Abstract
A heavy-metal-free chalcopyrite (CuFeS2) nanocrystal has been synthesized via microwave-assisted growth. Large-scale nanocrystals with an average particle size of 5 nm are fabricated by this technique within a very short period of time without any need for organic ligands. Scanning electron microscopy study (SEM) of individual synthesis steps indicates that aggregates of nanocrystals are formed as flakes during microwave-assisted synthesis. The colloidal solution of the CuFeS2 nanocrystal was prepared by sonicating these flakes. Transmission electron microscopy (TEM) study reveals the growth of sub-10 nm CuFeS2 nanocrystals that are further characterized by X-ray diffraction. UV–visible absorption spectroscopic study shows that the band gap of this nanocrystal is ∼1.3 eV. To investigate the photosensitive nature of this nanocrystal, a bilayer p–n heterojunction photodetector has been fabricated using this nontoxic CuFeS2 nanocrystal as a photoactive material and n-type ZnO as a charge-transport layer. The detectivity of this photodetector reaches above 1012 Jones in visible and near-infrared (NIR) regions under 10 V external bias, which is significantly high for a nontoxic nanocrystal-based photodetector.
1. Introduction
In the last two decades, a variety of colloidal nanocrystals have been developed, which have been used in different areas of electronics and optoelectronics including light-emitting diodes, photodetectors, solar cells, etc.1−4 However, most of these high-performing colloidal nanocrystals are heavy-metal based, which poses a big concern for the environment and health.5,6 Besides these electronic applications, different biomedical applications require nontoxic nanocrystals.7−10 Therefore, development of earth-abundant, nontoxic, and eco-friendly nanocrystal materials is of utmost importance.11−13 Accordingly, a large variety of different heavy-metal-free nanocrystals have been developed including Cu2ZnSnS4 (CZTS),14 CuInS2 (CIS),9,14CuInZnS (CIZS),15 Cu2SnS3,16 and CuFeS2.17 Among them, chalcopyrite (CuFeS2) is one of the important materials for both optoelectronic and biomedical applications. This CuFeS2 is one of the most earth-abundant ores showing semiconducting behavior. The optical band gap of this semiconductor is ∼0.55 eV, which can be increased above 1.0 eV by reducing the particle size of the nanostructured CuFeS2.17,18Therefore, there is a good opportunity to use CuFeS2 nanoparticles for photovoltaic and broad-band photodetector applications.19
Among the different optoelectronic devices, photodetectors have generated a great deal of interest due to their various applications in the fields of optical readers, photomultiplier tubes, remote sensing, photonic circuits, robotics, thermography, spectrometers, astronomy, cameras, optical communication, cell phones, etc.20,21 Currently, most of the nanocrystal-based photodetectors are fabricated either from toxic elements like lead (Pb),22,23 cadmium (Cd),24,25 and mercury (Hg)26 or from elements that are not earth-abundant.27,28 Chalcopyrite (CuFeS2) is an ore of copper and has elements in Cu1+/Cu2+, Fe3+/Fe2+, and S2– oxidation states that can overcome both these problems.29 CuFeS2 has a tetragonal crystal structure that is closely related to zinc blend (Figure 1a). Moreover, CuFeS2 is a direct band gap compound semiconductor with elements belonging to I–III–VI groups.30 These unique combinations of electrical and optical properties of CuFeS2 make it a versatile material for applications in different fields including batteries, solar cells, LEDs, and thermoelectric, spintronic, and photoelectrochemical cells.31−33 Nevertheless, there are very few reports on CuFeS2 as an active material in the fabrication of a solar cell or photodetector.34,35 This is due to the presence of the 3d orbitals of Fe that create an interband [IB] between the valence band [VB] and the conduction band [CB] of CuFeS2.33 The energy gap between VB and IB is 0.53 eV and indirect in nature, which is not efficient for light absorption.33
Figure 1.
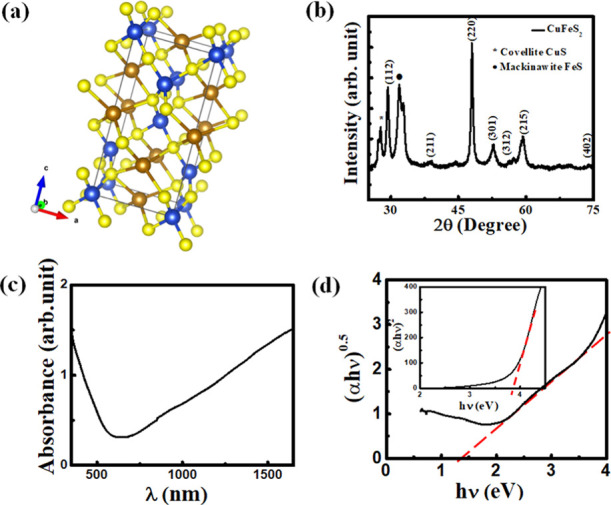
(a) Crystal structure of CuFeS2. Blue, brown, and yellow colors represent Cu, Fe, and S atoms, respectively. (b) X-ray diffraction (XRD) pattern of a CuFeS2 nanocrystal, (c) solution-phase absorption spectrum of a CuFeS2 nanocrystal, and (d) Tauc’s plot that has been used to determine the indirect band gap. Inset: Tauc’s plot that has been used to determine the direct band gap.
In this work, we present a scalable microwave-assisted synthesis technique that is capable of synthesizing a sub-10 nm CuFeS2 nanocrystal within a few minutes. This synthesized CuFeS2 nanocrystal does not contain any organic ligand and exhibits a strong quantum confinement effect with an enhanced optical band gap of 1.3 eV. A bilayer heterojunction photodetector fabricated using this CuFeS2 nanocrystal as an active material shows good photosensitivity in the visible region of light. In this device structure, a zinc oxide (ZnO) layer has been used as an electron-transport layer, whereas CuFeS2 works as a photoactive material.
2. Results and Discussion
2.1. X-ray Diffraction (XRD)
The crystal phase of a CuFeS2 nanocrystal has been identified by X-ray diffraction (XRD) study. For this study, an XRD sample has been prepared by drop-casting method on a Si substrate. Figure 1b shows the XRD pattern of the CuFeS2 nanocrystal, which indicates intense peaks at 2θ ∼ 29.32, 48.01, and 57.18 corresponding to the diffraction planes of (112), (220), and (312), respectively. These diffraction peaks confirm the formation of a tetragonal lattice structure of the CuFeS2 nanocrystal (JCPDS file no. 83-0983). Besides, impurity phases of FeS and CuS are observed in XRD data.
