Abstract
A demonstration experiment of the synthesis of a novel tetrazole derivative via a multicomponent reaction (Ugi tetrazole four component reaction, UT-4CR) bearing a luminol moiety and a subsequent exploitation of its chemiluminescent properties is described. A complex product is generated in just one simple step, so simple that children can do it: “kinderleicht”, German for dead easy. Students are stimulated, inspired, and involved in a multilevel active learning process using the Steps to Inquiry framework as a metacognitive tool that raises student awareness regarding scientific process and prompts them to ask their own questions discussing the merits of a mechanism and evaluating its effectiveness before they start their own cycles of inquiry.
Keywords: General Public, Elementary/Middle School Science, Demonstrations, Organic Chemistry, Hands-On Learning/Manipulatives, Photochemistry, Constructivism
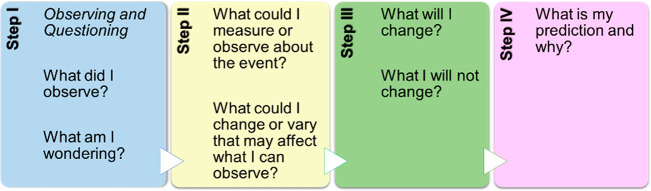
Students learn best if they are engaged in active learning; if they are motivated to deal with observations and concepts before the introduction of scientific concepts, jargon, and facts; and if they have the sense that they are part of a community of learners in a classroom environment that is very supportive of their learning.1−3 The inclusion of classroom activities that involve higher-order thinking skills4 and the use of metacognitive tools that encourage students to reflect on their observations are suggested by the National Science Standards (1996) guidelines.5 As such, successful demonstrations and discovery laboratories can pique students’ curiosity, enhance their understanding of scientific concepts, and motivate them when coupled with active learning practices in science. In this context, Rees et al.’s6 use of interactive diagrams following a demonstration prompting students to ask open-ended questions vital for scientific inquiry was followed. Coupled with Vygotski’s constructivist approach7 and Rees et al.’s6 Steps to Inquiry framework model,8 our students were scaffolded when answering questions in groups (Figure 1) such as “What did I observe?, How does this mechanism work?, What caused the observed change?, What components are responsible for what I am observing?, What are the advantages of such a mechanism?, What are potential applications?, What are the implications of such an approach?” before they conducted their own investigations (Supporting Information).
Figure 1.
Interactive diagram to facilitate our students’ inquiry with defined steps.
Particularly in chemistry, experiments with color and texture changes, odors, temperature changes, and more are often used to demonstrate the chemical principles involved after making sure that students are fascinated by what they observe.9−11 A chemiluminescent reaction is a typical chemical reaction with a “wow factor”12 due to its light-emitting product; thus, it is often employed for an interesting and fascinating lecture or for school demonstrations.13 In the course of the thermal transformation of reactants to products, bio- and chemiluminescence can occur when the chemical reaction energy of a so-called high-energy intermediate can be transformed into electronic excitation energy of the product, a process called chemiexcitation, in the appropriate energy range for visible light generation. The decay of this excited state to a lower energy level causes light emission. In chemiluminescence, this high-energy intermediate is normally man-made.14 Most chemiluminescence probes emit light which triggers student curiosity and urges them to pose questions in their attempt to make connections regarding this mechanism.
In fact, the reaction starts by the oxidation of the probe, by an oxidizing agent, into an unstable peroxide, bringing this light-emission material into its excited state; it then rapidly decays to the ground state with emitting light.15 Luminol is one of the most famous chemiluminescent chemicals (Figure 2).16 It exhibits chemiluminescence, with a blue glow, when mixed with an appropriate oxidizing agent. To exhibit its luminescence, luminol is activated with an oxidant, which is usually a solution of hydrogen peroxide in a basic environment (pH > 7). In the presence of a catalyst such as an iron, copper, or periodate compound, the hydrogen peroxide decomposes to form a superoxide anion and water. Upon reacting with the hydroxide due to its basic environment, luminol then forms a dianion whereas the superoxide produced from the hydrogen peroxide reacts with the luminol dianion.17,18 The product of this reaction, a peroxide, is very unstable and immediately decomposes with the loss of nitrogen to produce 5-aminophthalic acid with electrons in an excited state.19 As the excited state relaxes to the ground state, energy is liberated as a photon which is visible as blue light. The glow visibility lasts from seconds up to hours depending on the reaction conditions.20,21
Figure 2.
