Abstract
Due to the interest in using probiotic bacteria in poultry production, this research was focused on evaluating the effects of Lactobacillus fermentum Biocenol CCM 7514 administration on body weight gain and cytokine gene expression in chickens challenged with Campylobacter jejuni. One-hundred and eight 1-day old COBB 500 broiler chickens were equally assigned to four experimental groups at random. In the control group (C) chicks were left untreated, whereas in groups LB and LBCj a suspension of L. fermentum was administered. A suspension of C. jejuni was subsequently applied to groups Cj and LBCj. Body weight was registered, and the individuals were later slaughtered; cecum samples were collected at 12, 36 and 48 h post-infection (hpi). The entire experiment lasted seven days. Reverse transcription quantitative PCR (RT-qPCR) was used to determine expression levels of IL-1β, IL-15, IL-17, and IL-18 at each time point. Pathogen-infected individuals were observed to weigh significantly less than those fed with the probiotic. Significant differences were also found in transcript abundance; expression of IL-15 was downregulated by the probiotic and upregulated by C. jejuni. The effects of bacterial treatments were time-dependent, as the expression profiles differed at later stages. The present outcomes demonstrate that L. fermentum both reduces the impact of C. jejuni infection on chicken body weight and regulates positively pro-inflammatory cytokine expression, which ultimately increase bird well-being and improves production.
Keywords: broiler chicken, Lactobacillus fermentum, Campylobacter jejuni, cytokine gene expression, body weight
1. Introduction
Campylobacter jejuni has been considered one of the leading causes of human gastrointestinal diseases worldwide, with outbreaks registered both in industrialized and developing countries [1]. Campylobacter spp. colonizes the avian gut in high concentrations with few or no clinical symptoms. Hence, it has been traditionally considered commensal, although a revision of this bacteria–host interaction has been recently proposed [2]. Upon interaction with avian epithelial cells, C. jejuni stimulates the expression of various pro-inflammatory cytokines (TNF-α, IL-1β, IFN-γ, IL-6, and IL-2). Such conditions can lead to weight loss, intestinal damage and potential neurological disease, along with significant economic losses for poultry productivity [3,4]. The host immune response and the outcome of a C. jejuni colonization have been observed to be highly influenced by many factors, including chicken breed. It has been demonstrated that broiler-type birds mount a more vigorous response than layer-type birds [5]. However, some broiler lines have proved to be more susceptible than others to a C. jejuni infection, which is nonetheless characterized by an extended inflammatory response and an induction of lymphocyte activation [6,7]. Fast-growing broiler breeds, such as COBB 500, are widely used commercially. Several investigations have shown that this breed of broiler does indeed mount an important immune response against Campylobacter colonization [8,9,10,11]
Excessive use of antibiotics in food animals (e.g., fluoroquinolones) is not only known to promote the selection of resistant bacterial strains [12,13], but also to cause dysbiosis in chickens, which ultimately leads to a long-term diminished immune reaction [14,15]. The concept of early life programming, which assumes that the development of diseases later in life can be modulated by environmental exposures during critical pre- or early post-natal life, has recently arisen as particularly relevant because broilers are selected for rapid early growth, so the development of their immune system occurs mainly early in life [16]. For instance, chickens inoculated with three species of Lactobacillus (L. ingluviei, L. agilis, and L. reuteri), immediately post hatching, showed a significant increment in weight by 28 d of age. Furthermore, a reduction of pathogenic taxa such as the Shigella and Escherichia species was observed [17]. In addition, application of probiotics is known to promote an overall downregulation of pro-inflammatory mediators, including as IL-1β, TLR2, and TLR4 [18]. In particular, some species of Lactobacillus have proven useful for reducing the extent of C. jejuni colonization [19]. It has been demonstrated that early colonization of the gut (4 days of age) by Campylobacter spp. elicits an important immune reaction, including the regulation of various pro-inflammatory cytokines. However, this reaction can be significantly modulated by early treatment with probiotics [8,9,10,20]. IL-1β and IL-18 have been described as important players in the response to a C. jejuni infection in the presence of L. reuteri or L. salivarius [8,19]. These cytokines are important mediators in the initiation of inflammation, as their activation is known to increase the capacity of the innate immune system to react more efficiently against bacterial infections [21]. Various strains of L. fermentum have been regarded as promising probiotics, and have been suggested as potential tools for fighting C. jejuni infections, although these results come mainly from in vitro experiments [22,23]. Recently, we demonstrated that treatment with L. fermentum Biocenol CCM 7514 in chickens infected with C. coli promoted a heightened humoral response while dampening potential inflammation mediated by effector T cells in 1-week-old-chickens [20]. This study also revealed that the downregulation of IL-15 and IL-17, promoted by the probiotic, might be responsible for maintaining intraepithelial lymphocytes in an induced state and avoid their differentiation into effector cells. Moreover, early colonization by C. jejuni is known to induce a decrease in chicken growth rate, along with an increased pro-inflammatory response in caecal tissues, including upregulation of IL-17 [3]. In an attempt to assess the probiotic benefits of L. fermentum Biocenol CCM 7514 to a C. jejuni infection in vivo, we set up a pilot experiment to determine the effects of an early probiotic supplementation on body weight and cytokine expression (IL-1β, IL-15, IL-17, and IL-18) in chickens infected at 4 days of age.
2. Materials and Methods
2.1. Chickens and Experimental Design
Experiments were performed following the protocol no. 863/17-221, according to the guidelines established by the Ethical Commission of the University of Veterinary Medicine and Pharmacy in Košice, and approved by the National Veterinary and Food Administration of the Slovak Republic. A total of 108 broiler cock chickens (COBB 500) were used in the experiment, they had constant access to water and feed (free of probiotics, antibiotics, or coccidiostats) ad libitum. Individuals (27 per treatment) were allocated in four groups, following an experimental design used in previous research [20]. (i) the control group, in which neither of the bacteria were used, (ii) the probiotic group (L. fermentum, LB), (iii) the Campylobacter-challenged group (Cj), and (iv) the co-exposure group (LBCj). Within each group, chickens were subdivided into three separately-housed subgroups of nine birds, as measurements were taken at three different time points (Table 1). For sampling, three birds were weighted, sacrificed and caecal sections collected at a time, this process was performed in triplicate per time point (n = 9).
Table 1.
Schema of the methodology aimed at measuring cytokine transcript modulation in chickens exposed to L. fermentum and C. jejuni.
| Day of Experiment | Control (number of chickens) | LB Group (number of chickens) | Cj Group (number of chickens) | LBCj Group (number of chickens) |
|---|---|---|---|---|
| 0 d | 27 | 27 | 27 | 27 |
| 1 d | 27 | 27 L. fermentum dose 109 CFU/0.2 mL individually per os | 27 | 27 L. fermentum dose 109 CFU/0.2 mL individually per os |
| 2 d | 27 | 27 L. fermentum dose 109 CFU/0.2 mL individually per os | 27 | 27 L. fermentum dose 109 CFU/0.2 mL individually per os |
| 3 d | 27 | 27 L. fermentum dose 109 CFU/0.2 mL individually per os | 27 | 27 L. fermentum dose 109 CFU/0.2 mL individually per os |
| 4 d | 27 | 27 L. fermentum dose 109 CFU/0.2 mL individually per os | 27 C. jejuni dose 108 CFU/0.2 mL individually per os | 27 L. fermentum dose 109 CFU/0.2 mL + C. jejuni dose 108 CFU/0.2 mL individually per os |
| 5 d (12 hpi) sample collection | 18 | 18 L. fermentum dose 109 CFU/0.2 mL individually per os | 18 | 18 L. fermentum dose 109 CFU/0.2 mL individually per os |
| 6d (36 hpi) sample collection | 9 | 9 L. fermentum dose 109 CFU/0.2 mL individually per os | 9 | 9 L. fermentum dose 109 CFU/0.2 mL individually per os |
| 7d (48 hpi) sample collection | 9 | 9 L. fermentum dose 109 CFU/0.2 mL individually per os | 9 | 9 L. fermentum dose 109 CFU/0.2 mL individually per os |
C, Control group; LB, Lactobacillus fermentum; Cj, Campylobacter jejuni; hpi, hours post-infection.
Animals were floor reared (9 birds/m2) at an ambient temperature of 30–32 °C during the entire experiment. Chickens were exposed to 24 h of continuous light for the first two days of growth, and subsequently to a regime of 23 h of light and one of dark. Environmental conditions were maintained according to the broiler breeding standards [24].
L. fermentum Biocenol CCM 7514 was cultivated exactly as hitherto described [20]. C. jejuni CCM 6189 was grown in blood-free selective agar base CM0935 supplemented with SR0155 (containing Cefoperazone and Amphotericin B; Oxoid Ltd., United Kingdom) in a jar with microaerobic conditions (5% O2, 10% CO2, 85% N2, CampyGen; Oxoid Ltd.). Incubation was performed for 2 days at 42 °C. C. jejuni colonies were harvested and diluted in PBS to the specific viable concentration (1 × 108 CFU/mL). Bacterial number was controlled using optical density at 550 nm. Bacterial numbers were determined according to a standard microbiological method using serial dilution and posterior plating and prepared as previously detailed [9].
A L. fermentum suspension (109 colony-forming units (CFU)/0.2 mL) was administered daily for 7 days to the LB and LBCj groups per os. On the fourth day, chickens from groups Cj and LBCj were inoculated with a suspension of C. jejuni (108 CFU/0.2 mL). Samples were collected on day 5 (12 h post-infection (hpi)) from one of subgroup per group, on day 6 (36 hpi) and on day 7 (48 hpi) from the other two subgroups (Table 1); caecal sections were maintained in RNA-later (Thermo Scientific, Waltham, MA) and stored at –80 °C.
2.2. Body Weight of Chickens
The weight of each broiler chicken was recorded on a daily basis and on day 5 (12 h post-infection (hpi)), 6 (36 hpi) and 7 (48 hpi) using an analytical scale (BOECO, Germany) (Table 1).
2.3. RNA Extraction, Reverse Transcription, and Quantitative Polymerase Chain Reaction (PCR) Assays
Total RNA purification was performed using the RNeasy mini kit (Qiagen, Hilden, Germany) according to the instructions. Reverse transcription was carried out using iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA). The resulting cDNA was diluted in 10× in UltraPure™ DNase/RNase-Free distilled water (Invitrogen, Waltham, MA) and either employed as a template for Reverse transcription quantitative PCR (RT-qPCR) or stored at –20 °C. Primers used for determining the relative expression of IL-1β, IL-15, IL-17, and IL-18 are listed in Table 2. Amplification, detection, cycling conditions, melting curve assessment and data normalization were set as previously described [25]. Samples were analyzed in duplicate and mean values were used for subsequent analyses (Table S1).
Table 2.
