Abstract

The electrolysis of water is popular both as lab work and as a demonstration. In this activity, the electrolysis of water in the presence of a pH indicator is used to produce text and symbols. This report describes the design of an environmentally friendly setup of a writing board utilizing the electrolysis of water in a hydrogel environment. The activity can be performed by only using chemicals and materials that are easily accessible to everyone, with no special permit needed. The writing board has been developed mainly as an outreach activity for our faculty and has been assessed during visits from upper secondary school students.
Keywords: General Public, High School/Introductory Chemistry, Public Understanding/Outreach, Hands-On Learning/Manipulatives, Acids/Bases, Electrochemistry, Oxidation/Reduction
Introduction
Electrochemistry is an important subject as it is not only very common in everyday life but also important in our society. Its applications can be found in batteries, accumulators, fuel cells, study of the corrosion process, and industrial-scale production of important metals like aluminum.1 Some classic experiments and demonstrations on the subject include writing messages and designing art by exploiting the electrolysis of water.2 Many excellent examples on how to create laboratory work and how to visualize the products using color changes have been published frequently. Some of the examples have used electrolysis of water for demonstrating the chemical composition of water and/or for illustrating the redox reactions using color changes,3−7 while others have developed microscale laboratory exercises.8,9
In the electrolysis of water with an inert electrolyte, half-reactions occur at
| 1 |
| 2 |
The electrolytic products including H+ and OH– from eqs 1 and 2, respectively, show why pH indicators are a natural choice for illustration of the redox reaction. Examples of indicators used for this purpose include bromocresol green, bromothymol blue, and thymolphthalein.3,4,6 Another popular natural pH indicator is the juice of red cabbage due to its nontoxicity and broad changes in color. It is commonly used in Finnish schools, especially for the lower grades.10,11 The reason for the changes in color with pH are anthocyanins, the compounds that are present in red cabbage as well as in blueberries. The anthocyanins in red cabbage will change their color depending on pH of the environment such as a green at highly alkaline, blue at slightly alkaline, purple around neutral, pink at slightly acidic, and red at highly acidic conditions.11
Experiments in liquid media often require careful handling and uncontrollable contour of products; thus, gelatin and agar have been used to prepare hydrogels for electrochemistry by both Stauffer and Fox6 and Davis et al.8 Another interesting group of compounds for making gels are carrageenans, natural polysaccharides that can be extracted from red algae, some of which can be found also in parts of the Baltic Sea. Carrageenans are common in everyday life as they are used as stabilizers, thickeners, and gelling agents for example in the food industry. Aqueous solutions of carrageenans with specific cations will form physically cross-linked polyelectrolyte gels, that are thermoreversible.12−14
In this work, we have combined the teaching of pH and electrochemistry inspired by reported methods from others,2−11 focusing here on showing the wonders of everyday chemistry. In this paper, we describe the design of an environmentally friendly setup of a writing board utilizing the electrolysis of water in a hydrogel environment. The concept of using electrochemistry for producing text has been reported by others.2,15,16 The emphasis of the method reported in this paper has been on the electrolysis of water with the key factors being a safe and environmental friendly experiment. The reactions both at the cathode and at the anode can be performed without any special equipment, space, or ingredients. The setup has been developed for school visits to our Faculty of Natural Science and Engineering at Åbo Akademi University. Our aim is foremost to introduce chemistry in a fun and surprising way, showing that chemistry is present in our everyday lives and does not have to be dangerous. We have also taken this as an opportunity to show that chemistry does not have to be just test tubes; it is so much more and can also be a form of art. As an additional experiment, we also demonstrate how the hydrogel can be used for making stencil pictures.
Design
Materials
Reagent grade Κ-carrageenan, anhydrous magnesium sulfate, and ammonium hydroxide were acquired from Sigma-Aldrich. Reagent grade hydrochloric acid was acquired from Merck. Κ-carrageenan (SpecialIngredients) for cooking purposes and Epsom salt (magnesium sulfate, Nortembio) were bought through Amazon but are also available through pharmacies and health food stores. Red cabbage (Brassica oleraceae), bilberry (European blueberries, Vaccinium myrtillus), vinegar, citric acid, baking soda, and 9 V batteries were all purchased from a local supermarket. Petri dishes (d 90 mm) were acquired from VWR. The copper wire was bought from a local hardware store.
Hazards
All materials used in the writing board experiment are nonhazardous. For the stencil pictures (additional experiment), strong acids and bases are used. Proper lab safety measures should be taken, including working in a fume hood, wearing a lab coat, and using gloves and glasses.
