Abstract
This review presents the chemical diversity and pharmacological properties of secondary metabolites produced by endophytic fungi associated with various genera of Rubiaceae. Several classes of natural products are described for these endophytes, although, this study highlights the importance of some metabolites, which are involved in antifungal, antibacterial, anti-protozoal activities; neurodegenerative diseases; cytotoxic activity; anti-inflammatory and antioxidant activity; and hyperglycemic control.
Keywords: Rubiaceae, endophytes, fungi, antibacterial, antifungal, neurodegenerative diseases, anti-inflammatory
1. Introduction
Natural products are small molecules from primary and secondary metabolites naturally synthesized by microorganisms, plants, or animals [1,2]. They are a continuing source of novel bioactive metabolites and have a significant impact on modern medicine [3,4]. Currently, more than 70% of antibacterial and anticancer compounds are natural products or their derivatives [5,6].
Fungi-derived natural products are considered one of the most relevant sources discovery and molecular diversity for new drugs. They are valuable source of biological metabolites that find wide-ranging applications as antibiotics, antifungal, immunosuppressants, antiparasitic and anticancer agents [7,8,9,10,11,12]. Among the microorganisms, endophytes have aroused interest in the last decades mainly for the discovery of important secondary metabolites identified from them.
The term endophyte refers to the microorganism that colonizes interior organs of plants, generally inhabiting their aerial parts such as stems and leaves, but that does not have pathogenic effects on its host [1,7,13,14,15,16]. Endophytes are ubiquitously found in every plant species examined to date. It is worth mentioning that, of the nearly 300,000 species on earth, each plant hosts one or more endophytes, and approximately 1 million of different species of microorganisms can be found [1,17]. In their symbiotic association, the host plant protects and feeds the endophyte, which in return produces bioactive metabolites to enhance the growth and competitiveness of the host and to protect it from herbivores and plant pathogens [7,9,18].
Endophytic fungi are known to produce a wide range of bioactive secondary metabolites, emphasizing chemical diversity, molecules originality and their biological activities [1,7,9,15,16,17,19,20,21,22,23,24]. Some studies suggest that up to 51% of bioactive metabolites obtained from endophytic fungi have unknown chemical structure, which highlights the huge biotechnological potential of this microbial group to the discovery of new drugs [21].
This review will focus on secondary metabolites synthesized by endophytic fungi isolated from Rubiaceae species, as well as the biological activities described in the literature for these compounds. The bibliographic research was carried out until March 2020.
2. Secondary Metabolites Produced by Endophytic Fungi from Rubiaceae
Rubiaceae is the fourth largest angiosperm family and comprises about 617 genera and 13,000 species of herbs, shrubs, and trees, found worldwide, especially in tropical and warm regions [25,26,27]. This family presents a vast diversity of chemical substances such as iridoids, anthraquinones, indole alkaloids, terpenoids, flavonoids, and alkaloids [28,29,30,31,32]. Diverse species of Rubiaceae have widespread use in folk medicine, and some of them showed anti-inflammatory, analgesic, antibacterial, mutagenic, antiviral, and antioxidant activities. Besides, an effect on vascular diseases and action on the central nervous system were observed [25,27,33,34].
The research on microorganisms associated with the Rubiaceae family, for biotechnological applications, led to the isolation of endophytic fungi [35,36,37,38,39,40,41,42,43,44,45,46,47] and the discovery of several bioactive metabolites [38,48,49,50,51,52,53,54,55]. The diversity of chemical structures observed for secondary metabolites synthesized by fungi isolated from Rubiaceae species showed a dynamic range of metabolites pathways used by these microorganisms.
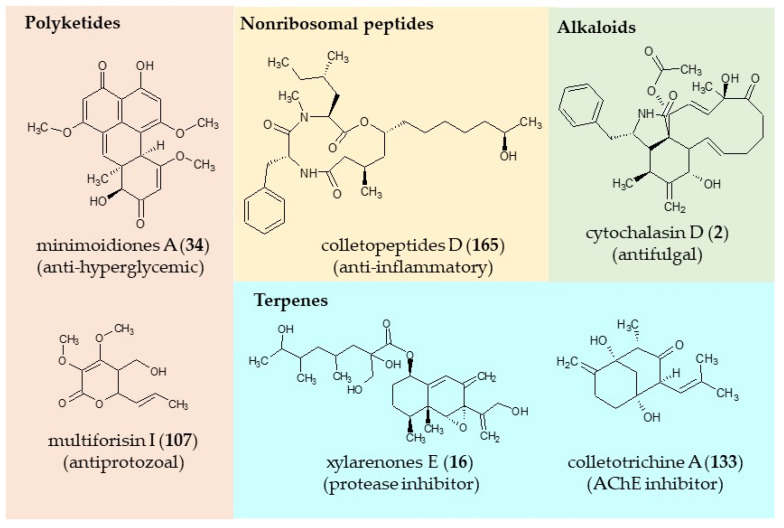
Fungi secondary metabolites are categorized in chemical classes: polyketides [56,57,58,59], non-ribosomal peptides [57,59,60,61,62], ribosomal peptides [62,63,64], terpenes [65,66,67], and hybrid metabolites [68,69,70,71,72,73,74]. These chemical classes are synthesized by specialized class-defining (backbone) enzymes such as polyketides synthases (PKSs), non-ribosomal peptide synthetases (NRPSs), terpene cyclases (TCs), and dimethylallyl tryptophan synthases (DMATSs), respectively. The endophytes isolated from Rubiaceae showed the ability to produce these chemical classes (Figure 1). The set of enzymes needed for the production of a secondary metabolite is encoded by a gene cluster (BGC). Interestingly the genes that are essential for the synthesis of a primary metabolite are dispersed throughout the fungal genome, while the genes encoding the enzymatic activities for metabolic pathways to produce any secondary metabolite are arranged in continuous fashion. In the last decades, significant advances have been observed in the identification, understanding, and engineering of fungal biosynthetic gene clusters (BGCs) [75,76,77,78,79,80,81,82,83,84].
Figure 1.
Examples of fungal natural products produced by endophytic fungi associated with various genera of Rubiaceae grouped by biosynthetic origin.
The endophytic fungi distribution and diversity in Rubiaceae have been reported since the 1950s [85,86,87], and studies performed with Coffea arabica stand out [88,89,90,91,92]. However, only in 1999 was the first study on the secondary metabolism of endophytic fungi isolated from Rubiaceae species published. In this work, Strobel related the occurrence of taxol (Figure 2), a potent anticancer drug, in the culture of the endophyte Seimatoantlerium tepuiense (Amphisphaeriaceae) isolated from Maguireothamnus speciosus [93]. The occurrence of taxol was described for other endophytes isolated from Rubiaceae species, Botryodiplodia theobromae (Botryosphaeriaceae) and Aspergillus oryzae (Trichomaceae), obtained from Morinda citrifolia and Tarenna asiatica, respectively [94,95,96]. The pharmacological properties of taxol, isolated from Botryodiplodia theobromae, were confirmed through the cytotoxicity assay [94].
Figure 2.
Taxol, the first compound isolated from an endophytic fungus from Rubiaceae.
The secondary metabolites study on microorganisms associated with Rubiaceae continued with Palicourea marcgravii St. Hil. It was popularly known as “erva de rato” and provided several endophytic fungi, including a Xylaria sp. (Xylariaceae) isolated from their leaves. The crude extract from Xylaria sp. showed a potential antifungal activity, and five compounds: 2-hexyl-3-methyl-butanodioic acid (1), cytochalasin D (2), 7-dechlorogriseofulvin (3), cytochalasin B (4) and griseofulvin (5) were obtained (Figure 3) [97].
Figure 3.
Five compounds extracted from Xylaria sp.
Oliveira et al. (2009) explored endophytic fungi living in plants of the Brazilian flora; two Penicillium (Trichocomaceae) species from leaves of Alibertia macrophylla were isolated. Penicillium sp.1 was cultivated in corn and potato dextrose broth produced three different compounds: orcinol (6), cyclo-(L-Pro-L-Val) (7), uracil (8). The acetonitrile fraction from Penicillium sp.2 led to three dihydroisocoumarins: 4-hydroxymellein (9), 8-methyl-mellein (10) and 5-hydroxymellein (11) [98]. Additionally, (R)-7-hydroxymellein (12) and (3R,4R)-4,7-dihydroxymellein (13) were also isolated from Penicillium sp. associated with A. macrophylla (Figure 4) [99].
Figure 4.
Compounds extracted from Penicillium species.
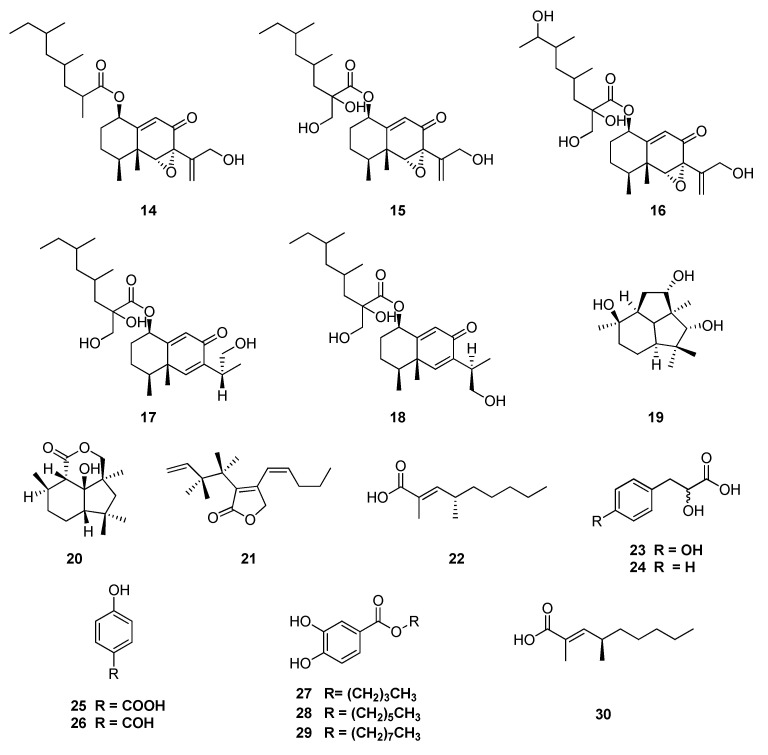
The continuing search for endophytes associated on A. macrophylla led to five new eremophilane sesquiterpenes: xylarenones C–G (14–18) isolated from solid cultures of Camarops sp. (Boliniaceae) [100,101]. This fungus was also able to produce two rearranged sesquiterpenes: 3,5,9-trihydroxy presilphiperfolane (19) and 4-deoxy-10-oxodihydrobotrydial (20); two branched polyketides: 4-((E)-pent-1-enyl)-3-((1’S,2’S)-1’,2’-dihydroxybut-3-enyl)-5H-furan-2-one (21) and (2E,4R)-2,4-dimethylnon-2-enoic acid (22); seven phenolic derivatives: p-hydroxyphenyllactic acid (23), phenyllactic acid (24), p-hydroxybenzoic acid (25), p-hydroxybenzaldehyde (26), n-butyl-3,4- dihydroxybenzoate (27), n-hexyl-3,4-dihydroxybenzoate (28) and n-octyl-3,4-dihydroxybenzoate (29); and the known compound (2E,4S)-2,4- dimethyloct-2-enoic acid (30) (Figure 5) [102].
Figure 5.
Compounds extracted from endophytes associated on A. macrophylla.