List of primers utilized in RT-qPCR for cytokine transcript detection.
| Primer | Sequence 5′-3′ | Reference |
|---|---|---|
| IL-1β For IL-1β Rev |
GAAGTGCTTCGTGCTGGAGT ACTGGCATCTGCCCAGTTC | [26] |
| IL-15 For IL-15 Rev |
TGGAGCTGATCAAGACATCTG CATTACAGGTTCCTGGCATTC | [27] |
| IL-17 For IL-17 Rev |
TATCAGCAAACGCTCACTGG AGTTCACGCACCTGGAATG | [26] |
| IL-18 For IL-18 Rev |
ACGTGGCAGCTTTTGAAGAT GCGGTGGTTTTGTAACAGTG | [25] |
| GAPDH For GAPDH Rev |
CCTGCATCTGCCCATTT GGCACGCCATCACTATC | [28] |
2.4. Statistical Analysis
A one-way analysis of variance, along with the Tukey post hoc test, was employed to determine the significant differences between the experimental groups. Relationships among the indicators were assessed using Pearson’s r correlation coefficient. Analyses were performed on MATLAB® version 9.9.9341360 (MathWorks, Natick, MA, USA) (R2016a); heatmaps were obtained using Python’s plotting library, Matplotlib 3.0.3 (Python Software Foundation, DE, USA).
3. Results
3.1. Body Weight of Chickens
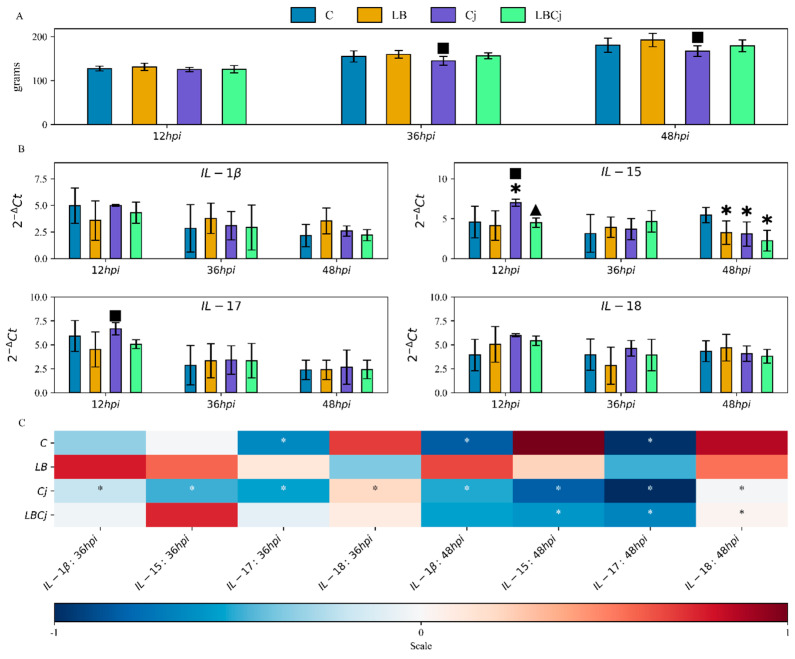
Compared to controls conditions, consumption of L. fermentum increased body weight at all time points, although not significantly (p > 0.05). Significant differences were observed between the probiotic and pathogen groups at 36 and 48 hpi (p < 0.05); chickens challenged with C. jejuni showed an overall decrease in body weight. Infection with C. jejuni did not induce such a response in the presence of the probiotic group, as the average body weight of these individuals was similar to those determined in chickens not inoculated with C. jejuni (Figure 1A).
Figure 1.
Effects on body weight and pro-inflammatory cytokine expression of L. fermentum and C. jejuni colonization at three different stages. (A) Average body weights of treated and untreated animals at 12, 36 and 48 hpi. (B) mRNA expression levels of caecal pro-inflammatory cytokines at three different time points. The Ct values of studied genes were normalized to a Ct value of the reference gene (GAPDH) (Delta -Δ- Ct), and calculated as 2−ΔCt. Values are mean ± standard error SE (n = 9). * denotes significant differences (Tukey’s test, p < 0.05) with the control group; ■ with the L. fermentum treatment, ▲ with C. jejuni treatment. C, control group; LB, L. fermentum group; Cj, C. jejuni group; LBcj, co-exposure group; hpi, hour post-infection. (C) Heat map denoting transcriptional fold change between time points. The colour scale, −1 (blue) to +1 (red), denotes mRNA levels relative to the values of the control group, with white designating down-regulated genes and black designating up-regulated genes. Relative expression was calculated and expressed as 2−ΔΔCt log2 fold change. Values are mean ± SE (n = 9). * denotes significant differences (Tukey’s test, p < 0.05). C, control group; LB, L. fermentum group; Cj, C. jejuni group; LBcj, co-exposure group; hpi, hour post-infection.
3.2. Cytokine Expression
One-day old broiler chickens were exposed to the probiotic, and later (4 days of age) inoculated with the pathogen to investigate their effects on pro-inflammatory cytokine expression. IL-1β is mainly produced by activated macrophages and has been linked to chronic conditions, high levels of this cytokine have been observed to enhance the intensity of inflammation [29]. During the experiment, the abundance of this cytokine was altered neither by the probiotic nor by the pathogen, or the administration of both (Figure 1B) (Table S1). IL-15 modulates natural killer (NK) cells, T cell activation and proliferation, and thus is considered a key mediator of inflammation [30]. The expression of IL-15 was modified by all bacterial treatments, especially at 12 and 48 hpi. In the first stage C. jejuni elicited a significant upregulation compared to control conditions (Figure 1B). Similarly, mRNA levels were significantly higher in C. jejuni-challenged individuals than in those treated with the probiotic in the presence and absence of the pathogen (Figure 1B) (Table S1). IL-17, synthesized by T helper 17 cells (Th17), triggers the production of chemokines that attract monocytes and neutrophils. Activation of this interleukin is normally detected in the pathogenesis of various bacterial and parasitic infection [31]. C. jejuni colonization, if compared to L. fermentum, promoted an active transcription of this factor at 12 hpi (Figure 1B) (Table S1). Interleukin-18, mainly synthesized by macrophages, promotes T helper cell (Th1) development and production of IFN-γ by CD4, CD8 T cells and NK cells [32]. The present investigation revealed no significant differences in relation to this factor between treatments at any time point (Figure 1B) (Table S1).
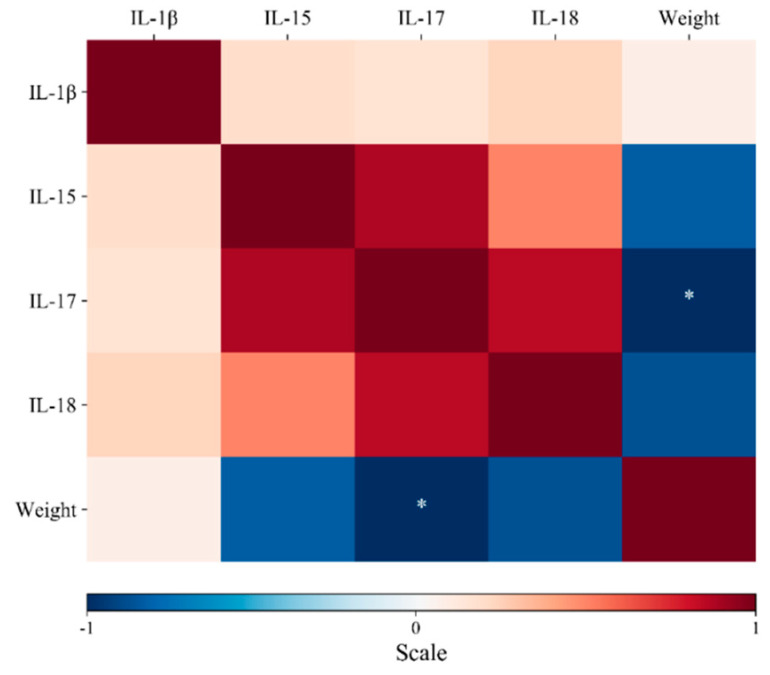
As shown in Figure 1B, administration of either the probiotic or the pathogen induced a different response in gene expression at 12 hpi; such expression varied at later stages depending on the treatment. In untreated animals, for instance, IL-17 was significantly downregulated at 36 (2-fold) and 48 hpi (2.4-fold), while IL-1β was significantly downregulated only at 48 hpi (2.3-fold), respectively. IL-18 and IL-15 were upregulated, although not significantly (Figure 1C) (Table S1). In L. fermentum-exposed individuals, IL-15 transcript abundance increased at 36 hpi. At 48 hpi, the transcriptional profile was similar to that of the first time point; all variations in expression were nonetheless not statistically significant (Figure 1C). On the other hand, in C. jejuni-infected chickens, expression of IL-15 was induced at 12 hpi (Figure 1B); this factor along with the others were significantly downregulated, especially at later stages (at least 2-fold) (Figure 1C). In the co-exposure group, initial conditions involved the expression of IL-18 followed by IL-17, IL-15 and IL-1β (Figure 1B). However, at the second time point IL-15 was the most abundant, while at the third time point cytokine levels returned to those observed at 12 hpi, although significantly lower for IL-15 (2-fold), IL-17 (2.1-fold), and IL-18 (1.4-fold) (Figure 1C). Pearson’s r analysis revealed high positive correlations between IL-15 and IL-17, although they were not significant. On the other hand, significant negative correlations (p < 0.05) were also found between IL-17 and body weight (Figure 2). The negative value of the coefficient (−0.97, blue color) implies that the two variables move in different directions; namely the increased abundance of IL-17 transcripts was directly associated with the decrease in body weight observed after pathogen colonization. The raw data can be found in Table S2.
Figure 2.
Heat map representing Pearson’s r correlation coefficient matrix among the indicators. Scores are denoted in color from -1 (blue) to 1 (red). * Indicates significant differences (Tukey’s test, p < 0.05). The negative value of the coefficient implies that IL-17 and body weight are inversely related; in other words, when this cytokine is upregulated, a loss in body weight might be expected.
4. Discussion
This investigation was conceived to gain a better understanding of the effects on body weight and pro-inflammatory cytokine expression of L. fermentum supplementation in chickens infected with C. jejuni. Moreover, in order to study the consequences of post-natal probiotic treatment to an early infection, L. fermentum was administered from the first day of the experiment, and samples were taken 12, 36 and 48 hpi. Increased inflammation has been linked to body weight loss in pathogen-challenged chickens [33]. Early colonization by C. jejuni results in a transient growth rate reduction and an increased pro-inflammatory response [3]. Body weight gain was determined following challenge with C. jejuni, but no significant differences were detected between the control and experimental groups at any point. However, animals exposed to the probiotic showed a slight significant increase in weight compared to those infected with the pathogen. Due to the reported beneficial effects of lactic acid bacteria on bird well-being and growth [34]. and the detrimental consequences of a C. jejuni infection [3], it would be reasonable to expect that the infected birds would not perform as well as those fed with the probiotic. Interestingly, the weight reduction caused by the pathogen was not observed in the co-exposure group, demonstrating that the probiotic was able to lessen the impact of C. jejuni negative effects.
As aforementioned, overexpression of pro-inflammatory cytokines has been related to pathogenicity and weight loss [3,33]. Thus, we decided to study the effects of bacterial treatments on the expression of key pro-inflammatory cytokines. It is well documented that microorganism-associated molecular patterns (e.g., LTA—lipoteichoic acid, wall teichoic acid, peptidoglycan) of Lactobacillus species are responsible for activation of specific receptors in macrophages as well as epithelial and dendritic cells, which activate downstream signaling inducing cytokine production [35]. IL-1β abundance was not altered by any of the treatments during the experiment. Exposure to various Lactobacillus species has promoted the upregulation of this factor, although in vitro [36]. This indicates that L. fermentum is not as effective as other species (e.g., L. acidophilus, L. reuteri, and L. salivarius) at inducing the expression of pro-inflammatory cytokines. Transcript abundance of this factor has been elicited by the purified lipooligosaccharide (LOS) of C. jejuni HS:10, although expression was determined from spleen samples [37]. Our results suggest that IL-1β is not directly involved in the response to an L. fermentum treatment in C. jejuni-challenged chickens, especially during the first two days after pathogen infection. The expression of IL-15 was modified by all bacterial treatments; C. jejuni, in particular, elicited an upregulation of this cytokine compared to what was observed in the control and probiotic groups. These outcomes corroborate previous findings showing that C. jejuni infection increases IL-15 expression, which has been linked to cellular necrosis and pro-inflammatory responses [38]. Similarly, probiotic treatments, making use of L. fermentum, have shown to induce an overall reduction of IL-15 abundance [20]. Conclusively, the probiotic treatment seemed to be quite effective at downregulating IL-15 expression, which could be otherwise increased by C. jejuni especially at 12 hpi.
C. jejuni administration, if compared to L. fermentum, elicited the expression of IL-17 uniquely at the first time point; upregulation of this cytokine has been previously reported in response to a C. jejuni infection [39]. Interestingly, other Lactobacillus species, particularly L. plantarum ZS2058, activated the IL-17 pathway [39]. These data reinforce the notion that L. fermentum is not as efficient at inducing expression of Th17 cytokines as other species of lactic acid bacteria. Lactobacilli synbiotics, composed of L. salivarius and galactooligosaccharides, were shown to induce a down-regulatory pattern over cytokine expression (IL-12, IL-8, IL-1β), while levels of IL-18 were in fact upregulated by such treatment [40]. In the present research, expression levels of IL-8 were not significantly modified by any of the treatments, which differ from previous findings, indicating a decrease in mRNA abundance after L. fermentum administration [20]. On the other hand, these outcomes agree with previous investigations revealing that C. jejuni administration could not modify IL-18 levels [6]. Arguably, IL-18 does not seem to play a critical role in the response to a C. jejuni infection in the presence or absence of this probiotic.
Exposure to the probiotic or pathogen modulated gene expression, especially at 12 hpi. In L. fermentum-exposed individuals, cytokine expression was not altered, implying that the probiotic did not promote transcription of the pro-inflammatory factors. On the contrary, C. jejuni did modify significantly the abundance of the evaluated interleukins. In the co-exposure group, cytokine expression was observed to vary importantly throughout the experiments, especially at 36 hpi. These outcomes demonstrate that L. fermentum is able to stabilize expression of the studied cytokines, which even in control conditions was observed to change significantly. Moreover, the probiotic treatment was proved to induce downregulation of pro-inflammatory factors in pathogen infected chickens. Arguably, L. fermentum seems capable of promoting intestinal homeostasis, at least in terms of pro-inflammatory cytokine abundance. Pearson’s r coefficient analysis revealed a significant inverse correlation between body weight and IL-17 abundance. Early colonization by C. jejuni induced a decrease in chicken growth rate along with an increased pro-inflammatory response in caecal tissues, including upregulation of IL-17 [3]. IL-17 is one of the strongest inflammatory mediators and is involved in the regulation of the immune response and the development of caecal lesions by recruiting neutrophils to the site of infection [41]. In fact, antibody-mediated neutralization of IL-17 resulted in reduced caecal lesions and enhanced body weight gains in pathogen-exposed chickens [41]. Our results suggest that L. fermentum treatment can maintain caecal IL-17 transcription levels unaltered, which in turn could have a positive effect on gut health and ultimately body weight. Actually, the registered body weight reduction in the C. jejuni treatment was not observed when infection occurred after probiotic supplementation, demonstrating the benefit of using lactic acid bacteria to promote gut integrity during pathogen invasion.
Recent experimentation by our group has revealed that levels of IL-15 and IL-17 are modulated by L. fermentum treatment in C. coli infected broilers [20]. These cytokines were downregulated after exposure to the probiotic. The low abundance of these cytokines may prevent intraepithelial lymphocytes (IEL) and memory CD8 T cells from differentiating into cytotoxic T cells, thus limiting the onset of inflammation. In fact, commensal bacteria are known to contribute to the maintenance of IELs, which are involved in wound healing and intestinal homeostasis restoration by secreting factors that promote growth [30]. Previous research has shown that Lactobacillus spp. administration reduces chicken pro-inflammatory cytokine expression and improves weight gain [34]. Here, we have revealed that L. fermentum dampens expression of pro-inflammatory factors and provides a slight advantage on weight gain in C. jejuni-challenged birds. The current results corroborate the above-mentioned findings, and emphasize the fact that IL-15 and IL-17 are key players in the response to an early Campylobacter spp. infection in the presence of the probiotic. Upregulation of these cytokines was elicited mainly by the presence of C. jejuni. Recent research has demonstrated that the abundance IL-1β and IL-18 increased significantly as a response to an early chicken infection by C. jejuni in the presence of L. reuteri [8]. The secretion of these factors is known to occur in response to bacterial infection or cellular damage, as they are key modulators of the initiation of inflammation [21]. The present outcomes showed that these cytokines are not upregulated by L. fermentum, which support the notion that this lactic acid bacteria have a mild effect, in terms of promoting pro-inflammatory cytokine expression, compared to other species of Lactobacillus [8,19].
The immune response to Campylobacter infection in chickens is complex, and starts with bacterial recognition by pattern recognition receptors (PRR), which upon activation promote expression of pro-inflammatory cytokines [42]. The present results show that the abundance of some these cytokines increased at 12 hpi, but later decreased at 48 hpi. This decline after the initial response has been reported previously after Campylobacter colonization [39], which implies that the host immune system deals with the initial colonization as an attack, although it later reaches a certain level of tolerance [43,44]. Arguably, chickens mount an immune response to infection by Campylobacter. Despite the fact that the initial response seems no different from those elicited by commensal organisms, various reports of pathology have demonstrated that Campylobacter might trigger a diseased-like state [7,41], which suggest that C. jejuni should not be regarded solely as commensal but instead as a pathogen [2].
5. Conclusions
The data presented herein highlight the benefits of utilizing probiotics for avoiding the negative influences of Campylobacter spp. infection on bird body weight and inflammation. However, further experiments must explore these effects at even later stages, and must include other cytokines (e.g., IL-12, IL-22, and IFN-γ), especially Th2 cytokines such as IL-4, IL-6 or IL-10. In addition, the expression of other inflammatory mediators, especially those involved in the prostaglandin signaling pathway, must be assessed in order to gain a better understanding of Campylobacter-induced intestinal inflammation and its modulation by lactic acid bacteria.
Supplementary Materials
The following are available online at https://www.mdpi.com/2306-7381/7/3/121/s1, Table S1: Relative mRNA expression of caecal pro-inflammatory cytokines in probiotic and pathogen-colonized chickens, Table S2: Raw data.
Author Contributions
M.Š.: conceptualization, methodology, supervision, writing—original draft preparation; M.L.-Á.: conceptualization, methodology, supervision, writing—original draft preparation; C.L.-Á.: formal analysis, methodology, software; V.R.: methodology, original draft preparation; V.K.: methodology, funding acquisition, RT-qPCR; J.K.: methodology, bacterial strain preparation; R.N.: methodology, bacterial strain preparation; D.O.-P.: methodology, RT-qPCR; C.V.-B.: conceptualization, supervision; M.L.: funding acquisition, conceptualization; R.H.: conceptualization, supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Grant Agency for Science of the Slovak Republic VEGA (1/0112/18, 1/0355/19) and Slovak Research and Developmental Agency (APVV-15-0165).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Roux F., Sproston E., Rotariu O., MacRae M., Sheppard S.K., Bessell P., Smith-Palmer A., Cowden J., Maiden M.C.J., Forbes K.J., et al. Elucidating the aetiology of human Campylobacter coli infections. PLoS ONE. 2013;8:e64504. doi: 10.1371/journal.pone.0064504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Awad W.A., Hess C., Hess M. Re-Thinking the chicken–Campylobacter jejuni interaction: A review. Avian Pathol. 2018;47:352–363. doi: 10.1080/03079457.2018.1475724. [DOI] [PubMed] [Google Scholar]
- 3.Connerton P.L., Richards P.J., Lafontaine G.M., O’kane P.M., Ghaffar N., Cummings N.J., Smith D.L., Fish N.M., Connerton I.F. The effect of the timing of exposure to Campylobacter jejuni 6 days vs. 20. Microbiome. 2018;6:1–17. doi: 10.1186/s40168-018-0477-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nyati K.K., Prasad K.N., Agrawal V., Husain N. Matrix metalloproteinases-2 and -9 in Campylobacter jejuni-induced paralytic neuropathy resembling Guillain-Barré syndrome in chickens. Microb. Pathog. 2017;111:395–401. doi: 10.1016/j.micpath.2017.09.018. [DOI] [PubMed] [Google Scholar]
- 5.Han Z., Willer T., Pielsticker C., Gerzova L., Rychlik I., Rautenschlein S. Differences in host breed and diet influence colonization by Campylobacter jejuni and induction of local immune responses in chicken. Gut Pathog. 2016;8:1–14. doi: 10.1186/s13099-016-0133-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li X., Swaggerty C.L., Kogut M.H., Chiang H.I., Wang Y., Genovese K.J., He H., Zhou H. Gene expression profiling of the local cecal response of genetic chicken lines that differ in their susceptibility to Campylobacter jejuni colonization. PLoS ONE. 2010;5:e11827. doi: 10.1371/journal.pone.0011827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Humphrey S., Chaloner G., Kemmett K., Davidson N., Williams N., Kipar A., Humphrey T., Wigley P. Campylobacter jejuni is not merely a commensal in commercial broiler chickens and affects bird welfare. MBio. 2014;5:1–7. doi: 10.1128/mBio.01364-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karaffová V., Revajová V., Koščová J., Gancarčíková S., Nemcová R., Ševčíková Z., Herich R., Levkut M., Sr. Local intestinal immune response including NLRP3 inflammasome in broiler chicken infected with Campylobacter jejuni after administration of Lactobacillus reuteri B1/1. Food Agric. Immunol. 2020;31:937–949. doi: 10.1080/09540105.2020.1788516. [DOI] [Google Scholar]
