Abstract
Cartilage defects are one of the most common symptoms of osteoarthritis (OA), a degenerative disease that affects millions of people world-wide and places a significant socio-economic burden on society. Hydrogels, which are a class of biomaterials that are elastic, and display smooth surfaces while exhibiting high water content, are promising candidates for cartilage regeneration. In recent years, various kinds of hydrogels have been developed and applied for the repair of cartilage defects in vitro or in vivo, some of which are hopeful to enter clinical trials. In this review, recent research findings and developments of hydrogels for cartilage defects repair are summarized. We discuss the principle of cartilage regeneration, and outline the requirements that have to be fulfilled for the deployment of hydrogels for medical applications. We also highlight the development of advanced hydrogels with tailored properties for different kinds of cartilage defects to meet the requirements of cartilage tissue engineering and precision medicine.
Keywords: Hydrogels, Articular cartilage defects, Tissue engineering, Clinical translation, Precision medicine
Graphical abstract
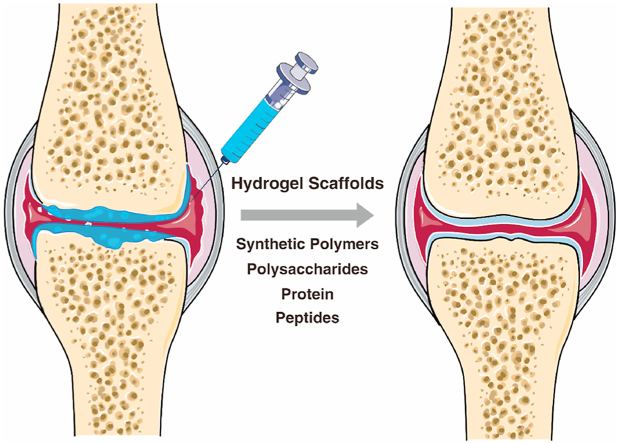
Hydrogels applied in joints for the repair of cartilage defects and cartilage regeneration. Hydrogels are three-dimensional hydrophilic polymer networks that can be fabricated from synthetic polymers, polysaccharides, protein, and peptides, and can be designed as injectable or in situ forming for practical clinical application.
Highlights
-
•
The biotechnology of developing hydrogels for cartilage defects repair is promising.
-
•
The principle for cartilage regeneration using hydrogels and requirements for clinical transformation are summarized.
-
•
Advanced hydrogels with tailored properties for different kinds of cartilage defects are discussed.
1. Introduction
Osteoarthritis (OA) is a widely occurring degenerative disease and a leading cause of disability that affects more than 303 million people globally [1]. Despite not being fatal, OA places a substantial burden on societies around the world, especially those with ageing populations. The pain and further mobility limitations impact both physical and mental wellbeing of patients. Although OA mostly occurs in elderly people, young people can be affected, especially after joint injuries. Due to the increase in the age of many societies, the predicted number of people with OA will increase by 50% over the next 20 years [2]. To ameliorate this situation, the development of new treatment strategies for OA are urgently needed, as current therapies are often not satisfactory for most patients. Development of new therapeutic strategies is difficult, as the pathogenesis of OA is heterogeneous and complex.
Based on studies over the last decade, one of the major pathological characteristics of OA are defects of articular cartilage [[3], [4], [5]]. The development of OA and cartilage defects is a vicious circle: catabolic and proinflammatory mediators in the OA joints lead to the excess production of proteolytic enzymes and break down the cartilage; the defect in the cartilage in turn amplifies the inflammation in the joint [6]. As cartilage is an important tissue that serves by reducing joint friction between bones, its breakdown may contribute to the deterioration of joint function [[7], [8], [9]]. Once cartilage defects occur, the limited self-repair capability of the tissue is insufficient and no significant regeneration will occur due to the complex structure of cartilage, where no blood vessels, nerves or lymphatic tissue exists [[10], [11], [12]].
In the clinic, osteochondral autografts, microfracture surgery, autologous chondrocyte implantation (ACI), and matrix-induced autologous chondrocyte implantation (MACI) are the most common strategies for the repair of small cartilage defects. Nevertheless, the success of these strategies is often complicated by the formation of fibrocartilage which impairs joint function [13]. Some other limitations such as shortage of chondrocyte source and low effectiveness, often observed in older patients, were summarized by Yang et al. in a recent review article [14]. To address these issues, improved and new strategies are needed to alleviate the problem. The chondrogenic capabilities of mesenchymal stem cells (MSC) have made them promising in cell therapy for cartilage defects. MSC, combined with materials such as hydrogels, can be delivered to the defect site of cartilage and promote cartilage regeneration. Also, hydrogels can be designed to enhance the chondrogenesis of MSC.
Because hydrogels possess cartilage tissue-like features, developing hydrogels is now the frontline of research to treat cartilage defects [15,16]. Hydrogels are three-dimensional hydrophilic polymer networks that can absorb large amounts of water while retaining their structure [17]. There is a wide range of different sources to choose from to create hydrogels (Table 1). Synthetic polymers such as polyethylene glycol (PEG), poly (vinyl alcohol) (PVA), and polyacrylamide (PAM) have been extensively used for making hydrogels in tissue engineering [[18], [19], [20]]. Hydrogels composed of synthetic polymers are able to exhibit high mechanical strength and good reproducibility but may be problematic due to biocompatibility issues, which have often been insufficiently investigated. Therefore, natural macromolecules including polysaccharides, proteins, and peptides that show excellent biocompatibility have continued attention as hydrogels for tissue engineering applications. In order to develop optimized protocols for cartilage regeneration, the sources of material as well as crosslinking methods have to be investigated in more detail. Several currently used methods for the preparation of natural hydrogels were summarized by Li et al. in a recent review [21].
Table 1.
Overview of hydrogels with different polymer types for cartilage tissue engineering: chemical structure and features for cartilage engineering.
| Name (Abbreviation) | Chemical structure | Features for cartilage engineering | Limitations | Refs | |
|---|---|---|---|---|---|
| Synthetic polymers | Polyethylene glycol (PEG) | 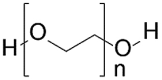 |
|
Biologically inert | [[47], [48], [49], [50], [51]] |
| |||||
| Poly (N-vinylcaprolactam) (PVCL) |  |
|
Moderate wettability, weak mechanical properties, low anti-biofouling | [42,157] | |
| |||||
| Polyvinyl alcohol (PVA) | 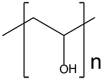 |
|
Biologically inert | [19,37,38,158] | |
| |||||
| Poly (2-methacryloyloxyethyl phosphorylcholine) (PMPC) | 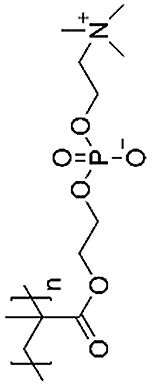 |
|
Do not support cell attachment | [[39], [40], [41],159] | |
| |||||
| |||||
| Polysaccharides | Chondroitin sulfate (CS) | 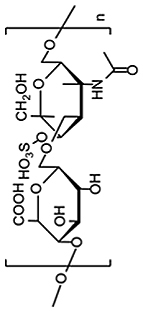 |
|
Rapid degradation | [11,34,[54], [55], [56]] |
| |||||
| |||||
| Hyaluronic Acid (HA) | 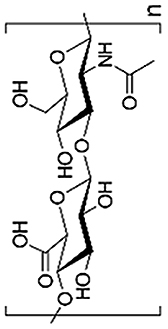 |
|
Do not support cell attachment | [62,64,68,160,161] | |
| |||||
| |||||
| Chitosan | 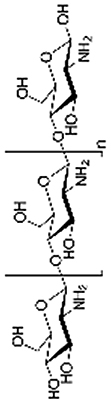 |
|
Low solubility and high viscosity | [[81], [82], [83],162] | |
| Alginate | 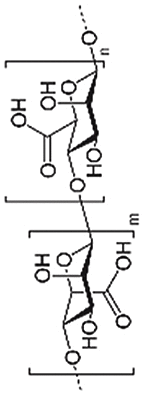 |
|
Non-biodegradable and elicit immunological responses | [[86], [87], [88], [89],91] | |
| |||||
| |||||
| Cellulose | 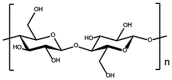 |
|
Lack of mechanical properties | [92,93,95] | |
| |||||
| Proteins | Collagen | 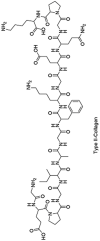 |
|
Limited number of functional groups for crosslinking | [30,99,100,103,163] |
| |||||
| Gelatin | 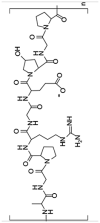 |
|
Poor mechanical properties and low thermal stability | [35,104,107,130] | |
| |||||
| |||||
| Silk Fibroin (SF) | 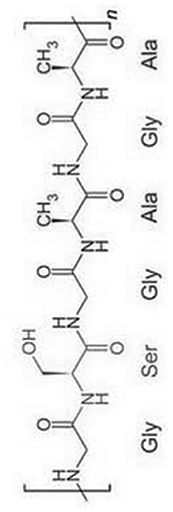 |
|
Limited options for anchoring growth factor | [111,113,114,164] | |
| |||||
| |||||
| |||||
| Sericin | 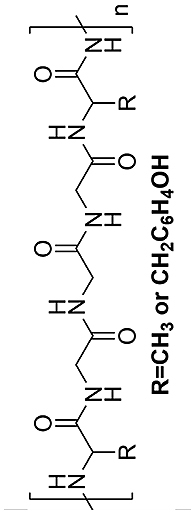 |
|
Low stability in aqueous solution | [117,165] | |
| |||||
| Fibrin | – |
|
Not chondro-permissive | [118,119] | |
| Peptides | – | – |
|
Need proper peptide design, synthesis, and purification | [125,128] |
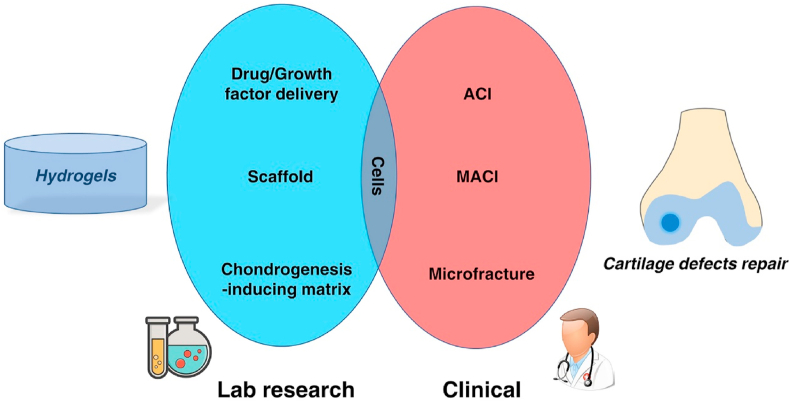
After decades of development, findings in basic research were translated into commercial hydrogel products used for cartilage defects repair. Several products have the potential to improve or even fully heal cartilage defects: NeoCART® is a type-I collagen matrix scaffold seeded with autogenous chondrocytes and was implanted 6 weeks following arthroscopic cartilage biopsy [22]; NovoCART®3D is a biphasic collagenous scaffold consists of a spongy part with pores arranged in columns of a set size and a dense and firm membrane [23]; CaReS® is a 3D type-I collagen hydrogel seeded with autologous chondrocytes [24]. These products represent hydrogel scaffolds for embedded autologous chondrocytes. ChonDux™ and BST-CarGel® are products designed for trapping cells from the bone marrow in microfracture surgery. An overview of hydrogel studies in research laboratories and their clinical translation for cartilage defects defines cells as an essential component (Fig. 1). From a top-down view, clinics found cells such as chondrocytes and bone marrow stromal cells (BMSCs) can be used to repair cartilage defects in ACI and microfracture surgery, then hydrogels were developed for acting as scaffolds or delivering factors to encapsulate chondrocytes and promote the chondrogenesis of stem cells. The outstanding hydrogels will finally serve for the clinics.
Fig. 1.
Schematic of relationship of hydrogel in lab and cartilage defects repair in clinical. ACI: autologous chondrocyte implantation; MACI: Matrix-induced autologous chondrocyte implantation.
In this review, we discuss the principle of developing hydrogels for cartilage defects repair. The properties and benefits of different hydrogels used for cartilage regeneration are analyzed and summarized to direct future research. Finally, we highlight the advantage of hydrogels that can be customized to fulfil different needs for cartilage defects to meet the high standards required in cartilage regeneration and precision medicine.
2. Principle for cartilage regeneration using hydrogels
Cartilage is hypocellular, avascular, aneural, and alymphatic, resulting in limited self-repair capacity after injuries. At the time of writing, hydrogels are being used for the repair of cartilage defects in two ways: One is to encapsulate autologous cells in the hydrogel (cell-laden hydrogel) which are then been implanted into the defect site [25,26]. The other way is to assist and induce surrounding stem cells to participate in repair [27,28]. Hydrogels with one or both above properties can be suitable for cartilage regeneration.
