Abstract
In some subjects, high-altitude hypobaric hypoxia leads to high-altitude pulmonary hypertension. The threshold for the diagnosis of high-altitude pulmonary hypertension is a mean pulmonary artery pressure of 30 mmHg, even though for general pulmonary hypertension is ≥25 mmHg. High-altitude pulmonary hypertension has been associated with high hematocrit findings (chronic mountain sickness), and although these are two separate entities, they have a synergistic effect that should be considered. In recent years, a new condition associated with high altitude was described in South America named long-term chronic intermittent hypoxia and has appeared in individuals who commute to work at high altitude but live and rest at sea level. In this review, we discuss the initial epidemiological pattern from the early studies done in Chile, the clinical presentation and possible molecular mechanism and a discussion of the potential management of this condition.
Keywords: high altitude pulmonary hypertension, epidemiology, chronic hypoxia, intermittent hypoxia, high altitude
Introduction
It is estimated that more than 140 million people worldwide currently live or work at an altitude over 2500 m,1,2 and the sequelae associated with this exposure represent a substantial public health burden.
Exposure to hypobaric hypoxia is accompanied by several changes in humans, with the most relevant changes occurring in the cardiopulmonary circuit, particularly in the right circuit.3 Moreover, in some individuals, these changes can lead to pathological disorders. The main recognized altitude disorders are acute mountain sickness and its variations, such as acute cerebral edema, high-altitude pulmonary edema (HAPE), high-altitude pulmonary hypertension (HAPH), and chronic mountain sickness (CMS).4
Similarly, the type of exposure to high altitude is usually classified or determined according to the duration of exposure. Thus, people who live permanently at high altitude (high-altitude residents) are considered to be exposed to chronic hypoxia, while people who visit high altitudes for hours or days for either leisure or work on a single occasion are considered to be exposed to acute hypoxia, and people who are exposed to high altitude for short periods are considered to be exposed to intermittent hypoxia. There are many types of models of intermittent or chronic intermittent hypoxia, and the classification and comparison of these models is confusing and misleading in experimental models and humans. Currently, a new type of hypoxia exposure that has appeared during the last 20 years must be added to these traditional types of exposure. In this type of exposure, subjects commute in shifts, spending their working days (ranging from 7 to 15 days) at altitudes higher than 3000 m and spending their resting periods at sea level (same days). They maintain this system for years. This particular condition was first recognized in South America, particularly in Chile, where mining and a variety of jobs related to frontier surveillance and civil support have led to settlements around these locations. This exposure has been termed long-term chronic intermittent exposure (originally called the Chilean miners’ model); it is completely different from other types of chronic intermittent exposure, such as training or obstructive sleep apnea.5–8 In Chile alone, this type of exposure affects approximately 100,000 individuals.7
This review mainly aims to provide an overview of HAPH in chronic and long-term chronic intermittent hypoxia in terms of its epidemiological, clinical, and molecular features.
High-altitude pulmonary hypertension
HAPH is classified as WHO Group 3.6 pulmonary hypertension in the general pulmonary hypertension classification.9,10 A cut-off point of ≥25 mmHg was established for all types of pulmonary hypertension.11 However, the definition of an altitude cut-off point has been a matter of discussion for years and was finally settled by an international consensus,4 providing a guideline for categorizing HAPH. Therefore, the key hallmark of HAPH is a mean pulmonary artery pressure (mPAP) of ≥30 mmHg, measured at altitude and at rest, in individuals without chronic obstructive lung disease or chronic cardiac disease.4 This condition is observed in subjects who are chronically exposed to hypobaric hypoxia due to hypoxic pulmonary vasoconstriction and subsequent vascular structural remodeling.3,12,13
Epidemiology of HAPE and HAPH
HAPE is a high-altitude disorder which occurs during the first day of acute exposure to hypoxia. It is a consequence of acute pulmonary hypertension along with impaired alveolar clearance, which manifests as uneven pulmonary patches of pulmonary edema that, if not treated, can lead to death.14,15 Its prevalence varies but may range from 1% to 14%,12 and the average prevalence was reported to be 4%.16 There is no Chilean national registry for chronic intermittent hypoxia exposure, but according to a survey of emergency departments in northern Chile, HAPE is a very rare entity that does not account for more than two to four cases per year. Therefore, we will focus this review on a major entity, HAPH.
Determining the actual prevalence of HAPH in chronic high-altitude residents is a difficult task because of the variations between different populations and the different methods used to measure mPAP.12,17 However, the compiled information for HAPH shows that its prevalence is 18% in the Kyrgyz population,18 while in other populations, such as those in South America, HAPH has been estimated to affect between 5% and 18% of the exposed population and to be more common in males than in females.19 In natives of the Spiti Valley in India, the prevalence of HAPH was reported as 3.23%.20 In China, HAPH affects mainly Han individuals who migrate to the high plateau.17 A consistent prevalence has been difficult to establish; even Ge and Helun21 do not mention any prevalence for HAPH. However, Neupane and Swenson12 estimated that the prevalence is approximately 8%. Additionally, several studies have suggested that HAPH is somewhat more prevalent in people who were not born and raised at high altitude but who come to live at high altitude later in life.12 Interestingly, two recent publications raised some doubts about the actual prevalence of a pathological mean systolic PAP for defining HAPH at rest in individuals with or without CMS; a systematic review performed by Soria et al.22 investigated mostly healthy male Andeans (Aymaras and Quechuas) who were high-altitude residents with CMS using mean systolic PAP, and posteriorly in the editorial by Naeije,23 he recalculated the mPAP. Soria et al.22 concluded that HAPH is less common than previously expected at rest but frequent during daily activities.22,23 However, according to Naeije,23 this provocative finding must be reconciled with further analysis and validation.
Given this disparity, to obtain an epidemiological reference, a consensus was reached in 2005, in which the general prevalence of HAPH was estimated to be between 10% and 15%.4 In addition, genetic adaptation has been demonstrated in some populations, such as Tibetans, in whom HAPH is almost nonexistent.24,25
Interestingly, the prevalence of HAPH during long-term chronic intermittent exposure has yet to be definitively determined. However, reports from Chile revealed an estimated prevalence of 9% in lowlanders7 (Table 1).
Table 1.
Prevalence of high-altitude pulmonary hypertension around the world and according to different types of exposure.
| Exposure type | Prevalence (%) | Population | Source |
|---|---|---|---|
| Chronic | |||
| 5–18 | Kirgyz | Aldashev et al.18 | |
| 5–18 | South America | Mirrakhimov and Strohl19 | |
| 3.2 | Spiti, India | Negi et al.20 | |
| 11 | Colorado | Robinson et al.26 | |
| 8 | Qinhai-Tibet | Neupane and Swenson,12 León-Velarde et al.4 | |
| 10–15 | Aymaras | ||
| Intermittent | |||
| 9 | Chile | Brito et al.7 | |
| Acute | Incidence | ||
| 4 | Overall | Maggiorini16 |
HAPE: high-altitude pulmonary edema.
Clinical presentation
The clinical presentation of individuals suffering from HAPH is not different from that of individuals suffering from any other type of pulmonary arterial hypertension: its features range from asymptomatic in some individuals to exertional dyspnea in the early stages, followed by general fatigue, intolerance to exercise, chest pain, mental alterations, dizziness, syncope, and ultimately cor pulmonale.12,17,19,26 Different mPAPs are found depending on altitude, and individual variations should be considered3; in addition, the potential contributory role of sleep apnea in HAPH, either central or obstructive, cannot be ignored, since the former is rather common and the latter is aggravated at high altitude.27
Functional capacity impairments (determined according to the New York Heart Association (NYHA) Functional Classification criteria and28 the six-minute walking test) are observed in people with HAPH, but there is still some disagreement.7 In some individuals and in some geographic locations, such as Colorado (USA), this type of hypertension does not seem to affect functional capacity.29 This observation was described by Grover30 as a “paradox of HAPH.” Moreover, a study performed in Kyrgyz highlanders demonstrated a modest reduction in exercise capacity.27 This led to the introduction of the concept of “pulmonary vascular reserve” instead of merely “exercise capacity.”7,31,32 Pulmonary vascular reserve is a concept that was introduced by some authors to explain why some patients with pulmonary hypertension have an increased aerobic exercise capacity. Therefore, “pulmonary vascular reserve” is defined as a combination of decreased pulmonary vascular reserve and increased lung diffusion capacity for nitric oxide that would allow for superior aerobic exercise capacity at a lower ventilatory cost at sea level and at high altitude.31–33
Strikingly, in some studies performed on subjects who have spent more than 10 years working intermittently at high altitude, no alteration in functional capacity was found (NYHA level I). Similarly, these workers at high altitude have been shown to exhibit prompt acclimation without exercise capacity impairment.7,34 The role of hypoxia dose in this model has not yet been defined; however, it has been seen in rats that at the morphologic35 and molecular levels, hypoxia dose could play a role. Therefore, it can be hypothesized that spending several days at sea level could play a role in long-term chronic intermittent hypoxia, since during intermittent exposure, the mechanisms and morphological changes are repeatedly turned on and off.36 Another hypothesis is that under long-term chronic intermittent hypoxia, the time spent at sea level could buffer the effects of hypoxia. Importantly, HAPH can be reversed when individuals return to live at sea level for a prolonged time.37
Main concepts in acclimatization at altitude and physiopathology in HAPH
Individuals exposed to high altitude respond to a low environmental partial pressure of oxygen by ultimately developing hypoxemia. The hypoxic stress responses observed in these individuals can be summarized as increases in ventilation (hypoxic ventilation response), cardiac output, and hemoglobin concentration. Concurrently, there is a reduction in the diffusive barriers between the lungs and tissue capillaries.12 Moreover, at the cellular level, there are changes in capillary and mitochondrial density as well as metabolic efficiency.38–40
