Abstract
There is growing evidence that exposure to hypoxia, regardless of the source, elicits several metabolic responses in individuals. These responses are constitutive and are usually observed under hypoxia but vary according to the type of exposure. The aim of this review was to describe the involvement of obesity and lipid metabolism in the development of high-altitude pulmonary hypertension and in the development of acute mountain sickness under chronic intermittent hypoxia. Overweight or obesity, which are common in individuals with long-term chronic intermittent hypoxia exposure (high-altitude miners, shift workers, and soldiers), are thought to play a major role in the development of acute mountain sickness and high-altitude pulmonary hypertension. This association may be rooted in the interactions between obesity-related metabolic and physical alterations, such as increased waist circumference and neck circumference, among others, which lead to critical ventilation impairments; these impairments aggravate hypoxemia at high altitude, thereby triggering high-altitude diseases. Overweight and obesity are strongly associated with higher mean pulmonary artery pressure in the context of long-term chronic intermittent hypoxia. Remarkably, de novo synthesis of triglycerides by the sterol regulatory element-binding protein-1c pathway has been demonstrated, mainly due to the upregulation of stearoyl-CoA desaturase-1, which is also associated with the same outcomes. Therefore, overweight, obesity, and other metabolic conditions may hinder proper acclimatization. The involved mechanisms include respiratory impairment, alteration of the nitric oxide pathways, inflammatory status, reactive oxygen species imbalance, and other metabolic changes; however, further studies are required.
Keywords: overweight, lipid profile, triglycerides, high altitude, pulmonary hypertension
Introduction
Exposure to high altitude results in several changes in an individual as part of their acclimatization processes. In some individuals, disease occurs as a result of high-altitude exposure because of reduced O2 availability and a failure to acclimatize. Hypobaric hypoxia occurs when ascending to high altitude because of the decrease in barometric pressure.1 Although the oxygen concentration remains constant (i.e. ∼21%), the partial pressure of inspired oxygen (PO2) and the saturation of arterial hemoglobin (SaO2) decrease.2
The latter generates changes in the oxygen transport cascade from ambient air to cellular mitochondria.3 At an altitude above 2000 m, humans may begin to suffer from high-altitude illnesses.4 The greater the altitude, the lower the possibility of life is because hypoxemia increases exponentially with altitude. Important advances have made it possible to live and work effectively over 5000 m using oxygen enrichment, such as at observatories and during the construction of the railway to Lhasa.5 However, the altitude limit for permanent human habitation can may be higher; in fact, La Rinconada (Perú), which is at 5100–5300 m, has been reported to be the highest permanently inhabited town in the world.6
There are different types of exposure to hypoxia according to the time spent at a given altitude. According to the duration of exposure, hypoxia is classified as acute (hours or days, principally tourists, trekkers, and alpinists), chronic hypoxia (CH, experienced by those living permanently at a high altitude), and chronic intermittent hypoxia (CIH), an interesting new phenomenon that has become more common in the last 20 years, which corresponds to spending a number of days alternating between high altitude and sea level over a long period. This pattern is particularly related to commercial activities, such as high-altitude mining; scientific activities, such as the use of high-altitude telescopes; frontier work; or defense forces operating in mountain areas.5 CIH is a relatively novel condition, and the term “Chilean miner model” was coined by Richalet et al.7
The main recognized high-altitude diseases that occur are acute mountain sickness (AMS), high-altitude cerebral edema, high-altitude pulmonary edema (HAPE), high-altitude pulmonary hypertension (HAPH), and chronic mountain sickness (CMS).
AMS, a common medical syndrome characterized by headache, loss of appetite, nausea, weakness, and dizziness, is the most common of these conditions.8 The prevalence of AMS depends strongly on the study setting. The prevalence of AMS varies between 40% and 90%, depending on altitude,9,10 the speed of ascent, the altitude attained, age, sex, ventilatory response to acute hypoxia,8 individual susceptibility, and the degree of acclimatization. It is important to note that the transient and mild or moderate nature of AMS is characterized by a rapid reversibility after descent and the administration of supplementary oxygen or full acclimatization.5,11 AMS (mild) is usually observed on day 1 (20–53%) in people experiencing chronic intermittent exposure, and regardless of the elapsed time in miners working shifts and soldiers, who are probably unable to reach full acclimatization.7,12,13
CMS is a disease characterized by excessive erythrocytosis that occurs in people who live permanently at high altitudes. It is characterized by hemoglobin levels ≥21 mg/dl in males and 19 mg/dl in females.14 However, CMS has not been described in people with CIH.13
The right cardiac circuits in high-altitude residents or lowlanders who go to work or live at high altitude and experience chronic hypobaric hypoxia present changes that are principally characterized by elevated pulmonary artery pressure (PAP), and some of these individuals may develop HAPH.13 Pulmonary hypertension (PH) is a disease defined by a mean pulmonary arterial pressure ≥25 mmHg at rest and is a syndrome resulting from restricted pulmonary arterial circulation, resulting in increased pulmonary vascular resistance and ultimately in right heart failure15 via multiple pathogenic mechanisms. PH has been classified into different categories according to its origins by the American College of Cardiology Foundation/American Heart Association, and the classification system was reviewed by the World Health Organization. PH symptoms include dizziness and exercise intolerance, with a prevalence of 20–50 per million persons.16 Therefore, PH pathogenesis is multifactorial and is characterized by an abnormally elevated PAP and a sustained increase in pulmonary vascular resistance and vascular remodeling.17 These characteristics may be due to a variety of causes and combinations of factors, such as endothelial dysfunction, vasoconstriction of small pulmonary arteries, and endothelial and smooth muscle cell proliferation.18
HAPH is classified into group 3, which includes PH associated with lung diseases and/or hypoxemia, or group 3.5 in the context of chronic exposure to high altitude.15 The characterization of HAPH has been defined by a consensus of experts in altitude studies as a median PAP (mPAP) ≥30 mmHg14 without excessive polycythemia. This cut-off value accounts for the fact that at high altitude, a higher mPAP is expected.
