Nanomedicine. 2010;5:523–528. doi: 10.2217/nnm.10.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cruje C., Yang C., Uertz J., Van Prooijen M., Chithrani B.D. Optimization of PEG coated nanoscale gold particles for enhanced radiation therapy. RSC Adv. 2015 doi: 10.1039/C5RA19104A. [DOI] [Google Scholar]
- 77.Cruje C., Chithrani D.B. Polyethylene Glycol Functionalized Nanoparticles for Improved Cancer Treatment. Rev. Nanosci. Nanotechnol. 2014 doi: 10.1166/rnn.2014.1042. [DOI] [Google Scholar]
- 78.Tenzer S., Docter D., Kuharev J., Musyanovych A., Fetz V., Hecht R., Schlenk F., Fischer D., Kiouptsi K., Reinhardt C., et al. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nat. Nanotechnol. 2013 doi: 10.1038/nnano.2013.181. [DOI] [PubMed] [Google Scholar]
- 79.Carnovale C., Bryant G., Shukla R., Bansal V. Metal Nanoparticles in Pharma. Springer International Publishing; Cham, Switzerland: 2017. Gold nanoparticle biodistribution and toxicity: Role of biological corona in relation with nanoparticle characteristics. [Google Scholar]
- 80.Yang C., Bromma K., Chithrani D. Peptide mediated in vivo tumor targeting of nanoparticles through optimization in single and multilayer in vitro cell models. Cancers (Basel) 2018;10:84. doi: 10.3390/cancers10030084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Manson J., Kumar D., Meenan B.J., Dixon D. Polyethylene glycol functionalized gold nanoparticles: The influence of capping density on stability in various media. Gold Bull. 2011 doi: 10.1007/s13404-011-0015-8. [DOI] [Google Scholar]
- 82.Zhang G., Yang Z., Lu W., Zhang R., Huang Q., Tian M., Li L., Liang D., Li C. Influence of anchoring ligands and particle size on the colloidal stability and in vivo biodistribution of polyethylene glycol-coated gold nanoparticles in tumor-xenografted mice. Biomaterials. 2009 doi: 10.1016/j.biomaterials.2008.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Milton Harris J., Chess R.B. Effect of pegylation on pharmaceuticals. Nat. Rev. Drug Discov. 2003;2:214–221. doi: 10.1038/nrd1033. [DOI] [PubMed] [Google Scholar]
- 84.Yin H.Q., Bi F.L., Gan F. Rapid synthesis of cyclic RGD conjugated gold nanoclusters for targeting and fluorescence imaging of melanoma A375 cells. Bioconjug. Chem. 2015 doi: 10.1021/bc500505c. [DOI] [PubMed] [Google Scholar]
- 85.Rieck K., Bromma K., Sung W., Bannister A., Schuemann J., Chithrani D.B. Modulation of gold nanoparticle mediated radiation dose enhancement through synchronization of breast tumor cell population. Br. J. Radiol. 2019;92 doi: 10.1259/bjr.20190283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cho W.S., Cho M., Jeong J., Choi M., Han B.S., Shin H.S., Hong J., Chung B.H., Jeong J., Cho M.H. Size-dependent tissue kinetics of PEG-coated gold nanoparticles. Toxicol. Appl. Pharmacol. 2010 doi: 10.1016/j.taap.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 87.Li X., Hu Z., Ma J., Wang X., Zhang Y., Wang W., Yuan Z. The systematic evaluation of size-dependent toxicity and multi-time biodistribution of gold nanoparticles. Colloids Surf. B Biointerfaces. 2018 doi: 10.1016/j.colsurfb.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 88.Fan M., Han Y., Gao S., Yan H., Cao L., Li Z., Liang X.J., Zhang J. Ultrasmall gold nanoparticles in cancer diagnosis and therapy. Theranostics. 2020;10:494–4957. doi: 10.7150/thno.42471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bugno J., Poellmann M.J., Sokolowski K., Hsu H.J., Kim D.H., Hong S. Tumor penetration of Sub-10 nm nanoparticles: Effect of dendrimer properties on their penetration in multicellular tumor spheroids. Nanomed. Nanotechnol. Biol. Med. 2019 doi: 10.1016/j.nano.2019.102059. [DOI] [PubMed] [Google Scholar]
