Abstract
Objective
We aimed to describe the baseline clinical characteristics and fracture history of patients taking teriparatide in routine clinical practice in the Middle East (ME) subregional cohort of the Asia and Latin America Fracture Observational Study (ALAFOS).
Methods
Herein, we report baseline clinical characteristics of patients who were prescribed teriparatide (20 µg/day, subcutaneous injection) in four participant ME countries (Saudi Arabia, United Arab Emirates, Kuwait, and Lebanon).
Results
The ME cohort included 707 patients mean (SD) age 69.3 (11.6) years. Mean (SD) bone mineral density (BMD) T-scores at baseline were −3.13 (1.28) for lumbar spine, −2.88 (0.94) for total hip, and −2.65 (1.02) for femoral neck. Osteoporotic fractures after age 40 years were reported in 45.8% (vertebral fracture 14.4%, hip fracture 18.4%) and comorbidities in 57.4% of patients. Before starting teriparatide, 19.9% of patients took other osteoporosis medications. The median (Q1; Q3) EuroQoL 5-Dimension 5-Level visual analog scale score for perceived overall health status was 70 (50; 80). Mean (SD) worst back pain in the previous 24 hours was 4.0 (3.2) using a 10-point numeric rating scale.
Conclusion
This analysis indicated that in ME countries, teriparatide is usually prescribed to patients with low BMD and high comorbidities, with prior fractures.
Keywords: Bone mineral density, non-vertebral fracture, observational study, osteoporosis, vertebral fracture, quality of life, teriparatide
Introduction
Globally, one in three women age >50 years and one in five men are likely to develop osteoporosis.1 In 2010, the estimated number of individuals (≥50 years of age) with a high risk of osteoporosis worldwide was 158 million, which is expected to double by 2040.2 Vertebral, hip, and wrist fractures are the most common complications of osteoporosis. Vertebral fractures occur in 30% to 50% of the population above the age of 50 years.3 In 1990, about 1.66 million people worldwide experienced a hip fracture, and this number is expected to rise to 6.26 million by 2050.4 However, most vertebral fractures are often asymptomatic and remain underdiagnosed. In 2000, the worldwide incidence of different osteoporotic fractures among individuals ≥50 years of age was estimated to be 1.6 million hip fractures, 1.7 million forearm fractures, and 1.4 million clinical vertebral fractures.5 In the Middle East (ME), about 25% of the population will be over 50 years of age in 2020, and this is expected to reach 40% by 2050.6 Age-standardized hip fracture incidence rates of 295 per 105 person-years in women and 200 per 105 person-years in men have been reported in a study from Kuwait.7,8 However, with increased life expectancy, this rate is expected to rise alarmingly. Further, demographic and lifestyle changes will also contribute to the higher incidence of osteoporosis in the near future.9,10
As a silent disease, diagnosing osteoporosis without a fracture remains a challenge, and nearly 50% to 75% of patients with osteoporosis subsequently experience a second fracture.11 Apart from disability, pain, and injuries associated with fractures, there are reports of early mortality, loss of independence, and decreased quality of life (QoL).11–14 The 1-year mortality rate after hip fracture is nearly 20%, which is on a par with that of cancer.15
Guidelines of the American Association of Clinical Endocrinologists (AACE) recommend alendronate, denosumab, risedronate, zoledronate in individuals with a high risk and no prior fractures, or abaloparatide, denosumab, romosozumab, teriparatide, zoledronate in individuals with a very high risk and prior fractures.16 Many recommended drugs, such as bisphosphonates, selective estrogen-receptor modulators, and denosumab (a receptor activator of nuclear factor-kB ligand inhibitor), which work by decreasing bone resorption, do not help with maintaining or restoring the cellular framework of bone and do not improve bone tissue volume.17,18
Teriparatide SC (Forteo®), a recombinant human parathyroid hormone [rhPTH (1-34); 20-μg once-daily subcutaneous injection] is the first approved anabolic or bone-building drug that stimulates bone formation (structurally similar to normal bone); it is prescribed to prevent vertebral and non-vertebral fractures (NVFs) in postmenopausal women with severe osteoporosis.19–22 Teriparatide SC is approved worldwide for treating osteoporosis in men and postmenopausal women who have a higher risk of fracture. The drug changes the macro- and microarchitecture by positively impacting bone stimulation while reducing resorption and increasing the size of the remodeling space and the number of bone basic multicellular units. This further increases bone strength by increasing the bone volume, bone width, and cortical thickness. Teriparatide given intermittently increases the number of osteoblasts, together with bone formation.20 These effects are mainly explained by the activation of preexisting osteoblasts, increased differentiation of bone-lining cells to become osteoblasts, and reduced osteoblast apoptosis; however, treatment also enhances differentiation of preosteoblasts. These actions help reverse the structural changes seen in osteoporotic bone, thereby improving bone quality and bone mass and contributing to reduced fracture rates.23,24 Although the efficacy of teriparatide is well documented in clinical trials,25–27 there is great interest in understanding its effectiveness in real-world settings. Silverman et al.28 (2019) conducted an integrated analysis of four observational studies to assess the incidence of new fractures for the categories of hip, NVFs, clinical vertebral fractures (CVFs), all clinical fractures, and wrist fractures in a total of 8828 patients (mean age 71 years) who were prescribed teriparatide in standard clinical practice. The first 6 months of treatment was considered a reference period, to compare fracture risk reduction. When hip-fracture rates during subsequent periods were compared with the 0- to 6-month reference period, rates for the >12- to 18-month and >18-month periods were significantly decreased. Similarly, NVFs, CVFs, and all clinical fractures also showed a significant decrease in each post-reference period, with maximum reduction in the >18-month period (NVFs: 52.7%, CVFs: 69.4%, and clinical fractures: 61.2%) versus 0 to 6 months.28
To our knowledge, no large-scale, prospective, observational studies have been performed in Asia, Latin America, or the ME with respect to teriparatide use in a real-world setting. The Asia and Latin America Fracture Observational Study (ALAFOS) assessed postmenopausal women with osteoporosis receiving treatment with teriparatide for a maximum of 24 months.29 In this article, we report the baseline clinical characteristics of patients who were prescribed teriparatide in routine clinical practice in the ME cohort of the ALAFOS.
Methods
Study design and ethical considerations
Our study cohort included teriparatide-naïve postmenopausal women with osteoporosis who were prescribed teriparatide (Forteo®, 20 μg/day) in four countries across the ME [Saudi Arabia, Kuwait, Lebanon, and the United Arab Emirates (UAE)], as per the clinical judgement of their treating physicians. Patients were enrolled between December 2015 and October 2017. Treating physicians were responsible for all decisions regarding the diagnosis and treatment of patients. The study drug, teriparatide (Forteo®) was not provided by the manufacturer but was obtained by the patients, as per each respective country’s prescribing policies.