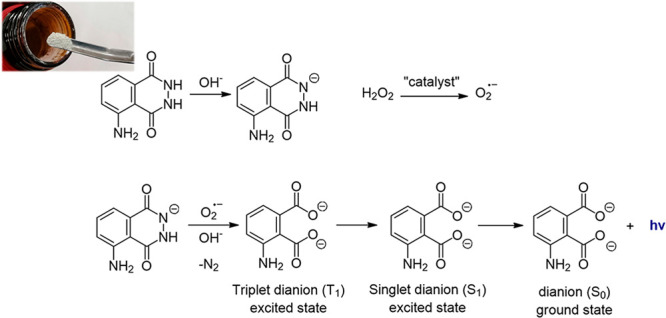
Activation of luminol (pale white powder; upper photo) with an oxidizing agent (hydrogen peroxide) in a basic environment in the presence of a catalyst. The excess energy is liberated as a photon that is visible as blue light.
Multicomponent reactions (MCRs) can be a very useful alternative to sequential, time-consuming multistep synthesis22 as they constitute one-pot reactions employing three or more starting materials, where most of the atoms of the starting materials are incorporated in the final product regardless the mechanisms involved.23,24 Several descriptive tags are regularly attached to MCRs (Figure 3) such as atom economy (the majority if not all of the atoms of the starting materials are incorporated in the product), efficiency (the product is formed in only one-step instead of multiple sequential steps), convergence, and sustainability. Due to their unique properties, we propose herein that MCRs can play an important role in the chemical education of junior or senior undergraduate students due to their ease to performance (no special equipment or experimental conditions such as inert atmosphere, dry solvents, or catalysts are needed), readily available building blocks, and demonstration of the principles of green chemistry.25−27 By applying deductive logic to the structure and outcomes of objective questions, students attempt to define multicomponent reactions and become witnesses of an alternative “way of thinking” which adopts a much more convergent approach to synthetic problems, rather than the classic, lengthy synthesis. This is evident in the student’s immediate response: “How is this happening?” (See interactive diagram in Supporting Information.)
Figure 3.
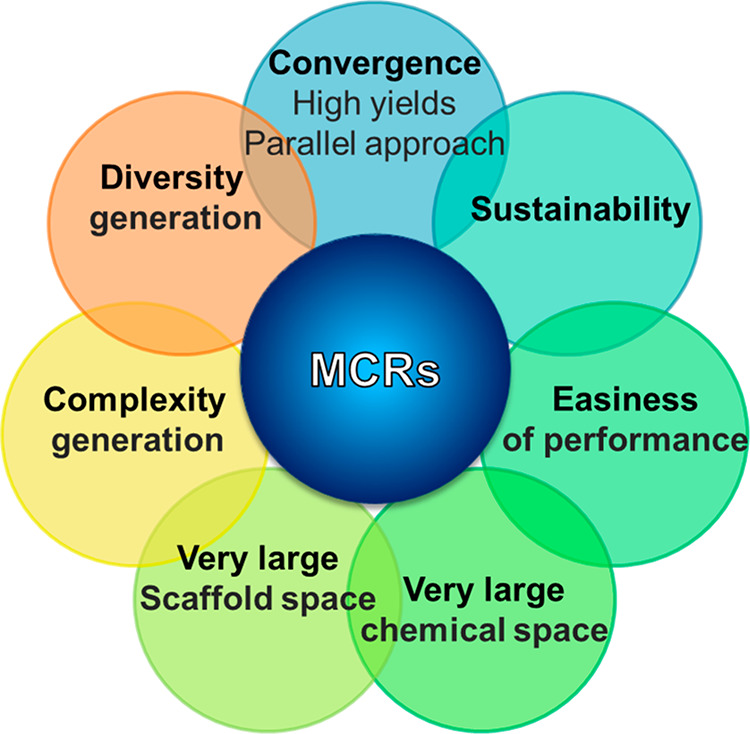
Basic characteristics of modern multicomponent reactions (MCRs).