- 90.Jing X., Yang F., Shao C., Wei K., Xie M., Shen H., Shu Y. Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol. Cancer. 2019;18:157. doi: 10.1186/s12943-019-1089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kharazian B., Lohse S.E., Ghasemi F., Raoufi M., Saei A.A., Hashemi F., Farvadi F., Alimohamadi R., Jalali S.A., Shokrgozar M.A., et al. Bare surface of gold nanoparticle induces inflammation through unfolding of plasma fibrinogen. Sci. Rep. 2018 doi: 10.1038/s41598-018-30915-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Elci S.G., Jiang Y., Yan B., Kim S.T., Saha K., Moyano D.F., Yesilbag Tonga G., Jackson L.C., Rotello V.M., Vachet R.W. Surface Charge Controls the Suborgan Biodistributions of Gold Nanoparticles. ACS Nano. 2016 doi: 10.1021/acsnano.6b02086. [DOI] [PubMed] [Google Scholar]
- 93.Riviere J.E., Jaberi-Douraki M., Lillich J., Azizi T., Joo H., Choi K., Thakkar R., Monteiro-Riviere N.A. Modeling gold nanoparticle biodistribution after arterial infusion into perfused tissue: Effects of surface coating, size and protein corona. Nanotoxicology. 2018 doi: 10.1080/17435390.2018.1476986. [DOI] [PubMed] [Google Scholar]
- 94.Geng F., Xing J.Z., Chen J., Yang R., Hao Y., Song K., Kong B. Pegylated glucose gold nanoparticles for improved in-vivo bio-distribution and enhanced radiotherapy on cervical cancer. J. Biomed. Nanotechnol. 2014 doi: 10.1166/jbn.2014.1855. [DOI] [PubMed] [Google Scholar]
- 95.Harding J., Burtness B. Cetuximab: An epidermal growth factor receptor chimeric human-murine monoclonal antibody. Drugs Today. 2005;41:107–127. doi: 10.1358/dot.2005.41.2.882662. [DOI] [PubMed] [Google Scholar]
- 96.Kao H.W., Lin Y.Y., Chen C.C., Chi K.H., Tien D.C., Hsia C.C., Lin W.J., Chen F.D., Lin M.H., Wang H.E. Biological characterization of cetuximab-conjugated gold nanoparticles in a tumor animal model. Nanotechnology. 2014 doi: 10.1088/0957-4484/25/29/295102. [DOI] [PubMed] [Google Scholar]
- 97.Ni X., Castanares M., Mukherjee A., Lupold S.E. Nucleic Acid Aptamers: Clinical Applications and Promising New Horizons. Curr. Med. Chem. 2011 doi: 10.2174/092986711797189600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jo H., Ban C. Aptamer-nanoparticle complexes as powerful diagnostic and therapeutic tools. Exp. Mol. Med. 2016;5 doi: 10.1038/emm.2016.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Byrne J.D., Betancourt T., Brannon-Peppas L. Active targeting schemes for nanoparticle systems in cancer therapeutics. Adv. Drug Deliv. Rev. 2008;15:1617–1626. doi: 10.1016/j.addr.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 100.Choi C.H.J., Alabi C.A., Webster P., Davis M.E. Mechanism of active targeting in solid tumors with transferrin-containing gold nanoparticles. Proc. Natl. Acad. Sci. USA. 2010 doi: 10.1073/pnas.0914140107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang Z., Jia J., Lai Y., Ma Y., Weng J., Sun L. Conjugating folic acid to gold nanoparticles through glutathione for targeting and detecting cancer cells. Bioorg. Med. Chem. 2010;18:5528–5534. doi: 10.1016/j.bmc.2010.06.045. [DOI] [PubMed] [Google Scholar]
