Abstract
Objectives
Heart failure (HF) is a common and potentially fatal condition. In 2015, HF affected approximately 40 million people globally. Evidence showing that the use of nitrates can improve clinical outcomes in patients with HF is limited. This study aimed to assess the effect of nitrates on functional capacity and exercise time in patients with HF.
Methods
PubMed, Cochrane Library, and Embase databases were reviewed for articles on the use of nitrates and other treatments for patients with HF. The primary endpoints were the 6-minute walk test distance, exercise time, and quality of life. Secondary endpoints were all-cause mortality, arrhythmia, hospitalization, and worsening HF. The weighted mean difference, risk ratio, and 95% confidence interval were calculated.
Results
A total of 14 related studies that comprised 26,321 patients were included. No significant differences were found in the 6-minute walk test distance, exercise time, and quality of life between the nitrate and control treatment groups. There were also no differences in all-cause mortality, the incidence of arrhythmia, hospitalization, and worsening HF between these two groups.
Conclusion
Patients with HF who receive nitrate treatment do not have better quality of life or exercise capacity compared with controls.
Keywords: Heart failure, meta-analysis, nitrate, 6-minute walk test, exercise, quality of life
Introduction
Cardiovascular disease (CVD) is the main cause of morbidity and mortality worldwide.1,2 CVD is associated with a decline in quality of life and an increase in disability, greatly increasing the cost of medical treatment.3,4 Heart failure (HF) is one of the most common CVDs in the world. HF continues to be a huge burden on patients, care givers, and health care systems. In Europe, approximately 6.5 million people are currently living with CVD,5 with a prevalence of ≥ 10% among patients aged 70 years and older.6 HF is the endpoint of all CVDs.7 The number of patients with HF has increased rapidly in most industrialized countries because of an increase in survival after acute myocardial infarction and aging of the population.6 HF is an important cause of death in the UK, where approximately 5% of people die from HF,5 and only 25% of patients survive beyond 5 years after their first admission.8
Organic nitrates are often used to treat HF.9–11 Short-term studies have shown that nitrate-induced venodilation significantly reduces right and left ventricular filling pressures, while arterial dilation is less obvious. Heart rate remains stable, while cardiac output usually slightly increases.12–14 Hemodynamic effects of nitrates might reduce pulmonary congestion and improve exercise ability of patients with preserved ejection fraction.15 Inadequate improvement in exercise endurance and its adverse effect on daily activity levels may be associated with the pathophysiology of HF with preserved ejection fraction. Vascular stiffness and ventricular contraction, chronotropic incompetence, autonomic nerve dysfunction, and changes in baroreflex sensitivity are usual and may restrict hemodynamic improvement of nitrates.16,17 Hydralazine and nitrate may work together in patients with congestive HF.18 While nitrate therapy appears to be beneficial for HF,19,20 hydralazine is also an effective vasodilator with some antioxidant effects that help prevent non-degradation.21 Although the exact cardiovascular properties of hydralazine are not fully understood, it directly reduces peripheral vascular resistance by relaxing vascular smooth muscle cells.22 Hydralazine might also inhibit activation of a membrane-related oxidase responsible for increasing production of superoxide, which causes nitrate tolerance.23
Several studies have compared nitrates with control treatments for HF, but no consistent outcomes have been reported.24–36 Therefore, this meta-analysis was conducted to assess the efficacy and safety of nitrates compared with control treatment for HF.
Material and methods
The present meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis guidelines37 and by following the published research protocol on PROSPERO (CRD: 42017069882). Ethics approval of the study protocol was not required because the study was a meta-analysis.
The following terms and their combinations were used to search PubMed, Embase, and Cochrane library databases for relevant studies up to September 2019: “nitrate/nitrates” and “heart failure.” All scanned citations, abstracts, and studies were reviewed. Additionally, references to retrieved manuscripts were cross-searched manually for further relevant publications.
The criteria for inclusion were as follows: (1) studies that involved patients with HF (regardless of ejection fraction or phenotype); (2) studies that had at least two groups, with one group receiving treatment with nitrates and another group receiving treatment without nitrates; and (3) studies that provided risk ratios (RRs) with 95% confidence intervals (CIs) or the ability to calculate these statistics from the data provided. The criteria for exclusion were as follows: (1) studies that used an overlapping database or the same population; and (2) studies that used cell or animal models.
All available data were extracted independently by two researchers on the basis of above-mentioned inclusion criteria. Subsequently, any differences were resolved through discussions with a third author. The following data were extracted from each study: first author’s name, year of publication, research design, country, sex, mean age, sample size, follow-up time, and outcomes assessed. The primary endpoints were the 6-minute walk test distance, exercise time, and quality of life. The secondary endpoints were all-cause mortality, arrhythmia, hospitalization, and worsening HF. The Cochrane Collaboration’s risk-of-bias tool was used to evaluate the quality of randomized, controlled trials (RCTs) for assessment of bias risk.38 The Newcastle–Ottawa 9-star system was used to assess the quality of cohort studies. A high-quality study was defined as a study with more than seven stars.39
The weighted mean difference (WMD) and 95% CI were calculated for continuous data, and the RR and 95% CI were calculated for binary data. Data were combined according to the random-effects (DerSimonian and Laird’s method) or fixed-effects model depending on the chi-square-based Q test and I2 statistic. A P value < 0.05 was considered statistically significant. If heterogeneity was significant, the random-effects model was adopted. Otherwise, a fixed-effects model was adopted. By removing one study for sensitivity analysis, the relative effect of each study on comprehensive assessment was evaluated. Publication bias was assessed by visual inspection of Begg’s funnel plot symmetry and evaluation of Egger’s test (P < 0.05). Statistical analysis was performed using Stata software version 12.0 (Stata Corp, College Station, TX, USA) and all tests were double sided.
Results
Studies and outcomes
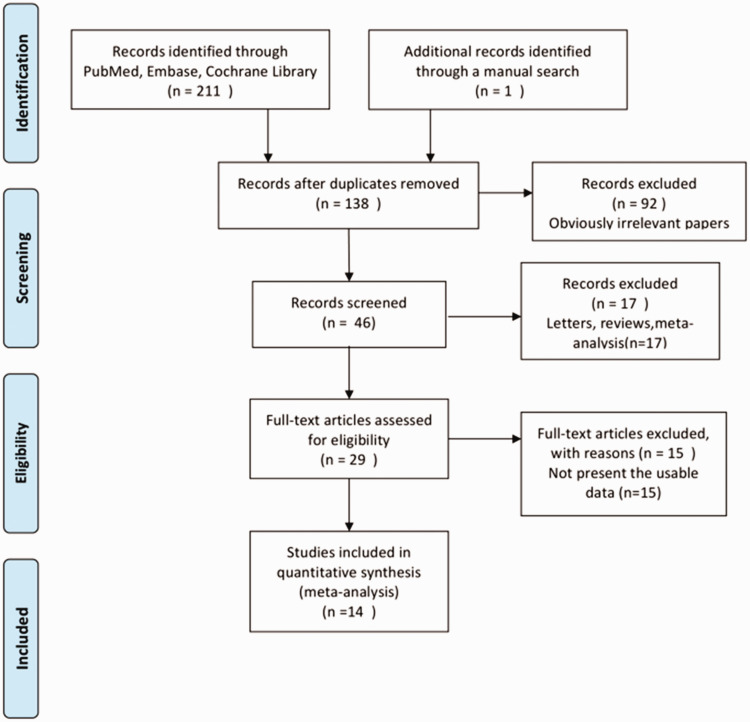
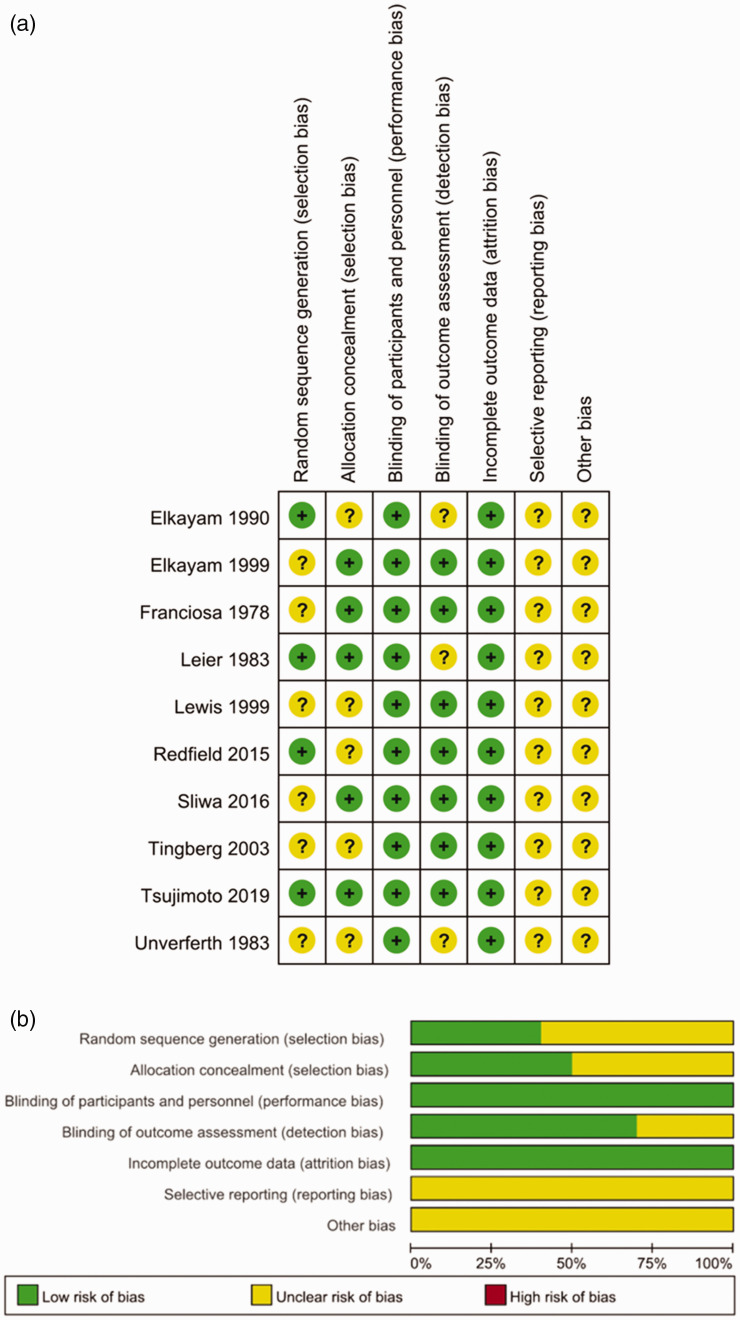
A total of 138 studies were related to the search terms and 92 unrelated studies were subsequently excluded. The remaining studies were systematically evaluated and 29 studies were full text. After reading the full text, 15 studies were considered inappropriate and excluded, and the remaining 14 studies were qualitatively analyzed. Finally, this meta-analysis included 14 articles, which involved 26,321 patients. The study selection process is shown in Figure 1. All studies were clinical studies, including nine RCTs and four cohort studies. The 14 studies represented 7647 and 18,674 patients with HF in the nitrate group and the control treatment group, respectively. The follow-up interval of these studies ranged from 1 to 57 months. A summary of biases determined in each RCT is shown in Figure 2. All of the included studies were of medium or high quality. The main features of the qualified studies are shown in Table 1. Table 2 lists the methodological quality of cohort studies based on the Newcastle–Ottawa Scale. The quality of the cohort studies was generally high; two studies had eight stars and two studies had seven stars.
Figure 1.
Flow diagram of identification of studies.
Figure 2.
Risk of bias assessments for randomized trials included in the meta-analysis. (a) Risk of bias summary; and (b) risk of bias graph. (+): Low risk of bias; (?): unclear risk of bias; and (–): high risk of bias.
Table 1.
Characteristics of the studies included in this meta-analysis.
| Author/year of publication | Country | Sex (male, %) | Mean age (years) | Phenotype |
Intervention |
Follow-up (months) | Study design | Outcomes assessed | |
|---|---|---|---|---|---|---|---|---|---|
| Nitrates | Control | ||||||||
| Franciosa/197818 | USA | 93.7 | 55.3 ± 1.5 | Congestive | ISDN 160 mg PO (N = 7) | Placebo (N = 7) | 4 | RCT | All-cause mortality, hospitalization, arrhythmia, worsening heart failure |
| Leier/198319 | USA | 80 | Nitrate: 51 ± 16 Control: 47 ± 15 |
Congestive | ISDN 160 mg PO (N = 13) | Placebo (N = 17) | 3 | RCT | All-cause mortality |
| Unverferth/198320 | USA | 75.5 | 57 ± 10 | Congestive | ISDN 160 mg PO (N = 6) | Placebo (N = 11) | 3 | RCT | All-cause mortality |
| Elkayam/199021 | USA | 89.3 | 55 ± 10 | Congestive | ISDN 160 mg PO (N = 20) | Nifedipine 80 mg PO (N = 21) | 6.5 | RCT | Treadmill exercise time |
| Lewis/199923 | Israel | 94.1 | 57 ± 8 | Congestive | IS-5-MN 50 mg PO (N = 67) | Placebo (N = 69) | 6 | RCT | All-cause mortality, arrhythmia, worsening heart failure |
| Elkayam/199922 | USA | 82.7 | Nitrate: 48 ± 3 Control: 48 ± 4 |
HFrEF | NTG 57 ± 5 mg transdermal patch OD (N = 14) | Placebo (N = 15) | 3 | RCT | All-cause mortality, hospitalization, quality of life, treadmill exercise time |
| Tingberg/200324 | Sweden | 75 | Nitrate: 63 ± 10 Control: 65 ± 9 |
HFrEF | ISMN 60 mg PO (N = 47) | Placebo (N = 45) | 12 | RCT | All-cause mortality, hospitalization, worsening heart failure |
| Herrero-Puente/201525 | Spain | 43.4 | Nitrate: 78.6 ± 10.2 Control: 79.8 ± 9.9 |
Acute | NTG 50 mg IV (N = 796) | Placebo (N = 2382) | 1 | Cohort | All-cause mortality |
| Redfield/201526 | USA | 42.7 | Nitrate: 69 ± 9 Control: 69 ± 10 |
HFpEF | ISMN 30–120 mg PO (N = 51) | Placebo (N = 59) | 1.5 | RCT | Arrhythmia, worsening heart failure, 6-minute walk test distance, quality of life |
| Ho/201627 | Canada | 51.7 | 77 | Acute | NTG (N = 3153) | No nitrates (N = 7925) | 12 | Cohort | All-cause mortality, hospitalization |
| Sliwa/201628 | South Africa | 59.9 | Nitrate: 56.7 ± 12.7 Control: 56.9 ± 13.3 |
Acute | Hydralazine 50 mg/ISDN 20 mg IV (N = 518) | Placebo (N = 532) | 6 | RCT | 6-minute walk test distance |
| Lim/201729 | Sweden | 51.9 | Nitrate: 79 ± 9 Control: 79 ± 8 |
HFpEF | ISDN/ISMN (N = 2235) | No nitrates (N = 4470) | 25 | Cohort | All-cause mortality |
| Ural/201730 | Turkey | 68.9 | 65.1 ± 11.6 | HFrEF | ISDN/ISMN 20–60 mg PO (N = 212) | No nitrates (N = 212) | 56 | Cohort | All-cause mortality, hospitalization |
| Tsujimoto/201946 | Japan | 48.4 | Nitrate: 69.6 ± 9.2 Control: 68.4 ± 9.6 |
HFpEF | Nitrates (N = 508) | No nitrates (N = 2909) | 57 | RCT | Hospitalization |
ISDN: isosorbide dinitrate; NTG: nitroglycerin; ISMN: isosorbide mononitrate; IS-5-MN: inosorbide-5-mononitrate; RCT: randomized, controlled trial; HFrEF: heart failure with reduced ejection fraction; HFpEF: heart failure with preserved ejection fraction; IV: intravenously; PO: per os (orally); OD: once a day.
Table 2.
Methodological quality of cohort studies included in the meta-analysis.*
| First author/year | Representativeness of the exposed cohort | Selection of the unexposed cohort | Ascertainment of exposure | Outcome of interest not present at the start of the study | Control for important factor or additional factor | Outcome assessment | Follow-up long enough for outcomes to occur | Adequacy of follow-up of cohorts | Total quality scores |
|---|---|---|---|---|---|---|---|---|---|
| Herrero-Puente/2015 | ⋆ | ⋆ | ⋆ | ⋆ | ⋆ | ⋆ | ⋆ | ⋆ | 8 |
| Ho/2016 | ⋆ | ⋆ | ⋆ | ⋆ | ⋆ | ⋆ | ⋆ | ⋆ | 8 |
| Lim/2017 | ⋆ | ⋆ | ⋆ | — | ⋆ | ⋆ | ⋆ | ⋆ | 7 |
| Ural/2017 | ⋆ | ⋆ | ⋆ | — | ⋆ | ⋆ | ⋆ | ⋆ | 7 |
*A study could be awarded a maximum of one star for each item, except for the item “control for important factor or additional factor”.
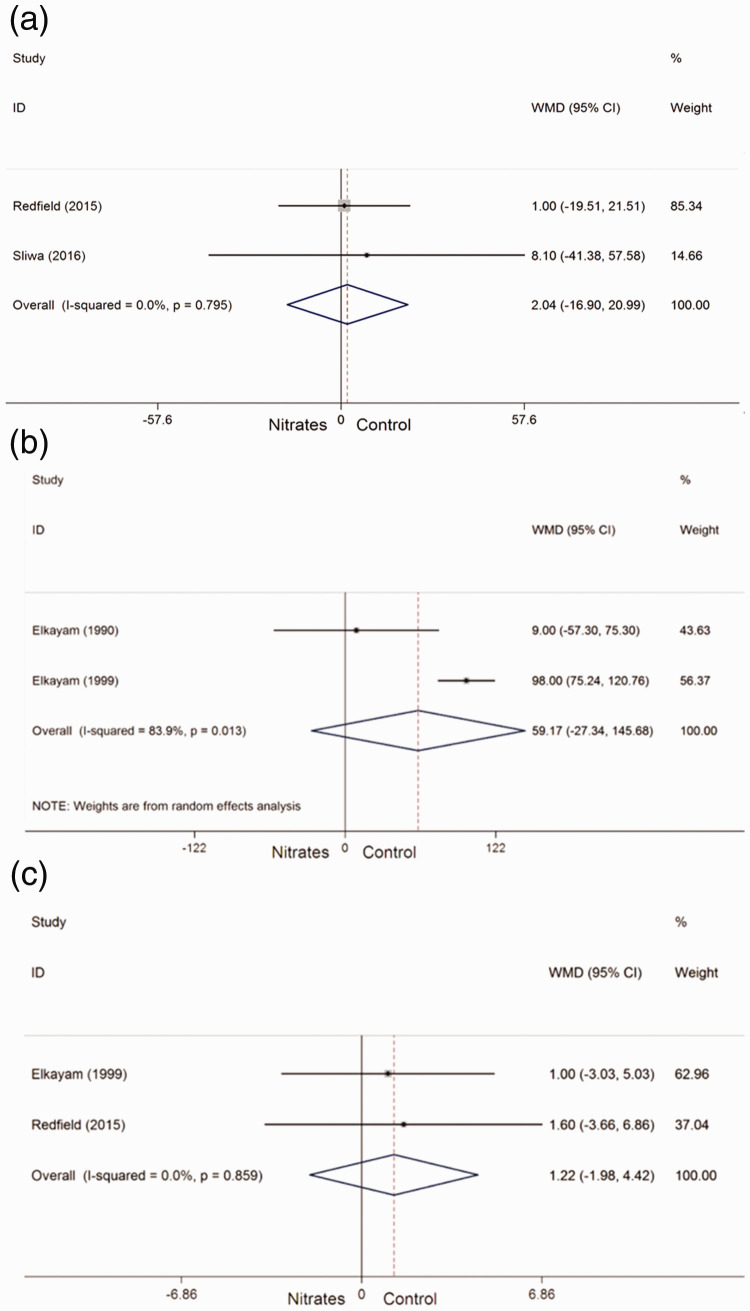
Two studies32,34 provided outcomes regarding the 6-minute walk test distance in patients who received nitrates and control treatments. No heterogeneity was found among the two studies. Therefore, a fixed-effects model was used. The 6-minute walk test distance was not significantly different between patients who received nitrates and those who received control treatment (WMD = 2.04, 95% CI: −16.90–20.99, Pheterogeneity = 0.795, I2 = 0%) (Figure 3a).
Figure 3.
Forest plots showing the effect of nitrates on primary outcomes in patients with heart failure. (a) Six-minute walk test distance; (b) exercise time; and (c) quality of life. WMD, weighted mean difference; CI, confidence interval.
Two studies27,28 provided outcomes regarding the exercise time in patients who received nitrates and control treatments, and were included in the meta-analysis. Significant heterogeneity was found among the two studies. Therefore, a random-effects model was used. No significant difference was observed in the exercise time between patients who received nitrates and those who received control treatment (WMD = 59.17, 95% CI: −27.34–145.68, Pheterogeneity = 0.013, I2 = 83.9%) (Figure 3b).
Two studies28,32 provided outcomes regarding quality of life in patients who received nitrates and control treatments. No significant heterogeneity was found among the two studies. Therefore, a fixed-effects model was used. Quality of life was not significantly different between patients who received nitrates and those who received control treatment (WMD = 1.22, 95% CI: −1.98–4.42, Pheterogeneity = 0.859, I2 = 0%) (Figure 3c).
All-cause mortality
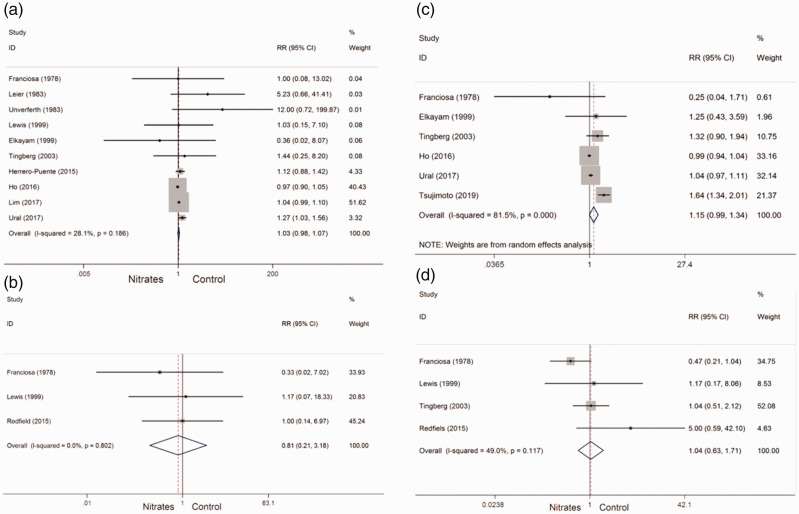
A total of 14 studies were included in the meta-analysis of adverse events. All-cause mortality was reported in 10 studies,18–20,22–25,27,29,30 which all compared nitrate with control treatments. A low heterogeneity between studies was found. Therefore, the fixed-effects model was used. The incidence of all-cause mortality was not significantly different between patients who received nitrates and those who received control treatment (RR = 1.03, 95% CI: 0.98–1.07, Pheterogeneity = 0.186, I2 = 28.1%) (Figure 4a).
Figure 4.
Pooled risk ratio of adverse events with nitrates in patients with heart failure. (a) All-cause mortality; (b) arrhythmia; (c) hospitalization; and (d) worsening heart failure. RR, risk ratio; CI, confidence interval.
Arrhythmia
Arrhythmia was reported in three studies.24,29,32 No significant heterogeneity between studies was found. Therefore, the fixed-effects model was used. The incidence of arrhythmia was not significantly different between patients who received nitrates and those who received control treatment (RR = 0.81, 95% CI: 0.21–3.18, Pheterogeneity = 0.802, I2 = 0%) (Figure 4b).
Hospitalization
Hospitalization was reported in six studies.24,28,30,33,36,40 A high heterogeneity between studies was found. Therefore, the random-effects model was used. The incidence of hospitalization was not significantly different between patients who received nitrates and those who received control treatment (RR = 1.15, 95% CI: 0.99–1.34, Pheterogeneity < 0.001, I2 = 81.5%) (Figure 4c).
Worsening HF
Worsening HF was reported in four studies.24,29,30,32 A low heterogeneity between studies was found. Therefore, the fixed-effects model was used. However, the incidence of worsening HF was not significantly different between patients who received nitrates and those who received control treatment (RR = 1.04, 95% CI: 0.63–1.71, Pheterogeneity = 0.117, I2 = 49%) (Figure 4d).
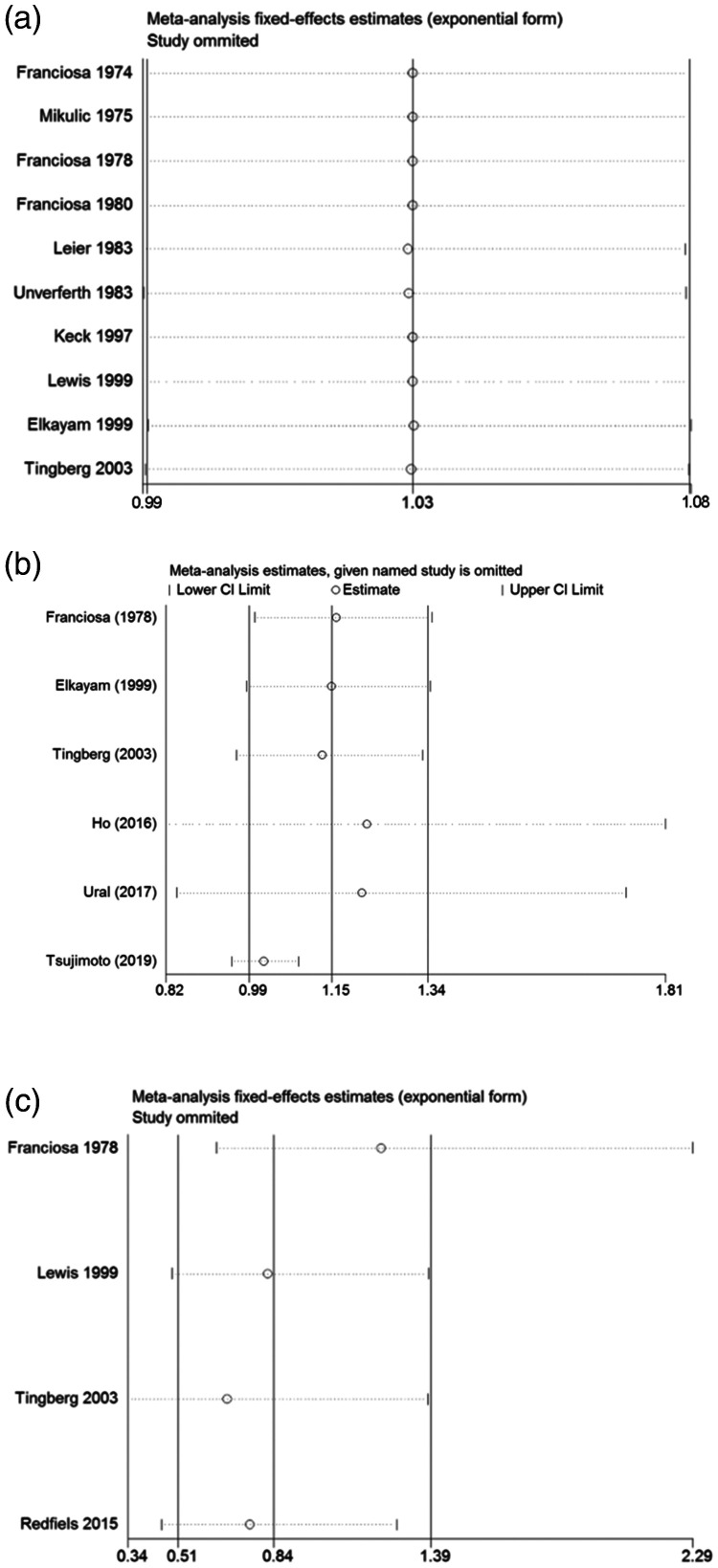
Sensitivity analysis was used to evaluate the effect of a single data set on the summary results by deleting each eligible study in turn. As shown in Figure 5a, 5b, and 5c, when any single study was removed, the overall statistical significance remained the same, which indicated that the results were statistically robust.
Figure 5.
Sensitivity analysis examining the effect of individual studies on pooled results. (a) All-cause mortality; (b) hospitalization; and (c) worsening heart failure. CI, confidence interval.
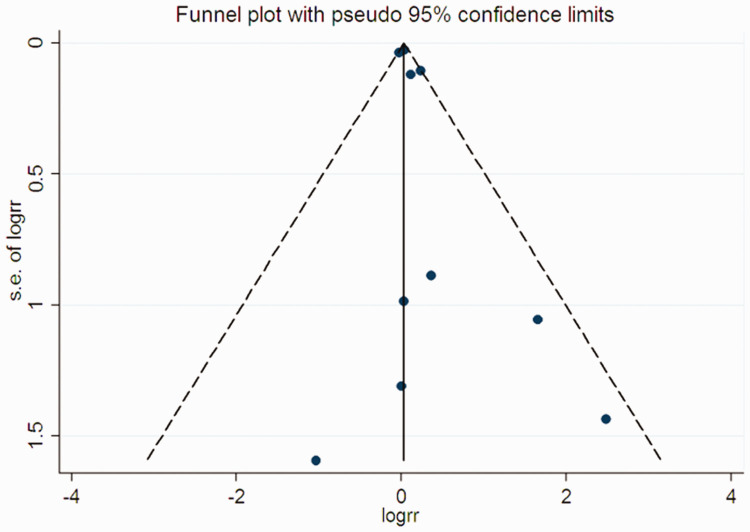
Finally, some degree of asymmetry in funnel plots was observed. However, Begg’s and Egger’s tests showed no significant publication bias in all-cause mortality (Begg’s test: P = 0.721; Egger’s test: P = 0.158) (Figure 6).
Figure 6.
Funnel plot for a publication bias test of all-cause mortality. Each point represents a separate study for the indicated association. s.e., standard error; logrr, logarithmic risk ratio.
Discussion
HF is a common and potentially fatal condition. Signs and symptoms of HF commonly include shortness of breath, excessive tiredness, and leg swelling.5,6 Patients suffer from exercise intolerance, which diminishes their ability to perform normal activities of daily living, and thus compromises their quality of life. Evidence showing that use of nitrates in HF can improve clinical outcomes is limited.5,6,28 Some studies compared treatments with nitrate and without nitrate in patients with HF to examine the benefits of nitrates.35,36 This systematic review and meta-analysis compared the efficacy and safety of nitrates for patients with HF. We found no significant difference in the 6-minute walk test distance, exercise time, and quality of life between the nitrate and control treatment groups. Moreover, no significant differences were found in all-cause mortality, the incidence of arrhythmia, hospitalization, and worsening HF between these two groups.
A meta-analysis is a method used to obtain quantitative information-related evidence based on a complete set of studies.41 A typical systematic review uses a meta-analysis method to combine the effects of a specific interest estimate and obtain a summary estimation effect.42 The present meta-analysis, which comprised 26,321 patients with HF from 14 studies, is the most extensive study to investigate the safety and efficacy of nitrates to date. Recently, Farag et al.43 conducted a systematic review of the efficacy and safety of nitrates in patients with HF. In the present meta-analysis, more eligible studies were identified and a detailed analysis was performed, whereas Farag et al.43 only analyzed a single study by systematic review.
In our analysis, the quality of randomized and nonrandomized studies was assessed using the Cochrane Collaboration’s risk-of-bias tool and the Newcastle–Ottawa scale, respectively. Using the Cochrane Collaboration’s risk-of-bias tool, we found only a moderate degree of confidence in the results. This finding is attributed to an unclear risk of selection bias observed among most of the included studies because of an unclear description of the exact randomization procedure and/or allocation concealment and also because of stratification during randomization. Begg’s funnel plot and Egger’s test were used to assess the publication bias of published studies in our study, and no publication bias was found.
According to the European Society of Cardiology Heart Failure Long-Term Registry,44 25% of patients with acute HF took nitrate orally before admission, and the use of prescription drugs increased to 32%. In patients with HF with reduced ejection fraction, the utilization rate of nitrate was 18%, while hydrazine was not used in combination with nitrate. Because 54% of patients with acute HF and 43% of patients with chronic HF have ischemic etiology, the relatively high use of nitrate may be attributed to related ischemic heart disease. However, limited data are available to support oral nitrate use in these patients. The efficacy of nitrate alone in treatment of ischemic HF was retrospectively investigated in a single-center study.44 During the 5-year follow-up period, the use of chronic nitrates (N = 83) was associated with a higher incidence of fatal or nonfatal myocardial infarction or stroke. Despite some limitations, such as few patients, a lack of tendency to match, and relatively few adverse events, this previous study showed that use of nitrate increased mortality. Farag et al.45 conducted a systematic review of RCTs to assess whether use of hydrazine and nitrate alone or in combination could improve survival rates. Although combined use of these two drugs reduced the total mortality and mortality rates of cardiovascular disease compared with placebo, both types of mortality rates were significantly higher compared with those with angiotensin-converting enzyme inhibition. The effects of nitrate alone on mortality were tested in seven studies, and a two-fold increase in total mortality compared with placebo was found. However, the incidence of events was small (N = 13), especially in the nitrate group, which was a reliable comparison. A strength of the present meta-analysis was that the number of patients was relatively high. However, our analysis showed that nitrate did not improve the daily activity level, submaximal exercise ability, and quality of life scores.
The effects of nitrate alone on the long-term prognosis of patients with HF with preserved ejection fraction using life-saving and disease-improved therapies are unclear. At present, the only trial on the use of nitrate was performed in patients with HF with preserved ejection fraction, and no beneficial effect on motor ability was shown in 6 weeks after nitrate treatment.32 Nevertheless, nitrates may have additional hemodynamic benefits for patients with HF with reduced ejection fraction who have ongoing symptoms, despite optimal management. Nitrate is used in combination with other vasodilators, such as enalapril, to help influence clinical outcomes by improving the nitrate–nitrite–nitric oxide pathway and reducing preload caused by venous vasodilation.
Our meta-analysis has some limitations follows. First, the analysis might have been compromised by extracting raw data from the included studies. Second, the follow-up time of 14 studies (from 1–57 months) greatly varied, limiting the assessment of long-term clinical efficacy of nitrates in patients with HF. Third, the language might also have introduced prejudice. Specifically, only English was selected to exclude other qualified researchers. Fourth, only two studies for each of the different outcomes met the inclusion criteria. Therefore, controlling for any significant heterogeneity was difficult if it existed. Finally, this study included HF with preserved and reduced ejection fractions. Therefore, clinical and pathophysiological differences might have been a source of clinical heterogeneity.
Conclusions
Despite the limitations, this meta-analysis shows no improvement in the quality of life or submaximal exercise capacity in patients with HF who have nitrate treatment compared with patients with HF receiving control treatment. Additionally, nitrate does not affect all-cause mortality and the hospitalization risk in patients with HF. Further research is required on larger data sets and well-designed models to validate our results.
Acknowledgments
We are grateful to Professor Licheng Zhao (Guangzhou University of Chinese Medicine) for his inspiring guidance in this work. We also thank Miss Jingyi Xu for her critical revision of the article.
Author contributions
ZY and SX contributed to the conception or design of the work. WL, JL, YN, ZZ, TW, and JK contributed to acquisition, analysis, or interpretation of data for the work. QL and WL drafted the manuscript. LW and HL critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This work was funded by the National Science Foundation of China (grant numbers: 8170151228, 81473621, and 817015128) and the National Key Research and Development Plan (No. 2018YFC1707401).
ORCID iD
Zhongqi Yang https://orcid.org/0000-0003-3582-2672
References
- 1.Cao B, Bray F, Beltran-Sanchez H, et al. Benchmarking life expectancy and cancer mortality: global comparison with cardiovascular disease 1981-2010. BMJ 2017; 357: j2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bansilal S, Castellano JM, Fuster V. Global burden of CVD: focus on secondary prevention of cardiovascular disease. Int J Cardiol 2015; 201: S1–S17. [DOI] [PubMed] [Google Scholar]
- 3.Hameed SS, Rawal I, Soni D, et al. Technology for diagnosis, treatment, and prevention of cardiometabolic disease in India. Prog Cardiovasc Dis 2016; 58: 620–629. [DOI] [PubMed] [Google Scholar]
- 4.Anchique Santos CV, Lopez-Jimenez F, Benaim B, et al. Cardiac rehabilitation in Latin America. Prog Cardiovasc Dis 2014; 57: 268–275. [DOI] [PubMed] [Google Scholar]
- 5.Tendera M. Epidemiology, treatment, and guidelines for the treatment of heart failure in Europe. Eur Heart J Suppl 2005; 7: J5–J9. [Google Scholar]
- 6.Mcmurray JJV, Stewart S. The burden of heart failure. Eur Heart J Suppl 2003; 5: 3–13. [Google Scholar]
- 7.Lane DA, Chong AY, Lip GY. Psychological interventions for depression in heart failure. Cochrane Database Syst Rev 2005; 5: CD003329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stewart S, MacIntyre K, Capewell S, et al. Heart failure and the aging population: an increasing burden in the 21st century? Heart 2003; 89: 49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohn JN. Nitrates for congestive heart failure. Am J Cardiol 1985; 56: 19A–23A. [DOI] [PubMed] [Google Scholar]
- 10.Yusuf S, Pitt B, Davis CE, et al. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991; 325: 293–302. [DOI] [PubMed] [Google Scholar]
- 11.Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med 1997; 336: 525–533. [DOI] [PubMed] [Google Scholar]
- 12.Dupuis J, Lalonde G, Lebeau R, et al. Sustained beneficial effect of a seventy-two hour intravenous infusion of nitroglycerin in patients with severe chronic congestive heart failure. Am Heart J 1990; 120: 625–637. [DOI] [PubMed] [Google Scholar]
- 13.Dupuis J, Lalonde G, Bichet D, et al. Captopril does not prevent nitroglycerin tolerance in heart failure. Can J Cardiol 1990; 6: 281–286. [PubMed] [Google Scholar]
- 14.Mehra A, Shotan A, Ostrzega E, et al. Potentiation of isosorbide dinitrate effects with N-acetylcysteine in patients with chronic heart failure. Circulation 1994; 89: 2595–2600. [DOI] [PubMed] [Google Scholar]
- 15.Zakeri R, Levine JA, Koepp GA, et al. Nitrate's effect on activity tolerance in heart failure with preserved ejection fraction trial: rationale and design. Circ Heart Fail 2015; 8: 221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwartzenberg S, Redfield MM, From AM, et al. Effects of vasodilation in heart failure with preserved or reduced ejection fraction implications of distinct pathophysiologies on response to therapy. J Am Coll Cardiol 2012; 59: 442–451. [DOI] [PubMed] [Google Scholar]
- 17.Von Lueder TG, Atar D. Comorbidities and polypharmacy. Heart Fail Clin 2014; 10: 367–372. [DOI] [PubMed] [Google Scholar]
- 18.Hare JM. Nitroso-redox balance in the cardiovascular system. N Engl J Med 2004; 351: 2112–2114. [DOI] [PubMed] [Google Scholar]
- 19.Macdonald P, Schyvens C, Winlaw D. The role of nitric oxide in heart failure. Potential for pharmacological intervention. Drugs Aging 1996; 8: 452–458. [DOI] [PubMed] [Google Scholar]
- 20.Dixon LJ, Morgan DR, Hughes SM, et al. Functional consequences of endothelial nitric oxide synthase uncoupling in congestive cardiac failure. Circulation 2003; 107: 1725–1728. [DOI] [PubMed] [Google Scholar]
