Abstract
Efficient microwave-assisted chemical processes were applied to the synthesis of an array of novel N-(4-methoxyphenylamino)-2-methyl benzo-, pyrido- or pyrazino-thieno[3,2-d]pyrimidin-4-amine derivatives. These heteroaromatic systems were envisioned as potent bioisosteric analogues of MPC-6827, an anticancer agent previously developed until phase II clinical studies. A brief evaluation and comparison of their antiproliferative activity on HT-29 and Caco-2, two human colorectal cancer cell lines, were also reported. At the tested concentrations (5 and 10 µM), thieno[3,2-d]pyrimidin-4-amines 4a and 4c exhibited an inhibitory effect similar to MPC-6827 on human colorectal cancer cell proliferation.
Keywords: microwave-assisted chemistry; thieno[3,2-d]pyrimidines; colorectal cancer; HT-29 cells; caco-2 cells; antiproliferative activity
1. Introduction
Due to their presence in numerous biologically active compounds, quinazoline derivatives are of particular interest for medicinal chemistry and remain a major research area in organic chemistry [1,2,3,4,5]. Among the numerous bioactive quinazolines, MPC-6827 (N-(4-methoxyphenylamino)-N,2-dimethylquinazoline) has been extensively studied for its therapeutic use against cancer [6,7]. MPC-6827, also named Azixa or verubulin, is a microtubule-destabilizing agent exhibiting a dual mode of action, leading to apoptosis by blocking cell cycle and to growth inhibition on several types of cancer such as breast, colon and ovarian cancers [8,9,10]. MPC-6827 is also known to reduce blood supply to the tumors [11]. Based on these data, this benzo[e]pyrimidine emerged as a good candidate for phase I and phase II clinical trials in patients with metastatic melanoma and glioblastoma multiforme [12,13,14]. Despite these investigations revealing some cardiotoxicity and leading to the suspension of clinical development in phase II [15], MPC-6827 remains an excellent model for the design of potential cytotoxic agents [16].
There are a few synthetic routes of MPC-6827 reported in the literature. The initial work of Sirisoma and his co-workers was carried out in three steps from anthranilic acid methyl ester [9]. In the last step, 4-chloro-2-methylquinazoline was reacted with N-methyl-4-methoxyaniline in anhydrous propanol to give the target product in an overall yield of 55% (Scheme 1) [9]. These methods usually require forcing conditions with long reaction times and, sometimes, conditions using toxic reagents (e.g., POCl3).
Scheme 1.
Proposed retrosynthetic route of the target tricyclic analogs of MPC-6827.
To develop sustainable and convenient multicomponent processes for the synthesis of quinazoline and quinazolinone derivatives [17,18,19], our group investigated a novel and efficient two-step synthesis of MPC-6827 [20]. Indeed, reaction of anthranilonitrile with N,N-dimethylacetamide dimethyl acetal (DMA-DMA) at 115 °C for 2 min gave acetimidamide intermediate in excellent yield (90%). It was intensely heated (200 °C for 2 h) with N-methyl-p-anisidine (1.5 equiv.), in N-methylpyrrolidone (NMP), in the presence of aluminium chloride (AlCl3, 1.5 equiv.). MPC-6827 was obtained in 71% yield, i.e., 64% using the two-step synthesis method (Figure 1).
Figure 1.
Synthetic routes of MPC-6827 described by Sirisoma et al. and our group a decade ago.
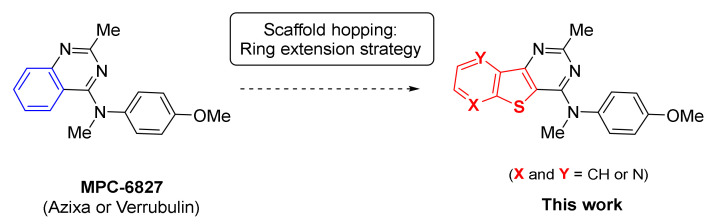
Combining our chemistry work with a scaffold hopping strategy, we envisioned to extend and replace the benzenic part of this small molecule into an aryl thiophene ring. Herein, we report the convenient synthesis of an array of novel N-(4-methoxyphenylamino)-2-methyl benzo-, pyrido- or pyrazino-thieno[3,2-d]pyrimidine derivatives, envisioned as bioisosteric analogs of MPC-6827 (Figure 2). A brief evaluation of their antiproliferative activity on two human colorectal cancer cell lines (human HT-29 and Caco-2) is also reported and compared with data obtained for the source of inspiration (MPC-6827).
Figure 2.
Structure of MPC-6827 and N-(4-methoxyphenylamino)-2-methyl benzo-, pyrido- or pyrazino-thieno[3,2-d]pyrimidine derivatives envisioned in this work.
2. Results and Discussion
2.1. Chemistry
The target compounds were N-(4-methoxyphenyl)-N,2-dimethylthieno[3,2-d]pyrimidin-4-amine derivatives bearing N-methyl-p-anisidine at C4 position of the pyrimidinyl ring. As depicted in Scheme 1, the retrosynthetic route to the novel heteroarenes was envisioned via intramolecular cyclization of the key N,N-dimethylacetimidamides (2), obtained by formylation of the corresponding cyanoenamines (1).
The first step of the process concerned the synthesis of N,N-dimethylacetimidamides (2a–d), which were obtained in good yields (61–83%) by heating the corresponding cyanoenamines (1a–d) with a large excess (10 equiv.) of DMA-DMA at 110–115 °C within 2–5 min of irradiation (Scheme 2).
Scheme 2.
Synthesis of compounds 2, 3 and 4 (see Table 1 for experimental conditions).