Most of these responses are elicited by the stabilization of hypoxia-inducible factors (HIFs). In an oxygenated environment, HIF subunits are hydroxylated at proline residues through prolyl hydroxylases (PHDs), and these subunits are destroyed by E3 ubiquitin ligase through the von Hippel-Lindau protein complex.41 The activity of PHDs is regulated by oxygen bioavailability; therefore, under hypoxic stress, the activity of PHD proteins is diminished, and thus, HIF proteins can induce the transcription of several genes, as discussed below.39
The first response of the pulmonary circulation to alveolar hypoxia is to redistribute the flow in the lung parenchyma to better oxygenated areas.12,42 This phenomenon is termed hypoxic pulmonary vasoconstriction (HPV) and was originally described by von Euler and Lijestrand in cats.3 HPV serves to optimize ventilation-perfusion matching in focal hypoxia and thereby enhances pulmonary gas exchange. When the bioavailability of oxygen is globally reduced, HPV induces general pulmonary vasoconstriction, which may lead to a rapid and reversible increase in mPAP, and under long-term chronic or intermittent hypobaric hypoxia, this process may trigger pulmonary hypertension, impair exercise capacity, and cause right heart failure and pulmonary edema.43
As mentioned above, the cardinal transcription factor involved in the control of oxygen-regulated genes such as HIF-1α is stabilized under hypoxic conditions and regulates many cellular responses that control hypoxia-induced pulmonary artery vasoconstriction in numerous cell lines.44–46 This notion is supported by a recent study that showed that the cell-specific deletion of HIF-1α suppressed hypoxia-induced pulmonary hypertension and the progression of pulmonary arterial remodeling.47
However, an imbalance among factors such as endothelial vasoconstrictors, vasodilators, reactive oxygen species, other molecules (e.g., insulin, asymmetric dimethylarginine (ADMA)), susceptibility, and genetic factors is clearly the cornerstone at the molecular level.7,48,49
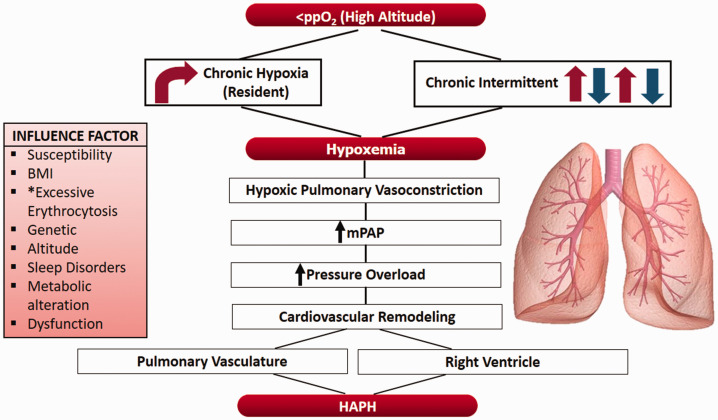
As has commonly been observed in the clinical setting, most responses are physiologically useful; however, in some cases, these beneficial responses are excessive and can be the source of a pathological response. The main examples of such cases are exaggerated polycythemia or CMS under chronic hypoxia and HPV leading to HAPH12 (Fig. 1).
Fig. 1.
A proposed scheme of events and factors involved in the development of high-altitude pulmonary hypertension; body mass index; partial pressure of O2; mean pulmonary artery pressure. *Notably, excessive erythrocytosis has not been demonstrated to play a role in long term chronic intermittent hypoxia.
Molecular mechanisms involved
During the HPV process, several mechanisms are activated in consecutive phases. Studies have suggested that the first phase is calcium-dependent smooth muscle cell (SMC) contraction followed by the escalation of numerous other mechanisms that act in SMCs.50–52
At the SMC level, the intracellular calcium concentration is increased mainly via the hypoxia-induced inhibition of potassium channels, which leads to membrane depolarization and induces the activation of l-type calcium channels. Additionally, another source of calcium is the sarcoplasmic reticulum, which leads to a subsequent additional influx through stored-operated calcium channels (SOCCs) and receptor-operated calcium channels (ROCCs), which are formed by several proteins, including transient receptor potential channels (TRPCs), Orai, acid-sensing ion channels (ASICs), and stromal interaction molecule (Stim).46,52
Interestingly, the expression of subunits of these proteins (Orai, TRPC, Stim, and ASIC) depends on the time of exposure to hypoxia. Since, in SMCs of the distal pulmonary arteries, under acute exposure to hypoxia, SOCC and ROCC are crucial for the HPV process through proteins such as Stim1 and 2, TRPC1 and 6, and TRPV4; however, under conditions of chronic hypoxia, SOCC are overexpressed in the pulmonary vasculature through proteins such as Orai1 and 2, TRPC1 and 6, Stim1 and 2, and ASIC1.46,53 Furthermore, recent studies have shown that under the condition of chronic hypoxia, the expression of specific Orai1 channels, TRPC6 and Stim1, plays a predominant role in the regulation of pulmonary hypertension, and their expression might be modulated by hydrogen peroxide (H2O2) production.54,55 In addition, recent studies have also shown that under chronic hypoxia, TRPC1 and 6, Orai2, and Stim2 could be regulated by HIF-1α.46,55,56
We hypothesize that many of these proteins might be related to long-term chronic intermittent hypobaric hypoxia-induced HAPH because there is little information on this particular condition, which seems to involve both acute and chronic responses. Therefore, further studies are needed.