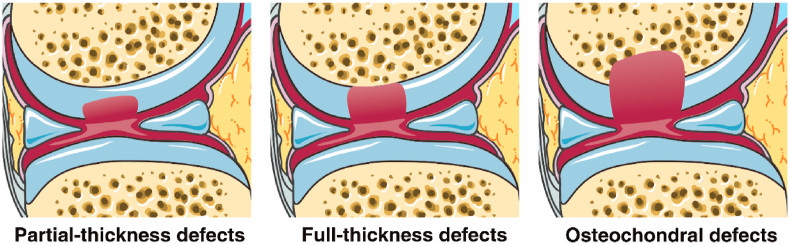
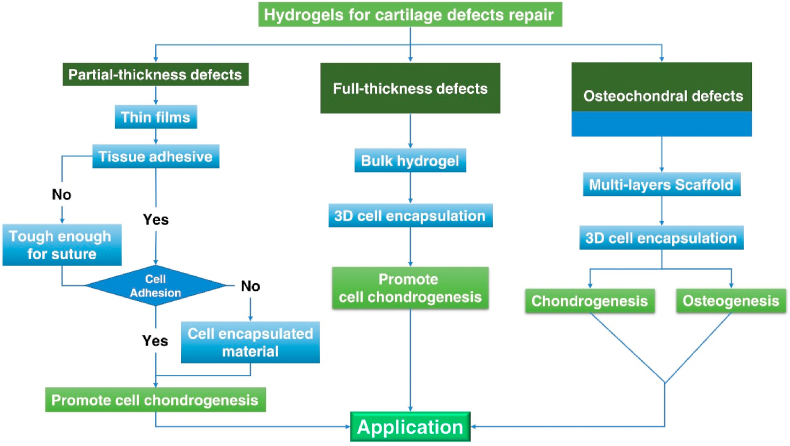
From the perspective of precision medicine, the methods of cartilage repair need to address different types of defects. Generally, cartilage defects can be divided into three classes, based on the depth of defects: partial-thickness defects, full-thickness defects, and osteochondral defects (Fig. 2) [29]. Partial-thickness defects are defects on the cartilage surface without penetrating the tidemark, while osteochondral defects penetrate into the bone marrow. The microenvironment of the three defects types is quite different: full-thickness defects and osteochondral defects can be partly repaired by bone marrow stromal cells (BMSCs) which are absent in the situation of partial-thickness defects due to the depth [30,31]. In addition, the mechanical characteristics are dissimilar between cartilage surface and osteochondral tissue. An approach that employs a multilayered scaffold with hierarchical organization might be superior for osteochondral defect repairs [3,32]. Due to the characteristics observed in defects and the difference in tissues, the materials used for the repair should be designed to accommodate for different kinds of defects.
Fig. 2.
Schematic diagram of three kinds of cartilage defects: partial-thickness defects, full-thickness defects, and osteochondral defects. Partial-thickness defects happen in the cartilage surface and do not penetrate the tidemark. No MSC from bone marrow will migrate or be recruited to the defect areas. Full-thickness defects are deeper cartilage defects to the tidemark but do not penetrate the subchondral bone. The environment of subchondral bone matrix is conducive to the migration and adhesion of MSC. Osteochondral defects penetrate the bone marrow and allow MSCs to be recruited to the defect area.
For the design of hydrogels aiming at cartilage regeneration and clinical translation, we summarized some criteria as well as suggestions for optimized outcomes. 1) Biocompatibility: Most materials for cartilage regeneration are designed to be used in the articular cavity; components that might cause inflammation and immune responses should be avoided. 2) Cell affinity: As the participation of cells is essential in the regeneration of cartilage, the material should facilitate easy cell attachment and/or embedding [[25], [26], [27], [28]]. Methods for cartilage regeneration include seeding exogenous cells into engineered products and the generation of a matrix environment that induce the migration of endogenous MSC towards the injured part. 3) Tissue integration property: The adhesion between hydrogels and tissue is crucial to avoid treatment failure caused by detachment of both components, and to promote integration of the material into the tissue during regeneration [[33], [34], [35]]. 4) Feasibility: Sprayable and injectable hydrogels hold great advantages in the practical applications especially in clinical settings.
3. Current state-of-art of hydrogels designed for cartilage regeneration
3.1. Hydrogel forming materials for cartilage regeneration and their sources
Hydrogels for tissue engineering can be made from various sources of both, natural and synthetic origin. This includes synthetic polymers, polysaccharides, proteins, and peptides. The features of these macromolecules for cartilage repair are listed in Table 1, and the details are described as follows.
3.1.1. Synthetic polymers
The synthetic polymers discussed in this review are industrial products. Generally, hydrogels made from synthetic polymers are mechanically strong and reproducible. In recent years, several kinds of synthetic polymers were investigated for their use in cartilage related applications.
Polyvinyl alcohol (PVA) is a reliable and high-performance carrier due to its excellent properties of film formation, emulsification, and adhesion [36]. PVA hydrogels formed by a cast-drying method were reported to have a mechanical performance similar to that of natural human cartilage, suggesting such hydrogels could be candidate for cartilage replacement [37]. Apart from the high strength, in vitro studies of PVA-chitosan hydrogels with mesenchymal stem cells (MSCs) showed chondrogenic differentiation and glycosaminoglycan (GAG) deposition, making it a promising material for cartilage repair [38].
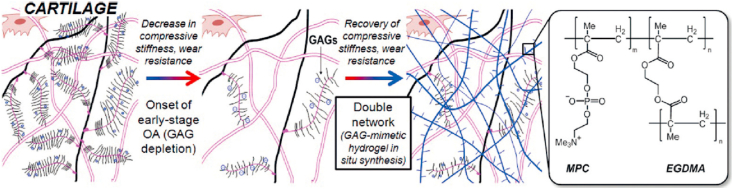
Poly(2-methacryloyloxyethyl phosphorylcholine) (PMPC) is a polymer that can function as a biomimetic boundary lubricant to imitate the properties of natural cartilage [39]. Milner et al. incorporated PMPC into a double network, forming a triple network hydrogel that could withstand a high amount of stress with low friction [39]. With these advantages, this hydrogel could reduce cartilage damage caused by partial implants. PMPC was considered as a biocompatible phospholipid polymer which, when polymerized, can be grafted onto a polyethylene surface to decreased friction [40]. Therefore, PMPC was used to form a double network hydrogel to restore the mechanical strength of damaged cartilage (Fig. 3) [41]. The results demonstrated that this kind of material is promising for early-stage osteoarthritis treatment.
Fig. 3.
Schematic representation of normal cartilage (left), osteoarthritic cartilage (middle), and hydrogel-reinforced cartilage (right). GAG depletion decreases compressive stiffness and wear resistance of cartilage. To recover lost properties, a GAG-mimetic hydrogel was made by polymerizing hydrophilic zwitterionic monomers 2-methacryloyloxyethyl phosphorylcholine (MPC) using ethylene glycol dimethacrylate (EGDMA) as a crosslinker [Reprinted from Refs. [41] with permission from Wiley].
Apart from mechanical properties, synthetic polymers can have versatile functions. Poly(N-vinylcaprolactam) (PVCL) is a cytocompatible thermosensitive polymer that can be used to fabricate injectable hydrogels for cartilage tissue engineering [42,43]. Sala et al. showed chondrocytes and MSCs exhibited high viability in PVCL hydrogels [42]. Both in vitro and in vivo tests showed that cartilage-specific extracellular matrix (ECM) was produced in a chondrocytes-laden PVCL hydrogel. Cartilage components including GAG and type II collagen increased over time with an even distribution throughout the material.
Polyethylene glycol (PEG): For a clinical application, PEG could be the most popular candidate as it was approved by Food and Drug Administration (FDA) for medical applications in pharmaceuticals and personal care products, and is considered safe even for oral consumption [44,45]. This polymer is easily modified while possessing good mechanical properties when forming hydrogels. Therefore, many research groups selected PEG as the basic material to design hydrogels for cartilage regeneration [46]. PEG hydrogels for clinical applications have been included in many “from bench to bed side” studies with promising results [28].
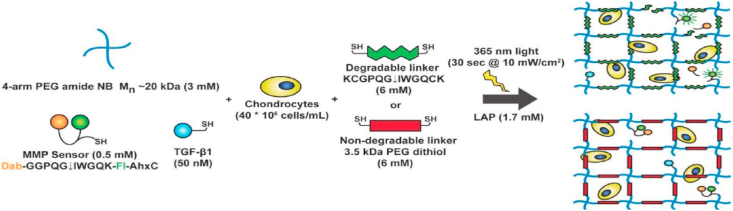
Recent research in this field focused on the interactions between PEG and cells, especially chondrocytes and MSCs. Basic problems here for PEG are the viability and the growth of the cells as PEG is biologically inert [47,48]. Therefore, other biological molecules are always used together with PEG to enhance the performance of the hydrogels. For example, a network of encapsulated hyaluronic acid (HA) in degradable PEG was designed to embed chondrocytes [49]. This network not only increased the number of chondrocytes and the effectiveness in deposition in tissues, but also decreased hypertrophy of cartilage. Sridhar et al. developed a PEG norbornene hydrogel with transforming growth factor beta 1 (TGF-β1) functionalized and crosslinked with an MMP-degradable peptide (Fig. 4) [50]. During tissue regeneration, the crosslinker peptides are cleaved by enzymes present in the surrounding tissue, allowing the degradation of the artificial matrix and the creation for an optimal environment for chondrocyte development and growth. Compared to non-degradable hydrogels, this hydrogel results in the production of appropriate amounts of matrix while maintaining the chondrocytes viable. Similarly, Skaalure et al. synthesized an enzyme-sensitive PEG hydrogel crosslinked by a peptide which is derived from aggrecanase-cleavable site in aggrecan [51]. This hydrogel system was also demonstrated to promote hyaline-like cartilage regeneration while avoiding the formation of hypertrophic cartilage.
Fig. 4.
Macromer solutions of 4-arm PEG norbornene, MMP fluorescent sensor, TGF-β1, and tethered growth factor were mixed with chondrocytes at 40 million cells/mL. The final hydrogel scaffold networks were UV cross-linked by degradable linker (MMP-degradable peptide sequence) or non-degradable linker (3.5 kDa PEG dithiol) [Reprinted from Refs. [50] with permission from Wiley].
Besides, in synthetic polymers, co-polymer can be specifically designed for cartilage tissue engineering. For example, poly-D,l-lactic acid/polyethylene glycol (PLA-PEG) was used to deliver bone morphogenetic protein-2 (rhBMP-2) for articular cartilage repair. In this system, PLA-PEG permits the release of rhBMP-2 for 3 weeks. In vivo results based on New Zealand White rabbits showed that subchondral defects were completely repaired after 6 weeks [52].
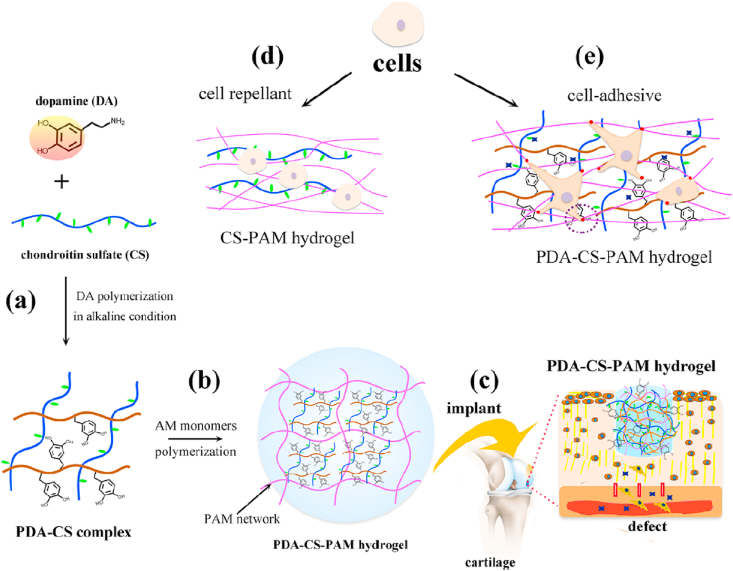
Above mentioned PVA, PMPC, PVCL, and PEG have been widely studied for cartilage tissue engineering. Hydrogels fabricated by such synthetic polymers are mechanically strong but most of them are biologically inert. The limitations of each polymer are summarized in Table 1. Therefore, native bio-macromolecules could be used to combine with synthetic polymers to simultaneously possess good mechanical strength and biological functions. For example, chondroitin sulfate (CS) is a sulfated GAG consisting of a chain of alternating N-acetylgalactosamine and glucuronic acid [53]. CS is also the main component of natural cartilage and can be used for the production of hydrogels serving as scaffolds in cartilage tissue engineering [34]. CS was reported to stimulate the secretion of glycoproteins and type II collagen by surrounding cells [54]. Although CS has good biocompatibility, it is rarely used alone due to its insufficient mechanical properties and fast degradation rates [11,55]. Recently, CS was combined with PEG to form a cartilage mimetic hydrogel, which was used to demonstrate the hypertrophy regulating capacity of CS during MSC chondrogenesis [56]. Han et al. incorporated CS into a polyacrylamide hydrogel, also demonstrating the cartilage regeneration ability of CS (Fig. 5) [57]. Besides, CS molecular chains can be modified to display physical or chemical-reactive moieties. An example is methacrylated chondroitin sulfate (CSMA) where CS chains have been modified with methacrylic anhydride. CSMA is degradable and can be UV-crosslinked to form a hydrogel scaffold that mimics the natural ECM in which chondrocytes were viable and multiplied [58]. CS is not only a macromolecule to form materials, but also is a regeneration factor for cartilage, and is worth to be studied in the future cartilage tissue engineering.
Fig. 5.
Schematic of CS containing hydrogel for cartilage tissue engineering. (a) CS was complexed with polydopamine (PDA). (b) CS was encapsulated in a PAM (polyacrylamide) hydrogel. (c) The CS containing hydrogel was implanted in a cartilage defect. (d) and (e) CS and polydopamine promoted cell adhesion on the hydrogel surfaces [Reprinted from Refs. [57] with permission from ACS Publications © 2018 American Chemical Society].
3.1.2. Polysaccharides
Hyaluronic acid (HA) is a linear biomacromolecule playing crucial roles in many cellular functions [59,60]. HA has fairly good biocompatibility when the molecular weight is high enough and is widely applied in cartilage tissue engineering because it is also a major component of cartilage ECM [49,[61], [62], [63], [64]]. Particularly, HA possesses biological activities and therefore commonly is used to fabricate hydrogel scaffolds interacting with cells [65]. Chondrocytes encapsulated within hydrogels containing 5% HA showed more than ten-folds increasing in the expression of cartilage marker genes like Aggrecan and Sox9 compared with 1.5%HA [62].
