Abstract
Purpose
Oral Lichen planus is a potentially malignant autoimmune disorder, characterized by burning and pain in the affected mucosa which reduces the quality and comfort of patient's life. Various treatment modalities have been documented for OLP but due its malignant potential the alternative therapeutic approaches with least or no side effects are being in demand. One of such, yet unexplored treatment is Platelet rich plasma (PRP). This study aims to evaluate the effectiveness of intralesional PRP as compared to the Intralesional corticosteroids in the management of erosive OLP.
Materials and methods
The study sample consisted of 20 clinically and histopathologically confirmed patients of Erosive OLP among which 10 patients were given intralesional corticosteroids and 10 patients were administered intralesional PRP. All the patients were given weekly injections for 2 months and were followed up till 4 months for assessing the parameters such as pain/burning, erythema and size of the lesions.
Results
The patients in both the groups showed a statistically significant reduction in all the assessed parameters of erosive lichen planus from baseline till 4 months of treatment and follow up. However, on comparison of the pain reduction, size of lesion and erythema scores between the two groups, the difference was found to be statistically insignificant.
Conclusion
The intralesional PRP was found to be of comparative effectiveness with respect to intralesional triamcinolone acetonide in the management of erosive OLP and with an added advantage of having less recurrence and no adverse effects.
Keywords: Erosive lichen planus, Corticosteroids, Platelet rich plasma, Potentially malignant disorder
1. Introduction
Lichen planus is a chronic, potentially malignant, mucocutaneous, inflammatory, immunological disease having preponderance towards the middle aged females, that frequently affects the oral mucosa and it usually tends to be more persistent and immune to the treatment. Oral lichen planus (OLP) has been estimated to affect 0.5%–2% of the general population.1 It can present clinically in 6 different patterns: papular, reticular, plaque, atrophic, erosive and bullous, each having specific characteristics. The erosive form, despite having lower prevalence, presents greater clinical significance and form a major concern for the dentists as these lesions are usually symptomatic and are reported to be potentially malignant.
OLP has multifactorial pathogenesis involving both antigen-specific and non-specific mechanisms like antigen specific keratinocyte killing by CD8+ cytotoxic T-cells and other mechanisms such as mast cell degranulation and matrix metalloproteinase (MMP) activation. The chronicity of this potentially malignant disease may be attributed to the deficient antigen specific TGF-B1 mediated immunosuppression.3
Various treatment modalities for OLP have been documented, such as corticosteroids, multivitamins, antioxidants, topical anesthetic agents and many more but no treatment modality has yet been proven to be a sole effective measure for controlling the disease. Corticosteroids have been considered the first-choice pharmacotherapy for managing OLP due to their anti-inflammatory and anti-immunologic properties.4 Intralesional corticosteroids (ILS) have been proven to be effective in managing OLP. Although, ILS maintains high concentration of the drug at the site, yet continuous and prolonged use is associated with many systemic adverse effects such as bad taste, nausea, facial swellings, dryness, mucosal atrophy, candidal infection, granuloma formation, hypersensitivity reactions, delayed wound healing and in later stages; hypothalamus pituitary adrenal suppression.5,6
Therefore, this is the need of an hour to establish an effective and efficient treatment modality for erosive OLP with lesser or no adverse effects. Platelet Rich Plasma is emerging as an increasingly demanding clinical application as an alternative source of growth factors for several dental procedures and oral mucosal lesion. PRP is concentrated plasma of the patient's blood that predominantly contains high concentration of platelets along with increased coagulation factors.7 Activated platelets release various growth factors such as platelet-derived growth factors, transforming growth factor, fibroblast growth factor and vascular endothelial growth factors, contributing and leading to cell proliferation, differentiation, neoangiogenesis, withdrawal of toxins and cellular regeneration.7,8 PRP decreases the associate morbidity and accelerates wound healing with an anti-inflammatory action. PRP is not associated with any adverse effects and is free from any immunological or allergic reactions.9
To best of our knowledge no study has yet been conducted to prove its potential role and therapeutic effect in the management of erosive OLP. In view of this; the present study was planned to assess and compare the effectiveness of PRP and intralesional steroids in the management of erosive lichen planus.