This study was conducted in accordance with the ethical principles of the Declaration of Helsinki and was consistent with good clinical practices and the applicable laws and regulations of the country or countries where the study was conducted. The study was approved by appropriate local bodies, and all eligible participants signed a consent form to release their information.
Objectives
The ALAFOS primarily focused on evaluating the incidence of new clinical fractures in postmenopausal women treated with teriparatide for up to 24 months.29 The main objective of the current subset analysis of the ALAFOS was to assess the baseline characteristics of women with postmenopausal osteoporosis who were prescribed teriparatide for the treatment of osteoporosis in the ME. Secondary aims of this study were to evaluate factors related to teriparatide adherence and to assess health-related quality of life (HRQoL), back pain, and physical function during the study.
Study methodology and assessment
The detailed study design and baseline patient characteristics of the overall ALAFOS cohort have been previously published.29 The following variables were recorded at baseline: (i) fracture history and details; (ii) patient demographics, osteoporosis risk factors, previous and concomitant medications, medical history and comorbidities, socioeconomic factors, and disease status; and (iii) patient-reported measures, osteoporosis knowledge, perception and beliefs, rheumatology attitude index, EuroQoL 5-Dimension 5-Level (EQ-5D-5L) scores, and patient-reported questionnaire responses.
Statistical analysis
Baseline data were analyzed for the entire ME cohort. We used descriptive statistics to describe the data obtained from this study subset. For continuous variables, mean and standard deviation (SD) or median and first and third quartiles (Q1, Q3) are presented. Categorical variables are summarized using frequency and percentage. No statistical comparisons were carried out among the four ME countries.
Results
Responses from 707 patients were included in the analysis (Saudi Arabia [n = 364], Kuwait [n = 225], Lebanon [n = 106], and UAE [n = 12]), constituting 23.3% of the total participants IN ALAFOS [N=3031]). Patient demographic characteristics and reproductive history are summarized in Table 1. The mean (SD) age of patients was 69.3 (11.6) years and mean (SD) body mass index was 29.9 (6.3) kg/m2. The mean (SD) age at onset of menopause was 47.3 (5.1) years. Surgical menopause was reported in 5.3% of patients in the ME subregional cohort, with Lebanon reporting the highest proportion (18.1%) (Table 1).
Table 1.
Patient baseline characteristics.
| Characteristics | Saudi Arabia(N = 364) | Kuwait(N = 225) | Lebanon(N = 106) | United Arab Emirates(N = 12) | Middle East cohort(N = 707) |
|---|---|---|---|---|---|
| Age (years), mean (SD) | 67.5 (13.6) | 70.5 (8.9) | 73.0 (8.2) | 72.8 (7.1) | 69.3 (11.6) |
| Body mass index (kg/m2), mean (SD) | 31.7 (7.2) | 30.0 (5.8) | 28.1 (5.7) | 31.2 (6.3) | 29.9 (6.3) |
| Bone mineral density (T-score), mean (SD) | |||||
| Lumbar spine | −3.86 (0.84) | −3.34 (0.69) | −2.61 (1.80) | −1.50 (1.30) | −3.13 (1.28) |
| (n) | 23 | 96 | 60 | 3 | 182 |
| Total hip | −3.36 (0.25) | −3.16 (0.89) | −2.60 (1.02) | −1.95 (0.64) | −2.88 (0.94) |
| (n) | 5 | 18 | 19 | 2 | 44 |
| Femoral neck | −3.65 (1.77) | −2.33 (0.67) | −2.95 (1.19) | −2.20 | −2.65 (1.02) |
| (n) | 2 | 37 | 35 | 1 | 75 |
| Patients with any osteoporotic fracture after age 40 years, n (%) | 126 (34.6) | 123 (54.7) | 71 (67.0) | 4 (33.3) | 324 (45.8) |
| Patients with total osteoporotic fractures after age 40 years, n (%) | |||||
| No fractures | 238 (65.4) | 102 (45.3) | 35 (33.0) | 8 (66.7) | 383 (54.2) |
| 1 fracture | 116 (31.9) | 114 (50.7) | 38 (35.8) | 4 (33.3) | 272 (38.5) |
| 2 fractures | 9 (2.5) | 9 (4.0) | 21 (19.8) | 0 (0.0) | 39 (5.5) |
| 3 fractures | 1 (0.3) | 0 (0.0) | 9 (8.5) | 0 (0.0) | 10 (1.4) |
| 4 fractures | 0 (0.0) | 0 (0.0) | 3 (2.8) | 0 (0.0) | 3 (0.4) |
| >5 fractures | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Previous fracture sites, n (%) | |||||
| Vertebral | 35 (9.6) | 40 (17.8) | 27 (25.5) | 0 (0.0) | 102 (14.4) |
| Non-vertebral | 94 (25.8) | 80 (35.6) | 48 (45.3) | 4 (33.3) | 226 (32.0) |
| Main non-vertebral* | 82 (22.5) | 67 (29.8) | 44 (41.5) | 3 (25.0) | 196 (27.7) |
| Hip | 65 (17.9) | 41 (18.2) | 23 (21.7) | 1 (8.3) | 130 (18.4) |
| Reproductive history | |||||
| Age at onset of menopause (years), mean (SD) | 46.9 (5.0) | 48.3 (3.6) | 47.1 (6.2) | NR | 47.3 (5.1) |
| Number of fertile years# | 33.9 (5.5) | 35.8 (4.2) | 33.8 (6.6) | NR | 34.6 (5.6) |
| Parity^, n (%) | |||||
| 0 | 30 (8.2) | 17 (7.6) | 11 (10.4) | 7 (58.3) | 65 (9.2) |
| 1 | 16 (4.4) | 3 (1.3) | 4 (3.8) | 0 (0.0) | 23 (3.3) |
| 2 | 19 (5.2) | 3 (1.3) | 15 (14.2) | 1 (8.3) | 38 (5.4) |
| 3 | 25 (6.9) | 15 (6.7) | 20 (18.9) | 0 (0.0) | 60 (8.5) |
| 4 | 36 (9.9) | 18 (8.0) | 13 (12.3) | 0 (0.0) | 67 (9.5) |
| 5 | 238 (65.4) | 169 (75.1) | 43 (40.6) | 4 (33.3) | 454 (64.2) |
| Type of menopause, n (%) | |||||
| Surgical menopause | 3 (1.1) | 7 (4.3) | 19 (18.1) | 0 (0.0) | 29 (5.3) |
N = total number of patients available; n = number of patients with valid (non-missing or unknown) values.