2.2. Ultraviolet–Visible (UV–vis) Absorption
The UV–vis absorption study of a CuFeS2 nanocrystal has been performed in a colloidal solution phase by dispersing in a dimethylformamide (DMF) solution. The absorption spectrum shown in Figure 1c indicates that CuFeS2 nanocrystals have strong absorption in the visible to the near-infrared (NIR) region. Considering the CuFeS2 nanocrystal as an indirect band gap semiconductor, the optical band gap of the synthesized nanocrystal has been calculated from Tauc’s plot (Figure 1d), which is ∼1.3 eV.18,34 The widening of the band gap of CuFeS2 nanocrystals with respect to its bulk structure is due to the strong quantum confinement effect. A Tauc’s plot is also shown in the inset of Figure 1d by considering the CuFeS2 nanocrystal as a direct band gap material that shows a band gap of 3.8 eV, can be responsible for the absorption of high energy photons only. Since the XRD data shows the existence of the CuS impurity phase, plasmonic absorption of the CuS impurity that normally exists within the range of 900–1500 nm can overlap with CuFeS2 absorption.36−38
2.3. Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM)
A scanning electron microscopy (SEM) study has been performed in an as-grown CuFeS2 nanocrystal, which is shown in Figure 2a. A thin-film sample for this SEM study has been prepared by spin-casting nanocrystals on a heavily doped Si substrate (p+-Si). This SEM image indicates that the microwave-assisted CuFeS2 nanostructures form flakes with a thickness of ∼10–12 nm due to the formation of two layers of CuFeS2 nanocrystals (inset, Figure 2a). In addition to this, transmission electron microscopy (TEM) study has been performed using an “as-grown” sample of CuFeS2 that is shown in Figure 2b, which shows a micron-sized cluster that is formed by agglomerated CuFeS2 nanocrystals. For this TEM study, a small amount of a powder sample of as-grown CuFeS2 was dispersed in DMF and stirred for 10 min. A drop of a diluted solution of this sample was taken on a TEM grid. The rest of this solution was sonicated in an ultrasonic bath for 15 min. A drop of this sonicated CuFeS2 sample was used for another set of TEM studies, which is shown in Figure 2c. Our TEM study of this sonicated sample indicates that the individual CuFeS2 nanocrystals have an average size of ∼5 nm (Figure 2d). This result also implies that the sonication method separates individual CuFeS2 flakes and then they are disintegrated into individual nanoparticles.
Figure 2.
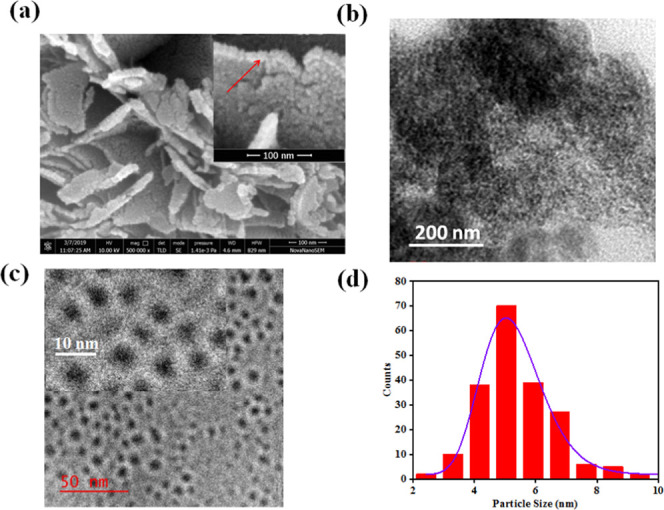
(a) SEM images of as-grown CuFeS2 powder. The inset shows the magnified image that indicates the formation of flakes with two layers of nanocrystals. (b) TEM image of as-grown CuFeS2. (c) TEM image of a dispersed colloidal CuFeS2 nanocrystal. The inset shows the higher magnification image. (d) Particle size distribution as obtained from the TEM study of a dispersed colloidal CuFeS2 nanocrystal.
2.4. Reaction Phenomena of a CuFeS2 Nanocrystal
Based on our electron microscopy studies, we propose a three-step growth process of a CuFeS2 nanoparticle. As mentioned earlier, at the beginning of the irradiation of microwaves, the color of the mixed precursor solution changes from bright yellow to orange. Irradiation for a longer time in the second step turns the color of the solution from orange to greenish-black. Our SEM and XRD studies have confirmed the formation of CuFeS2 flakes that are composed of CuFeS2 nanoparticles. In the third step, CuFeS2 flakes disintegrate into individual CuFeS2 nanoparticles under the sonication process. As a result, the nanoparticles remain in a colloidal form in the solvent. At the beginning of microwave irradiation, the temperature increases rapidly with time as the electric vector of the microwave of frequency 2.45 GHz starts interacting with the dipoles of an ethylene glycol solvent. Within thirty seconds of irradiation, the temperature of the mixture solution reaches 70 °C, whereas this temperature reaches ∼100 °C after around 80 s. When the temperature increases from 70 to 100 °C, the color of the solution changes from bright yellow to orange. It is observed that this orange color becomes more intense up to 110 s of irradiation. When the microwave irradiation is stopped at this stage, the temperature of this solution reaches close to room temperature within a couple of minutes and the color of the solution reverts to bright yellow. This observation indicates that the first step of this synthesis is a reversible process. However, the continuation of this microwave reaction shows another color change after 140 s of irradiation. This time, the deep orange color of the solution changes to greenish-black. At this stage, the temperature of the solution reaches 165 °C. After this, the solid product was separated from the solution by centrifugation at 10 000 rpm for 30 min. Then, it was dried in vacuum for 24 h at room temperature. The morphology of these powder nanoparticles observed by a high-resolution SEM (HRSEM) study shows a nanoplate structure, but after dispersion in DMF and sonication, the HRTEM image shows spherical nanoparticles with an average size of 5 nm. From these sequential observations, a growth process is proposed that is based on the “orientated attachment” model.39,40 As soon as CuFeS2 nanocrystals are formed at temperature ∼160 °C, the capping-free nanocrystals aggregate to form nanoplates spontaneously under the high-temperature condition. In this aggregated state, the nanoparticles come together and form a weak bridge that allows the solid–solid diffusion that leads to the formation of the nanoplate structure. The solution-phase ultrasonication process provides enough energy to break these nanoplates into individual nanoparticles. After sonication, the surface anion (S2–) of colloidal CuFeS2 nanocrystals may behave like a capping ligand around it, which restricts further agglomeration. Since the TEM samples are prepared from a sonicated solution of the nanocrystal, there is no possibility of agglomeration of nanoparticles and they are identified in TEM images.