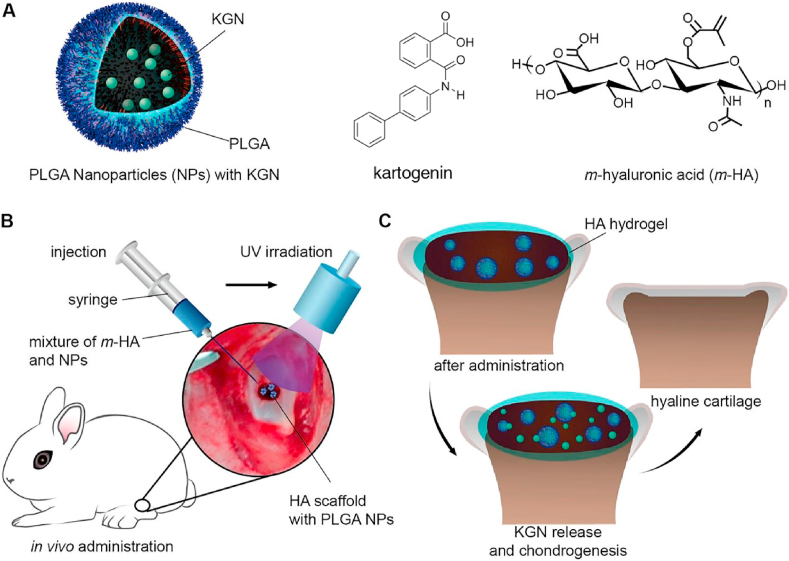
Like chondroitin sulfate, HA can be modified in order to become more versatile for cartilage tissue engineering. Methacrylated hyaluronic acid (HAMA) is an example of a modified HA. With a photoinitiator, HAMA can be crosslinked and form hydrogels under UV irradiation. For instance, Mouser et al. added HAMA to a thermosensitive copolymer solution to form a bio-ink [66]. When cultured with chondrocytes in vitro, a cartilage-like matrix was produced, and the amounts of matrix were dose-dependent to the HAMA concentration. Shi et al. used HAMA hydrogel encapsulating kartogenin (KGN)-loaded nanoparticles as scaffolds to provide a matrix for cell homing and cartilage regeneration (Fig. 6) [67]. KGN is a small molecule that can induce BMSCs to develop into chondrocytes. This technology has the advantage of being a one-step procedure, with regenerated tissues being similar to natural hyaline cartilage shown by the histological tests, specific markers analysis and biomechanical tests. Another approach was the development of a photo-sensitive hydrogel by creating o-nitrobenzyl alcohol moiety modified hyaluronic acids (HA-NB); HA-NB can form hydrogels on tissue surfaces in situ under light irradiation [68,69]. The material integrates well into existing cartilage and functions as a scaffold in cartilage defect sites in order to allow stem cell-derived exosomes to be retained at defect sites, which has the potential to replace stem cell therapy in cartilage regeneration [70].
Fig. 6.
(A) PLGA nanoparticles loaded with KGN, molecule structure of KGN, methacrylated HA (HAMA). (B) The brief operative treatment for cartilage defects repair. (C) The release of KGN from photo-crosslinked HA scaffold and the hyaline cartilage chondrogenesis [Reprinted from Ref. [67] with permission from ACS Publications © 2016 American Chemical Society].
In order to increase the affinity to bind proteins, HA can be sulfated, increasing the amount of negatively charged groups [71]. The sulfated HA hydrogel demonstrated slower degradation and enhanced growth factor retention as the original scaffold consisting of unmodified HA. Chen et al. also showed that 30% thiolated hyaluronic acid (HA-SH) and 70% collagen-I hybrid hydrogel could overcome the generally observed weak cell adhesion of HA while optimizing the gelation time to make the material suitable for injection [72]. In addition, the incorporation of HA in a chitosan-based hydrogel showed significantly increased proliferation of encapsulated chondrocytes and the deposition of cartilaginous extracellular matrix [73].
Chitosan is a polysaccharide extracted from the shells of shrimp and other crustaceans. The amino groups in chitosan make the molecule cationic. It shows antimicrobial properties and is highly bio-adhesive [26,[74], [75], [76], [77], [78]]. Liang et al. synthesized ampholytic chitosan/Carrageenan hybrid hydrogels which could induce chondrogenic differentiation of ATDC5 cells in vitro and showed good potential for medical applications for the regeneration of cartilage [79]. In order to construct a flexible hydrogel scaffold that can fill the different shapes of cartilage defects, Meng et al. blended chitosan with demineralized bone matrix particles modified with a phase display derived peptide E7 [80]. They demonstrated that the hybrid hydrogel scaffold improved MSCs survival, matrix production and chondrogenic differentiation. Methacrylated method also worked on Chitosan. Choi et al. mixed methacrylated glycol chitosan (MeGC) with type II collagen and TGF-β1, followed by exposure to visible blue light in presence of riboflavin, forming in a scaffold for mesenchymal stem cells (MSCs) in defect sites, for enhanced chondrogenic differentiation and integration into host tissue [81]. MeGC hydrogel is a good candidate to stabilize and control the release of TGF-β1 which is able to improve neocartilage formation, as well as being suitable as a drug delivery system [81,82]. Through Schiff base formation, glycol chitosan mixed with partially oxidized HA is able to form hydrogels, for the delivery of chondrocytes by non-invasive procedures [83].
Alginate is an anionic polysaccharide found in abundance in the cell walls of brown algae [17,84,85]. Because of its simple gelation with divalent cations like calcium ions Ca2+, alginate is often used as an injectable hydrogel, in a non-invasive approach for cartilage repair [86,87]. Alginate was also applied in 3D bioprinting techniques because of its fast cross-linking ability [88,89]. However, alginate alone lacks bio-functionality with respect to interactions with proteins and cells. Combined HA with alginate, Park et al. showed that hybrid scaffolds could retain the ability to form gels in the presence of Ca2+ and enhanced chondrogenic differentiation of ATDC5 [90]. Interestingly, alginate hydrogels can also be used as gene carriers for tissue engineering. For example, Gonzalez et al. encapsulated MSCs and nHA (nanohydroxyapatite) complexed with plasmid DNA (pDNA) encoding for pTGF-β3, pBMP2 or together into alginate hydrogels [91]. It was found that the pDNA was transported to MSCs, which did not occur in the control without alginate. This novel gene-activated alginate hydrogel successfully supported transfection of encapsulated MSCs and directed their differential orientation through different genes.
Cellulose is a linear chain polysaccharide consisting of d-glucose units and is the most abundant organic macromolecule on earth. For the construction of hydrogels, cellulose is often modified to enhance its properties. Sodium cellulose sulfate (NaCS) combined with gelatin is able to form a fibrous scaffold, in which hMSCs chondrogenesis was shown to be enhanced through increased while gene expression of type II collagen [92]. A new silated hydroxypropyl methylcellulose (Si-HPMC) hydrogel mixed with laponites, resulted in a hydrogel scaffold with improved mechanical strength that was cytocompatible and allowed oxygen diffusion [93]. When enriched with a biologically active marine exopolysaccharide (GY785), this Si-HPMC hydrogel produced an even more cartilage-like ECM [94]. Bacterial nanocellulose (BNC) is reported to possess nanofibrils similar to collagen fibrils found in tissue ECM; a bilayer BNC hydrogel has been shown to provide a suitable environment for chondrocytes to form cartilage in vitro and in vivo [95].
Polysaccharides are highly biocompatible nature macromolecules that resembles the glycan constituent of ECM. They have been widely investigated in the field of cartilage tissue engineering. However, single type of polysaccharide often has limitations to form ideal materials. For instance, CS can be biodegraded fast while chitosan has low solubility that is difficult to handle. The limitations of each polysaccharide are listed in Table 1. Combination with other category of polymers could be a solution to overcome these limitations.
3.1.3. Protein
Collagen is a type of ECM protein which naturally occurs in cartilage, supporting the growth of chondrocytes. Therefore, it is widely used in cartilage tissue engineering [[96], [97], [98]]. Collagen hydrogels reduce the risk of immune rejection by stimulating the ECM formation, reduce immunogenicity of exogenous cells that are seeded in engineered hydrogel constructs and promote cartilage regeneration [99]. In addition, Wong et al. found that type II collagen was capable of converting auricular chondrocytes into articular cartilage after dedifferentiation by monolayer passaging [100]. By using type I collagen as scaffold combined with stromal cell-derived factors-1 (SDF-1) as growth factor, Zhang et al. successfully induced the migration and adhesion of C-MSCs and SM-MSCs and promoted the self-repair of partial thickness defects [30]. Another study conducted by Jiang et al. indicated that collagen-based hydrogel encapsulated with MSCs could mediate the expression of Sox9 (a transcription factor regulates chondrogenesis), suggesting that appropriately designed hydrogel scaffold may work without growth factor [101]. Collagen can also be combined with other materials to form hybrid hydrogel scaffolds that have enhanced properties compared to the individual components. For instance, a native cartilage-mimic hydrogel was constructed using collagen, CS, and HA [102]. More interestingly, magnetic nanoparticles can be incorporated into type II collagen-HA-PEG hybrid hydrogels. This idea had not only beneficial effects on the engineered scaffold itself, but can also be used to transport the scaffold exactly to the cartilage defect [103], opening new avenues of cartilage tissue engineering. However, the effect of magnetic fields on the function of BMSCs remains to be investigated.
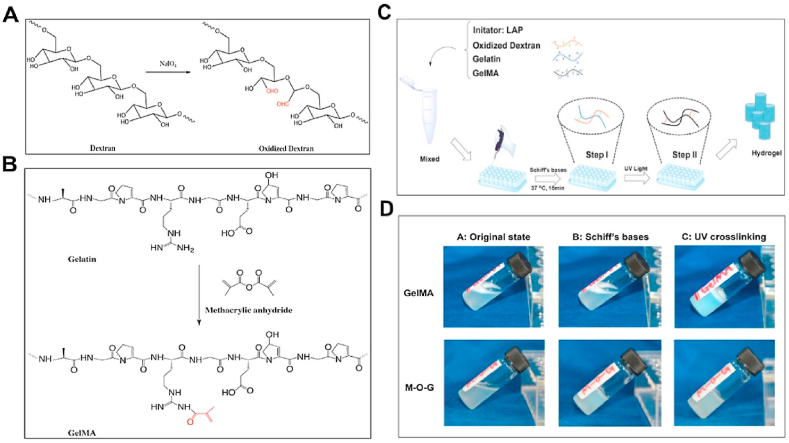
Gelatin, a kind of denatured collagen, is commonly used for cartilage repair as it is biocompatible, degradable, and shows good cell affinity, beside other advantageous properties [[104], [105], [106]]. However, major disadvantages of gelatin for cartilage repair are poor mechanical properties as well as low thermal stability [107]. To address these drawbacks, various methods have been developed. Wang et al. created a hybrid hydrogel consisting of gelatin and hydroxyphenylpropionic acid (HPA) [107]. The hydrogel was formed by using hydrogen peroxide (H2O2) and horseradish peroxidase (HRP) to catalyze HPA oxidative coupling. The advantage of this hydrogel is that the mechanical stiffness can be adjusted by changing the concentration of H2O2 or gelatin-HPA. Apart from combining gelatin with other materials, another way to form a stable hydrogel is to increase its crosslinking density. The formed hydrogel based on this modification showed increased mechanical strength and stability. Another study reports that a blended penta-block copolymer PNIPAAm-PCL-PEG-PCL-PNIPAAm with gelatin forms hydrogel scaffolds that possessed suitable hydrophilicity for growth, embedding, and ECM secretion of cells [108], while also increasing the material strength. Methacrylic anhydride (MA) easily reacts with gelatin to form photo-crosslinkable gelatin methacrylamide (GelMA) for hydrogel scaffolds. By combining acrylamide (AM) and GelMA, Han et al. obtained a hydrogel that showed increased mechanical strength and enhanced elasticity [104]. The compression strength and storage modulus of the AM and GelMA combining hydrogel can be up to 0.38 MPa and 1000 Pa, respectively. Zhou et al. recently reported a hydrogel scaffold composed of GelMA, oxidized dextran (ODex), and gelatin used for cartilage defect repair [35]. The formed hydrogels (M-O-G hydrogels) were crosslinked by a photo-initiator lithiumphenyl-2,4,6-trimethylbenzoylphosphinate (LAP) under UV irradiation (Fig. 7). In such a system, ODex not only partly crosslinked the network through reacting with gelatin, but also provided good tissue adhesive property by Schiff base reaction with amino groups found on the surface of the cartilage. Combining these properties together, the M-O-G hydrogels were formed in situ on the cartilage defects to enhance tissue integration. An in vivo study using a rabbit osteochondral defects model, showed that cartilage was regenerated at the defect site, and the new formed cartilage possessed comparable mechanical strength to the natural one. These GelMA hydrogels were reported to be promising candidates for cartilage tissue engineering. Rothrauff et al. demonstrated GelMA hydrogel had comparable efficacy to pepsin‐solubilized cartilage and tendon hydrogels in cartilage tissue engineering [109]. Similarly, Li et al. synthesized gelatin norbornene (GelNB) which can be used for encapsulating human mesenchymal stem cells [110]. This work provided researchers and clinicians with another choice of gelatin modification for articular cartilage tissue regeneration.
Fig. 7.
Schematic illustration of preparation of oxidized dextran (A) and GelMA(B). (A) Dextran was oxidized by NaIO4 to generate aldehyde groups (red) which can bond to amino groups through Schiff base reaction. (B) GelMA was formed by the reaction between gelatin and methacrylic anhydride. (C) Fabrication process of hydrogel scaffolds: Gelatin, GelMA, and oxidized dextran were firstly mixed with photoinitiator LAP. The mixed solution was pipetted into molds and allowed the Schiff base reaction. After final gelation by UV irradiation, hydrogels were formed and removed from molds. (D) Gross view of different phases of GelMA and M-O-G (Reprinted from Refs. [35] with permission from Elsevier).
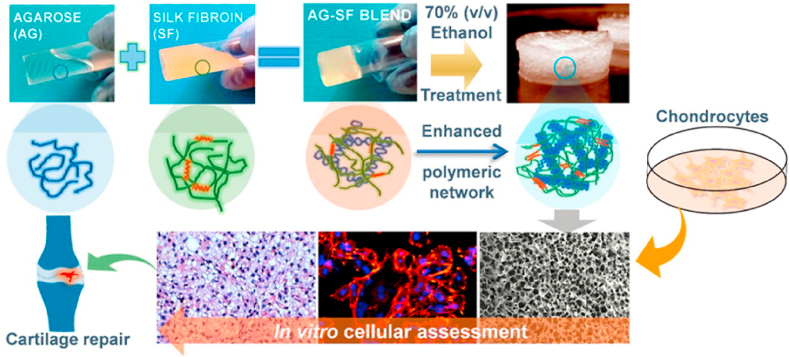
Silk fibroin (SF) is the main component of silk with excellent biocompatibility and biodegradability. Capable of mimicking the collagen structure of native cartilage, silk fibroin shows great potential in cartilage tissue engineering [111,112]. Zhou et al. synthesized methacrylated silk fibroin (MSF) which can be crosslinked by exposure to light [111]. The formed hydrogel was demonstrated to be compatible with mouse articular chondrocytes and suitable for cell adhesion. Horseradish peroxidase (HRP) mediated crosslinking was used to produce robust and interconnected porous silk fibroin scaffolds under physiological conditions [113]. Singh et al. fabricated a hydrogel composed of silk fibroin and agarose, which could be used for cartilage tissue engineering (Fig. 8) [114]. The hydrogel showed good immunocompatibility and was able to maintenance of chondrogenic phenotype. To further improve mechanical properties, Yodmuang et al. generated a silk microfiber-silk hydrogel which also resulted in a favorable chondrocyte response [115]. An overview of silk fibroin-based hydrogels for cartilage defects repair and regeneration was completed by Ribeiro et al. [116].
Fig. 8.
Agarose/silk fibroin blended hydrogels for cartilage tissue engineering [Reprinted from Refs. [114] with permission from ACS Publications © 2016 American Chemical Society].
Sericin is a protein extracted from silk fibers, and a byproduct in the silk industry, usually discarded as waste. However, like silk fibroin, sericin displays high biocompatibility. Thus, sericin can also be used to generate hydrogel for cartilage regeneration. By functionalizing the material with methacryloyl groups, the mechanical properties and degradation rates of sericin methacryloyl hydrogels can be adjusted across a wide range, making it possible to mimic native cartilage to suit different cartilage repair cases [117]. One of the advantages of using sericin is the nutrition-supplying property that allows chondrocytes proliferation even when lacking nutrition. Sericin methacryloyl hydrogels could be a promising scaffold for cartilage repair with molecular and mechanical resemblance to native cartilage and tunable properties.
Fibrin is a widely used biomaterial in the field of tissue engineering. Almeida et al. functionalized fibrin hydrogels with cartilage ECM for cartilage regeneration. Fibrin hydrogels could incorporate up to 2% (w/v) cartilage ECM particles which promoted the generation of cartilage-like tissue from freshly isolated stromal cells [118]. Snyder et al. reported a hydrogel made from chondrogenic fibrin and HA [119]. This hydrogel system could be injected, and it was possible to form a 3D network structure in situ. 3D cell culture of BMSCs in this hydrogel showed increasing Sox9 mRNA expression in quantitative polymerase chain reaction (qPCR) test, suggesting early chondrogenesis and thus a high potential for articular cartilage repair.
Proteins hold great potential as biomaterials for tissue engineering because of their excellent biocompatibility and biodegradability. The limitation for most proteins is the stability: high temperature or organic solvent can lead to the denaturation of protein. This limitation makes it difficult in material processing when use proteins. Mild processing method might be developed to solve this issue and lead to the revolution of biomaterials made from proteins.
3.1.4. Peptides
Due to their diversity, peptides are thought as one of the most promising molecules in cartilage regeneration because they can serve as components of permeable scaffolds or bio-functional factors [120]. For instance, Link protein N-terminal peptide (LPP) in ECM of cartilage was shown to stimulate the proliferation of cartilage stem/progenitor cells (CSPCs) [121]. Peptides can mimic some functions of proteins while they have the advantage to be adjustable in size. Peptides are readily synthesized in the lab and are relatively stable. This paves the way for developing peptide hydrogel scaffolds for cartilage tissue engineering. The challenge for peptide as cartilage regeneration materials is the proper design, synthesis, and purification. Another limitation to peptides, could also exist in polysaccharides, is the potential immunogenic and the effects of their degradation products.
Peptide based hydrogels have already shown their superiority in biocompatibility compared to many other materials. Callahan et al. synthesized an arginine-glycine-aspartic acid (RGD) peptide and used functionalized PEGDA hydrogel scaffold to enhance cell adhesion [122]. Based on this system, they found that mechanical properties of these hydrogel scaffolds were crucial for modulating human osteoarthritic chondrocyte behavior. Peptides can also be acrylated and simultaneously photopolymerized with acrylated PEG to form hydrogels [123]. When used bioprinting with this PEG-peptide hydrogel scaffold, hMSCs chondrogenic differentiation for cartilage formation was significantly enhanced compared with pure PEG hydrogel. Liu et al. synthesized copolymers consist of poly (l-alanine), PEG, and poly (l-alanine-co-l-phenylalanine) for cartilage tissue engineering [124]. The formed polypeptide hydrogels displayed thermo-sensitive properties, as well as mechanical strength which can be enhanced by increasing the content of phenylalanine, leading to hyaline-like cartilage formation in vivo macroscopically and histologically. Parmar et al. developed a biodegradable peptide hydrogel that significantly enhanced chondrogenic differentiation of hMSCs [125]. They found that, in this hydrogel system, CS-binding peptides help enhance MMP7 gene expression and activity, which are thought to be relevant to chondrogenesis through accelerating collagen type II maturation and promote functioning of chondrogenic factors. In another study, a photopolymerizing hydrogel based on CS and integrin binding RGD peptides was reported to increase lubricin gene expression of the encapsulated chondrocytes [126]. Diphenylalanine/serine (F2/S) peptide hydrogel, which is tunable in its properties, can be used to improve cartilage phenotype from differentiated pericytes. The content of type II collagen increased using this peptide hydrogel for chondrogenesis, without induction by components present in the media [127]. Lu et al. used bone marrow homing peptide (BMHP) functionalized with a self-assembling peptide (SAP) to fabricate a hydrogel scaffold for cartilage regeneration. The synthesized SAP hydrogels stimulate rabbit MSC attachment, proliferation, and chondrogenic differentiation. An in vivo test after 3 and 6 months showed that cartilage-like tissue with a smooth surface was formed on the articular cartilage defect. The study demonstrated that such hydrogel scaffolds are promising for cartilage repair without cell transplantation [128].
3.2. Additive manufacturing of hydrogels for cartilage regeneration
Generally, above mentioned source materials usually form hydrogels through mold-casting strategy if not delivered in situ. A novel technique is additive manufacturing or 3D printing/bioprinting which is increasingly being deployed to fabricate biomaterials for cartilage defects repair [27,[129], [130], [131]]. 3D printing is able to construct scaffolds precisely to match the defective area. Combining hydrogel and cell, 3D bioprinting could allow the precise 3D space depositing of living cells that then develop into functional artificial tissue. Using this approach, Zhu et al. produced a 3D bioprinted cell-laden cartilage tissue construct [130]. GelMA and PEGDA were selected and optimized as a printable resin for cell-hydrogel bioprinting. The printed hydrogel constructs sustained release TGF-β1 and improved chondrogenic differentiation of encapsulated MSCs. Aisenbrey et al. used stereolithography-based 3D printing with photopolymerizable hydrogel to treat focal chondral defects [129]. In this study, chondrocytes were incorporated in an injectable hydrogel precursor solution which can be infilled into a 3D printed support structure, promoting neotissue deposition. This kind of construct minimized the damage to the surrounding tissue when placed in focal chondral defects in an osteochondral model. Costantini et al. used two coaxial-needles system, another 3D bioprinting strategy, to print a 3D biomimetic hydrogel composed of similar biopolymers present in cartilage ECM [131]. BMSCs loaded and cultured in the printed hydrogels showed enhanced viability and chondrogenic differentiation. Although 3D bioprinting is an emerging technique for tissue engineering, it has not widely been applied for the repair of cartilage defects. The current development in bioprinting is to move from form to function [132]. Novel and innovative ideas need to be developed to take full advantage of this technique for cartilage applications.
4. Applications and clinical trial
After extensive lab-based research and testing, some of the developed materials mature into products with great potential for clinical applications. Currently, the use of hydrogels in the clinic is mainly to improve existing treatment strategies such as ACI, MACI, and microfracture surgery. A product consists of a 3-dimensional type I collagen scaffold and autologous chondrocytes and was developed for full-thickness cartilage defects [133]. This product proved to be effective albeit being simple and was demonstrated as a safe and effective treatment for full-thickness cartilage defects through to 5-year follow-up [134]. Another product (CaReS®) also based on collagen showed comparable results to microfracture for the treatment of patellofemoral articular cartilage lesions [135]. These results indicate collagen possesses advantages for cartilage defects repair in clinical.
Microfracture surgery is a clinical therapy to treat cartilage defects. In this method, small holes are created that penetrate subchondral bone to expose bone marrow and therein containing cells. However, about half of the procedures fail due to insufficient formation of new tissue and of fibrocartilage, but also due to unwanted bone invasion [14,28]. ChonDux hydrogel is a product that can improve this procedure. This hydrogel system consists of a PEG/HA network and a CS adhesive [28]. PEG was first modified to PEG diacrylate (PEGDA) which is able to photocrosslinked to hydrogel. Sharma et al. found HA incorporate with PEGDA could promote chondrogenesis of mesenchymal stem cells in vivo [28,136], while Wang et al. demonstrate CS adhesive helped the hydrogel retain on the cartilage [34]. With this hydrogel, the blood and bone marrow from the microfracture holes was accumulated around the defects and assists with the regeneration. A clinical trial of ChonDux for full-thickness femoral condyle defects showed 94.2% ± 16.3% of final defects filled over 24 months and the treated tissue was similar to uninjured cartilage, suggesting such a system is a safe supplementary product for microfracture therapy [137].
Stem cell therapy is a novel strategy to treat cartilage defects in OA. Cartistem® is a product composed of HA hydrogel and allogeneic human umbilical cord blood-derived mesenchymal stem cells (hUCB-MSCs). Over a 7-years clinical trial, this product was demonstrated to be safe and effective for cartilage regeneration [138]. Before this, in an animal study using the minipig model, it was shown that the chondrogenic differentiation capacity of hUCB-MSCs synergistically affected the degree of cartilage regeneration potential [139]. Given that HA is one of the main ingredients in ChonDux, we think HA is an important component for cartilage tissue engineering and holds great potential for clinical translation.
5. Challenges
It is well known that cartilage do not self-repair due to lack of blood vessels, nerves and lymphatic tissues [140,141]. Hydrogels can act as scaffolds, elastomers, drug/nutrition carriers to help the regeneration of cartilage. The challenge here is how to select the most suitable hydrogel for a procedure to help the patients in pain due to OA. To engineer a hydrogel for cartilage repair, the first thing that should be considered is the source of the material. We know that hydrogels made from PAM and its derivative double networks are extremely strong in mechanical like natural cartilage [142,143]. However, PAM hydrogels were reported causing inflammatory reactions in breast augmentation and were banned to use by Chinese State Food and Drug Administration in 2006 [144,145]. Therefore, it might be more challenging for a clinical application to use this material as an implant for cartilage repair. Currently, materials that are able to promote MSCs chondrogenesis and maintain the phenotype of chondrocytes in vitro, have shown to be the most promising types for cartilage regeneration. A mass plethora of studies demonstrated that such materials can be used for chondrogenic differentiation of cells (an incomplete list can be found in Table 1). Apart from the type of material, parameters such as matrix elasticity, matrix stiffness, and mechanical confinement can regulate the cell fate as well [13,146,147]. Due to the complexity of the tissue, mechanical and biological considerations in terms of cell development and medical as well as physical attributes or defects, an optimal solution has yet to be found. With the increasing use of machine learning and artificial intelligence (AI), we have tools to our disposal that could help to develop better materials in the future. These techniques may be able to help us accelerated the process of trial and error, and finally obtain the ideal material for cartilage regeneration.
Another problem for cartilage tissue engineering is the way to bond the biomaterials to cartilage [34]. Without efficient bonding, even newly formed cartilage around the introduced scaffold might not integrate well with the host tissue [35]. Thus, bio-adhesive properties for the design of novel hydrogel materials have to be considered. Recent advancements in the development of hydrogels with tough adhesive properties especially to wet surfaces, may pave the way in constructing biomaterials for cartilage tissue engineering [[148], [149], [150]]. However, when used for cartilage tissue engineering, the biocompatibility of the glue is needed to be considered. And the mechanism of the cells traverse and modify the glue to integrate the tissue should be investigated.
Till today, we are unable to achieve engineered cartilage as it is in the human body. Although cartilage has been investigated for many decades, new technologies such as cryo electron microscopy, solid-state NMR and mass spectrometry can be used to deepen our understanding of natural cartilage to reveal the exact components and molecular structure [151,152].