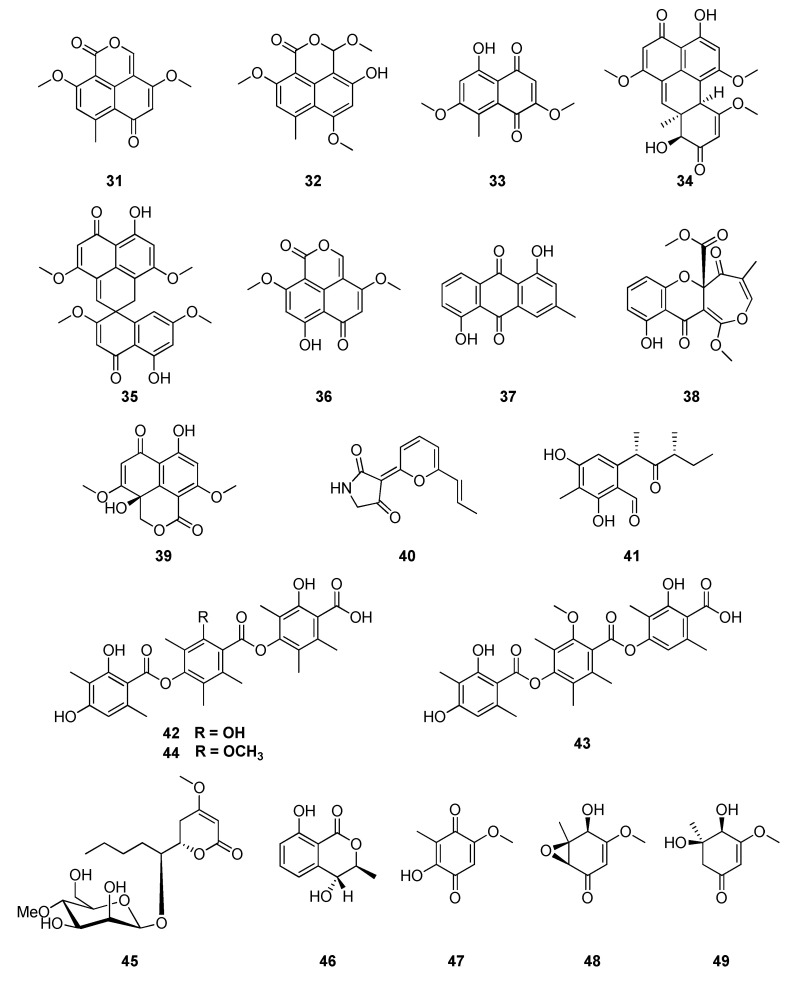
Extracts of solid cultures of Sporormiella minimoides (Sporormiaceae), isolated as an endophytic fungus from leaves Hintonia latiflora collected in Mexico, yielded five polyketides, 3,6-dimethoxy-8-methyl-1H,6Hbenzo[de]isochromene-1,9-dione (31), 3-hydroxy-1,6,10-trimethoxy-8-methyl-1H,3H-benzo[de]isochromen-9-one (32), 5-hydroxy-2,7-dimethoxy-8-methylnaphthoquinone (33), minimoidiones A (34) and B (35), along with four known compounds: corymbiferone (36), ziganein (37), brocaenol B (38) and preussochromone C (39) [103,104,105]. Two other compounds, 9S,11R(+)-ascosalitoxin (40) and vermelhotin (41), were also produced by endophytes from this plant [105,106]. The tridepsides, secondary metabolites produced by fungus Chaetomium sp. (Chaetomiaceae), also isolated from medicinal plant H. latiflora, were identified as thielavins A (42), J(43) and K (44) [107]. Two new compounds, pestalotin 4′-O-methyl-β-mannopyranoside (45) and 3S,4R-(+)-4-hydroxymellein (46), were isolated from an organic extract of X. feejeensis, which was isolated from this plant. In addition, the compounds (3S,4S)-4-hydroxymellein (9), (3S)-8-methylmellein (10), and the quinone derivatives 2-hydroxy-5-methoxy-3-methylcyclohexa-2,5-diene-1,4-dione (47), 4S,5S,6S-4-hydroxy-3-methoxy-5-methyl-5,6-epoxycyclohex-2-en-1-one (48), and 4R,5R-dihydroxy-3-methoxy-5-methylcyclohexen-2-en-1-one (49) were obtained (Figure 6) [108].
Figure 6.
Compounds extracted from endophytic fungus Sporormiella minimoides obtained from Hintonia latiflora.
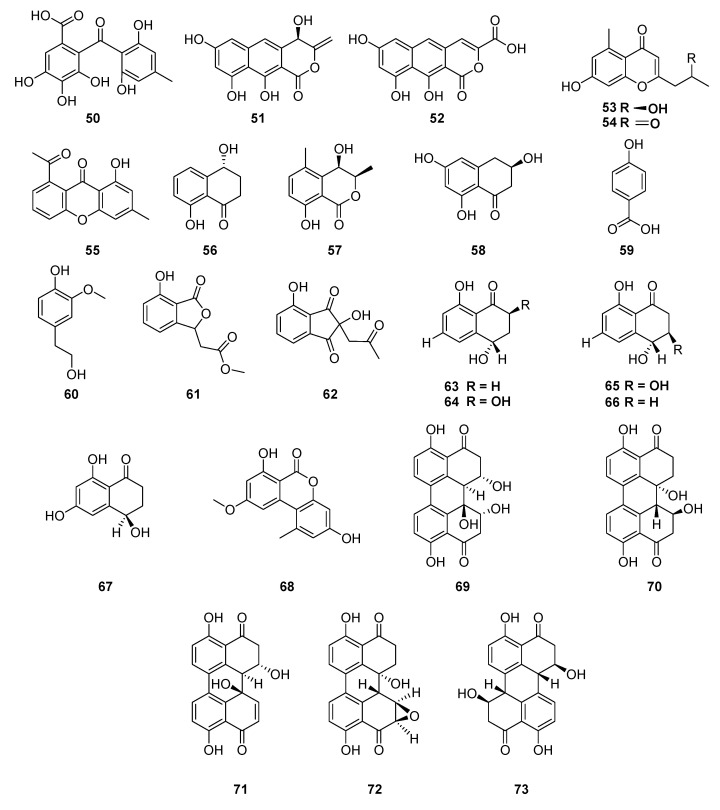
The ethyl acetate extract from Cytospora rhizophorae (Valsaceae), a fungus associated with Morinda officinalis, led to the isolation of three new compounds, named cytosporaphenones A–C (50–52), one new polyhydric benzophenone, and two new naphthopyrone derivatives, respectively. In addition to eight known compounds: 2-(2′S-hydroxypropyl)-5-methyl-7-hydroxychromone (53), 2-acetonyl-7-hydroxy-5-methylchromone (54), 8-hydroxy-6-methylxanthone-1-carboxylic acid (55), regiolone (56), (3R,4R)-cis-4-hydroxy-5-methylmellein (57), scytalone (58), p-hydroxybenzoic acid (59) and 4-hydroxy-3-methoxybenzene-ethanol (60). Interestingly, all of them were identified from this strain for the first time, and these three new compounds (50–52) were the most highly oxygenated metabolites of their families discovered in nature [109].
The endophytic fungal strain Alternaria sp. (Pleosporaceae) isolated from medicinal plant M. officinalis produced two new metabolites, isobenzofuranone A (61) and indandione B (62), together with eleven known compounds (63–73): isosclerone (63), 2,4,8-trihydroxy-1-tetralone (64), 3,4-dihydro-3,4,8-trihydroxy-1[2H]-naphthalenone (65), 6-hydroxyisosclerone (66), cis-4-hydroxyscytalone (67), alternariol-4-methyl ether (68), 6-epi-stemphytriol (69), dihydroalterperylenol (70), alterperylenol (71), altertoxin II (72) and stemphyperylenol (73). It is relevant emphasizing that indandione (62) showed a rarely occurring indanone skeleton in natural products (Figure 7) [110].
Figure 7.
Compounds extracted from endophytic fungal strain Alternaria sp. isolated from medicinal plant M. officinalis.
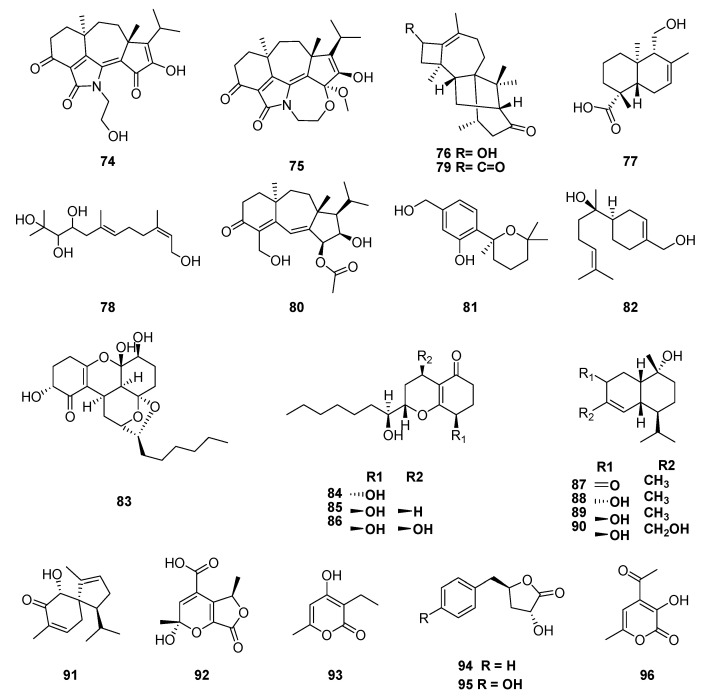
The chemical investigation of the endophytic fungus Trichoderma koningiopsis (Hypocreaceae), also isolated from M. officinalis yielded three new diterpenes: koninginols A–C (74–76); two new sesquiterpenoids, 11-hydroxy-15-drimeneoic acid (77) and koninginol D (78); as well as twelve known metabolites identified as harziandione 2 (79), radianspene B (80), (S)-(-)-5-(hydroxymethyl)-2-(2′,6′,6′-trimethyltetrahydro-2H-pyran-2-yl)phenol (81), hamanasol A (82), trichodermatide A (83), dihydropyran (84), ketodiol (85), 7-O-methylkoninginin D (86), (1S,6R,7S,10R)-10-hydroxy-4(5)-muurolen-3-one (87), 1R,3S,6S,7R,10S-7-isopropyl-4,10-dimethylbicyclo[4.4.0]dec-4-en-3,10-diol (88), 1R,3R,6S,7R,10S-7-isopropyl-4,10-dimethylbicyclo[4.4.0]dec-4-en-3,10-diol (89) and coprinol (90) [111]. Recently, six polyketides, 6-hydroxy-4-isopropyl-1,8-dimethylspiro[4.5]deca-1,8-dien-7-one (91), 2-hydroxy-2,5-dimethyl-7-oxo-5,7-dihydro-2H-furo[3,4-b]pyran-4-carboxylicacid (92), 3- ethyl-4-hydroxy-6-methyl-2H-pyran-2-one (93), harzialactone A (94), 3-hydroxy-5-(4-hydroxybenzyl)dihydrofuran-2(3H)-one (95), and 4-acetyl-3-hydroxy-6-methyl-pyran-2-one (96) were isolated from T. spirale, another endophytes from M. officinalis (Figure 8) [112].
Figure 8.
Compounds extracted from endophytic fungus Trichoderma koningiopsis (Hypocreaceae), also isolated from M. officinalis.
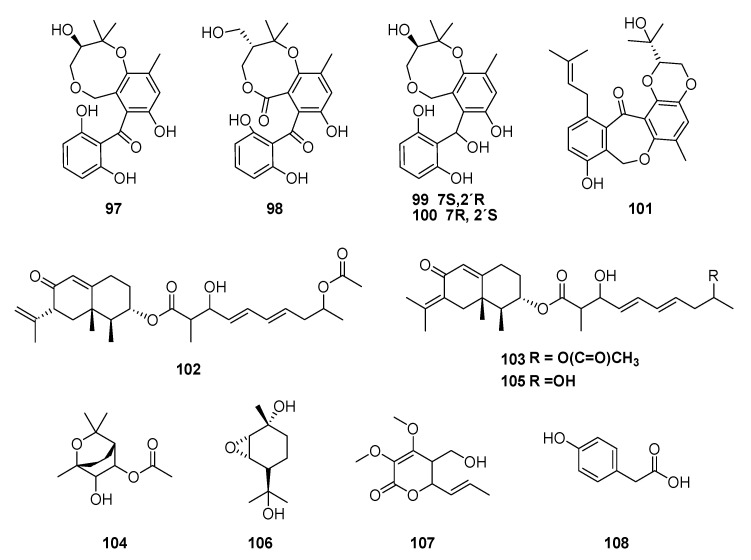
The endophytic fungi, Cytospora rhizophorae and Diaporthe lithocarpus (Diaporthaceae), were also obtained from M. officinalis. New metabolites isolated from C. rhizophorae included cytosporins A–D (97–100) meroterpenoids. These structures represent the first example of natural products that bear novel benzo[b][1,5]dioxocane framework embodying hemiterpene and benzophenone moieties [113]. Compounds 97–100 were evaluated for antimicrobial activities against Escherichia coli and Staphylococcus aureus. However, the compounds exhibited weak antibiotic activity with inhibition in concentrations above 250 μg mL−1. The endophytic fungus, D. lithocarpus, yielded tenllone I (101), a new benzophenone derivative; two new eremophilane derivatives, lithocarins B (102) and C (103); a new monoterpenoid, lithocarin D (104); tenellone H (105); and phomopene (106) [114]. Studies of endophytic fungus of Nigerian medicinal plants led to isolation of multiforisin I (107) and 4-hydroxyphenylacetic acid (108) of Neurospora discreta (Sordariaceae) from leaves of M. lucida (Figure 9) [115].
Figure 9.
Compounds extracted from endophytic fungi, Cytospora rhizophorae and Diaporthe lithocarpus obtained from M. officinalis.
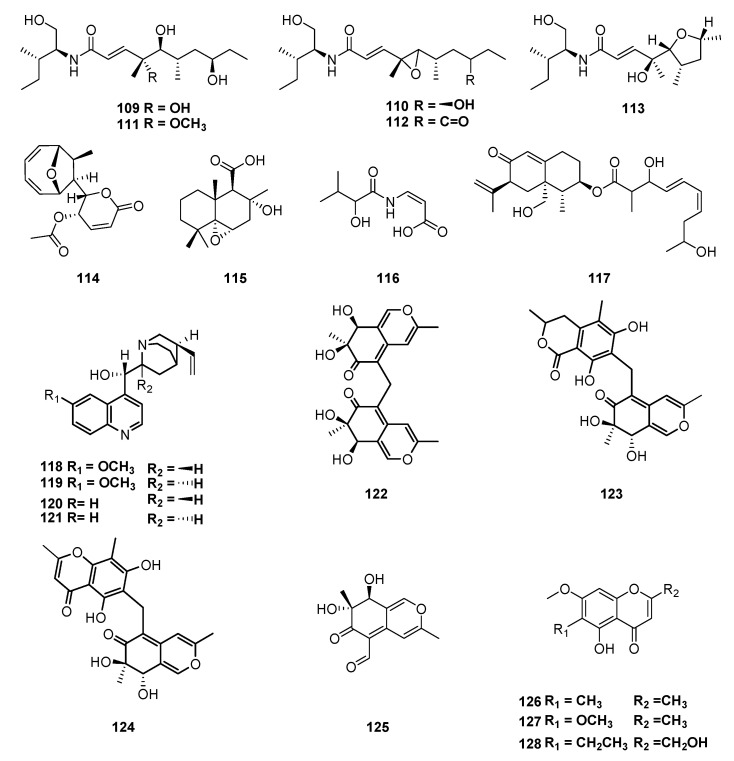
The curvularides A–E (109–113) are hybrid peptide–polyketides isolated from Curvularia geniculata (Pleosporaceae), an endophytic fungus obtained from the twigs of Catunaregam tomentosa. Their structures contain a 12-carbon atoms polyketide skeleton unit-linked, through an amide bond, with a derivative of L-isoleucine, a rare compound class [116]. The endophytic fungus D. pseudomangiferae retrieved from leaves of Sabicea cinerea species found along forest edges in the French Guiana, produces four metabolites: mycoepoxydiene (114) and altiloxin A (115), as well as enamidin (116) and eremofortin F (117) [117]. A filamentous fungus of the genus Diaporthe associated with the seeds of Cinchona ledgeriana, from West Java–Indonesia, produces cinchona alkaloids: quinine (118), quinidine (119), cinchonidine (120) and cinchonine (121), upon cultivation in a synthetic liquid medium [118,119,120,121,122]. Quinine (118), an antimalarial drug, has also been found in chloroform extracts of Colletotrichum spp. isolated from C. calisaya (Figure 10) [123].
Figure 10.
Compounds extracted from endophytic fungi, Curvularia geniculate, D. pseudomangiferae, Diaporthe, Colletotrichum spp and Mycoleptodiscus indicus obtained from Catunaregam tomentosa, Sabicea cinereal, Cinchona ledgeriana, C. calisaya and Borreria verticillate, respectively.
Three new azaphilones with an unusual methylene bridge, named mycoleptones A, B, and C (122–124), were obtained from cultures of Mycoleptodiscus indicus (Magnaporthaceae), a fungus isolated from South American medicinal plant Borreria verticillate (Figure 10) [124]. Besides, other polyketides, austidiol (125), eugenitin (126), 6-methoxieugenin (127), and 9-hydroxyeugenin (128), were also produced (Figure 10) [124,125].
A fungus endophyte from Uncaria rhynchophylla, C. gloeosporioides (Glomerellaceae), produced four novel lactams in culture broth, colletotrilactam A–D (129–132); colletotrichine A (133) and B (134); and eleven more compounds: 2-isopropyl-5-methyl-2,4-cyclohexadien-1-ol (135), cis-4-hydroxymellein (9), 8-methyl-mellein(10), hederagonic acid (136), mellein (137) and blumenol A (138), aspergiketone (139), djalonenol (140), (4S)-(+)-ascochin (141), 12,13-dihydroxyfumitremorgin C (142) and fumitremorgin C (143) [124,125,126]. On the other hand, when grown in wheat bran medium, C. gloeosporioides produced nine compounds: 4-epi-14-hydroxy-10, 23-dihydro-24, 25-dehydroaflavinine (144), 10, 23-dihydro-24,25-dehydro-21–oxoaflavinine (145), ergosterol (146), ergosterol peroxide (147), mellein (137), 4, 5-dihydroblumenol A (148), cyclo(L-leucyl-L-leucyl) (149), and brevianamide F (150). It was the first report of isolation of the compounds 144, 145, 148, 149, and 150 from the Colletotrichum genus (Figure 11) [127].
Figure 11.
Compounds extracted from endophytic fungi, C. gloeosporioides obtained from Uncaria rhynchophylla.
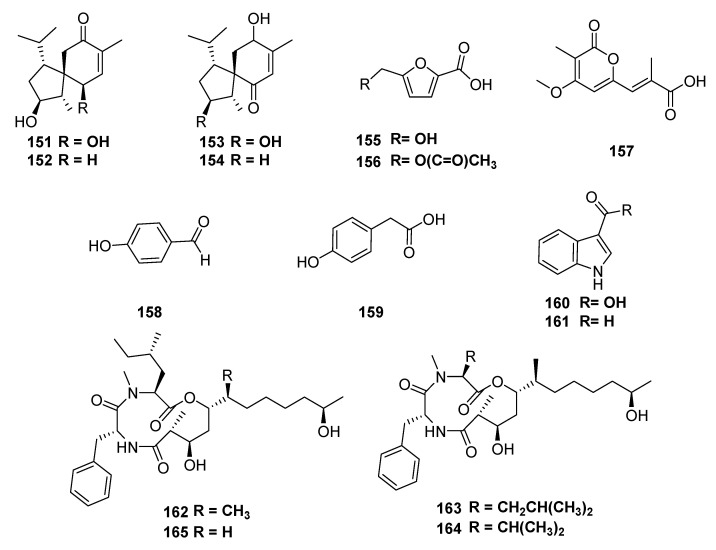
However, the chemical investigation of the C. gloeosporioides ethyl acetate extract, obtained from a solid culture, isolated from the leaves of Sabicea cinerea, led to the isolation of four new acoranes (151–154) and other seven known compounds: 5-hydroxymethyl-furan-2-carboxylic acid (155), 5-acetoxymethyl-furan-2-carboxylic acid (156), convolvulopyrone (157), p-hydroxybenzaldehyde (158), 4-hydroxyphenyl acetic acid (159), indole-3-carboxylic acid (160) and indole-3-carboxaldehyde (161) [128,129]. Recently, four cyclic tridepsipeptides, colletopeptides A−D (162–165), were isolated from Colletotrichum species from stems of Rubia pondantha(Figure 12) [130].
Figure 12.
Compounds extracted from endophytic fungi, Colletricnhum sp. obtained from Sabicea cinereal and Rubia pondantha.
Guignardia sp. (Botryosphaeriaceae) isolated from the leaves of the mangrove plant Scyphiphora hydrophyllacea Gaertn. F., produced six new meroterpenes, guignardones D–I (166–171); two known compounds, guignardones A (172) and B (173), and the fatty acid glucoside identified as (R)-3-hydroxyundecanoic acid methylester-3-O-α-L-rhamnopyranoside (174) [131,132,133]. Two other antibiotics, brefeldin A (175) and trichodermol (176), were isolated from endophytic fungus (code C22) from S. hydrophyllacea [134].
Analyzing the effect of the culture medium on the production of secondary metabolites by Panamanian endophytic fungi, an antiparasitic compound was obtained, cercosporin (177); and a new analog (178), isolated from endophytic fungus Mycosphaerella sp. (Mycosphaerellaceae), associated with the foliage of Psychotria horizontalis [135]. The structures of minor compounds in the extract were elucidated as 2-(2-butyl)-3-hydroxy-6-ethyl-6-methylcyclohex-2-ene-1,5-dione (179) and 3-(2-butyl)-6-ethyl-6- methyl-5-hydroxy-2-methoxy-cyclohex-2-eneone (180) (Figure 13) [136].
Figure 13.
Compounds extracted from endophytic fungi, Guignardia sp and Mycosphaerella sp. obtained from Scyphiphora hydrophyllacea and Psychotria horizontalis, respectively.
In continuous studies on the chemistry of the endophytic fungus P. griseoroseum (Trichocomaceae), an endophyte isolated from fruits of C. arabica, produced dimethylated tetraketide diclavatol (181), clavatol (182) and two benzylated flavonoids (183–184) [137,138]. The studies also resulted in the identification of two known tetronic acids, viridicatic acid (185) and terrestric acid (186), found in ethyl acetate and n-butanol extract [138]. Mycophenolic acid (187), 5-hydroxi-7-methoxy-4-methylphtalide (188) and ochratoxin A (189) were produced by P. crustosum obtained from coffee seeds [139,140]. After adding halides in a broth culture, two bromoroquefortines, 11-bromoroquefortine D (190) and 11-bromo-17-hydroxybromoroquefortine C (191), were produced by P. chrysogenum from leaves of C. arabica (Figure 14) [141].
Figure 14.
Compounds extracted from endophytic fungi, P. griseoroseum, P. crustosum and P. chrysogenum obtained from C. arabica.
An expedition to Yasuni National Park resulted in the isolation of endophytic Stelliosphaera formicum from Duroia hirsuta, an understory tree growing in Ecuador. Phylogenetic analysis of this organism describes it as a specimen of a new genus within the order Pleosporales. Besides this organism being an example of new taxonomic diversity, it also produced stelliophaerols A (192) and B (193), two new sesquiterpene-polyol conjugates 1 [142].
Chemical analyses of Phomopsis spp. (Valsaceae) isolated from tropical plants, including C. arabica, yielded alternariol (68), altenusin (194), altenuene (195), cytosporones C, O (196–197), and dothiorelones A–C (198–200) [143].
Recently, four secondary metabolites from C. cupreum associated with Mussaenda luteola were characterized as resorcinol (201), 6-(heptacosa-18′Z enyl)-2-(18”hydroxyl-1” enyl-19” oxy)-3-hydroxybenzoquinone (202), (3β–5α–dihydroxy–6β–phenylacetyloxy–ergosta–7, 22–diene) (203) and 2- dodecanol (204) (Figure 15) [144,145].
Figure 15.
Compounds extracted from endophytic fungi, Stelliosphaera formicum Phomopsis spp and C. cupreum obtained from Duroia hirsute, C. arabica and Mussaenda luteola, respectively.
3. Biological Activities
Endophyte fungi are capable of synthesizing bioactive compounds, including alkaloids, terpenoids, flavonoids and steroids. Hitherto, most of the secondary metabolites from endophytes are anticancer agents, antibiotics, biological control agents, and other bioactive compounds determined by their different functional roles. In this review, we highlight mainly bioactive natural products endophytically synthetized by endophytic fungi associated with various genera of Rubiaceae (Table 1).
Table 1.
Compounds produced by endophytic fungi associated with various genera of Rubiaceae and their respectively biological activities.
| Classification | Compound | Endophytic | Species | Biological Activities | Refrence |
|---|---|---|---|---|---|
| alkaloid | cytochalasin D (2) | Xylaria sp. | Palicourea marcgravii | antifungal | [97] |
| alkaloid | quinine (118) | Colletotrichum spp. | Cinchona ledgeriana | antiprotozoal | [120,121,146] |
| alkaloid | brevianamide F (150) | Colletotrichum gloeosporioides | Uncaria rhynchophylla | cytotoxic | [127] |
| alkaloid | 11-bromoroquefortine (190) | Penicillium chrysogenum | Coffea arabica | antibacterial, antiprotozoal, cytotoxic | [141] |
| coumarin | 4-hydroxy-mellein (9) | Penicillium sp. | Alibertia macrophylla | antifungal, acetylcholinesterase inhibitor, anti-hyperglycemic | [98] |
| coumarin | 8-methyl-mellein (10) | Penicillium sp. | Alibertia macrophylla | Antifungal | [98] |
| coumarin | (R)-7-hydroxymellein (12) | Penicillium sp. | Alibertia macrophylla | antifungal, acetylcholinesterase inhibitor | [99] |
| coumarin | (3R,4R)-4,7-dihydroxymellein (13) | Penicillium sp. | Alibertia macrophylla | antifungal, acetylcholinesterase inhibitor | [99] |
| coumarin | 3S,4R-(+)-4-hydroxymellein (46) | Xylaria feejeensis | Hintonia latiflora | anti-hyperglycemic | [108] |
| coumarin | mellein (137) | Colletotrichum gloeosporioides | Uncaria rhynchophylla | monoamine oxidase inhibitor | [127] |
| diketopiperazine | cyclo-(L-Pro-L-Val) (7) | Penicillium sp. | Alibertia macrophylla | acetylcholinesterase inhibitor | [98] |
| diketopiperazine | cyclo(L-Leu-L-Leu) (149) | Colletotrichum gloeosporioides | Uncaria rhynchophylla | cytotoxic | [127] |
| diterpene | koninginol A (74) | Trichoderma koningiopsis | Morinda officinalis | antifungal | [111] |
| diterpene | koninginol B (75) | Trichoderma koningiopsis | Morinda officinalis | antifungal, cytotoxic | [111] |
| fatty acid | (R)-3-hydroxyundecanoic acid methylester-3-O-α-L-rhamnopyranoside (174) | Guignardia sp. | Scyphiphora hydrophyllacea | antibacterial | [133] |
| meroterpene | guignardone I (171) | Guignardia sp. | Scyphiphora hydrophyllacea | antibacterial | [132] |
| meroterpene | guignardone B (173) | Guignardia sp. | Scyphiphora hydrophyllacea | antibacterial | [132] |
| phenolic compound | orcinol (6) | Penicillium sp. | Alibertia macrophylla | antifungal | [98] |
| phenolic compound | thielavins A (42) | Chaetomium sp. | Hintonia latiflora | anti-hyperglycemic | [107] |
| phenolic compound | thielavins J (43) | Chaetomium sp. | Hintonia latiflora | anti-hyperglycemic | [107] |
| phenolic compound | thielavins K (44) | Chaetomium sp. | Hintonia latiflora | anti-hyperglycemic | [107] |
| phenolic compound | cytosporaphenone A (50) | Cytospora rhizophorae | Morinda officinalis | cytotoxic | [109] |
| phenolic compound | resorcinol (201) | Chaetomium cupreum | Mussaenda luteola | antibacterial | [145] |
| polyketide | 2-hexyl-3-methyl-butanodioic acid (1) | Xylaria sp. | Palicourea marcgravii | antifungal | [97] |
| polyketide | (2E,4R)-2,4-dimethylnon-2-enoic acid (22) | Camarops sp. | Alibertia macrophylla | acetylcholinesterase inhibitor | [102] |
| polyketide | (2E,4S)-2,4-dimethyloct-2-enoic acid (30). | Camarops sp. | Alibertia macrophylla | acetylcholinesterase inhibitor | [102] |
| polyketide | 5-hydroxy-2,7-dimethoxy-8-methylnaphthoquinone (33) | Sporormiella minimoides | Hintonia latiflora | human calmodulin inhibitor | [103] |
| polyketide | minimoidione (34) | Sporormiella minimoides | Hintonia latiflora | anti-hyperglycemic | [104] |
| polyketide | vermelhotin (41) | Sporormiella minimoides | Hintonia latiflora | human calmodulin inhibitor | [106] |
| polyketide | 2,4,8-trihydroxy-1-tetralone (64) | Alternaria sp. | Morinda officinalis | anti-hyperglycemic | [110] |
| polyketide | 3,4-dihydro-3,4,8-trihydroxy-1[2H]-naphthalenone (65) | Alternaria sp. | Morinda officinalis | anti-hyperglycemic | [110] |
| polyketide | 6-hydroxy-4-isopropyl-1,8-dimethylspiro[4.5]deca-1,8-dien-7-one (91) | Trichoderma spirale | Morinda officinalis | cytotoxic | [112] |
| polyketide | 2-hydroxy-2,5-dimethyl-7-oxo-5,7-dihydro-2H-furo[3,4-b]pyran-4-carboxylicacid (92) | Trichoderma spirale | Morinda officinalis | cytotoxic | [112] |
| polyketide | 3-ethyl-4-hydroxy-6-methyl-2H-pyran-2-one (93) | Trichoderma spirale | Morinda officinalis | cytotoxic | [112] |
| polyketide | harzialactone A (94) | Trichoderma spirale | Morinda officinalis | cytotoxic | [112] |
| polyketide | 3-hydroxy-5-(4-hydroxybenzyl)dihydrofuran-2(3H)-one (95) | Trichoderma spirale | Morinda officinalis | cytotoxic | [112] |
| polyketide | 4-acetyl-3-hydroxy-6-methyl-pyran-2-one (96) | Trichoderma spirale | Morinda officinalis | cytotoxic | [112] |
| polyketide | multiforisin I (107) | Neurospora discrete | Morinda lucida | cytotoxic | [115] |
| polyketide | curvularide B (110) | Curvularia geniculata | Catunaregam tomentosa | antifungal | [116] |
| polyketide | mycoepoxydiene (114) | Diaporthe pseudomangiferae | Cinchona ledgeriana | cytotoxic | [117] |
| polyketide | mycoleptones A (122) | Mycoleptodiscus indicus | Borreria verticillata | antiprotozoal, cytotoxic | [146] |
| polyketide | mycoleptones B (123) | Mycoleptodiscus indicus | Borreria verticillata | antiprotozoal | [130] |
| polyketide | austidiol (125) | Mycoleptodiscus indicus | Borreria verticillata | antiprotozoal | [130] |
| polyketide | colletopeptide A (162) | Colletotrichum sp. | Rubia pondantha | anti-inflammatory, antioxidant | [130] |
| polyketide | colletopeptide B (163) | Colletotrichum sp. | Rubia pondantha | anti-inflammatory, antioxidant | [130] |
| polyketide | colletopeptide C (164) | Colletotrichum sp. | Rubia pondantha | anti-inflammatory, antioxidant | [130] |
| polyketide | colletopeptide D (165) | Colletotrichum sp. | Rubia pondantha | anti-inflammatory, antioxidant | [130] |
| quinone | 6-epi-stemphytriol (69) | Alternaria sp. | Morinda officinalis | anti-hyperglycemic | [110] |
| quinone | dihydroalterperylenol (70) | Alternaria sp. | Morinda officinalis | anti-hyperglycemic, cytotoxic | [110] |
| quinone | alterperylenol (71) | Alternaria sp. | Morinda officinalis | cytotoxic | [110] |
| quinone | altertoxin II (72) | Alternaria sp. | Morinda officinalis | anti-hyperglycemic | [110] |
| quinone | stemphyperylenol (73) | Alternaria sp. | Morinda officinalis | antifungal, anti-hyperglycemic | [110] |
| quinone | cercosporin (177) | Mycosphaerella sp. | Psychotria horizontalis | antiprotozoal, cytotoxic | [127,128,135] |
| quinone | 6-(heptacosa-18′Zenyl)-2-(18”hydroxyl-1”enyl-19” oxy)-3-hydroxybenzoquinone (202) | Chaetomium cupreum | Mussaenda luteola | antibacterial, cytotoxic | [145] |
| sesquiterpene | xylarenones G (18) | Camarops sp. | Alibertia macrophylla | anti-inflammatory | [100] |
| sesquiterpene | 1R,3S,6S,7R,10S-7-isopropyl-4,10-dimethylbicyclo[4.4.0]dec-4-en-3,10-diol (88) | Trichoderma koningiopsis | Morinda officinalis | cytotoxic | [111] |
| sesquiterpene | 1R,3R,6S,7R,10S-7-isopropyl-4,10-dimethylbicyclo[4.4.0]dec-4-en-3,10-diol (89) | Trichoderma koningiopsis | Morinda officinalis | cytotoxic | [111] |
| sesquiterpene | lithocarin B (102) | Diaporthe lithocarpus | Morinda officinalis | cytotoxic | [114] |
| sesquiterpene | lithocarin C (103) | Diaporthe lithocarpus | Morinda officinalis | cytotoxic | [114] |
| sesquiterpene | tenellone H (105) | Diaporthe lithocarpus | Morinda officinalis | cytotoxic | [114] |
| sesquiterpene | eremofortin F (117) | Diaporthe pseudomangiferae | Cinchona ledgeriana | cytotoxic | [117] |
| sesquiterpene | colletotrichine A (133) | Colletotrichum gloeosporioides | Uncaria rhynchophylla | acetylcholinesterase inhibitor | [125] |
| sesquiterpene | colletotrichine B (134) | Colletotrichum gloeosporioides | Uncaria rhynchophylla | acetylcholinesterase inhibitor | [126] |
| sesquiterpene | stelliophaerols A (192) | Stelliosphaera formicum | Duroia hirsuta | antibacterial | [142] |
| sesquiterpene | stelliophaerols B (193) | Stelliosphaera formicum | Duroia hirsuta | antibacterial | [142] |
| steroid | (3β–5α–dihydroxy–6β–phenylacetyloxy–ergosta–7, 22–diene) (203) | Chaetomium cupreum | Mussaenda luteola | antibacterial, cytotoxic | [145] |
3.1. Antifungal and Antibacterial Activity
Thin layer chromatography (TLC) bioautography indicated that compounds 1 and 2 (isolated from Xylaria sp.) and compounds 6, 9, 10, 12, and 13 (isolated from Penicillium sp.) display activity against Cladosporium cladosporioides and C. sphaerospermum. The most active compounds, orcinol (6), 4-hydroxymellein (9), (R)-7-hydroxymellein (12) and (3R,4R)-4,7-dihydroxymellein (13) showed a potent effect exhibiting a detection limit of 5.0 and 10.0 μg mL−1 against C. cladosporioides and C. sphaerospermum, respectively [97,98,99]. In a disk diffusion assay, curvularide B (110) exhibited activity against Candida albicans with an inhibition zone diameter of 12.1 mm; it also showed synergistic effect with a fluconazole drug [116].
Crude extracts of S. formicum from D. hirsuta showed specific activity against Staphylococcus aureus. Stelliosphaerols A (192) and B (193) were subsequently isolated by bioassay-guided isolation as causative agents of this activity. Following it, the growth inhibition assays revealed minimum inhibitory concentration (MIC) values for stelliosphaerols A and B of approximately 250 μg mL−1 [142]. On the other hand, the meroterpene guignardone I (171), guignardone B (173) and the fatty acid glucoside (174) produced by the endophytic fungal from S. hydrophyllacea showed modest inhibitory effects on S. aureus and methicillin-resistant S. aureus (MRSA) [132,133].
Two new diterpenes, koninginols A (74) and B (75), isolated from the endophytic fungus T. koningiopsis derived from M. officinalis, exhibited significant antibacterial activity against B. subtilis with MIC values of 10 and 2 μg mL−1, respectively [111]. Moreover, the alkaloid 11-bromoroquefortine D (190) was also able to inhibit this bacterium at a concentration of 15 mM [141]. Metabolites 201, 202 and 203 isolated from C. cupreum showed anti-mycobacterial activity against Mycobacterium tuberculosis, with MIC values of 6.3, 6.25 and 25 μg mL−1 [145].
3.2. Neurodegenerative Diseases
Acetylcholinesterase (AChE) and monoamine oxidase (MAO) are enzymatic targets for the search of new drugs for the treatment of neurodegenerative diseases [147,148,149,150]. Diketopiperazine 7 and the dihydroisocoumarins 12, 13, and 9, isolated from Penicillium sp. associated with A. macrophylla, exhibited AChE inhibitory activity and showed a detection limit of 10.0 μg (7, 12, 13) and 30.0 μg (9), respectively [98,99]. On the other hand, xylarenone C (14) isolated from Camarops sp., had a minimum AChE inhibitory concentration of 6.25 μg, while the others compound (15, 22, and 30) from Camarops sp., showed weak acetylcholinesterase (AChE) inhibitory activity [101]. Recently, the sesquiterpenoids colletotrichines A(133) and B (134) produced by C. gloeosporioides inhibited AChE activity with the half-maximal inhibitory concentration IC50 values of 28.0 and 38.0 μg mL−1, respectively [125,126].
Monoamine oxidase (MAO) is an enzyme that catalyzes the oxidative deamination of biogenic amines neurotransmitters. Besides, MAO plays an essential role in the central nervous system and peripheral organs [151,152]. Compound mellein (137), produced by C. gloeosporioides, showed potent MAO inhibitory activity with an IC50 value of 8.93 ± 0.34 μg mL−1, while the standard, iproniazid, was 1.80 ± 0.5 μg mL−1 [124].
3.3. Cytotoxic Activity
The eremophilane sesquiterpenes xylarenone C (14) and xylarenone D (15), isolated from Camarops sp., exhibited cytotoxic activity against human tumor cell lines, such as leukemia (HL-60), melanoma (MDA/MB-435), colon (HCT-8), and glioblastoma (SF-295). The antiproliferative effect was evaluated following 72h of treatment, and the compounds 14 and 15 were more active against MDA/MB-435 (IC50 = 2.4 μg mL−1) and HL-60 (IC50 = 1.2 μg mL−1) cells, respectively [101]. Eremophilane sesquiterpene compounds exhibit phytotoxic potential; antifungal, antibacterial, carcinostatic, and cytotoxic activities; and can act as a phytohormone [100,153].
Cytosporaphenone A (50), produced by the fungus C. rhizophorae, which is derived from M. officinalis, showed uncommon antiproliferative activity against MCF-7 and HepG-2 cell lines at a concentration of 100 μg mL−1, with inhibition rates of 91.0% and 80.5%, respectively [109]. The compounds 70 and 71 from the filamentous fungus Alternaria sp. reduced the viability of four human tumor cells lines: MCF-7, HepG-2, NCI-H460, and SF-268 with IC50 values ranging from 1.91 to 9.67 μM [110]. In addition, the multiforisin I (107) produced by N. discreta showed moderate activity against lymphoma cells, reducing 70% cell growth [115].
Bioassay-guided fractionation of ethyl acetate extract from D. pseudomangifera, by cytotoxic effects against mammalian cancer cells allowed the isolation of the mycoepoxydien (114), which showed cytotoxic activity with IC50 values of 7.5, 17.7, and 15.8 μM against against human uterine cervical carcinoma KB and MDA-MB-435 cells, respectively. The compound eremofortin F (117) was cytotoxic on KB (IC50 = 13.9 μM) and MRC5 (IC50 = 12.2 μM) cells [117]. The azaphilone mycoleptone B (122) isolated from M. indicus associated with B. verticillata presented cytotoxic activity against human prostate cancer (PC3) cells (IC50 = 7.1 ± 3.8 μM). However, when compared with doxorubicin, the reference compound for cytotoxicity assays, its activity was lower [146]. Cercosporin (177), produced by Mycosphaerella sp. associated with P. horizontalis, showed lower cytotoxicity to mammalian Vero cells (1.54 μM) and high potency against MCF7 cancer cell lines (IC50 = 4.68 μM). The analog compound (178) was not active in these assays [136].
The compounds isolated from the endophytic fungus T. koningiopsis were evaluated for cytotoxic activity against HepG-2, MCF-7, SF-268 and A549 cells lines. The compounds koninginol B (75), 1R,3S,6S,7R,10S-7-isopropyl-4,10-dimethylbicyclo[4.4.0]dec-4-en-3,10-diol (88) and 1R,3R,6S,7R,10S-7-isopropyl-4,10-dimethylbicyclo[4.4.0]dec-4-en-3,10-diol (89) showed antiproliferative activities against A549 with IC50 values of 46.6, 31.3 and 22.2 μM, respectively [111]. However, none of the metabolites isolated from T. spirale (91–96) presented cytotoxicity activity against cancer cell lines [112].
The compounds from D. lithocarpus, another endophyte also isolated from M. officinalis, were tested for their cytotoxic activity by the sulforhodamine B method on four human tumor cell lines (SF-268, MCF-7, HepG-2 and A549). The compounds lithocarin B (102), lithocarin C (103), and tenellone H (105) presented IC50 values ranging from 30 to 100 μM in the four tumor cell lines selected [114].
Cancer human cell proliferation (SF-295 and HTC-116) was inhibited by 11-bromo-roquefortine D (190) with rates of 63% and 6.7%, at a concentration of 5.3 μM, respectively [141]. The cytotoxicity activity of 6-(heptacosa-18′Zenyl)-2-(18”hydroxyl-1”enyl-19” oxy)-3-hydroxybenzoquinone (202) and (3β–5α–dihydroxy–6β–phenylacetyloxy–ergosta–7, 22–diene) (203) was evaluated against a breast cancer cell line (MCF-7). They reduced the cell viability by 52% and 49%, respectively, at a concentration of 100μg.mL−1 [145].
The PI3Kα inhibitory activity of compounds isolated from C. gloeosporioides, an endophytic fungus from U. rhynchophylla, was evaluated. The phosphoinositide 3-kinases (PI3Ks), a family of lipid kinases, showed a crucial regulatory role in many cellular processes, including cell proliferation, especially PI3Kα as one of the main targets for therapeutic intervention in cancer [154]. Hence, compounds from C. gloeosporioides were tested for their phosphoinositide 3-kinase (PI3Kα) inhibitory activity. The compounds cyclo(L-leucyl-l-leucyl) (149) and brevianamide F (150) showed potent PI3Kα inhibitory activity with IC50 values of 38.1 and 4.8 μM, respectively, while the other compounds showed weak activity at a concentration of 20 μg·mL−1 [127].
3.4. Anti-Inflammatory and Antioxidant Activity
Xylarenones C, D, F, and G (14, 15, 17, 18) obtained from broth cultures by Camarops sp. showed meaningful inhibitory effect of reactive oxygen species (ROS) produced by stimulated neutrophils. The inhibitory concentrations of 14 (IC50 = 6.13 ± 0.41 μM), 15 (IC50 = 5.73 ± 0.42 μM), 17 (IC50 = 5.90 ± 0.70 μM), and 18 (IC50 = 4.17 ± 0.81 μM) were similar to those of quercetin and apocynin, an efficient inhibitor of the NADPH (nicotinamide adenine dinucleotide phosphate) oxidase complex. Furthermore, the compounds 14, 15, 17 and 18 were also evaluated for their radical scavenging properties in different analytical methods, such as scavengers of superoxide anions (the first ROS produced via the NADPH oxidase complex by stimulated neutrophils), HOCl (the main strong oxidant produced by myeloperoxidase (MPO)), and MPO enzymatic activity, however, the compounds were inactive and had IC50 values of >100 μM [100].
Colletopeptide A (162) isolated from Colletotrichum sp. showed significant anti-inflammatory activity; it inhibited the effects of lipopolysaccharide-induced nitric oxide production with an IC50 value of 8.3 μM. The other colletopeptides, B (163), C (164) and D (165), also inhibited the lipopolysaccharide (LPS)-induced nitric oxide production, with IC50 values of 38.7, 13.5 and 22.2 μM, respectively [130].
3.5. Anti-Protozoal Activity
Azaphilones mycoleptones A and B (122–123) and the polyketide austidiol (125) isolated from M. indicus presented in vitro leishmanicidal activity, being active against Leishmania donovani, with IC50 values of 28.5, 21.7 and 20.5 μM, respectively [146].
The in vitro assay results suggest that cercosporin (177) is highly active against Plasmodium falciparum (IC50 = 1.03 μM), L. donovani (IC50 = 0.46 μM), and Trypanosoma cruzi (IC50 = 1.08 μM). Nevertheless, the bioactivity profile observed for cercosporin indicated that it was not specific for any the assayed parasites [127,128]. Compound 178, identified as a seven-membered dioxepane ring-opened analogue of cercosporin, showed a significant reduction in activity in all these biological assays (IC50 >10 μg mL−1), indicating the importance of the methylenedioxy functionality to the biological properties of compound 177 [136]. On the other hand, the alkaloid quinine (118) produced by Diaporthe sp. is a well-known antimalarial drug that is effective against the erythrocyte stage of the parasite P. falciparum [120,121,146].
3.6. Hyperglycemic Control
The best treatment for type 2 diabetes mellitus (TII-DM) involves hyperglycemic control using appropriate therapies. In recent years, substantial efforts to discover effective inhibitors of α-glucosidases from natural sources have been made [103,104]. The polyketide mimimoidione A (34) isolated from S. minimoides showed an excellent activity against Saccharomyces cerevisiae α-glucosidase (α-GHY), with an IC50 of 2.9 μM [104]. One the other hand, the tridepsides thielavins A (42), J (43) and K (44), isolated from Chaetomium sp. from H. latiflora, inhibited the α-GHY with IC50 values of 23.8, 15.8, and 22.1 μM, respectively. Their inhibitory action was better than the acarbose standard (IC50 = 545 μM). Thielavin J (43) inhibited the activity of α-glucosidase from B. stearothermophilus (αGHBs) with an IC50 = 30.5 μM, being less active than acarbose (IC50 = 0.015 μM) [107]. The thielavin K (44) reduced fasting and postprandial glucose levels in a TII-DM animal model. Therefore, thielavin-type tridepsides represent a new class of α-glucosidase inhibitors and can become hypoglycemic agents for the treatment of TII-DM [107].
The compounds 3S,4R-(+)-4-hydroxymellein (46) and 3S,4S-(+)-4-hydroxymellein (9) inhibited the activity of enzyme S. cerevisiae α-glucosidase, with IC50 values of 441 ± 23 and 549 ± 2.5 μM, respectively [108]. Six compounds from Alternaria sp., namely, 2,4,8-trihydroxy-1-tetralone (64), 3,4-dihydro-3,4,8-trihydroxy-1[2H]-naphthalenone (65), 6-epi-stemphytriol (69), dihydroalterperylenol (70), altertoxin II (72) and stemphyperylenol (73), demonstrated prominent inhibitory activities against α-glucosidase (αGHY). The IC50 values in the range of 12.05 to 166.13 μM, observed for these compounds, were clearly better and more significant when compared to the positive control of acarbose (IC50 = 427.34 μM) [110].
3.7. Other Activities
Proteases are relevant enzymatic targets because these proteins control the formation of functional peptides that participate in physiological processes [155]. The protease inhibitory activity of compounds xylarenones C–E (14–16) was evaluated in vitro using the enzymes subtilisin and pepsin. A potent inhibitory activity for the pepsin and subtilisin in protease assays was observed for compound 14 with an IC50 of 0.288 and 0.462 μM, respectively. However, compounds 15 and 16 displayed no inhibitory activity on subtilisin (<10%) at any of the four concentrations tested (1.00, 0.1, 0.01, and 0.001 μM) [153].
Metabolites produced by S. minimoides were evaluated as potential human calmodulin (hCaM) inhibitors, and two compounds, 5-hydroxy-2,7-dimethoxy-8-methylnaphthoquinone (33) and vermelhotin (41), quenched the extrinsic fluorescence of this biosensor significantly, with dissociation constant (Kd) values of 1.55 μM and 0.25 μM, respectively. The docking displayed studies to predict the interaction of 33 with hCaM and many hydrophobic interactions with Phe19, Phe68, Met51, Met71, Met72 and Ile52. However, vermelhotin (41) showed hydrophobic interactions with Phe92, Met109, Met124, Glu127, Ala128, and Met144 [103,106].
4. Conclusions
As demonstrated in this paper, an increasing number of publications revealed a significant interest in endophytes from the Rubiaceae family in recent years due to pharmacological activities. This review presents the chemical diversity and pharmacological properties of secondary metabolites produced by endophyte fungi associated with various genera of Rubiaceae. Several classes of natural products are described for this endophyte, although this study highlights the importance of some metabolites which are involved in antifungal, antibacterial, and anti-protozoal activities; neurodegenerative diseases, cytotoxic activity, anti-inflammatory and antioxidant activity; and hyperglycemic control.
Acknowledgments
The authors acknowledge the fellowship from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brasil (CAPES).
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Strobel G., Daisy B. Bioprospecting for microbial endophytes and their natural products. Microbiol. Mol. Biol. Rev. 2003;67:491–502. doi: 10.1128/MMBR.67.4.491-502.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calixto J.B. The role of natural products in modern drug discovery. An. Acad. Bras. Cienc. 2019;91(Suppl. 3):e20190105. doi: 10.1590/0001-3765201920190105. [DOI] [PubMed] [Google Scholar]
- 3.Singh N., Chandra R. Endophytes: Unexplored reservoir of bioactive natural products. In: Gupta N., editor. Handbook of Medicinal Plants and their Bioactive Compounds. Research Signpost; Kerala, India: 2014. pp. 1–10. [Google Scholar]
- 4.Kaul S., Gupta S., Ahmed M., Dhar M.K. Endophytic fungi from medicinal plants: A treasure hunt for bioactive metabolites. Phytochem. Rev. 2012;11:487–505. doi: 10.1007/s11101-012-9260-6. [DOI] [Google Scholar]
- 5.Newman D.J., Cragg G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016;79:629–661. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- 6.Newman D.J., Cragg G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020;83:770–803. doi: 10.1021/acs.jnatprod.9b01285. [DOI] [PubMed] [Google Scholar]
- 7.Gunatilaka A.A.L. Natural products from plant-associated microorganisms: Distribution, structural diversity, bioactivity, and implications of their occurrence. J. Nat. Prod. 2006;69:509–526. doi: 10.1021/np058128n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chandra S. Endophytic fungi: Novel sources of anticancer lead molecules. Appl. Microbiol. Biotechnol. 2012;95:47–59. doi: 10.1007/s00253-012-4128-7. [DOI] [PubMed] [Google Scholar]
- 9.Martinez-Klimova E., Rodriguez-Pena K., Sanchez S. Endophytes as sources of antibiotics. Biochem. Pharmacol. 2017;134:1–17. doi: 10.1016/j.bcp.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 10.Barbero M., Artuso E., Prandi C. Fungal anticancer metabolites: Synthesis towards drug discovery. Curr. Med. Chem. 2018;25:141–185. doi: 10.2174/0929867324666170511112815. [DOI] [PubMed] [Google Scholar]
- 11.Lima M.A., de Oliveira M.C., Pimenta A., Uchoa P. Aspergillus niger: A hundred years of contribution to the natural products chemistry. J. Braz. Chem. Soc. 2019;30:2029–2059. doi: 10.21577/0103-5053.20190080. [DOI] [Google Scholar]
- 12.Torres-Mendoza D., Ortega H.E., Cubilla-Rios L. Patents on endophytic fungi related to secondary metabolites and biotransformation applications. J. Fungi. 2020;6:58. doi: 10.3390/jof6020058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Azevedo J.L. Microrganismos endofíticos. In: Melo I.S., Azevedo J.L., editors. Ecologia Microbiana. Embrapa-CNPMA; Jaguariuna, Brazil: 1998. pp. 117–137. [Google Scholar]
- 14.Stone J., Bacon C.W., White J.F. An overview of endophytic microbes: Endophytism defined. In: Bacon C.W., White J.F., editors. Microbial Endophytes. Marcel Deker Inc.; New York, NY, USA: 2000. pp. 3–29. [Google Scholar]
- 15.Kusari S., Hertweck C., Spiteller M. Chemical ecology of endophytic fungi: Origins of secondary metabolites. Chem. Biol. 2012;19:792–798. doi: 10.1016/j.chembiol.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Vasundhara M., Reddy M.S., Kumar A. New and Future Developments in Microbial Biotechnology and Bioengineering. Elsevier; Amsterdam, The Nertherlands: 2019. Secondary metabolites from endophytic fungi and their biological activities; pp. 237–258. [DOI] [Google Scholar]
- 17.Guo B., Wang Y., Sun X., Tang K. Bioactive natural products from endophytes: A review. Appl. Biochem. Microbiol. 2008;44:136–142. doi: 10.1134/S0003683808020026. [DOI] [PubMed] [Google Scholar]
- 18.Dreyfuss M.M., Chapela I.H. Fungi as producers of secondary metabolites. In: Gullo V.P., editor. Discovery of Natural Products with Therapeutic Potencial. Butterworth-Heinemann; Boston, MA, USA: 1994. pp. 49–79. [Google Scholar]
- 19.Debbab A., Aly A.H., Proksch P. Bioactive secondary metabolites from endophytes and associated marine derived fungi. Fungal Divers. 2011;49:1–12. doi: 10.1007/s13225-011-0114-0. [DOI] [Google Scholar]
- 20.Chapla V.M., Biasetto C.R., Araujo A.R. Fungos endofíticos: Uma fonte inexplorada e sustentável de novos e bioativos produtos naturais. Rev. Virtual Quim. 2013;5:421–437. doi: 10.5935/1984-6835.20130036. [DOI] [Google Scholar]
- 21.Schulz B., Boyle C., Draeger S., Römmert A.K., Krohn K. Endophytic fungi: A source of novel biologically active secondary metabolites. Mycol. Res. 2002;106:996–1004. doi: 10.1017/S0953756202006342. [DOI] [Google Scholar]
- 22.Zhang H.W., Song Y.C., Tan R.X. Biology and chemistry of endophytes. Nat. Prod. Rep. 2006;23:753. doi: 10.1039/b609472b. [DOI] [PubMed] [Google Scholar]
- 23.Tan R.X., Zou W.X. Endophytes: A rich source of functional metabolites (1987 to 2000) Nat. Prod. Rep. 2001;18:448–459. doi: 10.1039/b100918o. [DOI] [PubMed] [Google Scholar]
- 24.Gallo M.B.C., Guimarães D.O., Momesso L.S., Pupo M.T. Natural products from endophytic fungi. In: Saikai R., Bezbaruah R.L., Bora T.C., editors. Microbial Biotechnology. New India Publishing Agency; New Deli, India: 2007. pp. 139–168. [Google Scholar]
- 25.Martins D., Nunez C. Secondary metabolites from Rubiaceae species. Molecules. 2015;20:13422–13495. doi: 10.3390/molecules200713422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pereira Z.V., Carvalho-Okano R.M., Garcia F.C.P. Rubiaceae Juss. da Reserva Florestal Mata do Paraíso, Viçosa, MG, Brasil. Acta Bot. Brasilica. 2006;20:207–224. doi: 10.1590/S0102-33062006000100020. [DOI] [Google Scholar]
- 27.Mongrand S., Badoc A., Patouille B., Lacomblez C., Chavent M., Bessoule J.J. Chemotaxonomy of the Rubiaceae family based on leaf fatty acid composition. Phytochemistry. 2005;66:549–559. doi: 10.1016/j.phytochem.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 28.Bolzani V.D.S., Young M.C.M., Furlan M., Cavalheiro A.J., Araújo A.R., Silva D.H.S., Lopes M.N. Secondary metabolites from brazilian Rubiaceae plant species: Chemotaxonomical and biolgical significance. In: Pandalai S.G., editor. Recent Research Developments in Phytochemistry. Volume 5. Research Signpost; Kerala, India: 2001. pp. 19–31. [Google Scholar]
- 29.Cardoso C.L., Silva D.H.S., Young M.C.M., Castro-Gamboa I., Bolzani V.D.S. Indole monoterpene alkaloids from Chimarrhis turbinata DC Prodr.: A contribution to the chemotaxonomic studies of the Rubiaceae family. Rev. Bras. Farmacogn. 2008;18:26–29. doi: 10.1590/S0102-695X2008000100007. [DOI] [Google Scholar]
- 30.Rosa E.A., Silva B.C., Silva F.M., Tanaka C.M.A., Peralta R.M., Oliveira C.M.A., Kato L., Ferreira H.D., Silva C.C. Flavonoides e atividade antioxidante em Palicourea rigida Kunth, Rubiaceae. Rev. Bras. Farmacogn. 2010;20:484–488. doi: 10.1590/S0102-695X2010000400004. [DOI] [Google Scholar]
- 31.Rosales P.F., Bordin G.S., Gower A.E., Moura S. Indole alkaloids: 2012 until now, highlighting the new chemical structures and biological activities. Fitoterapia. 2020;143:104558. doi: 10.1016/j.fitote.2020.104558. [DOI] [PubMed] [Google Scholar]
- 32.Soares D.B., Duarte L., Cavalcanti A., Silva F., Braga A., Lopes M.T., Takarashi J., Vieira-Filho S. Psychotria viridis: Chemical constituents from leaves and biological properties. An. Acad. Bras. Cienc. 2017;89:927–938. doi: 10.1590/0001-3765201720160411. [DOI] [PubMed] [Google Scholar]
- 33.Moreira V.F., Vieira I.J.C., Braz-Filho R. Chemistry and biological activity of Condamineeae tribe: A chemotaxonomic contribution of Rubiaceae family. Am. J. Plant Sci. 2015;6:2612–2631. doi: 10.4236/ajps.2015.616264. [DOI] [Google Scholar]
- 34.Sweelam H.M., Abd-Alla H.I., Abdelwahab A.B., Gabr M.M., Kirsch G. Secondary metabolites and biological activity of Pentas species: A minireview. J. Adv. Res. 2018;10:21–30. doi: 10.1016/j.jare.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dompeipen E.J., Srikandace Y., Suharso W.P., Cahyana H., Simanjunta P. Potential endophytic microbes selection for antidiabetic bioactive compounds production. Asian J. Biochem. 2011;6:465–471. doi: 10.3923/ajb.2011.465.471. [DOI] [Google Scholar]
- 36.Handayani D., Sandrawati N., Ruslan R., Nestianda O., Fajrina A., Tallei T.E. Cytotoxic and antimicrobial activities of ethyl acetate extract of mangrove plant Scyphiphora hydrophyllacea C. F. Gaertn—Associated fungi. J. Appl. Pharm. Sci. 2019;9:75–79. doi: 10.7324/JAPS.2019.90610. [DOI] [Google Scholar]
- 37.Qian X., Chen L., Guo X., He D., Shi M., Zhang D. Shifts in community composition and co-occurrence patterns of phyllosphere fungi inhabiting Mussaenda shikokiana along an elevation gradient. PeerJ. 2018;6:e5767. doi: 10.7717/peerj.5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nasr S., Lutz M., Amoozegar M.A., Eparvier V., Stien D., Fazeli S.A.S., Yurkov A. Graphiola fimbriata: The first species of Graphiolaceae (Exobasidiales, Basidiomycota) described only based on its yeast stage. Mycol. Prog. 2019;18:359–368. doi: 10.1007/s11557-018-1450-1. [DOI] [Google Scholar]
- 39.Oliveira C., Silva G., Bolzani V., Young M., Silva D., Lopes M., Araújo A. Two new metabolites of an undescribed endophytic fungus belonging to the order pleosporales isolated from Alibertia macrophylla (Rubiaceae) Planta Med. 2008;74:1057. doi: 10.1055/s-0028-1084439. [DOI] [Google Scholar]
- 40.Abdalla M.A., Aro A.O., Gado D., Passari A.K., Mishra V.K., Singh B.P., McGaw L.J. Isolation of endophytic fungi from South African plants, and screening for their antimicrobial and extracellular enzymatic activities and presence of type I polyketide synthases. South African J. Bot. 2020:1–7. doi: 10.1016/j.sajb.2020.03.021. [DOI] [Google Scholar]
- 41.Ferreira M.C., de Assis J.C.S., Rosa L.H. Diversity of endophytic fungi associated with Carapichea ipecacuanha from a native fragment of the Atlantic Rain Forest. South African J. Bot. 2020 doi: 10.1016/j.sajb.2019.12.031. [DOI] [Google Scholar]
- 42.Lemaire B., Lachenaud O., Persson C., Smets E., Dessein S. Screening for leaf-associated endophytes in the genus Psychotria (Rubiaceae) FEMS Microbiol. Ecol. 2012;81:364–372. doi: 10.1111/j.1574-6941.2012.01356.x. [DOI] [PubMed] [Google Scholar]
- 43.Conti R. Endophytic microorganisms from leaves of Spermacoce verticillata (L.): Diversity and antimicrobial activity. J. Appl. Pharm. Sci. 2012;2:17–22. doi: 10.7324/JAPS.2012.21204. [DOI] [Google Scholar]
- 44.de Lima T.E.F., Oliveira R.J.V., Neves R.P., Bezerra J.L., de Cavalcanti M.A.Q. Endophytic yeasts of Coffea arabica and Vitis labrusca cv. Isabel from Pernambuco, Brazil. Nov. Hedwigia. 2013;96:463–469. doi: 10.1127/0029-5035/2013/0080. [DOI] [Google Scholar]
- 45.Wu Y., Girmay S., da Silva V.M., Perry B., Hu X., Tan G.T. The role of endophytic fungi in the anticancer activity of Morinda citrifolia Linn. (Noni) Evidence-Based Complement. Altern. Med. 2015;2015:1–8. doi: 10.1155/2015/393960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sheik S., Chandrashekar K.R., Shirlal S. Endophytic fungi associated with Psychotria flavida Talbot., an endemic plant of Western Ghats (India) and their bioactive potential. Orient. Pharm. Exp. Med. 2018;18:149–158. doi: 10.1007/s13596-018-0308-z. [DOI] [Google Scholar]
- 47.Liu Y., Bai F., Li T., Yan H. An endophytic strain of genus Paenibacillus isolated from the fruits of Noni (Morinda citrifolia L.) has antagonistic activity against a Noni’s pathogenic strain of genus Aspergillus. Microb. Pathog. 2018;125:158–163. doi: 10.1016/j.micpath.2018.09.018. [DOI] [PubMed] [Google Scholar]
- 48.Freire E.S., Campos V.P., Pinho R.S.C., Oliveira D.F., Faria M.R., Pohlit A.M., Noberto N.P., Rezende E.L., Pfenning L.H., Silva J.R.C. Volatile substances produced by fusarium oxysporum from coffee rhizosphere and other microbes affect meloidogyne incognita and arthrobotrys conoides. J. Nematol. 2012;44:321–328. [PMC free article] [PubMed] [Google Scholar]
- 49.Chaves F.C., Gianfagna T.J., Aneja M., Posada F., Peterson S.W., Vega F.E. Aspergillus oryzae NRRL 35191 from coffee, a non-toxigenic endophyte with the ability to synthesize kojic acid. Mycol. Prog. 2012;11:263–267. doi: 10.1007/s11557-011-0745-2. [DOI] [Google Scholar]
- 50.Casella T.M., Eparvier V., Mandavid H., Bendelac A., Odonne G., Dayan L., Duplais C., Espindola L.S., Stien D. Antimicrobial and cytotoxic secondary metabolites from tropical leaf endophytes: Isolation of antibacterial agent pyrrocidine C from Lewia infectoria SNB-GTC2402. Phytochemistry. 2013;96:370–377. doi: 10.1016/j.phytochem.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 51.Araujo A., Habeck T., Gubiani J., Silva D., Cavalheiro A., Bolzani V. Phenolics compounds produced by Camarops sp. an endophytic fungus from Alibertia macrophylla (Rubiaceae) Planta Med. 2013;79:1182. doi: 10.1055/s-0033-1352098. [DOI] [Google Scholar]
- 52.Araujo A., Gubiani J., Cavalheiro A., Bolzani V.D.S., Nogueira C. A new compound from Camarops sp. an endophytic fungus in Alibertia macrophylla (Rubiaceae) Planta Med. 2014;80:775. doi: 10.1055/s-0034-1382412. [DOI] [Google Scholar]
- 53.Chow Y., Ting A.S.Y. Endophytic l-asparaginase-producing fungi from plants associated with anticancer properties. J. Adv. Res. 2015;6:869–876. doi: 10.1016/j.jare.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Monteiro M.C.P., Alves N.M., Queiroz M.V., Pinho D.B., Pereira O.L., Souza S.M.C., Cardoso P.G. Antimicrobial activity of endophytic fungi from coffee plants. Biosci. J. 2017:381–389. doi: 10.14393/BJ-v33n2-34494. [DOI] [Google Scholar]
- 55.Mata R., Figueroa M., Rivero-Cruz I., Macias-Rubalcava M.L. Insights in fungal bioprospecting in Mexico. Planta Med. 2018;84:594–605. doi: 10.1055/s-0044-101551. [DOI] [PubMed] [Google Scholar]
- 56.Helfrich E.J.N., Piel J. Biosynthesis of polyketides by trans-AT polyketide synthases. Nat. Prod. Rep. 2016;33:231–316. doi: 10.1039/C5NP00125K. [DOI] [PubMed] [Google Scholar]
- 57.Fisch K.M. Biosynthesis of natural products by microbial iterative hybrid PKS–NRPS. RSC Adv. 2013;3:18228. doi: 10.1039/c3ra42661k. [DOI] [Google Scholar]
- 58.Crawford J.M., Townsend C.A. New insights into the formation of fungal aromatic polyketides. Nat. Rev. Microbiol. 2010;8:879–889. doi: 10.1038/nrmicro2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Horsman M.E., Hari T.P.A., Boddy C. Polyketide synthase and non-ribosomal peptide synthetase thioesterase selectivity: Logic gate or a victim of fate? Nat. Prod. Rep. 2016;33:183–202. doi: 10.1039/C4NP00148F. [DOI] [PubMed] [Google Scholar]
- 60.Hur G.H., Vickery C.R., Burkart M. Explorations of catalytic domains in non-ribosomal peptide synthetase enzymology. Nat. Prod. Rep. 2012;29:1074. doi: 10.1039/c2np20025b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Singh M., Chaudhary S., Sareen D. Non-ribosomal peptide synthetases: Identifying the cryptic gene clusters and decoding the natural product. J. Biosci. 2017;42:175–187. doi: 10.1007/s12038-017-9663-z. [DOI] [PubMed] [Google Scholar]
- 62.Zhao P., Xue Y., Li J., Li X., Zu X., Zhao Z., Quan C., Gao W., Feng S. Non-lipopeptide fungi-derived peptide antibiotics developed since 2000. Biotechnol. Lett. 2019;41:651–673. doi: 10.1007/s10529-019-02677-3. [DOI] [PubMed] [Google Scholar]
- 63.Mishra A., Choi J., Choi S.J., Baek K.H. Cyclodipeptides: An overview of their biosynthesis and biological activity. Molecules. 2017;22:1796. doi: 10.3390/molecules22101796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nagano N., Umemura M., Izumikawa M., Kawano J., Ishii T., Kikuchi M., Tomii K., Kumagai T., Yoshimi A., Machida M. Class of cyclic ribosomal peptide synthetic genes in filamentous fungi. Fungal Genet. Biol. 2016;86:58–70. doi: 10.1016/j.fgb.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 65.Zhang C., Chen X., Orban A., Shukal S., Birk F., Too H.P., Rühl M. Agrocybe aegerita serves as a gateway for identifying sesquiterpene biosynthetic enzymes in higher fungi. ACS Chem. Biol. 2020;15:1268–1277. doi: 10.1021/acschembio.0c00155. [DOI] [PubMed] [Google Scholar]
- 66.Chen Y., Naresh A., Liang S., Lin C., Chein R., Lin H. Discovery of a dual function cytochrome P450 that catalyzes enyne formation in cyclohexanoid terpenoid biosynthesis. Angew. Chemie. 2020:ange.202004435. doi: 10.1002/ange.202004435. [DOI] [PubMed] [Google Scholar]
- 67.Yamaguchi S. Gibberellin metabolism and its regulation. Annu. Rev. Plant Biol. 2008;59:225–251. doi: 10.1146/annurev.arplant.59.032607.092804. [DOI] [PubMed] [Google Scholar]
- 68.Fraley A.E., Sherman D.H. Enzyme evolution in fungal indole alkaloid biosynthesis. FEBS J. 2020;287:1381–1402. doi: 10.1111/febs.15270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kishimoto S., Sato M., Tsunematsu Y., Watanabe K. Evaluation of biosynthetic pathway and engineered biosynthesis of alkaloids. Molecules. 2016;21:1078. doi: 10.3390/molecules21081078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Finefield J.M., Frisvad J.C., Sherman D.H., Williams R.M. Fungal origins of the bicyclo[2.2.2]diazaoctane ring system of prenylated indole alkaloids. J. Nat. Prod. 2012;75:812–833. doi: 10.1021/np200954v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li S.M. Prenylated indole derivatives from fungi: Structure diversity, biological activities, biosynthesis and chemoenzymatic synthesis. Nat. Prod. Rep. 2010;27:57–78. doi: 10.1039/B909987P. [DOI] [PubMed] [Google Scholar]
- 72.Steffan N., Grundmann A., Yin W.B., Kremer A., Li S.M. Indole prenyltransferases from fungi: A new enzyme group with high potential for the production of prenylated indole derivatives. Curr. Med. Chem. 2009;16:218–231. doi: 10.2174/092986709787002772. [DOI] [PubMed] [Google Scholar]
- 73.Xu W., Gavia D.J., Tang Y. Biosynthesis of fungal indole alkaloids. Nat. Prod. Rep. 2014;31:1474–1487. doi: 10.1039/C4NP00073K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Matsuda Y., Abe I. Biosynthesis of fungal meroterpenoids. Nat. Prod. Rep. 2016;33:26–53. doi: 10.1039/C5NP00090D. [DOI] [PubMed] [Google Scholar]
- 75.Mosunova O., Navarro-Muñoz J.C., Collemare J. Encyclopedia of Mycology. Elsevier; Amsterdam, The Netherlands: 2020. The biosynthesis of fungal secondary metabolites: From fundamentals to biotechnological applications; pp. 1–19. [DOI] [Google Scholar]
- 76.Keller N.P. Fungal secondary metabolism: Regulation, function and drug discovery. Nat. Rev. Microbiol. 2019;17:167–180. doi: 10.1038/s41579-018-0121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Skellam E. Strategies for Engineering Natural Product Biosynthesis in Fungi. Trends Biotechnol. 2019;37:416–427. doi: 10.1016/j.tibtech.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 78.Nielsen J.C., Nielsen J. Development of fungal cell factories for the production of secondary metabolites: Linking genomics and metabolism. Synth. Syst. Biotechnol. 2017;2:5–12. doi: 10.1016/j.synbio.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wisecaver J.H., Slot J.C., Rokas A. The evolution of fungal metabolic pathways. PLoS Genet. 2014;10:e1004816. doi: 10.1371/journal.pgen.1004816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lim F.Y., Keller N.P. Spatial and temporal control of fungal natural product synthesis. Nat. Prod. Rep. 2014;31:1277–1286. doi: 10.1039/C4NP00083H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wiemann P., Keller N.P. Strategies for mining fungal natural products. J. Ind. Microbiol. Biotechnol. 2014;41:301–313. doi: 10.1007/s10295-013-1366-3. [DOI] [PubMed] [Google Scholar]
- 82.Craney A., Ahmed S., Nodwell J. Towards a new science of secondary metabolism. J. Antibiot. (Tokyo) 2013;66:387–400. doi: 10.1038/ja.2013.25. [DOI] [PubMed] [Google Scholar]
- 83.Brakhage A.A., Schroeckh V. Fungal secondary metabolites—Strategies to activate silent gene clusters. Fungal Genet. Biol. 2011;48:15–22. doi: 10.1016/j.fgb.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 84.Hoffmeister D., Keller N.P. Natural products of filamentous fungi: Enzymes, genes, and their regulation. Nat. Prod. Rep. 2007;24:393–416. doi: 10.1039/B603084J. [DOI] [PubMed] [Google Scholar]
- 85.Lingappa B.T. Synchytrium borreriae, an endophytic alga. Mycologia. 1956;48:427. doi: 10.1080/00275514.1956.12024550. [DOI] [Google Scholar]
- 86.Shibuya H., Kitamura C., Maehara S., Nagahata M., Winarno H., Simanjuntak P., Kim H.-S., Wataya Y., Ohashi K. Transformation of Cinchona alkaloids into 1-N-oxide derivatives by endophytic Xylaria sp. isolated from Cinchona pubescens. Chem. Pharm. Bull. (Tokyo) 2003;51:71–74. doi: 10.1248/cpb.51.71. [DOI] [PubMed] [Google Scholar]
- 87.Souza A.Q.L., Souza A.D.L., Astolfi Filho S., Pinheiro M.L.B., Sarquis M.I.M., Pereira J.O. Atividade antimicrobiana de fungos endofíticos isolados de plantas tóxicas da amazônia: Palicourea longiflora (aubl.) rich e Strychnos cogens bentham. Acta Amaz. 2004;34:185–195. doi: 10.1590/S0044-59672004000200006. [DOI] [Google Scholar]
- 88.Jimenez-Salgado T., Fuentes-Ramirez L.E., Tapia-Hernandez A., Mascarua-Esparza M.A., Martinez-Romero E., Caballero-Mellado J. Coffea arabica L., a new host plant for Acetobacter diazotrophicus, and isolation of other nitrogen-fixing acetobacteria. Appl. Environ. Microbiol. 1997;63:3676–3683. doi: 10.1128/AEM.63.9.3676-3683.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ferreira J.B., de Abreu M.S., Pereira I.S. Incidência de Colletotrichum spp. em frutos de Coffea arabica L. em diferentes estádios fisiológicos e tecidos do fruto maduro. Ciência e Agrotecnologia. 2005;29:880–885. doi: 10.1590/S1413-70542005000400022. [DOI] [Google Scholar]
- 90.Peterson S.W., Vega F.E., Posada F., Nagai C. Penicillium coffeae, a new endophytic species isolated from a coffee plant and its phylogenetic relationship to P. fellutanum, P. thiersii and P. brocae based on parsimony analysis of multilocus DNA sequences. Mycologia. 2005;97:659–666. doi: 10.1080/15572536.2006.11832796. [DOI] [PubMed] [Google Scholar]
- 91.Santamaria J., Bayman P. Fungal epiphytes and endophytes of Coffee leaves (Coffea arabica) Microb. Ecol. 2005;50:1–8. doi: 10.1007/s00248-004-0002-1. [DOI] [PubMed] [Google Scholar]
- 92.Vega F.E., Pava-Ripoll M., Posada F., Buyer J.S. Endophytic bacteria in Coffea arabica L. J. Basic Microbiol. 2005;45:371–380. doi: 10.1002/jobm.200410551. [DOI] [PubMed] [Google Scholar]
- 93.Strobel G.A., Ford E., Li J.Y., Sears J., Sidhu R.S., Hess W.M. Seimatoantlerium tepuiense gen. nov., a unique epiphytic fungus producing taxol from the Venezuelan Guyana. Syst. Appl. Microbiol. 1999;22:426–433. doi: 10.1016/S0723-2020(99)80052-6. [DOI] [PubMed] [Google Scholar]
- 94.Pandi M., Manikandan R., Muthumary J. Anticancer activity of fungal taxol derived from Botryodiplodia theobromae Pat., an endophytic fungus, against 7, 12 dimethyl benz(a)anthracene (DMBA)-induced mammary gland carcinogenesis in Sprague dawley rats. Biomed. Pharmacother. 2010;64:48–53. doi: 10.1016/j.biopha.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 95.Pandi M., Kumaran R.S., Choi Y.K., Kim H.J., Muthumary J. Isolation and detection of taxol, an anticancer drug produced from Lasiodiplodia theobromae, an endophytic fungus of the medicinal plant Morinda citrifolia. African J. Biotechnol. 2011;10:1428–1435. doi: 10.5897/AJB10.950. [DOI] [Google Scholar]
- 96.Suresh G., Kokila D., Suresh T.C., Kumaran S., Velmurugan P., Vedhanayakisri K.A., Sivakumar S., Ravi A.V. Mycosynthesis of anticancer drug taxol by Aspergillus oryzae, an endophyte of Tarenna asiatica, characterization, and its activity against a human lung cancer cell line. Biocatal. Agric. Biotechnol. 2020;24:101525. doi: 10.1016/j.bcab.2020.101525. [DOI] [Google Scholar]
- 97.Cafeu M.C., Silva G.H., Teles H.L., Bolzani V.D.S., Araújo A.R., Young M.C.M., Pfenning L.H. Substâncias antifúngicas de Xylaria sp., um fungo endofítico isolado de Palicourea marcgravii (Rubiaceae) Quim. Nova. 2005;28:991–995. doi: 10.1590/S0100-40422005000600011. [DOI] [Google Scholar]
- 98.Oliveira C.M., Silva G.H., Regasini L.O., Zanardi L.M., Evangelista A.H., Young M.C.M., Bolzani V.D.S., Araujo A.R. Bioactive metabolites produced by Penicillium sp.1 and sp.2, two endophytes associated with Alibertia macrophylla (Rubiaceae) Zeitschrift für Naturforsch. C. 2009;64:824–830. doi: 10.1515/znc-2009-11-1212. [DOI] [PubMed] [Google Scholar]
- 99.Oliveira C.M., Regasini L.O., Silva G.H., Pfenning L.H., Young M.C.M., Berlinck R.G.S., Bolzani V.D.S., Araujo A.R. Dihydroisocoumarins produced by Xylaria sp. and Penicillium sp., endophytic fungi associated with Piper aduncum and Alibertia macrophylla. Phytochem. Lett. 2011;4:93–96. doi: 10.1016/j.phytol.2010.11.003. [DOI] [Google Scholar]
- 100.Gubiani J.R., Zeraik M.L., Oliveira C.M., Ximenes V.F., Nogueira C.R., Fonseca L.M., Silva D.H.S., Bolzani V.S., Araujo A.R. Biologically active eremophilane-type sesquiterpenes from Camarops sp., an endophytic fungus isolated from Alibertia macrophylla. J. Nat. Prod. 2014;77:668–672. doi: 10.1021/np400825s. [DOI] [PubMed] [Google Scholar]
- 101.Gubiani J.R., Nogueira C.R., Pereira M.D.P., Young M.C.M., Ferreira P.M.P., de Moraes M.O., Pessoa C., Bolzani V.S., Araujo A.R. Rearranged sesquiterpenes and branched polyketides produced by the endophyte Camarops sp. Phytochem. Lett. 2016;17:251–257. doi: 10.1016/j.phytol.2016.08.007. [DOI] [Google Scholar]
- 102.Gubiani J.R., Habeck T.R., Chapla V.M., Silva G., Bolzani V. da S.; Araújo, A.R. One stain-many compounds (OSMAC) method for production of phenolic compounds using Camarops sp., an endophytic fungus from Alibertia macrophylla (Rubiaceae) Quim. Nova. 2016;39:1221–1224. doi: 10.21577/0100-4042.20160169. [DOI] [Google Scholar]
- 103.Leyte-Lugo M., Figueroa M., Gonzalez M.C., Glenn A.E., González-Andrade M., Mata R. Metabolites from the entophytic fungus Sporormiella minimoides isolated from Hintonia latiflora. Phytochemistry. 2013;96:273–278. doi: 10.1016/j.phytochem.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 104.Rangel-Grimaldo M., Rivero-Cruz I., Madariaga-Mazon A., Figueroa M., Mata R. α-Glucosidase inhibitors from Preussia minimoides. J. Nat. Prod. 2017;80:582–587. doi: 10.1021/acs.jnatprod.6b00574. [DOI] [PubMed] [Google Scholar]
- 105.Leyte-Lugo M., Cerda-Garcia-Rojas C., Gonzalez M.C., Glenn A., Mata R. 9S,11R-(+)-Ascosalitoxin from an endophytic fungus isolated from Hintonia latiflora. Planta Med. 2012;78:1157. doi: 10.1055/s-0032-1320743. [DOI] [PubMed] [Google Scholar]
- 106.Leyte-Lugo M., Gonzalez-Andrade M., Gonzalez M.C., Glenn A.E., Cerda-Garcia-Rojas C.M., Mata R. (+)-Ascosalitoxin and vermelhotin, a calmodulin inhibitor, from an endophytic fungus isolated from Hintonia latiflora. J. Nat. Prod. 2012;75:1571–1577. doi: 10.1021/np300327y. [DOI] [PubMed] [Google Scholar]
- 107.Rivera-Chavez J., Gonzalez-Andrade M., Gonzalez M.C., Glenn A.E., Mata R. Thielavins A, J and K: α-Glucosidase inhibitors from MEXU 27095, an endophytic fungus from Hintonia latiflora. Phytochemistry. 2013;94:198–205. doi: 10.1016/j.phytochem.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 108.Rivera-Chavez J., Figueroa M., Gonzalez M.C., Glenn A.E., Mata R. α-Glucosidase inhibitors from a Xylaria feejeensis associated with Hintonia latiflora. J. Nat. Prod. 2015;78:730–735. doi: 10.1021/np500897y. [DOI] [PubMed] [Google Scholar]
- 109.Liu H.X., Tan H.B., Liu Y., Chen Y.C., Li S.N., Sun Z.H., Li H.H., Qiu S.X., Zhang W.M. Three new highly-oxygenated metabolites from the endophytic fungus Cytospora rhizophorae A761. Fitoterapia. 2017;117:1–5. doi: 10.1016/j.fitote.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 110.Wang Y., Liu H.X., Chen Y.C., Sun Z.H., Li H.H., Li S.N., Yan M.L., Zhang W.M. Two new metabolites from the endophytic fungus Alternaria sp. A744 derived from Morinda officinalis. Molecules. 2017;22:765. doi: 10.3390/molecules22050765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen S., Li H., Chen Y., Li S., Xu J., Guo H., Liu Z., Zhu S., Liu H., Zhang W. Three new diterpenes and two new sesquiterpenoids from the endophytic fungus Trichoderma koningiopsis A729. Bioorg. Chem. 2019;86:368–374. doi: 10.1016/j.bioorg.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 112.Chen S., Liu H., Liu Z., Li S., Chen Y., Li H., Li D., Zhang W. Two new polyketide compounds from the endophytic fungus Trichoderma spirale A725 of Morinda officinalis. Chin. J. Org. Chem. 2020 doi: 10.6023/cjoc201907041. [DOI] [Google Scholar]
- 113.Liu H.X., Tan H.B., Chen K., Zhao L.Y., Chen Y.C., Li S.N., Li H.H., Zhang W. Cytosporins A–D, novel benzophenone derivatives from the endophytic fungus Cytospora rhizophorae A761. Org. Biomol. Chem. 2019;17:2346–2350. doi: 10.1039/C8OB03223H. [DOI] [PubMed] [Google Scholar]
- 114.Liu H., Chen Y., Li H., Li S., Tan H., Liu Z., Li D., Liu H., Zhang W. Four new metabolites from the endophytic fungus Diaporthe lithocarpus A740. Fitoterapia. 2019;137:104260. doi: 10.1016/j.fitote.2019.104260. [DOI] [PubMed] [Google Scholar]
- 115.Sowemimo A.A., Edrada-Ebel R., Ebel R., Proksch P., Omobuwajo O.R., Adesanya S.A. Major constituents of the predominant endophytic fungi from the Nigerian plants Bryophyllum Pinnatum, Morinda Lucida and Jathropha Gossypiifolia. Nat. Prod. Commun. 2008;3:1934578X0800300. doi: 10.1177/1934578X0800300802. [DOI] [Google Scholar]
- 116.Chomcheon P., Wiyakrutta S., Aree T., Sriubolmas N., Ngamrojanavanich N., Mahidol C., Ruchirawat S., Kittakoop P. Curvularides A-E: Antifungal hybrid peptide-polyketides from the endophytic fungus Curvularia geniculata. Chem. A Eur. J. 2010;16:11178–11185. doi: 10.1002/chem.201000652. [DOI] [PubMed] [Google Scholar]
- 117.Mandavid H., Rodrigues A.M.S., Espindola L.S., Eparvier V., Stien D. Secondary metabolites isolated from the Amazonian endophytic fungus Diaporthe sp. SNB-GSS10. J. Nat. Prod. 2015;78:1735–1739. doi: 10.1021/np501029s. [DOI] [PubMed] [Google Scholar]
- 118.Maehara S., Simanjuntak P., Ohashi K., Shibuya H. Composition of endophytic fungi living in Cinchona ledgeriana (Rubiaceae) J. Nat. Med. 2010;64:227–230. doi: 10.1007/s11418-009-0380-2. [DOI] [PubMed] [Google Scholar]
- 119.Maehara S., Simanjuntak P., Kitamura C., Ohashi K., Shibuya H. Cinchona alkaloids are also produced by an endophytic filamentous fungus living in Cinchona plant. Chem. Pharm. Bull. (Tokyo) 2011;59:1073–1074. doi: 10.1248/cpb.59.1073. [DOI] [PubMed] [Google Scholar]
- 120.Maehara S., Simanjuntak P., Kitamura C., Ohashi K., Shibuya H. Bioproduction of Cinchona alkaloids by the endophytic fungus Diaporthe sp. associated with Cinchona ledgeriana. Chem. Pharm. Bull. 2012;60:1301–1304. doi: 10.1248/cpb.c12-00545. [DOI] [PubMed] [Google Scholar]
- 121.Maehara S., Agusta A., Kitamura C., Ohashi K., Shibuya H. Composition of the endophytic filamentous fungi associated with Cinchona ledgeriana seeds and production of Cinchona alkaloids. J. Nat. Med. 2016;70:271–275. doi: 10.1007/s11418-015-0954-0. [DOI] [PubMed] [Google Scholar]
- 122.Maehara S., Agusta A., Tokunaga Y., Shibuya H., Hata T. Endophyte composition and Cinchona alkaloid production abilities of Cinchona ledgeriana cultivated in Japan. J. Nat. Med. 2019;73:431–438. doi: 10.1007/s11418-018-1273-z. [DOI] [PubMed] [Google Scholar]
- 123.Radiastuti N., Mutea D., Sumarlin L.O. AIP Conference Proceedings 19–21 October 2016, East Kalimantan, Indonesia. AIP Publishing; Melville, NY, USA: 2017. Endophytic Colletrotrichum spp. from Cinchona calisaya wedd. and it’s potential quinine production as antibacterial and antimalaria; p. 020022. [DOI] [Google Scholar]
- 124.Wei B., Yang Z.D., Chen X.W., Zhou S.Y., Yu H.T., Sun J.Y., Yao X.J., Wang Y.G., Xue H.Y. Colletotrilactam A–D, novel lactams from Colletotrichum gloeosporioides GT-7, a fungal endophyte of Uncaria rhynchophylla. Fitoterapia. 2016;113:158–163. doi: 10.1016/j.fitote.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 125.Chen X.W., Yang Z.D., Sun J.H., Song T.T., Zhu B.Y., Zhao J.W. Colletotrichine A, a new sesquiterpenoid from Colletotrichum gloeosporioides GT-7, a fungal endophyte of Uncaria rhynchophylla. Nat. Prod. Res. 2018;32:880–884. doi: 10.1080/14786419.2017.1365071. [DOI] [PubMed] [Google Scholar]
- 126.Chen X.W., Yang Z.D., Li X.F., Sun J.H., Yang L.J., Zhang X.G. Colletotrichine B, a new sesquiterpenoid from Colletotrichum gloeosporioides GT-7, a fungal endophyte of Uncaria rhynchophylla. Nat. Prod. Res. 2019;33:108–112. doi: 10.1080/14786419.2018.1437437. [DOI] [PubMed] [Google Scholar]
- 127.Yang Z.D., Li Z.J., Zhao J.W., Sun J.H., Yang L.J., Shu Z.M. Secondary metabolites and PI3K inhibitory activity of Colletotrichum gloeosporioides, a fungal endophyte of Uncaria rhynchophylla. Curr. Microbiol. 2019;76:904–908. doi: 10.1007/s00284-019-01707-7. [DOI] [PubMed] [Google Scholar]
- 128.Andre A., Wojtowicz N., Toure K., Stien D., Eparvier V. New acorane sesquiterpenes isolated from the endophytic fungus Colletotrichum gloeosporioides SNB-GSS07. Tetrahedron Lett. 2017;58:1269–1272. doi: 10.1016/j.tetlet.2017.02.024. [DOI] [Google Scholar]
- 129.Numponsak T., Kumla J., Suwannarach N., Matsui K., Lumyong S. Biosynthetic pathway and optimal conditions for the production of indole-3-acetic acid by an endophytic fungus, Colletotrichum fructicola. PLoS ONE. 2018;13:e0205070. doi: 10.1371/journal.pone.0205070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Feng L., Wang J., Liu S., Zhang X.J., Bi Q.R., Hu Y.Y., Wang Z., Tan N.H. Colletopeptides A–D, anti-inflammatory cyclic tridepsipeptides from the plant endophytic fungus Colletotrichum sp. S8. J. Nat. Prod. 2019;82:1434–1441. doi: 10.1021/acs.jnatprod.8b00829. [DOI] [PubMed] [Google Scholar]
- 131.Zheng B., Zeng Y.B., Dai H.F., Zuo W.J., Guo Z.K., Yang T., Zhong H.M., Mei W.L. Two new meroterpenes from endophytic fungus A1 of Scyphiphora hydrophyllacea. J. Asian Nat. Prod. