Abstract
Objective
To investigate the relationship between Helicobacter pylori (H. pylori) infection and gallstones or gallbladder polyps.
Methods
This retrospective analysis included 27,881 individuals who underwent health examinations that included a H. pylori test and an abdominal ultrasound scan. Patients were divided into four groups: gallbladder polyp (P group), gallstone (S group), gallstone and gallbladder polyp (SP group), and no gallbladder disease (N group). Case–control matching was used to select the participants in the control group.
Results
The mean ages of participants in the P, S, and SP groups were all significantly higher than the mean age of participants in the N group. The proportions of participants with each type of body mass index significantly differed between the N and P groups, and between the N and S groups. In total 45.7% of participants exhibited H. pylori infection. After case-control matching, the proportion of participants with H. pylori infection did not significantly differ according to the presence or absence of gallbladder polyps. Similar results were observed regarding gallstones, as well as gallstones and gallbladder polyps.
Conclusion
H. pylori infection might not be related to gallbladder polyps or gallstones.
Keywords: Helicobacter pylori, gallstone, gallbladder polyp, body mass index, gallbladder disease, case-control study
Introduction
Approximately half of the world's population is infected with Helicobacter pylori (H. pylori), and the rate of infection is reportedly 55% in China.1 There is evidence that H. pylori is closely associated with gastric disease, but increasing numbers of studies have suggested that H. pylori is involved in extragastric diseases, such as obesity,2 osteoporosis,3 diabetes mellitus,4 chronic obstructive pulmonary disease,5 and chronic kidney disease.6
The incidence of gallbladder polyps has been reported to range from 3% to 7%, as determined by abdominal ultrasound examination.7–9 Although they are generally considered benign, approximately 2% of gallbladder polyps are reportedly related to gallbladder cancer.10 The main cause of gallbladder polyp formation is regarded as fibrosis due to cholesterol deposition in the gallbladder wall and chronic inflammation of the mucosa.11,12 Approximately 10% to 20% of the adult population exhibits gallstone disease.13 The formation of gallstones is primarily related to metabolic abnormalities.14
To the best of our knowledge, no study has focused on the relationship between H. pylori and gallbladder polyps. Notably, some studies have shown that H. pylori is a causative factor for gallstones, based on the finding of H. pylori DNA in the gallbladder mucosa or cholesterol gallstones.15–18 However, outcomes based on assessment of immunoglobulin G antibodies against H. pylori have been inconsistent.19,20 In this study, we investigated the relationship between H. pylori infection (determined by the 13C or 14C urease breath test) and the presence of gallbladder polyps or gallstones.
Methods
Participants and data collection
This retrospective analysis screened all individuals who underwent health examinations (including H. pylori infection test and abdominal ultrasound examination) in the health management center of Taizhou Hospital (Linhai, China) during the period from January 2017 to December 2019. Patients were excluded if they had invalid data (missing data for one or more study outcomes) or a history of cholecystectomy. H. pylori infection was determined by using a 13C or 14C urease breath test (Shenzhen Zhonghe Headway Bio-Sci & Tech Co., Ltd., Shenzhen, China). Body mass index (BMI) was calculated as weight/height squared (kg/m2). In accordance with Chinese standards,2 BMI values were categorized into the following four levels: underweight (<18.5 kg/m2), normal weight (18.5–23.9 kg/m2), overweight (24.0–27.9 kg/m2), and obesity (≥28.0 kg/m2). Gallbladder disease findings, as confirmed by abdominal ultrasound, were used to divide participants into the following four groups: gallbladder polyp (P group), gallstone (S group), gallstone and gallbladder polyp (SP group), and no gallbladder disease (N group).
Ethics approval
This study was approved by the Institutional Medical Ethics Review Board of Taizhou Hospital. The review board waived the requirement for informed consent because this was a retrospective study that did not include identifying information for any participant.
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics, version 26.0 (IBM Corp., Armonk, NY, USA). Continuous variables were expressed as mean ± standard deviation. Associations involving parametric data were analyzed using Student’s t-test. One-way analysis of variance or chi-squared tests were used for comparisons among groups. A 1:1 case–control matching protocol was implemented to match the essential confounding parameters (sex, match tolerance, 0; age, match tolerances, 2; BMI level, match tolerance, 0) between the P and N groups, between the S and residual N groups, and between the SP and residual N groups in a sequential manner. P < 0.05 was considered statistically significant.
Results
Participant characteristics
During the study period, 151,629 individuals (aged 7–103 years) underwent health examinations in our hospital. Following application of exclusion criteria, 27,881 individuals were included in the study; no individuals under the age of 18 years exhibited gallstones or gallbladder polyps. The numbers of participants in the N, P, S, and SP groups were 24,104 (86.5%), 2764 (9.9%), 926 (3.3%), and 87 (0.3%), respectively.
Comparisons of gallbladder disease status according to sex and age
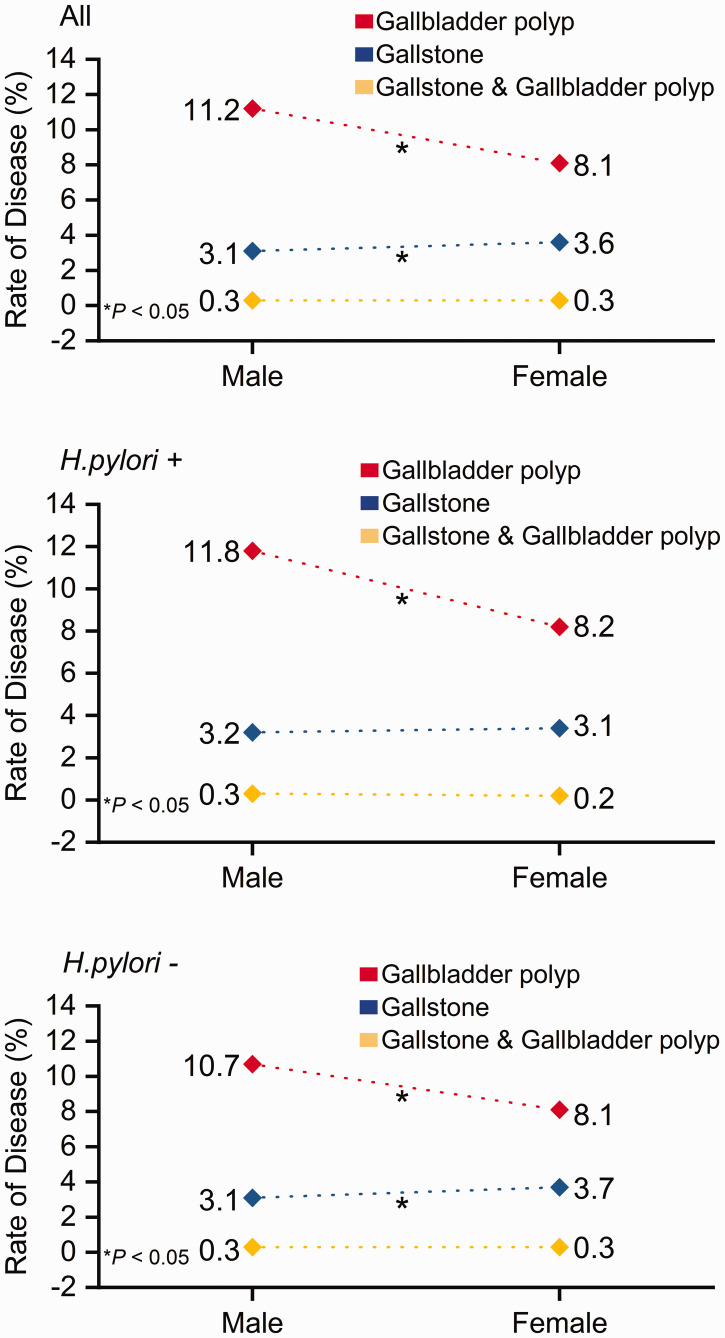
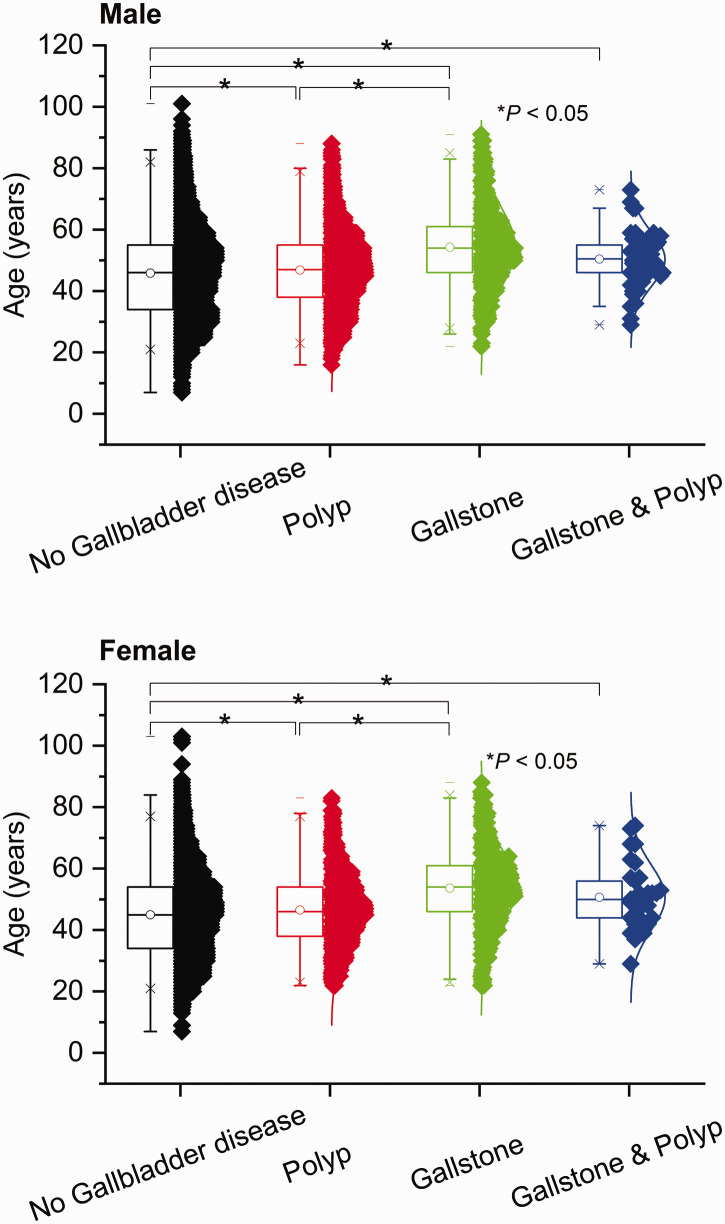
The incidence of gallbladder polyps was higher in men than in women (11.2% vs. 8.1%, P < 0.001), while the incidence of gallstones was lower in men than in women (3.1% vs. 3.6%, P = 0.031) (Figure 1). Among men in this study, the mean ages in the P, S, and SP groups were 46.7 ± 11.9 years, 54.0 ± 12.7 years, and 50.5 ± 9.4 years, respectively; these significantly differed from the mean age (45.5 ± 13.8 years) of men in the N group (P = 0.003, P < 0.001, and P = 0.016, respectively). The mean ages of women in the P, S, and SP groups were 46.5 ± 11.5 years, 53.6 ± 12.7 years, and 50.6 ± 10.5 years, respectively; these significantly differed from the mean age (45.0 ± 13.3 years) of women in the N group (P = 0.001, P < 0.001, and P = 0.014, respectively) (Figure 2). Furthermore, the mean ages significantly differed between the P and S groups in men (P < 0.001) and women (P < 0.001).
Figure 1.
Incidences of gallbladder polyps, gallstones, and gallstones and gallbladder polyps in men and women according to the status of Helicobacter pylori infection (top, all patients; middle, patients with H. pylori infection; bottom, patients without H. pylori infection). Red diamonds, gallbladder polyp group; blue diamonds, gallstone group; yellow diamonds, gallstone and gallbladder polyp group. *P < 0.05.
Figure 2.
Mean ages of participants with gallbladder polyps, gallstones, or gallstones and gallbladder polyps, compared with participants without gallbladder disease, stratified according to sex. *P < 0.05.
Comparison of BMI among groups
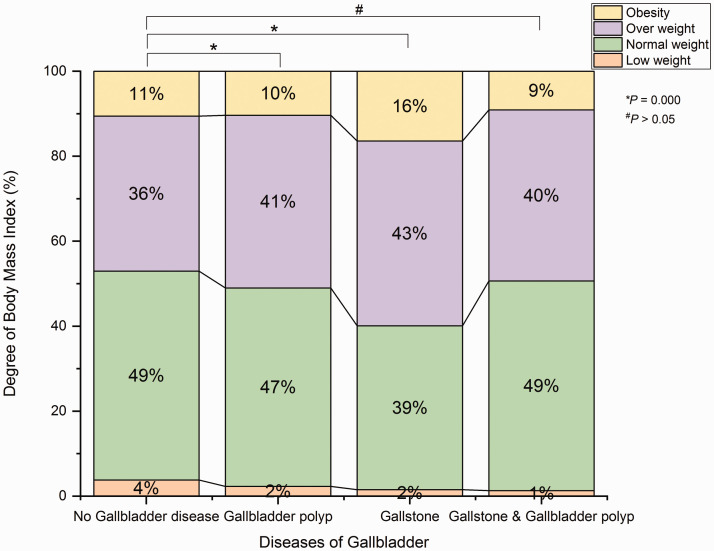
BMI data were missing for 10 participants (11.5%) in the SP group, 68 participants (7.3%) in the S group, 211 participants (7.6%) in the P group, and 1722 participants (7.1%) in the N group. According to the recorded BMI data, the proportions of participants with each BMI level were significantly different between the N and P groups (P < 0.001), and between the N and S groups (P < 0.001). However, the proportions of participants with each BMI level did not significantly differ between the N and SP groups (Figure 3).
Figure 3.
Body mass index levels, based on Chinese standards, among participants with gallbladder polyps, gallstones, or gallstones and gallbladder polyps, compared with participants without gallbladder disease. Yellow indicates BMI in obesity range; purple indicates BMI in overweight range; green indicates BMI in normal weight range; orange indicates BMI in underwent range. *P < 0.001.
BMI, body mass index; n.s., not significant.
Comparisons of H. pylori infection according to gallbladder disease status, sex, and age
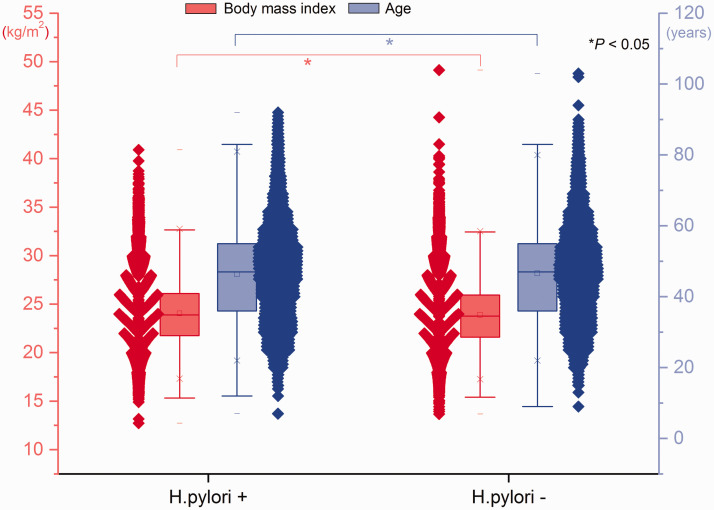
In total, 45.7% (12735/27881) of participants exhibited H. pylori infection. The proportion of participants with H. pylori infection was higher in the P group than in the N group (47.5% vs. 45.5%, P = 0.048); however, this proportion did not significantly differ between the S group (42.5%) and the N group, or between the SP group (45.5%) and the N group. The mean age was higher among participants with H. pylori infection than among participants without H. pylori infection (46.5 ± 13.5 years vs. 45.4 ± 13.8 years, P < 0.001). The mean BMI values were higher among participants with H. pylori infection than among participants without H. pylori infection (24.0 ± 3.3 kg/m2 vs. 23.9 ± 3.3 kg/m2, P = 0.001) (Figure 4). Among participants with H. pylori infection, the incidence of gallbladder polyps was higher in men than in women (11.8% vs. 8.2%, P < 0.001); in contrast, there were no significant sex-related differences in the incidences of gallstones or gallstones and gallbladder polyps (Figure 1). Among participants without H. pylori infection, the incidence of gallbladder polyps was higher in men than in women (10.7% vs. 8.1%, P < 0.001), whereas the incidence of gallstones was lower in men than in women (3.1% vs. 3.7%, P = 0.021).
Figure 4.
Ages and body mass indexes of participants with and without Helicobacter pylori infection. Red boxes, body mass index; blue boxes, age. Lines inside boxes indicate median values, boxes indicate interquartile ranges, and whiskers indicate minimum and maximum values. *P < 0.05.
Case-control matching analysis of H. pylori infection according to gallbladder disease status
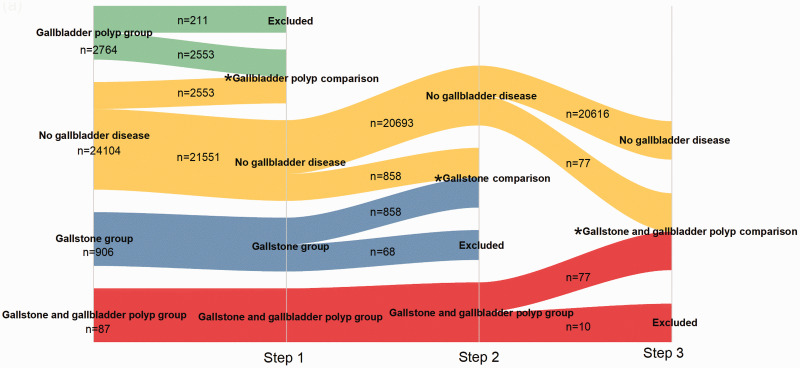
Based on the above outcomes, a 1:1 case-control matching protocol was implemented to match the essential confounding parameters among the P, S, and SP groups in a sequential manner. In step 1, there were 2764 participants in the P group. After matching, 211 participants were excluded from the P group; 2553 participants remained as the case group. In addition, 2553 participants from the N group were selected as the control group. At the end of the step 1, the remaining 21,551 participants in the N group were used as candidates for step 2. The same matching approach was used for the S and SP groups; the procedure is shown in Figure 5. Participants with missing BMI values were excluded from all groups. Participant characteristics in each group are shown in Table 1. The proportion of participants with H. pylori infection did not significantly differ between those with gallbladder polyps and those without gallbladder polyps. Similar results were observed concerning gallstones and gallstones and gallbladder polyps. These results did not show significant relationships between H. pylori infection and the presence of gallbladder polyps or gallstones.
Figure 5.
Schematic of 1:1 case-control matching protocol performed to match the essential confounding parameters for the gallbladder polyp, gallstone, and gallstone and gallbladder polyp groups.
*indicates outcome of each step.
Table 1.
Participant characteristics after case-control matching, stratified among gallbladder diseases.
|
Gallstone and gallbladder polyp disease |
Gallstone disease |
Gallbladder polyp disease |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Case group (n = 77) | Control group (n = 77) | P | Case group (n = 858) | Control group (n = 858) | P | Case group (n = 2553) | Control group (n = 2553) | P | |
| Age, years (mean ± standard deviation) | 50.5 ± 9.6 | 50.1 ± 10.1 | 0.769 | 54.1 ± 12.6 | 54 ± 12.9 | 0.876 | 46.8 ± 11.9 | 46.7 ± 12.1 | 0.943 |
| Sex, female (%) | 25 (32.5) | 25 (32.5) | 1.000 | 373 (43.5) | 373 (43.5) | 1.000 | 842 (33.0) | 842 (33.0) | 1.000 |
| H. pylori infection | 30 (39.0) | 33 (42.9) | 0.623 | 398 (46.4) | 420 (49.0) | 0.288 | 1214 (47.6) | 1157 (45.3) | 0.110 |
| Hypertension | 19 (24.7) | 10 (13.0) | 0.064 | 269 (31.4) | 217 (25.3) | 0.005 | 570 (22.3) | 484 (19.0) | 0.003 |
| Diabetes | 2 (2.6) | 5 (6.5) | 0.246 | 55 (6.4) | 49 (5.7) | 0.544 | 74 (2.9) | 78 (3.1) | 0.742 |
| Body mass index (kg/m2) | 24.1 ± 2.9 | 22.8 ± 2.8 | <0.001 | 25 ± 3.2 | 23.3 ± 3.1 | <0.001 | 24.3 ± 3 | 22.2 ± 3.1 | <0.001 |
| Underweight | 1 (1.3) | 1 (1.3) | 1.000 | 13 (1.5) | 13 (1.5) | 1.000 | 59 (2.3) | 59 (2.3) | 1.000 |
| Normal weight | 38 (49.4) | 38 (49.4) | 331 (38.6) | 331 (38.6) | 1192 (46.7) | 1192 (46.7) | |||
| Overweight | 31 (40.3) | 31 (40.3) | 373 (43.5) | 373 (43.5) | 1038 (40.7) | 1038 (40.7) | |||
| Obesity | 7 (9.1) | 7 (9.1) | 141 (16.4) | 141 (16.4) | 264 (10.3) | 264 (10.3) | |||
| Total bilirubin (µmol/L) | 13.1 ± 4.0 | 14.4 ± 6.7 | 0.134 | 14.4 ± 6.3 | 14.4 ± 5.9 | 0.793 | 14.4 ± 5.9 | 14.5 ± 5.8 | 0.711 |
| Direct bilirubin (µmol/L) | 3.8 ± 1.2 | 4.3 ± 2.1 | 0.069 | 4.2 ± 2.0 | 4.2 ± 1.8 | 0.948 | 4.2 ± 1.7 | 4.3 ± 1.7 | 0.559 |
| Indirect bilirubin (µmol/L) | 9.3 ± 3.1 | 10.1 ± 5.0 | 0.211 | 10.2 ± 4.6 | 10.2 ± 4.4 | 0.743 | 10.2 ± 4.4 | 10.2 ± 4.3 | 0.791 |
| Total bile acid (µmol/L) | 4.9 ± 4.7 | 3.6 ± 2.4 | 0.130 | 4.9 ± 5.0 | 4.5 ± 3.3 | 0.214 | 4.7 ± 6.7 | 4.5 ± 4.1 | 0.410 |
| Alanine aminotransferase (U/L) | 25.3 ± 16.8 | 26.7 ± 36.5 | 0.760 | 26.7 ± 23.4 | 23.1 ± 19.3 | 0.001 | 25.1 ± 23.3 | 22.7 ± 17.6 | <0.001 |
| Aspartate aminotransferase (U/L) | 22.7 ± 7.9 | 24.2 ± 17.2 | 0.498 | 24.8 ± 13.1 | 24.1 ± 11.9 | 0.258 | 23.3 ± 14.1 | 23.1 ± 9.4 | 0.494 |
| Alkaline phosphatase (U/L) | 30.8 ± 24.0 | 35.1 ± 57.6 | 0.540 | 36.6 ± 34 | 31.8 ± 36.8 | 0.005 | 32.2 ± 31.6 | 30.9 ± 37 | 0.188 |
| Triglyceride (mmol/L) | 1.8 ± 1.0 | 1.8 ± 1.6 | 0.998 | 2.0 ± 1.7 | 1.7 ± 1.9 | 0.007 | 1.8 ± 1.7 | 1.6 ± 1.4 | <0.001 |
| Total cholesterol (mmol/L) | 5.1 ± 0.9 | 5.1 ± 1.1 | 0.998 | 5.1 ± 1.0 | 5.1 ± 1.0 | 0.383 | 5.0 ± 1.0 | 5.0 ± 0.9 | 0.642 |
| High-density lipoprotein (mmol/L) | 1.4 ± 0.3 | 1.4 ± 0.3 | 0.533 | 1.4 ± 0.3 | 1.5 ± 0.3 | <0.001 | 1.4 ± 0.3 | 1.5 ± 0.3 | <0.001 |
| Low-density lipoprotein (mmol/L) | 2.8 ± 0.7 | 2.7 ± 0.8 | 0.824 | 2.7 ± 0.7 | 2.6 ± 0.7 | 0.167 | 2.6 ± 0.7 | 2.6 ± 0.7 | 0.005 |
| Leukocyte count (*109/L) | 6.6 ± 1.5 | 6.4 ± 1.6 | 0.376 | 6.5 ± 2.1 | 6.1 ± 1.6 | <0.001 | 6.3 ± 1.6 | 6.2 ± 1.6 | 0.014 |
| Neutrophil granulocyte count (*109/L) | 3.9 ± 1.2 | 3.7 ± 1.3 | 0.289 | 3.8 ± 1.3 | 3.6 ± 1.2 | <0.001 | 3.7 ± 1.2 | 3.6 ± 1.2 | 0.015 |
| Platelet count (*109/L) | 242.5 ± 56.1 | 238.6 ± 51.6 | 0.649 | 232.5 ± 55.8 | 228.8 ± 54.7 | 0.174 | 237.3 ± 53.1 | 233.9 ± 51.9 | 0.022 |
| Hemoglobin (g/L) | 147.8 ± 17.8 | 146.6 ± 13.2 | 0.631 | 143.8 ± 15.2 | 142.5 ± 15.1 | 0.076 | 146.3 ± 15.5 | 145.4 ± 15.1 | 0.036 |
Data are shown as n (%), except where otherwise indicated
Discussion
Multiple studies have shown that H. pylori species are present in patients with gallbladder diseases. Most studies involving detection of H. pylori species have demonstrated that the presence or absence of H. pylori is closely related to gallbladder disease status. For example, the proportion of patients with H. pylori in the biliary tract has reportedly ranged from 30% to 70%.21–23 In a meta-analysis that included 15 studies regarding hepatobiliary tract cancers, H. pylori species were found in 42.9% of patients with biliary diseases. In that study, specimens were obtained from one or more of the following sites: bile, gallbladder, liver, tumor tissue, and non-tumor tissue; they were assessed by 16s rRNA or DNA-based polymerase chain reaction, histology, genus-specific primers, and/or H. pylori primers, suggesting that the results were generalizable to other contexts.24 Another meta-analysis of 18 studies showed that H. pylori infection of the gallbladder was significantly associated with enhanced risks of chronic cholecystitis and cholecystitis.25 However, Rudi et al.26 found that no H. pylori DNA was present in 73 patients with acute or chronic cholecystitis. Moreover, H. pylori DNA was found in only one of 95 patients with chronic cholecystitis.27 All H. pylori isolates were obtained from patients (not from normal individuals) in those studies.
H. pylori can enter the bile duct by reflux from the duodenum when H. pylori is present in either gastric or gallbladder mucosa. Bansal et al.17 found that 81% of patients with H. pylori-positive bile had H. pylori in the gastric mucosa, based on urea breath test results. Bulajic et al.21 reported a strong association between the presence of H. pylori in the bile and the presence of H. pylori in the stomach. In our clinic, we have encountered barium reflux from the duodenum to the gallbladder in patients with duodenal ulcer due to H. pylori infection (Figure 6 shows a representative image of this reflux in a 32-year-old man). Hence, H. pylori is presumed to be present in the gallbladder or bile in such patients. However, this phenomenon does not indicate that H. pylori is a contributing factor in the onset of gallbladder diseases. Several types of bacteria have been detected in the bile duct, and microbial organisms contained in the bile from various biliary diseases are of intestinal origin. These species include isolates of Enterococcus, Klebsiella, Escherichia coli, Enterobacter, Pseudomonas, Proteus, and Streptococcus.28,29 The best objective evidence was obtained in a prospective study of C57L mice by Maurer et al. The results revealed no significant differences in formation of cholesterol monohydrate crystals, sandy stones, or true gallstones in H. pylori-infected mice, compared with uninfected mice, at the end of the 12-week study period. Therefore, the relationship between H. pylori and human gallbladder diseases requires further investigation.30
Figure 6.