Results and Discussion
Purpose of the Demonstration: A Student-Based Oriented Teaching
Following a spectacular reaction, the introduction to the often neglected MCR world created an unforgettable and meaningful experience for students. The objective of this exercise was to scaffold students so as to notice that, after incorporating luminol into a multicomponent reaction, they could visualize the assembly of the different building blocks before they could finally exploit the chemiluminescent properties of the new derivative. Upon experiencing for themselves the characteristic glow after the addition of the oxidative reagent,18,28 student interest was stimulated leading to the onset of a very fruitful discussion for the principles of chemiluminescence and MCRs (e.g., check the students answers to the following questions: “What did I observe?” “What could I measure or observe about the event?” “What could I change or vary that may affect what I can observe?” See the interactive diagram, steps I and II, SI).
Specific details regarding evidence of the teaching method and therefore evidence of learning might not seem initially evident in our approach. However, this is understandable as we followed an open inquiry approach following the demonstration. After watching the demonstration, a vast majority of our students were eager to ask questions that teachers would take note of but not directly answer. Following the key principles of open inquiry, teachers encouraged more questions, touching upon how we can acquire measurable and falsifiable data, dependent and independent variables, research methods, validity, and reliability. Then, they would ask students to make hypotheses as to how what was demonstrated worked. Being placed in the center of and taking ownership of their own learning, students were encouraged to work out how this works in a self-directed experimental sense following the demonstration. Challenging and active learning experimental environments do not entail following instructions recipe-wise. As such, teachers facilitated student pair work in the process of elucidating how this reaction works, in most cases by trial and error. This approach also required students to be resilient to failure and realize that there are dependent and independent variables in science that students can control or modify until they reach the desired outcome. A common practice among teachers, in our case, was to frame the questions and make students aware of the many variables that may be responsible for the final effect demonstrated. Teachers elicited the questions but did not answer them. They used common IBL (inquiry-based learning) tools like concept mapping in order to make the research process stages more visible to students. Visualization of experimental process, data, and progress is of paramount importance at this stage.6−8 Teachers only gave emphasis to inquiry skills following an inductive approach tailored to each student’s knowledge gaps, queries, and interests. They scaffolded the learning involved trying to hone students’ higher-order skills like creativity and analysis. Following such a student-centered approach, teachers were not always able to include more details as they attempted only to calibrate individual students’ needs by asking more open-ended questions and providing scaffolding in the sense of online resources.
Although many papers have covered chemiluminescence in the past, via luminol, mostly having luminol as the attention center,17,29−33 here we have a strong focus on the potential of multicomponent reactions in the synthesis of compounds of interest so as to render the MCR merits such as ease of performance, complexity, extreme diversity in a single step, sustainability, visibility, and testability. In the MCR world, students were encouraged to employ a different mentality in order to approach a synthetic problem and evaluate the “green-ness” of chemical processes. With the use of chemluminesence of the final adduct, students could visualize the assembly of four different building blocks to a unique, more complex, final compound and discussed the benefits of MCRs. In this context and although students did not measure per se the difference of chemiluminesence between luminol and the final product, the main pedagogical outcome of our work was achieved as students were actively engaged in the evaluation of the demonstrated mechanism within a working framework of viable green chemistry applications that aim to synthesize molecules with specific properties in order to solve current or future problems. In fact, the assembly of four different moieties with at least two points of diversification (see reaction 1) into one compound with chemiluminiscent properties can easily create enormous libraries that could be screened in a high-throughput fashion when compared to typical or no medicinal chemistry targets. These compounds can be utilized not only in biology, medicine, and imaging but also in material science, microwave irradiation, and green chemistry. All these important and interdisciplinary features were addressed with this demonstration experiment.
The Demonstration Experiment
Synthesis
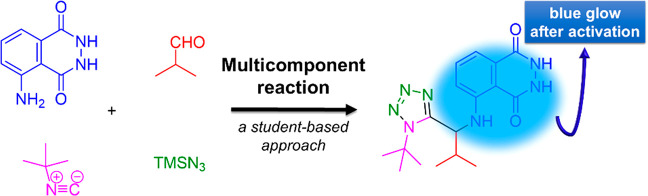
The luminol compound acts as the amine component in the Ugi tetrazole four component reaction (UT-4CR). This is a variant of the classical Ugi reaction with a great variability of building blocks and tolerability of orthogonal functional groups and is very easy to perform (reaction 1).34 Tetrazole derivatives are a prime class of heterocycles; they are very important in medicinal chemistry and drug design in which MCR chemistry offers convergent access to multiple of those scaffolds providing the three important elements of novelty, diversity, and complexity. After testing their own initial hypotheses regarding what other compounds luminοl (1) could react with (see SI), students realize that 1 can react with an aldehyde (2), isocyanide (3), and trimethylsilyl azide (4) in order to yield compound 5 which incorporates a tetrazole moiety along with luminol.
