Highlights
-
•
The cell-of-origin of early and late disseminating, metastatic clones determines tumor aggressiveness.
-
•
Cellular senescence and microenvironmental cues also promote collective invasion and acquired stemness as a dynamic state in non-senescent tumor cells.
-
•
Cancer stem cells instigate heterogeneity by existing in a reversible state of dormancy, quiescence, aneuploidy, stemness and drug resistance.
Keywords: Cancer stem cells, Metastasis, Minimal residual disease, Microenvironment, Tumor plasticity
Abstract
At the onset, few cancer cells amidst the tumor bulk, identified as cancer stem cells (CSCs) or early disseminated cancer cells (eDCCs) are capable of survival post conventional therapy and persist as minimal residual disease (MRD). Metastatic subclones emerge both early and late in the life of primary tumor ensuing an ongoing regional clonal evolution of progenitor cells in metastatic and primary tumors. In the last decade, multiple studies proposed various identities of stem-like cells that undergo transitions to adapt to the changing microenvironment as the disease progresses. This review advocates with substantial evidence the dynamic model of tumor propagation by exploring the specific cell types, reversible phenotypic plasticity between the tumorigenic leader seeds and the supporting follower cancer cells both in circulation and in solid tissue to accurately decipher tumor promoting clones and its role in metastatic dissemination and tumor re-growth. (142 words)
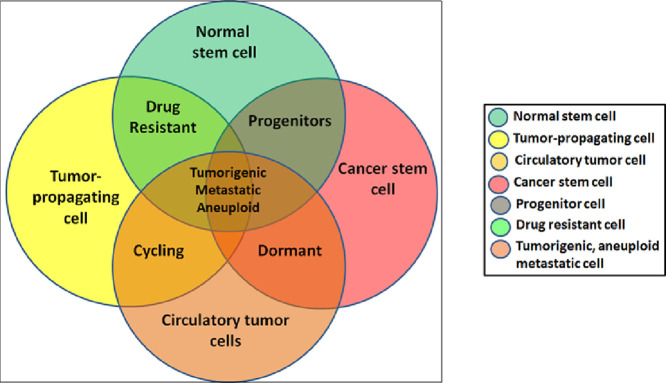
Graphical abstract: cell types transitions and stemness
Multiple cell types mark the three dimensional tissue hierarchy in solid epithelial tumors. From rare normal adult stem cells (NSCs) with restricted self-renewal capacity and low proliferative potential, altered cancer stem cells (CSCs) with an infinite self-renewal and varying proliferation, an intermediate transit amplifying (TA) or progenitor population with higher proliferative rates, tumor-initiating or propagating cells (T-ICs/TPCs) with enhanced drug resistance and shift between quiescence and active cycling conditions, dormant and cycling mix of circulatory tumor cells (CTCs) that have detached from the primary tumor and entered circulatory system, which upon extravasation to other tissue sites or organs form the disseminated tumor cells (DTCs) or metastasis (Mets). There co-exists a minor fraction or clone of cells that have inherited all malignant phenotypes of self-renewal, plasticity, tumorigenicity, aneuploidy and enhanced survival instincts forming the true seeds or “minimal residual disease”.
Introduction
Initially recorded by medical surgeons nearly 187 years ago [1] and albeit advancements in multiple therapeutic modalities, the disease of cancer is still an agonizing phenomenon with no complete cure in sight and is also the second leading cause of death worldwide [2]. The regressive nature after years of disease-free survival is the most dreadful characteristic of the disease. Cancer is capable of causing severe mortality when tissue-of-origin is in organs such as brain, lung and liver. In late stage of the disease, few localized transformed cancer cells evolve to move from amidst an encapsulated panel of similar cells, penetrate the stroma and blood vessels, survive in host milieu and effectively reestablish the primary disease state in a new locale as metastasis. Cancer cells abide by Newton's third law that every action results in an opposing reaction by instigating epigenetic transitions to counter the changing tumor microenvironment [3]. Ambiguity pertaining to the exact stage and time point when invasive cancer cells are released into body fluids facilitating its spread throughout the body remains an insurmountable challenge.
Metastasis persists as an unsolved issue accounting for 90% of deaths in patients with solid tumors [4]. Not all cancer cells have the ability to migrate/or metastasize and regenerate new tumors. Experiments have also shown that heterogeneous tumors can generate clones with different invasive and metastatic potential, two independent processes of tumor progression [5]. All cancer cells displaying an invasive phenotype may not be metastatic. Most malignant tumors inherit the morphology and aggressive kinetics of the embryonic layer from which its counterpart adult tissue differentiated [6,7]. Several pathways reported to play a role in migration of human embryonic stem (ES) cells are also recapitulated in malignant transformation that supported successful establishment of secondary tumors/ and recurrences in new sites. The presence of a tightly regulated and directed cell migration is a hallmark of embryogenesis or adult tissue renewal whereas tumor cell migration is non-coordinated and randomly oriented [6]. All of these migrations are known to occur by similar molecular mechanisms that involve elaborate cytoskeletal remodeling of cells from a non-migrating baso-apically polarized epithelial phenotype to a polarized migrating mesenchymal phenotype in response to signals in microenvironment [7]. Since these signaling molecules were specifically expressed in altered stem-like cancer cells and not in any other adult tissues, they also form ideal targets for therapeutic intervention.
Few questions remain elusive: What is the actual representation of stem cells amidst malignant tumor clones exhibiting plasticity in the migratory stage of the disease? Is interchangeability possible between different stem cell types? Are all transformed tumor cells genetically programmed to invade and survive? Is it possible to meticulously segregate and target metastatic from non-metastatic cancer cells? What determines the affinity of metastatic cancer cells to reseed unrelated organs specifically? Just as epithelial to mesenchymal transition (EMT) instigates metastasis, is it the reverse MET that successfully culminates the entire metastatic seeding of a malignant clone. If so, how to experimentally demonstrate MET in vivo? Though several queries are left unrequited owing to lack of substantial evidence, our intention is to compile reports outlining the metastatic cascade in light of developmental processes associating cancer stem cells. It addresses how CSCs shift between states of stemness, dormancy, aneuploidy and quiescence thereby imparting tumor heterogeneity through co-existence amidst multiple cell fractions. It is also important to know the impact of this complex heterogeneity in survival at time of treatment.
Evolution of the metastatic clone in solid tumor models
Variation between primary and metastatic tumors
Most solid tumors have metastasis prone clones of cancer cells that can emerge both early and late in the life of the primary tumor and subsequently surface as therapy resistant clones [8,9]. Retracing the source of origin of a metastatic clone (M) from numerous clones in primary tumor (P) based on mutational profile largely depends on accurate sampling of the minor subclone in the primary tumor that spawned the metastasis [9], [10], [11], [12]. The progeny of a single metastatic clone in advanced primary tumors manifest a striking growth advantage within the primary tumor site as well as for dissemination and growth at distant, secondary sites [11,12]. Despite harboring events shared in metastases, other clones failed to seed metastases signifying intratumor heterogeneity [9,10]. Intratumor heterogeneity calls for measures to accurately identify and target the metastatic clones for long term disease free survival.
Studies in primary and metastatic pancreatic tumors first explored the clonal relationships between primary and metastatic tumors [13,14]. Mutational analysis of a single tumor biopsy from renal-cell carcinoma failed to resemble the mutational landscape of the entire tumor bulk and revealed the presence of at least two clonal populations indicative of ongoing regional clonal evolution arising from progenitor cells of metastasis and primary tumor cells [15]. Primary tumor regions also showed a diploid profile, whereas the region most resembling the metastatic sites had a tetraploid profile [15]. A similar phenomenon was proven in an in vitro model of oral squamous cell carcinoma (OSCC) wherein four different fractions were identified from amidst a seemingly homogenous pool of oral cancer cells [16]. Each of these fractions represented distinct clones that have undergone transient epigenetic regulations and seemingly evolved from the same cell-of-origin [16,17]. Two coexisting subpopulations with differential invasion and tumorigenicity was observed in metastatic breast cancer cell line established of the same patient as a proof of the concept that invasion is necessary but not sufficient for metastasis [18]. Selective pressure from therapeutic intervention led to parallel evolution of six distinct PTEN mutations in the metastatic breast tumor clones [19]. Metastatic variants with enhanced drug resistance or metastatic outgrowth generated from heterogeneous cell populations also displayed a higher rate of chromosomal gene amplification events through unknown epigenetic mechanisms [20]. Another in vitro model of human ovarian cancer (malignant grade IV serous adenocarcinoma) was found to comprise of 19 immortalized stem and progenitor clones emerging from the single sample also indicative of intra-tumor heterogeneity. Five of these clones had a mutant mitochondrial DNA profile and were tumorigenic representing CSCs, while the remaining 14 clones had a germline mitochondrial DNA profile, expressed the stem cell marker CD133 and were non-tumorigenic. These CD133+ non CSCs could either remain quiescent, or commit towards endothelial differentiation and establish tumor vasculature thereby ensuring long-term tumor survival and CSC-mediated tumor progression [21]. This study provided evidence of tumor cell plasticity and tumor-derived endothelial hierarchy that established the auxiliary role of phenotypically similar non-stem cell clones in driving metastasis. The two caveats with immense clinical implications are the timing of metastatic dissemination and the cell-of-origin of metastatic clone.