2.5. I–V Characterization
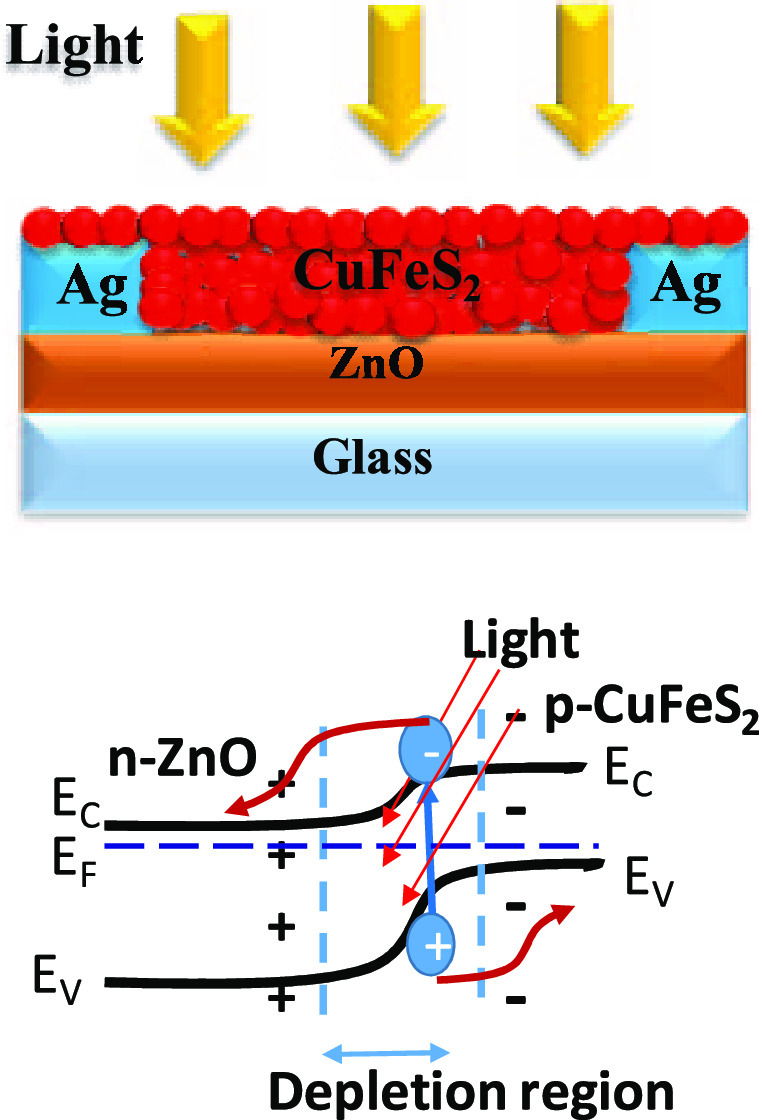
The device performance was examined by studying the current vs voltage (I–V) characterization in the presence of white light and under dark conditions at room temperature for bilayer heterojunction and single-layer devices as shown in Figure 3a,b, respectively. Figure 3c,d show the corresponding device structure. A xenon lamp was used as a white-light source that illuminated the top side of the devices with an intensity of 100 mW/m2. Figure 3a shows the I–V characteristics of heterojunction photoconductors. This data demonstrates that the difference of photo-to-dark current is more or less similar under different external biases with a value of ∼64, which implies excellent photosensitivity of the device. In this photodetector, ZnO and a CuFeS2 nanocrystal were used as the electron-transport layer and photoactive layer, respectively. Coating of ZnO significantly improves the current from 27 to 753 μA under light, keeping the dark current approximately the same as in the CuFeS2-only photodetector. The typical band alignments and charge transport of bilayer and single-layer devices are schematically presented in Figure 3e,f, respectively. From the XRD data, it can be seen that the impurity phases of CuS and FeS having band gaps of 2.2 and 1.0 eV, respectively, coexist with pure CuFeS2. However, these two impurity phases do not interfere much in the device performance for two reasons. First, both these materials are considered as semiconducting materials with reasonably good photosensitivity in the visible region of light. Besides, both these materials are p-type in nature and form a type II heterojunction with ZnO. Therefore, photogenerated electrons of the materials can easily transfer to ZnO layers like CuFeS2.41,42 Besides, due to the free “hole” carrier of CuS and FeS impurities, plasmonic absorption may arise, which is observed in the absorption spectra (Figure 1c). This plasmonic hole is metallic in nature and the plasmon oscillation frequency depends on the carrier concentration of holes according to the equation ωp = √Nhe2/εmh (where ωp is the plasma frequency, Nh is the density of free holes, mh is the effective mass of hole). Instead of exhibiting the metallic nature of this hole, the p–n heterostructure of p-CuS with an n-type semiconductor shows a superior photoresponse that has been observed earlier.43 Similar to this, plasmonic absorption of metal nanoparticles can also generate hot electrons that can easily transfer to the neighboring metal oxide layer and enhance the photosensitivity of the device.44
Figure 3.
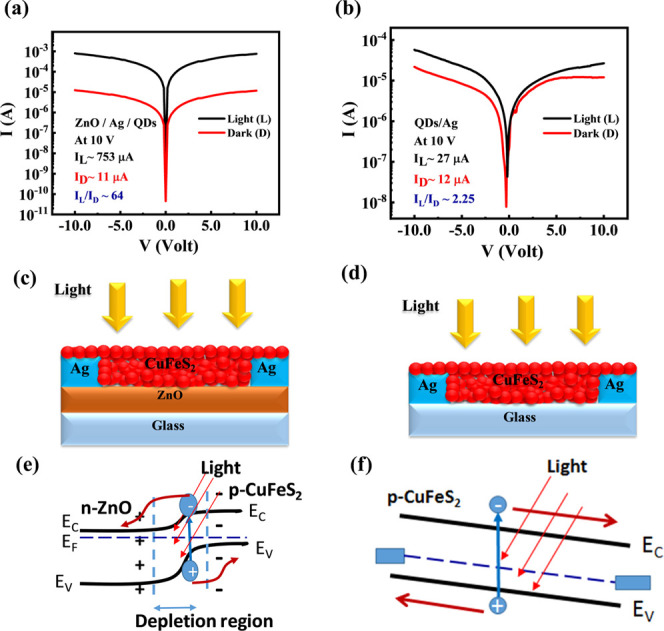
(a, b) Current vs voltage characteristics of the bilayer heterojunction photodetector (glass/ZnO/Ag/CuFeS2) and the single-layer photodetector (glass/CuFeS2/Ag). Schematic device structure of (c) the bilayer heterojunction and (d) the single-layer photodetector. Band diagram and charge transport of (e) the bilayer heterojunction device and (f) the single-layer device.