- 102.Charbgoo F., Nejabat M., Abnous K., Soltani F., Taghdisi S.M., Alibolandi M., Thomas Shier W., Steele T.W.J., Ramezani M. Gold nanoparticle should understand protein corona for being a clinical nanomaterial. J. Control. Release. 2018;272:39–53. doi: 10.1016/j.jconrel.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 103.de Carvalho T.G., Garcia V.B., de Araújo A.A., da Silva Gasparotto L.H., Silva H., Guerra G.C.B., de Castro Miguel E., de Carvalho Leitão R.F., da Silva Costa D.V., Cruz L.J., et al. Spherical neutral gold nanoparticles improve anti-inflammatory response, oxidative stress and fibrosis in alcohol-methamphetamine-induced liver injury in rats. Int. J. Pharm. 2018 doi: 10.1016/j.ijpharm.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 104.Shukla R., Bansal V., Chaudhary M., Basu A., Bhonde R.R., Sastry M. Biocompatibility of gold nanoparticles and their endocytotic fate inside the cellular compartment: A microscopic overview. Langmuir. 2005;23:10644–10654. doi: 10.1021/la0513712. [DOI] [PubMed] [Google Scholar]
- 105.Connor E.E., Mwamuka J., Gole A., Murphy C.J., Wyatt M.D. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small. 2005 doi: 10.1002/smll.200400093. [DOI] [PubMed] [Google Scholar]
- 106.Steckiewicz K.P., Barcinska E., Malankowska A., Zauszkiewicz–Pawlak A., Nowaczyk G., Zaleska-Medynska A., Inkielewicz-Stepniak I. Impact of gold nanoparticles shape on their cytotoxicity against human osteoblast and osteosarcoma in in vitro model. Evaluation of the safety of use and anti-cancer potential. J. Mater. Sci. Mater. Med. 2019 doi: 10.1007/s10856-019-6221-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang X.D., Wu D., Shen X., Chen J., Sun Y.M., Liu P.X., Liang X.J. Size-dependent radiosensitization of PEG-coated gold nanoparticles for cancer radiation therapy. Biomaterials. 2012 doi: 10.1016/j.biomaterials.2012.05.047. [DOI] [PubMed] [Google Scholar]
- 108.Fratoddi I., Venditti I., Cametti C., Russo M.V. How toxic are gold nanoparticles? The state-of-the-art. Nano Res. 2015 doi: 10.1007/s12274-014-0697-3. [DOI] [Google Scholar]
- 109.Jia Y.P., Ma B.Y., Wei X.W., Qian Z.Y. The in vitro and in vivo toxicity of gold nanoparticles. Chin. Chem. Lett. 2017 doi: 10.1016/j.cclet.2017.01.021. [DOI] [Google Scholar]
- 110.Haume K., Rosa S., Grellet S., Śmiałek M.A., Butterworth K.T., Solov’yov A.V., Prise K.M., Golding J., Mason N.J. Gold nanoparticles for cancer radiotherapy: A review. Cancer Nanotechnol. 2016;7 doi: 10.1186/s12645-016-0021-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nath R., Bongiorni P., Rockwell S. Iododeoxyuridine radiosensitization by low- and high-energy photons for brachytherapy dose rates. Radiat. Res. 1990 doi: 10.2307/3577836. [DOI] [PubMed] [Google Scholar]
- 112.Matsudaira H., Ueno A.M., Furuno I. Iodine contrast medium sensitizes cultured mammalian cells to X rays but not to γ rays. Radiat. Res. 1980 doi: 10.2307/3575225. [DOI] [PubMed] [Google Scholar]
- 113.Xie W.Z., Friedland W., Li W.B., Li C.Y., Oeh U., Qiu R., Li J.L., Hoeschen C. Simulation on the molecular radiosensitization effect of gold nanoparticles in cells irradiated by x-rays. Phys. Med. Biol. 2015 doi: 10.1088/0031-9155/60/16/6195. [DOI] [PubMed] [Google Scholar]
- 114.Khoo A.M., Cho S.H., Reynoso F.J., Aliru M., Aziz K., Bodd M., Yang X., Ahmed M.F., Yasar S., Manohar N., et al. Radiosensitization of Prostate Cancers in Vitro and in Vivo to Erbium-filtered Orthovoltage X-rays Using Actively Targeted Gold Nanoparticles. Sci. Rep. 2017 doi: 10.1038/s41598-017-18304-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yang C., Bromma K., Di Ciano-Oliveira C., Zafarana G., van Prooijen M., Chithrani D.B. Gold nanoparticle mediated combined cancer therapy. Cancer Nanotechnol. 2018 doi: 10.1186/s12645-018-0039-3. [DOI] [Google Scholar]