Preparation of pH Indicator Juice
Red cabbage and bilberry juices are chopped and crushed respectively after which newly boiled water (from a water boiler) is poured over and the mixture is left standing for an hour. To remove the solid pieces, the mixture was poured into a flask using a coffee press. For the red cabbage juice, we used 1200 mL of water and 450 g of chopped fresh red cabbage. For the bilberry juice, we used 1200 mL of water and 300 g of frozen bilberries. To have the juice readily available, a large batch can be made and stored in the freezer.
Preparation of the Responsive Hydrogel
To produce the hydrogel, 1.5 wt % of carrageenan is added to a 0.09 M solution of magnesium sulfate consisting of 60% 0.15 M magnesium sulfate solution and 40% indicator juice (prepared as described above). The carrageenan is added slowly and carefully under stirring and heating (70 °C). Care should be taken so that no clots, especially big ones, of carrageenan are formed. The pH of the solution can be adjusted using vinegar, citric acid, or baking soda in order to get the desired color of the gel. When all the carrageenan has been added, the solution can be removed from the heating plate. Upon cooling, the solution forms a hydrogel, and the hydrogel can be stored for more than a week in the fridge.
Preparation of the Writing Board
For writing purposes, the hot (70 °C) solution should be poured into a dish with low edges but a fairly large area (e.g., a Petri dish). The electrode setup for writing includes a ring of copper wire as the anode is placed at the edges of the dish, where one of the ends of the wire as the anode is sticking up so that a battery can be attached using alligator clip wires (Figure 1). The writing is done by attaching the copper wire in the hydrogel to one terminal of a 9 V battery while attaching another piece of straight copper wire (cathode) to the other terminal. We have used the negative terminal for writing as shown in the pictures in Figure 1. The straight copper wire is used as the pen; by making a small bend at the end, one can write by applying it gently and therefore without destroying the hydrogel. We have made copper pens by removing everything but the shell from a ball point pen and sticking the wire through it (Figure 2 and Figure S2, Supporting Information); detailed instructions can be found in the Supporting Information. The hydrogel can be used after a few hours but will work much better if left overnight at room temperature. The firmer the hydrogel is, the better the writing will be. If care is taken not to overheat the hydrogel, it can be reused after careful heating and stirring.
Figure 1.

Schematic three-step assembly and use of the writing board: (I) copper wire and Petri dish, (II) copper wire and pH indicator gel in Petri dish, and (III) final assembly of the writing board, with copper wire used as a writing tool attached to the negative terminal of the battery and copper wire in the gel attached to the positive terminal.
Figure 2.

(A) Design of our copper pen used as the tools for writing by visiting students, in the picture attached to an alligator clip. (B) The setup of the experiment as introduced to the visiting students (9 V battery, copper pen, alligator clips, pH indicator gel with copper wire).
Experimental Procedures
Writing Board Experiment
As mentioned in the Introduction, we have used our writing board when presenting the subject of chemistry at Åbo Akademi University to visiting upper secondary school students. The concept of the writing board has been introduced to the students as a challenge with three tasks:
Can you write your name in the hydrogel in such a way that scratching the gel is not your main form of producing the letters?
How many different colors can you produce in the hydrogel?
Can you tell which terminal of the battery you have used for writing?
The clues given for the task are eqs 1 and 2, and a colored pH scale for red cabbage juice along with a list of the materials available. For the purpose of the last task, the terminals of the battery are hidden beneath duct tape. A more detailed description can be found in Figure 2. Figure 3 and Figure S3 (Supporting Information) shows text produced using the negative terminal of the battery.
Figure 3.
