Abstract
In the United States adult T cell lymphoma–leukemia (ATLL) carries a dismal prognosis and mainly affects immigrants from human T cell lymphotropic virus 1 endemic areas. Allogeneic hematopoietic stem cell transplant (alloHSCT) can be effective and is recommended as an upfront treatment in the National Comprehensive Cancer Network guidelines. We studied the barriers to alloHSCT in 1 of the largest ATLL populations in the United States. Comprehensive chart and donor registry reviews were conducted for 88 ATLL patients treated at Montefiore Medical Center from 2003 to 2018. Among 49 patients with acute and 32 with lymphomatous subtypes, 48 (59.5%) were ineligible for alloHSCT because of early mortality (52%), loss to follow-up (21%), uninsured status (15%), patient declination (10%), and frailty (2%). Among 28 HLA-typed eligible patients (34.6%) matched related donors were identified for 7 (25%). A matched unrelated donor (MUD) search yielded HLA-matched in 2 patients (9.5%), HLA mismatched in 6 (28.5%), and no options in 13 (62%). Haploidentical donors were identified for 6 patients (46%) with no unrelated options. There were no suitable donors for 7 (25%) alloHSCT-eligible patients. The main limitation for alloHSCT after donor identification was death from progressive disease (82%). AlloHSCT was performed in 10 patients (12.3%) and was associated with better relapse-free survival (26 versus 11 months, P = .04) and overall survival (47 versus 10 months, P = .03). Early mortality and progressive disease are the main barriers to alloHSCT, but poor follow-up, uninsured status, and lack of suitable donor, including haploidentical, are also substantial limitations that might disproportionally affect this vulnerable population. AlloHSCT can achieve long-term remissions, and strategies aiming to overcome these barriers are urgently needed to improve outcomes in ATLL.
Keywords: Allogeneic hematopoietic stem cell transplant, Adult T cell, lymphoma-leukemia, Minority ethnicity donors
INTRODUCTION
Adult T cell lymphoma–leukemia (ATLL) is a rare mature peripheral T cell lymphoma caused by human T cell lymphotropic virus 1 (HTLV-1) [1]. It is highly resistant to chemotherapy and carries a dismal prognosis despite aggressive cytotoxic and antiviral treatment because of low rates of response, high rates of relapse, and no long-term efficacy of currently available treatments [2–4]. Clinical presentation is variable, and 4 subtypes are recognized [5]. Patients with the most common and aggressive variants, acute and lymphomatous, have a median overall survival (OS) of 6 to 12 months [5,6], whereas those with the more indolent forms, chronic and smoldering, can transform into aggressive disease and have 4-year OS rates of 36% and 52%, respectively [4].
ATLL is prevalent in HTLV-1 endemic regions, including Japan, the Caribbean, South America, West Africa, and the Middle East, but rare in Western countries [7,8]. The published experience on ATLL is largely limited to series out of Japan where it comprises up to 25% of T cell lymphomas [1,7]. Nonetheless, several cases are recognized in the United States every year, predominantly affecting Afro-Caribbean and South American immigrant populations [9–12]. Additionally, these patients present with more aggressive clinical features and have worse outcomes compared with those reported in Japanese ATLL cohorts and may represent a distinct clinical entity [9,10].
Allogeneic hematopoietic stem cell transplant (alloHSCT) is a promising treatment option with an increasing role in the management of ATLL [7]. The National Comprehensive Cancer Network guidelines recommend alloHSCT after 2 cycles of chemotherapy, but the obstacles to alloHSCT in the US population are under-recognized [13]. Most data favoring alloHSCT over chemotherapy alone originate from retrospective analyses, are limited by selection bias, and show a relatively high transplant-related mortality (TRM) [3,6]. Nevertheless, alloHSCT is the only treatment modality that can achieve long-term remissions or cure, especially for aggressive subtypes, and has demonstrated potential survival advantage [14,15]. Hence, upfront alloHSCT has become the preferred strategy in acute and lymphomatous subtype patients who are transplant-eligible [16,17].
Unfortunately, ATLL series in the United States demonstrates that only a small fraction of patients receive alloHSCT [9,10]. Because of the aggressive characteristic of ATLL seen in US patients, an alloHSCT in first complete remission (CR) is appropriate, and maximizing access to transplant is desirable. Nonetheless, the reasons for preventing alloHSCT for ATLL have not been carefully studied and reported. We sought to identify the barriers to alloHSCT in a tertiary care center in the Bronx with 1 of the largest ATLL populations in the United States.