- 116.Yang C., Bromma K., Sung W., Schuemann J., Chithrani D. Determining the radiation enhancement effects of gold nanoparticles in cells in a combined treatment with cisplatin and radiation at therapeutic megavoltage energies. Cancers (Basel) 2018;10:150. doi: 10.3390/cancers10050150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wolfe T., Chatterjee D., Lee J., Grant J.D., Bhattarai S., Tailor R., Goodrich G., Nicolucci P., Krishnan S. Targeted gold nanoparticles enhance sensitization of prostate tumors to megavoltage radiation therapy in vivo. Nanomed. Nanotechnol. Biol. Med. 2015 doi: 10.1016/j.nano.2014.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rosa S., Connolly C., Schettino G., Butterworth K.T., Prise K.M. Biological mechanisms of gold nanoparticle radiosensitization. Cancer Nanotechnol. 2017;8 doi: 10.1186/s12645-017-0026-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mikami Y., Dhakshinamoorthy A., Alvaro M., García H. Catalytic activity of unsupported gold nanoparticles. Catal. Sci. Technol. 2013;3:58–69. doi: 10.1039/C2CY20068F. [DOI] [Google Scholar]
- 120.Nel A., Xia T., Mädler L., Li N. Toxic potential of materials at the nanolevel. Science. 2006;311:622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- 121.Misawa M., Takahashi J. Generation of reactive oxygen species induced by gold nanoparticles under x-ray and UV Irradiations. Nanomed. Nanotechnol. Biol. Med. 2011 doi: 10.1016/j.nano.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 122.Butterworth K.T., McMahon S.J., Currell F.J., Prise K.M. Physical basis and biological mechanisms of gold nanoparticle radiosensitization. Nanoscale. 2012 doi: 10.1039/c2nr31227a. [DOI] [PubMed] [Google Scholar]
- 123.Pan Y., Leifert A., Ruau D., Neuss S., Bornemann J., Schmid G., Brandau W., Simon U., Jahnen-Dechent W. Gold nanoparticles of diameter 1.4 nm trigger necrosis by oxidative stress and mitochondrial damage. Small. 2009 doi: 10.1002/smll.200900466. [DOI] [PubMed] [Google Scholar]
- 124.Pawlik T.M., Keyomarsi K. Role of cell cycle in mediating sensitivity to radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2004 doi: 10.1016/j.ijrobp.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 125.Butterworth K.T., Coulter J.A., Jain S., Forker J., McMahon S.J., Schettino G., Prise K.M., Currell F.J., Hirst D.G. Evaluation of cytotoxicity and radiation enhancement using 1.9nm gold particles: Potential application for cancer therapy. Nanotechnology. 2010 doi: 10.1088/0957-4484/21/29/295101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Elbayoumi T.A. Nano drug-delivery systems in cancer therapy: Gains, pitfalls and considerations in DMPK and PD. Ther. Deliv. 2010;1 doi: 10.4155/tde.10.31. [DOI] [PubMed] [Google Scholar]
- 127.Paciotti G.F., Kingston D.G.I., Tamarkin L. Colloidal gold nanoparticles: A novel nanoparticle platform for developing multifunctional tumor-targeted drug delivery vectors. Drug Dev. Res. 2006;67:47–54. doi: 10.1002/ddr.20066. [DOI] [Google Scholar]
- 128.Paciotti G.F., Myer L., Weinreich D., Goia D., Pavel N., McLaughlin R.E., Tamarkin L. Colloidal gold: A novel nanoparticle vector for tumor directed drug delivery. Drug Deliv. J. Deliv. Target. Ther. Agents. 2004 doi: 10.1080/10717540490433895. [DOI] [PubMed] [Google Scholar]
- 129.Ghosh P., Han G., De M., Kim C.K., Rotello V.M. Gold nanoparticles in delivery applications. Adv. Drug Deliv. Rev. 2008;60 doi: 10.1016/j.addr.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 130.Spampinato V., Parracino M.A., La Spina R., Rossi F., Ceccone G. Surface Analysis of Gold Nanoparticles Functionalized with Thiol-Modified Glucose SAMs for Biosensor Applications. Front. Chem. 2016;4:8. doi: 10.3389/fchem.2016.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jazayeri M.H., Amani H., Pourfatollah A.A., Pazoki-Toroudi H., Sedighimoghaddam B. Various methods of gold nanoparticles (GNPs) conjugation to antibodies. Sens. Bio-Sens. Res. 2016;9:17–22. doi: 10.1016/j.sbsr.2016.04.002. [DOI] [Google Scholar]
- 132.Awotunde O., Okyem S., Chikoti R., Driskell J.D. The Role of Free Thiol on Protein Adsorption to Gold Nanoparticles. Langmuir. 2020 doi: 10.1021/acs.langmuir.0c01550. [DOI] [PubMed] [Google Scholar]
- 133.Paciotti G.F., Zhao J., Cao S., Brodie P.J., Tamarkin L., Huhta M., Myer L.D., Friedman J., Kingston D.G.I. Synthesis and Evaluation of Paclitaxel-Loaded Gold Nanoparticles for Tumor-Targeted Drug Delivery. Bioconjug. Chem. 2016 doi: 10.1021/acs.bioconjchem.6b00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Spicer C.D., Pashuck E.T., Stevens M.M. Achieving Controlled Biomolecule-Biomaterial Conjugation. Chem. Rev. 2018;118:7702–7743. doi: 10.1021/acs.chemrev.8b00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Brown S.D., Nativo P., Smith J.A., Stirling D., Edwards P.R., Venugopal B., Flint D.J., Plumb J.A., Graham D., Wheate N.J. Gold nanoparticles for the improved anticancer drug delivery of the active component of oxaliplatin. J. Am. Chem. Soc. 2010 doi: 10.1021/ja908117a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fratoddi I., Venditti I., Cametti C., Russo M.V. Gold nanoparticles and gold nanoparticle-conjugates for delivery of therapeutic molecules. Progress and challenges. J. Mater. Chem. B. 2014;2:4204–4220. doi: 10.1039/C4TB00383G. [DOI] [PubMed] [Google Scholar]
- 137.Patra C.R., Bhattacharya R., Wang E., Katarya A., Lau J.S., Dutta S., Muders M., Wang S., Buhrow S.A., Safgren S.L., et al. Targeted delivery of gemcitabine to pancreatic adenocarcinoma using cetuximab as a targeting agent. Cancer Res. 2008 doi: 10.1158/0008-5472.CAN-07-6102. [DOI] [PubMed] [Google Scholar]
- 138.Du Y., Xia L., Jo A., Davis R.M., Bissel P., Ehrich M.F., Kingston D.G.I. Synthesis and Evaluation of Doxorubicin-Loaded Gold Nanoparticles for Tumor-Targeted Drug Delivery. Bioconjug. Chem. 2018 doi: 10.1021/acs.bioconjchem.7b00756. [DOI] [PubMed] [Google Scholar]
- 139.François A., Laroche A., Pinaud N., Salmon L., Ruiz J., Robert J., Astruc D. Encapsulation of docetaxel into PEGylated gold nanoparticles for vectorization to cancer cells. ChemMedChem. 2011 doi: 10.1002/cmdc.201100311. [DOI] [PubMed] [Google Scholar]
- 140.Farooq M.U., Novosad V., Rozhkova E.A., Wali H., Ali A., Fateh A.A., Neogi P.B., Neogi A., Wang Z. Gold Nanoparticles-enabled Efficient Dual Delivery of Anticancer Therapeutics to HeLa Cells. Sci. Rep. 2018 doi: 10.1038/s41598-018-21331-y. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 141.Tan J., Cho T.J., Tsai D.H., Liu J., Pettibone J.M., You R., Hackley V.A., Zachariah M.R. Surface Modification of Cisplatin-Complexed Gold Nanoparticles and Its Influence on Colloidal Stability, Drug Loading, and Drug Release. Langmuir. 2018 doi: 10.1021/acs.langmuir.7b02354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rosenberg B., VanCamp L., Trosko J.E., Mansour V.H. Platinum compounds: A new class of potent antitumour agents. Nature. 1969;222:385–386. doi: 10.1038/222385a0. [DOI] [PubMed] [Google Scholar]
- 143.Wang D., Lippard S.J. Cellular processing of platinum anticancer drugs. Nat. Rev. Drug Discov. 2005;4:307–320. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