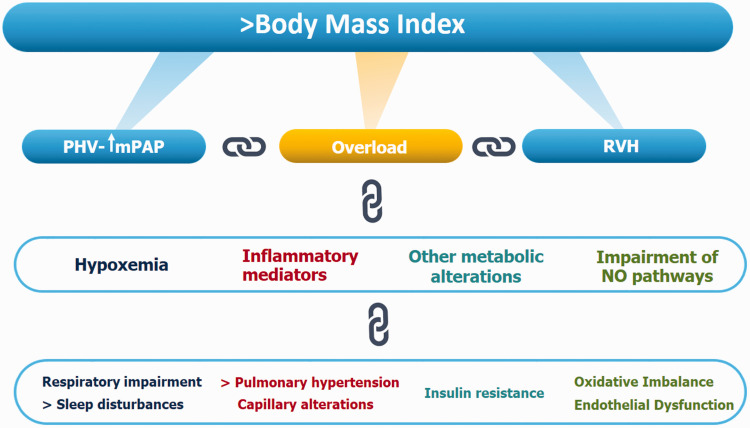
Regarding the mechanisms implicated in HAPH, the most widely accepted/discussed in the literature is hypoxic pulmonary vasoconstriction (HPV) due to hypoxemia and calcium release acting on RHO-kinase and the actin–myosin contractile apparatus,19,20 entailing a pressure overload that results in the development of right ventricular hypertrophy. This mechanism is the current one discussed in the literature, but there have recently been other contributors to HPV and the subsequent development of HAPH identified, such as respiratory impairment, inflammatory mediators, lower nitric oxide (NO) bioavailability, oxidative imbalance, and metabolic disorders (Fig. 1).
Fig. 1.
Schematic diagram showing the principal events associated with higher body mass index that lead to high-altitude pulmonary hypertension.
PHV: pulmonary hypoxic vasoconstriction; mPAP: median pulmonary artery pressure; RVH: right ventricular hypertrophy.
The aim of this review was to describe the involvement of obesity and lipid metabolism primarily on the development of HAPH and secondarily on AMS under CIH.
Main responses during acclimatization
Exposure to high altitude produces compensatory changes in individuals, with different responses according to many variables, such as the type of exposure, altitude, individual characteristics, and some genetic patterns.21 However, these same mechanisms may result in an inability to acclimatize, leading to disease in altitude-susceptible individuals.22
The main responses are ventilatory and cardiovascular because as soon as the PO2 falls, cardiac output is increased by sympathetic activation, and alveolar ventilation is increased through peripheral chemoreceptor activation. This ventilatory response is known as the hypoxic ventilatory response that is increased at the beginning of the exposure but is reduced over time as individuals become acclimatized. This later stage is regulated by central chemoreceptors; thus, regardless of the time spent, this response is maintained elevated showing a characteristic ventilatory pattern at high altitude.23,24
There is growing evidence that exposure to hypoxia, regardless of the source, elicits several metabolic responses in individuals. These responses are constitutive and are usually observed under hypoxia but vary according to the type of exposure. When these metabolic responses reach a steady state, acclimatization is achieved; if a steady state is not reached, the individual cannot become acclimatized. There are several conditioning factors that might preclude good acclimatization. Recently, attention has focused on the role of body weight as a conditioning factor.25 Therefore, it is important to evaluate the relationship between high-altitude hypoxia, body weight, and lipid metabolism and the contributions of body weight and lipid metabolism to acclimatization.