METHODS
We identified all patients diagnosed with ATLL who were treated between 2003 and 2018 at Montefiore Medical Center in the Bronx, New York. The diagnosis of ATLL was confirmed based on pathologic findings and HTLV-1 antibody positivity. ATLL subtype classification was based on the Shimoyama criteria [5]. Patient demographics, treatment records, and clinical data were obtained from a comprehensive manual review of the electronic medical records. Related, including haploidentical, and unrelated donor registry search results from the US National Marrow Donor Program were reviewed for all eligible patients who were HLA typed.
Demographic, clinical, and donor option results were summarized using descriptive statistics and analyzed with nonparametric statistics. Relapse-free survival (RFS) was the time from treatment initiation to relapse, last follow-up, or death. OS was the time from diagnosis to death or last follow-up. TRM was defined as death from transplant complications without relapse. The Kaplan-Meier method was used to compare survival, and statistical significance was examined with the log-rank test. All analyses were performed on IBM SPSS version 20.0.
RESULTS
We identified 88 patients, most of whom had aggressive ATLL subtypes, including 49 (55.7%) acute and 32 (36.4%) lymphomatous cases. Median age at presentation was 56 years (range, 28 to 87), and there was a female predominance (61.4%). The cohort largely included patients of Caribbean origin (84%); no patients of Asian descent were found. Almost all patients were immigrants born outside the United States (96.6%). Table 1 summarizes patient characteristics.
Table 1.
Patient Baseline Characteristics
| Characteristic | Value |
|---|---|
| Age at diagnosis | |
| ≤40 yr | 15 (17%) |
| 41–60 yr | 41 (46.6%) |
| ≥61 yr | 32 (36.4%) |
| Gender | |
| Male | 34 (38.6%) |
| Female | 54 (61.4%) |
| Geographic origin | |
| American | 80 (91%) |
| Caribbean | 74 (84%) |
| Central/South America | 6 (7%) |
| Africa | 8 (9%) |
| ATLL subtype | |
| Acute | 49 (55.7%) |
| Lymphomatous | 32 (36.4%) |
| Chronic | 4 (4.5%) |
| Smoldering | 3 (3.4%) |
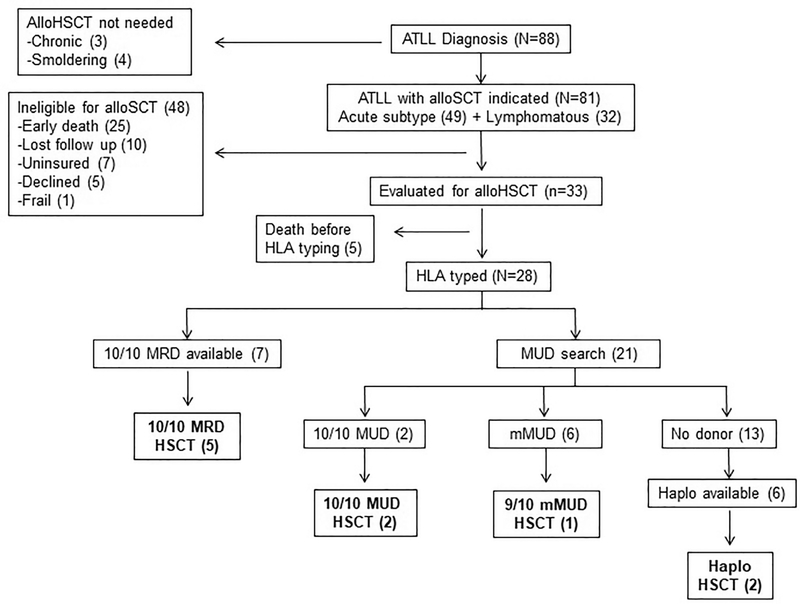
Most patients diagnosed with aggressive (acute or lymphomatous) subtypes (n = 81) were potential candidates for an alloHSCT treatment strategy at first CR; however, 48 (59.3%) were rendered ineligible for transplant. In these patients the most common reason for not considering alloHSCT was early death within 1 month of diagnosis date (n = 25, 52%). Other reasons included poor compliance with medical care and/or lost to follow-up during initial chemotherapy (n = 10, 21%), lack of health insurance that affords access to transplant (n = 7, 15%), patient personal preference and declination (n = 5, 10%), and poor performance status or frailty (n = 1, 2%). Uninsured patients received high-dose induction and maintenance chemotherapy at similar rates as their insured counterparts (78% versus 84%, P = .593).
