Abstract

Riboflavin (RF) is a well-known photosensitizer, responsible for the light-induced oxidation of methionine (Met) leading to the spoilage of wine. An NMR approach was used to investigate the role of gallic acid (GA) and sulfur dioxide (SO2) in the RF-mediated photo-oxidation of Met. Water solutions of RF and Met, with and without GA or SO2, were exposed to visible light for increasing time in both air and nitrogen atmospheres. Upon light exposure, a new signal appeared at 2.64 ppm that was assigned to the S(O)CH3 moiety of methionine sulfoxide. Its formation rate was lower in a nitrogen atmosphere and even lower in the presence of GA, supporting the ability of this compound in quenching the singlet oxygen. In contrast, SO2 caused relevant oxidation of Met, moderately observed even in the dark, making Met less available in donating electrons to RF. The competition of GA versus Met photo-oxidation was revealed, indicating effectiveness of this antioxidant against the light-dependent spoilage of wine. A pro-oxidant effect of SO2 toward Met was found as a possible consequence of radical pathways involving oxygen.
1. Introduction
Light can be detrimental to the chemical and sensory characteristics of many foods and beverages. A light-induced defect called sunlight flavor or light-struck taste (LST) is known in wine,1 beer,2 and milk.3,4 In wine, the photodegradative phenomena involve riboflavin (RF) and hit methionine (Met) with the consequent formation of sulfur-containing compounds, namely methanethiol and dimethyl disulfide.5,6 The presence of RF in wine is mostly due to yeast metabolism and its concentration can exceed 150 μg/L depending on the yeast strain.7 Like other amino acids, free Met is present in wine (average concentration 3–4 mg/L8,9) and it can also derive from the yeast metabolism and lysis.7 LST occurs most frequently in white wine bottled in clear glass and it can cause relevant economic losses and product waste.10 When wine is exposed to light, especially at wavelengths ranging from 370 to 450 nm, RF is promoted from the ground state (S0) to the reactive singlet state (S1) that can further reach the triplet state (T1) through an intersystem crossing, while generating singlet oxygen from molecular oxygen (pathway Type II).9,11 Singlet oxygen is a highly reactive electrophile able to react with amines, sulfides, and alkenes.12 Riboflavin in the T1 state can be reduced through the reaction with electron-donor compounds, such as Met, thus returning to a low energy state (pathway Type I).9,13−15 As a consequence, Met is oxidized to methional after the loss of two atoms of hydrogen. Methional is also unstable and sensitive to light and, through a retro Michael reaction, degrades to methanethiol. Eventually, two molecules of the latter yield dimethyl disulfide (Figure S1).16 Both Type I and II pathways may enhance oxidation through both the formation of reactive radical species and the production of singlet oxygen. The prevalence of one over the other depends on the concentration of oxygen and the type and concentration of electron-donors.17
Recently, Fracassetti and co-authors15 demonstrated the effectiveness of hydrolysable tannins, namely, gallotannins from nut gall and ellagitannin from oak and chestnut, against the formation of volatile sulfur compounds causing the light-induced fault. In these conditions, the sensory perception of LST was also avoided in model wine solution. The positive effect was comparable to that achieved by the addition of sulfur dioxide (SO2), the most common additive used in winemaking. To explain the protective effect of the hydrolysable tannins, the authors suggested the ability of these compounds to quench the singlet oxygen originating from pathway Type II. Moreover, it was proposed that the limited degradation of Met in presence of tannins might be due to their competition with Met in pathway Type I.
This study aimed to better clarify the role of tannins in the photo-oxidative reactions involving Met and RF. In particular, the possible singlet oxygen scavenging activity of gallic acid (GA), as a constitutive unit of hydrolysable tannins, and its competition with Met were evaluated in an aqueous solution containing RF and Met. Moreover, the role of SO2 was evaluated in dedicated experiments. In this study, an approach based on a nuclear magnetic resonance (NMR) technique was adopted. The NMR technique is a powerful tool to elucidate specific reaction mechanisms and highlight related chemical changes. Moreover, in the last few years, NMR methods have been increasingly applied for the analysis of complex matrices, such as liquid foods including fruit juices, beer, and wine, due to improvements in high-throughput automations, NMR sensitivity, and solvent-suppression routines.18−21 These aspects make NMR a versatile technique suitable for monitoring alcoholic fermentation as well.22 To the best of our knowledge, this is the first study applying an NMR approach for the evaluation of the light-dependent reaction mechanisms occurring between RF and Met in the presence of GA and SO2 in an aqueous environment.
2. Results and Discussion
The light-dependent reactions between RF and Met, also in presence of GA and SO2, were followed in aqueous solutions at pH 3.0 ± 0.1, which were carefully adjusted in order to minimize the effect of pH on the reaction rate and chemical shifts.24 Different conditions in terms of the presence of oxygen were also considered, indicated throughout the manuscript as “air condition” and “nitrogen condition”. In the latter, oxygen was removed from the solutions by extensive sparkling with nitrogen.
The laboratory-made illuminating apparatus allowed to reproduce the photodegradation reactions under standardized conditions with negligible variations of temperature (±2 °C),15 while running the experiments within the NMR tubes was the innovative approach that we adopted to monitor the trend of the reactions due to the possibility to perform spectra measurements immediately and with high throughput.
2.1. Kinetic Analysis of Relevant Chemical Reactions
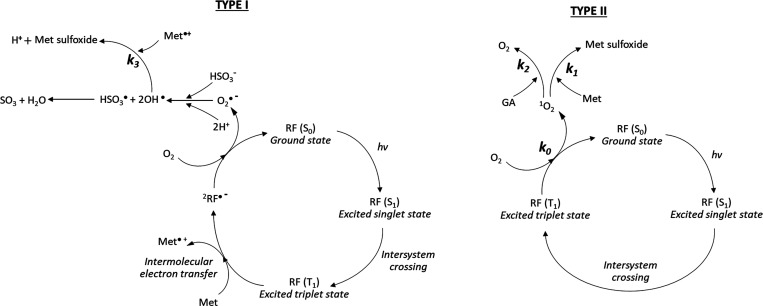
The scheme reported in Figure 1 shows the relevant light-dependent chemical reactions that Met may undergo in all our experimental conditions. This kinetic scheme is general, as it takes into account conditions like presence of GA and SO2 as well as air and nitrogen conditions. For the sake of discussion, we use the term “SO2” with a broad meaning, as generally accepted in oenology, as it considers the complex equilibria occurring in water solution among the metabilsulfite ion (S2O5–, the chemical species actually used in this study), sulfìte ion (HSO3–, the ion actually present in water solution at acidic pH), and SO2 itself. This scheme reflects what is known in the literature about the photodynamic activity of RF.9,13,15 Other side reactions, such as oxidation of GA25,26 and production of volatile sulfur compounds,15,16 might also occur but have not been represented here.