Stem cell origins of metastatic clone
Due to inaccessibility, the developmental origins of malignant solid tumors are vague in comparison to blood-borne malignancies [22]. Cancers may arise from mutations accumulating in long residing adult stem cells, in transit-amplifying progenitor cells or in terminally differentiated cells [23]. Evidence of tumors in tissues wherein the existence of stem cells is not yet confirmed (e.g. the kidney) proves that cancers may also arise from fully differentiated cells or from dormant tumor cells of unknown primary tumors [5]. Accumulation of mutations in early stem cells (or its progenitor cells) results in maturation arrest producing tumors with higher rates of metastasis also driven by a heterogeneous repertoire of chemokine receptors on the stem cells [22]. Tumors originating from late stem cells would have a more restricted chemokine-receptor profile, limited metastatic capacity and a homogeneous phenotype, whereas tumors originating from differentiated cells (eg, hyperplastic lesions) do not metastasize [23]. Deletion of Adenomatous polyposis coli (Apc) gene in the intestinal stem cells caused adenomas (premalignant lesions) and in the transient amplifying cells resulted in microadenomas implying that intestinal stem cells were more competent than the transient amplifying cell progeny to form tumors [24]. Distinct subtypes of prostate cancer varying in grades of malignancy were also found to arise from luminal and basal epithelial cell types subjected to the same oncogenic insults demonstrating that both primary human basal and luminal epithelial cells were the cells of origin [25].
Despite individual tissue-specific characteristics, a wide range of cancers including epithelial cancers, neural and hematopoietic malignancy shared a common set of transcriptional mechanisms with each other, as well as pluripotent and multipotent stem cells [26]. Tumors tend to retain the imprint of cell developmental stage as evidenced in blastomas, which are tumors arising from embryonic cell types before organ differentiation [27]. A large percentage of tumors were also found to recapitulate early developmental gene expression and the developmental signature in these cancers exhibited low tissue-specificity [28]. The aggressive behavior of tumors also depends on the processes of tissue formation and differentiation in early embryonic stages. The largest class of tumors causing death in man (bronchogenic carcinoma, colon adenocarcinoma, breast carcinoma and prostate carcinoma) along with the most frequently occurring tumors of man (squamous cell carcinoma of skin and basal cell carcinoma of skin) which are usually non-lethal, belongs to the ectoderm/endoderm class [27]. Ectoderm and endoderm-derived tumors were found to metastasize via lymphatics whereas mesenchyme-derived tumors tend to metastasize by hematogenous spread [27].
The global (2552 genes) features of human tumors resembled the early-developing cognate organ, whereas normal human tissues were closest to the late or mature stages of the developing organ [29]. Certain tumors express metastatic antigens such as β-human chorionic gonadotropin (β -HCG) and α-fetoprotein (AFP) which are stem-cell antigens normally expressed during embryogenesis but become re-manifested during carcinogenesis [22]. Additional stem cell antigens, c-kit was expressed in testicular carcinoma, malignant melanoma, small-cell lung carcinoma [22,30] and CD34 in dermatofibrosarcoma, epitheloid sarcoma and solitary fibrous tumors [31]. Epithelial tumor cells with an acquired expression of mesenchymal markers also showed many features of tumor-initiating cancer stem cells (CSCs), CD44hiCD24lo antigenic state and a heightened resistance to diverse cancer therapies, as well as enhanced invasive and metastatic properties [8,32]. The cell-of-origin of a tumor cell ultimately determines its biological attributes such as metastasis, heterogeneity, immortality, immune and drug resistance [22].
Cancer stem cells, EMT and metastasis
Initiation of EMT, invasion and metastasis phenomenon
With the current scenario of a single cancer cell or a de-differentiated stem cell harboring thousands of point mutations and chromosomal insertion deletions; it seems impossible for an altered cell to have a fine-tuned regulatory mechanism to guide EMT, migration and reestablish the disease via MET at a secondary site. Lineage-tracing studies using multicolored tumor cell clusters enabled the identification of five different stages of metastasis: collective invasion, local dissemination of clusters in the adjacent stroma, intravasation of tumor emboli, circulating-tumor cell (CTC) clusters and distant micro- and macrometastases [33]. Several morphological changes and cellular movements displayed during embryogenesis were unvaryingly recapitulated in tumors. EMT is one such process wherein normal epithelial cells of embryonic origin lose their apical-basal polarity, cell-cell adhesion and take on the motile features of fibroblasts facilitating gastrulation and neural crest formation; whereas epithelial tumor cells exploit this property to enhance their migratory phenotype [5,8]. Several reports timely reviewed [34] also suggest that stemness might not be a definitive cell phenotype but a transient state that could be acquired and shed based on the microenvironment and signaling cues indicative of a dynamic, tumor population that is non-targetable [35].
High-grade tumors reportedly contained cells that expressed molecular signatures pertaining to an EMT program and were associated with poor patient prognosis [32]. The expression of mesenchymal genes was typically enriched in the basal and triple-negative subtypes of breast cancer cells, with poor clinical outcomes [36]. Differentiated epithelial keratinocytes were found to initially undergo an EMT in wound healing to reconstitute epithelial cell sheets, followed by MET indicating that the reversible transitions between cell states are natural processes that are crucial to normal development and tissue homeostasis [37,38]. The morphologies of tumors of ectodermal/endodermal and mesodermal classes reveal that mesodermal tumors or sarcomas tend to have a spindle cell appearance while endodermal/ectodermal cells have an epithelial appearance also signifying diverse functional molecular pathways responsible for the malignant phenotype in sarcomas than the pathways followed for endodermal/ectodermal tumors [5,27].
In carcinomas, originating from breast, colon, prostate and the thyroid gland, distant metastases are typically similar in histology to the primary tumor, and cancer cells invade as cohesive multicellular strands as leaders while other cells follow behind indicating that invasion and metastasis can take place without EMT [33,39,40]. This collective invasion is displayed by cancers composed of heterogeneous subpopulations with distinct biologic properties pertaining to stemness, self-renewal, drug or immune resistance, secretome, senescence and tumorigenicity [41]. Cellular senescence which is rare in malignant tumors, promote collective invasion of non-senescent tumor cells without undergoing EMT by producing various growth factors, cytokines and proteases, forming the senescent-associated secretory phenotype (SASP). Senescent tumor cells are found to be frequently present in lymphatic channels, metastatic foci of lymph nodes and in the front region of collective invasion of papillary thyroid carcinoma (PTC) [41] although several other high-grade and mesenchymal tumors infiltrate by single-cell migration with EMT characteristics.
Studying EMT under physiologic conditions is critical for elucidating its role in tumor metastasis and a major hurdle is in accurately identifying tumor cells at the EMT stage that are scattered among the many stromal cells present within primary human tumor samples. Most of the EMT markers currently used are expressed in either epithelial or mesenchymal cells, and therefore are not specific. Several studies in mouse tumor models have suggested that tumor invasion and metastasis can also be achieved without an obvious EMT phenotype [42]. Partial or complete EMT and collective cell migration are not adequate to explain how individual tumor cells can intravasate into the blood circulation and travel to distant organs by breaching the dense basement membrane that also requires extracellular proteolysis followed by an epithelial-amoeboid transition that might enable cells to pass through thinner ECM [35].
Cancer-therapy regimen utilizing different classes of chemotherapeutic agents and ionizing radiation (IR) induce senescent-like phenotypes in tumor cells both in vitro and in vivo and may thus be a double-edged sword, capable of suppressing cancer as well accelerating premature aging and SASP [43]. Premature aging resulting from aggressive treatment might be one of the major factors that drive the onset of second malignancies among long-term childhood cancer survivors in later years after their cancer diagnosis [44]. Malignant cells capable of forming tumor emboli undergoes dermal lymphatic invasion, leading to high propensity for metastasis [45,46]. When the migration rate of a metastatic tumor cell is small, a single cancer stem cell generates a self-limited clone because of the finite life span of progeny, and spatial constraints whereas a higher migration rate could lead to seeding of new clones at sites further from older clones [47]. Micrometastases (2–50 cells) is from single disseminated tumor cells and a collection of tumor cells (>50 cells) forms macrometastases [22]. Blood-borne metastasis was found to be fueled by the generation of circulating tumor cells (CTCs) from a primary tumor deposit [48]. This accentuates the necessity of utilizing fundamentally different therapeutic approaches to reduce the adverse effect of senescence and collectively target multiple cell types and molecular pathways in all grades of tumors.
Transient phenotype of cancer stem cells
The expression of stem cell associated cell surface markers by cancer cells need not be a stable phenotype or these cells possess tumorigenic capacity [49]. Not all tumor cells with high tumor-initiation potential necessarily display tissue stem cell markers and properties indicating that under specific circumstances, high stem-cell marker expression can be a poor indicator of tumorigenicity [50]. Ideally a CSC must denote cancer cells able to propagate cancer regardless of the presence of stem cell markers. Multiple tumor cells coexisting in a state of permanent instability and expression of specific genes frequently appearing in cells that lacked expression initially makes CSCs and the stem cell state challenging for targeted therapy [16,51]. The hallmarks of a cancer stem cell irrespective of presence or absence of stemness related cell surface markers indicates a dynamic subset of cells with the features: (i) ability to undergo reversible tumor dormancy, (ii) reversible state of drug resistance, (iii) prolonged state of reversible proliferation arrest or quiescence and aneuploidy, (iv) stemness associated self-renewal and cell plasticity, (v) tumor regeneration potential and (vi) adeptness to sustain tumor heterogeneity.