- 144.Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer. 2007;7:574–584. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- 145.Reedijk J. New clues for platinum antitumor chemistry: Kinetically controlled metal binding to DNA. Proc. Natl. Acad. Sci. USA. 2003;100:3611–3616. doi: 10.1073/pnas.0737293100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Oliver T.G., Mercer K.L., Sayles L.C., Burke J.R., Mendus D., Lovejoy K.S., Cheng M.H., Subramanian A., Mu D., Powers S., et al. Chronic cisplatin treatment promotes enhanced damage repair and tumor progression in a mouse model of lung cancer. Genes Dev. 2010 doi: 10.1101/gad.1897010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Comenge J., Sotelo C., Romero F., Gallego O., Barnadas A., Parada T.G.C., Domínguez F., Puntes V.F. Detoxifying Antitumoral Drugs via Nanoconjugation: The Case of Gold Nanoparticles and Cisplatin. PLoS ONE. 2012;7:e47562. doi: 10.1371/journal.pone.0047562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhao X., Pan J., Li W., Yang W., Qin L., Pan Y. Gold nanoparticles enhance cisplatin delivery and potentiate chemotherapy by decompressing colorectal cancer vessels. Int. J. Nanomed. 2018 doi: 10.2147/IJN.S176928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lyseng-Williamson K.A., Fenton C. Docetaxel: A review of its use in metastatic breast cancer. Drugs. 2005;65:2513–2531. doi: 10.2165/00003495-200565170-00007. [DOI] [PubMed] [Google Scholar]
- 150.Kumar P. A new paradigm for the treatment of high-risk prostate cancer: Radiosensitization with docetaxel. Rev. Urol. 2003;5(Suppl. S3):S71–S77. [PMC free article] [PubMed] [Google Scholar]
- 151.Scagliotti G.V., Turrisi A.T. Docetaxel-Based Combined-Modality Chemoradiotherapy for Locally Advanced Non-Small Cell Lung Cancer. Oncologist. 2003;8:361–374. doi: 10.1634/theoncologist.8-4-361. [DOI] [PubMed] [Google Scholar]
- 152.Colevas A.D., Posner M.R. Docetaxel in head and neck cancer: A review. Am. J. Clin. Oncol. Cancer Clin. Trials. 1998;21:482–486. doi: 10.1097/00000421-199810000-00013. [DOI] [PubMed] [Google Scholar]
- 153.Fulton B., Spencer C.M. Docetaxel: A Review of its Pharmacodynamic and Pharmacokinetic Properties and Therapeutic Efficacy in the Management of Metastatic Breast Cancer. Drugs. 1996 doi: 10.2165/00003495-199651060-00011. [DOI] [PubMed] [Google Scholar]
- 154.Herbst R.S., Khuri F.R. Mode of action of docetaxel - A basis for combination with novel anticancer agents. Cancer Treat. Rev. 2003 doi: 10.1016/S0305-7372(03)00097-5. [DOI] [PubMed] [Google Scholar]
- 155.Nehmé A., Varadarajan P., Sellakumar G., Gerhold M., Niedner H., Zhang Q., Lin X., Christen R.D. Modulation of docetaxel-induced apoptosis and cell cycle arrest by all-trans retinoic acid in prostate cancer cells. Br. J. Cancer. 2001 doi: 10.1054/bjoc.2001.1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Loibl S., Denkert C., von Minckwitz G. Neoadjuvant treatment of breast cancer-Clinical and research perspective. Breast. 2015 doi: 10.1016/j.breast.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 157.Hennequin C., Giocanti N., Favaudon V. Interaction of ionizing radiation with paclitaxel (taxol) and docetaxel (taxotere) in HeLa and SQ20B cells. Cancer Res. 1996;56:1842–1850. [PubMed] [Google Scholar]
- 158.Mason K.A., Hunter N.R., Milas M., Abbruzzese J.L., Milas L. Docetaxel enhances tumor radioresponse in vivo. Clin. Cancer Res. 1997;3:2431–2438. [PubMed] [Google Scholar]
- 159.Kim J.A., Aberg C., Salvati A., Dawson K.A. Role of cell cycle on the cellular uptake and dilution of nanoparticles in a cell population. Nat. Nanotechnol. 2012 doi: 10.1038/nnano.2011.191. [DOI] [PubMed] [Google Scholar]