2. Methodology
The present study was conducted on clinically & histopathologically diagnosed patients of erosive OLP, reporting to the OPD of department of Oral medicine of a reputed dental college. The institutional ethical committee clearance was obtained before commencing the study. All the patients were well explained about the study and a written informed consent was taken. The study sample consisted of a total number of 20 patients of erosive OLP; randomly divided into two groups of 10 patients each. The study design was prospective, case control, randomized clinical trial.
Inclusion criteria consisted of clinically and histopathologically confirmed erosive OLP patients who consented to participate in the study and had not undergone any treatment in the past for the disease. Patients having history of drugs that could lead to Lichenoid reaction, anticoagulant therapy or non-steroidal anti-inflammatory drugs in the past 5 days were excluded. Patients with any Systemic disorders, immunocompromised patients and pregnant females were also excluded.
A complete clinical examination was performed and all the parameters were recorded in an evaluation sheet. The Complete blood count report of all the patients was assessed, especially in terms of platelet counts. The scores for the assessed parameters such as pain, burning, size/extent of lesion and erythema were determined by one blinded Oral medicine expert and the injection therapy was given by another blinded oral medicine expert.
Pain and Burning sensation on VAS was assessed with the grading of 0–10 in which 0 was counted as no burning sensation on VAS and 10 was counted as severe burning sensation. The ulceration and erythema were quantified based on severity and scores of 0 were given for normal.
Mucosa, 1 was given for mild erythema, 2 for moderate erythema and a score of 3 for severe erythema. Similarly, the size of the lesion was scored as 0 for normal mucosa, 1 for size up to 0.25 cm2, 2 for area up to 1 cm2and 3 for lesions larger than 1 cm2 area.10
Group 1 patients were given bilateral intralesional injections with 10 mg/ml triamcinolone acetonide weekly for a period of 8 weeks. For Group 2 patients, autologous Protein rich plasma was prepared from the patient's blood using the centrifugal machine, working on 220 V AC, 50 Hz with built in 5 speed regulator and maximum speed of 3500 rpm. Three percentage of calcium chloride was added to the obtained PRP in a ratio of 1:10 for the induction of platelet activation.
The injections in both groups were given after a field block with local anesthetic with vasoconstrictor. 0.5 ml of either corticosteroid or PRP was injected per 1cm2 of involved mucosa using a 25 gauge needle bilaterally. The same treatment protocol for each group was followed for consecutive 8 weeks and all the parameters were recorded at each visit. All the patients were followed up for next 2 months to assess the endpoint measures of the lesion. All the data obtained was subjected to Statistical analysis using SPSS (Statistical Package for Social Sciences) software.
3. Results
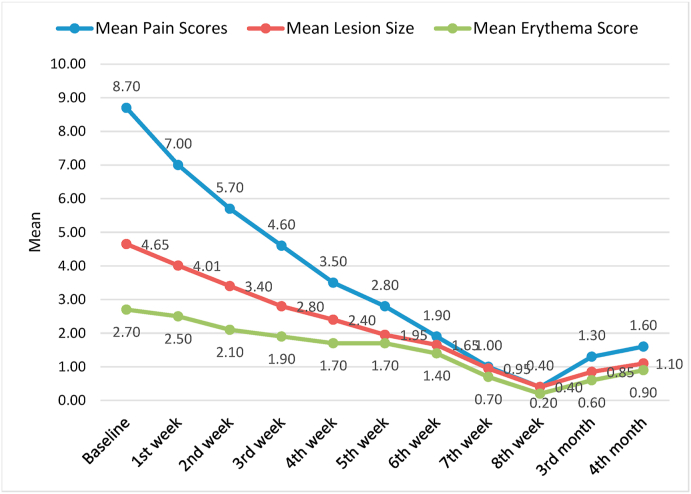
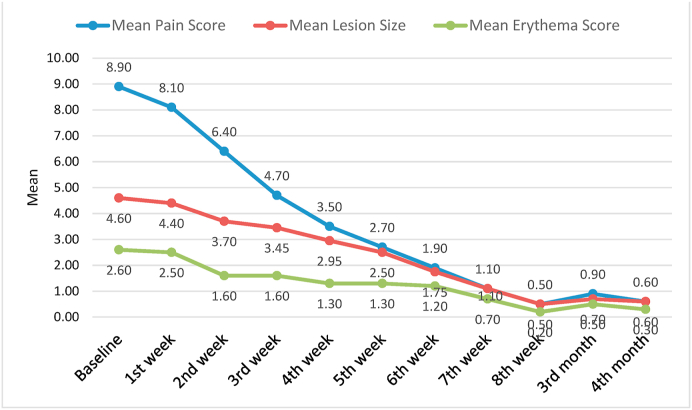
Table 1 depicts the mean values of pain scores based on VAS assessment in steroid group and PRP group at different follow up intervals starting from the baseline till the 4 months of follow up. The baseline mean pain scores of steroid and PRP group were 8.70 and 8.90 respectively.
Table 1.
Mean pain scores and p value at different follow up intervals in both the groups.
| Mean Pain Scores |
Unpaired T-test |
||||
|---|---|---|---|---|---|
| Group (N = 10) | Mean | Std. Deviation | P value | Significance | |
| Day 0 | Steriod Group | 8.70 | .94 | .651 | Not significant |
| PRP Group | 8.90 | .99 | .003 | ||
| 1st week | Steriod | 7.00 | .66 | .035 | |
| PRP | 8.10 | .73 | .791 | ||
| 2nd week | Steriod | 5.70 | .67 | 1.000 | |
| PRP | 6.40 | .69 | .736 | ||
| 3rd week | Steriod | 4.60 | .84 | 1.000 | |
| PRP | 4.70 | .82 | .722 | ||
| 4th week | Steroid | 3.50 | .70 | .673 | |
| PRP | 3.50 | .52 | .579 | ||
| 5th week | Steroid | 2.80 | .78 | .216 | |
| PRP | 2.70 | .48 | .703 | ||
| 6th week | Steroid | 1.90 | .56 | .561 | |
| PRP | 1.90 | .56 | .214 | ||
| 7th week | Steroid | 1.00 | .66 | .651 | |
| PRP | 1.10 | .56 | .003 | ||
| 8th week | Steroid | .40 | .51 | .035 | |
| PRP | .50 | .52 | .791 | ||
| 3rd month | Steroid | 1.30 | 1.6 | 1.000 | |
| PRP | .90 | 1.52 | .736 | ||
| 4th month | Steroid | 1.60 | 2.27 | 1.000 | |
| PRP | .60 | .96 | .722 | ||
| %Reduction Day 0–2month | Steroid | 95.2778 | 6.11 | .673 | |
| PRP | 94.2103 | 6.22 | .579 | ||
| %Reduction 0–3month | Steroid | 85.8611 | 17.38 | .216 | |
| PRP | 90.2103 | 15.36 | .703 | ||
| %Reduction 0–4month | Steriod | 82.5556 | 25.02153 | .561 | |
| PRP | 93.5278 | 9.91221 | .214 | ||
Percentage reduction of pain assessed at 4th month in group I and Group II compared to baseline was found to be 82.55% and 93.5% respectively. The table also depicts the comparison in both the groups where a trend of reduction in the mean pain scores was observed in both steroid and PRP group but the comparative p values were found to be insignificant.
Table 2 depicts the mean values of lesion size and percentage reduction of size in both steroid group and PRP group at different follow up intervals starting from the baseline till the 4 months of follow up. Table also depicts the comparison of mean size of the lesions in both the groups where a trend of reduction in the mean lesion size was observed in both steroid and PRP group but the comparative p values were found to be insignificant.
Table 2.