6. Conclusions and outlook
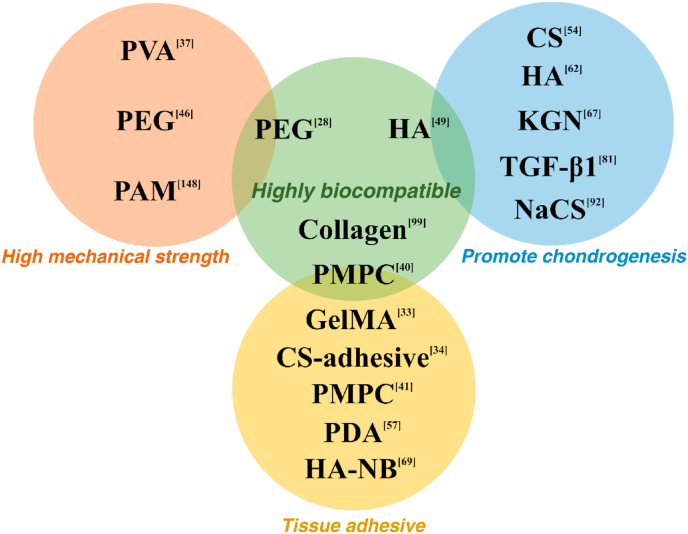
In this work, we have reviewed the progress of designing hydrogels for cartilage tissue engineering during the last 5 years. We provide an overview on hydrogel structure, most of which, in fact, act as a scaffold for cell development or drug delivery. Synthetic polymers such as PVA and PEG are biologically inert and have no direct effect on cells. Factors that help to promote MSC chondrogenesis and maintain the phenotype of chondrocytes are often included as cartilage-ECM like materials such as HA, CS, and also specific peptides like arginine-glycine-aspartic acid (RGD) and Link protein N-terminal peptide (LPP). Therefore, when producing hydrogels for articular cartilage tissue engineering, some suggestions are made: 1. if a mechanically strong scaffold is needed, synthetic polymers and silk related materials might be the best choice. 2. To enhance cell adhesion, proteins such as collagen and gelatin have been shown to be beneficial. 3. Chondrogenesis promoting factors such as HA, CS, and TGF-β1 should be added to the system to increase effectiveness of the product. 4. Hydrogels can be designed as tissue adhesive and injectable/paintable for practical applications and clinical translation. Some polymers and factors sorted by their characters are shown in Fig. 9.
Fig. 9.
Summary of the characteristics of some polymers and factors for cartilage tissue engineering applications. Abbreviations: PVA (Polyvinyl alcohol), PEG (Polyethylene glycol), PAM (Polyacrylamide), PMPC (Poly (2-methacryloyloxyethyl phosphorylcholine)), HA (Hyaluronic Acid), CS (Chondroitin sulfate), KGN (Kartogenin), TGF-β1 (Transforming growth factor beta 1), NaCS (Sodium cellulose sulfate), GelMA (Gelatin methacrylamide), PDA (Polydopamine), HA-NB (o-nitrobenzyl alcohol moiety modified hyaluronic acids).
In osteoarthritis, cartilage defects can be divided into three classes: partial-thickness defects, full-thickness defects, and osteochondral defects. In our opinion, it would be advantageous to consider that hydrogels should be specifically designed for their application, to treat different kinds of cartilage defects (Fig. 10). For example, scaffolds for full-thickness defects and osteochondral defects can be designed in bulk and are transplantable, with the 3D printing/bioprinting technique as an option to produce such scaffolds. Many studies focused on the treatment of full-thickness defects and osteochondral defects. But most of the cartilage defects observed in OA joints are partial-thickness, such defects need to be studied as well due to their different nature and extent of tissue damage [153,154]. For each kind of cartilage defect, the most suitable treatment strategy should be deployed.
Fig. 10.
Logic diagram of hydrogels designed for different kinds of cartilage defects.
In our opinion, cartilage defects in OA could be treated in two ways. One is to make an artificial cartilage which might be a tough and lubricated hydrogel. The artificial cartilage can be transplanted into the defect sites or replace the whole cartilage. This kind of materials could be designed and fabricated aiming at superior properties than natural cartilage. Some super tough hydrogels such as alginate-polyacrylamide (Alg-PAM) hydrogels and polyacrylamide-alginate double network hydrogels equilibrated with aqueous solutions of calcium chloride, which have been recently reported can be considered if biocompatibility and bioadhesion are satisfactory [148,155,156]. The other way is to design biomaterials to facilitate self-repair in the body. Stem cells from the synovial fluids cannot perform their task to repair injured cartilage when the micro-environment of the cartilage surface is not suitable for cell adhesion [30]. Therefore, hydrogels with proper mechanical strength and excellent stem cell adhesion properties are promising materials for cartilage repair. Many of the above-reviewed development strategies to construct various kinds of hydrogel scaffolds show the promising nature of such materials for the treatment of different kinds of cartilage defects in OA. With recent developments in stem cell therapy, harvesting the synergies of both fields has the potential to provide highly satisfactory solutions for OA patients.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgements
This work was supported by National key R&D program of China (2017YFA0104900), NSFC grants (31830029, 81630065, 81902187), and the Zhejiang Provincial Natural Science Foundation of China (No. LQ19E030019, LY19C070003), China Postdoctoral Science Foundation (2019M652112, 2018M642442, 2019M662084).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.James S.L., Abate D., Abate K.H., Abay S.M., Abbafati C., Abbasi N. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hunter D.J., Schofield D., Callander E. The individual and socioeconomic impact of osteoarthritis. Nat. Rev. Rheumatol. 2014;10:437–441. doi: 10.1038/nrrheum.2014.44. [DOI] [PubMed] [Google Scholar]
- 3.Wu Y., Zhu S.A., Wu C.T., Lu P., Hu C.C., Xiong S. A Bi-lineage conducive scaffold for osteochondral defect regeneration. Adv. Funct. Mater. 2014;24:4473–4483. [Google Scholar]
- 4.Glass K.A., Link J.M., Brunger J.M., Moutos F.T., Gersbach C.A., Guilak F. Tissue-engineered cartilage with inducible and tunable immunomodulatory properties. Biomaterials. 2014;35:5921–5931. doi: 10.1016/j.biomaterials.2014.03.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang D., Shin J., Cho Y., Kim H.S., Gu Y.R., Kim H. Stress-activated miR-204 governs senescent phenotypes of chondrocytes to promote osteoarthritis development. Sci. Transl. Med. 2019;11 doi: 10.1126/scitranslmed.aar6659. [DOI] [PubMed] [Google Scholar]
- 6.Sellam J., Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat. Rev. Rheumatol. 2010;6:625–635. doi: 10.1038/nrrheum.2010.159. [DOI] [PubMed] [Google Scholar]
- 7.Assenmacher A.T., Pareek A., Reardon P.J., Macalena J.A., Stuart M.J., AJJATJoA Krych. vol. 32. 2016. Long-term Outcomes after Osteochondral Allograft: a Systematic Review at Long-Term Follow-Up of 12.3 Years; pp. 2160–2168. [DOI] [PubMed] [Google Scholar]
- 8.Levingstone T.J., Thompson E., Matsiko A., Schepens A., Gleeson J.P., FJJAb O'Brien. vol. 32. 2016. Multi-layered Collagen-Based Scaffolds for Osteochondral Defect Repair in Rabbits; pp. 149–160. [DOI] [PubMed] [Google Scholar]
- 9.Gan D., Wang Z., Xie C., Wang X., Xing W., Ge X. Mussel-inspired tough hydrogel with in situ nanohydroxyapatite mineralization for osteochondral defect repair. Adv. Healthc. Materials. 2019;8 doi: 10.1002/adhm.201901103. [DOI] [PubMed] [Google Scholar]
- 10.Morgese G., Benetti E.M., Zenobi-Wong M. Molecularly engineered biolubricants for articular cartilage. Adv. Healthc. Mater. 2018;7 doi: 10.1002/adhm.201701463. [DOI] [PubMed] [Google Scholar]
- 11.Zhou F., Zhang X., Cai D., Li J., Mu Q., Zhang W. Silk fibroin-chondroitin sulfate scaffold with immuno-inhibition property for articular cartilage repair. Acta Biomater. 2017;63:64–75. doi: 10.1016/j.actbio.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 12.Radhakrishnan J., Subramanian A., Krishnan U.M., Sethuraman S. Injectable and 3D bioprinted polysaccharide hydrogels: from cartilage to osteochondral tissue engineering. Biomacromolecules. 2017;18:1–26. doi: 10.1021/acs.biomac.6b01619. [DOI] [PubMed] [Google Scholar]
- 13.Lee H.P., Gu L., Mooney D.J., Levenston M.E., Chaudhuri O. Mechanical confinement regulates cartilage matrix formation by chondrocytes. Nat. Mater. 2017;16:1243–1251. doi: 10.1038/nmat4993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang J., Zhang Y.S., Yue K., Khademhosseini A. Cell-laden hydrogels for osteochondral and cartilage tissue engineering. Acta Biomater. 2017;57:1–25. doi: 10.1016/j.actbio.2017.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han L., Liu K., Wang M., Wang K., Fang L., Chen H. Mussel-inspired adhesive and conductive hydrogel with long-lasting moisture and extreme temperature tolerance. Adv. Funct. Mater. 2018;28:1704195. [Google Scholar]
- 16.Wang H., Cai L., Paul A., Enejder A., Heilshorn S.C. Hybrid elastin-like polypeptide-polyethylene glycol (ELP-PEG) hydrogels with improved transparency and independent control of matrix mechanics and cell ligand density. Biomacromolecules. 2014;15:3421–3428. doi: 10.1021/bm500969d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y.S., Khademhosseini A. Advances in engineering hydrogels. Science. 2017;356 doi: 10.1126/science.aaf3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang X., Xu B., Puperi D.S., Yonezawa A.L., Wu Y., Tseng H. Integrating valve-inspired design features into poly(ethylene glycol) hydrogel scaffolds for heart valve tissue engineering. Acta Biomater. 2015;14:11–21. doi: 10.1016/j.actbio.2014.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qi X., Hu X., Wei W., Yu H., Li J., Zhang J. Investigation of Salecan/poly(vinyl alcohol) hydrogels prepared by freeze/thaw method. Carbohydr. Polym. 2015;118:60–69. doi: 10.1016/j.carbpol.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 20.Chen H., Yang F., Chen Q., Zheng J. A novel design of multi-mechanoresponsive and mechanically strong hydrogels. Adv. mater. 2017;29 doi: 10.1002/adma.201606900. [DOI] [PubMed] [Google Scholar]
- 21.Li L., Yu F., Zheng L., Wang R., Yan W., Wang Z. Natural hydrogels for cartilage regeneration: modification, preparation and application. J Orthop Translat. 2019;17:26–41. doi: 10.1016/j.jot.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crawford D.C., DeBerardino T.M., Williams R.J.I. vol. 94. 2012. NeoCart, an Autologous Cartilage Tissue Implant, Compared with Microfracture for Treatment of Distal Femoral Cartilage Lesions: an FDA Phase-II Prospective, Randomized Clinical Trial after Two Years; pp. 979–989. [DOI] [PubMed] [Google Scholar]
- 23.Niethammer T.R., Pietschmann M.F., Horng A., Rossbach B.P., Ficklscherer A., Jansson V. Graft hypertrophy of matrix-based autologous chondrocyte implantation: a two-year follow-up study of NOVOCART 3D implantation in the knee. Knee surgery, sports traumatology, arthroscopy : official journal of the ESSKA. 2014;22:1329–1336. doi: 10.1007/s00167-013-2454-7. [DOI] [PubMed] [Google Scholar]
- 24.Andereya S., Maus U., Gavenis K., Muller-Rath R., Miltner O., Mumme T. First clinical experiences with a novel 3D-collagen gel (CaReS) for the treatment of focal cartilage defects in the knee. Z. Orthop. Ihre Grenzgeb. 2006;144:272–280. doi: 10.1055/s-2006-933445. [DOI] [PubMed] [Google Scholar]
- 25.Feng Q., Li Q., Wen H., Chen J., Liang M., Huang H. Injection and self‐assembly of bioinspired stem cell‐laden gelatin/hyaluronic acid hybrid microgels promote cartilage repair in vivo. Adv. Funct. Mater. 2019:1906690. [Google Scholar]
- 26.Mellati A., Fan C.M., Tamayol A., Annabi N., Dai S., Bi J. Microengineered 3D cell-laden thermoresponsive hydrogels for mimicking cell morphology and orientation in cartilage tissue engineering. Biotechnol. Bioeng. 2017;114:217–231. doi: 10.1002/bit.26061. [DOI] [PubMed] [Google Scholar]
- 27.Guo T., Noshin M., Baker H.B., Taskoy E., Meredith S.J., Tang Q. 3D printed biofunctionalized scaffolds for microfracture repair of cartilage defects. Biomaterials. 2018;185:219–231. doi: 10.1016/j.biomaterials.2018.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharma B., Fermanian S., Gibson M., Unterman S., Herzka D.A., Cascio B. Human cartilage repair with a photoreactive adhesive-hydrogel composite. Sci. Transl. Med. 2013;5:167ra6. doi: 10.1126/scitranslmed.3004838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liao J., Tian T., Shi S., Xie X., Ma Q., Li G. The fabrication of biomimetic biphasic CAN-PAC hydrogel with a seamless interfacial layer applied in osteochondral defect repair. Bone Res. 2017;5:17018. doi: 10.1038/boneres.2017.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang W., Chen J., Tao J., Jiang Y., Hu C., Huang L. The use of type 1 collagen scaffold containing stromal cell-derived factor-1 to create a matrix environment conducive to partial-thickness cartilage defects repair. Biomaterials. 2013;34:713–723. doi: 10.1016/j.biomaterials.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 31.Hunziker E.B. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage. 2002;10:432–463. doi: 10.1053/joca.2002.0801. [DOI] [PubMed] [Google Scholar]
