Graphical abstract
Abbreviations: COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; HCQ, hydroxychloroquine; AZM, azithromycin; MTX, methotrexate; A20, TNF α-induced protein 3; MERS, middle east respiratory syndrome; ACE-2, angiotensin converting enzyme-2; TMPRSS, transmembrane protease serine; ARDS, acute respiratory distress syndrome; RLRs, RIG-I-like receptors; TLRs, toll-like receptors; HMGB1, high mobility group box 1; RSV, respiratory syncytial virus; DMARD, disease modifying anti-rheumatic drug; DHFR, dihydrofolate reductase; TS, thymidylate synthase; AICART, aminoimidazole carboxamide ribonucleotide formyl transferase enzyme
Keywords: COVID-19, SARS-CoV-2, Hydroxychloroquine, Methotrexate, Cytokine storm
Highlights
-
•
Coronavirus disease 2019 is characterized by acute respiratory distress syndrome.
-
•
The mortality rate of SARS-CoV-2 infection is linked to cytokine storm.
-
•
Methotrexate, is well known for its ability to mitigate overactive immune system.
-
•
Methotrexate could be an effective drug candidate in treating COVID-19 patients.
Abstract
Coronavirus disease 2019 (COVID-19) is a global health emergency caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). The rapid worldwide spread of SARS-CoV-2 infection has necessitated a global effort to identify effective therapeutic strategies in the absence of vaccine. Among the re-purposed drugs being tested currently, hydroxychloroquine (HCQ), without or with zinc ion (Zn++) and the antibiotic azithromycin (AZM), has been administered to prevent or treat patients with COVID-19. The outcome of multiple clinical studies on HCQ has been mixed. Zn++ interferes with viral replication by inhibiting replicative enzymes and its entry into cells may be facilitated by HCQ. Another immunomodulatory drug, methotrexate (MTX), is well known for its ability to mitigate overactive immune system by upregulating the anti-inflammatory protein, A20. However, its beneficial effect in treating COVID-19 has not drawn much attention. This review provides an overview of the virology of SARS-CoV-2 and an analysis of the mechanisms by which these anti-inflammatory agents may act in the treatment of COVID-19 patients. We propose a rationale for the combinatorial use of these re-purposed drugs that may help to combat this ongoing pandemic health emergency.
1. Introduction
In the past two decades, the world population witnessed the outbreak of infection by coronaviruses in 2002–2003 by Severe Acute Respiratory Syndrome (SARS) in China and in 2011 by Middle East Respiratory Syndrome (MERS) in Saudi Arabia [1]. After the first epidemic of SARS in China was over, a second interspecies-jumping event occurred in China, resulting in the re-emergence of SARS during the year 2003–2004 [2]. This observation prompted scientists to predict that a major severe respiratory syndrome pandemic caused by a modified coronavirus would infect humans, potentially at any time. This became a reality by the end of 2019 with the emergence of Coronavirus Disease 2019 (COVID-19; SARS-CoV-2) [1]. Each of these outbreaks were caused by viruses that belong to the family Coronaviridae within the order Nidovirales [1]. Thus far, 36 coronaviruses have been identified and are known to cause respiratory or intestinal infections of varying intensities in humans and other animals [2]. In 2003, 8,098 individuals were infected by SARS across 26 nations with a mortality rate of 9% [3]. The present novel coronavirus, SARS-CoV-2, has infected 29.5 million individuals to date (September 15, 2020) across 219 nations with an estimated mortality rate of 4%.
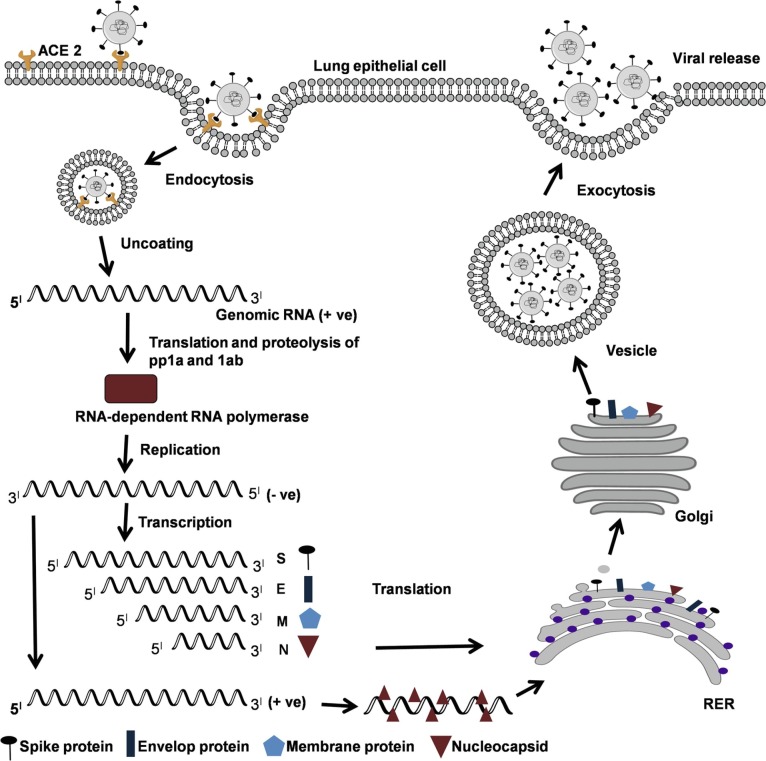
Coronaviruses are a large family of enveloped, positive-sense, single-stranded RNA viruses that infect target cells via angiotensin converting enzyme-2 (ACE-2) receptors (Fig. 1 ), a host transmembrane glycoprotein [4]. ACE-2 receptors are abundantly present in vascular endothelial cells, heart, lung, kidney and intestine. The viral genome encodes several structural proteins and non-structural proteins that are likely targets for the development of future drugs or vaccines and are actively being analyzed. The structural proteins include spike protein (S), nucleocapsid protein, membrane protein, and the envelope protein [4]. The S protein consists of an amino terminal S1 and carboxyl terminal S2 subunits connected by a fusion peptide. The interaction between the S protein and ACE-2 receptor is the primary determinant that enables the coronavirus to infect a host cell [5]. The receptor binding S1 subunit and the membrane-fusing carboxy terminal S2 subunit are critical regions of S protein for binding to the ACE-2 receptor [6]. Upon binding to the ACE-2 receptor, the S protein needs to be “primed” for fusion with host cell membrane to facilitate access to the host cell cytosol. This is generally accomplished by acid-dependent proteolytic cleavage at two specific sites of S protein by a cellular proteases, transmembrane protease serine 2 (TMPRSS2), TMPRSS4, and cysteine proteases cathepsin B and L [5], [6], [7], [8], [9]. After proteolytic cleavage, the S protein facilitates viral envelope fusion with the host cell membrane through the endosomal pathway [10], [11]. The coronavirus uses the vesicular trafficking system of the secretory pathway of the host cell to release newly synthesized viral particles by exocytosis [11]. In the cytoplasm, positive-sense, single-stranded RNA is translated with the help of host machinery to synthesize polyproteins pp1a and pp1ab [12] (Fig. 1). These protein complexes undergo proteolytic cleavage to produce the viral RNA-dependent RNA polymerase, helicase, and other nonstructural proteins [12]. RNA-dependent RNA polymerase replicates positive-sense, single-stranded RNA into negative-sense, single-stranded RNA. Using negative-sense, single-stranded RNA as template, positive-sense single-stranded RNA is synthesized as well as several mRNA to synthesize viral proteins. Protein assembly encapsulates positive-sense single stranded RNA to become viable coronaviruses [12].
Fig. 1.
Life cycle of coronavirus. (A) ACE-2-mediated entry of corona virus via endocytosis, followed by uncoating and synthesis of polyprotein 1a (pp1a) and pp1ab using genomic RNA (+ve) strand. Proteolysis of polyproteins with the help of host lysosomal proteases to make nonstructural protein RNA-dependent RNA polymerase (RdRP) that uses (+ve) strand genomic RNA as a template. The (+ve) strand genomic RNA is synthesized by the process of replication becomes the genome of the new virus particles. The transcription of (–ve) strand into subgenomic RNAs that are translated into structural proteins. Reassembly of viral particles in RER and secreted via Golgi vesicles as new viruses by exocytosis.
The symptoms of SARS-CoV-2 infection appear after an incubation period of approximately 5.2 days [13], [14]. The period from the onset of symptoms to death ranges from 6 to 41 days [14]. This time period is dependent on the age of the patient and status of the patient’s immune system. Generally, patients with a good immune system develop immunity against COVID-19 and survive the infection within two to three weeks. The primary clinical symptoms are characterized by a fever, cough, severe headache, loss of taste/smell, rash on skin/discoloration of fingers or toes. About 20 – 30% SARS-CoV-2-infected patients develop respiratory failure. A high mortality rate is strongly associated with the elderly, and particularly those individuals with co-morbidities including asthma, diabetes, obesity, heart disease, immune compromise, etc [15].