Overweight and obesity
Overweight and obesity are major public health problems and have become global health challenges due to an exponential increase in prevalence in recent decades.25,26 These conditions are associated with cardiovascular diseases and obesity-related respiratory function abnormalities, such as sleep-related breathing disorders and hypoxia-inducing respiratory abnormalities.27,28 Obesity is also related to the inflammatory status, metabolic syndrome, and insulin resistance.29
It must be highlighted that in recent years, a change has been observed regarding body weight in people who live permanently or work intermittently at high altitude. Currently, obesity in urban dwellers at high altitude has increased30 mostly because of changes in lifestyle (sedentarism and westernized alimentary habits),31 and people who commute to high altitude intermittently show marked levels of overweight or obesity.32 Additionally, overweight and obesity are related to AMS and HAPH. Therefore, obese individuals may be expected to have a greater risk for illness at higher altitudes.27
The connection between obesity and AMS is not clear. Despite the fact that some studies have suggested that there is no association between AMS and body mass index (BMI),33 many studies have supported the relationship. A study of obese and nonobese male railroad workers from He-bei (China) working in Tibet showed that the severity of symptoms was significantly different between obese and nonobese subjects, which indicated that the occurrence of AMS may be closely related to increased body weight.34 Likewise, another study found that obese participants had higher AMS scores than nonobese participants during a 24-h exposure to a simulated altitude of 3658 m;27 in Chinese workers at altitudes between 3500 and 5000 m, during the construction of the Qinghai–Tibet railroad, it was found that overweight workers were three times more likely to suffer from AMS than normal-weight workers.35 These results seem to conflict with those of a report from Mount Damavand; however, it should be noted that the Iranians in that report were mostly professional trekkers with good physical fitness, and although BMI was not reported, it can be surmised that their BMI was lower than that of the rail road workers. Therefore, obesity and body overweight must be considered a possible risk factor for AMS. Further support has been shown for this association in cohort studies performed by our laboratory that have shown body weight to be a risk factor for AMS and a lack of acclimatization. Additionally, it is difficult to find studies describing AMS in Andean populations, which are usually composed of high-altitude permanent dwellers. However, there is evidence that the Andean ancestry does not protect against AMS if those residing at sea level travel to high altitude.36,37
Overweight and obesity were reviewed since they are associated with cardiovascular diseases and respiratory abnormalities, including HAPH, a type of PH that occurs among residents and travelers at high altitudes.38 Therefore, it has been suggested that a combination of obesity and high altitude leads to the development of HAPH.39 In obese patients at an altitude of 2240 m, the combination of obesity and high altitude prompted the development of PAH.40 In morbidly obese patients living at moderate altitudes (2240 m), there is a high prevalence (96.5%) of HAPH, and alveolar hypoventilation and BMI are independent risk factors for HAPH severity.41
There is increasing evidence that under conditions of chronic intermittent exposure, overweight and obesity play major roles in the development of HAPH. It has been recognized that the CIH model contains both exposure conditions at high altitude: acute exposure (first days) and chronic exposure (for longer periods of time).13,42 In fact, recent studies have described higher BMI values in working populations exposed to CIH. In Kyrgyz, which is at 3800–4500 m, obesity and overweight are highly prevalent in workers who work shifts (15 days) in high-altitude mines.32 Additionally, soldiers working on a schedule of five days of work at high altitude (3550 m) followed by two days of rest at sea level showed increased BMIs.43 The same findings were demonstrated in miners: 52% of the miners were overweight, and 11% were obese.13 Additionally, a follow-up of young people at 3550 m (four at high altitude followed by three days at sea level) revealed the following prevalence rates: 42% of the young people were overweight, and 6% were obese.42 To explain the consistently higher prevalences of obesity and overweight under conditions of CIH, the following could be hypothesized: (1) AMS occurs on day 1 and is reversed on day 2 of the shift; therefore, there is no further anorexia;43 (2) mines and regiments provide ad libitum hypercaloric food; (3) the tasks performed mainly involve the use of joysticks, with limited physical effort; (4) during the days spent at sea level, they eat large amounts; and (5) the traditional sociocultural view supports overfeeding in miners.
There are two recent studies further supporting the relationship between HAPH and obesity. Both studies showed a prevalence of 9%. One of them that was performed in conscripts not only showed a prevalence of mPAP over 25 mmHg of 46% but also higher body weights and obesity rates.42 Another study conducted in miners clearly showed that HAPH is related to insulin resistance, overweight (BMI > 25), and higher waist circumference.13 Moreover, in an unpublished ongoing cohort study at our institution, the preliminary results demonstrate that BMI and waist circumference are correlated with elevated mPAP (BMI OR: 1.22; 95% CI: 1.011–1.51, p < 0.05), and BMI in month 1 of exposure could be used as a risk predictor of elevated mPAP at six months. Additionally, these subjects had a decrease in performance on the 6-min walk test that was positively correlated with BMI.
There are several possible explanations for the effects of overweight and obesity on the development of HAPH under conditions of CIH. The critical factor is that higher body mass leads to ventilation impairment. Thus, aggravating hypobaric hypoxia at high altitude leads to a greater degree of hypoxemia, which is a critical trigger for the development of high-altitude diseases.