The interactions of CSCs with the niche accounts for cancerous development, whereas the metastatic niche designates the specific locations, stromal cell types, diffusible signals, and ECM proteins bear consequences for the metastasis of disseminated tumor cells (DTCs) [52], [53], [54]. Cells with low tumorigenic potential can transit to a state of greater tumorigenicity in a dynamic manner that accounts for the higher frequency of tumor propagating cells (TPCs) in late-stage cancers [54]. Molecules released by dying cancer cells post therapy was found to induce mobilization and expansion of drug resistant TPCs [55] and also protect non-tumorigenic cells from anoikis through a bystander effect [56]. Anticancer drug‐resistant gastric cancer cells developed early‐phase drug tolerance to 5‐fluorouracil (5‐FU) by inducing a dynamic change in the cell heterogeneity generating a subset also termed as “persister” cells [57]. Reports state that commonly used chemotherapeutic agents and radiotherapy also accelerate the dynamic transition between non-tumorigenic to tumorigenic state [54,58,59]. Despite targeted therapeutic approaches in the treatment of chronic myeloid leukemia (CML), BCR-ABL-independent mechanisms seem to exist which sustain the survival of leukemic stem cells (LSCs) as minimal residual disease or persister cells with increased stem cell characteristics and quiescence [60]. Chronic inflammation and tumor necrosis factor α (TNFα), the major proinflammatory cytokine was found to induce malignant growth and stemness phenotype in HPV-infected oral cancer cells [61]. TIC population in pancreatic ductal adenocarcinoma (PDAC) was marked by high cell surface levels of the tetraspanin CD9 correlated with poorer survival, showed pronounced epithelial and mesenchymal cancer cell populations, re-initiated tumor formation and recapitulated the cellular heterogeneity of primary PDAC. Mechanistically, CD9 expression was found to augment glutamine uptake by increasing the cell surface expression of the glutamine transporter ASCT2, thereby enhancing PDAC growth [62]. CSCs in ovarian cancer contributed to tumor angiogenesis and metastasis by forming a proportion of tumor-derived endothelial cells, pericytes and lymphatic endothelial cells which are components of tumor blood vessel and lymphatic vessel [21,63]. Similarly, Glioblastomas (GBM) which are brain tumors with a poor prognosis reportedly contain GBM stem-like cells (GSC) found to contribute to tumor aggressiveness, resistance, and recurrence and are directly involved in the formation of new vessels by transdifferentiation into Tumor Derived Endothelial Cells (TDEC) [64]. The presence of CSCs mediates interaction with the microenvironment cues enabling emergence of survival features in respective tumor types [Table 1].
Table 1.
Major factors promoting metastasis and stemness in tumors.
| Tumor type | Stemness & Metastatic determinants | Cell-of-origin | Ref. |
|---|---|---|---|
| Renal cell carcinoma | Ongoing clonal evolution; de-differentiation and ploidy status | Progenitor cells of metastasis and primary tumor cells | 5, 15 |
| Oral squamous cell carcinoma | Drug transporter proteins and ploidy status | Transient shift between multiple tumorigenic fractions | 16 |
| Breast cancer | Evolution of six distinct PTEN mutations | Selective enrichment of metastatic, therapy resistant clones | 19 |
| Ovarian serous adenocarcinoma | Mutant mitochondrial DNA profile in tumorigenic CSC clones | 19 immortalized stem and progenitor clones from single tumor sample | 21 |
| Colon cancer | Deletion of APC gene | Intestinal stem cells form adenomas; transient amplifying cells result in microadenomas | 24 |
| Prostate cancer | MYC amplification and PTEN loss | Well-differentiated acinar adenocarcinoma, androgen receptor (AR) and prostate-specific antigen (PSA) positive originated from Luminal epithelial cells; Basal epithelial cells generated more aggressive tumors and low/absent AR and PSA expression | 25 |
| Papillary thyroid carcinoma | Senescent-associated secretory phenotype (SASP). | Promote collective invasion of non-senescent tumor cells | 41 |
| Leukemia | BCR-ABL-independent mechanisms | Survival of leukemic stem cells (LSCs) Minimal residual disease or Persister cells with increased stem cell characteristics and quiescence | 60 |
| Pancreatic ductal adenocarcinoma (PDAC) | High CD9 expression; high glutamine uptake and cell surface expression of the glutamine transporter ASCT2 | Tumor-initiating cells (TICs) enhanced tumorigenicity. | 62 |
| Ovarian cancer | Transdifferentiation to tumor-derived endothelial cells, pericytes and lymphatic endothelial cells | Ovarian CSCs | 63 |
| Glioblastoma (GBM) | Transdifferentiation into Tumor Derived Endothelial Cells (TDEC) | GBM stem-like cells (GSC) | 64 |
The "Cancer stem cell shift hypothesis" [23] stated that CSCs survives therapy by transiently alternating between drug resistant /or sensitive phase and a proliferative /or dormant cell cycle stage, highlighted by variable expressions of drug transporter proteins. Cancer cell populations also develop a dynamic survival strategy of reversibly drug-tolerant state [65]. Several reports also supported presence of a hybrid (epithelial to mesenchymal) E/M phenotype or hybrid E/M cells that are transient, promotes migration in cell clusters (CTCs) and are associated with aggressiveness of the disease [48,[66], [67], [68]] signifying this reversible state could be a highlight of tumor cells both in solid state and in circulation (ascites, pleural fluid etc.). The gene-expression profiles of breast cancer stem cells (BCSCs) that co-express mesenchymal (M) and epithelial (E) markers showed remarkable similarity across different molecular subtypes of breast cancer [69] and were also observed in human primary breast tumors and abundantly in metastatic circulating tumor cells (CTCs) in blood of (breast cancer) brca patients [67]. CTC clusters arising from oligoclonal tumor cell groupings and not from intravascular aggregation events, displayed 23- to 50-fold increased metastatic potential when compared with single CTCs [70]. Human CTCs found both as single cells and as clusters of cells held together by intercellular junctions, exhibit dynamic changes in expression of epithelial and mesenchymal markers during cancer progression. This dual (E/M) phenotypic plasticity is indicative of epigenetic modifications in transcriptome in line with the tumor milieu [67,69]. The presence of platelet-covered CTCs also plays important roles in metastasis by enhancing tumor cell survival, tumor-vascular interactions, and escape from immune surveillance [71].
Cancer stem cells (CSCs) in oral cancer was found to drive tumor spread, therapeutic resistance by undergoing EMT and MET to switch between epithelial and post-EMT sub-populations [72]. The post-EMT CSCs defined by a CD44highEpCAMlow/− CD24+ cell surface marker profile possessed enhanced therapeutic resistance [72]. Similarly, mesenchymal-like breast CSCs characterized as CD24-CD44+ were primarily quiescent and localized at the tumor invasive front, whereas epithelial-like BCSCs expressed aldehyde dehydrogenase (ALDH), were proliferative, and were located more centrally [69]. A DNA barcode tagged breast cancer cells that spawned metastases upon injection in mice displayed high expression of SERPINE2 and SLPI, genes that inhibit blood clotting and also programmed the cancer cells for vascular mimicry thereby acting as drivers of metastatic progression [73]. Temporal expression of specific cell surface protein markers in response to changing microenvironment are also adaptations displayed only by CSCs and not found in homeostatic normal intestinal epithelium. The L1 cell adhesion molecule (L1CAM) expression is induced and is required for orthotopic carcinoma propagation, liver metastatic colonization and chemoresistance in colorectal carcinoma (CRC), but not expressed during initiation of adenocarcinoma [74]. L1CAMhigh cells are found to partially overlap with LGR5high stem-like cells in human CRC organoids and when epithelial integrity is lost, chemoresistant CRC progenitors undergoes transition from an L1CAMlow to an L1CAMhigh state indicative of wound healing functionality deployed in metastasis-initiating cells [74]. Deployment of molecules such as L1CAM upon disruption of epithelial integrity instigates tumor regenerative trait and phenotypic plasticity in metastasis-initiating cells. Features of phenotypic and genotypic transitions and subsequent activation of alternate signaling pathways signifies the need for a dynamic screening of genomic and transcriptomic profile selectively enriched in tissue-specific and CTC-specific CSCs at various stages of tumor progression.
CSC survival traits - reversible quiescence and aneuploidy
The ability of residual tumor cells to persist without any clinical evidence of disease in the patient is cancer dormancy. CSCs are speculated to be a major player displaying the state of reversible quiescence. Reversible genetic alterations interconnect processes of dormancy, therapy resistance and plasticity of CSCs [75]. Irrespective of genetic differences, dormant cells are proliferatively quiescent in poorly vascularized hypoxic conditions, survive chemotherapy intertwined with angiogenic dormancy and reinitiate tumor formation or metastasis upon exit from dormancy [76]. Quiescence and its reversibility to resume proliferation are defining parameters of stem cells that prevents stem cell exhaustion in normal tissues in contrast to other nonproliferative conditions (terminal differentiation/senescence) [77,78]. Dormant cancer cells are known to harbor in metastatic, bone marrow, and perivascular niches [75]. Experiments using PKH67/PKH26 dyes have shown presence of therapy-refractory residual tumor stem cells persisting in a state of quiescence as label-retaining cells (LRCs) and have been isolated from primary ovarian tumors and metastases [78]. These label-retaining fractions were also identified as aneuploid cell populations with dormant cell status indicating that aneuploidy imparts selective advantages to survival of CSCs under stress conditions [78].