2. The role of the cytokine storm induced by SARS-CoV-2 in disease
The main pathogenic manifestations of COVID-19 infection as a respiratory system-targeting virus is severe pneumonia and acute cardiac injury. Several studies have analyzed cytokine profiles from COVID-19 patients and found that the host immune response to the SARS-CoV-2 virus is hyper inflammatory, resulting in the release of a large amount of pro-inflammatory cytokines in an event known as the “cytokine storm” [16], [17], [18], [19]. A cytokine storm refers to an over exuberant inflammatory response leads to the release of massive amounts of cytokines concurrently. The concomitant response to individual cytokines through cytokine-specific receptors, and the autocrine/paracrine action of newly induced mediators, leads to a degree of inflammation that is extremely difficult to counteract therapeutically. While the cytokine storm was first used in the context of sepsis, it has since been used to describe the overproduction of cytokines in response to many infectious diseases. The mortality rate of SARS-CoV-2 infection has been directly linked to the onset of massive inflammation due to the cytokine storm [20]. The cytokine storm was previously associated with avian H5N1 influenza virus infection in 2005 [21]. Similarly, the biological and clinical consequences of cytokine storms by immune system hyperactivity could be a primary reason for lung damage in patients infected with SARS-CoV-2. Individuals infected with SARS-CoV-2 showed increased numbers of immune cells and increased levels pro-inflammatory cytokines in the blood [20]. The cytokine storm causes an overwhelming inflammatory response that drives serious respiratory complications [22]. In the worst case, the cytokine storm initiates viral sepsis and inflammation-induced lung injury with complications such as pneumonitis, acute respiratory distress syndrome (ARDS), shock, organ failure, and potentially death [22]. At the time the individual develops ARDS, ventilators represent the only support system during the patient’s battle. The severe symptoms have been associated with increased fatalities.
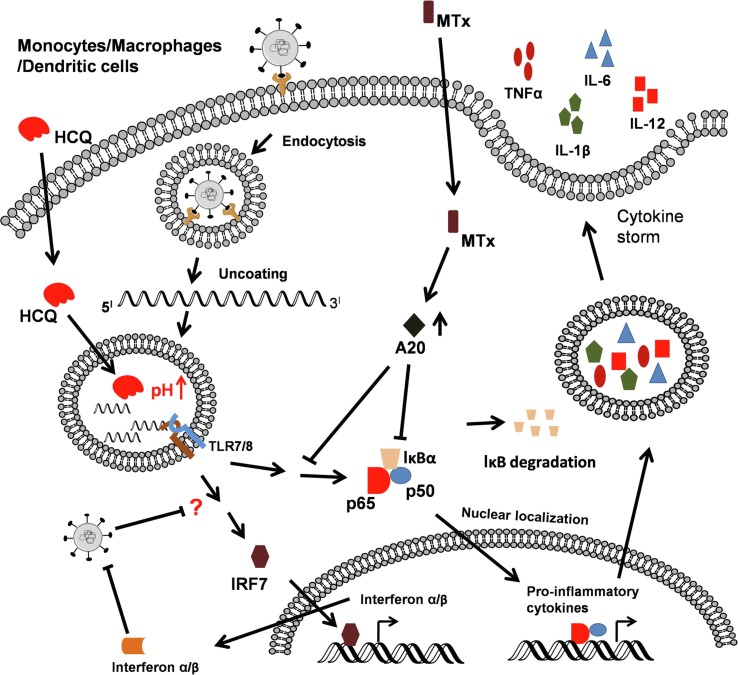
ARDS is the primary cause for mortality due to COVID-19 [22]. The increased coronavirus RNA load in lungs and blood is sensed by host antigen-presenting cells through multiple pattern recognition receptors including RIG-I-like Receptors (RLRs) and Toll-like receptors (TLRs) [23] (Fig. 2 ). TLR7 and TLR8 are SARS-CoV-2 single-stranded RNA-specific TLRs involved in inducing pro-inflammatory cytokines. Sustained activation of TLR7 and TLR8, along with other pattern recognition receptors (PRRs), leads to induction of the cytokine storm (Fig. 2). Cellular damage may lead to the release of host-derived “danger-associated molecular patterns” (DAMPs) that have been shown to trigger both TLR4 and Receptor for Advanced Glycation End products (RAGE) to elicit a potent proinflammatory response to viral infection [24], [25], [26]. The pathogenesis of ARDS is associated primarily with injury to the alveolo–capillary membrane that enhances lung permeability and the exudation of pulmonary edema fluid into the airspaces leading “lung leak” and to hypoxia [27].
Fig. 2.
Pathogenesis of corona virus and its interference by HCQ and MTX. (A) Phagocytosis of coronavirus by immune cells results in the activation PRR-mediated NF-κB and IRF7 signaling leading to induction of the cytokine storm. HCQ inhibits the engagement of viral nucleotides with endosomal TLRs by increasing pH. MTX inhibits cytokine storm by inducing an anti-inflammatory molecule, A20.
Huang et al. recently reported that patients infected with SARS-CoV-2 showed high levels of pro-inflammatory cytokines and chemokines [18]. They demonstrated increased levels of the proinflammatory cytokines IL-1β and IFN-γ, as well as the chemokines CXCL10 and CCL2, and they pointed out that the cytokine storm emerged as a main factor driving a more severe clinical course. Further, they observed that COVID-19 patients requiring ICU admission displayed higher concentrations of G-CSF, CXCL10, CCL2, and TNF-α compared to those in which the infection was less severe and did not require an ICU admission.
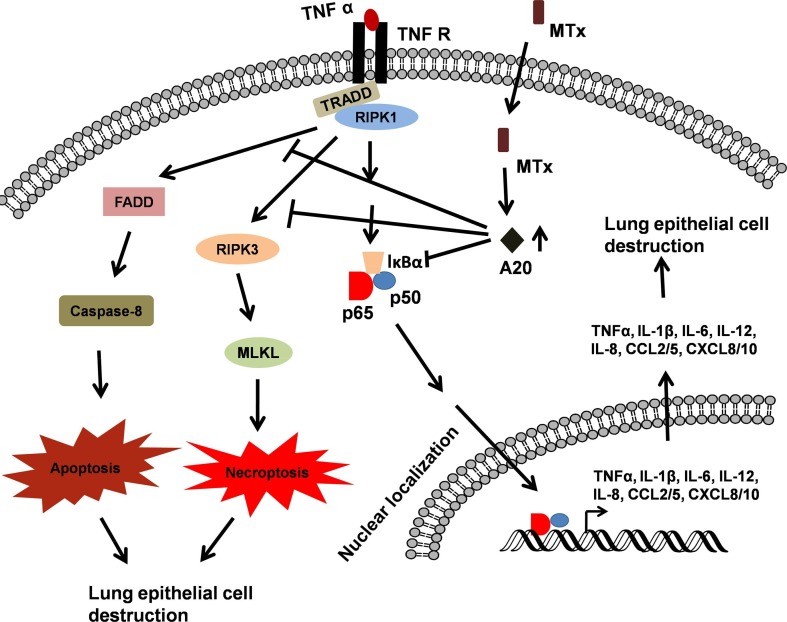
The uncontrolled release of large amounts of pro-inflammatory cytokines and chemokines by immune effector cells during SARS-CoV-2 infection has been reported, with particular association with IL-6 and IL-1β [15], [18], [28], [29]. Specifically, clinical studies showed that the serum levels of IL-6 are increased in COVID-19 patients and these high circulating serum levels are correlated to disease severity and suggested as predictors for disease severity [30], [31], [32], [33]. The hyperactive immune system-mediated cytokine storm attacks the body and causes ARDS, resulting in multiple organ failure and death in the most severe cases of SARS-CoV-2 infection [22]. For example, TNF-α released by immune effector cells contribute to maintenance of a pro-inflammatory signature, inducing necroptosis in lung epithelial and alveolar cells (leading to further release of DAMPs), and finally, death in severe cases of SARS-CoV-2 infection (Fig. 4).
Fig. 4.
MTX-mediated A20 upregulation inhibits TNF-α-induced signaling. TLR7/8-mediated TNF-α release from coronavirus-stimulated immune cells acts on lung epithelial cells, induces NF-κB activation, apoptosis and necroptosis that leads to the destruction of lung tissue. MTX-mediated A20 involves in the survival of lung tissue by inhibiting TNF-α-induced NF-κB activation, apoptosis and necroptosis.
Additionally, critically ill patients with COVID-19 are prone to thromboembolism/pulmonary embolism/pulmonary intravascular coagulopathy that correlates with disease severity [34], [35], [36]. Recent scientific reports on COVID-19 patients suggest that intravascular coagulopathy/fibrin deposition is mainly due to excessive inflammation, hypoxia, and immobilization of patients [36], [37]. Clinical reports from the Netherlands and France showed 31% and 20.6% incidence of thrombotic complications in ICU patients with COVID-19 infections, respectively [36], [38]. Another study from Wuhan, China reported that patients with d-dimer levels ≥2.0 µg/ml had a higher incidence of mortality compared to the patients with d-dimer levels <2.0 µg/ml [39].
Like SARS-CoV and MERS-CoV, SARS-CoV-2 may use multiple strategies to evade immune responses. One evasion mechanism may be the avoidance of the host immune system by the production of double-membrane vesicles that lack PRRs in which the virions replicate [40]. Studies in mice revealed that Type I IFN (IFN-α and IFN- β) has a protective effect against SARS-CoV and MERS-CoV infection, and more recently, in cases of SARS-CoV-2 [41]. Type I IFNs are key components of the immediate antiviral response and restrict viral replication through IFNAR signaling [42], [43]. Unlike patients infected with pathogenic influenza viruses, minimal amounts of IFNs have been detected in the peripheral blood or lungs of patients with severe COVID-19, although SARS-CoV-2 is capable of engaging the IFN-I and IFN-III systems [43], [44]. This clearly suggests that host response to SARS-CoV-2 fails to launch type I IFN and –III even though there is robust viral replication. Blanco-Melo et al. analyzed serum samples from COVID-19 patients from two cohorts of individuals and failed to detect type I IFN. On the other hand, sera from the same COVID-19 patients revealed the significant increase in circulating IL-6, IL1RA, CCL2, CCL8, CXCL2, CXCL8, CXCL9, and CXCL16 levels [43]. Both SARS-1 and MERS inhibit Type I IFN production by preventing nuclear transport of the transcription factor, IFN regulatory factor 3 (IRF3), and thereby inhibit activation of the IFN-β promoter by IRF3 [45], although this has not been established for SARS CoV-2. A recent report on COVID-19 patients confirmed that patients with undetectable IFN-α levels required invasive ventilation and longer stay in intensive care unit. Further, the viral load inversely correlated with the level of Type I IFN: as expected, the viral load was higher in IFN-negative/low patients with COVID-19 at disease diagnosis [46]. This indirectly suggests that SARS-CoV-2 may also have inhibitory effects on Type I IFN production (Fig. 2). Conversely, several studies have suggested the efficacy of treating COVID-19 patients with type I IFNs [41], [47], [48].