- 21.Cole RT, Gupta D, Butler J. Current perspectives on hydralazine and nitrate therapies in heart failure. Heart Fail Clin 2014; 10: 565–576. [DOI] [PubMed] [Google Scholar]
- 22.Knowles HJ, Tian YM, Mole DR, et al. Novel mechanism of action for hydralazine: induction of hypoxia-inducible factor-1alpha, vascular endothelial growth factor, and angiogenesis by inhibition of prolyl hydroxylases. Circ Res 2004; 95: 162–169. [DOI] [PubMed] [Google Scholar]
- 23.Munzel T, Kurz S, Rajagopalan S, et al. Hydralazine prevents nitroglycerin tolerance by inhibiting activation of a membrane-bound NADH oxidase. A new action for an old drug. J Clin Invest 1996; 98: 1465–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Franciosa JA, Nordstrom LA, Cohn JN. Nitrate therapy for congestive heart failure. JAMA 1978; 240: 443–446. [PubMed] [Google Scholar]
- 25.Leier CV, Huss P, Magorien RD, et al. Improved exercise capacity and differing arterial and venous tolerance during chronic isosorbide dinitrate therapy for congestive heart failure. Circulation 1983; 67: 817–822. [DOI] [PubMed] [Google Scholar]
- 26.Unverferth DV, Mehegan JP, Magorien RD, et al. Regression of myocardial cellular hypertrophy with vasodilator therapy in chronic congestive heart failure associated with idiopathic dilated cardiomyopathy. Am J Cardiol 1983; 51: 1392–1398. [DOI] [PubMed] [Google Scholar]
- 27.Elkayam U, Amin J, Mehra A, et al. A prospective, randomized, double-blind, crossover study to compare the efficacy and safety of chronic nifedipine therapy with that of isosorbide dinitrate and their combination in the treatment of chronic congestive heart failure. Circulation 1990; 82: 1954–1961. [DOI] [PubMed] [Google Scholar]
- 28.Elkayam U, Johnson JV, Shotan A, et al. Double-blind, placebo-controlled study to evaluate the effect of organic nitrates in patients with chronic heart failure treated with angiotensin-converting enzyme inhibition. Circulation 1999; 99: 2652–2657. [DOI] [PubMed] [Google Scholar]
- 29.Lewis BS, Rabinowitz B, Schlesinger Z, et al. Effect of isosorbide-5-mononitrate on exercise performance and clinical status in patients with congestive heart failure. Results of the Nitrates in Congestive Heart Failure (NICE) Study. Cardiology 1999; 91: 1–7. [DOI] [PubMed] [Google Scholar]
- 30.Tingberg E, Roijer A, Thilen U, et al. Randomized, double-blind, placebo-controlled long-term study of isosorbide-5-mononitrate therapy in patients with left ventricular dysfunction after acute myocardial infarction. Am Heart J 2003; 145: E1. [DOI] [PubMed] [Google Scholar]
- 31.Herrero-Puente P, Jacob J, Martin-Sanchez FJ, et al. Influence of Intravenous nitrate treatment on early mortality among patients with acute heart failure. NITRO-EAHFE Study. Rev Esp Cardiol (Engl Ed) 2015; 68: 959–967. [DOI] [PubMed] [Google Scholar]
- 32.Redfield MM, Anstrom KJ, Levine JA, et al. Isosorbide mononitrate in heart failure with preserved ejection fraction. N Engl J Med 2015; 373: 2314–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ho EC, Parker JD, Austin PC, et al. Impact of nitrate use on survival in acute heart failure: a propensity-matched analysis. J Am Heart Assoc 2016; 5: e002531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sliwa K, Damasceno A, Davison BA, et al. Bi treatment with hydralazine/nitrates vs. placebo in Africans admitted with acute HEart Failure (BA-HEF). Eur J Heart Fail 2016; 18: 1248–1258. [DOI] [PubMed] [Google Scholar]
- 35.Lim SL, Benson L, Dahlstrom U, et al. Association between use of long-acting nitrates and outcomes in heart failure with preserved ejection fraction. Circ Heart Fail 2017; 10: e003534. [DOI] [PubMed] [Google Scholar]
- 36.Ural D, Kandemir AS, Karauzum K, et al. Effect of oral nitrates on all-cause mortality and hospitalization in heart failure patients with reduced ejection fraction: a propensity-matched analysis. J Card Fail 2017; 23: 286–292. [DOI] [PubMed] [Google Scholar]
- 37.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009; 151: 264–269, w64. [DOI] [PubMed] [Google Scholar]
- 38.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010; 25: 603–605. [DOI] [PubMed] [Google Scholar]
- 40.Tsujimoto T, Kajio H. Use of nitrates and risk of cardiovascular events in patients with heart failure with preserved ejection fraction. Mayo Clin Proc 2019; 94: 1210–1220. [DOI] [PubMed] [Google Scholar]
- 41.Egger M, Smith GD. Meta-Analysis. Potentials and promise. BMJ 1997; 315: 1371–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zwahlen M, Renehan A, Egger M. Meta-analysis in medical research: potentials and limitations. Urol Oncol 2008; 26: 320–329. [DOI] [PubMed] [Google Scholar]
- 43.Farag M, Shoaib A, Gorog DA. Nitrates for the management of acute heart failure syndromes, A systematic review. J Cardiovasc Pharmacol Ther 2017; 22: 20–27. [DOI] [PubMed] [Google Scholar]
- 44.Maggioni AP, Anker SD, Dahlstrom U, et al. Are hospitalized or ambulatory patients with heart failure treated in accordance with European Society of Cardiology guidelines? Evidence from 12,440 patients of the ESC Heart Failure Long-Term Registry. Eur J Heart Fail 2013; 15: 1173–1184. [DOI] [PubMed] [Google Scholar]
- 45.Farag M, Mabote T, Shoaib A, et al. Hydralazine and nitrates alone or combined for the management of chronic heart failure: a systematic review. Int J Cardiol 2015; 196: 61–69. [DOI] [PubMed] [Google Scholar]