Aneuploid populations, akin to the CSCs, also constitute a dormant subset within tumors either through quiescence or a proliferation arrest [78]. An in vitro model of oral squamous cell carcinoma (OSCC) was reported to contain multiple tumor forming fractions in nod/scid mice [16]. Amidst these fractions, MP2 fraction of cells were highly tumorigenic, aneuploid with cells in G2/M stage of cell cycle and displayed mesenchymal phenotype (expressed more vimentin) indicative of cells undergoing EMT [16]. Induction of EMT by TGF-β secreted by tumor stroma transforms epithelial cancer cells to sustain mitotic abnormalities due to failed cytokinesis, failure of nuclear lamin formation and proliferation arrest, resulting in subsequent accumulation of aneuploidy status and genomic instability [79]. Eventhough TGF-β-induced mitotic defects are reversible on withdrawal, the acquired genomic abnormalities lead to increased mesenchymal marker expression and enhanced tumorigenic phenotypes within single circulating tumor cells (CTCs) in metastatic breast cancer patients [79]. Several signaling pathways such as overexpression of PLK1 and activation of FOXC2 protein was found to regulate G2/M transition leading to aneuploidy, acquired CSC properties and spontaneous metastasis in transformed human mammary epithelial cells reinforcing high therapeutic failure of triple-negative breast cancers (TNBCs) [69,72,80,81]. Conventional therapy mainly targets proliferative transit amplifying (TA) or progenitor cells and differentiated cells resulting in tumor debulking and emergence of dormant aneuploid CSCs and dormant therapy-refractive CSCs posing as minimal residual disease (MRD) [16,23,78]. An understanding of multiple subsets of stem cells and aneuploid clones comprising the variants within tumors is crucial to provide definitive targets for tumor progression.
Life cycle model of tumor cells piloted by CSCs
A normal epithelial tissue embodies a tightly regulated tissue hierarchy of rare adult stem cells with controlled proliferation and genomic integrity residing towards the basal layer. These cells interact with the microenvironment, whereas more densely populated differentiated and progenitor cells are organized to establish the three-dimensional architecture and functionality of the tissue. Any aberrant loss or gain of function and genomic instability resulted in cancer cells, generating a plethora of phenotypically and genetically diverse cell types within the tissue of origin. Based on the ability of mutated cancer cells to drive tumor progression, instigate metastasis and establish tumor reseeding in a different milieu, three models have been proposed; (i) CSC model or Hierarchy model, (ii) Stochastic model or Clonal evolution model and (iii) Dynamic model or Interconversion model [51,54]. Tumor progression is a dynamic process manifested by merger of tumor cells with varying degrees of heterogeneity, plasticity, dormancy, proliferation, DNA content, drug resistance, tumorigenicity and epigenetic profile. Early stage tumors are characterized by asymmetric division of altered normal stem cells or CSCs generating a tumorigenic stem cell and a non-tumorigenic (non-CSC) progeny that form well-differentiated tumors composed of a mix of differentiated states and follows the CSC model or Hierarchy model of cancer spread [51,82,83]. With progressive stages and grades of cancer (late-stage cancers), there is more generation of phenotypically similar clones of stem-like cells forming poorly differentiated or undifferentiated tumors depicting Stochastic or clonal evolution model [84]. In this model every cancer cell has the ability to regrow a tumor and can act as CSC, yet fail to address inter- and intra-tumor biological heterogeneity establishing the fact not all phenotypically similar clones are CSCs [16,51]. On the other hand, the concept of CSCs allows considering otherwise the emergence of therapeutic resistance and heterogeneity of cells within tumors. The dynamic model or Interconversion model aptly endorses the transient phenotypic shift evidenced by CSC fractions in several solid tumors [16,69,72]. Dynamic model integrates both stochastic and clonal evolution models to explain reversible epigenetic transformations favoring extensive phenotypic variations.
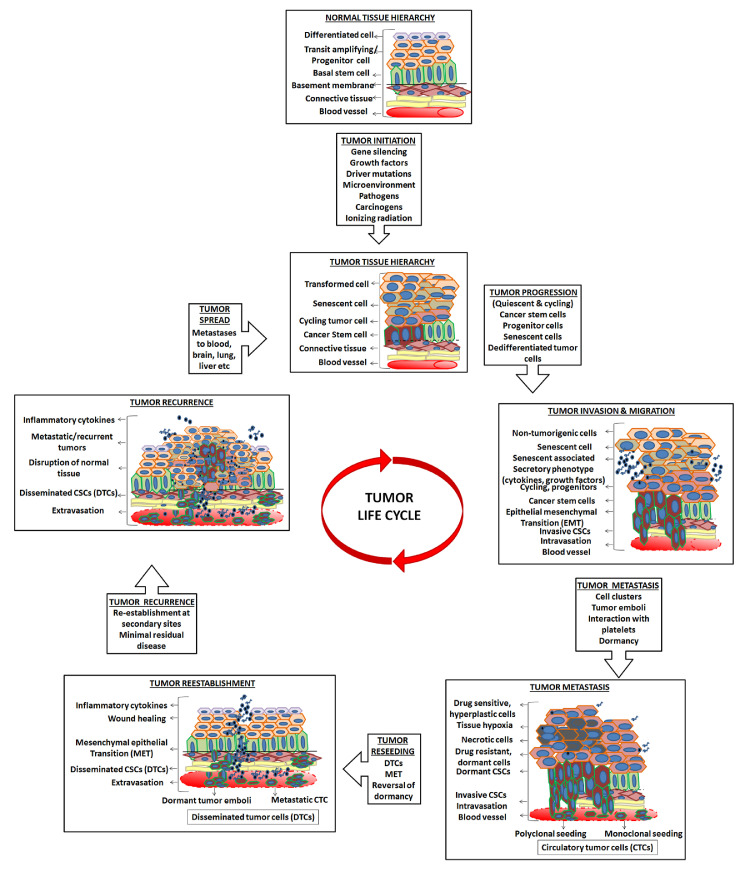
Whole-genome sequencing based characterization of multiple metastases in androgen-deprived metastatic prostate tumors revealed metastasis-to-metastasis spread, either through de novo monoclonal seeding of daughter metastases or through the transfer of multiple tumor clones (polyclonal seeding) between metastatic sites [85]. Multicolor lineage tracing studies in mouse model of breast cancer demonstrated that polyclonal seeding by cell clusters is a frequent mechanism accounting for >90% of metastases [33]. Polyclonally seeded metastases arising from heterotypic interactions between distinct tumor subclones were also reported in mouse models of breast [70], pancreas [86], and small cell carcinoma [87]. The metastatic spread from the primary tumor could be repeated or continuous metastatic seeding and removal of the primary source even after establishment of metastases could terminate further metastatic progression in some advanced cancers [88]. If metastasis occurs prior to detection of primary tumor then an early surgical resection may fail to reduce the risk of metastases in the future [8] and if seeds originated from cancer stem cells [16], an enhanced aggressiveness ensues [Fig. 1].
Fig. 1.
Events in the life trajectory of a tumor cell.
Transformation of a normal epithelial cell to a tumor cell followed by stages of tumor initiation, progression, malignancy, invasion and systemic spread are multi-step processes involving multiple cell types following the clonal evolution as well as CSC hierarchy model. The dynamic transition between these cycling/dormant and drug resistant/sensitive CSC phenotypes determines their survival in circulatory system and subsequent extravasation into congenial secondary organs or sites of inflammation. The emergence of secondary tumors or recurrences at new sites post metastatic events, marks the revival of an entire initiation phase and an unending journey of a tumorigenic cancer cell.
Evidence has shown that the presence of CTCs in the blood correlates with poor overall survival in patients with metastatic prostate, breast and colon cancers [89]. A fraction of CTCs are capable of entering distant sites and persist as disseminated tumor cells (DTCs) at the so‐called in situ stage [90,91]. Eventhough dissemination of tumor cells to the bone marrow is an early event in breast cancer, cells may lie dormant for many years before bone metastases that develop upon induction by bone marrow-derived IL1β which stimulated breast CSC colony formation [92]. Early‐stage DTCs have the ability to not only leave the primary site but also successfully seed a secondary distant site [90]. Substantial fraction of DTCs do not express molecular targets derived from the primary tumor indicative of heterogeneity between DTCs and the primary tumor originating from selective pressures of microenvironment [93]. Metastasis is a function not only of tumor cells but also involves cooperative interactions of those cells with normal cells of the body, in particular platelets and leukocytes. These other cell types alter the behavior of the tumor cells themselves and of endothelial cells lining the vasculature and assist in tumor cell arrest and extravasation at sites of metastasis and subsequently in the establishment of tumor cells in the early metastatic niche [94]. Several factors in tumor microenvironment and platelet-activated tumor cells tend to induce a prometastatic gene expression signature with an enhanced expression of various proteases, cytokines and growth factors which in turn maintains the stemness potential and inhibits the differentiation of CTCs in bone marrow niche as well in secondary metastatic sites [91].