The fundamental question is whether or not we will be prepared for such global health emergency in the future. In the absence of vaccination and proven antiviral drugs, the clinical management largely depends on supportive care. Clinical management with SARS in the year 2002 led to the identification of certain anti-malarial and anti-viral drugs that are effective against SARS infection and these have been tried in the management of COVID-19. Below, we present data on several potential therapeutics that have been or currently are being used in the treatment of various viral diseases in different parts of the world. The available literature and a basic understanding of their mechanism of action of each drug suggests that a combination of drugs are likely to be potential candidates for the treatment of current COVID-19, and perhaps, in a future outbreak by a novel coronavirus. Using the key words coronavirus, chloroquine (CQ), hydroxychloroquine (HCQ), azithromycin (AZM), Zn++, and methotrexate (MTX), we retrieved published, as well as preprinted, articles and their cross-references from the literature database.
3. Drug candidates against SARS-CoV-2 and other viral diseases
Although antibiotics are not effective against viral infections such as COVID-19, they can be used to combat secondary bacterial infections. Several biomedical research laboratories throughout the world are testing a variety of possible treatments. The United States Food and Drug Administration granted permission to use HCQ, an anti-malarial drug, and remdesivir, an anti-viral drug developed for Ebola, to treat severe COVID-19 [49], [50]. Along with HCQ, AZM, Zn++ supplements, and MTX have been proposed as possible treatments to combat severe COVID-19. A list of currently available drugs and their possible mechanisms of action that could be used to treat COVID-19 patients is provided in Table 1 .
Table 1.
Currently available possible drug candidates to treat COVID-19 patients.
| Drugs | Target | Pre-clinical/clinical trial | Results |
|---|---|---|---|
| Chloroquine (CQ) | Viral replication, TLR-7 and TLR-8 signaling | Clinical trial was conducted for 100 COVID-19 patients in China Randomized clinical trial: high dose 600 mg, twice daily for 10 days, low dose: 450 mg, twice daily on day 1 and once daily for next 4 days |
Increased viral clearance compared to control. Effective in inhibiting the exacerbation of pneumonia compared to control [70]. Lethality. High dose: 39%, Low dose: 15% [110]. |
| Hydroxychloroquine (HCQ) | Viral replication, TLR-7 and TLR-8 signaling | Randomized clinical trial: 400 mg/kg for 5 days Non-randomized clinical trial: 200 mg thrice daily for 10 days, 200 mg (thrice daily for 10 days) + azithromycin (500 mg on day1 followed by 250 mg per day for the next four days) 600 mg/day in the first 48 h after hospitalization |
Effective against pneumonia. HCQ: 80.6%, Control: 54.8% [72]. Significant reduction of viral load. HCQ: 57.1% HCQ + AZM: 100% [111]. No significant difference in mortality rate compared to control [112]. |
| Lopinavir and ritonavir | 3-chymotrypsin-like-protease | Randomized clinical trial: Lopinavir: 400 mg and ritonavir: 100 mg twice a day for 14 days | No significant difference in viral clearance, clinical improvement and mortality rate compared to control [113]. |
| Famotidine | Papain-like protease | Treatment options for non-hospitalized patients: 80 mg three times daily for a median of 11 days. Retrospective cohort study: 10 mg, 20 mg and 40 mg for a median of 5.8 days. |
Famotidine is well tolerated and associated with improved self-reported outcomes in non-hospitalized patients with COVID-19 [114]. Significantly reduced risk for the composite outcome of death or intubation [115]. |
| Umifenovir | Viral membrane lipids | Chinese clinical trial: 0.4 g/day for a median of 9 days Retrospective study: 0.2 g three times a day |
Improved the discharging rate and decreased the mortality rate [116]. No significant improvement of viral clearance [117]. |
| Ivermectin | Cell-transport protein | Preclinical study (in vitro): IC50 2.8uM Clinical trial: 600 µg/kg /day + standard care |
Reduction of virus (~5000 folds) in cell culture [118]. Reduction of viral load after ivermectin treatment in 1–5 days [119]. |
| Corticosteroid | Cytokines | CoDEX randomized clinical trial: Deamethasone 20 mg/day for 5 days, then 10 mg/d for 5 days + standard care Retrospective cohort study: methylprednisolone 40 mg/day for 5 days + immunoglobulin 20 g /day for 3–5 days |
Significant increase in the number of ventilator-free days compared to control [120]. Reduces disease progression, while having a negligible impact on the viral clearance [121]. |
| Remdesivir | Viral RNA-dependent RNA polymerase | Randomized clinical trial: 200 mg loading dose on day 1, followed by 100 mg daily for up to 9 additional days | Median recovery time of 10 days as compared with 15 days placebo received group [122]. |
3.1. HCQ and AZM
It is not unusual that a drug approved for a particular disease ultimately shows beneficial effects in other diseases as well. The best example is the use of anti-malarial drug HCQ in the treatment of rheumatoid arthritis, systemic lupus erythematosus, and currently, as a broad-spectrum anti-viral drug [51]. It has been well established that the HCQ increases the pH of cellular vesicles that are involved in the secretory pathways [51]. Increasing the pH in these acidic vesicles alters the biochemical events such as protein degradation by hydrolases in the lysosome, assembly of macromolecules in the lumen of the endoplasmic reticulum and endosomes, post-translational modification of proteins in the Golgi apparatus, and activation of zymogen formed in the secretory vesicles [51]. Since HCQ alters the pH of various vesicles in a cell, it is theoretically effective against any virus that uses the vesicular pathway for multiplication (Fig. 3 ). HCQ is well-known to modify cellular autophagy with the pH-dependent steps of endosome-mediated viral entry and late stages of replication of enveloped viruses such as retroviruses, flaviviruses, and coronaviruses [52], [53]. CQ or HCQ have been demonstrated to have an anti-HIV-1 effect when used in combination with anti-retroviral drugs in vitro or in patients [54], [55]. However, HCQ alone failed to reduce viral replication in HIV-infected patients [55]. Further, scientific reports have validated the anti-viral activity of HCQ that interferes with Japanese encephalitis virus internalization, yellow fever virus replication, and dengue virus maturation [56], [57], [58]. Again, HCQ failed to reduce viremia in patients with dengue disease [59]. In addition, CQ modulates biosynthesis of sialic acid by inhibiting quinone reductase-2, a key enzyme involved in the biosynthesis of sialic acid that is essential for receptor recognition and also by binding to the sialic acid-linked transmembrane proteins [60]. Moreover, CQ binds sialic acids with high affinity that may inhibit binding of SARS-CoV-2 [61]. Similarly, HCQ may inhibit the entry of SARS-CoV-2 by modulating biosynthesis of sialic acid and by interacting with sialic acid. Since HCQ has been shown to interfere at the entry level, transportation between vesicles, and at the post-entry level, it has been proposed to be beneficial as prophylactic as well as therapeutic treatment (Fig. 3).
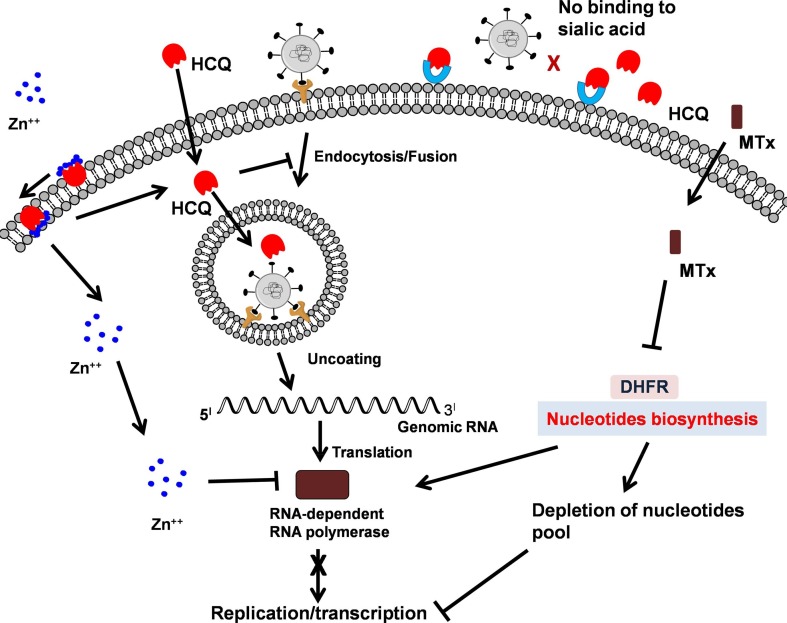
Fig. 3.
Possible sites of inhibition of viral replication by combination of HCQ, Zn++ and MTX. The combination of HCQ, Zn++ and MTX interferes with viral replication at several stages. HCQ interferes directly by inhibiting fusion of virus to host membrane receptors and indirectly acts as ionophore that transports Zn++ that inhibits viral RNA polymerase. HCQ directly interacts with sialic acid and interferes in the attachment of virus to membrane glycoproteins. MTX interferes at viral replication stage by depleting the nucleotide pool.
In the context of SARS CoV-2, the cytokine storm is highly detrimental and it may be initiated via single stranded RNA-sensing endosomal TLRs (e.g., TLR7 and TLR8) and RLRs (e.g., RIG-I), in monocyte-derived macrophages and dendritic cells [62], [63]. Activation of endosomal TLRs occurs within acidified endolysosomal compartments and leads to production of pro-inflammatory cytokines [64]. HCQ has been reported to abrogate endosomal TLR (TLR3, TLR7, TLR8, and TLR9) responses by preventing acidification [64]. Thus, in addition to its anti-viral actions, HCQ may also act as an anti-inflammatory molecule responsible for reduced pro-inflammatory cytokines by inactivating RNA-sensing TLRs in the endosomes [65] (Fig. 2). Furthermore, HCQ has been shown to prevent the release of the host protein and TLR4 agonist, High Mobility Group Box 1 (HMGB1), upon influenza infection [66]. Interestingly, both TLR4 and HMGB1 antagonists protect therapeutically against influenza-induced death in mice [67], [68], [69].