Figure 1.
Photocatalytic cycle for the riboflavin-mediated oxidation of methionine in the presence of gallic acid and sulfur dioxide through Type I and Type II mechanisms.
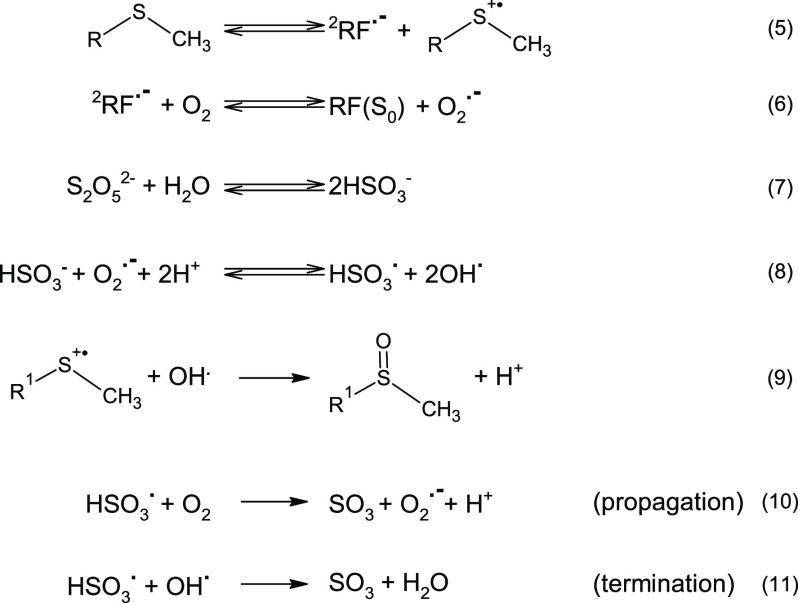
From the scheme in Figure 1, the following chemical reactions were considered, leading to the minimum set of kinetic differential equations able to describe the experimental data (equations 12–16). In this way, we have been able to discriminate between the experimental formation rates of Met sulfoxide, also reported in Table 1, and the corresponding kinetic constants, as their values are independent on the addition of antioxidants, singlet oxygen quenchers, or oxygen depletion:
 |
Table 1. Rate Constant Values (k) and Reaction Rate Values for Methionine Sulfoxide Formationa.
| antioxidant (mM) | air/nitrogen condition | [O2] (mM) | k0 (mM–1 s–1) | k1 × 103 (mM–1 s–1) | k2[antiox] (mM–1 s–1) | k3 (mM–2 s–1) | reaction rate × 103* (mM s–1) |
|---|---|---|---|---|---|---|---|
| no antioxidant | air | 0.26 | 0.20 | 0.28 | nd | nd | 0.17 ± 0.02 |
| gallic acid (2 mM) | air | 0.26 | 0.20 | 0.28 | 0.12 | nd | 0.030 ± 0.003 |
| sulfur dioxide (2 mM) | air | 0.26 | 0.20 | 0.28 | 0.034 | 0.015 | 3.18 ± 0.64 |
| sulfur dioxide (2 mM) + sodium azide (20 mM) | air | 0.28 | 0.20 | 0.28 | 0.14 | 0.0075 | 2.41 ± 0.26 |
| sulfur dioxide (1 mM) | air | 0.26 | 0.20 | 0.28 | 0.017 | 0.015 | 1.47 ± 0.16 |
| no antioxidant | nitrogen | 0.13 | 0.20 | 0.28 | nd | nd | 0.071 ± 0.010 |
| gallic acid (2 mM) | nitrogen | nd | nd | nd | nd | nd | < 0.005 |
| sulfur dioxide (2 mM) | nitrogen | 0.03 | 0.20 | 0.28 | 0.034 | 0.015 | 0.19 ± 0.01 |
In all reported experimental conditions, the light exposure was carried out with one fluorescent lamp up to 600 s. *: reaction rate was measured from d[Met-SO]/dt at t = 0. Estimated standard error for k values is ±10%. Legend: nd, not determined.
Reaction 1 describes the production of singlet oxygen by 1RF after light exposure, assuming that intersystem crossing is the rate-determining step (Type II mechanism). Singlet oxygen is going to oxidize Met to produce Met sulfoxide through a multistep mechanism. This reaction is governed by k1 (reaction 2). In the presence of an antioxidant and/or singlet oxygen quencher, such as GA or sodium azide, respectively, singlet oxygen converts back to molecular oxygen (k2 rate constant, reaction 3).
 |
5 |
Besides reaction 1–4, additional mechanisms can occur due to the addition of SO2. The chemistry of the most used additive in winemaking is quite complex, and it has been studied in the past years.27−29 The presence of SO2 can introduce additional oxidative pathways to the Type I mechanism (Figure 1). Met might act as electron donor to the excited RF triplet state, thus generating a Met radical cation and a RF radical anion,30 which further interacts with molecular oxygen producing superoxide radical (reaction 6). In the presence of HSO3–, generated by the hydrolysis equilibria of metabisulfite (reaction 7), superoxide ion is able to react with the sulfite ion generating HSO3 and OH· radical species (reaction 8).31 This introduces further radical chain reactions that increase the rate in Met sulfoxide release, collectively described by the k4 kinetic constant (reaction 4).32 As long as oxygen is present in the solution, the radical chain reaction is sustained by superoxide production (reaction 10). Eventually, the chain reaction is terminated by a reaction between two radical species, mainly OH·, HSO3, and Met·+ (reaction 9 and 11), ultimately producing additional Met sulfoxide and SO3. The overall reaction is actually given by reaction 4.
2.2. Assay with Gallic Acid
Gallic acid is the constitutive unit of gall nut tannins,33,34 which have already shown a protective effect against the appearance of LST.15 The light-dependent reactions involving RF and Met in the presence of GA were first monitored by irradiating the solutions in glass vials. Initial concentrations of the investigated compounds (RF, Met, GA) much higher than in real oenological conditions15 were chosen in order to have detectable NMR signals in a short time span during the kinetic experiments and to unambiguously highlight the involved reactions. A new signal was revealed at 2.64 ppm in the NMR spectra obtained for lightened samples, either in the absence (Figure 2B) and in the presence (Figure 2C) of GA. This signal, labeled as 3 in Figure 2, was assigned to the S(O)CH3 group of Met sulfoxide. The same signal, although with a much lower intensity (<1% of the main Met signal), could also be found in samples kept in the dark (Figure 2A), but it might be due to some unavoidable oxidation of Met during the sample preparation. After prolonged light exposure in the absence of GA, other resonances could be detected in the same spectral region (Figure 2B). The photoinduced oxidation of Met in presence of a photosensitizer compound, namely RF, could give origin to numerous compounds, with Met sulfoxide described as the major one produced in air conditions.35 The detected additional resonances might therefore originate by subsequent oxidative degradation of Met and/or Met sulfoxide. The actual chemical structure was not determined, but we could exclude the formation of methionine sulfone, methional, by means of spiking experiments (data not shown), or methanethiol, as the measured chemical shifts did not match with literature data. As shown later, these resonances did not appear when milder light conditions were applied (one fluorescent lamp, shorter exposure times).
Figure 2.
1H-NMR spectra (600 MHz) for 2 mM methionine (H2O/D2O 9:1 (v/v), pH 3.0 ± 0.1) exposed to light in the NMR tube in the presence of 0.2 mM riboflavin and 2 mM gallic acid (three fluorescent lamps) in an air condition. (A) control sample (dark condition); (B) after 15 min of light exposure; (C) after 15 min of light exposure with 2 mM gallic acid. Assignments are as follows: 1, methionine γ-CH2; 2, riboflavin 6-CH3 (low field) and 7-CH3 (high field); 3, methionine sulfoxide S(O)CH3.
A possible Met oxidation by other reagents, in particular, nitric acid, was excluded since neither a variation of Met signals nor extra resonances were detected in samples prepared with or without RF (data not shown).
The experimental evidence for the formation of Met sulfoxide derived not only from the resonance at δ 2.6 ppm but also from the characteristic multiplet resonating at δ 2.85–3.05 ppm assigned to the γ-CH2 moiety of Met sulfoxide (Figure S2). It should be noted that the sulfoxide group is actually a stereogenic center and thus may exist in two forms with opposite configurations (R/S). As Met itself has a stereogenic center at Cα (S configuration), Met sulfoxide might be produced in solution as a diastereomeric mixture (SR and SS configurations). The observed pattern of the γ-CH2 multiplet corresponds to the overlap of two separate γ-CH2 spin systems and, indeed, it is identical to the pattern described for a 1:1 mixture of the two diastereoisomers.36 The assignment was finally confirmed by the addition of the standard compound. During light exposure, the relative intensities of the γ-CH2 multiplets did not significantly change and were identical to those measured in the commercial compound. This indicates that both diastereoisomers increased at the same time, providing further evidence for the non-stereospecific formation of Met sulfoxide.37
The reaction rate constant of Met sulfoxide formation was preliminary determined by the initial curve slope (d[Met-S(O)CH3]/dt)t = 0. In air conditions, the reaction rate was 28.4 × 10–3 mM s–1 but was two-fold lower (12.54 × 10–3 mM s–1) in the presence of GA (Figure S3). We have related these results to the known ability of GA, actually observed for other phenolics, to act as a singlet oxygen quencher.25,26
Further experiments were carried out under milder conditions by changing the light exposure (one lamp, shorter irradiation time, up to 10 min). Most decisively, by directly exposing to light the solutions within the NMR tube, we were able to accurately monitor the Met-RF photo-oxidation reactions by acquiring NMR spectra every 30 s of light irradiation. Figure 3 shows the comparison of the NMR spectra obtained for samples after 120 s light exposure under air conditions in the absence (Figure 3B) and in the presence (Figure 3C) of GA. Even in such less severe lighting conditions, the signal corresponding to Met sulfoxide is clearly evident in the absence of GA, whereas it was negligible in the presence of GA. It is worth noting that the small signal appearing at 2.65 ppm in Figure 33A and C is actually due to the partial overlap with the C13 satellite signal of Met γ-CH2 (1JCH = 139 Hz).
Figure 3.
1H-NMR spectra (600 MHz) for 2 mM methionine (H2O/D2O 9:1 (v/v), pH 3.0 ± 0.1) exposed to light in the NMR tube in the presence of 0.2 mM riboflavin and 2 mM gallic acid (one fluorescent lamp) in an air condition. (A) control sample (dark condition); (B) after 120 s; (C) after 120 s of light exposure with 2 mM gallic acid. Assignments are as follows: 1, methionine γ-CH2; 2 riboflavin 6- CH3 (low field) and 7-CH3 (high field); 3, methionine sulfoxide S(O)CH3. *: C13 satellites of Met γ-CH2.
The overall oxidation of Met was indirectly evaluated by repeating the experiment in nitrogen conditions (Figure 4). In such conditions, the signal of Met-S(O)CH3 was detected in the experiment performed in the absence of GA (Figure 4B), whereas in the presence of GA, it resulted almost undetectable even after 10 min of light exposure (Figure 4C). We noticed that in the absence of GA after 420 s of light exposure, the signals of RF disappeared (Figure 4B). On the contrary, RF resonances were found even after 10 min of light exposure in the presence of GA (Figure 4C). Similar observation occurred for the aromatic RF signals at δ 7.89 ppm and δ 7.86 (data not shown). This is consistent with the suggested ability of GA to quench not only singlet oxygen but also the RF triplet state (T3).2 The loss of RF signals is related to a change in the electronic state of RF as discussed as follows (see Section 2.3).
Figure 4.
1H-NMR spectra (600 MHz) for 2 mM methionine (H2O/D2O 9:1 (v/v), pH 3.0 ± 0.1) exposed to light in the NMR tube in the presence of 0.2 mM riboflavin and 2 mM gallic acid (one fluorescent lamp) in a nitrogen condition. (A) control sample (dark condition); (B) after 420 s of light exposure; (C) after 600 s of light exposure with 2 mM gallic acid. Assignments are as follows: 1, methionine γ-CH2; 2, riboflavin 6-CH3 (low field) and 7-CH3 (high field); 3, methionine sulfoxide S(O)CH3. *: C13 satellites of Met γ-CH2.
Figure 5A reports the kinetics of Met sulfoxide formation during the above described experiments. The experimental data were fitted by directly solving the differential equations related to the reactions described in Section 2.1 (equations 12–16; see also Section 4.5). As no decrease of both GA and RF (ground state) was found during light exposure at least up to 240 s, their concentrations were kept fix to the initial conditions during the numerical simulation. We did not consider the time course of Met as its decrease was negligible. Actually, the detected amount of Met sulfoxide was about 2.5% of Met.
Figure 5.
Time course of methionine sulfoxide formation during light exposure (one fluorescent lamp) for a 2 mM methionine (Met) solution (H2O/D2O 9:1 (v/v), pH 3.0 ± 0.1) in the presence of 0.2 mM riboflavin under the following conditions: (A) air condition in the absence (circle open) and presence (box) of 2 mM gallic acid (GA); nitrogen condition in the absence of GA (box solid). (B) air condition in the presence of 1 mM (circle open) and 0.5 mM (box solid) Na2S2O5; air condition in the presence of 1 mM Na2S2O5 and 20 mM sodium azide (*); nitrogen conditions in the presence of 1 mM Na2S2O5 (box). Data are the average of experiments performed in triplicate. Standard deviations are reported as error bars. Lines represent the numerical simulations based on equations 12–16 using the kinetic constants reported in Table 1.
In the absence of GA, the formation of Met sulfoxide was two times higher in air conditions than in nitrogen conditions (d[Met-S(O)CH3]/dt = 0.17 × 10–3 mM s–1 vs 0.071 × 10–3 mM s–1) (Table 1, Figure 5A). In the presence of GA, the reaction rate of Met sulfoxide formation resulted in a six-fold decrease (d[Met-S(O)CH3]/dt = 0.030 × 10–3 mM s–1 vs 0.17 × 10–3 mM s–1) (Table 1, Figure 5A) in air conditions, while under nitrogen, the absence of oxygen obviously depressed the rate of formation to an undetectable level (estimate: <0.005 × 10–3 mM s–1, Table 1).
2.3. Assay with Sulfur Dioxide
The photo-oxidative reactions between RF and Met in presence of SO2 were measured by direct light exposure of solutions in the NMR tubes in both air and nitrogen conditions. As for the GA trials, concentrations were higher than those usually found in wine in order to follow the reactions by NMR easily and rapidly.
Surprisingly, in both cases, addition of metabisulfite caused a relevant formation of Met sulfoxide even after 60 s of light exposure (Figure 5B and Figure 6). Presence of this oxidative product was negligible in solutions kept in the dark (with or without SO2). We hypothesized that Met sulfoxide could arise from aerobic oxidation of bisulfite, leading to several radical species. These last might also be present in the dark;32 for this reason, we measured the concentration of Met-S(O)CH3 in a solution of Met with metabisulfite that was kept in the dark (see Section 4.3.2). After 3 h, Met sulfoxide was 0.05 mM, a concentration by far negligible when compared to that obtained after 60 s under light exposure (0.31 mM). Under this condition, Met sulfoxide formation was faster in the presence than in the absence of SO2, (e.g., d[Met-S(O)CH3]/dt = 3.18 × 10–3 mM s–1 vs 0.17 × 10–3 mM s–1, in air condition, Table 1) irrespective of whether in air or nitrogen conditions. Consistently, Met oxidation rate depends on the concentration of SO2. When the amount of SO2 was halved (1 mM vs 2 mM), the oxidation rate proportionately decreased (d[Met-S(O)CH3]/dt = 1.47 × 10–3 mM s–1 vs 3.18 × 10–3 mM s–1) (Table 1, Figure 5B). This finding gave evidence that the rate of Met sulfoxide formation is first order with respect to SO2.
Figure 6.
1H-NMR spectra (600 MHz) for 2 mM methionine (H2O/D2O 9:1 (v/v), pH 3.0 ± 0.1) exposed to light in the NMR tube in the presence of 0.2 mM riboflavin and 1 mM Na2S2O5 (one fluorescent lamp). (A) Without Na2S2O5; (B) with 1 mM Na2S2O5. Irradiation times are reported on top of spectra. Assignments are as follows: 1, methionine γ-CH2; 2, riboflavin 6-CH3 (low field) and 7-CH3 (high field); 3, methionine sulfoxide S(O)CH3.
The experimental data were used to directly solving the differential equations related to the reactions described in Section 2.1 (equations 12–16; see also Section 4.5). As described in the previous paragraph, RF concentration was kept constant during the numerical simulation (see below). The action of SO2 is governed by the k3 constant (equation 15) the value of which is 50-fold higher than Met oxidation by singlet oxygen (k3 = 15 × 10–3 mM–2 s–1 vs k1 = 0.28 × 10–3 mM–1 s–1) (Table 1, Figure 5B). This indicates that upon light exposure in our experimental conditions, SO2 has a relevant pro-oxidant behavior toward Met sulfoxide formation rather than behaving as an antioxidant. Oxygen is the ultimate oxidant reagent in all conditions, but SO2 promotes Met sulfoxide formation by introducing an alternative radical pathway.31,32 Radicals HSO3·, OH·, and O2·– were the dominant species that cause Met oxidation (see reactions 8 and 9).
In both air and nitrogen conditions, the two RF methyl signals resonating at δ 2.38–2.5 ppm experienced a severe line broadening and a remarkable decrease in intensity after 240 s of light exposure (Figure 6B). The same effect was observed in the absence of SO2, although after longer exposure (Figure 6A). This should not be interpreted as oxidative bleaching, responsible for irreversible destruction of RF, but rather as the accumulation of the 2RF·– radical anion produced from the RF excited triplet state during the Type I mechanism.9 The unpaired electron exerts a strong influence on NMR resonance transverse relaxation rates of neighbor protons (1/T2). This leads to detectable line broadening by chemical exchange with the flavin radical and eventually to complete signal disappearance (see Figure 7B and Figure 2 in reference (38)). Such flavin radical rapidly accumulates soon after the consumption of molecular oxygen during Met oxidation, which occurs more rapidly in the presence of SO2. A direct proof of the reversible formation of RF radical anions was given by detecting the restoration of RF signals and the 30% increase in Met sulfoxide concentration after bubbling air directly into the NMR solution for two min (Figure 7C).
Figure 7.
1H-NMR spectra (600 MHz) for 2 mM methionine (H2O/D2O 9:1 (v/v), pH 3.0 ± 0.1) exposed to light in the NMR tube in the presence of 0.2 mM riboflavin and 1 mM Na2S2O5 (one fluorescent lamp). (A) control sample (dark condition); (B) after light exposure under air conditions for 600 s; (C) after 600 s under light (air condition) and subsequent air bubbling for 2 min in the dark. Assignments are as follows: 1, methionine γ-CH2; 2, riboflavin 6-CH3 (low field) and 7-CH3 (high field); 3, methionine sulfoxide S(O)CH3; 4, 1,4-dioxane; 5, methionine α-H; 6, methionine SCH3; 7, methionine β-CH2; 8, methionine sulfoxide β-CH2; 9, methionine sulfoxide γ-CH2 [δ 2.88 ppm (S,R); δ 2.93 ppm and δ 2.83 ppm (S,S)].