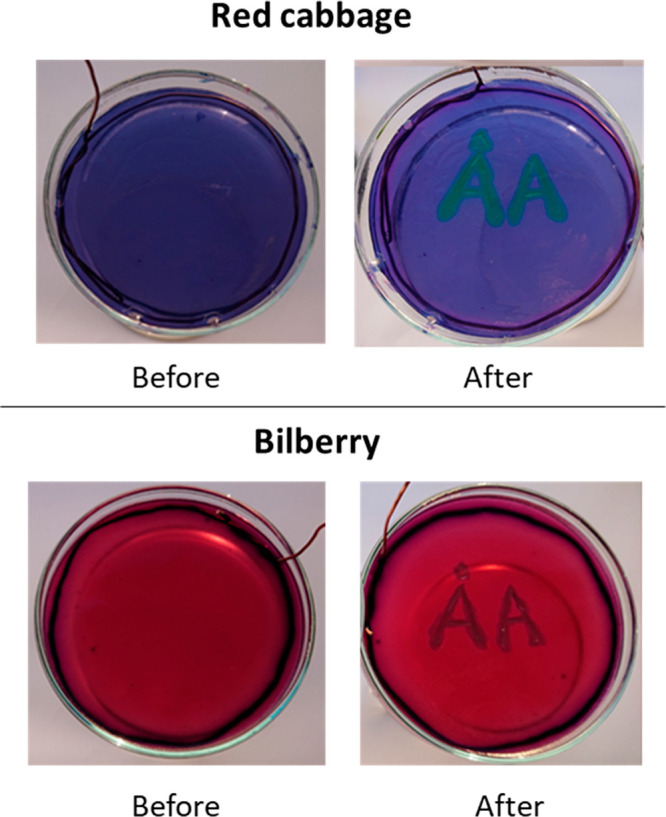
Red cabbage and bilberry hydrogels before and after writing “ÅA” using electrolysis.
Additional Experiments with the pH Responsive Hydrogel
Our main purpose for the hydrogel is using it as the presented writing board. Where electrochemistry has been used by others for writing messages, the pH reactivity of red cabbage has been used to make art in the form of patterns and paintings.17,18 To add to the versatility of the hydrogel, we have also used it as a substrate for making stencil pictures by exposing it to gases; examples of stencil pictures can be seen in Figure 4. The stencils used for the pictures presented here were made using parafilm. Using the stencils, the hydrogel was exposed to acidic or basic gases according to the desired color. For the included examples, we have used hydrochloric acid (red) and ammonia (green/yellow) (see also Figures S4 and S5, Supporting Information). The stencils can be made by students, but the gas exposure, which must be done in a fume hood, should be left to the teacher and only after taking proper safety precautions. The hydrogels should also be left in the fume hood for a while after the exposure. Since we have a hydrogel, the gases will easily penetrate and dissolve into the hydrogel, causing the color change. The ability to absorb additionally added water is not as good or as fast as that of paper. Therefore, you will not have results that are as nice by trying to paint with acid–base solutions. However, it gives instructors the opportunity to introduce another type of technique.
Figure 4.

Pictures of (A) letters “ÅA” and (B) random art made using stencils and exposure to hydrochloric acid (resulting red color) and ammonia (resulting green color) gases.
Integrating the Activity into the Curriculum
This activity can be part of an outreach activity and can also be integrated in the classroom on different levels as it combines many different concepts, for example, acids/bases, indicators, and electrochemistry. The concepts dealt with in the lab work make it suitable for both lower and upper secondary school in accordance with the Finnish curriculum for chemistry education. Something that is also highlighted in the Finnish curriculum is the importance of building a sustainable future, as this lab work builds on the ideas of biodegradability and fits well also with this important subject.
Evaluation
The experiment has been tested with 33 students from three different schools. After being introduced to the concept and finishing the three tasks mentioned earlier, the students were asked to evaluate their experience with the writing board. The students were asked about their earlier experiences with the electrolysis of water and about their opinions regarding the use of the writing board. Some questions had been left unanswered by some of the students, and some had filled in the form as a pair. The survey of their earlier experience with electrolysis of water was evaluated with yes or no questions. The survey regarding their opinions of working with the board was evaluated using open questions. The results of the survey can be found in Figure 5A,B, where the answers to these open questions could all be categorized as being either “yes” or “somewhat”. Some answers to the question “Other comments?” can be found in Box 1.
Figure 5.

Results of the questions regarding (A) earlier experience of the electrolysis of water and (B) the open questions about the experience of using the writing board.
Box 1. Examples of Comments Given by Visiting Students.
“it was fun”
“it was a very interesting technique”,
“it was a good way of making it interesting”
“I don’t like chemistry at all but this was a good way to make it interesting”
Discussion
There are multiple reasons regarding why we have chosen to use natural pH indicators and carrageenan. One of them is the opportunity to show the chemistry that is all around us. Choosing food items makes the experiment nontoxic, biodegradable, and environmentally friendly. In addition, it is also cost-effective both when it comes to purchase and waste handling. The average cost for a 1 cm thick hydrogel made with commercial chemicals in a 90 mm Petri dish, including the copper wire, is 0.28 euros. The Petri dish is not included in the price, nor is the battery. Most importantly, this makes the experiment accessible not only for teachers without a laboratory (e.g., in elementary school) but also for students who want to continue the experiment at home. We have chosen to use magnesium sulfate for the same reasons as we have chosen the other components. It is inexpensive, nontoxic, biodegradable, easily obtainable, and easy to handle, even though sodium sulfate is more commonly used for the electrolysis of water especially when pH determination is also important.8 The magnesium ion (Mg2+) also enhances the gelation of the carrageenan compared to the sodium ion (Na+).12 An increasing concentration of Mg2+ will, however, decrease the hardness of the hydrogel.19 Other gel-based electrolysis setups have been seen in this Journal earlier, using gelatin-based gels, both for illustrating color changes and for making microscale setups.6,8 Our hydrogel can be utilized also for these kinds of experiments and works well for a color changing reaction as it is highly transparent in its native form (i.e., without indicators present).
The visiting groups are usually very diverse, regarding both interest in and knowledge of chemistry. Some are very interested and have solid knowledge; others lack interest and have difficulties even with very basic concepts. This means that some students need more help than others; we have still decided not to give too many recipe-like instructions. Instead, we have made sure to have enough instructors present to enable discussion for those who need help. This also gives the instructor the opportunity to meet the students at their level. Suggestions for strategies on how to help the more unprepared students can be found in the second Lab Work heading (Lab Work for Classroom–Writing Board) in the Student Notes portion of the Supporting Information, as this work has been developed on the basis of our discussions with the visiting students. The laboratory time for our visiting groups varies between 30 and 60 min, with 30 min being the more common option. Due to the short time, we usually have very little time for further discussions. However, with more time on one’s hands or if someone is using this in a classroom setting, we see this as a great opportunity to take the demonstration further by discussing topics like green chemistry and the need for reducing waste, especially hazardous waste. The Petri dish used in the experiment, whether it is plastic or glass, can and should be cleaned and reused in other experiments. Plastic as a material has gained a lot of negative publicity in recent times and can be exchanged for something more recyclable like a drink carton. The battery can be exchanged for a small hand driven motor or solar cell.
We have used this experiment as an outreach activity for upper secondary students. However, it is possible to use it also with younger students as a more structured activity with more specific instructions. In this case, we recommend to at least start with using the cathode (the negative terminal of the battery) for writing as this reaction gives a much quicker response. The instructions for the outreach activity are based on limited time resources. We have noticed that some of the students are struggling with basic concepts, so we have therefore also developed instructions for classroom settings based on our experiences with the visiting students. These can be found in the Supporting Information.
The positive outcomes of combining science and art, as well as the benefits of using everyday items, have been addressed by several authors.2,20,21 Our overall impression is that, also, the students we have encountered really enjoyed both the artistic elements and the freedom of creativity of the experiment very much.
Acknowledgments
T.-P.H acknowledges financial support from the Magnus Ehrnrooth Foundation and the contribution of the COST Action CA17120 Chemobrionics. The authors would like to thank Svante Åberg, Umeå University, for giving us permission to use one of his figures in the Student Notes portion of the Supporting Information.
Supporting Information Available
The Supporting Information is available at https://pubs.acs.org/doi/10.1021/acs.jchemed.0c00440.
The authors declare no competing financial interest.