There are multiple reports supporting or refuting the use of HCQ in conjunction with AZM in COVID-19 patients. Early on, a Chinese group showed that chloroquine phosphate is effective in treating COVID-19-associated pneumonia in patients [70]. In this clinical study with more than 100 patients, the authors demonstrated that chloroquine phosphate treatment inhibited the exacerbation of pneumonia, improving lung pathology and shortening the disease course. In support of this, a study from France reported the efficacy of HCQ in clearing viral nasopharyngeal carriage of SARS-CoV-2 in COVID-19 patients within three to six days of infusion. They found a significant difference between HCQ-treated patients and controls [71]. According to this study, HCQ treatment cured virology in 70% of patients compared to 12.5% in the control group [71]. Recently, another report in support of HCQ treatment of COVID-19 patients for its anti-viral activity concluded that HCQ treatment significantly reduced the recovery time for body temperature and cough remission [72]. Interestingly, the comparative analysis of the chest CT of patients showed significant improvement in patients treated with HCQ [72]. Very recently, Catteau et al. from Belgium have shown the beneficial action of HCQ alone and HCQ with AZM in a large clinical trial. The authors have reported that fatality rate was lower in the HCQ group than in the group without HCQ. The significant decrease in mortality rate was observed in the patients group administered with HCQ monotherapy at a dosage of 2400 mg over 5 days compared with patients treated without HCQ [73].
The synergistic effect of HCQ and AZM has also been reported. AZM is known to prevent severe respiratory tract infections when administrated to patients suffering viral infection, although the mechanism is not well understood [74]. Very recently, the combinatorial effect of HCQ and AZM with good clinical outcome and decreased viral burden in a large population (1,061) of patients (91.7%) was reported [75]. While it has been surmised that the AZM acts by preventing the enhanced secondary bacterial infection after virus infection, it is possible that it also acts by inducing anti-inflammatory “alternatively activated” (M2) macrophages. Previously, it was shown that M2 macrophages were necessary for resolving the lung pathology associated with respiratory syncytial virus (RSV) infection [69], [76]. Administration of M2-inducing agents therapeutically, including AZM, resulted in resolution of RSV-induced pathology. This clearly suggests that AZM may not only prevent secondary bacterial infection, but also, blunts viral-induced pathology by creating anti-inflammatory environment.
In contrast to studies supporting the use of HCQ, other clinical studies failed to show significant difference between HCQ-treated and control responses. A study by Mallat et al. observed that the duration of hospital stay was longer in HCQ-treated with COVID-19 patients [77]. Another recent report with a larger patient population suggested that HCQ treatment did not provide beneficial support to use in patients with COVID-19 who require oxygen [78]. They found that additive HCQ treatment to standard care did not reduce patient admissions to the intensive care unit. Also, the rate of survival without ARDS did not increase in HCQ-treated patients compared to standard care alone. Very recently, Boulware and colleagues tested the efficacy of HCQ as COVID-19 post-exposure prophylaxis in a randomized clinical trial with asymptomatic individuals [79]. They found that HCQ failed to prevent illness with COVID-19 when used as post-exposure prophylaxis within 4 days after a high-risk or moderate-risk exposure [79]. In another clinical study with 807 veterans from the United States, HCQ treatment with or without co-administration of AZM did not improve mortality or reduce the need for mechanical ventilation [80]. In this report, they analyzed multiple parameters that are critical to assess the severity of COVID-19 patients including SpO2, respiratory rate, heart rate, temperature, blood pressure, liver enzymes, d-dimer, CRP, troponin I so on. Although some parameters were significantly different between HCQ alone or HCQ + AZM compared to the control group, no improvement in mortality compared to control group was observed [80]. Thus, while HCQ alone, or HCQ with AZM treatment of COVID-19 patients has been used in these desperate times, the efficacy of such treatment has not yet been confirmed in a placebo-controlled randomized clinical trial. Hopefully, such data will be forthcoming soon and will reveal if the mixed reports of efficacy are due to dosing, timing, or other environmental parameters not carefully studied. The paradoxical effect of HCQ monotherapy or combined with AZM could be due to the co-morbidities, such as cardiovascular complications of the COVID-19 patients. HCQ along with AZM might be beneficial to combat COVID-19 in patients without cardiovascular complications. For patients, especially in the elderly with a history of cardiovascular disease, HCQ and AZM may be detrimental that prolong QT interval [81], no instances of arrhythmogenic death were reported [82]. Additionally, HCQ treatment induces rash and headache in some patients. Also, the timing, severity of illness, and dose of HCQ will be very important to consider before treating COVID-19 patients. Finally, it is very unfortunate that the use of HCQ has become highly politicized. However, careful retrospective analysis of all studies reported may reveal specific populations or specific times during which this drug is most efficacious (https://www.tabletmag.com/sections/science/articles/hydroxychloroquine-morality-tale).
3.2. Zinc
Zinc deficiency is common in young children and elderly persons in the developing world and these individuals have suppressed immune responses and are more susceptible to a diverse range of infectious diseases [83], [84]. The common cold caused by rhinovirus is one of the most widespread illnesses that affects people of all ages and is more severe in zinc-deficient populations [85]. Administration of zinc for the treatment of the common cold is very well-established. Clinical studies have found that zinc reduces the duration of colds and decreases the upper respiratory infection in children [86]. In addition, zinc is an immunomodulator and helps the host fight infection and heal wounds [83]. Several studies revealed that the therapeutic effect of zinc is due to its interference with viral replication [87], [88], [89]. In cell culture studies, increased levels of intracellular zinc, along with the ionophores that facilitate zinc cellular import, were found to inhibit RNA replication of different RNA viruses, including influenza virus, RSV, polio virus, arteri virus, coronavirus, and several picornaviruses [87]. For some viruses, this effect has been attributed to the inhibition of certain proteolytic cleavages in the processing of the viral polyproteins. With arteri virus and coronaviruses, zinc interferes in viral replication by inhibition the RNA-dependent RNA polymerase enzyme [87]. Intracellular mobilization of zinc is crucial for its anti-viral activity and is facilitated by compounds like hinokitol, pyrrolidine dithicarbamate, and pyrithione that stimulate cellular import of zinc. Apart from affecting vesicular pH, HCQ is an excellent ionophore of zinc and increases the intracellular concentration of zinc within short time [90]. The combination of HCQ with zinc is more effective in regulating the viral multiplication by interfering at various steps of its life cycle (Fig. 3).
3.3. Methotrexate (MTX)
MTX, a disease modifying anti-rheumatic drug (DMARD), is used clinically for rheumatoid or psoriatic arthritis and systemic lupus erythematosis with good therapeutic results [91], [92], [93]. It is a folic acid analogue that is either used as a monotherapy or in combination with other synthetic DMARDs. MTX is polyglutamated inside the cell and MTX-polyglutamates (MTX-Pgl) constitute the active form of the drug. MTX-Pgl acts by inhibiting various enzymes such as dihydrofolate reductase (DHFR), thymidylate synthase (TS), and aminoimidazole carboxamide ribonucleotide formyl transferase enzyme (AICART) [94] that are involved in the synthesis of precursor nucleotides required for RNA and DNA synthesis (Fig. 3). AICART inhibition reduces inosine monophosphate and increases aminoimidazole carboxamide ribonucleotide (AICAR) which has anti-inflammatory effect. Impaired nucleic acid synthesis suppresses rapidly dividing cells such as immune cells, thereby disrupting the S phase of the cell cycle. Thus, in rheumatological diseases, MTX is an immunosuppressive drug that reduces inflammation and joint destruction [95]. Apart from inhibiting immune cell division, MTX also inhibits the synthesis of purine and pyrimidine pools in host cells. This decreased nucleotide pool impairs viral replication (Fig. 3).
In addition to interfering with the synthesis of precursor nucleotides, MTX has been shown to exert an immunomodulatory role [91]. Recently, Municio et al. [92] showed that long-term low-dose MTX treatment increased the expression of A20 in macrophages stimulated with TLR ligands or TNF-α (Fig. 2, Fig. 4). A20 is a TNF-inducible cytoplasmic protein that inhibits TNF-induced NF-κB activity [96]. It has been reported that A20 inhibits ubiquitin-dependent NF-κB signaling by exhibiting deubiquitinating capacity, ubiquitin binding, and E3 ligase activities [96]. Moreover, MTX reduced LPS/TNF-α-dependent pro-inflammatory cytokine production, MAPK activation, IκBα degradation, and NF-κB activity via A20 [92]. Therefore, the involvement of A20 in MTX response validates the anti-inflammatory role of MTX, widely used drug in inflammatory diseases with an excellent safety record. Moreover, TNFAIP3 gene single nucleotide polymorphisms (SNPs) are strongly associated with psoriasis, a well-studied inflammatory disease [97]. Interestingly, the reduction in A20 expression caused notably increased levels of IL-1β, IL-6, TNF-α, and IL-12 p40 in splenocytes, supporting the anti-inflammatory role of A20 in inflammatory diseases [97]. Overall, the immunomodulatory action of MTX might be exploited to treat COVID-19 patients since the cytokine storm is thought to be a major cause of morbidity and mortality (Fig. 4 ). Apart from these, MTX administration can provoke the release of an anti-inflammatory molecule, adenosine, that exerts its effects on immune cells [98]. Upon release, adenosine activates intracellular signaling cascade through specific receptors that results in the reduced production and release of pro-inflammatory cytokines [99]. It is well known that MTX modulates of reactive oxygen species (ROS) and reduces production of IL-6 which is associated with COVID-19 patient severity. In addition to reduced IL-6, MTX potently suppresses the JAK/STAT signaling pathway which is activated by multiple pro-inflammatory cytokines [100]. Further, High Mobility Group Box 1 (HMGB1), a host-derived “danger-associated molecular pattern,” has been associated with respiratory virus-mediated lung pathology by inducing inflammation through its receptors, receptor for advanced glycation end products (RAGE) and TLR4. Interestingly, MTX can inhibit the action of HMGB1 by directly binding to RAGE [99]. The range of MTX side effects is characterized by nausea, vomiting, diarrhea, myelosuppression, pancytopenia, liver dysfunction, acute renal failure, pulmonary symptoms, mucositis, stomatitis, ulceration of the gastrointestinal system and cutaneous ulcerations [101], [102].