CTC clusters isolated from the blood of patients with metastatic prostate cancer displayed reduced cell death and potential protection from shear stress than individual CTCs [95]. Incorporation of CTCs in heterotypic tumor–fibroblast aggregates in blood also had improved viability compared with single CTCs indicating that CTC aggregates with platelets, fibroblasts, tumor cell clones and leukocytes are most effective in metastasis than single cells [96]. More than 80% of tumor cells survive the circulatory phase of metastasis and successfully seed the lungs after 24 h [97]. Cancers characterized by a high rate of cell dissemination (10 to 150 cells seeded metastasis) are likely to give rise to highly heterogeneous metastases between initial formation, cancer progression and clinical detection [98]. Metastatic lung and breast cancer cells infiltrate and adapt to grow in cerebrospinal fluid (CSF), a remarkably acellular, mitogen-poor metastasis microenvironment by secretion of complement C3 that interacts with receptors in host and overcomes an epithelial barrier to actively enrich the CSF with plasma-derived components [99]. The efficiency of metastasis can increase if tumor cells maintain structural emboli, either homotypic (tumor cell–tumor cell) or heterotypic (tumor cell–leukocyte, -platelet, -fibrin) [100]. Endothelial cells in each tissue express unique cell surface markers that are recognized by CTCs leading to selective organotropism or selective tissue homing [101]. CTCs also adhere at sites of inflammation denoting rise of metastases at sites of tissue injury, attach to endothelium, survive, and grow intravascularly until intravascular foci expand disrupting vessel integrity and leading to extravasation, subsequent colonization and reestablishment of tumor initiation and progression at recurrent sites [100]. In addition to “seed and soil”, “climate” or the overall health of the individual is also a determining factor affecting the distribution of metastasis [100]. The majority (>75%) of human malignant tumors are already disseminated even before the primary tumor is detected and can reside dormant for decades in organs such as kidneys and the heart that are not usually the site of secondary tumors. These metastatic DTCs are capable of reversing dormancy leading to late recurrences [102].
Dedifferentiation, drug resistance and targeted purging of CSCs
Transformation of normal stem cells or dedifferentiation of mutated cancer cells can also lead to generation of induced cancer stem cells (iCSCs) that plays a major role in tumor recurrence and relapse [103]. Tumor microenvironment governs the enhanced stemness in cancer cells and subsequent dedifferentiation. Introduction of stemness factors (Oct-3/4, Sox-2 and Klf4), was found to significantly enhance CSC properties such as sphere formation, chemoresistance and tumorigenicity in colon cancer cells [104]. Cytokines and chemokines in tumor microenvironment and epigenetic and transcription factors are other commonly used tools to convert non-stem cancer cells into iCSCs. Inhibitor of differentiation 4 (ID4) was found to dedifferentiate non-stem glioma cells into glioma stem cells [105] and also associated with a stem-like poor prognosis phenotype in triple negative breast cancer [106]. Several reports indicate that dedifferentiation-redifferentiation process is critical for carcinogenesis, selective survival of tumor stem cells that drives invasion and metastases.
Since CSC mediated tumor progression is a dynamic process, targeting these heterogeneous fractions should also be dynamic. Mostly chemotherapeutic drugs target proliferating tumor cells, eluding dormant fractions which will revert to reseed tumors. Presence of drug transporter proteins and plasticity tend to promote survival of persister cell types. In vitro cell line studies and clinical samples have shown that ALDH1A1-positive [107] and drug-transporter proteins MDR1, ABCG2, and ABCB1 expressing fractions [108] are highly enriched for cancer stem cells with enhanced resistance to both EGFR-TKI (gefitinib) and other anticancer chemotherapy drugs (cisplatin, etoposide, and fluorouracil) than the negative fractions of cancer cells [109]. Alternative drugs that will effectively target all tumor cell types with reduced or trivial side effects are the need of the hour.
Mitaplatin drug is a conjugate of two Dicholroacetate (DCA) units appended to the axial positions of a six-coordinate Pt(IV) center that can target both nuclear DNA with Cisplatin and mitochondria with DCA selectively in cancer cells, while not harming normal cells [110]. DCA inhibits the activity of pyruvate dehydrogenase kinase (PDK), in turn shift cellular metabolism from glycolysis to glucose oxidation, decreasing Δψm and opens mitochondrial transition pores (MTPs) thereby driving cancer cells specifically to commit suicide by apoptosis [110]. Development of a T cell receptor (TCR) carrying the monomorphic MHC class I-related protein, MR1, was found to recognize and kill most human cancer types while remaining inert to noncancerous cells indicating emergence of a new pan-cancer, pan-population T cell–mediated cancer immunotherapy approach [111]. Mithramycin A (Mit-A) successfully inhibited CSC proliferation, in addition to inhibiting bulk cancer cells in a model of colorectal cancer (CRC) [112]. Simultaneous targeting of both cycling and dormant CSCs in solid tissues and disseminated as CTCs and DTCs in circulation alone would offer complete remission from metastasis and recurrence. The major goal is to eradicate the residual CSCs and simultaneously attain the state of treatment free response (TFR) to enhance the quality of living for the patients undergoing rigorous treatment regimen [113].
Conventional therapies fail to address the clinical pathology of malignant solid epithelial tumors that are marked by secondary changes such as rapid tumor growth, insufficient blood supply, breakage of protective anatomical barriers, higher infiltration of suppressed (tumor promoting) immune cells, tumor necrosis, scarring of surrounding tissues etc. In addition, residual surviving resistant tumor cells are an ever looming “Sword of Damocles”. Accurate identification of tumor promoting fractions or cancer stem cells enables development of multifunctional therapeutic intervention to treat patients at both ends of the clinical spectrum, early stages to late palliative or metastatic stages of cancer.
Declaration of Competing Interest
The authors declare no competing or financial interests.
Acknowledgments
Acknowledgments
We apologize to the many researchers in this field whose work we were unable to cite due to space restrictions.
Author contributions
VR – conceptualization and writing of review.
TR & MRP - conceptualization, design, editing, supervision and lab support.
Funding
This work was supported by grant from Indian Council for Medical Research (ICMR- DHR) Fellowship awarded to Dr. Vinitha Richard [No.DHR/HRD/Women Scientist/Type-V(2)/2014–2015] and fellowship extension support from Rajiv Gandhi center for Biotechnology (RGCB) Thiruvananthapuram. This work was also supported by Department of Biotechnology, Govt. of India, for translational research on TNBC (BT/01/CEIB/01CB/2016).
References
- 1.Carr I., Carr J. The origin of cancer metastasis. Can. Bull. Med. Hist. Bull. Can. d'Hist. Méd. 2005;22(1):353–358. doi: 10.3138/cbmh.22.2.353. [DOI] [PubMed] [Google Scholar]
- 2.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Arbab A.S., Jain M., Achyut B.R. Cancer therapeutics following Newton's third law. Biochem. Physiol. 2016;5(1):e145. doi: 10.4172/2168-9652.1000e145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta G.P., Massagué J. Cancer metastasis: building a framework. Cell. 2006;127(4):679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Aiello N.M., Stanger B.Z. Echoes of the embryo: using the developmental biology toolkit to study cancer. Dis. Models Mech. 2016;9(2):105–114. doi: 10.1242/dmm.023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurosaka S., Kashina A. Cell Biology of Embryonic Migration. Birth Defects Res. Part C Embryo Today Rev. 2008;84(2):102–122. doi: 10.1002/bdrc.20125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Etienne-Manneville S. Polarity proteins in migration and invasion. Oncogene. 2008;27(55):6970–6980. doi: 10.1038/onc.2008.347. [DOI] [PubMed] [Google Scholar]
- 8.Thiery J.P., Acloque H., Huang R.Y.J., Nieto M.A. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Hong M.K.H., Macintyre G., Wedge D.C., Van Loo P., Patel K., Lunke S., Alexandrov L.B., Sloggett C., Cmero M., Marass F. Tracking the origins and drivers of subclonal metastatic expansion in prostate cancer. Nat. Commun. 2015;6 doi: 10.1038/ncomms7605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haffner M.C., Mosbruger T., Esopi D.M., Fedor H., Heaphy C.M., Walker D.A., Adejola N., xFc, rel M, Hicks J. et al: Tracking the clonal origin of lethal prostate cancer. J. Clin. Invest., 123(11):4918–4922. [DOI] [PMC free article] [PubMed]