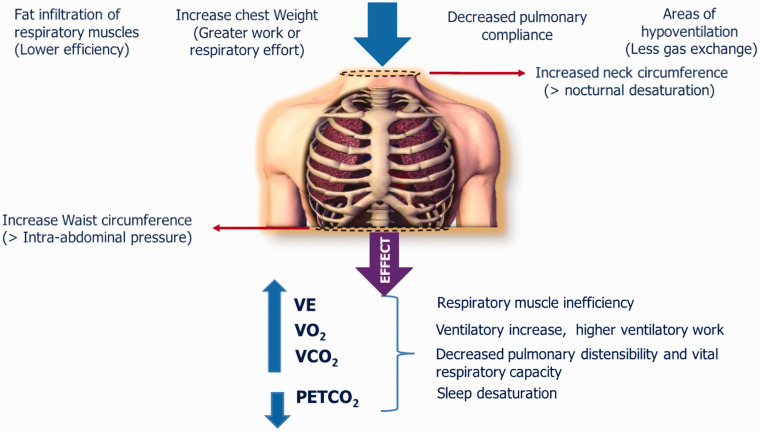
Anatomic alterations observed in obese individuals along with non-physiological decreases in total respiratory volume and compliance are important factors contributing to respiratory impairment.25 As a result of the accumulation of fat in and around the ribs, the diaphragm, and the abdomen, chest wall compliance is impaired.44 As BMI increases, there is evidence of a reduction in expiratory flow and decreases in forced expiratory volume in 1 s (FEV1) and forced vital capacity (FVC).45 Moreover, the pattern of body fat distribution may affect lung function46 since a higher ratio of abdominal circumference to waist circumference was negatively associated with FEV1 and FVC.47 Therefore, as suggested by the results of previous studies, central abdominal obesity has a greater impact on spirometric measures than back or lower body obesity. Moreover, lower muscle efficiency, increased chest weight, fat infiltration of the thoracic muscle, areas of hypoventilation and increased neck circumference lead to greater hypoxemia and, overall, general respiratory muscle inefficiency. In addition, the role of greater nocturnal sleep arterial oxygen desaturation and altered respiratory hypoxic response, which would aggravate hypoxemia and result in PH, cannot be ruled out,48 even in overweight subjects.49 The changes in respiratory function are summarized in the diagram in Fig. 2.
Fig. 2.
Schematic diagram of the factors involved in overweight and obesity that leads to respiratory impairment.
VE: minute ventilation; VO2: oxygen consumption; VCO2: carbon dioxide production; PETCO2: end-tidal PCO2.
Additionally, obese individuals have obesity-hypoventilation syndrome, greater nocturnal arterial oxygen desaturation, and periodic apneic breathing,48,50 eliciting severe hypoxemia and severe PH.41 It has also been demonstrated that a greater degree of alveolar hypoxia at altitude, which is aggravated by obesity, causes a further increase in the PAP.51
On the other hand, obese patients might have lung peripheral capillary alterations leading to greater permeability due to inflammatory mediators, such as interleukins (e.g. IL-6) and other cytokines.52 These inflammatory mediators are involved in the development of HAPE.53 Therefore, the abovementioned factors that have been linked to AMS and HAPE might also play a role in HAPH under conditions of CIH.
It also cannot be ruled out that other alterations associated with obesity might be contributors to HAPH, which could be supported by the role of adipose tissue itself and insulin resistance, that further contribute to inflammatory markers.54 Moreover, obesity has been shown to be accompanied by an impairment of the NO pathway42 and an imbalance in the redox state, leading to vascular remodeling.55
In summary, the evidence suggests that a high BMI is an important contributor to the development of HAPH, although neither all the mechanisms nor the contributory roles of other metabolic disturbances are well known.
Lipid metabolism
Total cholesterol, either esterified or free forms, and triglycerides (TGs) are the two main lipids present in the plasma. They are transported in pseudomicellar lipid–protein complexes, mainly as apolipoproteins.56,57 Regarding the blood lipid profile in those living permanently at high altitude (CH) and those undergoing CIH, there are two conclusions. One conclusion is that in general, most of the published works tend to agree that the values of total cholesterol and its fractions at high altitude are within normal ranges or lower than at sea level and have positive relationships with age,58 although this viewpoint is controversial. The other conclusion is that of all the components of the blood lipid profile, the only ones that are altered at high altitude under conditions of CH30 or CIH are TGs and very low-density lipoprotein (VLDL) cholesterol.58,59 The latter has been associated with HAPH13 and is inversely related to the SaO2.43 Therefore, we have demonstrated that under conditions of hypoxia, there is are increases in TGs and VLDL,58 and an association with higher mPAP has been found according to the results of an unpublished ongoing cohort study at our institution (area under the curve: 0.7; 95% CI: 0.53–0.86; p < 0.05).