3.4. Dexamethasone (DEX)
DEX is a synthetic glucocorticoid with 20 to 30 times the binding affinity for glucocorticoid receptors of endogenous cortisol. DEX is being used in a wide range of conditions for its anti-inflammatory and immunosuppressant effect [103]. Corticosteroids are widely used to treat autoimmune diseases such as rheumatoid arthritis, inflammatory bowel disease, multiple sclerosis, psoriasis and eczema. They are being used as immunosuppressant following organ transplant and also in treating certain cancers [104]. Very recently, clinical trial results from the United Kingdom claimed that a low dose of dexamethasone, a corticosteroid, reduces mortality in patients with COVID-19 on ventilators and requiring oxygen [105], [106]. In support of this, an earlier study by a team of physicians from China recommended short courses of corticosteroids at low to moderate doses for critically ill patients with COVID-19-induced pneumonia [107]. In contrast, a clinical study by Russell and colleagues provided the evidence that corticosteroid treatment did not support treatment for 2019-nCOV-induced lung injury [108]. With all these, dexamethasone is well known for its side effects [109]. Thus, we propose that low dose of MTX, alone or in combination with dexamethasone, could be an effective immunotherapy to protect health care workers and to treat COVID-19 patients.
4. Concluding remarks
SARS-CoV-2 has affected the human population worldwide by causing disease associated with ARDS. Although some clinical studies have supported the use of HCQ to treat patients with COVID-19, others have failed to support its effectiveness in treating SARS-CoV-2. HCQ might not be effective in critically ill COVID-19 patients, but it may help patients recover from SARS-CoV-2 infection if administered during very early stages along with AZM and Zn++. MTX is known to suppress TLR/TNF-α -induced NF-κB activation, and could be beneficial in combatting lung pathology caused by SARS-CoV-2. In conclusion, we suggest the combinatorial use of drug candidates, such as HCQ with AZM/Zn++ or MTX will require additional randomized, placebo-controlled clinical trials to unambiguously determine their efficacy as therapeutic approaches to combat COVID-19.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
The following funding sources supported this work:
DST-SERB EEQ/2017/000737 (RR).
NIH AI125215 (SNV).
UGC MRP-MAJOR-BIOC-2013-12157 (BSV).
References
- 1.Raoult D., Zumla A., Locatelli F., Ippolito G., Kroemer G. Coronavirus infections: Epidemiological, clinical and immunological features and hypotheses. Cell Stress. 2020;4:66–75. doi: 10.15698/cst2020.04.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng V.C., Lau S.K., Woo P.C., Yuen K.Y. Severe acute respiratory syndrome coronavirus as an agent of emerging and reemerging infection. Clin. Microbiol. Rev. 2007;20:660–694. doi: 10.1128/CMR.00023-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lam C.W., Chan M.H., Wong C.K. Severe acute respiratory syndrome: clinical and laboratory manifestations. Clin. Biochem. Rev. 2004;25:121–132. [PMC free article] [PubMed] [Google Scholar]
- 4.Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C., Choe H., Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Muller M.A., Drosten C., Pohlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181 doi: 10.1016/j.cell.2020.02.052. 271-280 e278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang J., Song W., Huang H., Sun Q. Pharmacological therapeutics targeting RNA-dependent RNA polymerase, proteinase and spike protein: from mechanistic studies to clinical trials for COVID-19. J. Clin. Med. 2020;9 doi: 10.3390/jcm9041131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simmons G., Gosalia D.N., Rennekamp A.J., Reeves J.D., Diamond S.L., Bates P. Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. Proc. Natl. Acad. Sci. USA. 2005;102:11876–11881. doi: 10.1073/pnas.0505577102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burkard C., Verheije M.H., Wicht O., van Kasteren S.I., van Kuppeveld F.J., Haagmans B.L., Pelkmans L., Rottier P.J., Bosch B.J., de Haan C.A. Coronavirus cell entry occurs through the endo-/lysosomal pathway in a proteolysis-dependent manner. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1004502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zang R., Gomez Castro M.F., McCune B.T., Zeng Q., Rothlauf P.W., Sonnek N.M., Liu Z., Brulois K.F., Wang X., Greenberg H.B., Diamond M.S., Ciorba M.A., Whelan S.P.J., Ding S. TMPRSS2 and TMPRSS4 promote SARS-CoV-2 infection of human small intestinal enterocytes. Sci. Immunol. 2020;5 doi: 10.1126/sciimmunol.abc3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xia S., Liu M., Wang C., Xu W., Lan Q., Feng S., Qi F., Bao L., Du L., Liu S., Qin C., Sun F., Shi Z., Zhu Y., Jiang S., Lu L. Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res. 2020;30:343–355. doi: 10.1038/s41422-020-0305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fung T.S., Liu D.X. Human coronavirus: host-pathogen interaction. Annu. Rev. Microbiol. 2019;73:529–557. doi: 10.1146/annurev-micro-020518-115759. [DOI] [PubMed] [Google Scholar]
- 12.van Hemert M.J., van den Worm S.H., Knoops K., Mommaas A.M., Gorbalenya A.E., Snijder E.J. SARS-coronavirus replication/transcription complexes are membrane-protected and need a host factor for activity in vitro. PLoS Pathog. 2008;4 doi: 10.1371/journal.ppat.1000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., Ren R., Leung K.S.M., Lau E.H.Y., Wong J.Y., Xing X., Xiang N., Wu Y., Li C., Chen Q., Li D., Liu T., Zhao J., Liu M., Tu W., Chen C., Jin L., Yang R., Wang Q., Zhou S., Wang R., Liu H., Luo Y., Liu Y., Shao G., Li H., Tao Z., Yang Y., Deng Z., Liu B., Ma Z., Zhang Y., Shi G., Lam T.T.Y., Wu J.T., Gao G.F., Cowling B.J., Yang B., Leung G.M., Feng Z. Early transmission dynamics in wuhan, china, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020;109 doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H.M. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ragab D., Salah Eldin H., Taeimah M., Khattab R., Salem R. The COVID-19 cytokine storm; what we know so far. Front. Immunol. 2020;11:1446. doi: 10.3389/fimmu.2020.01446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H., Wang T., Zhang X., Chen H., Yu H., Zhang X., Zhang M., Wu S., Song J., Chen T., Han M., Li S., Luo X., Zhao J., Ning Q. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 2020;130:2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ye Q., Wang B., Mao J. The pathogenesis and treatment of the ‘Cytokine Storm' in COVID-19. J. Infect. 2020;80:607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yuen K.Y., Wong S.S. Human infection by avian influenza A H5N1. Hong Kong Med. J. 2005;11:189–199. [PubMed] [Google Scholar]
- 22.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L., Tai Y., Bai C., Gao T., Song J., Xia P., Dong J., Zhao J., Wang F.S. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li G., Fan Y., Lai Y., Han T., Li Z., Zhou P., Pan P., Wang W., Hu D., Liu X., Zhang Q., Wu J. Coronavirus infections and immune responses. J. Med. Virol. 2020;92:424–432. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Zoelen M.A., Achouiti A., van der Poll T. The role of receptor for advanced glycation endproducts (RAGE) in infection. Crit. Care. 2011;15:208. doi: 10.1186/cc9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Zoelen M.A., van der Sluijs K.F., Achouiti A., Florquin S., Braun-Pater J.M., Yang H., Nawroth P.P., Tracey K.J., Bierhaus A., van der Poll T. Receptor for advanced glycation end products is detrimental during influenza A virus pneumonia. Virology. 2009;391:265–273. doi: 10.1016/j.virol.2009.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hall S.C., Agrawal D.K. Toll-like receptors, triggering receptor expressed on myeloid cells family members and receptor for advanced glycation end-products in allergic airway inflammation. Expert Rev. Respir. Med. 2016;10:171–184. doi: 10.1586/17476348.2016.1133303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coperchini F., Chiovato L., Croce L., Magri F., Rotondi M. The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020;53:25–32. doi: 10.1016/j.cytogfr.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kadkhoda K. COVID-19: an immunopathological view. mSphere. 2020;5 doi: 10.1128/mSphere.00344-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Russell B., Moss C., George G., Santaolalla A., Cope A., Papa S., Van Hemelrijck M. Associations between immune-suppressive and stimulating drugs and novel COVID-19-a systematic review of current evidence. Ecancermedicalscience. 2020;14:1022. doi: 10.3332/ecancer.2020.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang C., Wu Z., Li J.W., Zhao H., Wang G.Q. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int. J. Antimicrob. Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGonagle D., Sharif K., O'Regan A., Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun. Rev. 2020;19 doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henry B.M., de Oliveira M.H.S., Benoit S., Plebani M., Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin. Chem. Lab. Med. 2020;58:1021–1028. doi: 10.1515/cclm-2020-0369. [DOI] [PubMed] [Google Scholar]