Barium reflux from the duodenum to the gallbladder in a 32-year-old man with duodenal ulcer due to Helicobacter pylori infection (red arrows).
In the present study, we investigated the relationship between H. pylori and human gallbladder diseases by controlled epidemiological analysis. We found that the incidences of gallstones and gallbladder polyps differed according to sex (Figure 1). Moreover, the mean age and BMI levels significantly differed between participants with gallstones or gallbladder polyps, compared with participants who had no gallbladder diseases (Figures 2 and 3). Furthermore, the mean BMI values and mean age both significantly differed between participants, according to H. pylori infection status (Figure 4). The proportions of participants with H. pylori infection were similar between the gallbladder polyp and control groups after case–control matching with respect to age, sex, and BMI levels; similar results were observed gallstones and gallstones and gallbladder polyps. Although the proportion of participants with H. pylori infection was higher in the P group than in the N group, the P value was only 0.048 before case–control matching. Li et al.20 reported that the presence of H. pylori infection, based on 13C urease breath test results, was not associated with cholelithiasis in 8749 Chinese individuals.
There were several limitations in our study. First, selection bias was inevitable because this was a retrospective study without a rigorous design. Importantly, case–control matching was performed to reduce bias related to demographic factors. Second, the urease breath test results only indicated the presence of ongoing infection, so the length of the infection could not be confirmed. Notably, the formation of gallbladder polyps or gallstones is presumed to be a long-term process. Long-term follow-up studies may enable more robust conclusions. The results of our research are contrary to the conclusions of most published articles, possibly because this was a cross-sectional study. Finally, our study did not investigate the underlying mechanism involved in any potential relationship between H. pylori and human gallbladder diseases.
Our results suggest that H. pylori infection is a contributing factor in the onset of gallbladder polyps or gallstones. Further prospective cohort studies are needed to confirm our findings.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This study was supported by Science and Technology Bureau of Taizhou city (No. 1801ky01).
ORCID iD
Jinshun Zhang https://orcid.org/0000-0002-3961-2149
References
- 1.Nagy P, Johansson S, Molloy-Bland M. Systematic review of time trends in the prevalence of Helicobacter pylori infection in China and the USA. Gut Pathog 2016; 8: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu MY, Liu L, Yuan BS, et al. Association of obesity with Helicobacter pylori infection: a retrospective study. World J Gastroenterol 2017; 23: 2750–2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pan BL, Huang CF, Chuah SK, et al. Relationship between Helicobacter pylori infection and bone mineral density: a retrospective cross-sectional study. BMC Gastroenterol 2018; 18: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen YY, Fang WH, Wang CC, et al. Helicobacter pylori infection increases risk of incident metabolic syndrome and diabetes: a cohort study. PLoS One 2019; 14: e208913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peng YH, Chen CK, Su CH, et al. Increased risk of chronic obstructive pulmonary disease among patients with Helicobacter pylori infection: a population-based cohort study. Clin Respir J 2017; 11: 558–565. [DOI] [PubMed] [Google Scholar]
- 6.Wang JW, Hsu CN, Tai WC, et al. The association of Helicobacter pylori eradication with the occurrences of chronic kidney diseases in patients with peptic ulcer diseases. PLoS One 2016; 11: e164824. [Google Scholar]
- 7.Abdullah A, Rangaraj A, Rashid M, et al. Gallbladder polypoid lesions are inaccurately reported and undermanaged: a retrospective study of the management of gallbladder polypoid lesions detected at ultrasound in symptomatic patients during a 36-month period. Clin Radiol 2019; 74: 417–489. [DOI] [PubMed] [Google Scholar]
- 8.Lee KF, Wong J, Li JC, et al. Polypoid lesions of the gallbladder. Am J Surg 2004; 188: 186–190. [DOI] [PubMed] [Google Scholar]
- 9.Jorgensen T, Jensen KH. Polyps in the gallbladder. A prevalence study. Scand J Gastroenterol 1990; 25: 281–286. [PubMed] [Google Scholar]