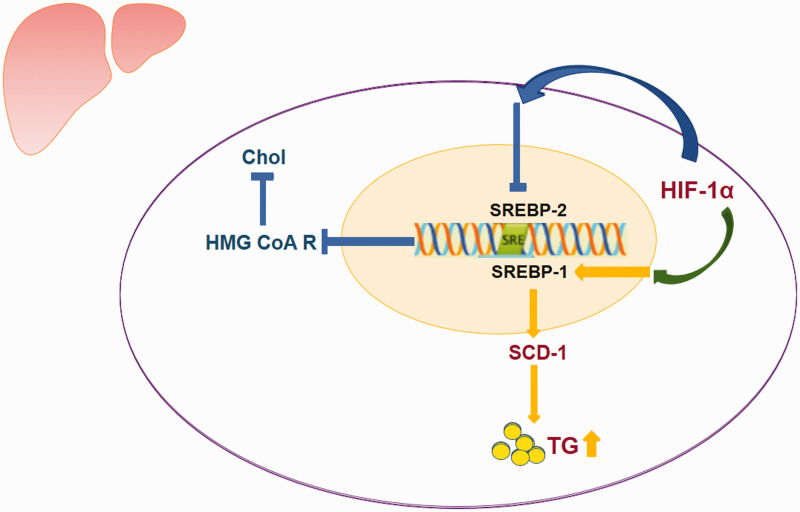
One molecular mechanism proposed to explain these changes in the lipid profile is hepatic de novo synthesis of TGs caused by the upregulation of stearoyl-CoA desaturase (SCD)-1 in the sterol regulatory element-binding protein (SREBP)-1c pathway that is induced by hypoxia and hypoxia-induced factor (HIF)-1α. Under normal physiological conditions, lipid biosynthesis in the liver is regulated by a family of transcription factors, namely, the SREBPs. The SREBPs include SREBP-1 and SREBP-2.60 SREBP-1 plays a crucial role in the dietary regulation of most hepatic lipogenic enzymes; this factor preferentially regulates enzymes involved in fatty acid SCD.61 SCD is a rate-limiting enzyme in the biosynthesis of monounsaturated fatty acids and TGs.62 SCD-1 is highly induced in the liver in response to a high-carbohydrate diet.62,63
Studies have demonstrated the important role of SCD-1 expression in lipid metabolism; this enzyme is not only involved in the pathology of obesity but also emerges as a key regulator in other types of conditions associated with inflammation and stress, such as hypoxia.64 Animal models have suggested that chronic hypobaric hypoxia could alter plasma lipid levels by upregulating TG biosynthesis without affecting the cholesterol biosynthetic pathway.59 It has been proposed that an increase in SCD-1 activity may be mediated by HIF-1α65 (Fig. 3). The mechanism by which an increase in TGs could predict or influence the development of HAPH is still unknown.
Fig. 3.
Schematic diagram of the pathway involved in the hepatic de novo biosynthesis of triglycerides.
Chol: total cholesterol; HMG CoA R: 3-hydroxy-3-methylglutaryl CoA reductase; SREBP-1: sterol regulatory element-binding proteins1; SREBP-2: sterol regulatory element-binding proteins 2; SCD-1: stearoyl-CoA desaturase; TG: triglycerides; HIF-1α: hypoxia-induced factor-1α.
On the other hand, to our knowledge, no other molecular mechanism has been proposed to be involved in TG elevation in individuals experiencing long-term CIH. However, in those experiencing short-term CIH, such as in those with obstructive sleep apnea, several mechanisms have been identified, such as a decrease in TGs due to enhanced lipid clearance, the inhibition of lipoprotein lipase activity in adipose tissue,66 the upregulation of lipid biosynthesis in the liver, and the increase of adipose tissue lipolysis and the subsequent free fatty acid influx in the liver.67,68 Therefore, further studies are needed regarding the molecular pathways involving TG and lipids under the long-term CIH condition described in this review.
Conclusion
During acclimatization to high altitude under a long-term regime of CIH pattern of exposure, overweight and obesity play relevant roles in the etiology of HAPH. One critical factor is the respiratory impairment caused by obesity-related anatomical and physiopathological alterations. Moreover, other involved mechanisms may include the alteration of NO pathways, inflammatory status, reactive oxygen species imbalance, and other metabolic changes; however, further studies are needed to confirm these mechanisms. The roles of TGs and VLDL are still unclear, but they could be predictors of HAPH in early stages.
Contributorship
P.S. presented at the conference; P.S., J.B., S.O., and E.P. contributed to drafting and critically revising the manuscript; and all agreed with the final version.
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
This work was funded by grants from FIC Tarapaca BIP 30477541-0, CONICYT/FONDEF/FONIS Sa 09I20007, and the German Federal Ministry of Education and Research under grant number 01DN17046 (DECIPHER).