NR = not reported; SD = standard deviation.
*Radius, hip, humerus, tibia, pelvis, and clavicle.
#Age at menopause − age at menstruation.
^Number of times given birth.
Note: Percentages are calculated using n, the number of valid (not missing or unknown) responses for each item as denominator.
BMD and fracture history
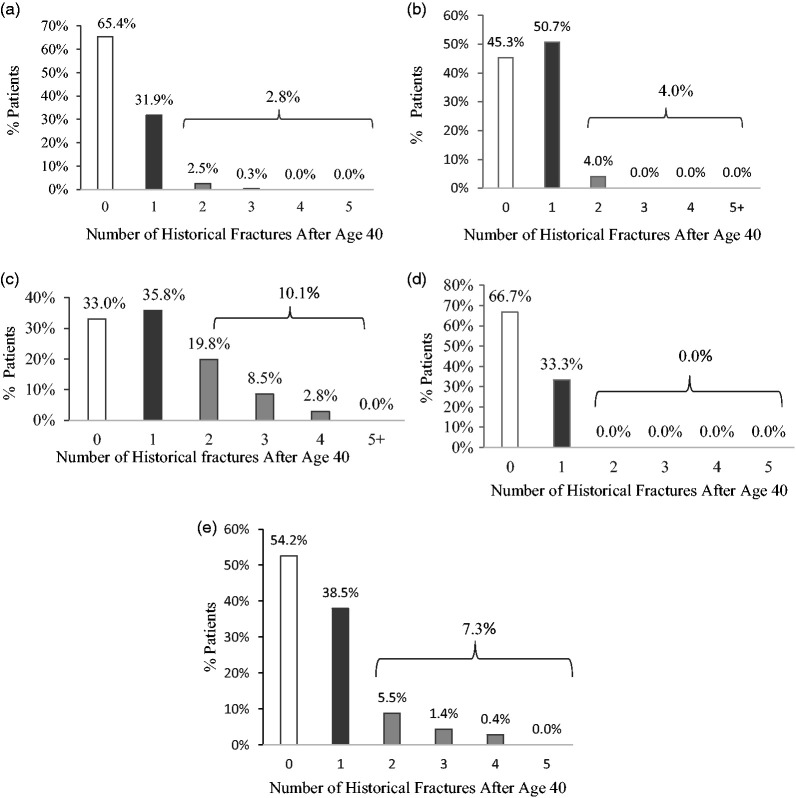
Mean (SD) bone mineral density (BMD) T-scores at baseline for ME countries were 3.13 (1.28), −2.88 (0.94), −2.65 (1.02) for the lumbar spine, total hip, and femoral neck, respectively. The UAE had the highest average BMD scores (ranging from −1.50 to −2.20) and Saudi Arabia the lowest (−3.36 to −3.86). At baseline, 45.8% of the patient cohort had experienced osteoporotic fractures after age 40 years (Lebanon [67.0%], Kuwait [54.7%], Saudi Arabia [34.6%], and the UAE [33.3%]) (Figure 1). More than one-third of ME patients experienced a single osteoporotic fracture (38.5%). Lebanon reported the highest frequency for non-vertebral (45.3%), vertebral (25.5%), and hip fractures (21.7%) followed by Kuwait (non-vertebral [35.6%], hip [18.2%], and vertebral [17.8%] fractures). Detailed data are presented in Table 1.
Figure 1.
Percent of Middle Eastern patients with osteoporotic fractures after 40 years of age
(a) Saudi Arabia (n = 364); (b) Kuwait (n = 225); (c) Lebanon (n = 106); (d) United Arab Emirates (n = 12); (e) Middle East cohort (n = 707).
Note: Percentages are calculated using n, the number of valid (not missing or unknown) responses for each item.
Risk factors for osteoporosis, fall history, comorbid conditions, and concomitant medications
Approximately 41.1% of the ME cohort had at least one recorded fall whereas 12.5% had a maternal history of osteoporosis. Comorbid conditions were present in 57.4% of patients at baseline, and 19.9% of patients had two comorbidities. Of these, current hypertension (HTN) (36.4%) and type 2 diabetes mellitus (T2DM) (20.8%) were the most commonly reported conditions. About 36.8% of the study population in the ME region were current smokers at baseline. Table 2 summarizes the risk factors for osteoporosis, fall history, comorbid conditions, and other medications used by patients in different ME countries.
Table 2.
Risk factors for osteoporosis and fractures at baseline, concomitant medications, and current comorbidities.
| Characteristics | Saudi Arabia (N = 364) |
Kuwait (N = 225) |
Lebanon (N = 106) |
United Arab Emirates (N = 12) |
Middle East cohort (N = 707) |
|---|---|---|---|---|---|
| Maternal history of osteoporosis or hip fracture, n (%) | 21 (7.3) | 11 (9.0) | 29 (37.2) | 0 (0.0) | 61 (12.5) |
| Number of falls in the past year, n (%) | |||||
| No falls | 84 (59.6) | 74 (63.8) | 54 (51.9) | 4 (66.7) | 216 (58.9) |
| 1 fall | 40 (28.4) | 19 (16.4) | 22 (21.2) | 2 (33.3) | 83 (22.6) |
| 2 falls | 11 (7.8) | 13 (11.2) | 17 (16.3) | 0 (0.0) | 41 (11.2) |
| ≥3 falls | 6 (4.3) | 10 (8.6) | 11 (10.6) | 0 (0.0) | 27 (7.4) |
| Smoking status, n (%) | |||||
| Current smokers | 164 (61.4) | 5 (3.0) | 31 (30.4) | 0 (0.0) | 200 (36.8) |
| Patients with current comorbidities*, n (%) | |||||
| Hypertension | 59 (16.3) | 138 (61.3) | 52 (49.1) | 6 (85.7) | 255 (36.4) |
| Type 2 diabetes mellitus | 27 (7.4) | 92 (41.1) | 21 (19.8) | 6 (85.7) | 146 (20.8) |
| Rheumatoid arthritis or other rheumatologic conditions | 0 (0.0) | 7 (3.1) | 10 (9.4) | 0 (0.0) | 17 (2.4) |
| Patients taking concomitant medications, n (%) | |||||
| Antihypertensives | 41 (11.3) | 46 (20.4) | 52 (49.1) | 5 (41.7) | 144 (20.4) |
| Insulin/oral hypoglycemics | 22 (6.0) | 34 (15.1) | 29 (27.4) | 6 (50.0) | 91 (12.9) |
| Glucocorticoids^ | 1 (1.7) | 2 (2.5) | 7 (8.8) | 0 (0.0) | 10 (4.5) |
N = total number of patients available; n=number of patients with valid (non-missing or unknown) values.
*The three most frequently reported comorbidities in the overall ALAFOS cohort 29 are listed here.
^Duration of therapy ≥3 months or a prednisone daily equivalent dose ≥7.5 mg.
Note: Percentages are calculated using n, the number of valid (not missing or unknown) responses for each item as denominator.
Osteoporosis and back pain therapies
At baseline, 19.9% of the ME patient cohort had taken osteoporosis medications before starting teriparatide therapy. The highest previous usage of osteoporosis drugs was observed in Lebanon (68.9%) whereas patients from Saudi Arabia reported the lowest use (4.4%). A total 11.0% and 7.9% of patients took vitamin D and calcium, respectively. Apart from vitamin D and calcium, alendronate was the most frequent previous osteoporosis medication (0.8% of patients). About 15.7% of patients in the ME cohort reported using at least one back pain medication in the previous 24 hours. The most commonly used pain-relieving drugs were paracetamol (9.9%) or non-steroidal anti-inflammatory drugs (NSAIDs, 4.8%). Individual country results showed that Lebanon reported the highest number of patients (58.5%) taking back pain medication whereas Saudi Arabia reported the lowest (1.6%) (Table 3).
Table 3.
Previous use of osteoporosis and analgesic medications.
| Characteristics | Saudi Arabia(N = 364) | Kuwait(N = 225) | Lebanon(N = 106) | United Arab Emirates(N = 12) | Middle East cohort(N = 707) |
|---|---|---|---|---|---|
| Number of previous osteoporosis medications, n (%) | |||||
| 0 | 348 (95.6) | 180 (80.0) | 33 (31.1) | 5 (41.7) | 566 (80.1) |
| 1 | 14 (3.8) | 27 (12.0) | 22 (20.8) | 3 (25.0) | 66 (9.3) |
| 2 | 0 (0.0) | 12 (5.3) | 26 (24.5) | 4 (33.3) | 42 (5.9) |
| 3 | 2 (0.5) | 6 (2.7) | 20 (18.9) | 0 (0.0) | 28 (4.0) |
| 4 | 0 (0.0) | 0 (0.0) | 2 (1.9) | 0 (0.0) | 2 (0.3) |
| 5 | 0 (0.0) | 0 (0.0) | 3 (2.8) | 0 (0.0) | 3 (0.4) |
| Previous pharmacotherapy for osteoporosis#, n (%) | 16 (4.4) | 45 (20.0) | 73 (68.9) | 7 (58.3) | 141 (19.9) |
| Vitamin D | 6 (1.6) | 21 (9.3) | 46 (43.4) | 5 (41.7) | 78 (11.0) |
| Calcium | 4 (1.1) | 17 (7.6) | 33 (31.1) | 2 (16.7) | 56 (7.9) |
| Any one or more bisphosphonates | 3 (0.8) | 5 (2.2) | 9 (8.5) | 0 (0.0) | 17 (2.4) |
| Alendronate | 3 (0.8) | 2 (0.9) | 1 (0.9) | 0 (0.0) | 6 (0.8) |
| Use of ≥1 back pain medication in the past 24 hours, n (%) | 6 (1.6) | 39 (17.3) | 62 (58.5) | 4 (33.3) | 111 (15.7) |
| Specific back pain medications, n (%) | |||||
| Paracetamol | 4 (1.1) | 15 (6.7) | 48 (45.3) | 3 (25.0) | 70 (9.9) |
| NSAIDS | 1 (0.3) | 18 (8.0) | 13 (12.3) | 2 (16.7) | 34 (4.8) |
| Celecoxib | 1 (0.3) | 15 (6.7) | 1 (0.9) | 0 (0.0) | 17 (2.4) |
| Diclofenac | 0 (0.0) | 3 (1.3) | 2 (1.9) | 0 (0.0) | 5 (0.7) |
| Ketoprofen | 0 (0.0) | 0 (0.0) | 4 (3.8) | 1 (8.3) | 5 (0.7) |
| Opioids | 0 (0.0) | 3 (1.3) | 7 (6.6) | 0 (0.0) | 10 (1.4) |
N = total number of patients available; n = number of patients with valid (non-missing or unknown) values.
NSAIDs = non-steroidal anti-inflammatory drugs; SERM = selective estrogen receptor modulator.
#Prior osteoporosis medications taken by >0.5% of all patients in the overall Middle East subregional cohort.
Note: Percentages are calculated using n, the number of valid (not missing or unknown) responses for each item.
Patient reported outcomes: Health-related quality of life (HRQoL) and back pain
At baseline, the median (Q1, Q3) of EQ-5D-5L visual analogue scale (VAS) score for perceived overall health status was 70 (50, 80) in the ME cohort. Individual frequencies of severe or extreme problems in the EQ-5D-5L domains for the entire ME subset were: mobility (n = 139; 23.1%), self-care (n = 104; 17.2%), usual activities (n = 140; 23.2%), pain/discomfort (n = 82; 14.1%), and anxiety/depression (n = 29; 5.6%). The mean (SD) score using a 10-point numeric rating scale (NRS) for back pain was 4.0 (3.2) for the average worst pain in the previous 24 hours and 3.5 (2.9) for mean average pain level in the previous 24 hours. Most enrolled patients from ME countries (>90%) were aware that they had osteoporosis and understood the importance of osteoporosis management (Table 4). The best patient ambulatory status was registered in the Lebanon subset, with 100% of patients being mobile with or without convalescent aid; Saudi Arabia had the highest number of bedbound patients (26.8%) (Table 4).
Table 4.
Health-related quality of life scores and osteoporosis knowledge at baseline.
| Characteristics | Saudi Arabia (N = 364) | Kuwait(N = 225) | Lebanon(N = 106) | United Arab Emirates(N = 12) | Middle East cohort(N = 707) |
|---|---|---|---|---|---|
| EQ-5D-5L-VAS score* | |||||
| Median (Q1, Q3) | 62.5 (50, 80) | 70.0 (60, 80) | 60.0 (40, 80) | 60.0 (35, 77.5) | 70.0 (50, 80) |
| Mean (SD) | 59.3 (26.4) | 68.9 (20.0) | 57.4 (25.8) | 58.1 (27.8) | 63.6 (23.7) |
| EQ-5D-5L severe/extreme problems#, n (%) | |||||
| Mobility | 45 (16.5) | 70 (31.8) | 23 (22.8) | 1 (12.5) | 139 (23.1) |
| Self-care | 29 (10.6) | 56 (25.6) | 18 (17.6) | 1 (12.5) | 104 (17.2) |
| Usual activities | 45 (16.3) | 69 (31.5) | 25 (24.5) | 1 (12.5) | 140 (23.2) |
| Pain/discomfort | 10 (3.9) | 47 (21.7) | 21 (20.6) | 4 (50) | 82 (14.1) |
| Anxiety/depression | 7 (3.5) | 11 (5.12) | 11 (11.3) | 0 (0.0) | 29 (5.6) |
| EQ-5D-5L – Utility Total Score, median (Q1, Q3) | 1.00 (0.59, 1.00) | 0.59 (0.31, 0.67) | 0.59 (0.32, 0.72) | 0.57 (0.17, 0.76) | 0.63 (0.48, 0.86) |
| Back Pain Numeric Rating Scale^ | |||||
| Worst pain in the past 24 hours | |||||
| Median (Q1, Q3) | 6.0 (4.0, 7.0) | 3.0 (0.0, 5.0) | 5.0 (2.0, 8.0) | 6.5 (1.5, 9.0) | 4.0 (0.0, 7.0) |
| Mean (SD) | 5.2 (2.6) | 3.0 (3.1) | 4.9 (3.2) | 5.4 (3.9) | 4.0 (3.2) |
| Average pain in the past 24 hours | |||||
| Median (Q1, Q3) | 5.0 (4.0, 7.0) | 2.0 (0.0, 4.0) | 4.0 (1.0, 7.0) | 6.5 (1.5, 8.5) | 3.0 (0.0, 6.0) |
| Mean (SD) | 5.1 (2.5) | 2.5 (2.5) | 3.9 (3.1) | 5.4 (3.9) | 3.5 (2.9) |
| Ambulatory status assessment, n (%) | |||||
| Ambulatory, no convalescent aid needed | 117 (54.9) | 101 (46.1) | 73 (70.2) | 5 (62.5) | 296 (54.4) |
| Ambulatory, with convalescent aid | 39 (18.3) | 95 (43.4) | 31 (29.8) | 2 (25.0) | 167 (30.7) |
| Non-ambulatory, bedbound/bedfast | 57 (26.8) | 23 (10.5) | 0 (0.0) | 1 (12.5) | 81 (14.9) |
| Osteoporosis knowledge and beliefs (agree/strongly agree), n (%) | |||||
| Worry about having osteoporosis | 257 (94.1) | 198 (91.6) | 92 (90.2) | 7 (87.5) | 554 (92.5) |
| With a fracture, I could end up disabled for a long time | 262 (94.6) | 198 (91.6) | 96 (93.2) | 7 (100) | 563 (93.4) |
| Osteoporosis medication can help me stay independent | 268 (96.1) | 188 (89.1) | 91 (88.4) | 6 (85.7) | 553 (92.2) |
| Osteoporosis medication is good for me | 273 (97.5) | 198 (92.5) | 93 (91.1) | 6 (85.7) | 570 (94.5) |
| Osteoporosis medication can help me stay active | 269 (96.4) | 179 (84.9) | 86 (85.1) | 6 (85.7) | 540 (90.3) |
N = total number of patients; n = number of patients with valid (non-missing or unknown) values.
Q1 = first quartile; Q3 = third quartile; VAS = Visual analog scale.
*A 100-mm visual analog scale was used to indicate perceived overall health status (0 = the worst health you can imagine; 100 = the best health you can imagine).
#For the “mobility,” “self-care,” and “usual activities” domains, extreme problems refer to inability to move, inability to take care of self, and inability to perform usual activities, respectively.
^Back pain was self-assessed by patients using a rating scale of 0 to 10 (0 = no back pain; 10 = worst possible back pain).
Note: Percentages are calculated using n, the number of valid (not missing or unknown) responses for each item.
Discussion
In this study, we provide a detailed description of the baseline characteristics of patients with postmenopausal osteoporosis from the ME region who were prescribed teriparatide treatment in real-world clinical practice. The data presented here indicate the mean (SD) age of enrolled patients was 69.3 (11.6) years, which is similar to other real-world evidence studies involving teriparatide: the European Forsteo Observational Study (EFOS), 71.5 (8.4) years; the Extended Forsteo Observational Study (ExFOS), 70.2 (9.8) years; and the Direct Assessment of Nonvertebral Fractures in Community Experience (DANCE), 68 (11.7) years,30–32 together with low BMD values and high osteoporotic fracture risk. BMD T-scores (lumbar spine, total hip, and femoral neck) were lowest in the Saudi cohort. The results of several other studies analyzing BMD among Saudi women support the findings of the present study.33–37
According to the 2017 Pan Arab Osteoporosis Society guidelines, teriparatide can be used in the following patients: a) postmenopausal women with severe osteoporosis (T-score less than −2.5 and at least one fragility fracture) who are unable to tolerate any available bisphosphonates; b) postmenopausal women with severe osteoporosis (T-score less than−2.5 and at least one fragility fracture) who continue to experience fractures after 1 year of taking bisphosphonates; and c) postmenopausal women with osteoporosis who are unable to tolerate bisphosphonates or who have relative contraindications to bisphosphonates (achalasia, scleroderma esophagus, esophageal strictures) in addition to relative contraindications to raloxifene (thrombosis, hot flashes) or for whom other osteoporosis therapies fail.38 In the present study, most of the population had BMD T-scores less than −2.5 (except the UAE cohort). In addition, only one-third of the population in Saudi Arabia and the UAE, around half that in Kuwait and the ME, and two-thirds of the population in Lebanon had at least one fracture. Furthermore, less than 10% of patients were previously prescribed bisphosphonates. The tolerance to bisphosphonates was not recorded before administering teriparatide in the present study. In contrast to the Pan Arab Osteoporosis Society guidelines, the 2015 guidelines for osteoporosis in Saudi Arabia state that rhPTH therapy should be restricted to patients with multiple fractures, low bone density (T-score less than −2.7), and glucocorticoid-induced osteoporosis (GIO).39,40 However, in our Saudi cohort, only 2.5% of patients had more than one fracture. The Kuwait osteoporosis guidelines (2018) recommend the use of teriparatide in GIO and in patients with a history of fragility fractures (spine, hip, or ≥2 other fractures).41 In contrast to these guidelines, the Kuwait cohort in our study showed few cases (4.0%) with a history of multiple fractures at baseline. These deviations from the guidelines might be owing to a lack of awareness and familiarity with the current guidelines among physicians, a lack of motivation and agreement in applying these guidelines, and factors related to patients and the environment.42
Previous randomized clinical trials across several countries have demonstrated the efficacy and safety of teriparatide along with sustained vertebral fracture risk reduction, even after withdrawal of teriparatide.24,26 Several prior observational studies have evaluated the use of teriparatide in routine clinical practice across different parts of the world. Studies including the EFOS, ExFOS, and DANCE have shown that the incidence of osteoporotic fractures decreased over time in patients receiving teriparatide in routine clinical practice in Europe and the United States.30–32 Results of the recently published Japan Fracture Observational Study (JFOS) indicate that 20 µg/day teriparatide is also effective in reducing the risk of clinical fractures among Japanese patients in a real-world setting.43 No large-scale, prospective, observational studies have been conducted to assess the use of teriparatide in real-world settings in Asia, Latin America, or the ME region.30–32 ALAFOS gathered real-life evidence regarding the use of teriparatide in a global study using a large sample size and broad geographical representation. In this study, the second largest after DANCE, we assessed the real-world use and outcomes of teriparatide in the ME subset, which has exhibited an increasing prevalence of osteoporosis.