Other molecular pathways are also involved in HPV since the endothelium generates several vasoactive mediators that act on the surrounding vascular SMCs.52 These molecules include nitric oxide (NO) and prostacyclin, which act as vasodilators, and endothelin-1 (ET-1), which acts as a vasoconstrictor.57
It is important to highlight that ET-1 presents dual activity since it can activate vasoconstriction via endothelin A receptor (ETA) binding or activate the vasodilator pathway by binding to the endothelin B receptor (ETB), which leads to NO release.50 However, studies have shown that the vasoconstrictor and pro-remodeling effects of ET-1 are triggered in SMCs, while vasodilation and anti-remodeling effects are observed in the endothelium. This is supported by studies (both in vitro and in vivo) in which treatment with a dual ETA and ETB blocker (macitentan) and a selective ETB receptor antagonist (BQ788) prevented the pro-proliferative phenotype, perfusion, remodeling, and hemodynamic effects in Sugen/hypoxic rats, suggesting that under hypoxic conditions, vasoconstriction and remodeling are predominant at the pulmonary artery SMC level.40
However, it is important to highlight the effect of macitentan administration on cardiac tissue, specifically on increasing perfusion of the right ventricle and microvessels and improving cardiac output and systolic pressure and function.40
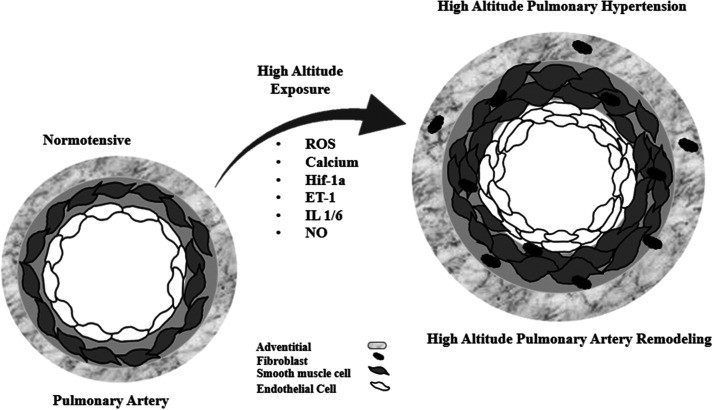
The vasoconstrictor molecule ET-1 has a promoter that is highly responsive to hypoxia, which is associated with HIF-1, as shown by a study in which ET-1 was found to possess a binding site for HIF-1 at the 118-bp position.58 A scheme with the main molecular pathways involved the development of HAPH is shown in Fig. 2. Additionally, studies have shown that under chronic hypoxia, ET-1 can be modulated by HIF-1α-induced TRPC1 and 6, Orai2 and Stim2.46,55,56
Fig. 2.
A proposed scheme for high-altitude pulmonary hypertension progression and some of its mechanisms in the pulmonary vasculature; endothelin-1; reactive oxygen species; hypoxia-inducible factor-1α; nitric oxid; and interleukin 1 and 6.
NO activity seems to play a critical role in the development of HPV and subsequent HAPH since it has been demonstrated that at higher altitudes, the NO pathway is impaired. Studies have shown that both chronic and long-term intermittent hypobaric hypoxia lead to a notable increase in superoxide radicals, which can impair and decrease NO bioavailability.49,59,60 Conversely, Tibetan high-altitude residents, who are genetically adapted to high altitude, have been shown to release more NO than do lowlanders.61
Interestingly, although a higher hematocrit might contribute to HPV, it has also been reported that deoxygenated red cells release ATP, which activates endothelial cell NO production via purinergic receptor binding62 and thus promotes vasodilation.52 This latter observation deserves further research.
Working at high altitude under chronic intermittent hypoxia and HAPH
Very little information is available about HAPH during exposure to long-term chronic intermittent hypobaric hypoxia at high altitude, mainly because this is a new epidemiological and biological condition that has been recognized for fewer than 20 years. Its main features, determined over time, can be summarized as follows: it has a prevalence of 9%, it is similar to chronic hypoxia, it exhibits cardiac and pulmonary vasculature remodeling that manifests as a certain degree of right ventricular hypertrophy and vascular remodeling, and it is associated with preservation of the left ventricular ejection fraction and right ventricular function, almost normal pulmonary vascular resistance, and a lack of functional repercussions or impairment of work capacity.7,63 Notably, excessive erythrocytosis, which plays a role in the development of HAPH,52 is rare in this condition.7 This latter phenomenon may be a consequence of shifting in the model because, in this model, every mechanism can be turned on and turned off according to exposure to hypoxia.36 Additionally, some risk or conditioning factors, such as insulin resistance, overweight, and elevated ADMA, have been demonstrated to be associated with this condition.7 In addition, this working condition poses a burden for worker safety programs and epidemiological surveillance.64
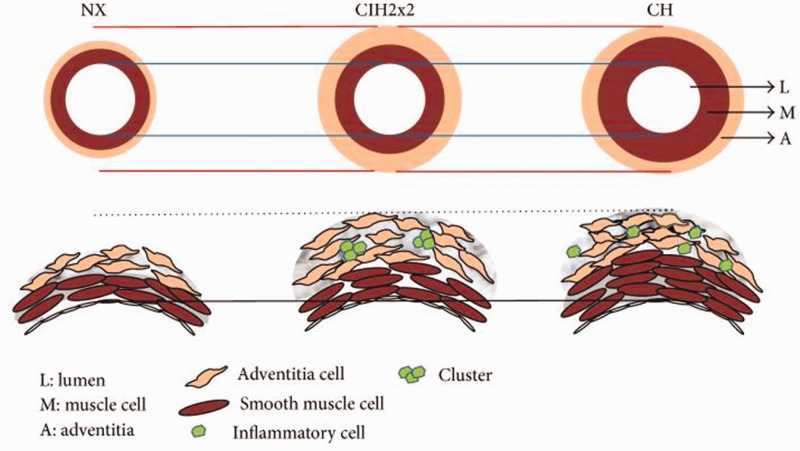
Experimental work has demonstrated that vascular remodeling and right ventricular hypertrophy65 as well as oxidative and nitrosative stress49,60 play roles in this condition. Additionally, endothelial dysfunction,66 alteration of the coagulation–fibrinolysis system and a decrease in myocardial contractility67 have been demonstrated. Finally, all morphological changes that occur in affected individuals and/or animals are similar to but manifest to a lesser extent than those found in chronic hypoxia7,65 (Fig. 3). Further validation is required to determine whether the difference between this condition and chronic hypoxia is due to the commuting system.7,68
Fig. 3.
Scheme showing differences in pulmonary vasculature remodeling between chronic intermittent hypoxia and chronic hypoxia. (Reproduced with permission from Brito et al.65)
Treatment
There is no definite and suitable treatment for HAPH caused by chronic and intermittent hypoxia. The obvious treatment is to bring the patient to sea level since this course of action has been demonstrated to lead to an almost full reversion over time.37 However, the cost associated with bringing residents, mining workers, or other professionals away from their work place, with regard for social and economic implications, may be insurmountable and prevent this measure from being considered. Several trials of drug monotherapy for pulmonary arterial hypertension have been conducted and have included calcium channel blockers, ET-1 receptor antagonists, phosphodiesterase type 5 inhibitors, prostacyclin analogs, inositol triphosphate agonists,11 and acetazolamide.17,19 However, none of these drugs have been used for the long-term treatment of HAPH.
As both Neupane and Swenson12 and Basnyat and Murdoch69 proposed, treatment should be focused on the prevention of risk factors, such as sleep disorders and disorders that provoke low nocturnal arterial oxygen saturation. In fact, the use of oxygen in dormitories for long-term chronic intermittent hypoxia in workers is strongly supported by the literature and by West.70
Conclusion and future perspectives
In summary, HAPH is a disease that occurs as a result of hypoxemia due to high-altitude exposure. It affects a portion of individuals who either live or work intermittently at high altitudes. Although its prevalence has been estimated to be rather low and variable, the large number of people exposed to this condition poses an important disease burden and represents a public health concern. Further measures need to be taken to ameliorate its impact and prevalence. We emphasize that this new condition, which results from the exposure to high altitudes in work shifts and exposure to sea level for rest in lowlanders for days and long term, also merits further study.
There are some courses of action that should be taken to reduce the burden of this disease, and future avenues for research are clear. Among the more important steps, researchers should 1) establish a national registry for HAPH, 2) determine and assess early biomarkers, 3) revisit a cut-off point for mPAP that designates physiological versus pathological status, and 4) obtain a more in-depth understanding of the molecular mechanisms involved in this process, identify reliable and effective targets for HAPH, and attempt to reduce the impact of alterations or pathologies that contribute to increases mPAP.
Contributorship
JB addressed the conference, and JB, PS, and EP contributed to drafting and critical revision of the manuscript, and accepted the final version of this manuscript.
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
This work was funded by grants from FIC Tarapaca (BIP 30477541-0), AECID and the German Federal Ministry of Education and Research (01DN17046 (DECIPHER)).