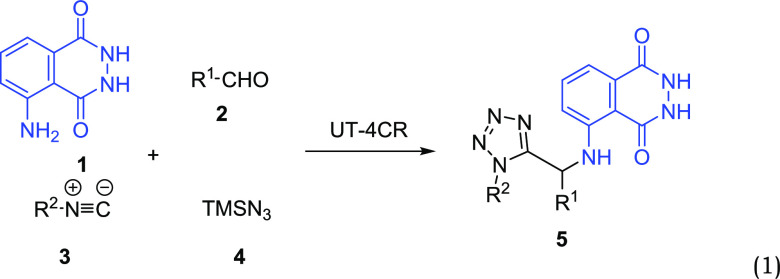 |
1 |
Indeed, in a very facile setup, we have reacted, under microwave conditions (see Experimental Section for more details), isobutyraldehyde, tert-butyl isocyanide, trimethylsilyl azide, and the luminol in methanol as solvent, affording the targeted compound 5 as foreseen (reaction 2). Compound 5 is a gray solid, very easy to transfer, weigh, and store (Figure 4).
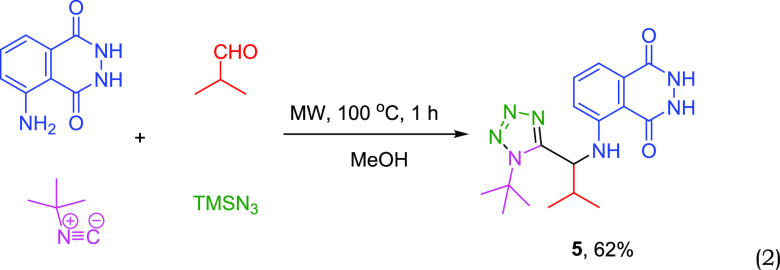 |
2 |
Figure 4.
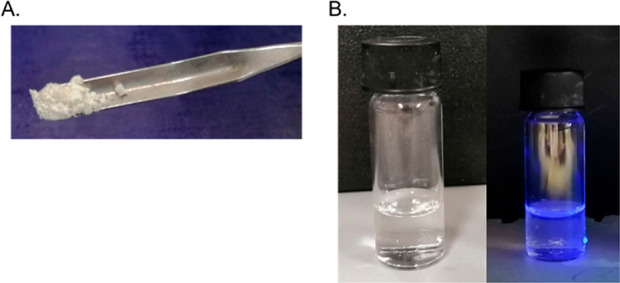
(A) Tetrazole adduct 5 as gray solid. (B) Solution of compound 5 in MeOH (left), with same solution under UV light at 366 nm (right).
Chemiluminescence
In order to carry out the demonstration, we proceed to its final and conclusive part. Therefore, we mixed novel compound 5 with an oxidizing agent such as hydrogen peroxide under the appropriate conditions (reaction 3, see Experimental Section and SI for more details).35 Then, the characteristic blue glow gradually appears and lasts about 40 s. Interestingly, students immediately notice the color change while being in a dark room. The effect is very strong and characteristic/typical of the reaction (Figures 5 and 6).
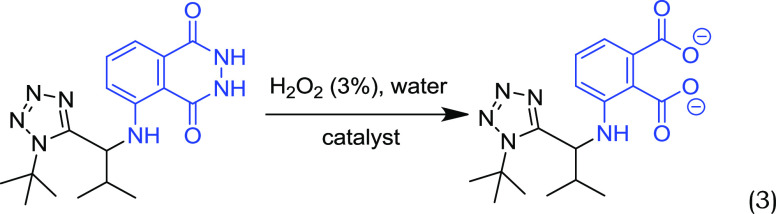 |
3 |
Figure 5.
Slow addition of the solution of the hydrogen peroxide to the aqueous basic solution of compound 5. The characteristic blue glow is gradually produced.
Figure 6.
Students having fun in the dark while they are learning the basic concepts of chemluminesence through the novel luminol-tetrazole derivative. They are slowly adding the solution of hydrogen peroxide with a Pasteur pipet and observe the phenomenon.