More than half of the cohort in our study had other comorbid conditions, with HTN and T2DM being most common. The number of patients with comorbid conditions was highest in Kuwait and lowest in the UAE. A recent study conducted by Puth et al.44 (2018) reported that more than 95% of the study population with osteoporosis had at least one comorbidity. Al Quaiz et al.37 (2014) reported that among women with low BMD in Saudi Arabia, 37% had a history of diabetes. A similar study in Oman by Menon et al.45 (2018) found that comorbidities like DM, HTN, ischemic heart disease, thyroid dysfunction, and respiratory disorders were present in most (79.9%) patients with osteoporosis. Another study conducted in an Indian population with severe osteoporosis stated that most patients treated with teriparatide and other antiresorptive medications had HTN at baseline.46 A similar study conducted in Japan reported that nearly half of the study population (48.5%) treated with teriparatide had one or more comorbidities at baseline.25
In this study, about 12% of all patients from the ME region had a maternal history of osteoporosis and hip fractures, and many had more than one fall. Lebanon registered the highest number of patients with a history of fall (48.1%) and maternal osteoporosis (37.2%). This is much higher than previously reported in a cross-sectional study from Riyadh in 2009 where 1.7% of patients diagnosed with low BMD (women ≥40 years of age) had a history of osteoporosis and 1.5% had a family history of fractures.37 We also found that 22.6% of the total ME cohort had a history of at least one fall, which is similar to a study by Rouzi et al.47 (2015) using the same sample size in a Saudi cohort (23.2%). A high percentage of women (45.8%) had osteoporotic fractures after age 40 years, which is far earlier than the observed age globally (>50 years).48
Among all cohorts in the ME region, we found vertebral fractures and NVFs to be most prevalent in the Lebanon subset. Another study conducted in 2013 also showed a high annual incidence of hip fractures among Lebanese women, and these rates were comparable to those of their southern European counterparts; the authors also noticed an exponential increase in hip fractures with age and stated that this occurrence can be expected to increase in the coming decades.49
We observed a high proportion of osteoporosis treatment-naïve patients. Despite being the most commonly used medications, the use of calcium and vitamin D was low. A similar osteoporosis medication history at baseline in the ME has been found in other studies.45,50–52
Eighty percent of patients in the ME cohort of the ALAFOS did not receive any prior osteoporosis medication, which is much more than the values obtained in the global ALAFOS (43.7%).29 Observational studies in the United States, Europe, and Japan have reported 14.3%, 11.3%, and 48.7%, of the native cohort to be treatment naïve, respectively.31,32,43 This can be explained by differences in the incidence rate of fragility fractures among postmenopausal women, comorbidities, as well as lifestyle and awareness about osteoporosis between the ME and European countries.32 This also reflects the variations in clinical practice across different regions.
Our study showed that 5% to 10% of patients used analgesics or NSAIDs. Back pain medications were taken by 15.7% of the population across ME countries. The mean back pain score (NRS) in the ME cohort was 3.5, which was comparable with the global ALAFOS score.29 Celecoxib (2.4%) was the most common NSAID used by patients and paracetamol (9.9%) was the most common analgesic. The use of back pain medication was lower in the ME cohort than in the East Asian and Latin American cohorts, which may be attributed to lower prevalence of back pain in the ME region.29
Most patients with osteoporosis (>90%) were aware of their condition and understood the importance of osteoporosis management. The highest rate of patient awareness was in Saudi Arabia. A similar study conducted in Saudi Arabia in 2012, including both male and female patients, assessed knowledge, attitude and behavior, to understand awareness of the Saudi subset regarding osteoporosis and related factors.53 Interestingly, the study reported that a considerable number of patients were unaware of their disease. However, another cross-sectional study conducted in Saudi Arabia in 2018 reported a substantial level of knowledge and a positive attitude toward osteoporosis.54 A study conducted in Lebanon to assess patient awareness about osteoporosis revealed that osteoporosis knowledge and perception is low among Lebanese women age 50 years and older.1,55
The median value of the EQ-5D-5L total utility score in the ME region (0.63) was similar to that in the ExFOS (0.62).31 However, the median value was higher than values in the global ALAFOS (0.59), EFOS (0.59), and JFOS (0.55).29,30,43 Several studies have reported a reduction in QoL with both osteoporosis-associated vertebral and hip fractures.56
Strengths and limitations
The strength of this study is that, being observational in nature, our findings provide real-world data from clinical settings and reflect the actual use of teriparatide in the ME region. However, there are several limitations to the study. As a single-arm study, there is no comparator, which is a general limitation of all observational studies. In addition, the patient distribution and health care models among the four countries are not uniform, and prescription patterns may differ among these countries. As this was a cross-sectional, self-reported survey, there is a high likelihood that patients failed to recall all data or correct data in terms of the number of fractures, falls, comorbid conditions, and concomitant medications. Finally, when reporting back pain and HRQoL domains, perception bias might be present.
Conclusion
In this ME subregional analysis of the ALAFOS, we found that patients who were prescribed teriparatide in the four participating countries (Saudi Arabia, Kuwait, Lebanon, and the UAE) had a mean age similar to their counterparts in Europe and the United States, according to previous real-world evidence studies. Patients had compromised QoL, low BMD values along with comorbid conditions, and a history of falls and maternal osteoporosis. Most patients in the present study did not receive any previous osteoporosis medications, as compared with other regions in the ALAFOS. However, patients were aware of their disease and understood the importance of osteoporosis management.
Acknowledgement
The authors would like to thank Dr. Hossam Othman for his contributions in the study. Dr. Othman unfortunately passed away in August 2018. May his soul rest in peace. We would also like to acknowledge Turacoz Healthcare Solutions (https://www.turacoz.com) for medical writing assistance.