When a photon of incident light has higher energy (hυ) than the band gap (Eg) of the CuFeS2 nanocrystal, the energy of a photon can be absorbed by the valence electrons of the nanocrystal. This absorption leads to the formation of an e––h+ pair (exciton) inside the nanocrystal that experiences a force due to the existing electric field created in the depletion region formed by the heterojunction of the bilayer photodetector (Figure 3e). Due to this electric field, the electrons and holes get separated from each other, and electrons are transported through the ZnO layer, whereas holes remain in the CuFeS2 layer (Figure 3e). Because of this physical separation of charge carriers, the recombination of photogenerated carriers is considerably reduced. Moreover, the ZnO layer possesses higher carrier mobility that can transport electrons to the electrode at a much faster rate. Therefore, the bilayer heterojunction photodetector enhanced the overall conductivity of the photodetector in comparison to only CuFeS2 in the photodetector. A similar phenomenon has been observed in PbS nanocrystal- and CdS nanocrystal-based photodetectors.25,39
2.6. Responsivity, Detectivity, and Response Time
Three important parameters of a photoconductor are responsivity (Rλ), detectivity (D*), and external quantum efficiency (EQE) that determine the overall performance of the device in a range of electromagnetic spectra and provide the crucial information to decide on its application. The responsivity (Rλ) is the ratio of the photocurrent (Iph) produced by the device to the power of the incident light (P) illuminating the effective surface area (A) of the photodetector and EQE is the number of photoelectrons generated per unit photon. On the other hand, detectivity is related to the sensitivity of the photodetector, and it is inversely proportional to the “noise equivalent power” (NEP), normalized to the per-unit detector’s photosensitive area in the range of 1 Hz bandwidth. In other words, detectivity is a measure of the lowest possible value of radiant power that can generate a signal. The following mathematical relations show the interrelationship among Rλ, D*, and EQE
| 1 |
| 2 |
and
| 3 |
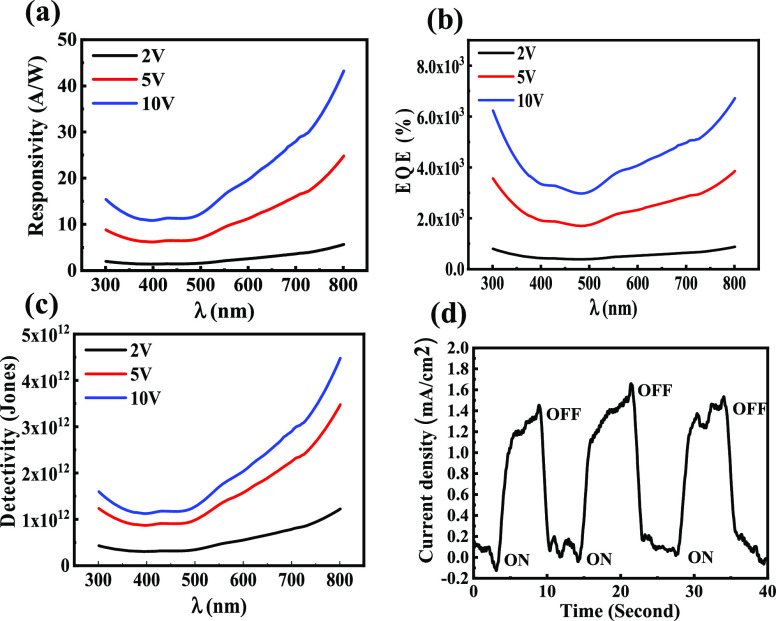
where Iph is the photocurrent at a particular applied voltage, P is the intensity of the incident light per unit area, h is Planck’s constant, c is the speed of light,39e is the electronic charge, Jd is the dark current per unit effective area of the photodetector, and λ is the wavelength of light. In the expression of detectivity (eq 3), we have considered that the noise current is dominated by dark current only, which is a good approximation for low-intensity photodetection. Figure 4a–c show the graph of Rλ, EQE, and D* respectively at different biasing values. Figure 4a has demonstrated its excellent photosensitivity in the range of 350–800 nm. This data also indicates that the responsivity increases with wavelength, which is a very similar trend to the absorption of the device. Moreover, this Rλ increases with external biasing, which is due to an increase in the gain of the photocurrent. The maximum value of the responsivity of this device is 43 A/W at 800 nm with the biasing of 10 V, which is a reasonably high value with respect to nanocrystal-based photodetectors.
Figure 4.
(a) Responsivity, (b) EQE, and (c) detectivity of a bilayer heterojunction photodetector at different biases in the spectral range of 300–800 nm. (d) Transient photoresponse of the photodetector under 5 V external bias and one sun white pulse light illumination with a repetition of 7 s.
The measured EQE values for the devices at different external biases show a very high multispectral photoresponse in the visible–NIR range as shown in Figure 4b. The EQE follows a pattern similar to that shown by the responsivity of the device. Similar to responsivity data, the EQE value is also enhanced with external bias due to the enhancement of photocurrent. This data indicates that the highest value of EQE for this photodetector is ∼6717% at 800 nm under 10 V external bias. Figure 4c shows the detectivity of the device under different external biases. The highest D* value exhibited by the device is ∼4.48 × 1012 Jones at 800 nm for 10 V bias, which is reasonably high for a nanocrystal-based photodetector. These device parameters have been compared with some earlier reports that are presented in Table 1. This comparison indicates that the device performance of this photodetector is superior to those of a number of earlier studies. Therefore, this effort will be a strong addition to heavy-metal-free photodetector research.