- 11.Turajlic S., Swanton C. Metastasis as an evolutionary process. Science. 2016;352(6282):169–175. doi: 10.1126/science.aaf2784. [DOI] [PubMed] [Google Scholar]
- 12.Kerbel R.S., Waghorne C., Korczak B., Lagarde A., Breitman M.L. Clonal dominance of primary tumours by metastatic cells: genetic analysis and biological implications. Cancer Surv. 1988;7(4):597–629. [PubMed] [Google Scholar]
- 13.Yachida S., Jones S., Bozic I., Antal T., Leary R., Fu B., Kamiyama M., Hruban R.H., Eshleman J.R., Nowak M.A. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467(7319):1114–1117. doi: 10.1038/nature09515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campbell P.J., Yachida S., Mudie L.J., Stephens P.J., Pleasance E.D., Stebbings L.A., Morsberger L.A., Latimer C., McLaren S., Lin M.-.L. The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature. 2010;467(7319):1109–1113. doi: 10.1038/nature09460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerlinger M., Rowan A.J., Horswell S., Larkin J., Endesfelder D., Gronroos E., Martinez P., Matthews N., Stewart A., Tarpey P. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med. 2012;366(10):883–892. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richard V., Sebastian P., Nair M.G., Nair S.N., Malieckal T.T., Santhosh Kumar T.R., Pillai M.R. Multiple drug resistant, tumorigenic stem-like cells in oral cancer. Cancer Lett. 2013;338(2):300–316. doi: 10.1016/j.canlet.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 17.Richard V., Raju R., Paul A.M., Girijadevi R., Santhosh Kumar T.R., Pillai M.R. Analysis of microRNA‐mRNA interactions in stem cell-enriched fraction of oral squamous cell carcinoma. Oncol. Res. Featur. Preclin. Clin. Cancer Therapeutics. 2018;26(1):17–26. doi: 10.3727/096504017X14881490607028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amaro A., Angelini G., Mirisola V., Esposito A.I., Reverberi D., Matis S., Maffei M., Giaretti W., Viale M., Gangemi R. A highly invasive subpopulation of MDA-MB-231 breast cancer cells shows accelerated growth, differential chemoresistance, features of apocrine tumors and reduced tumorigenicity in vivo. Oncotarget. 2016;7(42):68803–68820. doi: 10.18632/oncotarget.11931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Juric D., Castel P., Griffith M., Griffith O.L., Won H.H., Ellis H., Ebbesen S.H., Ainscough B.J., Ramu A., Iyer G. Convergent loss of PTEN leads to clinical resistance to a PI(3)K[agr] inhibitor. Nature. 2015;518(7538):240–244. doi: 10.1038/nature13948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cillo C., Dick J.E., Ling V., Hill R.P. Generation of drug-resistant variants in metastatic B16 mouse melanoma cell lines. Cancer Res. 1987;47(10):2604–2608. [PubMed] [Google Scholar]
- 21.Kusumbe A.P., Mali A.M., Bapat S.A. CD133-expressing stem cells associated with ovarian metastases establish an endothelial hierarchy and contribute to tumor vasculature. Stem Cells. 2009;27(3):498–508. doi: 10.1634/stemcells.2008-0868. [DOI] [PubMed] [Google Scholar]
- 22.Tu S.-.M., Lin S.-.H., Logothetis C.J. Stem-cell origin of metastasis and heterogeneity in solid tumours. Lancet Oncol. 2002;3(8):508–513. doi: 10.1016/s1470-2045(02)00820-3. [DOI] [PubMed] [Google Scholar]
- 23.Richard V., Pillai M.R. The stem cell code in oral epithelial tumorigenesis: ‘The cancer stem cell shift hypothesis’. Biochim. Biophys. Acta (BBA) Rev. Cancer. 2010;1806(2):146–162. doi: 10.1016/j.bbcan.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 24.Barker N., Ridgway R.A., van Es J.H., van de Wetering M., Begthel H., van den Born M., Danenberg E., Clarke A.R., Sansom O.J., Clevers H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457(7229):608–611. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- 25.Park J.W., Lee J.K., Phillips J.W., Huang P., Cheng D., Huang J., Witte O.N. Prostate epithelial cell of origin determines cancer differentiation state in an organoid transformation assay. Proc. Natl. Acad. Sci. 2016;113(16):4482–4487. doi: 10.1073/pnas.1603645113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palmer N.P., Schmid P.R., Berger B., Kohane I.S. A gene expression profile of stem cell pluripotentiality and differentiation is conserved across diverse solid and hematopoietic cancers. Genome Biol. 2012;13(8):1–13. doi: 10.1186/gb-2012-13-8-r71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berman J.J. Tumor classification: molecular analysis meets Aristotle. BMC Cancer. 2004;4(1):1–9. doi: 10.1186/1471-2407-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naxerova K., Bult C.J., Peaston A., Fancher K., Knowles B.B., Kasif S., Kohane I.S. Analysis of gene expression in a developmental context emphasizes distinct biological leitmotifs in human cancers. Genome Biol. 2008;9(7):1–19. doi: 10.1186/gb-2008-9-7-r108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kho A.T., Zhao Q., Cai Z., Butte A.J., Kim J.Y.H., Pomeroy S.L., Rowitch D.H., Kohane I.S. Conserved mechanisms across development and tumorigenesis revealed by a mouse development perspective of human cancers. Genes Dev. 2004;18(6):629–640. doi: 10.1101/gad.1182504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takeshima H., Kaji M., Uchida H., Hirano H. Kuratsu J-i: Expression and distribution of c-kit receptor and its ligand in human CNS germ cell tumors: a useful histological marker for the diagnosis of germinoma. Brain Tumor Pathol. 2004;21(1):13–16. doi: 10.1007/BF02482171. [DOI] [PubMed] [Google Scholar]
- 31.Natkunam Y., Rouse R.V., Zhu S., Fisher C., van de Rijn M.: Immunoblot analysis of CD34 expression in histologically diverse neoplasms. Am. J. Pathol., 156(1):21–27. [DOI] [PMC free article] [PubMed]
- 32.Tam W.L., Weinberg R.A. The epigenetics of epithelial-mesenchymal plasticity in cancer. Nat. Med. 2013;19(11):1438–1449. doi: 10.1038/nm.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheung K.J., Padmanaban V., Silvestri V., Schipper K., Cohen J.D., Fairchild A.N., Gorin M.A., Verdone J.E., Pienta K.J., Bader J.S. Polyclonal breast cancer metastases arise from collective dissemination of keratin 14-expressing tumor cell clusters. Proc. Natl. Acad. Sci. 2016;113(7):E854–E863. doi: 10.1073/pnas.1508541113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poleszczuk J., Enderling H. Cancer stem cell plasticity as tumor growth promoter and catalyst of population collapse. Stem Cells Int. 2016;2016 doi: 10.1155/2016/3923527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lim J., Thiery J.P. Epithelial-mesenchymal transitions: insights from development. Development. 2012;139(19):3471. doi: 10.1242/dev.071209. [DOI] [PubMed] [Google Scholar]
- 36.Carey L., Winer E., Viale G., Cameron D., Gianni L. Triple-negative breast cancer: disease entity or title of convenience? Nat. Rev. Clin. Oncol. 2010;7(12):683–692. doi: 10.1038/nrclinonc.2010.154. [DOI] [PubMed] [Google Scholar]
- 37.Arnoux V., Nassour M., L'Helgoualc'h A., Hipskind R.A., Savagner P. Erk5 controls slug expression and keratinocyte activation during wound healing. Mol. Biol. Cell. 2008;19(11):4738–4749. doi: 10.1091/mbc.E07-10-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang J., Weinberg R.A. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev. Cell. 2008;14(6):818–829. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 39.Westcott J.M., Prechtl A.M., Maine E.A., Dang T.T., Esparza M.A., Sun H., Zhou Y., Xie Y., Pearson G.W. An epigenetically distinct breast cancer cell subpopulation promotes collective invasion. J. Clin. Invest. 2015;125(5):1927–1943. doi: 10.1172/JCI77767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Friedl P., Locker J., Sahai E., Segall J.E. Classifying collective cancer cell invasion. Nat. Cell Biol. 2012;14(8):777–783. doi: 10.1038/ncb2548. [DOI] [PubMed] [Google Scholar]
- 41.Kim Y.H., Choi Y.W., Lee J., Soh E.Y., Kim J.-.H., Park T.J. Senescent tumor cells lead the collective invasion in thyroid cancer. Nat. Commun. 2017;8(1):15208. doi: 10.1038/ncomms15208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Han G., Lu S.-.L., Li A.G., He W., Corless C.L., Kulesz-Martin M., Wang X.-.J. Distinct mechanisms of TGF-β1–mediated epithelial-to-mesenchymal transition and metastasis during skin carcinogenesis. J. Clin. Invest. 2005;115(7):1714–1723. doi: 10.1172/JCI24399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qin S., Schulte B.A., Wang G.Y. Role of senescence induction in cancer treatment. World J. Clin. Oncol. 2018;9(8):180–187. doi: 10.5306/wjco.v9.i8.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Y., Gundelach J., Lindquist L.D., Baker D.J., van Deursen J., Bram R.J. Chemotherapy-induced cellular senescence suppresses progression of Notch-driven T-ALL. PLoS One. 2019;14(10) doi: 10.1371/journal.pone.0224172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsoi D.T., Rowsell C., McGregor C., Kelly C.M., Verma S., Pritchard K.I. Disseminated tumor embolism from breast cancer leading to multiorgan failure. J. Clin. Oncol. 2010;28(12):e180–e183. doi: 10.1200/JCO.2009.25.1009. [DOI] [PubMed] [Google Scholar]