According to our preceding work (Figure 1) [20], (E)-N′-(2-cyanobenzo[b]thiophen-3-yl)-N,N-dimethylacetimidamide (2a) was treated with N-methyl-p-anisidine and 1.5 equiv. of AlCl3 in N-methylpyrrolidone (NMP), and was heated at 200 °C for 2 h under microwave irradiation (Scheme 2). These operating conditions allowed the synthesis of MPC-6827; however, we were unable to generate its thiophenic analogue (4a) or the corresponding enamine intermediate resulting from the attack of the aromatic secondary amine on the activated carbonitrile group (Scheme 2).
Thus, an alternative two-step procedure starting from acetimidamide 2a was considered (Scheme 2). Starting acetimidamide (2a) was heated at 118 °C with p-anisidine (1 equiv.) in acetonitrile/acetic acid (2:1; v/v) as solvent. However, after 30 min or 1 h of irradiation (400 W), the formation of N-(4-methoxyphenyl)-2-methyl-benzo[4,5]thieno[3,2-d]pyrimidin-4-amine (3a) was not complete and reagents were recovered in the final mixture. The addition of AlCl3 (1 equiv.) and an increase of reaction temperature until 160 °C allowed access to the attempted cyclized compound (3a) in good yield (78%). Alkylation of the exocyclic amine was realized under usual conditions by stirring (3a) with a large excess of iodomethane (CH3I) in the presence of sodium hydride (NaH), at 0 °C for 2 h. The expected N-(4-methoxyphenyl)-N,2-dimethylbenzo[4,5]thieno[3,2-d]pyrimidin-4-amine (4a) was then obtained in very good yield (86%) (Scheme 2, Table 1). This two-step sequence was applied to N′-(2-cyanothieno[3,2-b]pyridin-3-yl)-, N′-(2-cyanothieno[2,3-b]pyridin-3-yl)- and N′-(6-cyanothieno[2,3-b]pyrazin-7-yl)-N,N-dimethylacetimidamides (2b, 2c and 2d, respectively) to yield the final products 4b, 4c and 4d, respectively (Scheme 2, Table 1).
Table 1.
Experimental conditions for the synthesis of compounds 2, 3 and 4.
| Compound 2 | Temp 1 (°C) |
Time 2 (min) |
Yield 3 (%) |
Compound 3 | Temp 1 (°C) |
Yield 3 (%) |
Compound 4 | Yield 3 (%) 1 |
|---|---|---|---|---|---|---|---|---|
| a | 115 | 5 | 72 | a | 160 | 78 | a | 86 |
| b | 115 | 5 | 74 | b | 160 | 37 | b | 90 |
| c | 115 | 5 | 83 | c | 160 | 27 | c | 83 |
| d | 100 | 2 | 61 | d | 140 | 59 | d | 74 |
1 Temperature; 2 reaction time; 3 isolated yields.
Microwave-assisted heating is an efficient technology that allows reproducible and safe operating conditions for convenient access to various molecules when traditional multistep processes would need long reaction times and unstable and toxic reagents (e.g., formamide and POCl3) [21,22,23,24]. The innovative conditions previously described for the synthesis of MPC-6827 failed to provide compounds 4a–d and a more traditional approach was investigated.
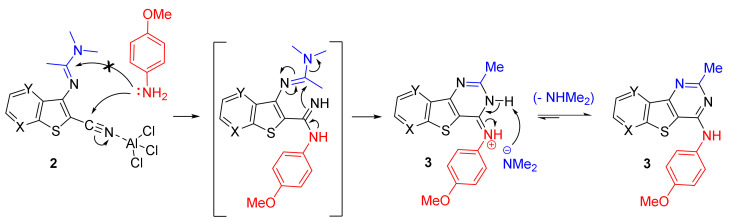
The crucial part of our synthetic pathway was the cyclization step in which cyanoenamines 2 were converted into tricyclic compounds 3. Despite our efforts, the synthesis of thienopyridines 3b and 3c remained difficult, as demonstrated by the low yields described in Table 1. The suggested mechanism is described in Scheme 3.
Scheme 3.
Suggested mechanism for reaction of 2 series with p-anisidine and access to 3, then 4.
Based on the electrophilic character of the cyano group, the reaction starts by nucleophilic attack of the primary aromatic amine on the carbon of the cyano group, which is activated by AlCl3 [25]. The intermediate p-methoxyphenylamidine can then undergo intramolecular cyclization via the attack of the more nucleophilic amidino secondary amine to enamine function, generating the pyrimidine ring present in the expected N-(4-methoxyphenyl)-2-methyl-benzo[4,5]thieno[3,2-d]pyrimidin-4-amines (3). Interestingly, the release of the dimethylamino group, during the cyclisation step, would favor afterwards the aromatization step.
2.2. Antiproliferative Activity on Colorectal Cancer Cell Lines (Caco-2 and HT-29)
In preliminary experiments, the antiproliferative effect of compounds (3b–d) and (4a–d) was evaluated on Caco-2 cells and compared with that of MPC-6827. Each molecule was tested at two concentrations (5 and 10 µM) for 1, 24, 48 and 72 h. The benzo[4,5]thieno[3,2-d]pyrimidine 3a was not tested because of its insolubility in the conditions used. The proliferation of Caco-2 cells was not altered with molecules 3b, 3c, 3d, 4b and 4d (data not shown). The two most interesting compounds (4a and 4c) were also tested on HT-29 cells. Results obtained on Caco-2 and HT-29 cells for 4a, 4c and MPC-6827 are presented in Figure 3 and Figure 4, respectively.
Figure 3.
Proliferation of Caco-2 cells treated with MPC-6827 (5 and 10 µM), 4a (5 and 10 µM) and 4c (5 and 10 µM) after 1, 24, 48 and 72 h of treatment. Proliferation was evaluated with an MTT assay. Data are reported as the mean ± SD in percentage from three independent experiments. * P < 0.05 vs. control that represented 100% of proliferation.
Figure 4.
Proliferation of HT-29 cells treated with MPC-6827 (5 and 10 µM), 4a (5 and 10 µM) and 4c (5 and 10 µM) after 1, 24, 48 and 72 h of treatment. Proliferation was evaluated with an MTT assay. Data are reported as the mean ± SD in percentage from three independent experiments. * P < 0.05 vs. control that represented 100% of proliferation.