- 33.Ulhaq Z.S., Soraya G.V. Interleukin-6 as a potential biomarker of COVID-19 progression. Med. Mal. Infect. 2020;50:382–383. doi: 10.1016/j.medmal.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levi M., Thachil J., Iba T., Levy J.H. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7:e438–e440. doi: 10.1016/S2352-3026(20)30145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fogarty H., Townsend L., Ni Cheallaigh C., Bergin C., Martin-Loeches I., Browne P., Bacon C.L., Gaule R., Gillett A., Byrne M., Ryan K., O'Connell N., O'Sullivan J.M., Conlon N., O'Donnell J.S. COVID19 coagulopathy in Caucasian patients. Br. J. Haematol. 2020 doi: 10.1111/bjh.16749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klok F.A., Kruip M., van der Meer N.J.M., Arbous M.S., Gommers D., Kant K.M., Kaptein F.H.J., van Paassen J., Stals M.A.M., Huisman M.V., Endeman H. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Magro C., Mulvey J.J., Berlin D., Nuovo G., Salvatore S., Harp J., Baxter-Stoltzfus A., Laurence J. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl. Res. 2020 doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poissy J., Goutay J., Caplan M., Parmentier E., Duburcq T., Lassalle F., Jeanpierre E., Rauch A., Labreuche J., Susen S. Pulmonary embolism in COVID-19 patients: awareness of an increased prevalence. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- 39.Zhang L., Yan X., Fan Q., Liu H., Liu X., Liu Z., Zhang Z. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J. Thromb. Haemost. 2020 doi: 10.1111/jth.14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Snijder E.J., van der Meer Y., Zevenhoven-Dobbe J., Onderwater J.J., van der Meulen J., Koerten H.K., Mommaas A.M. Ultrastructure and origin of membrane vesicles associated with the severe acute respiratory syndrome coronavirus replication complex. J. Virol. 2006;80:5927–5940. doi: 10.1128/JVI.02501-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou Q., Chen V., Shannon C.P., Wei X.S., Xiang X., Wang X., Wang Z.H., Tebbutt S.J., Kollmann T.R., Fish E.N. Interferon-alpha2b Treatment for COVID-19. Front. Immunol. 2020;11:1061. doi: 10.3389/fimmu.2020.01061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Acharya D., Liu G., Gack M.U. Dysregulation of type I interferon responses in COVID-19. Nat. Rev. Immunol. 2020;20:397–398. doi: 10.1038/s41577-020-0346-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blanco-Melo D., Nilsson-Payant B.E., Liu W.C., Uhl S., Hoagland D., Moller R., Jordan T.X., Oishi K., Panis M., Sachs D., Wang T.T., Schwartz R.E., Lim J.K., Albrecht R.A., tenOever B.R. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181:1036–1045. doi: 10.1016/j.cell.2020.04.026. e1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hadjadj J., Yatim N., Barnabei L., Corneau A., Boussier J., Smith N., Pere H., Charbit B., Bondet V., Chenevier-Gobeaux C., Breillat P., Carlier N., Gauzit R., Morbieu C., Pene F., Marin N., Roche N., Szwebel T.A., Merkling S.H., Treluyer J.M., Veyer D., Mouthon L., Blanc C., Tharaux P.L., Rozenberg F., Fischer A., Duffy D., Rieux-Laucat F., Kerneis S., Terrier B. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369:718–724. doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang Y., Zhang L., Geng H., Deng Y., Huang B., Guo Y., Zhao Z., Tan W. The structural and accessory proteins M, ORF 4a, ORF 4b, and ORF 5 of Middle East respiratory syndrome coronavirus (MERS-CoV) are potent interferon antagonists. Protein Cell. 2013;4:951–961. doi: 10.1007/s13238-013-3096-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trouillet-Assant S., Viel S., Gaymard A., Pons S., Richard J.C., Perret M., Villard M., Brengel-Pesce K., Lina B., Mezidi M., Bitker L., Belot A. C.H.S. group, Type I IFN immunoprofiling in COVID-19 patients. J. Allergy Clin. Immunol. 2020 doi: 10.1016/j.jaci.2020.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee J.S., Shin E.C. The type I interferon response in COVID-19: implications for treatment. Nat. Rev. Immunol. 2020 doi: 10.1038/s41577-020-00429-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zare-Zardini H., Soltaninejad H., Ferdosian F., Hamidieh A.A., Memarpoor-Yazdi M. Coronavirus disease (COVID-19) in children: prevalence diagnosis, clinical symptoms, and treatment. Int. J. Gen. Med. 2019;13(2020):477–482. doi: 10.2147/IJGM.S262098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Y., Zhang D., Du G., Du R., Zhao J., Jin Y., Fu S., Gao L., Cheng Z., Lu Q., Hu Y., Luo G., Wang K., Lu Y., Li H., Wang S., Ruan S., Yang C., Mei C., Wang Y., Ding D., Wu F., Tang X., Ye X., Ye Y., Liu B., Yang J., Yin W., Wang A., Fan G., Zhou F., Liu Z., Gu X., Xu J., Shang L., Zhang Y., Cao L., Guo T., Wan Y., Qin H., Jiang Y., Jaki T., Hayden F.G., Horby P.W., Cao B., Wang C. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gordon C.J., Tchesnokov E.P., Woolner E., Perry J.K., Feng J.Y., Porter D.P., Gotte M. Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency. J. Biol. Chem. 2020;295:6785–6797. doi: 10.1074/jbc.RA120.013679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hashem A.M., Alghamdi B.S., Algaissi A.A., Alshehri F.S., Bukhari A., Alfaleh M.A., Memish Z.A. Therapeutic use of chloroquine and hydroxychloroquine in COVID-19 and other viral infections: A narrative review. Travel Med. Infect. Dis. 2020;101735 doi: 10.1016/j.tmaid.2020.101735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vincent M.J., Bergeron E., Benjannet S., Erickson B.R., Rollin P.E., Ksiazek T.G., Seidah N.G., Nichol S.T. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Savarino A., Boelaert J.R., Cassone A., Majori G., Cauda R. Effects of chloroquine on viral infections: an old drug against today's diseases? Lancet. Infect. Dis. 2003;3:722–727. doi: 10.1016/S1473-3099(03)00806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Savarino A., Gennero L., Chen H.C., Serrano D., Malavasi F., Boelaert J.R., Sperber K. Anti-HIV effects of chloroquine: mechanisms of inhibition and spectrum of activity. AIDS. 2001;15:2221–2229. doi: 10.1097/00002030-200111230-00002. [DOI] [PubMed] [Google Scholar]
- 55.Paton N.I., Goodall R.L., Dunn D.T., Franzen S., Collaco-Moraes Y., Gazzard B.G., Williams I.G., Fisher M.J., Winston A., Fox J., Orkin C., Herieka E.A., Ainsworth J.G., Post F.A., Wansbrough-Jones M., Kelleher P., T. Hydroxychloroquine Trial Effects of hydroxychloroquine on immune activation and disease progression among HIV-infected patients not receiving antiretroviral therapy: a randomized controlled trial. JAMA. 2012;308:353–361. doi: 10.1001/jama.2012.6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhu Y.Z., Xu Q.Q., Wu D.G., Ren H., Zhao P., Lao W.G., Wang Y., Tao Q.Y., Qian X.J., Wei Y.H., Cao M.M., Qi Z.T. Japanese encephalitis virus enters rat neuroblastoma cells via a pH-dependent, dynamin and caveola-mediated endocytosis pathway. J. Virol. 2012;86:13407–13422. doi: 10.1128/JVI.00903-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Randolph V.B., Winkler G., Stollar V. Acidotropic amines inhibit proteolytic processing of flavivirus prM protein. Virology. 1990;174:450–458. doi: 10.1016/0042-6822(90)90099-d. [DOI] [PubMed] [Google Scholar]
- 58.Brandriss M.W., Schlesinger J.J. Antibody-mediated infection of P388D1 cells with 17D yellow fever virus: effects of chloroquine and cytochalasin B. J. Gen. Virol. 1984;65(Pt 4):791–794. doi: 10.1099/0022-1317-65-4-791. [DOI] [PubMed] [Google Scholar]
- 59.Wang L.F., Lin Y.S., Huang N.C., Yu C.Y., Tsai W.L., Chen J.J., Kubota T., Matsuoka M., Chen S.R., Yang C.S., Lu R.W., Lin Y.L., Chang T.H. Hydroxychloroquine-inhibited dengue virus is associated with host defense machinery. J. Interferon Cytokine Res. 2015;35:143–156. doi: 10.1089/jir.2014.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Devaux C.A., Rolain J.M., Colson P., Raoult D. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int. J. Antimicrob. Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fantini J., Di Scala C., Chahinian H., Yahi N. Structural and molecular modeling studies reveal a new mechanism of action of chloroquine and hydroxychloroquine against SARS-CoV-2 infection. Int. J. Antimicrob. Agents. 2020;105960 doi: 10.1016/j.ijantimicag.2020.105960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Diebold S.S., Kaisho T., Hemmi H., Akira S., Reise Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 63.Saito T., Owen D.M., Jiang F., Marcotrigiano J., Gale M., Jr. Innate immunity induced by composition-dependent RIG-I recognition of hepatitis C virus RNA. Nature. 2008;454:523–527. doi: 10.1038/nature07106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hacker H., Mischak H., Miethke T., Liptay S., Schmid R., Sparwasser T., Heeg K., Lipford G.B., Wagner H. CpG-DNA-specific activation of antigen-presenting cells requires stress kinase activity and is preceded by non-specific endocytosis and endosomal maturation. EMBO J. 1998;17:6230–6240. doi: 10.1093/emboj/17.21.6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fujita Y., Matsuoka N., Temmoku J., Furuya M.Y., Asano T., Sato S., Kobayashi H., Watanabe H., Suzuki E., Urano T., Kozuru H., Yatsuhashi H., Koga T., Kawakami A., Migita K. Hydroxychloroquine inhibits IL-1beta production from amyloid-stimulated human neutrophils. Arthritis Res. Ther. 2019;21:250. doi: 10.1186/s13075-019-2040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schierbeck H., Wahamaa H., Andersson U., Harris H.E. Immunomodulatory drugs regulate HMGB1 release from activated human monocytes. Mol. Med. 2010;16:343–351. doi: 10.2119/molmed.2010.