- 46.Kondo J., Endo H., Okuyama H., Ishikawa O., Iishi H., Tsujii M., Ohue M., Inoue M. Retaining cell–cell contact enables preparation and culture of spheroids composed of pure primary cancer cells from colorectal cancer. PNAS. 2011;108(15):6235–6240. doi: 10.1073/pnas.1015938108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Enderling H., Hlatky L., Hahnfeldt P. Migration rules: tumours are conglomerates of self-metastases. Br. J. Cancer. 2009;100(12):1917–1925. doi: 10.1038/sj.bjc.6605071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aceto N., Toner M., Maheswaran S., Haber D.A. En route to metastasis: circulating tumor cell clusters and epithelial-to-mesenchymal transition. Trends Cancer. 2015;1(1):44–52. doi: 10.1016/j.trecan.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 49.Dirks P. Cancer stem cells: Invitation to a second round. Nature. 2010;466(7302):40–41. doi: 10.1038/466040a. [DOI] [PubMed] [Google Scholar]
- 50.Barrett Lindy E., Granot Z., Coker C., Iavarone A., Hambardzumyan D., Holland Eric C., H-s Nam, Benezra R. Self-renewal does not predict tumor growth potential in mouse models of high-grade glioma. Cancer Cell. 2012;21(1):11–24. doi: 10.1016/j.ccr.2011.11.025. [DOI] [PubMed] [Google Scholar]
- 51.Capp J.-.P. Cancer stem cells: from historical roots to a new perspective. J. Oncol. 2019;2019 doi: 10.1155/2019/5189232. 5189232-5189232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oskarsson T., Batlle E., Massagué J. Metastatic stem cells: sources, niches, and vital pathways. Cell Stem Cell. 2014;14(3):306–321. doi: 10.1016/j.stem.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lapouge G., Beck B., Nassar D., Dubois C., Dekoninck S., Blanpain C. Skin squamous cell carcinoma propagating cells increase with tumour progression and invasiveness. EMBO J. 2012;31(24):4563–4575. doi: 10.1038/emboj.2012.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vessoni A.T., Filippi-Chiela E.C., Lenz G., Batista L.F.Z. Tumor propagating cells: drivers of tumor plasticity, heterogeneity, and recurrence. Oncogene. 2020;39(10):2055–2068. doi: 10.1038/s41388-019-1128-4. [DOI] [PubMed] [Google Scholar]
- 55.Kurtova A.V., Xiao J., Mo Q., Pazhanisamy S., Krasnow R., Lerner S.P., Chen F., Roh T.T., Lay E., Ho P.L. Blocking PGE2-induced tumour repopulation abrogates bladder cancer chemoresistance. Nature. 2015;517(7533):209–213. doi: 10.1038/nature14034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim S.-.Y., Hong S.-.H., Basse P.H., Wu C., Bartlett D.L., Kwon Y.T., Lee Y.J. Cancer stem cells protect non-stem cells from anoikis: bystander effects. J. Cell. Biochem. 2016;117(10):2289–2301. doi: 10.1002/jcb.25527. [DOI] [PubMed] [Google Scholar]
- 57.Kawakami R., Mashima T., Kawata N., Kumagai K., Migita T., Sano T., Mizunuma N., Yamaguchi K., Seimiya H. ALDH1A3-mTOR axis as a therapeutic target for anticancer drug-tolerant persister cells in gastric cancer. Cancer Sci. 2020 doi: 10.1111/cas.14316. n/a(n/a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Auffinger B., Tobias A.L., Han Y., Lee G., Guo D., Dey M., Lesniak M.S., Ahmed A.U. Conversion of differentiated cancer cells into cancer stem-like cells in a glioblastoma model after primary chemotherapy. Cell Death Differ. 2014;21(7):1119–1131. doi: 10.1038/cdd.2014.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lagadec C., Vlashi E., Della Donna L., Dekmezian C., Pajonk F. Radiation-induced reprogramming of breast cancer cells. Stem Cells. 2012;30(5):833–844. doi: 10.1002/stem.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kinstrie R., Horne G.A., Morrison H., Irvine D., Munje C., Castañeda E.G., Moka H.A., Dunn K., Cassels J.E., Parry N. CD93 is expressed on chronic myeloid leukemia stem cells and identifies a quiescent population which persists after tyrosine kinase inhibitor therapy. Leukemia. 2020 doi: 10.1038/s41375-019-0684-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hong H.S., Akhavan J., Lee S.H., Kim R.H., Kang M.K., Park N.-.H., Shin K.-.H. Proinflammatory cytokine TNFα promotes HPV-associated oral carcinogenesis by increasing cancer stemness. Int. J. Oral Sci. 2020;12(1):3. doi: 10.1038/s41368-019-0069-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang V.M.Y., Ferreira R.M.M., Almagro J., Evan T., Legrave N., Zaw Thin M., Frith D., Carvalho J., Barry D.J., Snijders A.P. CD9 identifies pancreatic cancer stem cells and modulates glutamine metabolism to fuel tumour growth. Nat. Cell Biol. 2019;21(11):1425–1435. doi: 10.1038/s41556-019-0407-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Krishnapriya S., Sidhanth C., Manasa P., Sneha S., Bindhya S., Nagare R.P., Ganesan T.S. Abstract 182: cancer stem cells and tumor angiogenesis in serous adenocarcinoma of ovary. Cancer Res. 2019;79(13 Supplement):182. doi: 10.1007/s10456-019-09669-x. [DOI] [PubMed] [Google Scholar]
- 64.Deshors P., Toulas C., Arnauduc F., Malric L., Siegfried A., Nicaise Y., Lemarié A., Larrieu D., Tosolini M., Cohen-Jonathan Moyal E. Ionizing radiation induces endothelial transdifferentiation of glioblastoma stem-like cells through the Tie2 signaling pathway. Cell Death Dis. 2019;10(11):816. doi: 10.1038/s41419-019-2055-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sharma S.V., Lee D.Y., Li B., Quinlan M.P., Takahashi F., Maheswaran S., McDermott U., Azizian N., Zou L., Fischbach M.A. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell. 2010;141(1):69–80. doi: 10.1016/j.cell.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jolly M.K., Tripathi S.C., Jia D., Mooney S.M., Celiktas M., Hanash S.M., Mani S.A., Pienta K.J., Ben-Jacob E., Levine H. Stability of the hybrid epithelial/mesenchymal phenotype. Oncotarget. 2016;7(19):27067–27084. doi: 10.18632/oncotarget.8166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yu M., Bardia A., Wittner B.S., Stott S.L., Smas M.E., Ting D.T., Isakoff S.J., Ciciliano J.C., Wells M.N., Shah A.M. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science. 2013;339(6119):580–584. doi: 10.1126/science.1228522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jordan N.V., Bardia A., Wittner B.S., Benes C., Ligorio M., Zheng Y., Yu M., Sundaresan T.K., Licausi J.A., Desai R. HER2 expression identifies dynamic functional states within circulating breast cancer cells. Nature. 2016;537(7618):102–106. doi: 10.1038/nature19328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu S., Cong Y., Wang D., Sun Y., Deng L., Liu Y., Martin-Trevino R., Shang L., McDermott S.P., Landis M.D. Breast cancer stem cells transition between epithelial and mesenchymal states reflective of their normal counterparts. Stem Cell Rep. 2013;2(1):78–91. doi: 10.1016/j.stemcr.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aceto N., Bardia A., Miyamoto David T., Donaldson Maria C., Wittner Ben S., Spencer Joel A., Yu M., Pely A., Engstrom A., Zhu H. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell. 2014;158(5):1110–1122. doi: 10.1016/j.cell.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jiang X., Wong K.H.K., Khankhel A.H., Zeinali M., Reategui E., Phillips M.J., Luo X., Aceto N., Fachin F., Hoang A.N. Microfluidic isolation of platelet-covered circulating tumor cells. Lab Chip. 2017;17(20):3498–3503. doi: 10.1039/c7lc00654c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Biddle A., Gammon L., Liang X., Costea D.E., Mackenzie I.C. Phenotypic plasticity determines cancer stem cell therapeutic resistance in oral squamous cell carcinoma. EBioMedicine. 2016;4:138–145. doi: 10.1016/j.ebiom.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wagenblast E., Soto M., Gutiérrez-Ángel S., Hartl C.A., Gable A.L., Maceli A.R., Erard N., Williams A.M., Kim S.Y., Dickopf S. A model of breast cancer heterogeneity reveals vascular mimicry as a driver of metastasis. Nature. 2015;520(7547):358–362. doi: 10.1038/nature14403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ganesh K., Basnet H., Kaygusuz Y., Laughney A.M., He L., Sharma R., O'Rourke K.P., Reuter V.P., Huang Y.-.H., Turkekul M. L1CAM defines the regenerative origin of metastasis-initiating cells in colorectal cancer. Nat. Cancer. 2020;1(1):28–45. doi: 10.1038/s43018-019-0006-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Talukdar S., Bhoopathi P., Emdad L., Das S., Sarkar D., Fisher P.B. Chapter two - dormancy and cancer stem cells: an enigma for cancer therapeutic targeting. In: Civin CI, Kingsbury TJ, Kim M, Fisher PB, editors. Volume 141. Academic Press; 2019. pp. 43–84. (Advances in Cancer Research). Edited by. Edited by. [DOI] [PubMed] [Google Scholar]
- 76.Cabarcas S.M., Mathews L.A., Farrar W.L. The cancer stem cell niche—There goes the neighborhood? Int. J. Cancer. 2011;129(10):2315–2327. doi: 10.1002/ijc.26312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sang L., Coller H.A., Roberts J.M. Control of the reversibility of cellular quiescence by the transcriptional repressor HES1. Science. 2008;321(5892):1095. doi: 10.1126/science.1155998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kusumbe A.P., Bapat S.A. Cancer stem cells and aneuploid populations within developing tumors are the major determinants of tumor dormancy. Cancer Res. 2009;69(24):9245. doi: 10.1158/0008-5472.CAN-09-2802. [DOI] [PubMed] [Google Scholar]
- 79.Comaills V., Kabeche L., Morris R., Buisson R., Yu M., Madden M.W., LiCausi J.A., Boukhali M., Tajima K., Pan S. Genomic instability is induced by persistent proliferation of cells undergoing epithelial-to-mesenchymal transition. Cell Rep. 2016;17(10):2632–2647. doi: 10.1016/j.celrep.2016.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pietilä M., Vijay G.V., Soundararajan R., Yu X., Symmans W.F., Sphyris N., Mani S.A. FOXC2 regulates the G2/M transition of stem cell-rich breast cancer cells and sensitizes them to PLK1 inhibition. Sci. Rep. 2016;6(1):23070. doi: 10.1038/srep23070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hollier B.G., Tinnirello A.A., Werden S.J., Evans K.W., Taube J.H., Sarkar T.R., Sphyris N., Shariati M., Kumar S.V., Battula V.L. FOXC2 expression links epithelial–mesenchymal transition and stem cell properties in breast cancer. Cancer Res. 2013;73(6):1981. doi: 10.1158/0008-5472.CAN-12-2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shackleton M. Normal stem cells and cancer stem cells: similar and different. Semin. Cancer Biol. 2010;20(2):85–92. doi: 10.1016/j.semcancer.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 83.Dirkse A., Golebiewska A., Buder T., Nazarov P.V., Muller A., Poovathingal S., Brons N.H.C., Leite S., Sauvageot N., Sarkisjan D. Stem cell-associated heterogeneity in Glioblastoma results from intrinsic tumor plasticity shaped by the microenvironment. Nat. Commun. 2019;10(1):1787. doi: 10.1038/s41467-019-09853-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bu P., Chen K.-.Y., Lipkin S.M., Shen X. Asymmetric division: a marker for cancer stem cells? Oncotarget. 2013;4(7) doi: 10.18632/oncotarget.1029. July 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gundem G., Van Loo P., Kremeyer B., Alexandrov L.B., Tubio J.M.C., Papaemmanuil E., Brewer D.S., Kallio H.M.L., Hognas G., Annala M. The evolutionary history of lethal metastatic prostate cancer. Nature. 2015;520(7547):353–357. doi: 10.1038/nature14347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Maddipati R., Stanger B.Z. Pancreatic cancer metastases harbor evidence of polyclonality. Cancer Discov. 2015;5(10):1086–1097. doi: 10.1158/2159-8290.CD-15-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McFadden David G., Papagiannakopoulos T., Taylor-Weiner A., Stewart C., Carter Scott L., Cibulskis K., Bhutkar A., McKenna A., Dooley A., Vernon A. Genetic and clonal dissection of murine small cell lung carcinoma progression by genome sequencing. Cell. 2014;156(6):1298–1311. doi: 10.1016/j.cell.2014.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Swanton C. Intratumour heterogeneity: evolution through space and time. Cancer Res. 2012;72(19):4875–4882. doi: 10.1158/0008-5472.CAN-12-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kapeleris J., Kulasinghe A., Warkiani M.E., Vela I., Kenny L., O'Byrne K., Punyadeera C. The prognostic role of circulating tumor cells (CTCs) in lung cancer. Front. Oncol. 2018;8 doi: 10.3389/fonc.2018.00311. 311-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dasgupta A., Lim A.R., Ghajar C.M. Circulating and disseminated tumor cells: harbingers or initiators of metastasis? Molecular oncology. 2017;11(1):40–61. doi: 10.1002/1878-0261.12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hen O., Barkan D. Dormant disseminated tumor cells and cancer stem/progenitor-like cells: similarities and opportunities. Semin. Cancer Biol. 2019 doi: 10.1016/j.semcancer.2019.09.002. [DOI] [PubMed] [Google Scholar]
- 92.Eyre R., Alférez D.G., Santiago-Gómez A., Spence K., McConnell J.C., Hart C., Simões B.M., Lefley D., Tulotta C., Storer J. Microenvironmental IL1β promotes breast cancer metastatic colonisation in the bone via activation of Wnt signalling. Nat. Commun. 2019;10(1):5016. doi: 10.1038/s41467-019-12807-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ghajar C.M. Metastasis prevention by targeting the dormant niche. Nat. Rev. Cancer. 2015;15(4):238–247. doi: 10.1038/nrc3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Labelle M., Hynes R.O. The initial hours of metastasis: the importance of cooperative host–tumor cell interactions during hematogenous dissemination. Cancer Discov. 2012;2(12):1091. doi: 10.1158/2159-8290.CD-12-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yu M., Stott S., Toner M., Maheswaran S., Haber D.A. Circulating tumor cells: approaches to isolation and characterization. J. Cell Biol. 2011;192(3):373–382. doi: 10.1083/jcb.201010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Duda D.G., Duyverman A.M.M.J., Kohno M., Snuderl M., Steller E.J.A., Fukumura D., Jain R.K. Malignant cells facilitate lung metastasis by bringing their own soil. Proc. Natl. Acad. Sci. 2010;107(50):21677. doi: 10.1073/pnas.1016234107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Luzzi K.J., MacDonald I.C., Schmidt E.E., Kerkvliet N., Morris V.L., Chambers A.F., Groom A.C. Multistep nature of metastatic inefficiency: dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Am. J. Pathol. 1998;153(3):865–873. doi: 10.1016/S0002-9440(10)65628-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Heyde A., Reiter J.G., Naxerova K., Nowak M.A. Consecutive seeding and transfer of genetic diversity in metastasis. Proc. Natl. Acad. Sci. 2019;116(28):14129. doi: 10.1073/pnas.1819408116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Boire A., Zou Y., Shieh J., Macalinao D.G., Pentsova E., Massagué J. Complement component 3 adapts the cerebrospinal fluid for leptomeningeal metastasis. Cell. 2017;168(6):1101–1113. doi: 10.1016/j.cell.2017.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Welch D.R., Hurst D.R. Defining the hallmarks of metastasis. Cancer Res. 2019;79(12):3011. doi: 10.1158/0008-5472.CAN-19-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chambers A.F., Groom A.C., MacDonald I.C. Dissemination and growth of cancer cells in metastatic sites. Nat. Rev. Cancer. 2002;2(8):563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 102.Friberg S., Nyström A. Cancer Metastases: Early Dissemination and Late Recurrences. Cancer Growth Metastasis. 2015;8:43–49. doi: 10.4137/CGM.S31244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen X., Liao R., Li D., Sun J. Induced cancer stem cells generated by radiochemotherapy and their therapeutic implications. Oncotarget. 2017;8(10):17301–17312. doi: 10.18632/oncotarget.14230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Oshima N., Yamada Y., Nagayama S., Kawada K., Hasegawa S., Okabe H. Induction of cancer stem cell properties in colon cancer cells by defined factors. PLoS One. 2014;9(7) doi: 10.1371/journal.pone.0101735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jeon H.M., Jin X., Lee J.S. Inhibitor of differentiation 4 drives brain tumor-initiating cell genesis through cyclin E and notch signaling. Genes Dev. 2008;22(15):2028–2033. doi: 10.1101/gad.1668708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Baker L.A., Holliday H., Roden D. Proteogenomic analysis of inhibitor of differentiation 4 (ID4) in basal-like breast cancer. Breast Cancer Res. 2020;22:63. doi: 10.1186/s13058-020-01306-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Huang C.P., Tsai M.F., Chang T.H. ALDH-positive lung cancer stem cells confer resistance to epidermal growth factor receptor tyrosine kinase inhibitors. Cancer Lett. 2013 Jan 1;328(1):144–151. doi: 10.1016/j.canlet.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 108.Hirschmann-Jax C., Foster A.E., Wulf G.G. et al. A distinct “side population” of cells with high drug efflux capacity in human tumor cells. PNAS. 2004;101(39):14228–14233. doi: 10.1073/pnas.0400067101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Phi L.T.H., Sari I.N., Yang Y.G. Cancer stem cells (CSCs) in drug resistance and their therapeutic implications in cancer treatment. Stem Cells Int. 2018;2018 doi: 10.1155/2018/5416923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dhar S., Lippard S.J. Mitaplatin, a potent fusion of cisplatin and the orphan drug dichloroacetate. Proc. Natl. Acad. Sci. 2009;106(52):22199. doi: 10.1073/pnas.0912276106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Crowther M.D., Dolton G., Legut M., Caillaud M.E., Lloyd A., Attaf M., Galloway S.A.E., Rius C., Farrell C.P., Szomolay B. Genome-wide CRISPR–Cas9 screening reveals ubiquitous T cell cancer targeting via the monomorphic MHC class I-related protein MR1. Nat. Immunol. 2020;21(2):178–185. doi: 10.1038/s41590-019-0578-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Quarni W., Dutta R., Green R., Katiri S., Patel B., Mohapatra S.S., Mohapatra S. Mithramycin A inhibits colorectal cancer growth by targeting cancer stem cells. Sci. Rep. 2019;9(1):15202. doi: 10.1038/s41598-019-50917-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mahon F.-.X. Treatment-free remission in CML: who, how, and why? Hematol. Am. Soc. Hematol. Educ. Program. 2017;2017(1):102–109. doi: 10.1182/asheducation-2017.1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]