- 32.Jia S., Wang J., Zhang T., Pan W., Li Z., He X. Multilayered scaffold with a compact interfacial layer enhances osteochondral defect repair. ACS Appl. Mater. Interfaces. 2018;10:20296–20305. doi: 10.1021/acsami.8b03445. [DOI] [PubMed] [Google Scholar]
- 33.Shirzaei Sani E., Kheirkhah A., Rana D., Sun Z., Foulsham W., Sheikhi A. Sutureless repair of corneal injuries using naturally derived bioadhesive hydrogels. Sci Adv. 2019;5 doi: 10.1126/sciadv.aav1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang D.A., Varghese S., Sharma B., Strehin I., Fermanian S., Gorham J. Multifunctional chondroitin sulphate for cartilage tissue-biomaterial integration. Nat. Mater. 2007;6:385–392. doi: 10.1038/nmat1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou F., Hong Y., Zhang X., Yang L., Li J., Jiang D. Tough hydrogel with enhanced tissue integration and in situ forming capability for osteochondral defect repair. Appl. Mater. Today. 2018;13:32–44. [Google Scholar]
- 36.Lu Y., Liu X., Luo G. Synthesis of polystyrene latex via emulsion polymerization with poly(vinyl alcohol) as sole stabilizer. J. Appl. Polym. Sci. 2017;134:45111. [Google Scholar]
- 37.Oliveira A.S., Seidi O., Ribeiro N., Colaco R., Serro A.P. Tribomechanical comparison between PVA hydrogels obtained using different processing conditions and human cartilage. Materials. 2019;12 doi: 10.3390/ma12203413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dashtdar H., Murali M.R., Abbas A.A., Suhaeb A.M., Selvaratnam L., Tay L.X. PVA-chitosan composite hydrogel versus alginate beads as a potential mesenchymal stem cell carrier for the treatment of focal cartilage defects. Knee Surg. Sports Traumatol. Arthrosc. : official journal of the ESSKA. 2015;23:1368–1377. doi: 10.1007/s00167-013-2723-5. [DOI] [PubMed] [Google Scholar]
- 39.Milner P.E., Parkes M., Puetzer J.L., Chapman R., Stevens M.M., Cann P. A low friction, biphasic and boundary lubricating hydrogel for cartilage replacement. Acta Biomater. 2018;65:102–111. doi: 10.1016/j.actbio.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 40.Moro T., Takatori Y., Ishihara K., Konno T., Takigawa Y., Matsushita T. Surface grafting of artificial joints with a biocompatible polymer for preventing periprosthetic osteolysis. Nat. Mater. 2004;3:829–836. doi: 10.1038/nmat1233. [DOI] [PubMed] [Google Scholar]
- 41.Cooper B.G., Stewart R.C., Burstein D., Snyder B.D., Grinstaff M.W. A tissue-penetrating double network restores the mechanical properties of degenerated articular cartilage. Angew. Chem. 2016;55:4226–4230. doi: 10.1002/anie.201511767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sala R.L., Kwon M.Y., Kim M., Gullbrand S.E., Henning E.A., Mauck R.L. Thermosensitive poly(N-vinylcaprolactam) injectable hydrogels for cartilage tissue engineering. Tissue Eng. 2017;23:935–945. doi: 10.1089/ten.tea.2016.0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lynch B., Crawford K., Baruti O., Abdulahad A., Webster M., Puetzer J. The effect of hypoxia on thermosensitive poly(N-vinylcaprolactam) hydrogels with tunable mechanical integrity for cartilage tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2017;105:1863–1873. doi: 10.1002/jbm.b.33705. [DOI] [PubMed] [Google Scholar]
- 44.Bhattarai N., Matsen F.A., Zhang M. PEG-grafted chitosan as an injectable thermoreversible hydrogel. Macromol. Biosci. 2005;5:107–111. doi: 10.1002/mabi.200400140. [DOI] [PubMed] [Google Scholar]
- 45.Chin S.Y., Poh Y.C., Kohler A.C., Sia S.K. An additive manufacturing technique for the facile and rapid fabrication of hydrogel-based micromachines with magnetically responsive components. J. Vis. Exp. 2018;(137) doi: 10.3791/56727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin C.C. Recent advances in crosslinking chemistry of biomimetic poly(ethylene glycol) hydrogels. RSC Adv. 2015;5:39844–39853. doi: 10.1039/C5RA05734E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mehrali M., Thakur A., Pennisi C.P., Talebian S., Arpanaei A., Nikkhah M. Nanoreinforced hydrogels for tissue engineering: biomaterials that are compatible with load-bearing and electroactive tissues. Adv. Mater. 2017;29(8) doi: 10.1002/adma.201603612. [DOI] [PubMed] [Google Scholar]
- 48.Gaharwar A.K., Dammu S.A., Canter J.M., Wu C.J., Schmidt G. Highly extensible, tough, and elastomeric nanocomposite hydrogels from poly(ethylene glycol) and hydroxyapatite nanoparticles. Biomacromolecules. 2011;12:1641–1650. doi: 10.1021/bm200027z. [DOI] [PubMed] [Google Scholar]
- 49.Skaalure S.C., Dimson S.O., Pennington A.M., Bryant S.J. Semi-interpenetrating networks of hyaluronic acid in degradable PEG hydrogels for cartilage tissue engineering. Acta Biomater. 2014;10:3409–3420. doi: 10.1016/j.actbio.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 50.Sridhar B.V., Brock J.L., Silver J.S., Leight J.L., Randolph M.A., Anseth K.S. Development of a cellularly degradable PEG hydrogel to promote articular cartilage extracellular matrix deposition. Advanced healthcare materials. 2015;4:702–713. doi: 10.1002/adhm.201400695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Skaalure S.C., Chu S., Bryant S.J. An enzyme-sensitive PEG hydrogel based on aggrecan catabolism for cartilage tissue engineering. Adv. Healthc. Mater. 2015;4:420–431. doi: 10.1002/adhm.201400277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tamai N., Myoui A., Hirao M., Kaito T., Ochi T., Tanaka J. A new biotechnology for articular cartilage repair: subchondral implantation of a composite of interconnected porous hydroxyapatite, synthetic polymer (PLA-PEG), and bone morphogenetic protein-2 (rhBMP-2) Osteoarthritis Cartilage. 2005;13:405–417. doi: 10.1016/j.joca.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 53.Balakrishnan B., Banerjee R. Biopolymer-based hydrogels for cartilage tissue engineering. Chem. Rev. 2011;111:4453–4474. doi: 10.1021/cr100123h. [DOI] [PubMed] [Google Scholar]
- 54.Yu F., Cao X., Zeng L., Zhang Q., Chen X. An interpenetrating HA/G/CS biomimic hydrogel via Diels-Alder click chemistry for cartilage tissue engineering. Carbohydr. Polym. 2013;97:188–195. doi: 10.1016/j.carbpol.2013.04.046. [DOI] [PubMed] [Google Scholar]
- 55.Chang K.Y., Hung L.H., Chu I.M., Ko C.S., Lee Y.D. The application of type II collagen and chondroitin sulfate grafted PCL porous scaffold in cartilage tissue engineering. J. Biomed. Mater. Res. 2010;92:712–723. doi: 10.1002/jbm.a.32198. [DOI] [PubMed] [Google Scholar]
- 56.Aisenbrey E.A., Bryant S.J. The role of chondroitin sulfate in regulating hypertrophy during MSC chondrogenesis in a cartilage mimetic hydrogel under dynamic loading. Biomaterials. 2019;190–191:51–62. doi: 10.1016/j.biomaterials.2018.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Han L., Wang M., Li P., Gan D., Yan L., Xu J. Mussel-inspired tissue-adhesive hydrogel based on the polydopamine-chondroitin sulfate complex for growth-factor-free cartilage regeneration. ACS Appl. Mater. Interfaces. 2018;10:28015–28026. doi: 10.1021/acsami.8b05314. [DOI] [PubMed] [Google Scholar]
- 58.Liao J., Qu Y., Chu B., Zhang X., Qian Z. Biodegradable CSMA/PECA/Graphene porous hybrid scaffold for cartilage tissue engineering. Sci. Rep. 2015;5:9879. doi: 10.1038/srep09879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Song E., Han W., Li C., Cheng D., Li L., Liu L. Hyaluronic acid-decorated graphene oxide nanohybrids as nanocarriers for targeted and pH-responsive anticancer drug delivery. ACS Appl. Mater. Interfaces. 2014;6:11882–11890. doi: 10.1021/am502423r. [DOI] [PubMed] [Google Scholar]
- 60.Yang Y., Zhang J., Liu Z., Lin Q., Liu X., Bao C. Tissue-integratable and biocompatible photogelation by the imine crosslinking reaction. Adv. mater. 2016;28:2724–2730. doi: 10.1002/adma.201505336. [DOI] [PubMed] [Google Scholar]
- 61.Li L., Wang N., Jin X., Deng R., Nie S., Sun L. Biodegradable and injectable in situ cross-linking chitosan-hyaluronic acid based hydrogels for postoperative adhesion prevention. Biomaterials. 2014;35:3903–3917. doi: 10.1016/j.biomaterials.2014.01.050. [DOI] [PubMed] [Google Scholar]
- 62.Zhu D., Wang H., Trinh P., Heilshorn S.C., Yang F. Elastin-like protein-hyaluronic acid (ELP-HA) hydrogels with decoupled mechanical and biochemical cues for cartilage regeneration. Biomaterials. 2017;127:132–140. doi: 10.1016/j.biomaterials.2017.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yu Y., Brouillette M.J., Seol D., Zheng H., Buckwalter J.A., Martin J.A. Use of recombinant human stromal cell-derived factor 1 alpha-loaded fibrin/hyaluronic acid hydrogel networks to achieve functional repair of full-thickness bovine articular cartilage via homing of chondrogenic progenitor cells. Arthritis & rheumatology. 2015;67:1274–1285. doi: 10.1002/art.39049. [DOI] [PubMed] [Google Scholar]
- 64.Chen P., Zhu S., Wang Y., Mu Q., Wu Y., Xia Q. The amelioration of cartilage degeneration by ADAMTS-5 inhibitor delivered in a hyaluronic acid hydrogel. Biomaterials. 2014;35:2827–2836. doi: 10.1016/j.biomaterials.2013.12.076. [DOI] [PubMed] [Google Scholar]
- 65.Lam J., Truong N.F., Segura T. Design of cell-matrix interactions in hyaluronic acid hydrogel scaffolds. Acta Biomater. 2014;10:1571–1580. doi: 10.1016/j.actbio.2013.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mouser V.H., Abbadessa A., Levato R., Hennink W.E., Vermonden T., Gawlitta D. Development of a thermosensitive HAMA-containing bio-ink for the fabrication of composite cartilage repair constructs. Biofabrication. 2017;9 doi: 10.1088/1758-5090/aa6265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shi D., Xu X., Ye Y., Song K., Cheng Y., Di J. Photo-cross-linked scaffold with kartogenin-encapsulated nanoparticles for cartilage regeneration. ACS Nano. 2016;10:1292–1299. doi: 10.1021/acsnano.5b06663. [DOI] [PubMed] [Google Scholar]
- 68.Liu X., Yang Y., Niu X., Lin Q., Zhao B., Wang Y. An in situ photocrosslinkable platelet rich plasma - complexed hydrogel glue with growth factor controlled release ability to promote cartilage defect repair. Acta Biomater. 2017;62:179–187. doi: 10.1016/j.actbio.2017.05.023. [DOI] [PubMed] [Google Scholar]
- 69.Hong Y., Zhou F., Hua Y., Zhang X., Ni C., Pan D. A strongly adhesive hemostatic hydrogel for the repair of arterial and heart bleeds. Nat. Commun. 2019;10:2060. doi: 10.1038/s41467-019-10004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu X., Yang Y., Li Y., Niu X., Zhao B., Wang Y. Integration of stem cell-derived exosomes with in situ hydrogel glue as a promising tissue patch for articular cartilage regeneration. Nanoscale. 2017;9:4430–4438. doi: 10.1039/c7nr00352h. [DOI] [PubMed] [Google Scholar]
- 71.Feng Q., Lin S., Zhang K., Dong C., Wu T., Huang H. Sulfated hyaluronic acid hydrogels with retarded degradation and enhanced growth factor retention promote hMSC chondrogenesis and articular cartilage integrity with reduced hypertrophy. Acta Biomater. 2017;53:329–342. doi: 10.1016/j.actbio.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 72.Chen Y., Sui J., Wang Q., Yin Y., Liu J., Wang Q. Injectable self-crosslinking HA-SH/Col I blend hydrogels for in vitro construction of engineered cartilage. Carbohydr. Polym. 2018;190:57–66. doi: 10.1016/j.carbpol.2018.02.057. [DOI] [PubMed] [Google Scholar]
- 73.Park H., Choi B., Hu J., Lee M. Injectable chitosan hyaluronic acid hydrogels for cartilage tissue engineering. Acta Biomater. 2013;9:4779–4786. doi: 10.1016/j.actbio.2012.08.033. [DOI] [PubMed] [Google Scholar]
- 74.Choi B., Kim S., Lin B., Wu B.M., Lee M. Cartilaginous extracellular matrix-modified chitosan hydrogels for cartilage tissue engineering. ACS Appl. Mater. Interfaces. 2014;6:20110–20121. doi: 10.1021/am505723k. [DOI] [PubMed] [Google Scholar]
- 75.Zhou Y., Gao H.L., Shen L.L., Pan Z., Mao L.B., Wu T. Chitosan microspheres with an extracellular matrix-mimicking nanofibrous structure as cell-carrier building blocks for bottom-up cartilage tissue engineering. Nanoscale. 2016;8:309–317. doi: 10.1039/c5nr06876b. [DOI] [PubMed] [Google Scholar]
- 76.Man Z., Hu X., Liu Z., Huang H., Meng Q., Zhang X. Transplantation of allogenic chondrocytes with chitosan hydrogel-demineralized bone matrix hybrid scaffold to repair rabbit cartilage injury. Biomaterials. 2016;108:157–167. doi: 10.1016/j.biomaterials.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 77.Mellati A., Kiamahalleh M.V., Madani S.H., Dai S., Bi J., Jin B. Poly(N-isopropylacrylamide) hydrogel/chitosan scaffold hybrid for three-dimensional stem cell culture and cartilage tissue engineering. J. Biomed. Mater. Res. 2016;104:2764–2774. doi: 10.1002/jbm.a.35810. [DOI] [PubMed] [Google Scholar]