The results of the biological evaluation highlighted that the two compounds (4a and 4c) that possessed antiproliferative properties on Caco-2 and HT-29 were as interesting as MPC-6827 (Azixa or verubulin).
Overall, the number of surviving Caco-2 cancer cells decreased in a time-dependent manner. More precisely, inhibition of the proliferation of Caco-2 colon cancer cells was 25% after 24 h of treatment with 10 µM concentrations of 4c. Higher inhibitory effects measured after 48 h of incubation were close to the final results observed after 72 h of experiment. In this case, proliferation of Caco-2 cells was significantly inhibited in the presence of 4a and 4c, in a manner equivalent to that of MPC-6827, with inhibitory effects of 30% and 45% at 5 µM and 10 µM, respectively (Figure 3).
Similar to Caco-2 cells, HT-29 cancer cell proliferation was significantly inhibited in the presence of MPC-6827 and compounds 4a and 4c, after 72 h of treatment (Figure 4). The growth inhibition of the HT-29 colorectal cancer cell line also appeared to be time-dependent. In addition, more important effects were observed compared to those described for Caco-2 cells (around 50% and 60% inhibition at 5 µM and 10 µM concentration, respectively) (Figure 3 and Figure 4).
3. Conclusions
This work demonstrated that the novel thieno[3,2-d]pyrimidin-4-amines 4a and 4c exhibited a similar inhibitory effect on colon cancer cell proliferation as MPC-6827. The exchange of the carbon at position 6 of 4a by a nitrogen atom, as in 4c, appeared to maintain the biological activity studied. Furthermore, a comparison of results described for 4a and 4c with those obtained for 4b and 4d suggested that modifying the atom at position 9 of the heteroaromatic scaffold strongly decreased the biological effect. These preliminary results encourage us to carry on the development of such compounds in the hope of identifying new leads.
4. Materials and Methods
4.1. Chemistry
4.1.1. General Information
All reagents were purchased from commercial suppliers and were used without further purification. All reactions were monitored by thin-layer chromatography with aluminium plates (0.25 mm) precoated with silica gel 60 F254 (Merck KGaA, Darmstadt, Germany). Visualization was performed with UV light at a wavelength of 254 nm. Purifications were conducted with a flash column chromatography system (PuriFlash, Interchim, Montluçon, France) using stepwise gradients of petroleum ether (PE)/dichloromethane (DCM) or ethyl acetate (EtOAc) as the eluent. Melting points were measured with an SMP3 Melting Point instrument (STUART, Bibby Scientific Ltd., Roissy, France) with a precision of 1.5 °C. IR spectra were recorded with a Spectrum 100 Series FTIR spectrometer (PerkinElmer, Villebon S/Yvette, France). NMR spectra (1H and 13C) were acquired at 295 K using an AVANCE 300 MHz spectrometer (Bruker, Wissembourg, France) at 300 and 75.4 MHz. Coupling constant J was in Hz and chemical shifts were given in ppm. Mass (ESI, EI and field desorption (FD) were recorded with an LCP 1er XR spectrometer (WATERS, Guyancourt, France). Microwave experiments at atmospheric pressure were carried out in RotoSYNTH (0–1200 W) (Milestone Srl, Italy). Microwave reactions in sealed tubes (10 mL) were performed with an Initiator microwave synthesis instrument (0–400W) (Biotage, Uppsala, Sweden). The percentage of purity of all tested products was more than 95% (determined by HPLC analysis). 1H-NMR and 13C-NMR spectra of new compounds are available in Supplementary Materials (Section Figures S1–S12).
All details concerning the synthesis of cyanoenamines (1a–d) are described in preceding work [26,27,28]. MPC-6827 was synthesized according to our previous methods [20].
4.1.2. General Procedure for the Synthesis of N,N-dimethylacetimidamide Derivatives (2a–d)
A mixture of the appropriate cyanoenamine (3.0 mmol) and DMA-DMA (4 mL, 30 mmol) was heated under microwave irradiation (800 W). On completion, the reaction was cooled to room temperature and crude products were extracted 3 times with EtOAc (5 mL). The organic layers were washed with cold water, dried over Na2SO4, filtered and evaporated in vacuo. The crude product was purified by silica gel column chromatography using PE/DCM (100:0–0:100, v/v) as the eluent to give the desired products.
(E)-N′-(2-Cyanobenzo[b]thiophen-3-yl)-N,N-dimethylacetimidamide (2a): Orange powder (0.502 g, 72%) obtained from 3-aminobenzothiophene-2-carbonitrile (1a) after 5 min at 115 °C according to the general procedure; mp: 92–93 °C; IR (neat) νmax (cm−1): 2199 (CN), 1591, 1557, 1476, 1456, 1426, 1414, 1397, 1365, 1346, 1318, 1188, 1059, 1024, 1016, 934, 887, 755, 737; 1H NMR (300 MHz, DMSO-d6): δ 7.98 (dd, 1H, J1 = 1 Hz, J2 = 8 Hz, H-7), 7.66 (dd, 1H, J1 = 1 Hz, J2 = 8 Hz, H-4), 7.58 (td, 1H, J1 = 1 Hz, J2 = 8 Hz, H-6), 7.45 (td, 1H, J1 = 1 Hz, J2 = 8 Hz, H-5), 3.34 (s, 3H, NCH3), 3.12 (s, 3H, NCH3), 1.97 (s, 3H, CH3); 13C NMR (75 MHz, DMSO-d6): δ 159.6, 156.8, 138.8, 133.9, 128.5, 125.1, 123.6, 123.3, 115.9, 97.0, 40.0 (2C), 15.9; HRMS calculated for C13H14N3S [M + H]+ 244.0908 found 244.0908.