- 160.Bannister A.H., Bromma K., Sung W., Monica M., Cicon L., Howard P., Chow R.L., Schuemann J., Chithrani D.B. Modulation of nanoparticle uptake, intracellular distribution, and retention with docetaxel to enhance radiotherapy. Br. J. Radiol. 2020;93 doi: 10.1259/bjr.20190742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Rawla P., Sunkara T., Gaduputi V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J. Oncol. 2019;10:10–27. doi: 10.14740/wjon1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Burris H.A., Moore M.J., Andersen J., Green M.R., Rothenberg M.L., Modiano M.R., Cripps M.C., Portenoy R.K., Storniolo A.M., Tarassoff P., et al. Improvements in survival and clinical benefit with gemcitabine as first- line therapy for patients with advanced pancreas cancer: A randomized trial. J. Clin. Oncol. 1997 doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 163.Shelley M.D., Jones G., Cleves A., Wilt T.J., Mason M.D., Kynaston H.G. Intravesical gemcitabine therapy for non-muscle invasive bladder cancer (NMIBC): A systematic review. BJU Int. 2012;109:496–505. doi: 10.1111/j.1464-410X.2011.10880.x. [DOI] [PubMed] [Google Scholar]
- 164.Kroep J.R., Giaccone G., Tolis C., Voorn D.A., Loves W.J.P., Van Groeningen C.J., Pinedo H.M., Peters G.J. Sequence dependent effect of paclitaxel on gemcitabine metabolism in relation to cell cycle and cytotoxicity in non-small-cell lung cancer cell lines. Br. J. Cancer. 2000 doi: 10.1054/bjoc.2000.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Peters G.J., Van Der Wilt C.L., Van Moorsel C.J.A., Kroep J.R., Bergman A.M., Ackland S.P. Basis for effective combination cancer chemotherapy with antimetabolites. Pharmacol. Ther. 2000;87:227–253. doi: 10.1016/S0163-7258(00)00086-3. [DOI] [PubMed] [Google Scholar]
- 166.Peters G.J., Clavel M., Noordhuis P., Geyssen G.J., Laan A.C., Guastalla J., Edzes H.T., Vermorken J.B. Clinical phase I and pharmacology study of gemcitabine (2′, 2′-difluorodeoxycytidine) administered in a two-weekly schedule. J. Chemother. 2007 doi: 10.1179/joc.2007.19.2.212. [DOI] [PubMed] [Google Scholar]
- 167.Huai Y., Zhang Y., Xiong X., Das S., Bhattacharya R., Mukherjee P. Gold Nanoparticles sensitize pancreatic cancer cells to gemcitabine. Cell Stress. 2019 doi: 10.15698/cst2019.08.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Shibue T., Weinberg R.A. EMT, CSCs, and drug resistance: The mechanistic link and clinical implications. Nat. Rev. Clin. Oncol. 2017;14:611–629. doi: 10.1038/nrclinonc.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Pal K., Al-Suraih F., Gonzalez-Rodriguez R., Dutta S.K., Wang E., Kwak H.S., Caulfield T.R., Coffer J.L., Bhattacharya S. Multifaceted peptide assisted one-pot synthesis of gold nanoparticles for plectin-1 targeted gemcitabine delivery in pancreatic cancer. Nanoscale. 2017 doi: 10.1039/C7NR03172F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Loehrer P.J., Feng Y., Cardenes H., Wagner L., Brell J.M., Cella D., Flynn P., Ramanathan R.K., Crane C.H., Alberts S.R., et al. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: An Eastern Cooperative Oncology Group trial. J. Clin. Oncol. 2011 doi: 10.1200/JCO.2011.34.8904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Davidi E.S., Dreifuss T., Motiei M., Shai E., Bragilovski D., Lubimov L., Kindler M.J.J., Popovtzer A., Don J., Popovtzer R. Cisplatin-conjugated gold nanoparticles as a theranostic agent for head and neck cancer. Head Neck. 2018 doi: 10.1002/hed.24935. [DOI] [PubMed] [Google Scholar]