Table 1. Comparison of Various Device Structures of Nanocrystal-Based Photodetectors.
| device | responsivity [A W–1] | EQE [%] | detectivity [cm Hz1/2 W–1] | wavelength [nm] | ref |
|---|---|---|---|---|---|
| ZnO/CuFeS2 | 43 A/W | 6717 | 4.48 × 1012 | 300–800 | this work |
| ZnO/PbS | 4.19 | 742 | 1.07 × 1011 | 350–900 | ACS Appl. Nano Mater 1, 6063–6072 (2018)39 |
| Bi2Te3–SnSe–Bi2Te3 | 5.5 | 1833 | 6 × 1010 | 370–808 | Adv. Funct. Mater. 27, 1701823 (2017)45 |
| Gr–WS2–Gr | 3.5 | 933 | 1.6 × 1010 | 532 | ACS Appl. Mater. Interfaces 9, 5392–5398 (2017)46 |
| Gr–WS2–Gr | 0.1 | 30 | NA | 488–633 | Science (80). 275, 1102–1106 (1997)47 |
| Gr–MoTe2–Gr | 0.205 | 53.8 | NA | 473–1064 | Nat. Nanotechnol. 6, 45–50 (2011)48 |
| Ag–SnSe–Ag | 8.8 × 10–8 | 2.3 × 10–7 | 8.7 × 105 | 473 | Nanotechnology 25, 105705 (2014)49 |
| ITO–SnSe–ITO | 6 × 10–6 | NA | 1.8 × 107 | white light | Mater. Res. Express 3, 105038 (2016)50 |
| Ti–SnSe/WS2–Ti | 0.099 | 26 | 1.2 × 108 | 473–1064 | ACS Appl. Mater. Interfaces 8, 4781–4788 (2016)51 |
The response time is another important characteristic of a photodetector and to characterize the speed of our device. For this study, the photodetector has been illuminated with a pulsed white-light source of one sun with a repetition interval of 7 s under 5 V external bias. The resulting transient photoresponse of the photoconductor is displayed in Figure 4d, which shows a rising and decay time of this device of 1.4 and 1.0 s, respectively. This data indicates that as a photoconductor device it has an excellent recovery time (Table 2).
Table 2. Comparison of Device Response Time with Reported CuFeS2 Nanocrystal-Based Photodetectors.
| type of CuFeS2 nanocrystal photodetector | rise time | decay time | references |
|---|---|---|---|
| Si/CuFeS2 photodiode | 18 s | 20 s | ACS Appl. Mater. Interfaces 2015, 7, 2235–224152 |
| CuFeS2 photoconductor | 5 min | 3 min | J. Phys. Chem. Lett. 2018, 9, 696–70118 |
| CuFeS2 photoconductor | 30 s | 30 s | Adv. Mater. Interfaces 2020, 7, 200005634 |
| CuFeS2/ZnO photoconductor | 1.4 s | 1.6 s | this work |
3. Conclusions
In summary, we have successfully synthesized a heavy-metal-free CuFeS2 nanocrystal in a short period of time via the microwave-assisted synthesis technique without using an organic ligand. TEM and SEM studies of individual steps in this synthesis indicate that nanocrystals are attached to each other during this microwave synthesis, forming nanoflakes, which is attributed to the growth of nanocrystals at high temperatures (∼150 °C) and the absence of an organic ligand. However, the TEM study of this nanocrystal indicates that the ultrasonication process can successfully separate them to form a stable solution of colloidal CuFeS2 nanocrystals. This data also indicates that the nanocrystals are quite uniform in size, with an average particle size of ∼5 nm. Using these nanocrystals, we have successfully fabricated a bilayer p–n heterojunction photodetector with ZnO as an electron-transport layer showing excellent photosensitivity in the visible and near-infrared regions. The detectivity of this photodetector reaches above 1012 Jones in the visible–NIR region under 10 V external bias, which is significantly high for a nontoxic nanocrystal-based photodetector.
4. Experimental Section
4.1. Synthesis of a CuFeS2 Nanocrystal and a ZnO Thin Film
To synthesize a CuFeS2 nanocrystal by the microwave-assisted technique, cupric acetate monohydrate (98%) and anhydrous ferric chloride were used as precursor materials. In the beginning, 50 mM copper acetate monohydrate [Cu (CH3COO)2·H2O], 150 mM ferric chloride [FeCl3], and 100 mM thiourea [CH4N2S] were simultaneously dissolved in 20 mL of ethylene glycol (EG). The mixture was stirred using a magnetic stirrer (1000 rpm) until all of the precursors dissolved, resulting in a dark yellow solution. The homogeneous precursor solution was subjected to 400 W microwave radiation for 5 min until the color of the solution changed from bright yellow to greenish-black. The initial color change in this synthesis process is observed to be reversible. After microwave irradiation, the precipitate was separated by centrifugation (10 000 rpm for 30 min) three times to remove larger particles and the unreacted precursors. In the first cycle, the entire product was centrifuged, which mostly removed the ethylene glycol solvent and unreacted materials. After collecting the solid product, the sample was dried and redispersed in a mixture of ethanol and acetone (1:1) and was centrifuged under the same conditions, which was repeated one more time. After this, purified CuFeS2 nanocrystals were kept for 24 h for drying at room temperature in vacuum and then dissolved in dimethylformamide (DMF) at a concentration of 8 mg/mL, as it is observed that CuFeS2 nanocrystals have better solubility in DMF compared to EG. Before thin-film deposition by spin-coating, the solution was agitated via a sonication process for 15 min.
To deposit a ZnO thin film, a precursor solution of zinc acetate (dihydrate) was prepared using 2-methoxyethanol as a solvent. The solution was stirred for 1 h using a magnetic stirrer to obtain a clear transparent solution of zinc acetate. After that, monoethanolamine (MEA) was added to the zinc acetate solution with a volume ratio of 1:15 to obtain the final solution with a concentration of 300 mM. The solution was kept under stirring for 6 more hours. Finally, the resultant solution was filtered with a polyvinylidene difluoride syringe filter with 0.22 μm pore size to remove bigger particles. The filtered solution was coated on a cleaned glass substrate using a spin coater, followed by an annealing process at 500° C to form a uniform thin film of ZnO.
4.2. Material Characterization
The crystal structure and phase identification of the samples were analyzed using a Rigaku X-ray diffractometer (Rigaku SmartLab 9 kW Powder type) with Cu Kα radiation (λ = 1.54 Å) in the 2θ range of 10–90° with a scan rate of 2°/min. The characterization of the microstructure of these samples was carried out by high-resolution scanning electron microscopy (HR-SEM, Nova Nano SEM 450, 30 kV) and transmission electron microscopy (TEM, Tecnai G2 20 TWIN, 200 kV). The UV–visible spectra (DRS) of different samples were recorded by a UV–visible spectrophotometer (Shimadzu UV-3600).