Mean Lesion size at different follow up intervals in both the groups with comparative p-values.
| Mean Lesion size |
Unpaired T-test |
||||
|---|---|---|---|---|---|
| Group (N = 10) | Mean | Std. Deviation | P value | Significance | |
| Day 0 | Steriod | 4.650 | .6258 | .892 | Not significant |
| PRP Group | 4.60 | .9661 | .455 | ||
| 1st week | Steriod | 4.01 | .633 | .464 | |
| PRP | 4.40 | 1.487 | .083 | ||
| 2nd week | Steriod | 3.400 | .6992 | .049 | |
| PRP | 3.700 | 1.0593 | .027 | ||
| 3rd week | Steriod | 2.800 | .6749 | .695 | |
| PRP | 3.450 | .8960 | .572 | ||
| 4th week | Steroid | 2.40 | .615 | .673 | |
| PRP | 2.95 | .550 | .719 | ||
| 5th week | Steroid | 1.95 | .497 | .318 | |
| PRP | 2.50 | .527 | .566 | ||
| 6th week | Steroid | 1.650 | .6687 | .744 | |
| PRP | 1.750 | .4249 | .329 | ||
| 7th week | Steroid | .950 | .5986 | .892 | |
| PRP | 1.100 | .5676 | .455 | ||
| 8th week | Steroid | .40 | .516 | .464 | |
| PRP | .50 | .527 | .083 | ||
| 3rd month | Steroid | .85 | .883 | .049 | |
| PRP | .70 | .949 | .027 | ||
| 4th month | Steroid | 1.10 | 1.197 | .695 | |
| PRP | .60 | .966 | .572 | ||
| %Reduction Day 0–2month | Steroid | 91.7778 | 10.63421 | .673 | |
| PRP | 88.7121 | 12.70907 | .719 | ||
| %Reduction 0–3month | Steroid | 82.1692 | 17.94899 | .318 | |
| PRP | 84.8939 | 18.73786 | .566 | ||
| %Reduction 0–4month | Steriod | 76.3788 | 25.30927 | .744 | |
| PRP | 86.4899 | 19.37902 | .329 | ||
Table 3 depicts the mean erythema and ulceration scores in steroid and PRP group at different follow up intervals. Percentage reduction of erythema at 2 months follow up in group I and Group II was found to be 91.66% and 93.33% respectively. When the comparison of mean erythema reduction was assessed in both groups the p values were found to be insignificant.
Table 3.
Mean erythema scores at different follow up intervals in both the groups.
| Mean Erythema scores |
Unpaired T-test |
||||
|---|---|---|---|---|---|
| Group (N = 10) | Mean | Std. Deviation | P value | Significance | |
| Day 0 | Steriod Group | 2.70 | .483 | 1.000 | Not significant |
| PRP Group | 2.60 | .483 | 1.000 | ||
| 1st week | Steriod | 2.50 | .527 | .018 | |
| PRP | 2.50 | .527 | .135 | ||
| 2nd week | Steriod | 2.10 | .316 | .081 | |
| PRP | 1.60 | .516 | .202 | ||
| 3rd week | Steriod | 1.90 | .316 | .449 | |
| PRP | 1.60 | .516 | 1.000 | ||
| 4th week | Steroid | 1.70 | .483 | 1.000 | |
| PRP | 1.30 | .483 | .777 | ||
| 5th week | Steroid | 1.70 | .823 | .132 | |
| PRP | 1.30 | .483 | .820 | ||
| 6th week | Steroid | 1.40 | .516 | .789 | |
| PRP | 1.20 | .632 | .132 | ||
| 7th week | Steroid | .70 | .483 | 1.000 | |
| PRP | .70 | .675 | 1.000 | ||
| 8th week | Steroid | .20 | .422 | .018 | |
| PRP | .20 | .422 | .135 | ||
| 3rd month | Steroid | .60 | .843 | .081 | |
| PRP | .50 | .707 | .202 | ||
| 4th month | Steroid | .90 | 1.101 | .449 | |
| PRP | .30 | .483 | 1.000 | ||
| %Reduction Day 0–2month | Steroid | 91.6667 | 18.00206 | 1.000 | |
| PRP | 93.3333 | 14.05457 | .777 | ||
| %Reduction 0–3month | Steroid | 78.3333 | 29.44969 | .132 | |
| PRP | 81.6667 | 25.39807 | .820 | ||
| %Reduction 0–4month | Steriod | 70.0000 | 36.68350 | .789 | |
| PRP | 90.0000 | 16.10153 | .132 | ||
The graph 1 and graph 2 shows the comparative assessment of all the three parameters at different time intervals in the steriod and PRP group respectively.
Graph 1.
Mean scores of pain, lesion size and erythema at different follow up intervals in steriod group.
Graph 2.
Mean scores of pain, lesion size and erythema at different follow up intervals in PRP group.
4. Discussion
OLP is one of the most common potentially malignant oral disorder believed to be an autoimmune pathology mainly characterized as a white lesion with wickham's stria causing burning and pain in the affected oral mucosa leading to discomfort and morbidity of the patients. Being a multifactorial disease; lichen planus currently has no singe established cure or treatment.
Out of various treatment modalities available for the Lichen planus; corticosteroids have been effectively and extensively been used but have a major disadvantage of potential side effects. On the contrary to corticosteroids; PRP has better safety profile and least or no adverse effects. It has a diversity of therapeutic properties including antioxidant, anti-inflammatory and Immunomodulatory effect justifying its usage in cases of Erosive OLP.9,11
A total of 20 clinically and histopathologically confirmed erosive OLP patients were divided into control (corticosteroid) and study groups (PRP group) with 10 patients each. Study sample consisted of 18 females and 2 males with one male patient in each group. Present study reported 90% of the patients to be females, which is in accordance with the literature where maximum studies report female predominance of lichen planus which may be attributed to high proneness to stress and hormonal disturbances in women.1,3
The age range of the study sample was found to be 28–60 years with a mean age of 44.5 years. According to the studies of Chitturi et al.,12 and Mostafa and Ahmed13 the mean age was found to be 45.72 years and 43 years respectively. This slight difference in mean age could be attributed to study sample belonging to different geographic region and ethnicity. Maximum cases in our study involved the buccal mucosa as the affected site.
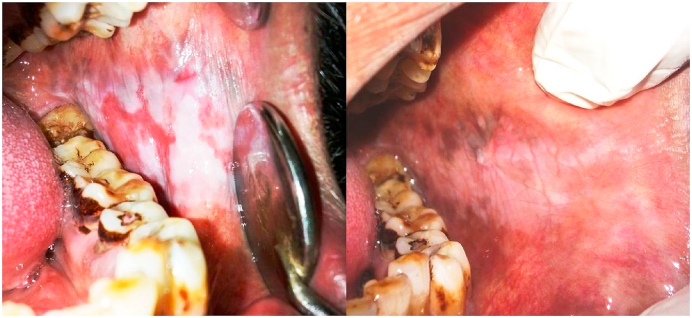
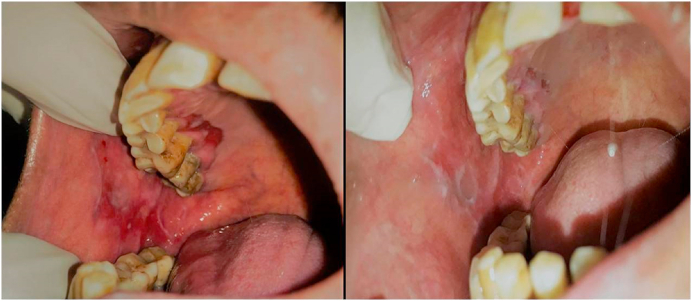
The patients in both the Steroid and PRP group showed a statistically significant improvement (P < 0.000) in all the assessed parameters from the baseline to second month of treatment and further 2 months of follow up visits. Both the modalities were observed to be highly effective in management of erosive lichen planus (Fig. 1, Fig. 2).
Fig. 1.
Erosive OLP; Pre and post treatment after 8 weeks with Corticosteroids.
Fig. 2.
Erosive OLP; Pre and post treatment after 8 weeks with PRP.
Extensive past research has proven effectiveness of intralesional steroids in treatment of Oral Lichen planus such as studies conducted by Liu, Chuanxia et al. and Lee, Young Chan et al. Xia, and Juan et al. which observed similar positive results as the present study.5,14,15
However, on comparing both the groups, no statistically significant differences were observed between the two groups. In other words, when two groups were compared, both the steroid and PRP therapy was found to be equally effective in managing erosive lichen planus.
Among 10 patients in the PRP group, 9 patients reported complete resolution of erythema by the 8th week of treatment. Among 10 patients in the steriod group, 7 patients reported complete resolution of erythema by the end of treatment i.e 2 months. Elisabetta Merigo et al.,16 reported a case of non responding Lichen planus treated with rinses of platelet rich plasma. The patient was relieved of symptoms after PRP therapy where other management modalities like steroids, Lasers and hydroxychloroquine fail to provide relief. Thus PRP could be an effective treatment in patients resistant to conventional therapies in OLP.
The use PRP in erosive OLP was found to be comparative and equivalent in its effectiveness to corticosteroids. Although, during follow up for next 2 months after treatment; the patients treated with PRP showed no or less recurrence with only 1 patient out of 10 showed mild erythema and slight burning in the 15th week. In the corticosteroid group, during follow up, 3 patients out of 10 showed recurrence of the lesion with increased VAS scores of pain and erythema as compared to 8th week.
Also, there were mild side effects noted in 2 patients in steroid group but the acknowledging fact is that none of the patients treated with PRP accorded any kind of adverse effects. One more interesting observation was that during the initial weeks of treatment, PRP showed slightly less reduction in assessed parameters as compared to steroid group although statistically insignificant but during later phase of treatment PRP gave equally or better comparative results than steroids which could be due to difference in mechanism of action of both modalities.
As all the patients were given intralesional injections, one of the important advantage of the study was non dependence on the patient compliance. All the patients were followed up for duration of four months thus recurrences and any side effects could be elicited.
Further studies with a larger sample size would allow for stronger prediction effectiveness of PRP in OLP and will further authenticate the effectiveness of PRP in erosive OLP. In the present study, the blood was collected every week for fresh PRP preparation. For some patients, it might be a concern to provide blood sample every week for PRP preparation. Thus in future, some methodology which can allow collected blood to be used multiple times for preparing PRP could prove to be more beneficial.
5. Conclusion
To the best of our knowledge, this is the first clinical trial to be conducted using intralesional PRP in the management of OLP. PRP was found to be of equal comparative effectiveness with respect to corticosteroids in managing potentially malignant erosive OLP. Owing to the anti-inflammatory effects, healing stimulating properties and biologically safe nature of PRP, it could be used as a novel alternative management therapy in Oral Lichen planus.
Source of funding
This research did not receive any specific grant from any funding agencies in public, commercial or not for profit sector.
Declaration of competing interest
None.
Acknowledgements
None.
Contributor Information
Upasana Sethi Ahuja, Email: upasanasethi03@gmail.com.
Nidhi Puri, Email: drnidhipuri16@gmail.com.
Chandramani B. More, Email: drchandramanimore@gmail.com.
Ritu Gupta, Email: drritupcd@gmail.com.
Deepak Gupta, Email: drdeepak_26@rediffmail.com.