- 172.Peng C., Xu J., Yu M., Ning X., Huang Y., Du B., Hernandez E., Kapur P., Hsieh J.T., Zheng J. Tuning the In Vivo Transport of Anticancer Drugs Using Renal-Clearable Gold Nanoparticles. Angew. Chem.-Int. Ed. 2019 doi: 10.1002/anie.201903256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kalimuthu K., Lubin B.C., Bazylevich A., Gellerman G., Shpilberg O., Luboshits G., Firer M.A. Gold nanoparticles stabilize peptide-drug-conjugates for sustained targeted drug delivery to cancer cells. J. Nanobiotechnol. 2018 doi: 10.1186/s12951-018-0362-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Alamzadeh Z., Beik J., Mirrahimi M., Shakeri-Zadeh A., Ebrahimi F., Komeili A., Ghalandari B., Ghaznavi H., Kamrava S.K., Moustakis C. Gold nanoparticles promote a multimodal synergistic cancer therapy strategy by co-delivery of thermo-chemo-radio therapy. Eur. J. Pharm. Sci. 2020 doi: 10.1016/j.ejps.2020.105235. [DOI] [PubMed] [Google Scholar]
- 175.Safwat M.A., Soliman G.M., Sayed D., Attia M.A. Gold nanoparticles enhance 5-fluorouracil anticancer efficacy against colorectal cancer cells. Int. J. Pharm. 2016 doi: 10.1016/j.ijpharm.2016.09.076. [DOI] [PubMed] [Google Scholar]
- 176.Fathy M.M., Mohamed F.S., Elbialy N.S., Elshemey W.M. Multifunctional Chitosan-Capped Gold Nanoparticles for enhanced cancer chemo-radiotherapy: An invitro study. Phys. Med. 2018 doi: 10.1016/j.ejmp.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 177.Wilhelm S., Tavares A.J., Dai Q., Ohta S., Audet J., Dvorak H.F., Chan W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016;1 doi: 10.1038/natrevmats.2016.14. [DOI] [Google Scholar]
- 178.Verma M. Personalized medicine and cancer. J. Pers. Med. 2012;2:1–14. doi: 10.3390/jpm2010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Krzyszczyk P., Acevedo A., Davidoff E.J., Timmins L.M., Marrero-Berrios I., Patel M., White C., Lowe C., Sherba J.J., Hartmanshenn C., et al. The growing role of precision and personalized medicine for cancer treatment. Technology. 2018 doi: 10.1142/S2339547818300020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Fan H., Demirci U., Chen P. Emerging organoid models: Leaping forward in cancer research. J. Hematol. Oncol. 2019;12 doi: 10.1186/s13045-019-0832-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Fatehullah A., Tan S.H., Barker N. Organoids as an in vitro model of human development and disease. Nat. Cell Biol. 2016;18:246–254. doi: 10.1038/ncb3312. [DOI] [PubMed] [Google Scholar]
- 182.Driehuis E., Clevers H. CRISPR/Cas 9 genome editing and its applications in organoids. Am. J. Physiol.-Gastrointest. Liver Physiol. 2017;312:G257–G265. doi: 10.1152/ajpgi.00410.2016. [DOI] [PubMed] [Google Scholar]
- 183.Yao Y., Xu X., Yang L., Zhu J., Wan J., Shen L., Xia F., Fu G., Deng Y., Pan M., et al. Patient-Derived Organoids Predict Chemoradiation Responses of Locally Advanced Rectal Cancer. Cell Stem Cell. 2020 doi: 10.1016/j.stem.2019.10.010. [DOI] [PubMed] [Google Scholar]
- 184.Vives J., Batlle-Morera L. The challenge of developing human 3D organoids into medicines. Stem Cell Res. Ther. 2020;11:1–4. doi: 10.1186/s13287-020-1586-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Dossena M., Piras R., Cherubini A., Barilani M., Dugnani E., Salanitro F., Moreth T., Pampaloni F., Piemonti L., Lazzari L. Standardized GMP-compliant scalable production of human pancreas organoids. Stem Cell Res. Ther. 2020 doi: 10.1186/s13287-020-1585-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Quereda V., Hou S., Madoux F., Scampavia L., Spicer T.P., Duckett D. A Cytotoxic Three-Dimensional-Spheroid, High-Throughput Assay Using Patient-Derived Glioma Stem Cells. SLAS Discov. 2018 doi: 10.1177/2472555218775055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Brüningk S.C., Rivens I., Box C., Oelfke U., ter Haar G. 3D tumour spheroids for the prediction of the effects of radiation and hyperthermia treatments. Sci. Rep. 2020 doi: 10.1038/s41598-020-58569-4. [DOI] [PMC free article] [PubMed] [Google Scholar]