Declaration of conflicting interest
A.A.A., H.A., S.A.A., G.M., S.S., Y.Y., N.S.A. have received consulting and lecture fees from Eli Lilly and Company. A.M. and M.T. are employees of Eli Lilly and Company.
Funding
Eli Lilly and Company sponsored the ALAFOS (unique study identifier: B3D-MC-B026). The sponsor was involved in the conduct of the study, including the design of the study and collection, analysis and interpretation of data, and writing the manuscript.
ORCID iDs
Mir Sadat-Ali https://orcid.org/0000-0001-8590-0830
Ahmed Mahmoud https://orcid.org/0000-0003-1859-1569
Mohamed Taher https://orcid.org/0000-0002-1793-581X
References
- 1.Sozen T, Ozisik L, Basaran NC. An overview and management of osteoporosis. Eur J Rheumatol 2017; 4: 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oden A, McCloskey EV, Kanis JA, et al. Burden of high fracture probability worldwide: secular increases 2010-2040. Osteoporos Int 2015; 26: 2243–2248. [DOI] [PubMed] [Google Scholar]
- 3.Ballane G, Cauley JA, Luckey MM, et al. Worldwide prevalence and incidence of osteoporotic vertebral fractures. Osteoporos Int 2017; 28: 1531–1542. [DOI] [PubMed] [Google Scholar]
- 4.Cooper C, Campion G, Melton LJ., 3rd. Hip fractures in the elderly: a world-wide projection. Osteoporos Int 1992; 2: 285–289. [DOI] [PubMed] [Google Scholar]
- 5.Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 2006; 17: 1726–1733. [DOI] [PubMed] [Google Scholar]
- 6.Fuleihan GEH, Adib G. Epidemiology, costs & burden of osteoporosis in 2011; 2019: [1–19 pp.]. Available from: https: //www.iofbonehealth.org/sites/default/files/PDFs/Audit%20Middle%20East_Africa/ME_audit-executive_summary.pdf
- 7.Dhanwal DK, Cooper C, Dennison EM. Geographic variation in osteoporotic hip fracture incidence: the growing importance of Asian influences in coming decades. J Osteoporos 2010; 2010: 757102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Memon A, Pospula WM, Tantawy AY, et al. Incidence of hip fracture in Kuwait. Int J Epidemiol 1998; 27: 860–865. [DOI] [PubMed] [Google Scholar]
- 9.Alwahhabi BK. Osteoporosis in Saudi Arabia: are we doing enough? Saudi Med J 2015; 36: 1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maalouf G, Gannage-Yared MH, Ezzedine J, et al. Middle East and North Africa consensus on osteoporosis. J Musculoskelet Neuronal Interact 2007; 7: 131–143. [PubMed] [Google Scholar]
- 11.What you need to know about Osteoporosis. Report. Osteoporosis Australia; 2014.
- 12.Lips P, Van Schoor NM. Quality of life in patients with osteoporosis. Osteoporos Int 2005; 16: 447–455. [DOI] [PubMed] [Google Scholar]
- 13.Center JR, Nguyen TV, Schneider D, et al. Mortality after all major types of osteoporotic fracture in men and women: an observational study. Lancet 1999; 353: 878–882. [DOI] [PubMed] [Google Scholar]
- 14.Pisani P, Renna MD, Conversano F, et al. Major osteoporotic fragility fractures: risk factor updates and societal impact. World J Orthop 2016; 7: 171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee YK, Lee YJ, Ha YC, et al. Five-year relative survival of patients with osteoporotic hip fracture. J Clin Endocrinol Metab 2014; 99: 97–100. [DOI] [PubMed] [Google Scholar]
- 16.Endocrinologists AAoC. AACE Clinical Practice Guidelines for the Diagnosis and Treatment of Postmenopausal Osteoporosis - 2020 Update. Available from: https: //www.aace.com/disease-state-resources/reproductive-and-gonad/clinical-practice-guidelines-recent-news-and-updates (accessed 9 June 2020).
- 17.Riggs BL, Parfitt AM. Drugs used to treat osteoporosis: the critical need for a uniform nomenclature based on their action on bone remodeling. J Bone Miner Res 2005; 20: 177–184. [DOI] [PubMed] [Google Scholar]
- 18.Seeman E, Delmas PD. Bone quality–the material and structural basis of bone strength and fragility. N Engl J Med 2006; 354: 2250–2261. [DOI] [PubMed] [Google Scholar]
- 19.Tu KN, Lie JD, Wan CKV, et al. Osteoporosis: a Review of Treatment Options. P T 2018; 43: 92–104. [PMC free article] [PubMed] [Google Scholar]
- 20.Brixen KT, Christensen B, Ejersted C, et al. Teriparatide (biosynthetic human parathyroid hormone 1–34): a new paradigm in the treatment of osteoporosis. Basic Clin Pharmacol Toxicol 2004; 94: 260–270. [DOI] [PubMed] [Google Scholar]
- 21.Tella SH, Gallagher JC. Prevention and treatment of postmenopausal osteoporosis. J Steroid Biochem Mol Biol 2014; 142: 155–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bodenner D, Redman C, Riggs A. Teriparatide in the management of osteoporosis. Clin Interv Aging 2007; 2: 499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang Y, Zhao JJ, Mitlak BH, et al. Recombinant human parathyroid hormone (1–34) [teriparatide] improves both cortical and cancellous bone structure. J Bone Miner Res 2003; 18: 1932–1941. [DOI] [PubMed] [Google Scholar]
- 24.Lindsay R, Scheele WH, Neer R, et al. Sustained vertebral fracture risk reduction after withdrawal of teriparatide in postmenopausal women with osteoporosis. Arch Intern Med 2004; 164: 2024–2030. [DOI] [PubMed] [Google Scholar]
- 25.Nishikawa A, Ishida T, Taketsuna M, et al. Safety and effectiveness of daily teriparatide in a prospective observational study in patients with osteoporosis at high risk of fracture in Japan. Clin Interv Aging 2016; 11: 913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 2001; 344: 1434–1441. [DOI] [PubMed] [Google Scholar]
- 27.Kendler DL, Marin F, Zerbini CAF, et al. Effects of teriparatide and risedronate on new fractures in post-menopausal women with severe osteoporosis (VERO): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet 2018; 391: 230–240. [DOI] [PubMed] [Google Scholar]
- 28.Silverman S, Langdahl BL, Fujiwara S, et al. Reduction of Hip and Other Fractures in Patients Receiving Teriparatide in Real-World Clinical Practice: integrated Analysis of Four Prospective Observational Studies. Calcif Tissue Int 2019; 104: 193–200. [DOI] [PubMed] [Google Scholar]