Following the observations and results of this demonstration, students not only experienced and reflected on fundamental principles of organic chemistry and photochemistry but also discussed and evaluated a modern approach of how to synthesize a functionalized molecule. (For example, check the student replies to the following questions: “What will I change?” “What I will not change?” “What is my prediction and why?” See the interactive diagram, steps III and IV, in SI.) As such, students with hands-on real synthesis of an unprecedented compound (such as compound 5) became more familiar with a series of principles such as microwave irradiation, basic organic synthesis, and green and sustainable chemistry. Moreover, they reported that they were inspired and stimulated, having as a driving force their own curiosity (Figure S2, Supporting Information). In this context, we support that chemistry teaching can be considerably more effective when it involves reflection and evaluation of the methods employed by scientists exploring a reaction or properties of compounds rather than a dry conversion of symbols or numbers. The above demonstration could be performed either by the tutor or students themselves depending on the presence of basic laboratory safety skills and student competences.
This demonstration can have further pedagogical applications depending on the content knowledge of university students. When we piloted this experiment with undergraduate students, we noticed the implications of incorporating four different compounds (all commercially available) into a new, complex molecule that had never been described in the literature. Not surprisingly, students reported that they found that novelty factor fascinating. At a more advanced level (college or graduate students), this example could also serve as an NMR exercise by correctly assigning the peaks (see SI). In the same vein, students might not always make accurate observations or explanations, but through active engagement3 they become familiar with the principles of microwave-assisted organic synthesis.36−38 Last but not least, compound 5 could be developed as an imaging probe or utilized in material sciences.
Hazards
Before the commencement of any chemical demonstration, it is advisible to review the American Chemical Society Safety Guidelines for Chemical Demonstrations.39 Standard safety procedures should be followed; when handling the chemicals and preparing the solutions, chemical splash goggles, nitrile gloves, and proper lab attire should be worn. All reagents and solvents have to be stored in a well-ventilated place and be kept cool. In addition, contact of chemicals with skin and eyes should be avoided. Luminol is not a hazardous substance and does not contain hazardous ingredients (Article 2 REACH Regulation (EC) 1907/2006). Isobutyraldehyde, trimethylsilyl azide, and tert-butyl isocyanide are highly flammable. All sources of ignition (heat, spark, open flames, and hot surfaces) should be kept away. tert-Butyl isocyanide has a strong smell and should be handled in a suitable fume hood. Although the scale of the proposed experiments is safe and convenient to work with, in terms of the smell, it is proposed to keep an open beaker or flask with 25–50 mL of 1 M HCl or 1 M NaOCl inside the fume hood to extinguish all the vapors. Additionally, but not necessarily all the glassware could be washed with a solution of 1 M HCl or 1 M NaOCl after usage to hydrolyze the isocyanide residues. Important information on the SDS files is included in the SI.
Experimental Section
General Methods
All the reagents and solvents were purchased from Sigma-Aldrich, AK Scientific, Fluorochem, Abcr GmbH, and Acros and were used without further purification. All microwave irradiation reactions were carried out in a Biotage Initiator microwave synthesizer. Thin layer chromatography was performed on Millipore precoated silica gel plates (0.20 mm thick, particle size 25 μm). Nuclear magnetic resonance spectra were recorded on Bruker Avance 500 spectrometers {1H NMR (500 MHz), 13C NMR (126 MHz)}. Chemical shifts for 1H NMR were reported as δ values, and coupling constants were in hertz (Hz). The following abbreviations were used for spin multiplicity: s = singlet, br s = broad singlet, d = doublet, t = triplet, q = quartet, quin = quintet, dd = double of doublets, ddd = double doublet of doublets, m = multiplet. Chemical shifts for 13C NMR were reported in ppm relative to the solvent peak.
Synthesis of the 5-((1-(1-(tert-Butyl)-1H-tetrazol-5-yl)-2-methylpropyl)amino)-2,3-dihydrophthalazine-1,4-dione (5)
Luminol (0.177 g, 1.0 mmol) dissolved in methanol (1 mL), isobutyraldehyde (0.09 mL, 1.0 mmol), tert-butyl isocyanide (0.12 mL, 1.1 mmol), and trimethylsilyl azide (0.14 mL, 1.1 mmol) were added into a 5 mL microwave vial. The solution was irradiated in a microwave oven for 1 h at 100 °C. After completion of the reaction (TLC control), the residue was dried under reduced pressure via rotary evaporation and was purified using flash chromatography (dichloromethane–methanol, 4:1) to afford the desired product as a gray solid in 62% yield.