As depicted in Figure 1, both Type I and Type II mechanisms might co-exist in the presence of SO2. In order to evaluate the role played by singlet oxygen (Type I mechanism) in Met oxidation in comparison with the above described radical pathway, further experiments were devised in the presence of sodium azide. Figure 8A shows the NMR spectrum obtained in the presence of SO2 in nitrogen conditions at 240 s of light exposure. As previously discussed, a little formation of Met sulfoxide was observed due to the limited amount of oxygen leading to the accumulation of an RF radical intermediate. As a consequence, the signals assigned to RF were not detected. In the presence of oxygen (Figure 8B), we observed the formation of Met sulfoxide, leading to a complete consumption of molecular oxygen. As a consequence, the accumulation of the RF radical intermediate occurred and, like in nitrogen conditions, the signals assigned to RF were not detected. The addition of sodium azide had only a little effect on preventing Met oxidation with about a 25% decrease in the oxidation rate (Table 1). However, in this case, the characteristic NMR signals of RF were preserved (Figure 8C), suggesting that the RF radical ion did not accumulate. Sodium azide is a well-known singlet oxygen quencher,39,40 but the little difference between the formation rates might suggest that only a small fraction of the overall Met oxidation relates to Type I mechanism with a major role played by the SO2-catalyzed radical pathway. In addition, it has been suggested a possible role of sodium azide in quenching the RF triplet state.41,42 This is consistent with our findings that the RF signals were still detected even after 600 s of light exposure in presence of sodium azide (Figure 8C).
Figure 8.
1H-NMR spectra (600 MHz) for 2 mM methionine (H2O/D2O 9:1 (v/v), pH 3.0 ± 0.1) exposed to light in the NMR tube for 240 s in the presence of 0.2 mM riboflavin and 1 mM Na2S2O5 (one fluorescent lamp). (A) Without sodium azide in nitrogen conditions; (B) without sodium azide in air conditions; (C) with 20 mM sodium azide in air conditions. Assignments are as follows: 1, methionine γ-CH2; 2, riboflavin 6-CH3 (low field) and 7-CH3 (high field); 3, methionine sulfoxide S(O)CH3.
3. Conclusions
The photo-oxidation reactions involving RF and Met brought to formation of Met sulfoxide, which allows pointing out specific behaviors for both GA and SO2. The first compound acts as a very effective photoprotector inhibiting the increase of Met sulfoxide in both air and nitrogen conditions. On the contrary, SO2 behaves as pro-oxidant under light conditions, promoting a fast and quantitative oxidation of Met to Met sulfoxide mainly through radical pathways using the dissolved molecular oxygen. The oxidation of Met, however, ends up once the molecular oxygen is completely consumed.
In this work, a model system was conceived where Met was the only electron donor. Both GA and SO2 were added at levels higher than those usually found in wine and this has to be considered in terms of their effect against the photo-oxidative reactions. However, we believe that the mechanisms outlined here are still valid in a real oenological condition. A lower amount of Met sulfoxide could be expected to form in bottled wine also depending on dissolved oxygen at bottling. However, the radical species originated as a consequence of the light exposure can oxidize other wine components, such as aromatic compounds and amino acids other than Met.
NMR was found to be a suitable technique, with reference to Met sulfoxide, to investigate the photoinduced oxidation mechanisms occurring between RF and Met. The NMR approach allows elucidating such reactions in an aqueous environment in a short time.
From an oenological perspective, the addition of phenolics can exert a protective effect against the photo-oxidative reactions representing an appropriate tool to avoid the light-dependent spoilage of wine. Further researches will be carried out in order to understand the effectiveness of phenolics at a wine concentration level as well as their effect on wine composition and occurrence of LST depending on wine storage conditions.
4. Experimental Section
4.1. Chemicals and Reagents
Riboflavin (RF), l-methionine (Met), methionine sulfoxide (Met sulfoxide), methionine sulfone, methional, nitric acid, sodium 4,4-dimethyl-2-silapentane-1-sulfonate (DSS), deuterium oxide (99.8% isotopic purity), and 5 mm O.D. NMR tubes (Wilmad 535-PP type) were purchased from Sigma-Aldrich (Milan, Italy). Gallic acid (GA) was purchased from Carlo Erba (Rodano, Milan, Italy) and sodium metabisulfite from Baker (Deventer, Holland). All solutions were prepared using water purified through a Milli-Q system (Millipore Filter Corp., Bedford, MA, USA).
4.2. Conditions of Light Exposure
The light treatment was carried out using a laboratory-made apparatus previously described15 and consisting of three fluorescence light bulbs placed 40 cm from each other. The glass vials (10 mL) containing the sample were positioned in order to be fully illuminated. The compact fluorescent light bulbs (Philips) emitted cold light (6500 K) with a luminous flux of 3172 Lumen with high emission in the absorption wavelengths of riboflavin (370 and 440 nm). The apparatus was kept in a dark room with air conditioning set at 20 ± 2 °C. The temperature was monitored in the middle of the apparatus using a thermometer dipped in a vial containing water.
A lamp of the same type was mainly used for the light exposure of the samples into the NMR tube. The tube was filled with the sample and placed in front of the lamp at the distance of about 30 cm.
The samples were maintained at 20 ± 2 °C and protected from light before and after the controlled light exposure.
4.3. Experimental Plan
Experiments carried out under both air and nitrogen atmospheres were designed with the purpose of evaluating the ability of GA to (i) scavenging the singlet oxygen and (ii) competing with Met in donating electrons to RF. A separate set of experiments was designed to evaluate the effect of SO2 on RF and Met photo-oxidation under both air and nitrogen atmospheres.
4.3.1. Assays with Gallic Acid
In a preliminary assay, a water solution containing RF (0.2 mM; 75.3 mg/L) and Met (2 mM; 298.4 mg/L) in the absence and in the presence of GA (2 mM; 340.2 mg/L) and adjusted at pH 3 ± 0.1 with 0.01 N nitric acid was exposed to light up to 120 min in 10 mL clear glass vials closed with a screw cap (Fisher Scientific, Rodano, MI, Italy). The solution was poured into six vials, five of which were exposed to light, and one was maintained at dark. The light exposure lasted 120 min in total, and every 15 min, one vial was collected, the solution was transferred in a NMR tube protected from light, and the NMR spectrum immediately registered (Table S1). Both the signal found at 2.64 ppm assigned to the S(O)CH3 moiety and the characteristic multiplet at 2.85–3.05 ppm assigned to the γ-CH2 moiety pointed to the identification of Met sulfoxide (Figure S2). Compound identity was confirmed through the addition of pure Met-sulfoxide (50 μL of a 10 mM solution) into the NMR tube. In order to identify the other resonances not ascribable to Met sulfoxide, the addition into separate NMR tubes of 50 μL of standard 10 mM methionine sulfone and methional were carried out. The above described solutions were used for further experiments directly carried out into the NMR tube (550 μL). The tube was filled with the solution, exposed to up to eight lighting cycles (10 min each), as detailed in Table S1, and then the NMR spectrum was immediately recorded. Additional experiments (referred to as “nitrogen conditions” throughout the text) were carried out after removal of oxygen from the stock solutions by nitrogen bubbling for 15 min and introducing the solutions under nitrogen into the NMR tube followed by nitrogen sparging of headspace for 5 min. Further details of the experiments are reported in Table S1. In order to exclude Met oxidation not directly linked to light exposure, a 2 mM water solution of Met with and without addition of RF (0.2 mM) and adjusted at pH 3.0 ± 0.1 by addition of nitric acid 0.01 N was kept under dark conditions and analyzed by NMR every 15 min up to 3 h.