ORCID iDs
Brito Julio https://orcid.org/0000-0002-0050-8774
Siques Patricia https://orcid.org/0000-0002-4963-195X
Pena Eduardo https://orcid.org/0000-0003-2664-1722
References
- 1.Moore LG, Niermeyer S, Zamudio S. Human adaptation to high altitude: regional and life-cycle perspectives. Am J Phys Anthropol 1998; 27: 25–64. [DOI] [PubMed] [Google Scholar]
- 2.West JB, Schoene RB, Luks AM, et al. High altitude medicine and physiology, 5th ed London: CRC Press, 2012. [Google Scholar]
- 3.Penaloza D, Arias-Stella J. The heart and pulmonary circulation at high altitudes: healthy highlanders and chronic mountain sickness. Circulation 2007; 115: 1132–1146. [DOI] [PubMed] [Google Scholar]
- 4.Leon-Velarde F, Maggiorini M, Reeves JT, et al. Consensus statement on chronic and subacute high altitude diseases. High Alt Med Biol 2005; 6: 147–157. [DOI] [PubMed] [Google Scholar]
- 5.Richalet JP, Donoso MV, Jimenez D, et al. Chilean miners commuting from sea level to 4500 m: a prospective study. High Alt Med Biol 2002; 3: 159–166. [DOI] [PubMed] [Google Scholar]
- 6.Brito J, Siques P, Leon-Velarde F, et al. Chronic intermittent hypoxia at high altitude exposure for over 12 years: assessment of hematological, cardiovascular, and renal effects. High Alt Med Biol 2007; 8: 236–244. [DOI] [PubMed] [Google Scholar]
- 7.Brito J, Siques P, Lopez R, et al. Long-term intermittent work at high altitude: right heart functional and morphological status and associated cardiometabolic factors. Front Physiol 2018; 9: 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.West JB. Intermittent exposure to high altitude. High Alt Med Biol 2002; 3: 141–143. [DOI] [PubMed] [Google Scholar]
- 9.McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American college of cardiology foundation task force on expert consensus documents and the American heart association developed in collaboration with the American college of chest physicians; American thoracic society, Inc.; and the pulmonary hypertension association. J Am Coll Cardiol 2009; 53: 1573–1619. [DOI] [PubMed] [Google Scholar]
- 10.Galie N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the task force for the diagnosis and treatment of pulmonary hypertension of the European society of cardiology (ESC) and the European respiratory society (ERS), endorsed by the International society of heart and lung transplantation (ISHLT). Eur Heart J 2009; 30: 2493–2537. [DOI] [PubMed] [Google Scholar]
- 11.Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016; 37: 67–119. [DOI] [PubMed] [Google Scholar]
- 12.Neupane M, Swenson ER. High-altitude pulmonary vascular diseases. Adv Pulm Hypertens 2017; 15: 149–157. [Google Scholar]
- 13.Sommer N, Dietrich A, Schermuly RT, et al. Regulation of hypoxic pulmonary vasoconstriction: basic mechanisms. Eur Respir J 2008; 32: 1639–1651. [DOI] [PubMed] [Google Scholar]
- 14.Bartsch P, Swenson ER. Acute high-altitude illnesses. N Engl J Med 2013; 369: 1666–1667. [DOI] [PubMed] [Google Scholar]
- 15.Wilkins MR, Ghofrani HA, Weissmann N, et al. Pathophysiology and treatment of high-altitude pulmonary vascular disease. Circulation 2015; 131: 582–590. [DOI] [PubMed] [Google Scholar]
- 16.Maggiorini M. High altitude-induced pulmonary oedema. Cardiovasc Res 2006; 72: 41–50. [DOI] [PubMed] [Google Scholar]
- 17.Xu XQ, Jing ZC. High-altitude pulmonary hypertension. Eur Respir Rev 2009; 18: 13–17. [DOI] [PubMed] [Google Scholar]
- 18.Aldashev AA, Sarybaev AS, Sydykov AS, et al. Characterization of high-altitude pulmonary hypertension in the Kyrgyz: association with angiotensin-converting enzyme genotype. Am J Respir Crit Care Med 2002; 166: 1396–1402. [DOI] [PubMed] [Google Scholar]
- 19.Mirrakhimov AE, Strohl KP. High-altitude pulmonary hypertension: an update on disease pathogenesis and management. Open Cardiovasc Med J 2016; 10: 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Negi PC, Marwaha R, Asotra S, et al. Prevalence of high altitude pulmonary hypertension among the natives of Spiti Valley–a high altitude region in Himachal Pradesh, India. High Alt Med Biol 2014; 15: 504–510. [DOI] [PubMed] [Google Scholar]
- 21.Ge RL, Helun G. Current concept of chronic mountain sickness: pulmonary hypertension-related high-altitude heart disease. Wilderness Environ Med 2001; 12: 190–194. [DOI] [PubMed] [Google Scholar]
- 22.Soria R, Egger M, Scherrer U, et al. Pulmonary arterial pressure at rest and during exercise in chronic mountain sickness: a meta-analysis. Eur Respir J 2019; 53: 1802040. [DOI] [PubMed] [Google Scholar]
- 23.Naeije R. Pulmonary hypertension at high altitude. Eur Respir J 2019; 53: 1900985. [DOI] [PubMed] [Google Scholar]