- 29.Chen CH, Elsalmawy AH, Ish-Shalom S, et al. Study description and baseline characteristics of the population enrolled in a multinational, observational study of teriparatide in postmenopausal women with osteoporosis: the Asia and Latin America Fracture Observational Study (ALAFOS). Curr Med Res Opin 2019; 35: 1041–1049. [DOI] [PubMed] [Google Scholar]
- 30.Rajzbaum G, Jakob F, Karras D, et al. Characterization of patients in the European Forsteo Observational Study (EFOS): postmenopausal women entering teriparatide treatment in a community setting. Curr Med Res Opin 2008; 24: 377–384. [DOI] [PubMed] [Google Scholar]
- 31.Langdahl BL, Ljunggren Ö, Benhamou CL, et al. Fracture rate, quality of life and back pain in patients with osteoporosis treated with teriparatide: 24-month results from the Extended Forsteo Observational Study (ExFOS). Calcif Tissue Int 2016; 99: 259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silverman S, Miller P, Sebba A, et al. The Direct Assessment of Nonvertebral Fractures in Community Experience (DANCE) study: 2-year nonvertebral fragility fracture results. Osteoporos Int 2013; 24: 2309–2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.El-Desouki MI. Osteoporosis in postmenopausal Saudi women using dual x-ray bone densitometry. Saudi Med J 2003; 24: 953–956. [PubMed] [Google Scholar]
- 34.Sadat-Ali M, Al-Habdan IM, Al-Mulhim FA, et al. Bone mineral density among postmenopausal Saudi women. Saudi Med J 2004; 25: 1623–1625. [PubMed] [Google Scholar]
- 35.Ardawi MS, Maimany AA, Bahksh TM, et al. Bone mineral density of the spine and femur in healthy Saudis. Osteoporos Int 2005; 16: 43–55. [DOI] [PubMed] [Google Scholar]
- 36.Rouzi AA, Al-Sibiani SA, Al-Senani NS, et al. Independent predictors of all osteoporosis-related fractures among healthy Saudi postmenopausal women: the CEOR Study. Bone 2012; 50: 713–722. [DOI] [PubMed] [Google Scholar]
- 37.AlQuaiz AM, Kazi A, Tayel S, et al. Prevalence and factors associated with low bone mineral density in Saudi women: a community based survey. BMC Musculoskelet Disord 2014; 15: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jassim NA, Adib G, Younis AAR, et al. Pan Arab Osteoporosis Society Guidelines for Osteoporosis Management. Mediterr J Rheumatol 2017; 28: 37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alshahrani F, Aidarous SA, Alamri F, et al. National Plan for Osteoporosis Prevention and Management in the Kingdom of Saudi Arabia. In: Ministry of Health, editor. 2018. pp.3–27.
- 40.Al-Saleh Y, Sulimani R, Sabico S, et al. 2015 guidelines for osteoporosis in Saudi Arabia: recommendations from the Saudi Osteoporosis Society. Ann Saudi Med 2015; 35: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.FRAX Based Kuwait Osteoporosis Guidelines. Kuwait Osteoporosis Society; 2018.
- 42.Fischer F, Lange K, Klose K, et al. (eds). Barriers and strategies in guideline implementation—a scoping review. Healthcare 2016; 4: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soen S, Fujiwara S, Takayanagi R, et al. Real-world effectiveness of daily teriparatide in Japanese patients with osteoporosis at high risk for fracture: final results from the 24-month Japan Fracture Observational Study (JFOS). Curr Med Res Opin 2017; 33: 2049–2056. [DOI] [PubMed] [Google Scholar]
- 44.Puth MT, Klaschik M, Schmid M, et al. Prevalence and comorbidity of osteoporosis– a cross-sectional analysis on 10,660 adults aged 50 years and older in Germany. BMC Musculoskelet Disord 2018; 19: 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Venugopal Menon K, Al Harthy HHS, Al Habsi KSK, et al. Are we treating osteoporotic fractures of the hip adequately? A Middle Eastern cohort study. Arch Osteoporos 2018; 13: 6. [DOI] [PubMed] [Google Scholar]
- 46.Chhabra H, Malhotra R, Marwah S, et al. An observational study to assess back pain in patients with severe osteoporosis treated with teriparatide versus antiresorptives: an Indian subpopulation analysis. Indian J Endocrinol Metab 2015; 19: 483–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rouzi AA, Ardawi MS, Qari MH, et al. Risk factors for falls in a longitudinal cohort study of Saudi postmenopausal women: the Center of Excellence for Osteoporosis Research Study. Menopause 2015; 22: 1012–1020. [DOI] [PubMed] [Google Scholar]
- 48.Marik PE, Corwin HL. Efficacy of red blood cell transfusion in the critically ill: a systematic review of the literature. J Crit Care Med 2008; 36: 2667–2674. [DOI] [PubMed] [Google Scholar]
- 49.Maalouf G, Bachour F, Hlais S, et al. Epidemiology of hip fractures in Lebanon: a nationwide survey. Orthop Traumatol Surg Res 2013; 99: 675–680. [DOI] [PubMed] [Google Scholar]
- 50.Hreybe H, Salamoun M, Badra M, et al. Hip fractures in Lebanese patients: determinants and prognosis. J Clin Densitom 2004; 7: 368–375. [DOI] [PubMed] [Google Scholar]
- 51.Tabatabaei-Malazy O, Salari P, Khashayar P, et al. New horizons in treatment of osteoporosis. DARU 2017; 25: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gombotz H, Rehak PH, Shander A, et al. Blood use in elective surgery: the Austrian benchmark study. Transfusion 2007; 47: 1468–1480. [DOI] [PubMed] [Google Scholar]
- 53.Barzanji AT, Alamri FA, Mohamed AG. Osteoporosis: a study of knowledge, attitude and practice among adults in Riyadh, Saudi Arabia. J Community Health 2013; 38: 1098–1105. [DOI] [PubMed] [Google Scholar]
- 54.Tripathi R, Makeen HA, Albarraq AA, et al. Knowledge, attitude and practice about osteoporosis in south-western Saudi Arabia: a cross-sectional survey. Int J Health Promot Educ 2019; 57: 13–22. [Google Scholar]
- 55.Ahmadieh H, Basho A, Chehade A, et al. Perception of peri-menopausal and postmenopausal Lebanese women on osteoporosis: a cross-sectional study. J Clin Transl Endocrinol 2018; 14: 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martin AR, Sornay-Rendu E, Chandler JM, et al. The impact of osteoporosis on quality-of-life: the OFELY cohort. Bone 2002; 31: 32–36. [DOI] [PubMed] [Google Scholar]