4.3.2. Assays with Sulfur Dioxide
A water solution (550 μL) containing RF (0.2 mM; 75.3 mg/L), Met (2 mM; 298.4 mg/L), and 1 mM sodium metabisulfite equivalent to 2 mM SO2 (128 mg/L) was adjusted at pH 3.0 ± 0.1 with 0.01 N nitric acid, poured into the NMR tube and exposed up to 10 lighting cycles for a total of 10 min under light. Both air and nitrogen conditions were tested (see Section 4.3.1). The NMR spectra were acquired immediately after light exposure. Moreover, after 10 min (600 s) of light exposure, air was bubbled directly into the NMR tube protected from light for 2 min and NMR spectra were registered. As a control, the same solution was kept in the dark and NMR spectra were registered every 15 min up to 3 h.
In order to evidence the impact of SO2 concentration on the Met sulfoxide formation rate, further experiments were carried out under air conditions in presence of 1 mM SO2 equivalent (0.5 mM sodium metabisulfite), RF (0.2 mM), and Met (2 mM) at a pH value adjusted to 3.0 ± 0.1 with nitric acid 0.01 N.
To clarify the role of singlet oxygen in Met oxidation in the presence of SO2, an experiment was repeated by adding sodium azide (20 mM) under air conditions (see Table S2).
4.4. NMR Analysis
The 1H-NMR spectra were recorded on a Bruker AV600 spectrometer (Bruker Spectrospin AG, Rheinstetten, Germany), operating at 600.1 MHz for the 1H nucleus and equipped with a standard triple-resonance probe with z-axis gradients and temperature control unit. 1H- chemical shifts (δ) were measured in ppm, used as a reference external DSS set at 0.00 ppm. All NMR samples were prepared in 550 μL H2O:D2O (9:1, v/v). Water signal suppression was achieved by excitation sculpting with gradients.23 Other relevant acquisition parameters were: time-domain, 16 K; number of scans, 196; relaxation delay, 4 s; total acquisition time, 13 min. All NMR experiments were performed in triplicate, except for that in glass vials, on solutions prepared from daily-made stock solutions that had undergone the relevant treatment. NMR acquisition was carried out at 25 ± 1 °C.
4.5. Data Treatment
Raw NMR data were Fourier transformed after 0.3 Hz exponential multiplication and baseline correction. Spectra were processed using Bruker software TOPSPIN v.1.3.
For each NMR experiment, all concentrations were measured upon integration and referred on the response of 1,4-dioxane 0.09 mM (δ 3.58 ppm) added as internal standard. The quantification of Met sulfoxide (S(O)CH3, δ 2.64 ppm) took into account the partial overlap of C13 satellites of Met (δ 2.64 ppm and 2.44 ppm). The intensity of C13 satellite defined the limit of detection (LOD) of Met sulfoxide as 3.37 μM (0.55%). Apparent kinetic rate constants (k) were determined by solving with numerical methods the following general kinetic differential equations (see Section 2.1). As Type I and Type II reaction cycles depicted in Figure 1 are rather complex to be completely described by formal differential equations, we tried to limit the number of kinetic parameters to a minimum and devise a minimum number of rate-determining steps that are able to numerically describe the experimental data:
| 12 |
| 13 |
| 14 |
| 15 |
| 16 |
where k0 – k3 have the following meaning:
k0 (mM–1 s–1): photodynamic singlet oxygen production by RF;
k1 (mM–1 s–1): Met sulfoxide production by singlet oxygen (Type II mechanism);
k2 (mM–1 s–1): singlet oxygen quenching by antioxidant or sodium azide;
k3 (mM–2 s–1): Met sulfoxide production by radicals originated from molecular oxygen and sulfite ion (superoxide radical-mediated Type I mechanism).
“[antiox]” indicates the molar concentration of GA, sodium azide, or SO2 according to the actual experimental conditions reported in Table 1.
Calculations were performed with the aid of wxMaxima (Maxima, a Computer Algebra System, version19.01.2x (2019) http://wxmaxima.sourceforge.net/). Rate constant were optimized using a “grid-search” least-squares fitting, using 5% spacing for each parameter.
Initial conditions for the differential equations were defined as the actual experimental concentrations of RF, Met, and all relevant antioxidants. The initial concentration values of Met sulfoxide were introduced as measured by NMR at t = 0. For air conditions, the concentration of oxygen was assumed to be equal to 0.26 mM. In nitrogen conditions, it varied between 0.03 and 0.12 mM, the actual value being optimized during fitting as reported in Table 1.
The data are expressed as the concentration of Met sulfoxide (mM) calculated from the integral of the methyl resonance assigned to Met sulfoxide-S(O)CH3, using 1,4-dioxane as internal reference. The reaction rate (mM s–1) was determined as the concentration of Met sulfoxide as function of time (d[Met-S(O)CH3]/dt).
Besides Met sulfoxide and IS, the signals followed through the light exposure were those assigned to RF (δ 2.48 and 2.38 ppm for 6-CH3 (low field) and 7-CH3 (high field), respectively; δ 7.89 and δ 7.86 ppm for 9-H and 6-H, respectively), methionine γ-CH2 (δ 2.55 ppm), and GA (δ 7.05 ppm).
Acknowledgments
The study was supported by the European Agricultural Fund for Rural Development (Enofotoshield project; D.d.s. 1 luglio 2019 - n. 9551, B.U.R.L. Serie Ordinaria n. 27 - 04 luglio 2019) and Piano di Sostegno alla Ricerca 2017-2018 – Linea 2 – Università degli Studi di Milano.
Glossary
Abbreviation Used
- RF
riboflavin
- Met
methionine
- Met sulfoxide
methionine sulfoxide
- GA
gallic acid
- SO2
sulfur dioxide
- LST
light-struck taste
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c03845.