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shirey K.A., Lai W., Scott A.J., Lipsky M., Mistry P., Pletneva L.M., Karp C.L., McAlees J., Gioannini T.L., Weiss J., Chen W.H., Ernst R.K., Rossignol D.P., Gusovsky F., Blanco J.C., Vogel S.N. The TLR4 antagonist Eritoran protects mice from lethal influenza infection. Nature. 2013;497:498–502. doi: 10.1038/nature12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Andersson U., Ottestad W., Tracey K.J. Extracellular HMGB1: a therapeutic target in severe pulmonary inflammation including COVID-19? Mol. Med. 2020;26:42. doi: 10.1186/s10020-020-00172-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shirey K.A., Lai W., Patel M.C., Pletneva L.M., Pang C., Kurt-Jones E., Lipsky M., Roger T., Calandra T., Tracey K.J., Al-Abed Y., Bowie A.G., Fasano A., Dinarello C.A., Gusovsky F., Blanco J.C., Vogel S.N. Novel strategies for targeting innate immune responses to influenza. Mucosal Immunol. 2016;9:1173–1182. doi: 10.1038/mi.2015.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gao J., Tian Z., Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14:72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 71.Gautret P., Lagier J.C., Parola P., Hoang V.T., Meddeb L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Vieira V.E., Tissot Dupont H., Honore S., Colson P., Chabriere E., La Scola B., Rolain J.M., Brouqui P., Raoult D. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020;56 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 72.Chen Z., Hu J., Zhang Z., Jiang S., Han S., Yan D., Zhuang R., Hu B., Zhang Z. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. MedRxiv. 2020 [Google Scholar]
- 73.Catteau L., Dauby N., Montourcy M., Bottieau E., Hautekiet J., Goetghebeur E., van Ierssel S., Duysburgh E., Van Oyen H., Wyndham-Thomas C., Van Beckhoven D., C.-H.S. Belgian Collaborative Group on Low-dose hydroxychloroquine therapy and mortality in hospitalised patients with COVID-19: a nationwide observational study of 8075 participants. Int. J. Antimicrob. Agents. 2020:106144. doi: 10.1016/j.ijantimicag.2020.106144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bacharier L.B., Guilbert T.W., Mauger D.T., Boehmer S., Beigelman A., Fitzpatrick A.M., Jackson D.J., Baxi S.N., Benson M., Burnham C.D., Cabana M., Castro M., Chmiel J.F., Covar R., Daines M., Gaffin J.M., Gentile D.A., Holguin F., Israel E., Kelly H.W., Lazarus S.C., Lemanske R.F., Jr., Ly N., Meade K., Morgan W., Moy J., Olin T., Peters S.P., Phipatanakul W., Pongracic J.A., Raissy H.H., Ross K., Sheehan W.J., Sorkness C., Szefler S.J., Teague W.G., Thyne S., Martinez F.D. Early Administration of Azithromycin and Prevention of Severe Lower Respiratory Tract Illnesses in Preschool Children With a History of Such Illnesses: A Randomized Clinical Trial. JAMA. 2015;314:2034–2044. doi: 10.1001/jama.2015.13896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Million M., Lagier J.C., Gautret P., Colson P., Fournier P.E., Amrane S., Hocquart M., Mailhe M., Esteves-Vieira V., Doudier B., Aubry C., Correard F., Giraud-Gatineau A., Roussel Y., Berenger C., Cassir N., Seng P., Zandotti C., Dhiver C., Ravaux I., Tomei C., Eldin C., Tissot-Dupont H., Honore S., Stein A., Jacquier A., Deharo J.C., Chabriere E., Levasseur A., Fenollar F., Rolain J.M., Obadia Y., Brouqui P., Drancourt M., La Scola B., Parola P., Raoult D. Early treatment of COVID-19 patients with hydroxychloroquine and azithromycin: A retrospective analysis of 1061 cases in Marseille, France. Travel Med. Infect. Dis. 2020:101738. doi: 10.1016/j.tmaid.2020.101738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shirey K.A., Lai W., Pletneva L.M., Finkelman F.D., Feola D.J., Blanco J.C., Vogel S.N. Agents that increase AAM differentiation blunt RSV-mediated lung pathology. J. Leukoc. Biol. 2014;96:951–955. doi: 10.1189/jlb.4HI0414-226R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mallat J., Hamed F., Balkis M., Mohamed M.A., Mooty M., Malik A., Nusair A., Bonilla F. Hydroxychloroquine is associated with slower viral clearance in clinical COVID-19 patients with mild to moderate disease: A retrospective study. medRxiv. 2020 doi: 10.1097/MD.0000000000023720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mahevas M., Tran V.T., Roumier M., Chabrol A., Paule R., Guillaud C., Fois E., Lepeule R., Szwebel T.A., Lescure F.X., Schlemmer F., Matignon M., Khellaf M., Crickx E., Terrier B., Morbieu C., Legendre P., Dang J., Schoindre Y., Pawlotsky J.M., Michel M., Perrodeau E., Carlier N., Roche N., de Lastours V., Ourghanlian C., Kerneis S., Menager P., Mouthon L., Audureau E., Ravaud P., Godeau B., Gallien S., Costedoat-Chalumeau N. Clinical efficacy of hydroxychloroquine in patients with covid-19 pneumonia who require oxygen: observational comparative study using routine care data. BMJ. 2020;369 doi: 10.1136/bmj.m1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Boulware D.R., Pullen M.F., Bangdiwala A.S., Pastick K.A., Lofgren S.M., Okafor E.C., Skipper C.P., Nascene A.A., Nicol M.R., Abassi M., Engen N.W., Cheng M.P., LaBar D., Lother S.A., MacKenzie L.J., Drobot G., Marten N., Zarychanski R., Kelly L.E., Schwartz I.S., McDonald E.G., Rajasingham R., Lee T.C., Hullsiek K.H. A randomized trial of hydroxychloroquine as postexposure prophylaxis for covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2016638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Magagnoli J., Narendran S., Pereira F., Cummings T.H., Hardin J.W., Sutton S.S., Ambati J. Outcomes of hydroxychloroquine usage in United States Veterans Hospitalized with COVID-19. Med (N Y) 2020 doi: 10.1016/j.medj.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ramireddy A., Chugh H., Reinier K., Ebinger J., Park E., Thompson M., Cingolani E., Cheng S., Marban E., Albert C.M., Chugh S.S. Experience With Hydroxychloroquine and Azithromycin in the Coronavirus Disease 2019 Pandemic: Implications for QT Interval Monitoring. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.017144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Saleh M., Gabriels J., Chang D., Soo Kim B., Mansoor A., Mahmood E., Makker P., Ismail H., Goldner B., Willner J., Beldner S., Mitra R., John R., Chinitz J., Skipitaris N., Mountantonakis S., Epstein L.M. Effect of chloroquine, hydroxychloroquine, and azithromycin on the corrected QT interval in patients with SARS-CoV-2 infection. Circ Arrhythm Electrophysiol. 2020;13 doi: 10.1161/CIRCEP.120.008662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Roohani N., Hurrell R., Kelishadi R., Schulin R. Zinc and its importance for human health: An integrative review. J Res Med Sci. 2013;18:144–157. [PMC free article] [PubMed] [Google Scholar]
- 84.Lowe N.M., Fekete K., Decsi T. Methods of assessment of zinc status in humans: a systematic review. Am. J. Clin. Nutr. 2009;89:2040S–2051S. doi: 10.3945/ajcn.2009.27230G. [DOI] [PubMed] [Google Scholar]
- 85.Jacobs S.E., Lamson D.M., St George K., Walsh T.J. Human rhinoviruses. Clin. Microbiol. Rev. 2013;26:135–162. doi: 10.1128/CMR.00077-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Skalny A.V., Rink L., Ajsuvakova O.P., Aschner M., Gritsenko V.A., Alekseenko S.I., Svistunov A.A., Petrakis D., Spandidos D.A., Aaseth J., Tsatsakis A., Tinkov A.A. Zinc and respiratory tract infections: Perspectives for COVID19 (Review) Int. J. Mol. Med. 2020 doi: 10.3892/ijmm.2020.4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.te Velthuis A.J., van den Worm S.H., Sims A.C., Baric R.S., Snijder E.J., van Hemert M.J. Zn(2+) inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Perry D.K., Smyth M.J., Stennicke H.R., Salvesen G.S., Duriez P., Poirier G.G., Hannun Y.A. Zinc is a potent inhibitor of the apoptotic protease, caspase-3. A novel target for zinc in the inhibition of apoptosis. J. Biol. Chem. 1997;272:18530–18533. doi: 10.1074/jbc.272.30.18530. [DOI] [PubMed] [Google Scholar]
- 89.Hojyo S., Fukada T. Roles of zinc signaling in the immune system. J. Immunol. Res. 2016;2016:6762343. doi: 10.1155/2016/6762343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xue J., Moyer A., Peng B., Wu J., Hannafon B.N., Ding W.Q. Chloroquine is a zinc ionophore. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0109180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bedoui Y., Giry C., Jaffar-Bandjee M.C., Selambarom J., Guiraud P., Gasque P. Immunomodulatory drug methotrexate used to treat patients with chronic inflammatory rheumatisms post-chikungunya does not impair the synovial antiviral and bone repair responses. PLoS Negl. Trop Dis. 2018;12 doi: 10.1371/journal.pntd.0006634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Municio C., Dominguez-Soto A., Fuentelsaz-Romero S., Lamana A., Montes N., Cuevas V.D., Campos R.G., Pablos J.L., Gonzalez-Alvaro I., Puig-Kroger A. Methotrexate limits inflammation through an A20-dependent cross-tolerance mechanism. Ann. Rheum. Dis. 2018;77:752–759. doi: 10.1136/annrheumdis-2017-212537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McLean-Tooke A., Aldridge C., Waugh S., Spickett G.P., Kay L. Methotrexate, rheumatoid arthritis and infection risk: what is the evidence? Rheumatology (Oxford) 2009;48:867–871. doi: 10.1093/rheumatology/kep101. [DOI] [PubMed] [Google Scholar]