- 78.Kashi M., Baghbani F., Moztarzadeh F., Mobasheri H., Kowsari E. Green synthesis of degradable conductive thermosensitive oligopyrrole/chitosan hydrogel intended for cartilage tissue engineering. Int. J. Biol. Macromol. 2018;107:1567–1575. doi: 10.1016/j.ijbiomac.2017.10.015. [DOI] [PubMed] [Google Scholar]
- 79.Liang X., Wang X., Xu Q., Lu Y., Zhang Y., Xia H. Rubbery chitosan/carrageenan hydrogels constructed through an electroneutrality system and their potential application as cartilage scaffolds. Biomacromolecules. 2018;19:340–352. doi: 10.1021/acs.biomac.7b01456. [DOI] [PubMed] [Google Scholar]
- 80.Meng Q., Man Z., Dai L., Huang H., Zhang X., Hu X. A composite scaffold of MSC affinity peptide-modified demineralized bone matrix particles and chitosan hydrogel for cartilage regeneration. Sci. Rep. 2015;5:17802. doi: 10.1038/srep17802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Choi B., Kim S., Lin B., Li K., Bezouglaia O., Kim J. Visible-light-initiated hydrogels preserving cartilage extracellular signaling for inducing chondrogenesis of mesenchymal stem cells. Acta Biomater. 2015;12:30–41. doi: 10.1016/j.actbio.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 82.Choi B., Kim S., Fan J., Kowalski T., Petrigliano F., Evseenko D. Covalently conjugated transforming growth factor-beta 1 in modular chitosan hydrogels for the effective treatment of articular cartilage defects. Biomater Sci. 2015;3:742–752. doi: 10.1039/c4bm00431k. [DOI] [PubMed] [Google Scholar]
- 83.Kim D.Y., Park H., Kim S.W., Lee J.W., Lee K.Y. Injectable hydrogels prepared from partially oxidized hyaluronate and glycol chitosan for chondrocyte encapsulation. Carbohydr. Polym. 2017;157:1281–1287. doi: 10.1016/j.carbpol.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 84.Caliari S.R., Burdick J.A. A practical guide to hydrogels for cell culture. Nat. Methods. 2016;13:405–414. doi: 10.1038/nmeth.3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Samorezov J.E., Morlock C.M., Alsberg E. Dual ionic and photo-crosslinked alginate hydrogels for micropatterned spatial control of material properties and cell behavior. Bioconjugate Chem. 2015;26:1339–1347. doi: 10.1021/acs.bioconjchem.5b00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Balakrishnan B., Joshi N., Jayakrishnan A., Banerjee R. Self-crosslinked oxidized alginate/gelatin hydrogel as injectable, adhesive biomimetic scaffolds for cartilage regeneration. Acta Biomater. 2014;10:3650–3663. doi: 10.1016/j.actbio.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 87.Yan S., Wang T., Feng L., Zhu J., Zhang K., Chen X. Injectable in situ self-cross-linking hydrogels based on poly(L-glutamic acid) and alginate for cartilage tissue engineering. Biomacromolecules. 2014;15:4495–4508. doi: 10.1021/bm501313t. [DOI] [PubMed] [Google Scholar]
- 88.Markstedt K., Mantas A., Tournier I., Martinez Avila H., Hagg D., Gatenholm P. 3D bioprinting human chondrocytes with nanocellulose-alginate bioink for cartilage tissue engineering applications. Biomacromolecules. 2015;16:1489–1496. doi: 10.1021/acs.biomac.5b00188. [DOI] [PubMed] [Google Scholar]
- 89.Muller M., Ozturk E., Arlov O., Gatenholm P., Zenobi-Wong M. Alginate sulfate-nanocellulose bioinks for cartilage bioprinting applications. Ann. Biomed. Eng. 2017;45:210–223. doi: 10.1007/s10439-016-1704-5. [DOI] [PubMed] [Google Scholar]
- 90.Park H., Lee H.J., An H., Lee K.Y. Alginate hydrogels modified with low molecular weight hyaluronate for cartilage regeneration. Carbohydr. Polym. 2017;162:100–107. doi: 10.1016/j.carbpol.2017.01.045. [DOI] [PubMed] [Google Scholar]
- 91.Gonzalez-Fernandez T., Tierney E.G., Cunniffe G.M., O'Brien F.J., Kelly D.J. Gene delivery of TGF-beta 3 and BMP2 in an MSC-laden alginate hydrogel for articular cartilage and endochondral bone tissue engineering. Tissue Eng. 2016;22:776–787. doi: 10.1089/ten.TEA.2015.0576. [DOI] [PubMed] [Google Scholar]
- 92.Huang G.P., Molina A., Tran N., Collins G., Arinzeh T.L. Investigating cellulose derived glycosaminoglycan mimetic scaffolds for cartilage tissue engineering applications. J Tissue Eng Regen Med. 2018;12:e592–e603. doi: 10.1002/term.2331. [DOI] [PubMed] [Google Scholar]
- 93.Boyer C., Figueiredo L., Pace R., Lesoeur J., Rouillon T., Visage C.L. Laponite nanoparticle-associated silated hydroxypropylmethyl cellulose as an injectable reinforced interpenetrating network hydrogel for cartilage tissue engineering. Acta Biomater. 2018;65:112–122. doi: 10.1016/j.actbio.2017.11.027. [DOI] [PubMed] [Google Scholar]
- 94.Rederstorff E., Rethore G., Weiss P., Sourice S., Beck-Cormier S., Mathieu E. Enriching a cellulose hydrogel with a biologically active marine exopolysaccharide for cell-based cartilage engineering. J Tissue Eng Regen Med. 2017;11:1152–1164. doi: 10.1002/term.2018. [DOI] [PubMed] [Google Scholar]
- 95.Martinez Avila H., Feldmann E.M., Pleumeekers M.M., Nimeskern L., Kuo W., de Jong W.C. Novel bilayer bacterial nanocellulose scaffold supports neocartilage formation in vitro and in vivo. Biomaterials. 2015;44:122–133. doi: 10.1016/j.biomaterials.2014.12.025. [DOI] [PubMed] [Google Scholar]
- 96.Zhang L., Zheng L., Fan H.S., Zhang X.D. A scaffold-filter model for studying the chondrogenic differentiation of stem cells in vitro. Mater Sci Eng C Mater Biol Appl. 2017;70:962–968. doi: 10.1016/j.msec.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 97.Lazarini M., Bordeaux-Rego P., Giardini-Rosa R., Duarte A.S.S., Baratti M.O., Zorzi A.R. Natural type II collagen hydrogel, fibrin sealant, and adipose-derived stem cells as a promising combination for articular cartilage repair. Cartilage. 2017;8:439–443. doi: 10.1177/1947603516675914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Parmar P.A., St-Pierre J.P., Chow L.W., Spicer C.D., Stoichevska V., Peng Y.Y. Enhanced articular cartilage by human mesenchymal stem cells in enzymatically mediated transiently RGDS-functionalized collagen-mimetic hydrogels. Acta Biomater. 2017;51:75–88. doi: 10.1016/j.actbio.2017.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yuan T., Zhang L., Li K., Fan H., Fan Y., Liang J. Collagen hydrogel as an immunomodulatory scaffold in cartilage tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2014;102:337–344. doi: 10.1002/jbm.b.33011. [DOI] [PubMed] [Google Scholar]
- 100.Wong C.C., Chen C.H., Chiu L.H., Tsuang Y.H., Bai M.Y., Chung R.J. Facilitating in vivo articular cartilage repair by tissue-engineered cartilage grafts produced from auricular chondrocytes. Am. J. Sports Med. 2018;46:713–727. doi: 10.1177/0363546517741306. [DOI] [PubMed] [Google Scholar]
- 101.Jiang X., Huang X., Jiang T., Zheng L., Zhao J., Zhang X. The role of Sox9 in collagen hydrogel-mediated chondrogenic differentiation of adult mesenchymal stem cells (MSCs) Biomater Sci. 2018;6:1556–1568. doi: 10.1039/c8bm00317c. [DOI] [PubMed] [Google Scholar]
- 102.Jiang X., Liu J., Liu Q., Lu Z., Zheng L., Zhao J. Therapy for cartilage defects: functional ectopic cartilage constructed by cartilage-simulating collagen, chondroitin sulfate and hyaluronic acid (CCH) hybrid hydrogel with allogeneic chondrocytes. Biomater Sci. 2018;6:1616–1626. doi: 10.1039/c8bm00354h. [DOI] [PubMed] [Google Scholar]
- 103.Zhang N., Lock J., Sallee A., Liu H. Magnetic nanocomposite hydrogel for potential cartilage tissue engineering: synthesis, characterization, and cytocompatibility with bone marrow derived mesenchymal stem cells. ACS Appl. Mater. Interfaces. 2015;7:20987–20998. doi: 10.1021/acsami.5b06939. [DOI] [PubMed] [Google Scholar]
- 104.Han L., Xu J.L., Lu X., Gan D.L., Wang Z.X., Wang K.F. Biohybrid methacrylated gelatin/polyacrylamide hydrogels for cartilage repair. J. Mater. Chem. B. 2017;5:731–741. doi: 10.1039/c6tb02348g. [DOI] [PubMed] [Google Scholar]
- 105.Song K., Li L., Li W., Zhu Y., Jiao Z., Lim M. Three-dimensional dynamic fabrication of engineered cartilage based on chitosan/gelatin hybrid hydrogel scaffold in a spinner flask with a special designed steel frame. Mater Sci Eng C Mater Biol Appl. 2015;55:384–392. doi: 10.1016/j.msec.2015.05.062. [DOI] [PubMed] [Google Scholar]
- 106.Lin H., Cheng A.W., Alexander P.G., Beck A.M., Tuan R.S. Cartilage tissue engineering application of injectable gelatin hydrogel with in situ visible-light-activated gelation capability in both air and aqueous solution. Tissue Eng. 2014;20:2402–2411. doi: 10.1089/ten.tea.2013.0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang L.S., Du C., Toh W.S., Wan A.C., Gao S.J., Kurisawa M. Modulation of chondrocyte functions and stiffness-dependent cartilage repair using an injectable enzymatically crosslinked hydrogel with tunable mechanical properties. Biomaterials. 2014;35:2207–2217. doi: 10.1016/j.biomaterials.2013.11.070. [DOI] [PubMed] [Google Scholar]
- 108.Saghebasl S., Davaran S., Rahbarghazi R., Montaseri A., Salehi R., Ramazani A. Synthesis and in vitro evaluation of thermosensitive hydrogel scaffolds based on (PNIPAAm-PCL-PEG-PCL-PNIPAAm)/Gelatin and (PCL-PEG-PCL)/Gelatin for use in cartilage tissue engineering. J. Biomater. Sci. Polym. Ed. 2018;29:1185–1206. doi: 10.1080/09205063.2018.1447627. [DOI] [PubMed] [Google Scholar]
- 109.Rothrauff B.B., Coluccino L., Gottardi R., Ceseracciu L., Scaglione S., Goldoni L. Efficacy of thermoresponsive, photocrosslinkable hydrogels derived from decellularized tendon and cartilage extracellular matrix for cartilage tissue engineering. J Tissue Eng Regen Med. 2018;12:e159–e170. doi: 10.1002/term.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li F., Truong V.X., Thissen H., Frith J.E., Forsythe J.S. Microfluidic encapsulation of human mesenchymal stem cells for articular cartilage tissue regeneration. ACS Appl. Mater. Interfaces. 2017;9:8589–8601. doi: 10.1021/acsami.7b00728. [DOI] [PubMed] [Google Scholar]
- 111.Zhou Y., Liang K., Zhao S., Zhang C., Li J., Yang H. Photopolymerized maleilated chitosan/methacrylated silk fibroin micro/nanocomposite hydrogels as potential scaffolds for cartilage tissue engineering. Int. J. Biol. Macromol. 2018;108:383–390. doi: 10.1016/j.ijbiomac.2017.12.032. [DOI] [PubMed] [Google Scholar]
- 112.Lee J.M., Sultan M.T., Kim S.H., Kumar V., Yeon Y.K., Lee O.J. Artificial auricular cartilage using silk fibroin and polyvinyl alcohol hydrogel. Int. J. Mol. Sci. 2017;18 doi: 10.3390/ijms18081707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ribeiro V.P., da Silva Morais A., Maia F.R., Canadas R.F., Costa J.B., Oliveira A.L. Combinatory approach for developing silk fibroin scaffolds for cartilage regeneration. Acta Biomater. 2018;72:167–181. doi: 10.1016/j.actbio.2018.03.047. [DOI] [PubMed] [Google Scholar]
- 114.Singh Y.P., Bhardwaj N., Mandal B.B. Potential of agarose/silk fibroin blended hydrogel for in vitro cartilage tissue engineering. ACS Appl. Mater. Interfaces. 2016;8:21236–21249. doi: 10.1021/acsami.6b08285. [DOI] [PubMed] [Google Scholar]
- 115.Yodmuang S., McNamara S.L., Nover A.B., Mandal B.B., Agarwal M., Kelly T.A. Silk microfiber-reinforced silk hydrogel composites for functional cartilage tissue repair. Acta Biomater. 2015;11:27–36. doi: 10.1016/j.actbio.2014.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ribeiro V.P., Pina S., Oliveira J.M., Reis R.L. Silk fibroin-based hydrogels and scaffolds for osteochondral repair and regeneration. Adv. Exp. Med. Biol. 2018;1058:305–325. doi: 10.1007/978-3-319-76711-6_14. [DOI] [PubMed] [Google Scholar]
- 117.Qi C., Liu J., Jin Y., Xu L., Wang G., Wang Z. Photo-crosslinkable, injectable sericin hydrogel as 3D biomimetic extracellular matrix for minimally invasive repairing cartilage. Biomaterials. 2018;163:89–104. doi: 10.1016/j.biomaterials.2018.02.016. [DOI] [PubMed] [Google Scholar]
- 118.Almeida H.V., Eswaramoorthy R., Cunniffe G.M., Buckley C.T., O'Brien F.J., Kelly D.J. Fibrin hydrogels functionalized with cartilage extracellular matrix and incorporating freshly isolated stromal cells as an injectable for cartilage regeneration. Acta Biomater. 2016;36:55–62. doi: 10.1016/j.actbio.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 119.Snyder T.N., Madhavan K., Intrator M., Dregalla R.C., Park D. A fibrin/hyaluronic acid hydrogel for the delivery of mesenchymal stem cells and potential for articular cartilage repair. J. Biol. Eng. 2014;8:10. doi: 10.1186/1754-1611-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hastar N., Arslan E., Guler M.O., Tekinay A.B. Peptide-based materials for cartilage tissue regeneration. In: Sunna A., Care A., Bergquist P.L., editors. Peptides and Peptide-Based Biomaterials and Their Biomedical Applications. Springer International Publishing; Cham: 2017. pp. 155–166. [Google Scholar]