Comprehensive evaluation for alloHSCT was started in 33 patients, but 5 (15%) experienced early progression of disease and died from medical complications before completing proper workups. Of 28 alloHSCT-eligible patients who completed HLA typing and pretransplant testing, a 10/10 matched related donor (MRD) was identified in 7 (25%). In the remaining 21 patients the best available unrelated option in the National Marrow Donor Program registry search was a 10/10 matched unrelated donor (MUD) for 2 (9.5%), a 9/10 mismatched unrelated donor (mMUD) for 4 (19%), and an 8/10 mMUD for 2 patients (9.5%).
No unrelated donor option was identified for most patients for whom a registry search was started (n = 13, 62%). In this group a potential half-matched first-degree relative who met all criteria to serve as a haploidentical donor was identified for only 6 patients (46%). After accounting for all potential donor sources in our center (which excludes umbilical cord blood), no suitable donor option of any kind was available for 7 of 28 (25%) alloHSCT-eligible HLA-typed patients who completed all pretransplant testing.
AlloHSCT was performed in 10 patients, representing 12.3% of all ATLL cases and 35.7% of the alloHSCT-eligible HLA-typed group. The reasons for preventing alloHSCT in 18 eligible patients who had completed all pretransplant requisites were patient preference or declination (n = 2, 11%), lack of an available suitable donor (n = 7, 39%), and disease progression or relapse leading to death (n = 9, 50%). For most patients in the latter group (n = 8, 89%), the best available donor option was mMUD or haploidentical, which precluded consideration for salvage alloHSCT. Figure 1 summarizes the donor search and alloHSCT experience.
Figure 1.
Summary of donor search and alloHSCT experience.
Survival analysis in the transplant-eligible subgroup (n = 28) showed that alloHSCT was associated with superior outcomes. Median RFS was 26 months in patients treated with alloHSCT and 10 months in transplant-eligible patients who did not proceed to alloHSCT (P = .04). Similarly, median OS was 47 months in alloHSCT patients and 11 months in those who did not proceed to transplant (P = .03). After alloHSCT, 5 patients (50%) achieved CR and were in remission at the time of this report, including 3 patients with long-term remissions lasting over 12 months. Patients transplanted in CR with a fully matched donor (MRD or MUD) had better outcomes and constitute 80% of patients who remain alive.
TRM occurred in 4 patients (40%), all early within 100 days of transplant. Acute grade IV graft-versus-host-disease was diagnosed and contributed to mortality in 2 patients. The other 2 patients died of infectious complications. Severe disease-free marrow aplasia was confirmed in 1 patient, whereas early relapse could not be ruled out in the other. Table 2 summarizes the characteristics and outcomes of all transplanted patients.
Table 2.
Summary of Characteristics and Outcomes of All Transplanted Patients
| Patient No. | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Age, yr | 43 | 51 | 42 | 38 | 42 | 74 | 40 | 65 | 54 | 56 |
| Gender | Male | Male | Male | Female | Male | Female | Male | Male | Female | Female |
| Subtype | Acute | Acute | Acute | Acute | Lymphomatous | Lymphomatous | Lymphomatous | Acute | Lymphomatous | Lymphomatous |
| Lines pre-HSCT | 5 | 1 | 3 | 1 | 3 | 2 | 1 | 4 | 1 | 1 |
| Status | Progressive disease | PR | PR | CR | PR | Progressive disease | CR | PR | CR | CR |
| Graft | MRD | MRD | Haploidentical | MUD | MRD | MUD | MRD | Haploidentical | MUD | MRD |
| Conditioning | RIC (Mel/Flu) | MA(Cy/TBI) | RIC (Mel/Flu) | RIC (Mel/Flu) | RIC (Mel/Flu) | RIC (Mel/Flu) | RIC (Mel/Flu) | RIC (Flu/Cy) | RIC (Flu/Mel/ATG) | RIC (Mel/Flu) |
| Acute GVHD (grade) | GI (I) | GI (IV) | Skin (II) | GI, Skin (I) | GI (IV) | GI (I) | Skin (I) | No | GI (IV) | No |
| Chronic GVHD (grade) | No | NA | Skin (II) | Skin | NA | NA | GI (II) | NA | GI (III) | NA |
| 4 | 1.5 | 55 | 60 | 3 | <1 | 14 | 2 | 7 | 4 | |
| OS | 10 | 8 | 67 | 71 | 47 | 5 | 22 | 12 | 19 | 10 |
| Alive | No, relapse | No, TRM | Yes | Yes | No, TRM | No, TRM | Yes | No, TRM | Yes | Yes |
PR indicates partial remission; RIC, reduced-intensity conditioning; Mel, melphalan; Flu, fludarabine; MA, myeloablative; Cy, cyclophosphamide; TBI, total body irradiation; ATG, antithymocyte globulin; GI, gastrointestinal; NA.