ORCID iDs
Patricia Siques https://orcid.org/0000-0002-4963-195X
Julio Brito https://orcid.org/0000-0002-0050-8774
Stefany Ordenes https://orcid.org/0000-0002-5658-5533
Eduardo Pena https://orcid.org/0000-0003-2664-1722
References
- 1.Heath D and Williams DR. The carotid bodies. In: Heath D and Williams DR (eds) Man at high altitude. New York, NY: Churchill Livingstone, 1977, pp.60–82.
- 2.Tymko MM, Tremblay JC, Bailey DM, et al. The impact of hypoxaemia on vascular function in lowlanders and high altitude indigenous populations. J Physiol 2019; 597: 5759–5776. [DOI] [PubMed] [Google Scholar]
- 3.Luks AM, Swenson ER, Bärtsch P. Acute high-altitude sickness. Eur Respir Rev 2017; 26: 160096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bärtsch P, Swenson ER. Clinical practice: acute high-altitude illnesses. N Engl J Med 2013; 368: 2294–2302. [DOI] [PubMed] [Google Scholar]
- 5.West J, Schoene R, Luks A, et al. High altitude medicine and physiology 5E, Boca Raton, FL: CRC Press, 2013. [Google Scholar]
- 6.West JB. Highest permanent human habitation. High Alt Med Biol 2002; 3: 401–407. [DOI] [PubMed] [Google Scholar]
- 7.Richalet J-P, Donoso MV, Jiménez D, et al. Chilean miners commuting from sea level to 4500 m: a prospective study. High Alt Med Biol 2002; 3: 159–166. [DOI] [PubMed] [Google Scholar]
- 8.Hackett PH, Roach RC. High-altitude illness. N Engl J Med 2001; 345: 107–114. [DOI] [PubMed] [Google Scholar]
- 9.Kayser B, Dumont L, Lysakowski C, et al. Reappraisal of acetazolamide for the prevention of acute mountain sickness: a systematic review and meta-analysis. High Alt Med Biol 2012; 13: 82–92. [DOI] [PubMed] [Google Scholar]
- 10.Schneider M, Bernasch D, Weymann JR, et al. Acute mountain sickness: influence of susceptibility, preexposure, and ascent rate. Med Sci Sports Exerc 2002; 34: 1886–1891. [DOI] [PubMed] [Google Scholar]
- 11.Richalet JP, Keromes A, Dersch B, et al. Caractéristiques physiologiques des alpinistes de haute altitude. Sci Sports 1988; 3: 89–108. [Google Scholar]
- 12.Siqués P, Brito J, Banegas JR, et al. Blood pressure responses in young adults first exposed to high altitude for 12 months at 3550 m. High Alt Med Biol 2009; 10: 329–335. [DOI] [PubMed] [Google Scholar]
- 13.Brito J, Siques P, López R, et al. Long-term intermittent work at high altitude: right heart functional and morphological status and associated cardiometabolic factors. Front Physiol 2018; 9: 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.León-Velarde F, Maggiorini M, Reeves JT, et al. Consensus statement on chronic and subacute high altitude diseases. High Alt Med Biol 2005; 6: 147–157. [DOI] [PubMed] [Google Scholar]
- 15.McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol 2009; 53: 1573–1619. [DOI] [PubMed] [Google Scholar]
- 16.Potus F, Graydon C, Provencher S, et al. Vascular remodeling process in pulmonary arterial hypertension, with focus on miR-204 and miR-126 (2013 Grover Conference series). Pulm Circ 2014; 4: 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crosswhite P, Sun Z. Molecular mechanisms of pulmonary arterial remodeling. Mol Med 2014; 20: 191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lai Y-C, Potoka KC, Champion HC, et al. Pulmonary arterial hypertension: the clinical syndrome. Circ Res 2014; 115: 115–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Von Euler US, Liljestrand G. Observations on the pulmonary arterial blood pressure in the cat. Acta Physiol Scand 1946; 12: 301–320. [Google Scholar]
- 20.Moudgil R, Michelakis ED, Archer SL. Hypoxic pulmonary vasoconstriction. J Appl Physiol 2005; 98: 390–403. [DOI] [PubMed] [Google Scholar]
- 21.Niermeyer S, Zamudio S and Moore IG. The people. In: Hornbein T and Schoene R (eds) High altitude: an exploration of human adaptation. New York, NY: Marcel Dekker, 2001, pp.43–100.