4.3. Device Fabrication
All photodetector devices have been fabricated on glass substrates of size 15 × 15 mm. In the beginning, all substrates were cleaned with a piranha solution that removes the organic impurity from the surface of substrates and makes them hydrophilic. After that, these substrates were cleaned by distilled water, followed by isopropyl alcohol by keeping them in an ultrasonic bath for 10 min each. Finally, the substrates were dried by blowing dry air. To deposit ZnO thin films, a precursor solution of 300 mM concentration was spin-coated at a speed of 2500 rpm for 60 s. The coated glass slides were annealed at a temperature of 350 °C for 10 min. This process was repeated two more times to obtain the desired thickness of ZnO. Finally, this ZnO thin film was kept at 500 °C for 30 min to obtain a crystalline thin film of ZnO. After ZnO deposition, parallel silver (Ag) electrodes (separation 0.45 mm and length 9 mm) of thickness 60 nm were deposited by a thermal evaporator. After the electrode deposition, a colloidal solution of a CuFeS2 nanocrystal was spin-coated over the ZnO-coated substrate at a speed of 2000 rpm for 300 s. For better stacking of nanocrystal layers, ethylene-dithiol (EDT) was spin-coated over them, which also reduces the number of electron trap states of the nanocrystals. This process was repeated two times to achieve the desired thickness of nanocrystals. The schematic of the final device structure bilayer heterojunction is shown in Figure 3c. A reference photodetector has been fabricated without using a ZnO layer, which is shown in Figure 3d.
4.4. Electrical Characterizations and External Quantum Efficiency (EQE) Measurement
All electrical characterizations have been performed under open atmospheric conditions. During the measurements, all electrical contacts were made by a manual probe station. Electrical data was obtained by a dual-source meter (Keysight B2902A). White light was illuminated from a xenon light source. The intensity of white light was measured by a calibrated Si photodetector (Thorlabs, Inc.). The EQE of the device under bias condition was measured by illuminating the device with different wavelengths of light from a monochromator.
Acknowledgments
This work was supported by the “Science and Engineering Research Board”, India (EMR/2015/000689). The authors are grateful to the Central Instrument Facility Centre, IIT(BHU), for providing the SEM and AFM measurement facility. S.V.S. and B.K. thank IIT(BHU) for providing a Ph.D. fellowship. S.B. acknowledges the financial support from the Ministry of Science and Technology, Taiwan (MOST 109-2221-E-131-002 and 107-2221-E-131-029-MY2).
Author Contributions
§ B.K. and S.V.S. contributed equally to this work.
The authors declare no competing financial interest.
References
- Litvin A. P.; Martynenko I. V.; Purcell-Milton F.; Baranov A. V.; Fedorov A. V.; Gun’ko Y. K. Colloidal quantum dots for optoelectronics. J. Mater. Chem. A 2017, 5, 13252–13275. 10.1039/C7TA02076G. [DOI] [PubMed] [Google Scholar]
- Rauch T.; Böberl M.; Tedde S. F.; Fürst J.; Kovalenko M. V.; Hesser G.; Lemmer U.; Heiss W.; Hayden O. Near-infrared imaging with quantum-dot-sensitized organic photodiodes. Nat. Photonics 2009, 3, 332–336. 10.1038/nphoton.2009.72. [DOI] [Google Scholar]
- Cho K.-S.; Lee E. K.; Joo W.-J.; Jang E.; Kim T.-H.; Lee S. J.; Kwon S.-J.; Han J. Y.; Kim B.-K.; Choi B. L.; Kim J. M. High-performance crosslinked colloidal quantum-dot light-emitting diodes. Nat. Photonics 2009, 3, 341–345. 10.1038/nphoton.2009.92. [DOI] [Google Scholar]
- Sargent E. H. Colloidal quantum dot solar cells. Nat. Photonics 2012, 6, 133–135. 10.1038/nphoton.2012.33. [DOI] [Google Scholar]
- Kovalenko M. V. Opportunities and challenges for quantum dot photovoltaics. Nat. Nanotechnol. 2015, 10, 994–997. 10.1038/nnano.2015.284. [DOI] [PubMed] [Google Scholar]
- Zhang Q.; Yin Y. All-Inorganic Metal Halide Perovskite Nanocrystals: Opportunities and Challenges. ACS Cent. Sci. 2018, 4, 668–679. 10.1021/acscentsci.8b00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes P. D.; Chandrawati R.; Stevens M. M. Colloidal nanoparticles as advanced biological sensors. Science 2014, 346, 1247390 10.1126/science.1247390. [DOI] [PubMed] [Google Scholar]
- Rosenthal S. J.; Chang J. C.; Kovtun O.; McBride J. R.; Tomlinson I. D. Biocompatible Quantum Dots for Biological Applications. Chem. Biol. 2011, 18, 10–24. 10.1016/j.chembiol.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medintz I. L.; Uyeda H. T.; Goldman E. R.; Mattoussi H. Quantum dot bioconjugates for imaging, labelling and sensing. Nat. Mater. 2005, 4, 435–446. 10.1038/nmat1390. [DOI] [PubMed] [Google Scholar]
- Kataria M.; Yadav K.; Haider G.; Liao Y. M.; Liou Y.-R.; Cai S.-Y.; Lin H.-i.; Chen Y. H.; Paul Inbaraj C. R.; Bera K. P.; Lee H. M.; Chen Y.-T.; Wang W.-H.; Chen Y. F. Transparent, Wearable, Broadband, and Highly Sensitive Upconversion Nanoparticles and Graphene-Based Hybrid Photodetectors. ACS Photonics 2018, 5, 2336–2347. 10.1021/acsphotonics.8b00141. [DOI] [Google Scholar]