- 24.Simonson TS, Huff CD, Witherspoon DJ, et al. Adaptive genetic changes related to haemoglobin concentration in native high-altitude Tibetans. Exp Physiol 2015; 100: 1263–1268. [DOI] [PubMed] [Google Scholar]
- 25.Young JM, Williams DR, Thompson AAR. Thin air, thick vessels: historical and current perspectives on hypoxic pulmonary hypertension. Front Med 2019; 6: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robinson JC, Abbott C, Meadows CA, et al. Long-term health outcomes in high-altitude pulmonary hypertension. High Alt Med Biol 2017; 18: 61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Latshang TD, Furian M, Aeschbacher SS, et al. Association between sleep apnoea and pulmonary hypertension in Kyrgyz highlanders. Eur Respir J 2017; 49: 1601530. [DOI] [PubMed] [Google Scholar]
- 28.The Criteria Committee of the New York Heart Association. Nomenclature and criteria for diagnosis of diseases of the heart and great vessels, Boston: Little, Brown & Co, 1994. [Google Scholar]
- 29.Penaloza D, Banchero N, Sime F, et al. The heart in chronic hypoxia. Biochem Clin 1963; 2: 283–298. [PubMed] [Google Scholar]
- 30.Grover RF. The paradox of hypoxic pulmonary hypertension (2013 Grover Conference series). Pulm Circ 2014; 4: 151–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pavelescu A, Faoro V, Guenard H, et al. Pulmonary vascular reserve and exercise capacity at sea level and at high altitude. High Alt Med Biol 2013; 14: 19–26. [DOI] [PubMed] [Google Scholar]
- 32.Naeije R, Dedobbeleer C. Pulmonary hypertension and the right ventricle in hypoxia. Exp Physiol 2013; 98: 1247–1256. [DOI] [PubMed] [Google Scholar]
- 33.Groepenhoff H, Overbeek MJ, Mule M, et al. Exercise pathophysiology in patients with chronic mountain sickness exercise in chronic mountain sickness. Chest 2012; 142: 877–884. [DOI] [PubMed] [Google Scholar]
- 34.Farias JG, Jimenez D, Osorio J, et al. Acclimatization to chronic intermittent hypoxia in mine workers: a challenge to mountain medicine in Chile. Biol Res 2013; 46: 59–67. [DOI] [PubMed] [Google Scholar]
- 35.Brito J, Siques P, León-Velarde F, et al. Varying exposure regimes to long term chronic intermittent hypoxia exert different outcomes and morphological effects on Wistar rats at 4600 m. Toxicol Environ Chem 2008; 90: 169–179. [Google Scholar]
- 36.Powell FL, Garcia N. Physiological effects of intermittent hypoxia. High Alt Med Biol 2000; 1: 125–136. [DOI] [PubMed] [Google Scholar]
- 37.Grover RF, Vogel JH, Voigt GC, et al. Reversal of high altitude pulmonary hypertension. Am J Cardiol 1966; 18: 928–932. [DOI] [PubMed] [Google Scholar]
- 38.Mathieu-Costello O. Muscle adaptation to altitude: tissue capillarity and capacity for aerobic metabolism. High Alt Med Biol 2001; 2: 413–425. [DOI] [PubMed] [Google Scholar]
- 39.Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell 2010; 40: 294–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nadeau V, Potus F, Boucherat O, et al. Dual ET(A)/ET(B) blockade with macitentan improves both vascular remodeling and angiogenesis in pulmonary arterial hypertension. Pulm Circ 2018; 8: 2045893217741429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Demidenko ZN, Blagosklonny MV. The purpose of the HIF-1/PHD feedback loop: to limit mTOR-induced HIF-1alpha. Cell Cycle 2011; 10: 1557–1562. [DOI] [PubMed] [Google Scholar]
- 42.Motley HL, Cournand A, Werko L, et al. The influence of short periods of induced acute anoxia upon pulmonary artery pressures in man. Am J Physiol 1947; 150: 315–320. [DOI] [PubMed] [Google Scholar]
- 43.Kylhammar D, Radegran G. The principal pathways involved in the in vivo modulation of hypoxic pulmonary vasoconstriction, pulmonary arterial remodelling and pulmonary hypertension. Acta Physiol 2017; 219: 728–756. [DOI] [PubMed] [Google Scholar]
- 44.Wang J, Weigand L, Lu W, et al. Hypoxia inducible factor 1 mediates hypoxia-induced TRPC expression and elevated intracellular Ca2+ in pulmonary arterial smooth muscle cells. Circ Res 2006; 98: 1528–1537. [DOI] [PubMed] [Google Scholar]
- 45.Palazon A, Goldrath AW, Nizet V, et al. HIF transcription factors, inflammation, and immunity. Immunity 2014; 41: 518–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reyes RV, Castillo-Galan S, Hernandez I, et al. Revisiting the role of TRP, orai, and ASIC channels in the pulmonary arterial response to hypoxia. Front Physiol 2018; 9: 486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kojima H, Tokunou T, Takahara Y, et al. Hypoxia-inducible factor-1 alpha deletion in myeloid lineage attenuates hypoxia-induced pulmonary hypertension. Physiol Rep 2019; 7: e14025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Richalet JP and Pichon A. Right ventricle and high altitude. In: Gaine SP, Naeije R and Peacock AJ (eds) The right heart. London: Springer, 2014, pp.117–129.