Chemiluminescence of the Tetrazole Derivative 5
To a stirred solution of the previously synthesized compound 5 (0.357 g, 1 mmol) in deionized water (1 L) in an Erlenmeyer flask, Na2CO3 (4.0 g), NaHCO3 (24.0 g), (NH4)2CO3 (0.5 g), and CuSO4·5H2O (0.4 g) were added and dissolved under stirring. In another flask, a solution of 3% H2O2 (50 mL) in deionized water (950 mL, total volume 1 L) was prepared. Mixing of the two solutions gives the characteristic blue glow. The novel luminol-tetrazole derivative was visibly glowing for approximately 40 s.
Conclusions
Inquiry, reflection, and critical evaluation processes are deemed essential skills prior to researchers creating new knowledge and subjecting it to peer review, before they can make adaptations and apply it in new settings. This demonstration combined with Rees et al.’s6 reflection prompts can provide a model for how college or school teaching can likewise engage students in active learning. Chemists ask questions, and they search for answers by gathering, collating, and interpreting data, sharing proposed explanations and solutions, and then trying these new proposals out in different contexts. This may raise new questions, and so the process continues in a cyclic fashion. Here, we take advantage of all the vital components of scientific inquiry upon demonstrating the synthesis and properties of a novel chemiluminescent compound by a multicomponent assembly process.
Acknowledgments
This research has been supported (A.D.) by the National Institute of Health (NIH) (2R01GM097082-05), the European Lead Factory (IMI) under Grant 115489, and the Qatar National Research Foundation (NPRP6-065-3-012). Moreover, funding was received through ITN “Accelerated Early stage drug dIScovery” (AEGIS, Grant 675555) and COFUND ALERT (Grant 665250), Hartstichting (ESCAPE-HF, 2018B012), and a KWF Kankerbestrijding grant (Grant 10504). We would like also to thank Markella Konstantinidou for her help in spectra recording.
Supporting Information Available
The Supporting Information is available at https://pubs.acs.org/doi/10.1021/acs.jchemed.0c00290.
Author Present Address
∥ Alzheimer’s Research UK Oxford Drug Discovery Institute, University of Oxford, NDM Research Building, Old Road Campus, Roosevelt Drive, Oxford OX3 7FZ, UK (T.Z.-T.)
Author Contributions
§ C.G.N. and T.Z.-T. contributed equally.
The authors declare no competing financial interest.
Supplementary Material
References
- Bybee R.; Taylor J.; Gardner A.; Scotter P.; Carlson J.; Westbrook A.; Landes N. The BSCS 5E Instructional Model: Origins, Effectiveness, and Applications. BSCS 2006, 5, 88–98. [Google Scholar]
- Brown A. L.; Campione J. C.. Guided Discovery in a Community of Learners. Classroom Lessons: Integrating Cognitive Theory and Classroom Practice; The MIT Press: Cambridge, MA, 1994; pp 229–270. [Google Scholar]
- Freeman S.; Eddy S. L.; McDonough M.; Smith M. K.; Okoroafor N.; Jordt H.; Wenderoth M. P. Active Learning Increases Student Performance in Science, Engineering, and Mathematics. Proc. Natl. Acad. Sci. U. S. A. 2014, 111 (23), 8410–8415. 10.1073/pnas.1319030111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krathwohl D. R. A. Revision of Bloom’s Taxonomy: An Overview. Theory Pract. 2002, 41 (4), 212–218. 10.1207/s15430421tip4104_2. [DOI] [Google Scholar]
- Shea J. H. National Science Education Standards. J. Geol. Educ. 1995, 43 (2), 102–102. 10.5408/0022-1368-43.2.102. [DOI] [Google Scholar]
- Rees C.; Pardo R.; Parker J. Steps to Opening Scientific Inquiry: Pre-Service Teachers’ Practicum Experiences with a New Support Framework. J. Sci. Teacher Educ. 2013, 24 (3), 475–496. 10.1007/s10972-012-9315-y. [DOI] [Google Scholar]
- Vygotsky L. S.Mind in Society: Development of Higher Psychological Processes; Cole M., Jolm-Steiner V., Scribner S., Souberman E., Eds.; Harvard University Press, 1980. 10.2307/j.ctvjf9vz4. [DOI] [Google Scholar]
- Youth Science Canada. Steps to Inquiry interactive posters. https://smarterscience.youthscience.ca/posters-and-pdfs.