Reaction scheme of methional formation due to light exposure; 1H-NMR spectra of methionine sulfoxide in light-exposed sample and commercial sample; kinetics of methionine sulfoxide formation for the preliminary experiment; experimental conditions applied in the presence of gallic acid; and experimental conditions applied in the presence of sulfur dioxide (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Mattivi F.; Monetti A.; Vrhovšek U.; Tonon D.; Andrés-Lacueva C. High-performance liquid chromatographic determination of the riboflavin concentration in white wines for predicting their resistance to light. J. Chromatogr. A. 2000, 888, 121–127. 10.1016/S0021-9673(00)00561-6. [DOI] [PubMed] [Google Scholar]
- Goldsmith M. R.; Rogers P. J.; Cabral N. M.; Ghiggino K. P.; Roddick F. A. Riboflavin triplet quenchers inhibit lightstruck flavor formation in beer. J. Amer. Soc. Brew. Chem. 2005, 63, 177–184. 10.1094/ASBCJ-63-0177. [DOI] [Google Scholar]
- Fracassetti D.; Limbo S.; D’Incecco P.; Tirelli A.; Pellegrino L. Development of a HPLC method for the simultaneous analysis of riboflavin and other flavin compounds in liquid milk and milk products. Eur. Food Res. Technol. 2018, 244, 1545–1554. 10.1007/s00217-018-3068-6. [DOI] [Google Scholar]
- Limbo S.; Pellegrino L.; D’Incecco P.; Gobbi S.; Rosi V.; Fracassetti D. Storage of pasteurized milk in clear PET bottles combined with light exposure on a retail display case: A possible strategy to define the shelf life. Food Chem. 2020, 329, 127116. 10.1016/j.foodchem.2020.127116. [DOI] [PubMed] [Google Scholar]
- Maujean A.; Seguin N. Contribution à l’étude des goúts de lumière dans les vins de Champagne. 4. Approaches a une solution œnologique des moyens de prévention des goúts de lumière. Sci. Aliment. 1983, 3, 603–613. [Google Scholar]
- Andrés-Lacueva C.; Mattivi F.; Tonon D. Determination of riboflavin, flavin mononucleotide and flavin-adenine dinucleotide in wine and other beverages by high-performance liquid chromatography with fluorescence detection. J. Chromatogr. A. 1998, 823, 355–363. 10.1016/S0021-9673(98)00585-8. [DOI] [PubMed] [Google Scholar]
- Fracassetti D.; Gabrielli M.; Encinas J.; Manara M.; Pellegrino I.; Tirelli A. Approaches to prevent the light-struck taste in white wine. Aust. J. Grape Wine Res. 2017, 23, 329–333. 10.1111/ajgw.12295. [DOI] [Google Scholar]
- Amerine M A; Ough C S.. Alcohols. In Methods for Wine and Must Analysis; Amerine M. A., Ough C. S., Eds.; John Wiley and Sons: New York, 1980. [Google Scholar]
- Grant-Preece P.; Barril C.; Schmidtke L. M.; Scollary G. R.; Clark A. C. Light-induced changes in bottled white wine and underlying photochemical mechanisms. Crit. Rev. Food Sci. Nutr. 2017, 57, 743–754. 10.1080/10408398.2014.919246. [DOI] [PubMed] [Google Scholar]
- Cáceres-Mella A.; Flores-Valdivia D.; Laurie V. F.; López-Solís R.; Peña-Neira Á. Chemical and sensory effects of storing Sauvignon Blanc wine in colored bottles under artificial light. J. Agric. Food Chem. 2014, 62, 7255–7262. 10.1021/jf501467f. [DOI] [PubMed] [Google Scholar]
- Choe E.; Huang R.; Min D. B. Chemical reactions and stability of riboflavin in foods. J. Food Sci. 2005, 70, R28–R36. 10.1111/j.1365-2621.2005.tb09055.x. [DOI] [Google Scholar]
- DeRosa M. C.; Crutchley R. J. Photosensitized singlet oxygen and its applications. Coord. Chem. Rev. 2002, 233-234, 351–371. 10.1016/S0010-8545(02)00034-6. [DOI] [Google Scholar]
- Cardoso D. R.; Libardi S. H.; Skibsted L. H. Riboflavin as a photosensitizer. Effects on human health and food quality. Food Funct. 2012, 3, 487–502. 10.1039/c2fo10246c. [DOI] [PubMed] [Google Scholar]
- Sheraz M. A.; Kazi S. H.; Ahmed S.; Anwar Z.; Ahmad I. Photo, thermal and chemical degradation of riboflavin. Beilstein J. Org. Chem. 2014, 10, 1999–2012. 10.3762/bjoc.10.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fracassetti D.; Limbo S.; Pellegrino L.; Tirelli A. Light-induced reactions of methionine and riboflavin in model wine: Effects of hydrolysable tannins and sulfur dioxide. Food Chem. 2019, 298, 124952. 10.1016/j.foodchem.2019.124952. [DOI] [PubMed] [Google Scholar]
- Maujean A.; Seguin N. Contribution à l’étude des goúts de lumière dans les vins de Champagne. 3. Les réactions photochimiques responsables des goúts de lumière dans le vin de Champagne. Sci. Aliment. 1983, 3, 589–601. [Google Scholar]
- Min D. B.; Boff J. M. Chemistry and reaction of singlet oxygen in foods. Compr. Rev. Food Sci. Food Saf. 2002, 1, 58–72. 10.1111/j.1541-4337.2002.tb00007.x. [DOI] [PubMed] [Google Scholar]
- Godelmann R.; Fang F.; Humpfer E.; Schütz B.; Bansbach M.; Schäfer H.; Spraul M. Targeted and nontargeted wine analysis by 1H NMR spectroscopy combined with multivariate statistical analysis. Differentiation of important parameters: grape variety, geographical origin, year of vintage. J. Agric. Food Chem. 2013, 61, 5610. 10.1021/jf400800d. [DOI] [PubMed] [Google Scholar]
- Ragone R.; Crupi P.; Piccinonna S.; Bergamini C.; Mazzone F.; Fanizzi F. P.; Schena F. P.; Antonacci D. Classification and chemometric study of Southern Italy monovarietal wines based on NMR and HPLC-DAD-MS. Food Sci. Biotechnol. 2015, 24, 817–826. 10.1007/s10068-015-0106-z. [DOI] [Google Scholar]
- Amargianitaki M.; Spyros A. NMR-based metabolomics in wine quality control and authentication. Chem. Biol. Technol. Agric. 2017, 4, 9. 10.1186/s40538-017-0092-x. [DOI] [Google Scholar]