Supplementary Material
References
- Fine L. W.; Beall H.; Stuehr J.. Chemistry for Scientists and Engineers, preliminary ed.; Saunders College: Philadelphia, 2000; p 633. [Google Scholar]
- Kuntzleman T. S. Electrochemistry with Simple Materials to Create Designs and Write Messages. J. Chem. Educ. 2019, 96 (6), 1178–1181. 10.1021/acs.jchemed.9b00012. [DOI] [Google Scholar]
- Heideman S.; Wollaston G. The Electrolysis of Water: An Improved Demonstration Procedure. J. Chem. Educ. 1986, 63 (9), 809–810. 10.1021/ed063p809.2. [DOI] [Google Scholar]
- Eggen P.-O.; Kvittingen L. A Small-Scale and Low-Cost Apparatus for the Electrolysis of Water. J. Chem. Educ. 2004, 81 (9), 1337–1338. 10.1021/ed081p1337. [DOI] [Google Scholar]
- Hendricks L. J.; Williams J. T. Demonstration of Electrochemical Cell Properties by a Simple, Colorful Oxidation-Reduction Experiment. J. Chem. Educ. 1982, 59 (7), 586–587. 10.1021/ed059p586. [DOI] [Google Scholar]
- Stauffer M. T.; Fox J. P. Yet Another Variation on the Electrolysis of Water at Iron Nails. J. Chem. Educ. 2008, 85 (4), 523. 10.1021/ed085p523. [DOI] [Google Scholar]
- Zhou R. E. How to Offer the Optimal Demonstration of the Electrolysis of Water. J. Chem. Educ. 1996, 73 (8), 786–787. 10.1021/ed073p786. [DOI] [Google Scholar]
- Davis T. A.; Athey S. L.; Vandevender M. L.; Crihfield C. L.; Kolanko C. C. E.; Shao S.; Ellington M. C. G.; Dicks J. K.; Carver J. S.; Holland L. A. Electrolysis of Water in The Secondary School Science Laboratory with Inexpensive Microfluidics. J. Chem. Educ. 2015, 92 (1), 116–119. 10.1021/ed400757m. [DOI] [Google Scholar]
- Kamata M.; Yajima S. Microscale Electrolysis Using Coin-Type Lithium Batteries and Filter Paper. J. Chem. Educ. 2013, 90 (2), 228–231. 10.1021/ed300365d. [DOI] [Google Scholar]
- Fortman J. J.; Stubbs K. M. Demonstrations with Red Cabbage Indicator. J. Chem. Educ. 1992, 69 (1), 66. 10.1021/ed069p66.1. [DOI] [Google Scholar]
- Stoddard R. L.; McIndoe J. S. The Color-Changing Sports Drink: An Ingestible Demonstration. J. Chem. Educ. 2013, 90 (8), 1032–1034. 10.1021/ed3007346. [DOI] [Google Scholar]
- Robal M.; Brenner T.; Matsukawa S.; Ogawa H.; Truus K.; Rudolph B.; Tuvikene R. Monocationic salts of carrageenans: Preparation and physico-chemical properties. Food Hydrocolloids 2017, 63, 656–667. 10.1016/j.foodhyd.2016.09.032. [DOI] [Google Scholar]
- Takemasa M.; Chiba A.; Date M. Gelation Mechanism of κ- and ι-Carrageenan Investigated by Correletion between the Strain-Optical Coefficient and the Dynamic Shear Modulus. Macromolecules 2001, 34 (21), 7427–7434. 10.1021/ma0102924. [DOI] [Google Scholar]
- Pereira L.Edible Seaweeds of the World, 1st ed.; CRC Press: Boca Raton, 2016; pp 76–81. [Google Scholar]
- Harakas G. N. Production of Colorful Aluminum Keepsakes and Gas Sensing Smart Materials: Anodizing, Dyeing, and Etching Small Aluminum Parts on a Budget. J. Chem. Educ. 2018, 95 (7), 1187–1191. 10.1021/acs.jchemed.7b00817. [DOI] [Google Scholar]
- Valetaud M.; Loget G.; Roche J.; Hüsken N.; Fattah Z.; Badets V.; Fontaine O.; Zigah D. The EChemPen: A Guiding Hand To Learn Electrochemical Surface Modifications. J. Chem. Educ. 2015, 92 (10), 1700–1704. 10.1021/acs.jchemed.5b00149. [DOI] [Google Scholar]
- Suzuki C. Making Colorful Patterns on Paper Dyed with Red Cabbage Juice. J. Chem. Educ. 1991, 68 (7), 588. 10.1021/ed068p588. [DOI] [Google Scholar]
- Lech J.; Dounin V. JCE Classroom Activity #110: Artistic Anthocyanins and Acid-Base Chemistry. J. Chem. Educ. 2011, 88 (12), 1684–1686. 10.1021/ed1011647. [DOI] [Google Scholar]
- Kadota K.; Nogami S.; Uchiyama H.; Tozuka Y. Controlled release behavior of curcumin from kappa-carrageenan gels with flexible texture by the addition of metal chlorides. Food Hydrocolloids 2020, 101, 105564. 10.1016/j.foodhyd.2019.105564. [DOI] [Google Scholar]
- Moore J. Science and Art. J. Chem. Educ. 2001, 78 (10), 1295. 10.1021/ed078p1295. [DOI] [Google Scholar]
- Danipog D. L.; Ferido M. B. Using Art-Based Chemistry Activities To Improve Students’ Conceptual Understanding in Chemistry. J. Chem. Educ. 2011, 88 (12), 1610–1615. 10.1021/ed100009a. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