- Orna M. V.Sputnik to Smartphones: A Half-Century of Chemistry Education; Orna M. V., Ed.; ACS Symposium Series; American Chemical Society, Washington, DC, 2015; Vol. 1208. 10.1021/bk-2015-1208. [DOI] [Google Scholar]
- Muzyka J. L. Visualization Tools for Organic Chemistry. J. Chem. Educ. 2009, 86 (2), 254. 10.1021/ed086p254. [DOI] [Google Scholar]
- Jones L. L.; Jordan K. D.; Stillings N. A. Molecular Visualization in Chemistry Education: The Role of Multidisciplinary Collaboration. Chem. Educ. Res. Pract. 2005, 6 (3), 136–149. 10.1039/B5RP90005K. [DOI] [Google Scholar]
- Bornstein M. H.; Gardner H. Frames of Mind: The Theory of Multiple Intelligences. J. Aesthetic Educ. 1986, 20 (2), 120. 10.2307/3332707. [DOI] [Google Scholar]
- Makishima A.Topics of Bioluminescence and Chemoluminescence. In Biochemistry for Materials Science; Elsevier: New York, 2019; pp 77–83. 10.1016/B978-0-12-817054-0.00005-9. [DOI] [Google Scholar]
- Adam W.; Cilento G.. Chemical and Biological Generation of Excited States; Elsevier: San Diego, 1982. 10.1016/B978-0-12-044080-1.X5001-9. [DOI] [Google Scholar]
- Rauhut M. M. Chemiluminescence from Concerted Peroxide Decomposition Reactions. Acc. Chem. Res. 1969, 2 (3), 80–87. 10.1021/ar50015a003. [DOI] [Google Scholar]
- Khan P.; Idrees D.; Moxley M. A.; Corbett J. A.; Ahmad F.; Von Figura G.; Sly W. S.; Waheed A.; Hassan M. I. Luminol-Based Chemiluminescent Signals: Clinical and Non-Clinical Application and Future Uses. Appl. Biochem. Biotechnol. 2014, 173, 333. 10.1007/s12010-014-0850-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panzarasa G. Shining Light on Nanochemistry Using Silver Nanoparticle-Enhanced Luminol Chemiluminescence. J. Chem. Educ. 2014, 91 (5), 696–700. 10.1021/ed400736k. [DOI] [Google Scholar]
- Krawczyk T.; Słupska R.; Baj S. Applications of Chemiluminescence in the Teaching of Experimental Design. J. Chem. Educ. 2015, 92 (2), 317–321. 10.1021/ed5002587. [DOI] [Google Scholar]
- Merenyi G.; Lind J. S. Role of a Peroxide Intermediate in the Chemiluminescence of Luminol. A Mechanistic Study. J. Am. Chem. Soc. 1980, 102 (18), 5830–5835. 10.1021/ja00538a022. [DOI] [Google Scholar]
- Huntress E.; Stanley L.; Parker A. The Preparation of 3-Aminophthalhydrazide for Use in the Demonstration of Chemiluminescence. J. Am. Chem. Soc. 1934, 56 (1), 241–242. 10.1021/ja01316a077. [DOI] [Google Scholar]
- Huntress E. H.; Stanley L. N.; Parker A. S. The Oxidation of 3-Aminophthalhydrazide (“luminol”) as a Lecture Demonstration of Chemiluminescence. J. Chem. Educ. 1934, 11 (3), 142–145. 10.1021/ed011p142. [DOI] [Google Scholar]
- Zarganes-Tzitzikas T.; Chandgude A. L.; Dömling A. Multicomponent Reactions, Union of MCRs and Beyond. Chem. Rec. 2015, 15 (5), 981–996. 10.1002/tcr.201500201. [DOI] [PubMed] [Google Scholar]
- Dömling A. Recent Developments in Isocyanide Based Multicomponent Reactions in Applied Chemistry. Chem. Rev. 2006, 106 (1), 17–89. 10.1021/cr0505728. [DOI] [PubMed] [Google Scholar]