(E)-N′-(2-Cyanothieno[3,2-b]pyridin-3-yl)-N,N-dimethylacetimidamide (2b): Orange oil (0.513 g, 74%) obtained from 3-aminothieno[3,2-b]pyridine-2-carbonitrile (1b) after 5 min at 115 °C according to the general procedure; IR (neat) νmax (cm−1): 2210 (CN), 1576, 1413, 1391, 1369, 1348, 1191, 1062, 1022, 794, 784; 1H NMR (300 MHz, DMSO-d6): δ 8.73 (dd, 1H, J1 = 2 Hz, J2 = 5 Hz, H-5), 8.50 (dd, 1H, J1 = 2 Hz, J2 = 8 Hz, H-7), 7.56 (dd, 1H, J1 = 5 Hz, J2 = 8 Hz, H-6), 3.11 (s, 6H, N(CH3)2), 1.89 (s, 3H, CH3); 13C NMR (75 MHz, DMSO-d6): δ 160.3, 156.4, 148.2, 147.3, 133.8, 132.1, 122.1, 115.7, 91.5, 40.2 (2C), 17.3; HRMS calculated for C12H13N4S [M + H]+ 245.0861 found 245.0852.
(E)-N′-(2-Cyanothieno[2,3-b]pyridin-3-yl)-N,N-dimethylacetimidamide (2c): Grey powder (0.578 g, 83%) obtained from 3-aminothieno[2,3-b]pyridine-2-carbonitrile (1c) after 5 min at 115 °C according to the general procedure; mp: 136–137 °C; IR (neat) νmax (cm−1): 2201 (CN), 1575, 1553, 1448, 1429, 1417, 1399, 1381, 1369, 1342, 1186, 1058, 1024, 934, 809, 755, 736; 1H NMR (300 MHz, DMSO-d6): δ 8.76 (dd, 1H, J1 = 2 Hz, J2 = 5 Hz, H-4), 8.08 (dd, 1H, J1 = 2 Hz, J2 = 8 Hz, H-6), 7.52 (dd, 1H, J1 = 5 Hz, J2 = 8 Hz, H-5), 3.12 (s, 6H, N(CH3)2), 2.01 (s, 3H, CH3); 13C NMR (75 MHz, DMSO-d6): δ 160.1, 159.4, 154.9, 150.9, 132.0, 127.8, 120.6, 115.5, 86.2, 39.8 (2C), 16.1; HRMS calculated for C12H13N4S [M + H]+ 245.0861 found 245.0853.
(E)-N′-(6-Cyanothieno[2,3-b]pyrazin-7-yl)-N,N-dimethylacetimidamide (2d): Orange powder (0.424 g, 61%) obtained from 7-aminothieno[2,3-b]pyrazine-6-carbonitrile (1d) after 2 min at 100 °C according to the general procedure; mp: 139–140 °C; IR (neat) νmax (cm−1): 2209 (CN), 1575, 1478, 1431, 1413, 1393, 1377, 1365, 1341, 1334, 1180, 1081, 1072, 1041, 1024, 946, 865, 745; 1H NMR (300 MHz, DMSO-d6): δ 8.83 (d, 1H, J = 8 Hz, H-5), 8.79 (d, 1H, J = 8 Hz, H-6), 3.12 (s, 6H, N(CH3)2), 1.94 (s, 3H, CH3); 13C NMR (75 MHz, DMSO-d6): δ 160.9, 154.5, 154.3, 144.3, 143.2, 141.8, 115.1, 91.1, 39.9 (2C), 17.3; HRMS calculated for C11H12N5S [M + H]+ 246.0813 found 246.0815.
4.1.3. General Procedure for the Synthesis of N-(4-methoxyphenyl)-2-methylthieno[3,2-d]pyrimidin-4-amines (3a–d)
A mixture of N,N-dimethylacetimidamide derivatives (2a–d) (1.0 mmol), p-anisidine (1.0 mmol) and aluminium chloride (1.0 mmol) in MeCN (4 mL)/AcOH (2 mL) was heated under microwave irradiation (400 W). On completion, the reaction was cooled to room temperature and water was added. The solid was filtered off, washed twice with water and dried. The crude solid was purified by silica gel column chromatography using PE/EtOAc (100:0–0:100, v/v) as the eluent to give the desired products.
N-(4-Methoxyphenyl)-2-methylbenzo[4,5]thieno[3,2-d]pyrimidin-4-amine (3a): Pale yellow powder (0.250 g, 78%) obtained from 2a after 60 min at 160 °C according to the general procedure; mp: 263–264 °C; IR (neat) νmax (cm−1): 1605, 1582, 1569, 1509, 1432, 1388, 1301, 1250, 1170, 1124, 1098, 1023, 894, 826, 774, 743; 1H NMR (300 MHz, DMSO-d6): δ 8.78 (s, 1H, NH), 8.22 (dd, 1H, J1 = 1 Hz, J2 = 7 Hz, H-9), 7.75 (dd, 1H, J1 = 1 Hz, J2 = 7 Hz, H-8), 7.68-7.58 (m, 4H, H-6, H-7 and H-ar), 7.04 (d, 2H, J = 9 Hz, H-ar), 3.82 (s, 3H, OCH3), 2.73 (s, 3H, CH3); 13C NMR (75 MHz, DMSO-d6): δ 161.3, 158.6, 157.0, 156.1, 140.3 (2C), 130.4 (2C), 125.6 (2C), 123.8 (2C), 114.1 (3C), 112.2, 55.3, 23.5; HRMS calculated for C18H16N3OS [M + H]+ 322.1014 found 322.1026.
N-(4-Methoxyphenyl)-2-methylpyrido[2’,3’:4,5]thieno[3,2-d]pyrimidin-4-amine (3b): Yellow powder (0.119 g, 37%) obtained from 2b after 60 min at 160 °C according to the general procedure; mp: >300 °C; IR (neat) νmax (cm−1): 1561, 1546, 1515, 1497, 1441, 1410, 1372, 1337, 1296, 1247, 1231, 1176, 1090, 1006, 813, 771, 742; 1H NMR (300 MHz, DMSO-d6): δ 9.62 (s, 1H, NH), 8.81 (dd, 1H, J1 = 2 Hz, J2 = 5 Hz, H-9), 8.63 (dd, 1H, J1 = 2 Hz, J2 = 8 Hz, H-7), 7.65-7.59 (m, 3H, H-8 and H-ar), 6.97 (d, 2H, J = 9 Hz, H-ar), 3.79 (s, 3H, OCH3), 2.58 (s, 3H, CH3); 13C NMR (75 MHz, DMSO-d6): δ 163.7, 161.6, 156.2, 155.5, 154.3, 151.5, 131.3 (2C), 127.4, 124.9, 120.8, 113.7 (3C), 110.9, 55.2, 25.6; HRMS calculated for C17H15N4OS [M + H]+ 323.0967 found 323.0960.
N-(4-Methoxyphenyl)-2-methylpyrido[3’,2’:4,5]thieno[3,2-d]pyrimidin-4-amine (3c): Yellow powder (0.087 g, 27%) obtained from 2c after 60 min of irradiation at 160 °C according to the general procedure; mp: 221–222 °C; IR (neat) νmax (cm−1): 1579, 1539, 1506, 1410, 1381, 1260, 1067, 982, 824, 748; 1H NMR (300 MHz, DMSO-d6): δ 9.65 (s, 1H, NH), 8.83 (dd, 1H, J1 = 2 Hz, J2 = 5 Hz, H-8), 8.63 (dd, 1H, J1 = 2 Hz, J2 = 8 Hz, H-6), 7.65-7.62 (m, 3H, H-7 and H-ar), 6.98 (d, 2H, J = 9 Hz, H-ar), 3.79 (s, 3H, OCH3), 2.60 (s, 3H, CH3); HRMS calculated for C17H15N4OS [M + H]+ 323.0967 found 323.0975.
N-(4-Methoxyphenyl)-2-methylpyrazino[2’,3’:4,5]thieno[3,2-d]pyrimidin-4-amine (3d): Yellow powder (0.191 g, 59%) obtained from 2d after 60 min at 140 °C according to the general procedure; mp: 266–267 °C; IR (neat) νmax(cm−1): 1551, 1515, 1500, 1436, 1412, 1372, 1333, 1299, 1238, 1176, 1096, 1088, 1032, 1002, 861, 839, 765, 724; 1H NMR (300 MHz, DMSO-d6): δ 9.76 (s, 1H, NH), 8.93 (d, 1H, J = 8 Hz, H-7), 8.92 (d, 1H, J = 8 Hz, H-8), 7.65 (d, 2H, J = 9 Hz, H-ar), 6.99 (d, 2H, J = 9 Hz, H-ar), 3.79 (s, 3H, OCH3), 2.54 (s, 3H, CH3); 13C NMR (75 MHz, DMSO-d6): δ 164.4, 156.6, 156.4, 155.7, 152.7, 145.0, 143.5, 143.0, 131.1, 125.0, 113.8 (3C), 113.4, 55.2, 25.6; HRMS calculated for C16H14N5OS [M + H]+ 324.0919 found 324.0917.
4.1.4. General Procedure for the Synthesis of N-(4-methoxyphenyl)-N,2-dimethylthieno[3,2-d]pyrimidin-4-amines (4a–d)
A suspension of N-(4-methoxyphenyl)-2-methylthieno[3,2-d]pyrimidin-4-amine (4.0 mmol) in DMF (5 mL) was cooled to 0 °C. Then, NaH (60% in mineral oil, 8.0 mmol) and methyl iodide (96 mmol) were added. The reaction mixture was stirred at 0 °C for 1 h and warmed to room temperature. The reaction was quenched by adding water. EtOAc was added and the organic phase was washed twice with H2O and brine, dried over MgSO4, filtered and evaporated under vacuum. The crude solid was purified by silica gel column chromatography using PE/EtOAc (100:0–0:100, v/v) as the eluent to give the desired product.
N-(4-Methoxyphenyl)-N,2-dimethylbenzo[4,5]thieno[3,2-d]pyrimidin-4-amine (4a): White powder (0.090 g, 86%) obtained from 3a according to the general procedure; mp: 147–148 °C; IR (neat) νmax (cm−1): 1539, 1525, 1508, 1456, 1440, 1390, 1369, 1346, 1247, 1165, 1145, 1093, 1032, 1008, 838, 754, 734; 1H NMR (300 MHz, DMSO-d6): δ 8.26 (dd, 1H, J1 = 1 Hz, J2 = 7 Hz, H-9), 7.87 (dd, 1H, J1 = 1 Hz, J2 = 7 Hz, H-8), 7.57-7.43 (m, 4H, H-6, H-7 and H-ar), 7.08 (d, 2H, J = 9 Hz, H-ar), 3.86 (s, 3H, OCH3), 3.52 (s, 3H, CH3), 2.65 (s, 3H, CH3); 13C NMR (75 MHz, DMSO-d6): δ 163.2, 159.2, 157.2, 156.7, 140.5, 135.4, 132.8, 130.6, 129.3 (2C), 124.8, 123.0, 122.6, 114.6 (2C), 111.8, 55.4, 39.6, 25.5; HRMS calculated for C19H18N3OS [M + H]+ 336.1171 found 336.1163.