- 22.West JB. Are permanent residents of high altitude fully adapted to their hypoxic environment?. High Alt Med Biol 2017; 18: 135–139. [DOI] [PubMed] [Google Scholar]
- 23.Teppema LJ, Dahan A. The ventilatory response to hypoxia in mammals: mechanisms, measurement, and analysis. Physiol Rev 2010; 90: 675–754. [DOI] [PubMed] [Google Scholar]
- 24.Richalet J-P, Larmignat P, Poitrine E, et al. Physiological risk factors for severe high-altitude illness. Am J Respir Crit Care Med 2012; 185: 192–198. [DOI] [PubMed] [Google Scholar]
- 25.San Martin R, Brito J, Siques P, et al. Obesity as a conditioning factor for high-altitude diseases. Obes Facts 2017; 10: 363–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.James WPT. The fundamental drivers of the obesity epidemic. Obes Rev 2008; 9: 6–13. [DOI] [PubMed] [Google Scholar]
- 27.Ri-Li G, Chase PJ, Witkowski S, et al. Obesity: associations with acute mountain sickness. Ann Intern Med 2003; 139: 253–257. [DOI] [PubMed] [Google Scholar]
- 28.Kopelman PG. Obesity as a medical problem. Nature 2000; 404: 635–643. [DOI] [PubMed] [Google Scholar]
- 29.Huicho L, Trelles M, Gonzales F, et al. Mortality profiles in a country facing epidemiological transition: an analysis of registered data. BMC Public Health 2009; 9: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miele C, Schwartz AR, Gilman RH, et al. Increased cardiometabolic risk and worsening hypoxemia at high altitude. High Alt Med Biol 2016; 17: 93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mohanna S, Baracco R, Seclén S. Lipid profile, waist circumference, and body mass index in a high altitude population. High Alt Med Biol 2006; 7: 245–255. [DOI] [PubMed] [Google Scholar]
- 32.Esenamanova MK, Kochkorova FA, Tsivinskaya TA, et al. Chronic intermittent high altitude exposure, occupation, and body mass index in workers of mining industry. High Alt Med Biol 2014; 15: 412–417. [DOI] [PubMed] [Google Scholar]
- 33.Ziaee V, Yunesian M, Ahmadinejad Z, et al. Acute mountain sickness in Iranian trekkers around Mount Damavand (5671m) in Iran. Wilderness Environ Med 2003; 14: 214–219. [DOI] [PubMed] [Google Scholar]
- 34.Yang B, Li N, Sun ZJ, et al. Obesity is a risk factor for acute mountain sickness: a prospective study in Tibet railway construction workers on Tibetan plateau. Eur Rev Med Pharmacol Sci 2013; 19: 119–122. [PubMed] [Google Scholar]
- 35.Wu TY, Ding SQ, Liu JL, et al. Who are more at risk for acute mountain sickness: a prospective study in Qinghai-Tibet railroad construction workers on Mt. Tanggula. Chin Med J (Engl) 2012; 125: 1393–1400. [PubMed] [Google Scholar]
- 36.West JB. Acute mountain sickness. High Alt Med Biol 2002; 3: 415–419. [DOI] [PubMed] [Google Scholar]
- 37.Cabello G, Fariña E, Jeldres A, et al. Andean high-altitude ancestry does not protect from acute mountain sickness and altitude-induced arterial hypoxemia. Rev Cienc Tecnol Am 2017; 42: 39–43. [Google Scholar]
- 38.He Q, Nan X, Li S, et al. Tsantan sumtang alleviates chronic hypoxia-induced pulmonary hypertension by inhibiting proliferation of pulmonary vascular cells. Biomed Res Int 2018; 2018: 9504158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raw R. Obesity and pulmonary hypertension. What's the link?. BJMP 2009; 2: 4–5. [Google Scholar]
- 40.Lupi-Herrera E, Seoane M, Sandoval J, et al. Behavior of the pulmonary circulation in the grossly obese patient. Chest 1980; 78: 553–558. [DOI] [PubMed] [Google Scholar]
- 41.Valencia-Flores M, Rebollar V, Santiago V, et al. Prevalence of pulmonary hypertension and its association with respiratory disturbances in obese patients living at moderately high altitude. Int J Obes 2004; 28: 1174–1180. [DOI] [PubMed] [Google Scholar]
- 42.Siques P, Brito J, Schwedhelm E, et al. Asymmetric dimethylarginine at sea level is a predictive marker of hypoxic pulmonary arterial hypertension at high altitude. Front Physiol 2019; 10: 651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brito J, Siqués P, León-Velarde F, et al. Chronic intermittent hypoxia at high altitude exposure for over 12 years: assessment of hematological, cardiovascular, and renal effects. High Alt Med Biol 2007; 8: 236–244. [DOI] [PubMed] [Google Scholar]
- 44.Naimark A, Cherniack RM. Compliance of the respiratory system and its components in health and obesity. J Appl Physiol 1960; 15: 377–382. [DOI] [PubMed] [Google Scholar]