- Dömling A.; Wang W.; Wang K. Chemistry and Biology Of Multicomponent Reactions. Chem. Rev. 2012, 112 (6), 3083–3135. 10.1021/cr100233r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafin M.; Priest O. P. Identifying Passerini Products Using a Green, Guided-Inquiry, Collaborative Approach Combined with Spectroscopic Lab Techniques. J. Chem. Educ. 2015, 92 (3), 579–581. 10.1021/ed5007184. [DOI] [Google Scholar]
- Hooper M. M.; DeBoef B. A. Green Multicomponent Reaction for the Organic Chemistry Laboratory. The Aqueous Passerini Reaction. J. Chem. Educ. 2009, 86 (9), 1077–1079. 10.1021/ed086p1077. [DOI] [Google Scholar]
- Marcos C. F.; Bossio R.; Marcaccini S.; Pepino R. Multicomponent Reactions: A Convenient Undergraduate Organic Chemistry Experiment. J. Chem. Educ. 2000, 77 (3), 382–384. 10.1021/ed077p382. [DOI] [Google Scholar]
- O’Hara P. B.; St. Peter W.; Engelson C. Turning on the Light: Lessons from Luminescence. J. Chem. Educ. 2005, 82 (1), 49. 10.1021/ed082p49. [DOI] [Google Scholar]
- Merényi G.; Lind J.; Eriksen T. E. Luminol Chemiluminescence: Chemistry, Excitation, Emitter. J. Biolumin. Chemilumin. 1990, 5 (1), 53–56. 10.1002/bio.1170050111. [DOI] [PubMed] [Google Scholar]
- Chalmers J. H.; Bradbury M. W.; Fabricant J. D. A. Multicolored Luminol-Based Chemiluminescence Demonstration. J. Chem. Educ. 1987, 64 (11), 969. 10.1021/ed064p969.1. [DOI] [Google Scholar]
- Burke B. A.; Golestaneh K.; Samson H. Luminosity, My Dear Watson, Luminosity! - Or, Are Those Bloodstains?. J. Chem. Educ. 1999, 76 (1), 65. 10.1021/ed076p65. [DOI] [Google Scholar]
- Thomas N. C.; Faulk S. Colorful Chemical Fountains. J. Chem. Educ. 2008, 85 (8), 1061. 10.1021/ed085p1061. [DOI] [Google Scholar]
- Ibanez J. G.; Zavala-Araiza D.; Sotomayor-Martinez Barranco B.; Torres-Perez J.; Camacho-Zuniga C.; Bohrmann-Linde C.; Tausch M. W. A. Demonstration of Simultaneous Electrochemiluminescence. J. Chem. Educ. 2013, 90 (4), 470–472. 10.1021/ed300701a. [DOI] [Google Scholar]
- Neochoritis C. G.; Zhao T.; Dömling A. Tetrazoles via Multicomponent Reactions. Chem. Rev. 2019, 119 (3), 1970–2042. 10.1021/acs.chemrev.8b00564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakhashiri B. Z.Chemical Demonstrations: A Handbook for Teachers of Chemistry; The University of Wisconsin Press: Madison, WI, 2011; Vol. 5. [Google Scholar]
- Gawande M. B.; Shelke S. N.; Zboril R.; Varma R. S. Microwave-Assisted Chemistry: Synthetic Applications for Rapid Assembly of Nanomaterials and Organics. Acc. Chem. Res. 2014, 47 (4), 1338–1348. 10.1021/ar400309b. [DOI] [PubMed] [Google Scholar]
- Kappe C. O. Controlled Microwave Heating in Modern Organic Synthesis. Angew. Chem., Int. Ed. 2004, 43 (46), 6250–6284. 10.1002/anie.200400655. [DOI] [PubMed] [Google Scholar]
- Sharma N.; Sharma U. K.; Van der Eycken E. V. Microwave-Assisted Organic Synthesis: Overview of Recent Applications. Green Techniques for Organic Synthesis and Medicinal Chemistry; January 22, 2018; pp 441–468. 10.1002/9781119288152.ch17. [DOI] [Google Scholar]
- ACS Safety Guidelines for Chemical Demonstrations . https://www.acs.org/content/dam/acsorg/education/policies/safety/divched_2018_safetyflyer2pager_proof1.pdf (accessed November 18, 2019)..
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.