DISCUSSION
AlloHSCT has a positive impact on the prognosis of ATLL patients and is the only treatment option with potential for long-term remissions or cure [1,2]. Although prospective randomized studies are lacking, expert consensus dictates that, when feasible, upfront alloHSCT constitutes the optimal strategy for aggressive ATLL subtypes, preferably in first CR, as recommended in the National Comprehensive Cancer Network guidelines (category 2A) [1,13,16]. Unfortunately, only a small fraction of patients undergo alloHSCT [18,19]; however, the barriers preventing alloHSCT have not been largely studied, and all available data are derived from Japanese series.
Our center in the Bronx, New York has 1 of the largest ATLL cohorts in the United States. Our experience and results from another cohort in Miami, Florida suggests that ATLL in the United States represents a distinct, more aggressive clinical entity that mostly affects Afro-Caribbean and South American immigrants. Compared with Japanese series higher rates of acute or lymphomatous subtypes; more frequent aggressive features like hypercalcemia, complex cytogenetics, or central nervous system involvement; and poorer survival are described in US patients [9,10]. These patients could benefit significantly from alloHSCT, but very few receive transplant [10,12]. In this study we reviewed the issues limiting implementation of alloHSCT for ATLL in the United States, within the context of our center’s experience.
Over half of the patients with aggressive ATLL were not eligible for alloHSCT promptly after presentation. Surprisingly, age and frailty were not significant factors. The median age at diagnosis in this and other US series is younger than that of Japanese patients [10,11]. Regardless of younger age, early mortality due to treatment refractoriness was the single most important contributing factor to transplant disqualification. Furthermore, a relatively high proportion of patients for whom transplant was considered after initial assessment had rapid progressive disease leading to death and precluding proper evaluation. It is clear that the aggressive nature of the disease is the main limitation for any significant intervention because of a high rate of early mortality.
In the United States ATLL occurs most frequently in a socioeconomically challenged migrant population, and healthcare access disparities might play a role in their dismal outcomes [10,11]. Indeed, limited financial or familial support, low heath literacy, and poor rates of follow-up are common among these vulnerable patients and, at least in part, contributed to transplant ineligibility in some of our patients. Additionally, irrespective of chemotherapy efficacy or patient characteristics, uninsured status, which disproportionally impacts immigrants, was a relatively frequent factor precluding alloHSCT in our patient cohort.
In our center the practice is to HLA type all transplant-eligible ATLL patients, and the median time to identify a suitable donor was 97.5 days but varied significantly based on type of donor. It is possible that other healthcare- and provider-related barriers were undetected by limiting the analysis to a cohort treated uniformly with early transplant consideration in an experienced center. Notably, late presentation with advanced disease due to limited access to healthcare and delayed referral and underestimation of transplant importance by less experienced providers are possible.
A third of patients in our cohort were alloHSCT eligible; however, donor recruitment was problematic, and a lack of available donors was a significant barrier to alloHSCT. In Japan a third of patients have access to an MRD, although high rates of HTLV-1 carriers are found among siblings [20]. In our predominantly migrant cohort only 25% of patients had access to a sibling MRD. Moreover, an unrelated donor registry search found a full match for only 2 of 21 patients, and a 1-antigen mismatched donor was available for very few. Contrarily, in Japan a suitable unrelated donor is found in one-third of the cases [7]. This reflects a major under-representation of the ethnicities affected in the United States among donor registries in Western countries. As an example, in the United Kingdom African/Afro-Caribbean ethnicities account for only 2.4% of donors [21]. The absence of a matched donor is a major problem for patients in the United States because alloHSCT from an HLA-mismatched donor is a known adverse prognostic factor in ATLL [22].
The experience with alternative allografts is sparse in ATLL, but some studies favor it over no transplant [1,23]. Unfortunately, a suitable half-matched haploidentical donor was only identified for about half of our patients with no unrelated donor options. Given that the rate of haploidentical donor identification is expected to be much higher, this represents another unique problem of our vulnerable population that is partially influenced by socioeconomic challenges. Overall, our series shows that one-fourth of transplant-eligible patients had no suitable donor and suggests that all efforts to maximize the possibility of finding a donor are warranted early after identification of an alloHSCT candidate.
TRM rates of 34% to 45% have been reported in similar ATLL series, and in our study this was 40% [3,6]. AlloHSCT carries a high risk of TRM, but this should be balanced with the poor salvage options available for relapsed disease. Fuji et al. [16], using a Markov decision analysis, showed that chemotherapy followed by alloHSCT was the better treatment strategy in patients with aggressive ATLL. One major limitation, which was acknowledged by the authors, was that this decision analysis model was based on a retrospective cohort. Because it would be difficult to conduct randomized studies of alloHSCT in this rare disease, the evidence is likely to be limited to retrospective analysis or prospective series that include alloHSCT as a treatment option.
Although the TRM rate in our cohort rate was high, survival was significantly better in patients who underwent alloHSCT, with the best outcomes achieved in patients transplanted in CR with a matched allograft. A previous report from the University of Miami also showed favorable results in 5 patients transplanted in CR, with RFS of 7, 2, 28, and 24 months, whereas 1 patient died of graft failure [10]. The retrospective nature, selection bias for alloHSCT, and small sample size are significant limitations of this analysis; however, the difference is noticeable even after exclusion of all patients with early death or those who were transplant ineligible and further demonstrates the dismal outcomes of ATLL in US patients.
In conclusion, our results show that only 12.3% of ATLL patients undergo alloHSCT in a highly experienced tertiary center in the United States. Treatment refractoriness and early mortality are the main barriers to alloHSCT, as has been described in Japanese series. However, paucity of suitable donors (including haploidentical) and socioeconomic barriers to transplant, including poor follow-up rates and uninsured status, are additional significant barriers to successful alloHSCT that have not been previously highlighted and are unique to the vulnerable migrant population that constitutes most ATLL cases in the United States. These patients represent a distinct clinical entity with more aggressive disease and worse outcomes than their Japanese counterparts, for which alloHSCT can achieve long-term remission, especially in patients with successful induction chemotherapy who receive transplant from a matched donor. Hence, better upfront treatment strategies to achieve higher CR rates, in addition to collective efforts to maximize access to early HLA-matched alloHSCT and minimize TRM, are urgently needed to improve the outcomes of patients with ATLL in the United States.
Footnotes
Conflict of interest statement: M.J. has served on advisory boards for Seattle Genetics and miRagen.
Financial disclosure: The authors have nothing to disclose.
REFERENCES
- 1.Phillips AA, Harewood JCK. Adult T cell leukemia-lymphoma (ATL): state of the art. Curr Hematol Malig Rep. 2018;13:300–307. [DOI] [PubMed] [Google Scholar]
- 2.Katsuya H, Ishitsuka K. Treatment advances and prognosis for patients with adult T-cell leukemia-lymphoma. J Clin Exp Hematop. 2017;57:87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hishizawa M, Kanda J, Utsunomiya A, et al. Transplantation of allogeneic hematopoietic stem cells for adult T-cell leukemia: a nationwide retrospective study. Blood. 2010;116:1369–1376. [DOI] [PubMed] [Google Scholar]
- 4.Katsuya H, Ishitsuka K, Utsunomiya A, et al. Treatment and survival among 1594 patients with ATL. Blood. 2015;126:2570–2577. [DOI] [PubMed] [Google Scholar]
- 5.Shimoyama M Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. A report from the Lymphoma Study Group (1984–87). Br J Haematol. 1991;79:428–437. [DOI] [PubMed] [Google Scholar]
- 6.Kawada H, Yoshimitsu M, Nakamura D, et al. A retrospective analysis of treatment outcomes in adult T cell leukemia/lymphoma patients with aggressive disease treated with or without allogeneic stem cell transplantation: A single-center experience. Biol Blood Marrow Transplant. 2015;21:696–700. [DOI] [PubMed] [Google Scholar]
- 7.Phillips EH, Hodson A, Hermine O, Bazarbachi A, Cwynarski K. Striving to cure adult T-cell leukaemia/lymphoma: a role for allogeneic stem cell transplant? Bone Marrow Transplant. 2016;51:1549–1555. [DOI] [PubMed] [Google Scholar]
- 8.Ishitsuka K, Tamura K. Human T-cell leukaemia virus type I and adult T-cell leukaemia-lymphoma. Lancet Oncol. 2014;15:e517–e526. [DOI] [PubMed] [Google Scholar]
- 9.Zell M, Assal A, Derman O, et al. Adult T-cell leukemia/lymphoma in the Caribbean cohort is a distinct clinical entity with dismal response to conventional chemotherapy. Oncotarget. 2016;7:51981–51990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malpica L, Pimentel A, Reis IM, et al. Epidemiology, clinical features, and outcome of HTLV-1-related ATLL in an area of prevalence in the United States. Blood Adv. 2018;2:607–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phillips AA, Willim RD, Savage DG, et al. A multi-institutional experience of autologous stem cell transplantation in North American patients with human T-cell lymphotropic virus type-1 adult T-cell leukemia/lymphoma suggests ineffective salvage of relapsed patients. Leuk Lymph. 2009;50:1039–1042. [DOI] [PubMed] [Google Scholar]
- 12.Roe C, Komrokji R, Zhang L, Price S, Sokol L. Adult T-cell leukemia/lymphoma: rarely encountered in the United States. Clin Lymph Myeloma Leuk. 2016;16(Suppl):S191–S194. [DOI] [PubMed] [Google Scholar]
- 13.National Comprehensive Cancer Network. T-cell lymphomas (version 2.2019); 2019. Available at: https://www.nccn.org/professionals/physi-cian_gls/pdf/t-cell.pdf.
- 14.Yoshimitsu M, Tanosaki R, Kato K, et al. Risk assessment in adult T cell leukemia/lymphoma treated with allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2018;24:832–839. [DOI] [PubMed] [Google Scholar]
- 15.Kamiunten A, Sekine M, Kameda T, et al. Outcome of allogeneic hematopoietic cell transplantation in patients with adult T-cell leukemia. Hematol Oncol. 2018;36:651–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuji S, Kurosawa S, Inamoto Y, et al. Role of up-front allogeneic hematopoietic stem cell transplantation for patients with aggressive adult T-cell leukemia-lymphoma: a decision analysis. Bone Marrow Transplant. 2018;53:905–908. [DOI] [PubMed] [Google Scholar]
- 17.Phillips AA. Hematopoeitic stem cell transplantation for adult T cell leukemia-lymphoma: who is the best candidate? Leuk Lymph. 2017;58:1–3. [DOI] [PubMed] [Google Scholar]
- 18.Utsunomiya A, Choi I, Chihara D, Seto M. Recent advances in the treatment of adult T-cell leukemia-lymphomas. Cancer Sci. 2015;106:344–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marcais A, Suarez F, Sibon D, Bazarbachi A, Hermine O. Clinical trials of adult T-cell leukaemia/lymphoma treatment. Leuk Res Treatment. 2012;2012 932175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Momita S, Ikeda S, Amagasaki T, et al. Survey of anti-human T-cell leukemia virus type I antibody in family members of patients with adult T-cell leukemia. Jpn J Cancer Res. 1990;81:884–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nolan A Transplant NBa. Together for better. Stem Cell Registry Annual Review NHS Blood and Transplant. 2014. [Google Scholar]
- 22.Tokunaga M, Uto H, Takeuchi S, et al. Newly identified poor prognostic factors for adult T-cell leukemia-lymphoma treated with allogeneic hematopoietic stem cell transplantation. Leuk Lymph. 2017;58:37–44. [DOI] [PubMed] [Google Scholar]
- 23.Hermine O, Ramos JC, Tobinai K. A review of new findings in adult T-cell leukemia-lymphoma: a focus on current and emerging treatment strategies. Adv Ther. 2018;35:135–152. [DOI] [PMC free article] [PubMed] [Google Scholar]