- 45.Ray CS, Sue DY, Bray G, et al. Effects of obesity on respiratory function. Am Rev Respir Dis 1983; 128: 501–506. [DOI] [PubMed] [Google Scholar]
- 46.Collins LC, Hoberty PD, Walker JF, et al. The effect of body fat distribution on pulmonary function tests. Chest 1995; 107: 1298–1302. [DOI] [PubMed] [Google Scholar]
- 47.Lazarus R, Sparrow D, Weiss ST. Effects of obesity and fat distribution on ventilatory function. Chest 1997; 111: 891–898. [DOI] [PubMed] [Google Scholar]
- 48.Parameswaran K, Todd DC, Soth M. Altered respiratory physiology in obesity. Can Respir J 2006; 13: 203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sahebjami H. Dyspnea in obese healthy men. Chest 1998; 114: 1373–1377. [DOI] [PubMed] [Google Scholar]
- 50.Ge R-L, Stone JA, Levine BD, et al. Exaggerated respiratory chemosensitivity and association with level at 3568m in obesity. Respir Physiol Neurobiol 2005; 146: 47–54. [DOI] [PubMed] [Google Scholar]
- 51.Toff NJ. Hazards of air travel for the obese: miss pickwick and the boeing 747. J R Coll Physicians Lond 1993; 27: 375–376. [PMC free article] [PubMed] [Google Scholar]
- 52.Kayser B, Verges S. Hypoxia, energy balance and obesity: from pathophysiological mechanisms to new treatment strategies. Obes Rev 2013; 14: 579–592. [DOI] [PubMed] [Google Scholar]
- 53.Swenson ER, Bärtsch P. High-altitude pulmonary edema. Compr Physiol 2012; 2: 2753–2773. [DOI] [PubMed] [Google Scholar]
- 54.Goossens GH, Bizzarri A, Venteclef N, et al. Increased adipose tissue oxygen tension in obese compared with lean men is accompanied by insulin resistance, impaired adipose tissue capillarization, and inflammation. Circulation 2011; 124: 67–76. [DOI] [PubMed] [Google Scholar]
- 55.El Kasmi KC, Pugliese SC, Riddle SR, et al. Adventitial fibroblasts induce a distinct proinflammatory/profibrotic macrophage phenotype in pulmonary hypertension. J Immunol 2014; 193: 597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chapman MJ, Ginsberg HN, Amarenco P, et al. Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: evidence and guidance for management. Eur Heart J 2011; 32: 1345–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Itabe H, Yamaguchi T, Nimura S, et al. Perilipins: a diversity of intracellular lipid droplet proteins. Lipids Health Dis 2017; 16: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Siqués P, Brito J, León-Velarde F, et al. Hematological and lipid profile changes in sea-level natives after exposure to 3550-m altitude for 8 months. High Alt Med Biol 2007; 8: 286–295. [DOI] [PubMed] [Google Scholar]
- 59.Siques P, Brito J, Naveas N, et al. Plasma and liver lipid profiles in rats exposed to chronic hypobaric hypoxia: changes in metabolic pathways. High Alt Med Biol 2014; 15: 388–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shimano H. Sterol regulatory element-binding proteins (SREBPs): transcriptional regulators of lipid synthetic genes. Prog Lipid Res 2001; 40: 439–452. [DOI] [PubMed] [Google Scholar]
- 61.Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest 2002; 109: 1125–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Miyazaki M, Kim H-J, Man WC, et al. Oleoyl-CoA is the majorde novoproduct of stearoyl-CoA desaturase 1 gene isoform and substrate for the biosynthesis of the harderian gland 1-Alkyl-2,3-diacylglycerol. J Biol Chem 2001; 276: 39455–39461. [DOI] [PubMed] [Google Scholar]
- 63.Ntambi JM, Miyazaki M. Regulation of stearoyl-CoA desaturases and role in metabolism. Prog Lipid Res 2004; 43: 91–104. [DOI] [PubMed] [Google Scholar]
- 64.Liu X, Strable MS, Ntambi JM. Stearoyl CoA desaturase 1: role in cellular inflammation and stress. Adv Nutr 2011; 2: 15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Semenza GL. HIF-1 and mechanisms of hypoxia sensing. Curr Opin Cell Biol 2001; 13: 167–171. [DOI] [PubMed] [Google Scholar]
- 66.Drager LF, Li J, Shin M-K, et al. Intermittent hypoxia inhibits clearance of triglyceride-rich lipoproteins and inactivates adipose lipoprotein lipase in a mouse model of sleep apnoea. Eur Heart J 2012; 33: 783–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li J, Thorne LN, Punjabi NM, et al. Intermittent hypoxia induces hyperlipidemia in lean mice. Circ Res 2005; 97: 698–706. [DOI] [PubMed] [Google Scholar]
- 68.Drager LF, Jun JC, Polotsky VY. Metabolic consequences of intermittent hypoxia: relevance to obstructive sleep apnea. Best Pract Res Clin Endocrinol Metab 2010; 24: 843–851. [DOI] [PMC free article] [PubMed] [Google Scholar]