- 49.Luneburg N, Siques P, Brito J, et al. Long-term chronic intermittent hypobaric hypoxia in rats causes an imbalance in the asymmetric dimethylarginine/nitric oxide pathway and ROS activity: a possible synergistic mechanism for altitude pulmonary hypertension? Pulm Med 2016; 2016: 6578578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aaronson PI, Robertson TP, Ward JP. Endothelium-derived mediators and hypoxic pulmonary vasoconstriction. Respir Physiol Neurobiol 2002; 132: 107–120. [DOI] [PubMed] [Google Scholar]
- 51.Sylvester JT, Shimoda LA, Aaronson PI, et al. Hypoxic pulmonary vasoconstriction. Physiol Rev 2012; 92: 367–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Swenson ER. Hypoxic pulmonary vasoconstriction. High Alt Med Biol 2013; 14: 101–110. [DOI] [PubMed] [Google Scholar]
- 53.He X, Song S, Ayon RJ, et al. Hypoxia selectively upregulates cation channels and increases cytosolic [Ca(2+)] in pulmonary, but not coronary, arterial smooth muscle cells. Am J Physiol Cell Physiol 2018; 314: C504–C517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen TX, Xu XY, Zhao Z, et al. Hydrogen peroxide is a critical regulator of the hypoxia-induced alterations of store-operated Ca(2+) entry into rat pulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 2017; 312: L477–L487. [DOI] [PubMed] [Google Scholar]
- 55.Rode B, Bailey MA, Marthan R, et al. ORAI channels as potential therapeutic targets in pulmonary hypertension. Physiology 2018; 33: 261–268. [DOI] [PubMed] [Google Scholar]
- 56.Wang J, Xu C, Zheng Q, et al. Orai1, 2, 3 and STIM1 promote store-operated calcium entry in pulmonary arterial smooth muscle cells. Cell Death Discov 2017; 3: 17074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Houde M, Desbiens L, D’Orleans-Juste P. Endothelin-1: biosynthesis, signaling and vasoreactivity. Adv Pharmacol 2016; 77: 143–175. [DOI] [PubMed] [Google Scholar]
- 58.Yamashita K, Discher DJ, Hu J, et al. Molecular regulation of the endothelin-1 gene by hypoxia. Contributions of hypoxia-inducible factor-1, activator protein-1, GATA-2, AND p300/CBP. J Biol Chem 2001; 276: 12645–12653. [DOI] [PubMed] [Google Scholar]
- 59.Barman SA, Chen F, Su Y, et al. NADPH oxidase 4 is expressed in pulmonary artery adventitia and contributes to hypertensive vascular remodeling. Arterioscler Thromb Vasc Biol 2014; 34: 1704–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Siques P, de Pablo ALL, Brito J, et al. Nitric oxide and superoxide anion balance in rats exposed to chronic and long term intermittent hypoxia. Biomed Res Int 2014; 2014: 610474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Beall CM, Laskowski D, Erzurum SC. Nitric oxide in adaptation to altitude. Free Radic Biol Med 2012; 52: 1123–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sprague RS, Olearczyk JJ, Spence DM, et al. Extracellular ATP signaling in the rabbit lung: erythrocytes as determinants of vascular resistance. Am J Physiol Heart Circ Physiol 2003; 285: H693–H700. [DOI] [PubMed] [Google Scholar]
- 63.Moraga FA, Osorio J, Jiménez D, et al. Aerobic capacity, lactate concentration, and work assessment during maximum exercise at sea level and high altitude in miners exposed to chronic intermittent hypobaric hypoxia (3,800 m). Front Physiol 2019; 10: 1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reveco R, Velásquez M, Bustos L, et al. Determining the operating costs of a medical surveillance program for copper miners exposed to high altitude–induced chronic intermittent hypoxia in chile using a combination of microcosting and time-driven activity-based costing. Value Health Reg Issues 2019; 20: 115–121. [DOI] [PubMed] [Google Scholar]
- 65.Brito J, Siques P, Arribas SM, et al. Adventitial alterations are the main features in pulmonary artery remodeling due to long-term chronic intermittent hypobaric hypoxia in rats. Biomed Res Int 2015; 2015: 169841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Calderon-Gerstein WS, Lopez-Pena A, Macha-Ramirez R, et al. Endothelial dysfunction assessment by flow-mediated dilation in a high-altitude population. Vasc Health Risk Manag 2017; 13: 421–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhu J, Kang J, Li X, et al. Chronic intermittent hypoxia vs chronic continuous hypoxia: effects on vascular endothelial function and myocardial contractility. Clin Hemorheol Microcirc 2020; 74(4): 417–427. [DOI] [PubMed] [Google Scholar]
- 68.Navarrete-Opazo A, Mitchell GS. Therapeutic potential of intermittent hypoxia: a matter of dose. Am J Physiol Regul Integr Comp Physiol 2014; 307: R1181–R1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Basnyat B, Murdoch DR. High-altitude illness. Lancet 2003; 361: 1967–1974. [DOI] [PubMed] [Google Scholar]
- 70.West JB. Oxygen conditioning: a new technique for improving living and working at high altitude. Physiology 2016; 31: 216–222. [DOI] [PubMed] [Google Scholar]