- 9.Karaffová V., Marcinková E., Bobíková K., Herich R., Revajová V., Stašová D., Kavuľová A., Levkutová M., Levkut M., Lauková A., et al. TLR4 and TLR21 expression, MIF, IFN-β, MD-2, CD14 activation, and SIgA production in chickens administered with EFAL41 strain challenged with Campylobacter jejuni. Folia Microbiol. 2017;62:89–97. doi: 10.1007/s12223-016-0475-6. [DOI] [PubMed] [Google Scholar]
- 10.Laukova A., Pogany Simonova M., Kubasova I., Gancarcikova S., Placha I., Scerbova J., Revajova V., Herich R., Levkut M., Strompfova V. Pilot experiment in chickens challenged with Campylobacter jejuni CCM6191 administered enterocin M-producing probiotic strain Enterococcus faecium CCM8558 to check its protective effect. Czech J. Anim. Sci. 2017;62:491–500. doi: 10.17221/12/2017-CJAS. [DOI] [Google Scholar]
- 11.Mortada M., Cosby D.E., Shanmugasundaram R., Selvaraj R.K. In vivo and in vitro assessment of commercial probiotic and organic acid feed additives in broilers challenged with Campylobacter coli. J. Appl. Poult. Res. 2020;29:435–446. doi: 10.1016/j.japr.2020.02.001. [DOI] [Google Scholar]
- 12.Elhadidy M., Miller W.G., Arguello H., Álvarez-Ordóñez A., Duarte A., Dierick K., Botteldoorn N. Genetic basis and clonal population structure of antibiotic resistance in Campylobacter jejuni isolated from broiler carcasses in Belgium. Front. Microbiol. 2018;9:1–9. doi: 10.3389/fmicb.2018.01014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mäesaar M., Meremäe K., Ivanova M., Roasto M. Antimicrobial resistance and multilocus sequence types of Campylobacter jejuni isolated from Baltic broiler chicken meat and Estonian human patients. Poult. Sci. 2018;97:3645–3651. doi: 10.3382/ps/pey219. [DOI] [PubMed] [Google Scholar]
- 14.Simon K., Verwoolde M.B., Zhang J., Smidt H., De Vries Reilingh G., Kemp B., Lammers A. Long-term effects of early life microbiota disturbance on adaptive immunity in laying hens. Poult. Sci. 2016;95:1543–1554. doi: 10.3382/ps/pew088. [DOI] [PubMed] [Google Scholar]
- 15.Schokker D., Jansman A.J.M., Veninga G., de Bruin N., Vastenhouw S.A., de Bree F.M., Bossers A., Rebel J.M.J., Smits M.A. Perturbation of microbiota in one-day old broiler chickens with antibiotic for 24 hours negatively affects intestinal immune development. BMC Genom. 2017;18:1–14. doi: 10.1186/s12864-017-3625-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rubio L.A. Possibilities of early life programming in broiler chickens via intestinal microbiota modulation. Poult. Sci. 2019;98:695–706. doi: 10.3382/ps/pey416. [DOI] [PubMed] [Google Scholar]
- 17.Baldwin S., Hughes R.J., Van T.T.H., Moore R.J., Stanley D. At-hatch administration of probiotic to chickens can introduce beneficial changes in gut microbiota. PLoS ONE. 2018;13:e0194825. doi: 10.1371/journal.pone.0194825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang L., Luo L., Zhang Y., Wang Z., Xia Z. Effects of the dietary probiotic, Enterococcus Faecium NCIMB11181, on the intestinal barrier and system immune status in Escherichia Coli O78-challenged broiler chickens. Probiotics Antimicrob. Proteins. 2019;11:946–956. doi: 10.1007/s12602-018-9434-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saint-Cyr M.J., Haddad N., Taminiau B., Poezevara T., Quesne S., Amelot M., Daube G., Chemaly M., Dousset X., Guyard-Nicodème M. Use of the potential probiotic strain Lactobacillus salivarius SMXD51 to control Campylobacter jejuni in broilers. Int. J. Food Microbiol. 2017;247:9–17. doi: 10.1016/j.ijfoodmicro.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 20.Šefcová M., Larrea-Álvarez M., Larrea-Álvarez C., Karaffová V., Revajová V., Gancarčíková S., Ševčíková Z., Herich R. Lactobacillus fermentum Administration Modulates Cytokine Expression and Lymphocyte Subpopulation Levels in Broiler Chickens Challenged with Campylobacter coli. Foodborne Pathog. Dis. 2020;17:485–493. doi: 10.1089/fpd.2019.2739. [DOI] [PubMed] [Google Scholar]
- 21.Kelley N., Jeltema D., Duan Y., He Y. The NLRP3 inflammasome: An overview of mechanisms of activation and regulation. Int. J. Mol. Sci. 2019;20:3328. doi: 10.3390/ijms20133328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stašová D., Husáková E., Bobíková K., Karaffová V., Levkutová M., Levkut M. Expression of cytokines in chicken peripheral blood mononuclear cells after stimulation by probiotic bacteria and Campylobacter jejuni in vitro. Food Agric. Immunol. 2015;26:813–820. doi: 10.1080/09540105.2015.1036356. [DOI] [Google Scholar]
- 23.Lehri B., Seddon A.M., Karlyshev A.V. Lactobacillus fermentum 3872 as a potential tool for combatting Campylobacter jejuni infections. Virulence. 2017;8:1753–1760. doi: 10.1080/21505594.2017.1362533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cobb-Vantress Broiler Management Guide. [(accessed on 23 August 2020)]; Available online: https://cobb-vantress.com.