- Fan S.; Zhong Q.; Fauhl-Hassek C.; Pfister M. K.-H.; Horn B.; Huang Z. Classification of Chinese wine varieties using 1H NMR spectroscopy combined with multivariate statistical analysis. Food Control 2018, 88, 113–122. 10.1016/j.foodcont.2017.11.002. [DOI] [Google Scholar]
- Herbert-Pucheta J. E.; Mejía-Fonseca I.; Zepeda-Vallejo L. G.; Milmo-Brittingham D.; Maya G. P. The “Wine-T1” NMR experiment for novel wine-metabolome fingerprinting with nuclear-spin relaxation. BIO Web Conf. 2019, 12, 02029 10.1051/bioconf/20191202029. [DOI] [Google Scholar]
- Hwang T.-L.; Shaka A. J. Water suppression that works. Excitation sculpting using arbitrary wave-forms and pulsed-field gradients. J. Magn. Reson., Ser. A 1995, 112, 275–279. 10.1006/jmra.1995.1047. [DOI] [Google Scholar]
- Bharti S. K.; Roy R. Quantitative 1H NMR spectroscopy. TrAC Trends Anal. Chem. 2012, 35, 5–26. 10.1016/j.trac.2012.02.007. [DOI] [Google Scholar]
- Briviba K.; Devasagayam T. P. A.; Sies H.; Steenken S. Selective para hydroxylation of phenol and aniline by singlet molecular oxygen. Chem. Res. Toxicol. 1993, 6, 548–553. 10.1021/tx00034a025. [DOI] [PubMed] [Google Scholar]
- Lagunes I.; Trigos Á. Photo-oxidation of ergosterol: indirect detection of antioxidants photosensitizers or quenchers of singlet oxygen. J. Photochem. Photobiol., B 2015, 145, 30–34. 10.1016/j.jphotobiol.2015.02.014. [DOI] [PubMed] [Google Scholar]
- Usseglio-Tomasset L. Properties and use of sulphur dioxide. Food Addit. Contam. 1992, 9, 399–404. 10.1080/02652039209374090. [DOI] [PubMed] [Google Scholar]
- Ribereau-Gayon P.; Glories Y.; Maujean A.; Dubourdieu D.. Handbook of enology, The microbiology of wine and vinifications; 2nd ed.; John Wiley & Sons: Chichester, 2006; 193–222. [Google Scholar]
- Waterhouse A. L.; Sacks G. L.; Jeffery D. W.. Understanding wine chemistry; John Wiley & Sons, Inc.: Chichester, West Sussex, 2016; 140–148. [Google Scholar]
- Silva E.; Barrias P.; Fuentes-Lemus E.; Tirapegui C.; Aspee A.; Carroll L.; Davies M. J.; López-Alarcón C. Riboflavin-induced Type 1 photo-oxidation of tryptophan using a high intensity 365 nm light emitting diode. Free Radical Biol. Med. 2019, 131, 133–143. 10.1016/j.freeradbiomed.2018.11.026. [DOI] [PubMed] [Google Scholar]
- Yang S.-F. Sulfoxide formation from methionine or its sulfide analogs during aerobic oxidation of sulfite. Biochemistry 1970, 9, 5008–5014. 10.1021/bi00827a027. [DOI] [PubMed] [Google Scholar]
- Inoue M.; Hayatsu H. The interactions between bisulfite and amino acids. The formation of methionine sulfoxide from methionine in the presence of oxygen. Chem. Pharm. Bull. 1971, 19, 1286–1289. 10.1248/cpb.19.1286. [DOI] [Google Scholar]
- Obreque-Slíer E.; Peña-Neira A.; López-Solís R.; Ramírez-Escudero C.; Zamora-Marín F. Phenolic characterization of commercial enological tannins. Eur. Food Res. Technol. 2009, 229, 859–866. 10.1007/s00217-009-1121-1. [DOI] [Google Scholar]
- Vignault A.; González-Centeno M. R.; Pascual O.; Gombau J.; Jourdes M.; Moine V.; Iturmendi N.; Canals J. M.; Zamora F.; Teissedre P. L. Chemical characterization, antioxidant properties and oxygen consumption rate of 36 commercial oenological tannins in a model wine solution. Food Chem. 2018, 268, 210–219. 10.1016/j.foodchem.2018.06.031. [DOI] [PubMed] [Google Scholar]
- Barata-Vallejo S.; Ferreri C.; Postigo A.; Chatgilialoglu C. Radiation chemical studies of methionine in aqueous solution: understanding the role of molecular oxygen. Chem. Res. Toxicol. 2010, 23, 258–263. 10.1021/tx900427d. [DOI] [PubMed] [Google Scholar]
- Raskatov J. A.; Virgil S.; Lee H.-W.; Henling L. M.; Chan K.; Kuhn A. J.; Foley A. R. A facile method for the separation of methionine sulfoxide diastereomers, structural assignment, and DFT analysis. Chem. – Eur. J. 2020, 26, 4467–4470. 10.1002/chem.201904848. [DOI] [PubMed] [Google Scholar]
- Gennaris A.; Ezraty B.; Henry C.; Agrebi R.; Vergnes A.; Oheix E.; Bos J.; Leverrier P.; Espinosa L.; Szewczyk J.; Vertommen D.; Iranzo O.; Collet J.-F.; Barras F. Repairing oxidized proteins in the bacterial envelope using respiratory chain electrons. Nature 2015, 528, 409–412. 10.1038/nature15764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmeier C.; Bartling H.; Magerl K.; Gschwind R. M. LED-illuminated NMR studies of flavin-catalyzed photooxidations reveal solvent control of the electron-transfer mechanism. Angew. Chem. Int. Ed. 2015, 54, 1347–1351. 10.1002/anie.201409146. [DOI] [PubMed] [Google Scholar]
- Yang S. O.; Lee J. M.; Lee J. C.; Lee J. H. Effects of riboflavin-photosensitization on the formation of volatiles in linoleic acid model systems with sodium azide or D2O. Food Chem. 2007, 105, 1375–1381. 10.1016/j.foodchem.2007.05.002. [DOI] [Google Scholar]
- Lee J. H.; Min D. B. Changes of headspace volatiles in milk with riboflavin photosensitization. J. Food Sci. 2009, 74, C563–C568. 10.1111/j.1750-3841.2009.01295.x. [DOI] [PubMed] [Google Scholar]
- Boscá F.; Miranda M. A.; Morera I. M.; Samadi A. Involvement of type I and type II mechanisms in the linoleic acid peroxidation photosensitized by tiaprofenic acid. J. Photochem. Photobiol., B 2000, 58, 1–5. 10.1016/S1011-1344(00)00102-0. [DOI] [PubMed] [Google Scholar]
- Navaratnam S.; Hamblett I.; Tonnesen H. H. Photoreactivity of biologically active compounds. XVI. Formation and reactivity of free radicals in mefloquine. J. Photochem. Photobiol., B 2000, 56, 25–38. 10.1016/S1011-1344(00)00056-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.