References
- 1.Edwards P.C., Kelsch R. Oral lichen planus: clinical presentation and management. Journal j can dental association. 2002;68(8):494–499. [PubMed] [Google Scholar]
- 2.Payeras M.R., Cherubini K., Figueiredo M.A., Salum F.G. Oral lichen planus: focus on etiopathogenesis. Arch Oral Biol. 2013 Sep;58(9):1057–1069. doi: 10.1016/j.archoralbio.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Sugerman P.B., Savage N.W., Walsh L.J., Zhao Z.Z., Zhou X.J., Khan A. Etal pathogenesis of oral lichen planus. Crit Rev Oral Biol Med. 2002;13(4):350–365. doi: 10.1177/154411130201300405. [DOI] [PubMed] [Google Scholar]
- 4.Lodi G., Scully C., Carrozzo M., Griffiths M., Sugerman P.B., Thongprasom K. Current controversies in oral lichen planus: report of an international consensus meeting. Clinical management and malignant transformation. OOOE. 2005;100(2):164–178. doi: 10.1016/j.tripleo.2004.06.076. [DOI] [PubMed] [Google Scholar]
- 5.Lee Y.C., Shin S.Y., Kim S.W., Eun Y.G. vol. 148. 2013. pp. 443–449. (Intralesional Injection versus Mouth Rinse of Triamcinolone Acetonide in Oral Lichen Planus: A Randomized Controlled Study Otolaryngology-Head and Neck Surgery). 3. [DOI] [PubMed] [Google Scholar]
- 6.EL-Komy M.H.M., Saleh N.A., Saleh M.A. Autologous platelet-rich plasma and triamcinolone acetonide intralesional injection in the treatment of oral erosions of pemphigus vulgaris: a pilot study. Arch Dermatol Res. 2018;310(4):375–381. doi: 10.1007/s00403-018-1824-x. [DOI] [PubMed] [Google Scholar]
- 7.Pietrzak W.S., Eppley B.L. Platelet rich plasma: biology and new technology. J Craniofac Surg. 2005;16(6):1043–1054. doi: 10.1097/01.scs.0000186454.07097.bf. 2005. [DOI] [PubMed] [Google Scholar]
- 8.Dhillon R.S., Schwarz E.M., Maloney M.D. Platelet-rich plasma therapy—future or trend? Arthritis Res Ther. 2012;14:219. doi: 10.1186/ar3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knezevic N.N., Candido K.D., Desai R., Kaye A.D. Is platelet-rich plasma a future therapy in pain management? Med. Clin. N. Am. 2016;100:199–217. doi: 10.1016/j.mcna.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 10.Wang J., van der Waal I. Disease scoring systems for oral lichen planus; a critical appraisal. Med Oral Patol Oral Cir Bucal. 2015;20(2):e199–e204. doi: 10.4317/medoral.20524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernandez-Moure J.S., Van Eps J.L., Cabrera F.J. Platelet-rich plasma: a biomimetic approach to enhancement of surgical wound healing. J Surg Res. 2017;207:33–44. doi: 10.1016/j.jss.2016.08.063. [DOI] [PubMed] [Google Scholar]
- 12.Chitturi R.T., Sindhuja P., Parameswar R.A. A clinical study on oral lichen planus with special emphasis on hyperpigmentation. J Pharm BioAllied Sci. 2015;7 doi: 10.4103/0975-7406.163513. S495-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mostafa B., Ahmed E. Prevalence of oral lichen planus among a sample of the Egyptian population. J Clin Exp Dent. 2015;7 doi: 10.4317/jced.51875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu C., Xie B., Yang Y. Efficacy of intralesional betamethasone for erosive oral lichen planus and evaluation of recurrence: a randomized, controlled trial. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116(5):584–590. doi: 10.1016/j.oooo.2013.07.023. [DOI] [PubMed] [Google Scholar]
- 15.Xia J., Li C., Hong Y., Yang L., Huang Y., Cheng B. Short-term clinical evaluation of intralesional triamcinolone acetonide injection for ulcerative oral lichen planus. J Oral Pathol Med. 2006;35(6):327–331. doi: 10.1111/j.1600-0714.2006.00441.x. [DOI] [PubMed] [Google Scholar]
- 16.Merigo E., Oppici A., Parlatore A. Platelet-rich plasma (PRP) rinses for the treatment of non-responding oral lichen planus: a case report. Biomedicines. 2018;6(1):15. doi: 10.3390/biomedicines6010015. [DOI] [PMC free article] [PubMed] [Google Scholar]