- 25.Šefcová M., Levkut M., Bobíková K., Karaffová V., Revajová V., Cingeľová Maruščáková I., Levkutová M., Ševčíková Z., Herich R., Levkut M. Cytokine response after stimulation of culture cells by zinc and probiotic strain. In Vitro Cell. Dev. Biol. Anim. 2019;55:830–837. doi: 10.1007/s11626-019-00401-z. [DOI] [PubMed] [Google Scholar]
- 26.Crhanova M., Hradecka H., Faldynova M., Matulova M., Havlickova H., Sisak F., Rychlik I. Immune response of chicken gut to natural colonization by gut microflora and to Salmonella enterica serovar Enteritidis infection. Infect. Immun. 2011;79:2755–2763. doi: 10.1128/IAI.01375-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kolesarova M., Spisakova V., Matulova M., Crhanova M., Sisak F., Rychlik I. Characterisation of basal expression of selected cytokines in the liver, spleen, and respiratory, reproductive and intestinal tract of hens. Vet. Med. 2011;56:325–332. doi: 10.17221/1586-VETMED. [DOI] [Google Scholar]
- 28.De Boever S., Vangestel C., De Backer P., Croubels S., Sys S.U. Identification and validation of housekeeping genes as internal control for gene expression in an intravenous LPS inflammation model in chickens. Vet. Immunol. Immunopathol. 2008;122:312–317. doi: 10.1016/j.vetimm.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 29.Bhat I.A., Naykoo N.A., Qasim I., Ganie F.A., Yousuf Q., Bhat B.A., Rasool R., Aziz S.A., Shah Z.A. Association of interleukin 1 Beta (IL-1β) polymorphism with mRNA expression and risk of non small cell lung cancer. Meta Gene. 2014;2:123–133. doi: 10.1016/j.mgene.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Konjar Š., Ferreira C., Blankenhaus B., Veldhoen M. Intestinal barrier interactions with specialized CD8 T cells. Front. Immunol. 2017;8:1–15. doi: 10.3389/fimmu.2017.01281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mensikova M., Stepanova H., Faldyna M. Interleukin-17 in veterinary animal species and its role in various diseases: A review. Cytokine. 2013;64:11–17. doi: 10.1016/j.cyto.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 32.He H., Genovese K.J., Kogut M.H. Modulation of chicken macrophage effector function by TH1/TH2 cytokines. Cytokine. 2011;53:363–369. doi: 10.1016/j.cyto.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 33.Oh S., Lillehoj H.S., Lee Y., Bravo D., Lillehoj E.P. Dietary antibiotic growth promoters down-regulate intestinal inflammatory cytokine expression in chickens challenged with LPS or co-infected with Eimeria maxima and Clostridium perfringens. Front. Vet. Sci. 2019;6:1–13. doi: 10.3389/fvets.2019.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sureshkumar S., Jung S.K., Kim D., Oh K.B., Yang H., Lee H.C., Jin J.Y., Sun L.H., Lee S., Byun S.J. Oral administration of Lactobacillus reuteri expressing a 3D8 single-chain variable fragment (ScFv) enhances chicken growth and conserves immune homeostasis. 3 Biotech. 2019;9:1–7. doi: 10.1007/s13205-019-1811-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bron P.A., Van Baarlen P., Kleerebezem M. Emerging molecular insights into the interaction between probiotics and the host intestinal mucosa. Nat. Rev. Microbiol. 2012;10:66–78. doi: 10.1038/nrmicro2690. [DOI] [PubMed] [Google Scholar]
- 36.Brisbin J.T., Gong J., Parvizi P., Sharif S. Effects of lactobacilli on cytokine expression by chicken spleen and cecal tonsil cells. Clin. Vaccine Immunol. 2010;17:1337–1343. doi: 10.1128/CVI.00143-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barjesteh N., Hodgins D.C., St. Paul M., Quinteiro-Filho W.M., DePass C., Monteiro M.A., Sharif S. Induction of chicken cytokine responses in vivo and in vitro by lipooligosaccharide of Campylobacter jejuni HS:10. Vet. Microbiol. 2013;164:122–130. doi: 10.1016/j.vetmic.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 38.Sanad Y.M., Kwoni J., Kashoma I., Zhang X., Kassem I.I., Saif Y.M., Rajashekara G. Insights into potential pathogenesis mechanisms associated with Campylobacter jejuni-induced abortion in ewes. BMC Vet. Res. 2014;10:1–13. doi: 10.1186/s12917-014-0274-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shaughnessy R.G., Meade K.G., McGivney A.B., Allan B., O’Farrelly C. Global gene expression analysis of chicken caecal response to Campylobacter jejuni. Vet. Immunol. Immunopathol. 2011;142:64–71. doi: 10.1016/j.vetimm.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 40.Dunislawska A., Slawinska A., Stadnicka K., Bednarczyk M., Gulewicz P., Jozefiak D., Siwek M. Synbiotics for broiler chickens—In vitro design and evaluation of the influence on host and selected microbiota populations following in ovo delivery. PLoS ONE. 2017;12:e0168587. doi: 10.1371/journal.pone.0168587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang L., Liu R., Song M., Hu Y., Pan B., Cai J., Wang M. Eimeria tenella: Interleukin 17 contributes to host immunopathology in the gut during experimental infection. Exp. Parasitol. 2013;133:121–130. doi: 10.1016/j.exppara.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 42.Fonseca B.B., Fernandez H., Rossi D.A., editors. Campylobacter spp. and Related Organisms in Poultry. Springer; Cham, Switzerland: 2016. pp. 1–206. [Google Scholar]
- 43.Smith C.K., Abuoun M., Cawthraw S.A., Humphrey T.J., Rothwell L., Kaiser P., Barrow P.A., Jones M.A. Campylobacter colonization of the chicken induces a proinflammatory response in mucosal tissues. FEMS Immunol. Med. Microbiol. 2008;54:114–121. doi: 10.1111/j.1574-695X.2008.00458.x. [DOI] [PubMed] [Google Scholar]
- 44.Hermans D., Pasmans F., Heyndrickx M., Van Immerseel F., Martel A., Van Deun K., Haesebrouck F. A Tolerogenic Mucosal Immune Response Leads to Persistent Campylobacter Jejuni Colonization in the Chicken Gut. Crit. Rev. Microbiol. 2012;38:17–29. doi: 10.3109/1040841X.2011.615298. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.