- 10.Park JY, Hong SP, Kim YJ, et al. Long-term follow up of gallbladder polyps. J Gastroenterol Hepatol 2009; 24: 219–222. [DOI] [PubMed] [Google Scholar]
- 11.Chatterjee A, Lopes VC, Nikolaidis P, et al. Uncommon intraluminal tumors of the gallbladder and biliary tract: spectrum of imaging appearances. Radiographics 2019; 39: 388–412. [DOI] [PubMed] [Google Scholar]
- 12.Seretis C, Lagoudianakis E, Gemenetzis G, et al. Metaplastic changes in chronic cholecystitis: implications for early diagnosis and surgical intervention to prevent the gallbladder metaplasia-dysplasia-carcinoma sequence. J Clin Med Res 2014; 6: 26–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lammert F, Gurusamy K, Ko CW, et al. Gallstones. Nat Rev Dis Primers 2016; 2: 16024. [DOI] [PubMed] [Google Scholar]
- 14.Portincasa P, Moschetta A, Palasciano G. Cholesterol gallstone disease. Lancet 2006; 368: 230–239. [DOI] [PubMed] [Google Scholar]
- 15.Monstein HJ, Jonsson Y, Zdolsek J, et al. Identification of Helicobacter pylori DNA in human cholesterol gallstones. Scand J Gastroenterol 2002; 37: 112–119. [DOI] [PubMed] [Google Scholar]
- 16.Tajeddin E, Sherafat SJ, Majidi MRS, et al. Association of diverse bacterial communities in human bile samples with biliary tract disorders: a survey using culture and polymerase chain reaction-denaturing gradient gel electrophoresis methods. Eur J Clin Microbiol 2016; 35: 1331–1339. [DOI] [PubMed] [Google Scholar]
- 17.Bansal VK, Misra MC, Chaubal G, et al. Helicobacter pylori in gallbladder mucosa in patients with gallbladder disease. Indian J Gastroenterol 2012; 31: 57–60. [DOI] [PubMed] [Google Scholar]
- 18.Lee J, Lee DH, Lee JI, et al. Identification of Helicobacter pylori in gallstone, bile, and other hepatobiliary tissues of patients with cholecystitis. Gut Liver 2010; 4: 60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takahashi Y, Yamamichi N, Shimamoto T, et al. Helicobacter pylori infection is positively associated with gallstones: a large-scale cross-sectional study in Japan. J Gastroenterol 2014; 49: 882–889. [DOI] [PubMed] [Google Scholar]
- 20.Li T, Cong P, Zhao S, et al. Correlation between Helicobacter pylori and cholelithiasis in Qingdao, Shandong. Chinese Journal of Current Advances in General Surgery 2019; 3: 192–194, 199. [Google Scholar]
- 21.Bulajic M, Maisonneuve P, Schneider-Brachert W, et al. Helicobacter pylori and the risk of benign and malignant biliary tract disease. Cancer 2002; 95: 1946–1953. [DOI] [PubMed] [Google Scholar]
- 22.Abayli B, Colakoglu S, Serin M, et al. Helicobacter pylori in the etiology of cholesterol gallstones. J Clin Gastroenterol 2005; 39: 134–137. [PubMed] [Google Scholar]
- 23.Kawaguchi M, Saito T, Ohno H, et al. Bacteria closely resembling Helicobacter pylori detected immunohistologically and genetically in resected gallbladder mucosa. J Gastroenterol 1996; 31: 294–298. [DOI] [PubMed] [Google Scholar]
- 24.Pandey M, Shukla M. Helicobacter species are associated with possible increase in risk of hepatobiliary tract cancers. Surg Oncol 2009; 18: 51–56. [DOI] [PubMed] [Google Scholar]
- 25.Cen L, Pan J, Zhou B, et al. Helicobacter pylori infection of the gallbladder and the risk of chronic cholecystitis and cholelithiasis: a systematic review and meta-analysis. Helicobacter 2018; 23. [DOI] [PubMed] [Google Scholar]
- 26.Rudi J, Rudy A, Maiwald M, et al. Helicobacter sp. are not detectable in bile from German patients with biliary disease. Gastroenterology 1999; 116: 1016–1017. [DOI] [PubMed] [Google Scholar]
- 27.Mendez-Sanchez N, Pichardo R, Gonzalez J, et al. Lack of association between Helicobacter sp. colonization and gallstone disease. J Clin Gastroenterol 2001; 32: 138–141. [DOI] [PubMed] [Google Scholar]
- 28.Chang WT, Lee KT, Wang SR, et al. Bacteriology and antimicrobial susceptibility in biliary tract disease: an audit of 10-year's experience. Kaohsiung J Med Sci 2002; 18: 221–228. [PubMed] [Google Scholar]
- 29.Kaya M, Bestas R, Bacalan F, et al. Microbial profile and antibiotic sensitivity pattern in bile cultures from endoscopic retrograde cholangiography patients. World J Gastroenterol 2012; 18: 3585–3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maurer KJ, Rogers AB, Ge Z, et al. Helicobacter pylori and cholesterol gallstone formation in C57L/J mice: a prospective study. Am J Physiol Gastrointest Liver Physiol 2006; 290: G175–G182. [DOI] [PubMed] [Google Scholar]