- 121.He R., Wang B., Cui M., Xiong Z., Lin H., Zhao L. Link protein N-terminal peptide as a potential stimulating factor for stem cell-based cartilage regeneration. Stem Cell. Int. 2018;2018:3217895. doi: 10.1155/2018/3217895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Callahan L.A., Ganios A.M., Childers E.P., Weiner S.D., Becker M.L. Primary human chondrocyte extracellular matrix formation and phenotype maintenance using RGD-derivatized PEGDM hydrogels possessing a continuous Young's modulus gradient. Acta Biomater. 2013;9:6095–6104. doi: 10.1016/j.actbio.2012.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gao G., Yonezawa T., Hubbell K., Dai G., Cui X. Inkjet-bioprinted acrylated peptides and PEG hydrogel with human mesenchymal stem cells promote robust bone and cartilage formation with minimal printhead clogging. Biotechnol. J. 2015;10:1568–1577. doi: 10.1002/biot.201400635. [DOI] [PubMed] [Google Scholar]
- 124.Liu H., Cheng Y., Chen J., Chang F., Wang J., Ding J. Component effect of stem cell-loaded thermosensitive polypeptide hydrogels on cartilage repair. Acta Biomater. 2018;73:103–111. doi: 10.1016/j.actbio.2018.04.035. [DOI] [PubMed] [Google Scholar]
- 125.Parmar P.A., Chow L.W., St-Pierre J.P., Horejs C.M., Peng Y.Y., Werkmeister J.A. Collagen-mimetic peptide-modifiable hydrogels for articular cartilage regeneration. Biomaterials. 2015;54:213–225. doi: 10.1016/j.biomaterials.2015.02.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kim H.D., Heo J., Hwang Y., Kwak S.Y., Park O.K., Kim H. Extracellular-matrix-based and Arg-Gly-Asp-modified photopolymerizing hydrogels for cartilage tissue engineering. Tissue Eng. 2015;21:757–766. doi: 10.1089/ten.tea.2014.0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Alakpa E.V., Jayawarna V., Burgess K.E.V., West C.C., Peault B., Ulijn R.V. Improving cartilage phenotype from differentiated pericytes in tunable peptide hydrogels. Sci. Rep. 2017;7:6895. doi: 10.1038/s41598-017-07255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lu J., Shen X., Sun X., Yin H., Yang S., Lu C. Increased recruitment of endogenous stem cells and chondrogenic differentiation by a composite scaffold containing bone marrow homing peptide for cartilage regeneration. Theranostics. 2018;8:5039–5058. doi: 10.7150/thno.26981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Aisenbrey E.A., Tomaschke A., Kleinjan E., Muralidharan A., Pascual-Garrido C., McLeod R.R. A stereolithography-based 3D printed hybrid scaffold for in situ cartilage defect repair. Macromol. Biosci. 2018;18 doi: 10.1002/mabi.201700267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhu W., Cui H., Boualam B., Masood F., Flynn E., Rao R.D. 3D bioprinting mesenchymal stem cell-laden construct with core-shell nanospheres for cartilage tissue engineering. Nanotechnology. 2018;29:185101. doi: 10.1088/1361-6528/aaafa1. [DOI] [PubMed] [Google Scholar]
- 131.Costantini M., Idaszek J., Szoke K., Jaroszewicz J., Dentini M., Barbetta A. 3D bioprinting of BM-MSCs-loaded ECM biomimetic hydrogels for in vitro neocartilage formation. Biofabrication. 2016;8 doi: 10.1088/1758-5090/8/3/035002. 035002. [DOI] [PubMed] [Google Scholar]
- 132.Levato R., Jungst T., Scheuring R.G., Blunk T., Groll J., Malda J. From Shape to Function: the next step in bioprinting. Adv. mater. 2020 doi: 10.1002/adma.201906423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Crawford D.C., Heveran C.M., Cannon W.D., Jr., Foo L.F., Potter H.G. An autologous cartilage tissue implant NeoCart for treatment of grade III chondral injury to the distal femur: prospective clinical safety trial at 2 years. Am. J. Sports Med. 2009;37:1334–1343. doi: 10.1177/0363546509333011. [DOI] [PubMed] [Google Scholar]
- 134.Anderson D.E., Williams R.J., 3rd, DeBerardino T.M., Taylor D.C., Ma C.B., Kane M.S. Magnetic resonance imaging characterization and clinical outcomes after NeoCart surgical therapy as a primary reparative treatment for knee cartilage injuries. Am. J. Sports Med. 2017;45:875–883. doi: 10.1177/0363546516677255. [DOI] [PubMed] [Google Scholar]
- 135.Petri M., Broese M., Liodakis E., Guenther D., Krettek C., Jagodzinski M. CaReS® (MACT) versus microfracture in treating symptomatic patellofemoral cartilage defects: a retrospective matched-pair analysis. J. Orthop. Sci. 2013;18:38–44. doi: 10.1007/s00776-012-0305-x. [DOI] [PubMed] [Google Scholar]
- 136.Sharma B., Williams C.G., Khan M., Manson P., Elisseeff J.H. In vivo chondrogenesis of mesenchymal stem cells in a photopolymerized hydrogel. Plast. Reconstr. Surg. 2007;119:112–120. doi: 10.1097/01.prs.0000236896.22479.52. [DOI] [PubMed] [Google Scholar]
- 137.Wolf M.T., Zhang H., Sharma B., Marcus N.A., Pietzner U., Fickert S. Cartilage; 2018. Two-year Follow-Up and Remodeling Kinetics of ChonDux Hydrogel for Full-Thickness Cartilage Defect Repair in the Knee. 1947603518800547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Park Y.-B., Ha C.-W., Lee C.-H., Yoon Y.C., Park Y.-G. Cartilage regeneration in osteoarthritic patients by a composite of allogeneic umbilical cord blood-derived mesenchymal stem cells and hyaluronate hydrogel: results from a clinical trial for safety and proof-of-concept with 7 years of extended follow-up. Stem Cells Transl. Med. 2017;6:613–621. doi: 10.5966/sctm.2016-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ha C.-W., Park Y.-B., Chung J.-Y., Park Y.-G. Cartilage repair using composites of human umbilical cord blood-derived mesenchymal stem cells and hyaluronic acid hydrogel in a minipig model. Stem Cells Transl. Med. 2015;4:1044–1051. doi: 10.5966/sctm.2014-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nie X., Chuah Y.J., Zhu W., He P., Peck Y., Wang D.A. Decellularized tissue engineered hyaline cartilage graft for articular cartilage repair. Biomaterials. 2020;235:119821. doi: 10.1016/j.biomaterials.2020.119821. [DOI] [PubMed] [Google Scholar]
- 141.Xuan H., Hu H., Geng C., Song J., Shen Y., Lei D. Biofunctionalized chondrogenic shape-memory ternary scaffolds for efficient cell-free cartilage regeneration. Acta Biomater. 2020;105:97–110. doi: 10.1016/j.actbio.2020.01.015. [DOI] [PubMed] [Google Scholar]
- 142.Gong J.P., Katsuyama Y., Kurokawa T., Osada Y. Double-network hydrogels with extremely high mechanical strength. Adv. Mater. 2003;15:1155. [Google Scholar]
- 143.Sun J.Y., Zhao X., Illeperuma W.R., Chaudhuri O., Oh K.H., Mooney D.J. Highly stretchable and tough hydrogels. Nature. 2012;489:133–136. doi: 10.1038/nature11409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Peters W., Fornasier V. Complications from injectable materials used for breast augmentation. Can. J. Plast. Surg. 2009;17:89–96. doi: 10.1177/229255030901700305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Amin S.P., Marmur E.S., Goldberg D.J. vol. 30. 2004. Complications from Injectable Polyacrylamide Gel, a New Nonbiodegradable Soft Tissue Filler; pp. 1507–1509. [DOI] [PubMed] [Google Scholar]
- 146.Huebsch N., Arany P.R., Mao A.S., Shvartsman D., Ali O.A., Bencherif S.A. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat. Mater. 2010;9:518–526. doi: 10.1038/nmat2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Engler A.J., Sen S., Sweeney H.L., Discher D.E. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 148.Li J., Celiz A.D., Yang J., Yang Q., Wamala I., Whyte W. Tough adhesives for diverse wet surfaces. Science. 2017;357:378–381. doi: 10.1126/science.aah6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhao Y., Wu Y., Wang L., Zhang M., Chen X., Liu M. Bio-inspired reversible underwater adhesive. Nat. Commun. 2017;8:2218. doi: 10.1038/s41467-017-02387-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pei X., Zhang H., Zhou Y., Zhou L., Fu J. Stretchable, self-healing and tissue-adhesive zwitterionic hydrogels as strain sensors for wireless monitoring of organ motions. Mater. Horiz. 2020;7(7) [Google Scholar]
- 151.Melin Furst C., Ahrman E., Bratteby K., Waldemarson S., Malmstrom J., Blom A.M. Quantitative mass spectrometry to study inflammatory cartilage degradation and resulting interactions with the complement system. J. Immunol. 2016;197:3415–3424. doi: 10.4049/jimmunol.1601006. [DOI] [PubMed] [Google Scholar]
- 152.Chow W.Y., Norman B.P., Roberts N.B., Ranganath L.R., Teutloff C., Bittl R. Pigmentation chemistry and radical-based collagen degradation in alkaptonuria and osteoarthritic cartilage. Angew Chem. Int. Ed. Engl. 2020;59(29):11937–11942. doi: 10.1002/anie.202000618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Men Y.T., Jiang Y.L., Chen L., Zhang C.Q., Ye J.D. On mechanical mechanism of damage evolution in articular cartilage. Mater Sci Eng C Mater Biol Appl. 2017;78:79–87. doi: 10.1016/j.msec.2017.03.289. [DOI] [PubMed] [Google Scholar]
- 154.Guermazi A., Hayashi D., Roemer F.W., Niu J., Quinn E.K., Crema M.D. Brief report: partial- and full-thickness focal cartilage defects contribute equally to development of new cartilage damage in knee osteoarthritis: the multicenter osteoarthritis study. Arthritis & rheumatology. 2017;69:560–564. doi: 10.1002/art.39970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tong X., Du L., Xu Q. Tough, adhesive and self-healing conductive 3D network hydrogel of physically linked functionalized-boron nitride/clay/poly(N-isopropylacrylamide) J. Mater. Chem. 2018;6:3091–3099. [Google Scholar]
- 156.Morelle X.P., Illeperuma W.R., Tian K., Bai R., Suo Z., Vlassak J.J. Highly stretchable and tough hydrogels below water freezing temperature. Adv. mater. 2018 doi: 10.1002/adma.201801541. [DOI] [PubMed] [Google Scholar]
- 157.Yang B., Wang C., Zhang Y., Ye L., Qian Y., Shu Y. A thermoresponsive poly(N-vinylcaprolactam-co-sulfobetaine methacrylate) zwitterionic hydrogel exhibiting switchable anti-biofouling and cytocompatibility. Polym. Chem. 2015;6:3431–3442. [Google Scholar]
- 158.Li B., Gao Y., Guo L., Fan Y., Kawazoe N., Fan H. Synthesis of photo-reactive poly (vinyl alcohol) and construction of scaffold-free cartilage like pellets in vitro. Regen. Biomater. 2018;5:159–166. doi: 10.1093/rb/rby009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Liu W., Deng C., McLaughlin C.R., Fagerholm P., Lagali N.S., Heyne B. Collagen-phosphorylcholine interpenetrating network hydrogels as corneal substitutes. Biomaterials. 2009;30:1551–1559. doi: 10.1016/j.biomaterials.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 160.Chua P.H., Neoh K.G., Kang E.T., Wang W. Surface functionalization of titanium with hyaluronic acid/chitosan polyelectrolyte multilayers and RGD for promoting osteoblast functions and inhibiting bacterial adhesion. Biomaterials. 2008;29:1412–1421. doi: 10.1016/j.biomaterials.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 161.Kim J., Kim I.S., Cho T.H., Lee K.B., Hwang S.J., Tae G. Bone regeneration using hyaluronic acid-based hydrogel with bone morphogenic protein-2 and human mesenchymal stem cells. Biomaterials. 2007;28:1830–1837. doi: 10.1016/j.biomaterials.2006.11.050. [DOI] [PubMed] [Google Scholar]
- 162.Yang Y., Wang X., Yang F., Shen H., Wu D. A universal soaking strategy to convert composite hydrogels into extremely tough and rapidly recoverable double-network hydrogels. Adv. Mater. 2016;28(33):7178–7184. doi: 10.1002/adma.201601742. [DOI] [PubMed] [Google Scholar]
- 163.Ravichandran R., Islam M.M., Alarcon E.I., Samanta A., Wang S., Lundström P. Functionalised type-I collagen as a hydrogel building block for bio-orthogonal tissue engineering applications. J. Mater. Chem. B. 2016;4:318–326. doi: 10.1039/c5tb02035b. [DOI] [PubMed] [Google Scholar]
- 164.Sawatjui N., Damrongrungruang T., Leeanansaksiri W., Jearanaikoon P., Hongeng S., Limpaiboon T. Silk fibroin/gelatin-chondroitin sulfate-hyaluronic acid effectively enhances in vitro chondrogenesis of bone marrow mesenchymal stem cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2015;52:90–96. doi: 10.1016/j.msec.2015.03.043. [DOI] [PubMed] [Google Scholar]
- 165.Liu J., Qi C., Tao K., Zhang J., Zhang J., Xu L. Sericin/dextran injectable hydrogel as an optically trackable drug delivery system for malignant melanoma treatment. ACS Appl. Mater. Interfaces. 2016;8:6411–6422. doi: 10.1021/acsami.6b00959. [DOI] [PubMed] [Google Scholar]