- Reiss P.; Carrière M.; Lincheneau C.; Vaure L.; Tamang S. Synthesis of Semiconductor Nanocrystals, Focusing on Nontoxic and Earth-Abundant Materials. Chem. Rev. 2016, 116, 10731–10819. 10.1021/acs.chemrev.6b00116. [DOI] [PubMed] [Google Scholar]
- Tang J.; Konstantatos G.; Hinds S.; Myrskog S.; Pattantyus-Abraham A. G.; Clifford J.; Sargent E. H. Heavy-Metal-Free Solution-Processed Nanoparticle-Based Photodetectors: Doping of Intrinsic Vacancies Enables Engineering of Sensitivity and Speed. ACS Nano 2009, 3, 331–338. 10.1021/nn800718u. [DOI] [PubMed] [Google Scholar]
- Xiang H.; Hu Z.; Billot L.; Aigouy L.; Zhang W.; McCulloch I.; Chen Z. Heavy-Metal-Free Flexible Hybrid Polymer-Nanocrystal Photodetectors Sensitive to 1.5 μm Wavelength. ACS Appl. Mater. Interfaces 2019, 11, 42571–42579. 10.1021/acsami.9b14034. [DOI] [PubMed] [Google Scholar]
- Zhou H.; Hsu W.-C.; Duan H.-S.; Bob B.; Yang W.; Song T.-B.; Hsu C.-J.; Yang Y. CZTS nanocrystals: a promising approach for next generation thin film photovoltaics. Energy Environ. Sci. 2013, 6, 2822–2838. 10.1039/c3ee41627e. [DOI] [Google Scholar]
- Liu Z.; Tang A.; Wang M.; Yang C.; Teng F. Heating-up synthesis of cadimum-free and color-tunable quaternary and five-component Cu–In–Zn–S-based semiconductor nanocrystals. J. Mater. Chem. C 2015, 3, 10114–10120. 10.1039/C5TC02469B. [DOI] [Google Scholar]
- Dias S.; Kumawat K.; Biswas S.; Krupanidhi S. B. Solvothermal Synthesis of Cu2SnS3 Quantum Dots and Their Application in Near-Infrared Photodetectors. Inorg. Chem. 2017, 56, 2198–2203. 10.1021/acs.inorgchem.6b02832. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya B.; Pandey A. CuFeS2 Quantum Dots and Highly Luminescent CuFeS2 Based Core/Shell Structures: Synthesis, Tunability, and Photophysics. J. Am. Chem. Soc. 2016, 138, 10207–10213. 10.1021/jacs.6b04981. [DOI] [PubMed] [Google Scholar]
- Sugathan A.; Bhattacharyya B.; Kishore V. V. R.; Kumar A.; Rajasekar G. P.; Sarma D. D.; Pandey A. Why Does CuFeS2 Resemble Gold?. J. Phys. Chem. Lett. 2018, 9, 696–701. 10.1021/acs.jpclett.7b03190. [DOI] [PubMed] [Google Scholar]
- Bastola E.; Bhandari K. P.; Subedi I.; Podraza N. J.; Ellingson R. J. Structural, optical, and hole transport properties of earth-abundant chalcopyrite (CuFeS2) nanocrystals. MRS Commun. 2018, 8, 970–978. 10.1557/mrc.2018.117. [DOI] [Google Scholar]
- García de Arquer F. P.; Armin A.; Meredith P.; Sargent E. H. Solution-processed semiconductors for next-generation photodetectors. Nat. Rev. Mater 2017, 2, 16100. 10.1038/natrevmats.2016.100. [DOI] [Google Scholar]
- Barve A. V.; Lee S. J.; Noh S. K.; Krishna S. Review of current progress in quantum dot infrared photodetectors. Laser Photonics Rev. 2010, 4, 738–750. 10.1002/lpor.200900031. [DOI] [Google Scholar]
- Konstantatos G.; Sargent E. H. Colloidal quantum dot photodetectors. Infrared Phys. Techn. 2011, 54, 278–282. 10.1016/j.infrared.2010.12.029. [DOI] [Google Scholar]
- Pal B. N.; Robel I.; Mohite A.; Laocharoensuk R.; Werder D. J.; Klimov V. I. High-Sensitivity p–n Junction Photodiodes Based on PbS Nanocrystal Quantum Dots. Adv. Funct. Mater. 2012, 22, 1741–1748. 10.1002/adfm.201102532. [DOI] [Google Scholar]
- Deng K.; Li L. CdS Nanoscale Photodetectors. Adv. Mater. 2014, 26, 2619–2635. 10.1002/adma.201304621. [DOI] [PubMed] [Google Scholar]
- Maity P.; Singh S. V.; Biring S.; Pal B. N.; Ghosh A. K. Selective near-infrared (NIR) photodetectors fabricated with colloidal CdS:Co quantum dots. J. Mater. Chem. C 2019, 7, 7725–7733. 10.1039/C9TC01871A. [DOI] [Google Scholar]
- Deng Z.; Jeong K. S.; Guyot-Sionnest P. Colloidal Quantum Dots Intraband Photodetectors. ACS Nano 2014, 8, 11707–11714. 10.1021/nn505092a. [DOI] [PubMed] [Google Scholar]
- Lim H.; Tsao S.; Zhang W.; Razeghi M. High-performance InAs quantum-dot infrared photodetectors grown on InP substrate operating at room temperature. Appl. Phys. Lett. 2007, 90, 131112 10.1063/1.2719160. [DOI] [Google Scholar]
- Gunapala S. D.; Levine B. F.; Ritter D.; Hamm R. A.; Panish M. B. Lattice-matched InGaAsP/InP long-wavelength quantum well infrared photodetectors. Appl. Phys. Lett. 1992, 60, 636–638. 10.1063/1.106577. [DOI] [Google Scholar]
- Conejeros S.; Alemany P.; Llunell M.; Moreira I. dP. R.; Sánchez V.; Llanos J. Electronic Structure and Magnetic Properties of CuFeS2. Inorg. Chem. 2015, 54, 4840–4849. 10.1021/acs.inorgchem.5b00399. [DOI] [PubMed] [Google Scholar]
- Zhang Z.; Xu B.; Zhang L.; Ren S. Hybrid Chalcopyrite–Polymer Magnetoconducting Materials. ACS Appl. Mater. Interfaces 2016, 8, 11215–11220. 10.1021/acsami.6b03362. [DOI] [PubMed] [Google Scholar]
- Wu Y.; Zhou B.; Yang C.; Liao S.; Zhang W.-H.; Li C. CuFeS2 colloidal nanocrystals as an efficient electrocatalyst for dye sensitized solar cells. Chem. Commun. 2016, 52, 11488–11491. 10.1039/C6CC06241E. [DOI] [PubMed] [Google Scholar]
- Gabka G.; Zybała R.; Bujak P.; Ostrowski A.; Chmielewski M.; Lisowski W.; Sobczak J. W.; Pron A. Facile Gram-Scale Synthesis of the First n-Type CuFeS2 Nanocrystals for Thermoelectric Applications. Eur. J. Inorg. Chem. 2017, 2017, 3150–3153. 10.1002/ejic.201700611. [DOI] [Google Scholar]