N-(4-Methoxyphenyl)-N,2-dimethylpyrido[2’,3’:4,5]thieno[3,2-d]pyrimidin-4-amine (4b): Yellow powder (0.094 g, 90%) obtained from 3b according to the general procedure; mp: >300 °C; IR (neat) νmax (cm−1): 2921, 1383, 1248, 1077, 1033, 860; 1H NMR (300 MHz, DMSO-d6): δ 8.76 (dd, 1H, J1 = 2 Hz, J2 = 5 Hz, H-8), 8.39 (dd, 1H, J1 = 2 Hz, J2 = 8 Hz, H-6), 7.53 (dd, 1H, J1 = 5 Hz, J2 = 8 Hz, H-7), 7.45 (d, 2H, J = 9 Hz, H-ar), 7.09 (d, 2H, J = 9 Hz, H-ar), 3.86 (s, 3H, OCH3), 3.53 (s, 3H, CH3), 2.67 (s, 3H, CH3); HRMS calculated for C18H17N4OS [M + H]+ 337.1123 found 337.1133.
N-(4-Methoxyphenyl)-N,2-dimethylpyrido[3’,2’:4,5]thieno[3,2-d]pyrimidin-4-amine (4c): White powder (0.087 g, 83%) obtained from 3c according to the general procedure; mp: 161–162 °C; IR (neat) νmax (cm−1): 1539, 1510, 1443, 1415, 1395, 1375, 1349, 1303, 1252, 1169, 1097, 1029, 979, 873, 835, 772, 748; 1H NMR (300 MHz, DMSO-d6): δ 8.70 (dd, 1H, J1 = 2 Hz, J2 = 5 Hz, H-9), 8.59 (dd, 1H, J1 = 2 Hz, J2 = 8 Hz, H-7), 7.54 (dd, 1H, J1 = 5 Hz, J2 = 8 Hz, H-8), 7.45 (d, 2H, J = 9 Hz, H-ar), 7.09 (d, 2H, J = 9 Hz, H-ar), 3.88 (s, 3H, OCH3), 3.54 (s, 3H, CH3), 2.65 (s, 3H, CH3); 13C NMR (75 MHz, DMSO-d6): δ 163.7, 161.9, 159.5, 157.1, 154.6, 151.5, 135.1, 131.4, 130.9 (2C), 127.0, 120.6, 115.0 (2C), 111.0, 55.6, 40.7, 25.8; HRMS calculated for C18H17N4OS [M + H]+ 337.1123 found 337.1119.
N-(4-Methoxyphenyl)-N,2-methylpyrazino[2’,3’:4,5]thieno[3,2-d]pyrimidin-4-amine (4d): Brown powder (0.077 g, 74%) obtained from 3d according to the general procedure; mp: 238–239 °C; IR (neat) νmax (cm−1): 1531, 1505, 1480, 1458, 1439, 1394, 1355, 1344, 1244, 1179, 1173, 1159, 1108, 1090, 1031, 1011, 864, 834, 800, 764; 1H NMR (300 MHz, DMSO-d6): δ 8.83 (d, 1H, J = 8 Hz, H-7), 8.81 (d, 1H, J = 8 Hz, H-8), 7.47 (d, 2H, J = 9 Hz, H-ar), 7.10 (d, 2H, J = 9 Hz, H-ar), 3.87 (s, 3H, OCH3), 3.54 (s, 3H, CH3), 2.67 (s, 3H, CH3); 13C NMR (75 MHz, DMSO-d6): δ 164.2, 159.6, 157.2, 156.7, 153.0, 145.0, 142.8 (2C), 134.7, 131.0 (2C), 114.8 (2C), 113.2, 55.5, 39.1, 25.7; HRMS calculated for C17H16N5OS [M + H]+ 338.1076 found 338.1073.
4.2. Antiproliferative Activity
The antiproliferative activity was estimated according to methods previously described [21].
Human HT-29 and Caco-2 colorectal adenocarcinoma cells were purchased from American Type Culture Collection (Manassas, VA, USA). HT-29 and Caco-2 colorectal cancer cell lines possess different p53 mutations [29]. The cells were grown in Dulbecco’s modified Eagle’s medium supplemented with 20% fetal bovine serum (Invitrogen, France), 2 mM L-glutamine (Invitrogen,) and penicillin (10 U/mL)/streptomycin (10 µg/mL) at 37 °C with 5% CO2 and 90% relative humidity. Dimethyl sulfoxide (DMSO) was purchased from Sigma-Aldrich, France.
MTT proliferation assay: Caco-2 and HT-29 cells were seeded at a density of 8000 cells/well in 96-well microplates. Cells were treated after 24 h and allowed to proliferate with or without molecules, for 4 days. Molecules were tested at indicated concentrations after extemporaneous dilution in a medium of a starting solution (100 mmol/L in DMSO). The final concentration of DMSO in culture medium was maintained at 0.1%. Molecule 3a was not tested because of its poor solubility in the conditions used. The MTT test was carried out daily after treatment (1, 24, 48 or 72 h).
For the tested molecules, data of statistical analysis were presented as mean ± SD from at least three independent experiments. At least six different replicates were conducted for each compound. A statistical analysis was performed with non-parametric test (Mann–Whitney U test between two groups) using the SigmaStat software. Differences were considered to be statistically significant at a P value < 0.05. No effect of the solvent was observed. The results obtained for the treated cells were compared with those obtained with cells cultured in the presence of DMSO, which represented 100% of proliferation (control).
Acknowledgments
Financial support from the MESR (French: Ministère de l’Enseignement Supérieur & de la Recherche) is gratefully acknowledged for the doctoral fellowships to Y.L. T.B. and his co-workers thank the University of Rouen-Normandie for its support. The authors thank Carole Dubouilh-Benard and Catherine Buquet for technical support on chemistry and biological experiments, respectively.
Supplementary Materials
The following materials are available online at https://www.mdpi.com/1424-8247/13/9/202/s1, 1H-NMR and 13C-NMR spectra of new compounds.
Author Contributions
T.B. and P.M. conceived the project and designed the experiments. Y.L. performed the chemical experimental work, accompanied by M.-R.N. C.C. designed and supervised the overall aspect of biological experiments. T.B. wrote the manuscript with the cooperation of C.C., C.F. and P.M. All authors have commented on the manuscript and agreed to the published version of the manuscript.
Funding
T.B. thanks LABEX SynOrg (ANR-11-LABX-0029) for financial support.