- 94.Boccalatte F.E., Voena C., Riganti C., Bosia A., D'Amico L., Riera L., Cheng M., Ruggeri B., Jensen O.N., Goss V.L., Lee K., Nardone J., Rush J., Polakiewicz R.D., Comb M.J., Chiarle R., Inghirami G. The enzymatic activity of 5-aminoimidazole-4-carboxamide ribonucleotide formyltransferase/IMP cyclohydrolase is enhanced by NPM-ALK: new insights in ALK-mediated pathogenesis and the treatment of ALCL. Blood. 2009;113:2776–2790. doi: 10.1182/blood-2008-06-161018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Noack M., Miossec P. Effects of methotrexate alone or combined with arthritis-related biotherapies in an in vitro co-culture model with immune cells and synoviocytes. Front. Immunol. 2019;10:2992. doi: 10.3389/fimmu.2019.02992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wertz I.E., O'Rourke K.M., Zhou H., Eby M., Aravind L., Seshagiri S., Wu P., Wiesmann C., Baker R., Boone D.L., Ma A., Koonin E.V., Dixit V.M. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-kappaB signalling. Nature. 2004;430:694–699. doi: 10.1038/nature02794. [DOI] [PubMed] [Google Scholar]
- 97.Tejasvi T., Stuart P.E., Chandran V., Voorhees J.J., Gladman D.D., Rahman P., Elder J.T., Nair R.P. TNFAIP3 gene polymorphisms are associated with response to TNF blockade in psoriasis. J, Invest. Dermatol. 2012;132:593–600. doi: 10.1038/jid.2011.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bedoui Y., Guillot X., Selambarom J., Guiraud P., Giry C., Jaffar-Bandjee M.C., Ralandison S., Gasque P. Methotrexate an old drug with new tricks. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20205023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Frohman E.M., Villemarette-Pittman N.R., Cruz R.A., Longmuir R., Rowe V., Rowe E.S., Varkey T.C., Steinman L., Zamvil S.S., Frohman T.C. Part II. High-dose methotrexate with leucovorin rescue for severe COVID-19: An immune stabilization strategy for SARS-CoV-2 induced 'PANIC' attack. J. Neurol. Sci. 2020;415 doi: 10.1016/j.jns.2020.116935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Thomas S., Fisher K.H., Snowden J.A., Danson S.J., Brown S., Zeidler M.P. Methotrexate is a JAK/STAT pathway inhibitor. PLoS One. 2015;10 doi: 10.1371/journal.pone.0130078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tan K.W., Tay Y.K. A case of acute methotrexate toxicity. Ann. Acad. Med. Singap. 2011;40:97–99. [PubMed] [Google Scholar]
- 102.Al-Dawsari N.A., Croke J., Yaar M. Sclerotic atrophic plaques associated with a tattoo. Dermatol. Online J. 2014;20 [PubMed] [Google Scholar]
- 103.Zabirowicz E.S., Gan T.J. Elsevier; 2019. Pharmacology of Postoperative Nausea and Vomiting, Pharmacology and Physiology for Anesthesia; pp. 671–692. [Google Scholar]
- 104.Coutinho A.E., Chapman K.E. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol. Cell. Endocrinol. 2011;335:2–13. doi: 10.1016/j.mce.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.R.C. Group, Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L., Staplin N., Brightling C., Ustianowski A., Elmahi E., Prudon B., Green C., Felton T., Chadwick D., Rege K., Fegan C., Chappell L.C., Faust S.N., Jaki T., Jeffery K., Montgomery A., Rowan K., Juszczak E., Baillie J.K., Haynes R., Landray M.J. Dexamethasone in hospitalized patients with covid-19 – preliminary report. N. Engl. J. Med. 2020 [Google Scholar]
- 106.Ledford H. Coronavirus breakthrough: dexamethasone is first drug shown to save lives. Nature. 2020;582:469. doi: 10.1038/d41586-020-01824-5. [DOI] [PubMed] [Google Scholar]
- 107.Shang L., Zhao J., Hu Y., Du R., Cao B. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet. 2020;395:683–684. doi: 10.1016/S0140-6736(20)30361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395:473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mattos-Silva P., Felix N.S., Silva P.L., Robba C., Battaglini D., Pelosi P., Rocco P.R.M., Cruz F.F. Pros and cons of corticosteroid therapy for COVID-19 patients. Respir. Physiol. Neurobiol. 2020;280 doi: 10.1016/j.resp.2020.103492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Borba M.G.S., Val F.F.A., Sampaio V.S., Alexandre M.A.A., Melo G.C., Brito M., Mourao M.P.G., Brito-Sousa J.D., Baia-da-Silva D., Guerra M.V.F., Hajjar L.A., Pinto R.C., Balieiro A.A.S., Pacheco A.G.F., Santos J.D.O., Jr., Naveca F.G., Xavier M.S., Siqueira A.M., Schwarzbold A., Croda J., Nogueira M.L., Romero G.A.S., Bassat Q., Fontes C.J., Albuquerque B.C., Daniel-Ribeiro C.T., Monteiro W.M., Lacerda M.V.G., CloroCovid T. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. JAMA Netw. Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gautret P., Lagier J.C., Parola P., Hoang V.T., Meddeb L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Vieira V.E., Dupont H.T., Honore S., Colson P., Chabriere E., La Scola B., Rolain J.M., Brouqui P., Raoult D. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020;105949 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 112.Mahevas M., Tran V.T., Roumier M., Chabrol A., Paule R., Guillaud C., Gallien S., Lepeule R., Szwebel T.A., Lescure X. No evidence of clinical efficacy of hydroxychloroquine in patients hospitalized for COVID-19 infection with oxygen requirement: results of a study using routinely collected data to emulate a target trial. MedRxiv. 2020 [Google Scholar]
- 113.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., Ruan L., Song B., Cai Y., Wei M., Li X., Xia J., Chen N., Xiang J., Yu T., Bai T., Xie X., Zhang L., Li C., Yuan Y., Chen H., Li H., Huang H., Tu S., Gong F., Liu Y., Wei Y., Dong C., Zhou F., Gu X., Xu J., Liu Z., Zhang Y., Li H., Shang L., Wang K., Li K., Zhou X., Dong X., Qu Z., Lu S., Hu X., Ruan S., Luo S., Wu J., Peng L., Cheng F., Pan L., Zou J., Jia C., Wang J., Liu X., Wang S., Wu X., Ge Q., He J., Zhan H., Qiu F., Guo L., Huang C., Jaki T., Hayden F.G., Horby P.W., Zhang D., Wang C. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. N. Engl. J. Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Janowitz T., Gablenz E., Pattinson D., Wang T.C., Conigliaro J., Tracey K., Tuveson D. Famotidine use and quantitative symptom tracking for COVID-19 in non-hospitalised patients: a case series. Gut. 2020;69:1592–1597. doi: 10.1136/gutjnl-2020-321852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Freedberg D.E., Conigliaro J., Wang T.C., Tracey K.J., Callahan M.V., Abrams J.A., G. Famotidine Research Famotidine use is associated with improved clinical outcomes in hospitalized COVID-19 patients: a propensity score matched retrospective cohort study. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang Z., Yang B., Li Q., Wen L., Zhang R. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin. Infect. Dis. 2020;71:769–777. doi: 10.1093/cid/ciaa272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lian N., Xie H., Lin S., Huang J., Zhao J., Lin Q. Umifenovir treatment is not associated with improved outcomes in patients with coronavirus disease 2019: a retrospective study. Clin. Microbiol. Infect. 2020;26:917–921. doi: 10.1016/j.cmi.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Caly L., Druce J.D., Catton M.G., Jans D.A., Wagstaff K.M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020;178 doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gupta D., Sahoo A.K., Singh A. Ivermectin: potential candidate for the treatment of Covid 19. Braz. J. Infect. Dis. 2020;24:369–371. doi: 10.1016/j.bjid.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tomazini B.M., Maia I.S., Cavalcanti A.B., Berwanger O., Rosa R.G., Veiga V.C., Avezum A., Lopes R.D., Bueno F.R., Silva M., Baldassare F.P., Costa E.L.V., Moura R.A.B., Honorato M.O., Costa A.N., Damiani L.P., Lisboa T., Kawano-Dourado L., Zampieri F.G., Olivato G.B., Righy C., Amendola C.P., Roepke R.M.L., Freitas D.H.M., Forte D.N., Freitas F.G.R., Fernandes C.C.F., Melro L.M.G., Junior G.F.S., Morais D.C., Zung S., Machado F.R., Azevedo L.C.P., Investigators C.C.-B.I. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA. 2020 doi: 10.1001/jama.2020.17021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hu Z., Lv Y., Xu C., Sun W., Chen W., Peng Z., Chen C., Cui X., Jiao D., Cheng C., Chi Y., Wei H., Hu C., Zeng Y., Zhang X., Yi Y. Clinical use of short-course and low-dose corticosteroids in patients with non-severe COVID-19 during pneumonia progression. Front. Public Health. 2020;8:355. doi: 10.3389/fpubh.2020.00355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Beigel John, Tomashek Kay, Dodd Lori, Mehta Aneesh, Zingman Barry, Kalil Andre, Hohmann Elizabeth, Chu Helen, Luetkemeyer Annie, Kline Susan, ACTT-1 Study Group Members Remdesivir for the treatment of Covid-19 — final report. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2007764. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]