- Ghosh S.; Avellini T.; Petrelli A.; Kriegel I.; Gaspari R.; Almeida G.; Bertoni G.; Cavalli A.; Scotognella F.; Pellegrino T.; Manna L. Colloidal CuFeS2 Nanocrystals: Intermediate Fe d-Band Leads to High Photothermal Conversion Efficiency. Chem. Mater. 2016, 28, 4848–4858. 10.1021/acs.chemmater.6b02192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugathan A.; Saigal N.; Rajasekar G. P.; Pandey A. Copper Iron Sulfide Nanocrystal-Bulk Silicon Heterojunctions for Broadband Photodetection. Adv. Mater. Interfaces 2020, 7, 2000056 10.1002/admi.202000056. [DOI] [Google Scholar]
- Layek A.; Middya S.; Dey A.; Das M.; Datta J.; Banerjee C.; Ray P. P. Study of resonance energy transfer between MEH-PPV and CuFeS2 nanoparticle and their application in energy harvesting device. J. Alloys Compd. 2014, 613, 364–369. 10.1016/j.jallcom.2014.06.007. [DOI] [Google Scholar]
- Xie Y.; Riedinger A.; Prato M.; Casu A.; Genovese A.; Guardia P.; Sottini S.; Sangregorio C.; Miszta K.; Ghosh S.; Pellegrino T.; Manna L. Copper Sulfide Nanocrystals with Tunable Composition by Reduction of Covellite Nanocrystals with Cu+ Ions. J. Am. Chem. Soc. 2013, 135, 17630–17637. 10.1021/ja409754v. [DOI] [PubMed] [Google Scholar]
- Luther J. M.; Jain P. K.; Ewers T.; Alivisatos A. P. Localized surface plasmon resonances arising from free carriers in doped quantum dots. Nat. Mater. 2011, 10, 361–366. 10.1038/nmat3004. [DOI] [PubMed] [Google Scholar]
- Liu X.; Wang X.; Zhou B.; Law W.-C.; Cartwright A. N.; Swihart M. T. Size-Controlled Synthesis of Cu2-xE (E = S, Se) Nanocrystals with Strong Tunable Near-Infrared Localized Surface Plasmon Resonance and High Conductivity in Thin Films. Adv. Funct. Mater. 2013, 23, 1256–1264. 10.1002/adfm.201202061. [DOI] [Google Scholar]
- Paliwal A.; Singh S. V.; Sharma A.; Sugathan A.; Liu S.-W.; Biring S.; Pal B. N. Microwave-Polyol Synthesis of Sub-10-nm PbS Nanocrystals for Metal Oxide/Nanocrystal Heterojunction Photodetectors. ACS Appl. Nano Mater. 2018, 1, 6063–6072. 10.1021/acsanm.8b01194. [DOI] [Google Scholar]
- Thanh N. T. K.; Maclean N.; Mahiddine S. Mechanisms of Nucleation and Growth of Nanoparticles in Solution. Chem. Rev. 2014, 114, 7610–7630. 10.1021/cr400544s. [DOI] [PubMed] [Google Scholar]
- Basu M.; Garg N.; Ganguli A. K. A type-II semiconductor (ZnO/CuS heterostructure) for visible light photocatalysis. J. Mater. Chem. A 2014, 2, 7517–7525. 10.1039/C3TA15446G. [DOI] [Google Scholar]
- Wang D.-Y.; Jiang Y.-T.; Lin C.-C.; Li S.-S.; Wang Y.-T.; Chen C.-C.; Chen C.-W. Solution-Processable Pyrite FeS2 Nanocrystals for the Fabrication of Heterojunction Photodiodes with Visible to NIR Photodetection. Adv. Mater. 2012, 24, 3415–3420. 10.1002/adma.201200753. [DOI] [PubMed] [Google Scholar]
- Hassan M. S.; Bera S.; Gupta D.; Ray S. K.; Sapra S. MoSe2–Cu2S Vertical p–n Nanoheterostructures for High-Performance Photodetectors. ACS Appl. Mater. Interfaces 2019, 11, 4074–4083. 10.1021/acsami.8b16205. [DOI] [PubMed] [Google Scholar]
- Singh S. V.; Kumar M. P.; Anantharaj S.; Mukherjee B.; Kundu S.; Pal B. N. Direct Evidence of an Efficient Plasmon-Induced Hot-Electron Transfer at an in Situ Grown Ag/TiO2 Interface for Highly Enhanced Solar H2 Generation. ACS Appl. Energy Mater. 2020, 3, 1821–1830. 10.1021/acsaem.9b02267. [DOI] [Google Scholar]
- Yao J.; Zheng Z.; Yang G. All-Layered 2D Optoelectronics: A High-Performance UV–vis–NIR Broadband SnSe Photodetector with Bi2Te3 Topological Insulator Electrodes. Adv. Funct. Mater. 2017, 27, 1701823 10.1002/adfm.201701823. [DOI] [Google Scholar]
- Zhang K.; Fang X.; Wang Y.; Wan Y.; Song Q.; Zhai W.; Li Y.; Ran G.; Ye Y.; Dai L. Ultrasensitive Near-Infrared Photodetectors Based on a Graphene–MoTe2–Graphene Vertical van der Waals Heterostructure. ACS Appl. Mater. Interfaces 2017, 9, 5392–5398. 10.1021/acsami.6b14483. [DOI] [PubMed] [Google Scholar]
- Nie S.; Emory S. R. Probing Single Molecules and Single Nanoparticles by Surface-Enhanced Raman Scattering. Science 1997, 275, 1102. 10.1126/science.275.5303.1102. [DOI] [PubMed] [Google Scholar]
- Shimizu T.; Haruyama J.; Marcano D. C.; Kosinkin D. V.; Tour J. M.; Hirose K.; Suenaga K. Large intrinsic energy bandgaps in annealed nanotube-derived graphene nanoribbons. Nat. Nanotechnol. 2011, 6, 45. 10.1038/nnano.2010.249. [DOI] [PubMed] [Google Scholar]
- Cao J.; Wang Z.; Zhan X.; Wang Q.; Safdar M.; Wang Y.; He J. Vertical SnSe nanorod arrays: from controlled synthesis and growth mechanism to thermistor and photoresistor. Nanotechnology 2014, 25, 105705 10.1088/0957-4484/25/10/105705. [DOI] [PubMed] [Google Scholar]
- Pawbake A. S.; Jadkar S. R.; Late D. J. High performance humidity sensor and photodetector based on SnSe nanorods. Mater. Res. Express 2016, 3, 105038 10.1088/2053-1591/3/10/105038. [DOI] [Google Scholar]
- Jia Z.; Xiang J.; Wen F.; Yang R.; Hao C.; Liu Z. Enhanced Photoresponse of SnSe-Nanocrystals-Decorated WS2 Monolayer Phototransistor. ACS Appl. Mater. Interfaces 2016, 8, 4781–4788. 10.1021/acsami.5b12137. [DOI] [PubMed] [Google Scholar]
- Wang W.; Jiang J.; Ding T.; Wang C.; Zuo J.; Yang Q. Alternative Synthesis of CuFeSe2 Nanocrystals with Magnetic and Photoelectric Properties. ACS Appl. Mater. Interfaces 2015, 7, 2235–2241. 10.1021/am508844w. [DOI] [PubMed] [Google Scholar]