Conflicts of Interest
The authors declare no conflict of interest. The funding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- 1.Khan I., Ibrar A., Ahmed W., Saeed A. Synthetic approaches, functionalization and therapeutic potential of quinazoline and quinazolinone skeletons: The advances continue. Eur. J. Med. Chem. 2015;90:124–169. doi: 10.1016/j.ejmech.2014.10.084. [DOI] [PubMed] [Google Scholar]
- 2.Alagarsamy V., Chitra K., Saravanan G., Raja Solomon V., Sulthana M.T., Narendhar B. An overview of quinazolines: Pharmacological significance and recent developments. Eur. J. Med. Chem. 2018;151:628–685. doi: 10.1016/j.ejmech.2018.03.076. [DOI] [PubMed] [Google Scholar]
- 3.Hameed A., Al-Rashida M., Uroos M., Abid Ali S., Marium A., Khalid I., Khan M. Quinazoline and quinazolinone as important medicinal scaffolds: A comparative patent review (2011–2016) Expert Opin. Ther. Pat. 2018;28:281–297. doi: 10.1080/13543776.2018.1432596. [DOI] [PubMed] [Google Scholar]
- 4.Li Y., Xiao J., Zhang Q., Yu W., Liu M., Guo Y., He J., Liu Y. The association between anti-tumor potency and structure-activity of protein-kinases inhibitors based on quinazoline molecular skeleton. Bioorg. Med. Chem. 2019;27:568–577. doi: 10.1016/j.bmc.2018.12.032. [DOI] [PubMed] [Google Scholar]
- 5.Das D., Hong J. Recent advancements of 4-aminoquinazoline derivatives as kinase inhibitors and their applications in medicinal chemistry. Eur. J. Med. Chem. 2019;170:55–72. doi: 10.1016/j.ejmech.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Kasibhatla S., Baichwal V., Cai S.X., Roth B., Skvortsova I., Skvortsov S., Lukas P., English N.M., Sirisoma N., Drewe J., et al. MPC-6827: A small-molecule inhibitor of microtubule formation that is not a substrate for multidrug resistance pumps. Cancer Res. 2007;67:5865–5871. doi: 10.1158/0008-5472.CAN-07-0127. [DOI] [PubMed] [Google Scholar]
- 7.Sirisoma N., Kasibhatla S., Pervin A., Zhang H., Jiang S., Willardsen J.A., Anderson M.B., Baichwal V., Mather G.G., KJessing K., et al. Discovery of 2-chloro-N-(4-methoxyphenyl)-N-methylquinazolin-4-amine (EP128265, MPI-0441138) as a potent inducer of apoptosis with high in vivo activity. J. Med. Chem. 2008;51:4771–4779. doi: 10.1021/jm8003653. [DOI] [PubMed] [Google Scholar]
- 8.Kemnitzer W., Sirisoma N., May C., Tseng B., Drewe J., Cai S.X. Discovery of 4-anilino-N-methylthieno[3,2-d]pyrimidines and 4-anilino-N-methylthieno[2,3-d]pyrimidines as potent apoptosis inducers. Bioorg. Med. Chem. Lett. 2009;19:3536–3540. doi: 10.1016/j.bmcl.2009.04.145. [DOI] [PubMed] [Google Scholar]
- 9.Sirisoma N., Pervin A., Zhang H., Jiang S., Willardsen J.A., Anderson M.B., Mather G.G., Jessing K., Pleiman C.M., Kasibhatla S., et al. Discovery of N-(4-methoxyphenyl)-N,2-dimethylquinazolin-4-amine, a potent apoptosis inducer and efficacious anticancer agent with high blood brain barrier penetration. J. Med. Chem. 2009;52:2341–2351. doi: 10.1021/jm801315b. [DOI] [PubMed] [Google Scholar]
- 10.Sirisoma N., Pervin A., Zhang H., Jiang S., Adam Willardsen J., Anderson M.B., Mather G., Pleiman C.M., Kasibhatla S., Tseng B., et al. Discovery of N-methyl-4-(4-methoxyanilino)quinazolines as potent apoptosis inducers. Structure-activity relationship of the quinazoline ring. Bioorg. Med. Chem. Lett. 2010;20:2330–2334. doi: 10.1016/j.bmcl.2010.01.155. [DOI] [PubMed] [Google Scholar]
- 11.Mahal K., Resch M., Ficner R., Schobert R., Biersack B., Mueller T. Effects of the tumor-vasculature-disrupting agent Verubulin and two heteroaryl analogues on cancer cells, endothelial cells, and blood vessels. ChemMedChem. 2014;9:847–854. doi: 10.1002/cmdc.201300531. [DOI] [PubMed] [Google Scholar]
- 12.Tsimberidou A.-M., Akerley W., Schabel M.C., Hong D.S., Uehara C., Chhabra A., Warren T., Mather G.G., Evans B.A., Woodland D.P., et al. Phase I clinical trial of MPC-6827 (Azixa), a microtubule destabilizing agent, in patients with advanced cancer. Mol. Cancer Ther. 2010;9:3410–3419. doi: 10.1158/1535-7163.MCT-10-0516. [DOI] [PubMed] [Google Scholar]
- 13.Grossmann K.F., Colman H., Akerley W.A., Glantz M., Matsuoko Y., Beelen A.P., Yu M., De Groot J.F., Aiken R.D., Olsen J.J., et al. Phase I trial of verubulin (MPC-6827) plus carboplatin in patients with relapsed glioblastoma multiform. J. Neurooncol. 2012;110:257–264. doi: 10.1007/s11060-012-0964-7. [DOI] [PubMed] [Google Scholar]
- 14.Chamberlain M.C., Grimm S., Phuphanich S., Recht L., Zhu J.Z., Kim L., Rosenfeld S., Fadul C.E. A phase 2 trial of verubulin for recurrent glioblastoma: A prospective study by the brain tumor investigational consortium (BTIC) J. Neurooncol. 2014;118:335–343. doi: 10.1007/s11060-014-1437-y. [DOI] [PubMed] [Google Scholar]
- 15.Subbiaha I.M., Lenihanb D.J., Tsimberidouc A.M. Cardiovascular toxicity profiles of vascular-disrupting agents. Oncologist. 2011;16:1120–1130. doi: 10.1634/theoncologist.2010-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li W., Yin Y., Shuai W., Xu F., Yao H., Liu J., Cheng K., Xu J., Zhu Z., Xu S. Discovery of novel quinazolines as potential anti-tubulin agents occupying three zones of colchicine domain. Bioorg. Chem. 2019;83:380–390. doi: 10.1016/j.bioorg.2018.10.027. [DOI] [PubMed] [Google Scholar]
- 17.Loidreau Y., Besson T. Microwave-assisted thermal decomposition of formamide: A tool for coupling a pyrimidine ring with an aromatic partner. Tetrahedron. 2011;67:4852–4857. doi: 10.1016/j.tet.2011.05.010. [DOI] [Google Scholar]
- 18.Hédou D., Deau E., Dubouilh-Benard C., Sanselme M., Martinet A., Chosson E., Levacher V., Besson T. Microwave-assisted (3 + 2) cycloaddition and Suzuki-Miyaura cross-coupling for a concise access to novel polyaromatic scaffolds. Eur. J. Org. Chem. 2013:7533–7545. doi: 10.1002/ejoc.201301014. [DOI] [Google Scholar]
- 19.Deau E., Hédou D., Chosson E., Levacher V., Besson T. Convenient one-pot synthesis of N3-substituted pyrido[2,3-d]-, pyrido[3,4-d]-, pyrido[4,3-d]-pyrimidin-4(3H)-ones, and quinazolin-4(3H)-ones analogs. Tetrahedron Lett. 2013;54:3518–3521. doi: 10.1016/j.tetlet.2013.04.096. [DOI] [Google Scholar]
- 20.Foucourt A., Dubouilh-Benard C., Chosson E., Corbière C., Buquet C., Iannelli M., Leblond B., Marsais F., Besson T. Microwave-accelerated Dimroth rearrangement for the synthesis of 4-anilino-6-nitroquinazolines. Application to an efficient synthesis of a microtubule destabilizing agent. Tetrahedron. 2010;66:4495–4502. doi: 10.1016/j.tet.2010.04.066. [DOI] [Google Scholar]
- 21.Loidreau Y., Dubouilh-Benard C., Marchand P., Nourrisson M.-R., Duflos M., Buquet C., Corbière C., Besson T. Efficient new synthesis of N-arylbenzofuro[3,2-d]pyrimidin-4-amines and their novel benzo[b]thieno[3,2-d]pyrimidin-4-amine analogues via a microwave-assisted Dimroth rearrangement. J. Heterocycl. Chem. 2013;50:1187–1197. doi: 10.1002/jhet.1716. [DOI] [Google Scholar]
- 22.Brocklesby K.L., Waby J.S., Cawthorne C., Smith G. An alternative synthesis of Vandetanib (CaprelsaTM) via a microwave accelerated Dimroth rearrangement. Tetrahedron Lett. 2017;58:1467–1469. doi: 10.1016/j.tetlet.2017.02.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marinho E., Proença M.F. The Reaction of 2-(acylamino)benzonitriles with primary aromatic amines: A convenient synthesis of 2-substituted 4-(arylamino)quinazolines. Synthesis. 2015;47:1623–1632. doi: 10.1055/s-0034-1380322. [DOI] [Google Scholar]
- 24.Lemaire L., Leleu-Chavain N., Tourteau A., Abdul-Sada A., Spencer J., Millet R. A rapid route for the preparation of pyrimido[5,4-d]- and pyrido[3,2-d]oxazoles. Tetrahedron Lett. 2015;56:2448–2450. doi: 10.1016/j.tetlet.2015.03.082. [DOI] [Google Scholar]
- 25.Koutentis P.A., Mirallai S.I. Reinvestigating the synthesis of N-arylbenzamidines from benzonitriles and anilines in the presence of AlCl3. Tetrahedron. 2010;66:5134–5139. doi: 10.1016/j.tet.2010.04.103. [DOI] [Google Scholar]
- 26.Loidreau Y., Marchand P., Dubouilh-Benard C., Nourrisson M.-R., Duflos M., Lozach O., Loaëc N., Meijer L., Besson T. Synthesis and biological evaluation of N-arylbenzo[b]thieno[3,2-d]pyrimidin-4-amines and their pyrido and pyrazino analogues as Ser/Thr kinase inhibitors. Eur. J. Med. Chem. 2012;58:171–183. doi: 10.1016/j.ejmech.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 27.Loidreau Y., Dubouilh-Benard C., Nourrisson M.-R., Loaëc N., Meijer L., Besson T., Marchand P. Exploring Kinase Inhibition Properties of 9H-pyrimido[5,4-b]- and [4,5-b]indol-4-amine Derivatives. Pharmaceuticals. 2020;13:89. doi: 10.3390/ph13050089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loidreau Y., Melissen S., Levacher V., Logé C., Graton J., Le Questel J.Y., Besson T. Study of N1-alkylation of indoles from the reaction of 2(or 3)-aminoindole-3-(or 2)carbonitriles with DMF-dialkylacetals. Org. Biomol. Chem. 2012;20:4916–4925. doi: 10.1039/c2ob25747e. [DOI] [PubMed] [Google Scholar]
- 29.Lin-Lee Y.C., Tatebe S., Savaraj N., Ishikawa T., Tien Kuo M. Differential sensitivities of the MRP gene family and γ-glutamylcysteine synthetase to prooxidants in human colorectal carcinoma cell lines with different p53 status. Biochem. Pharmacol. 2001;61:555–563. doi: 10.1016/S0006-2952(00)00592-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.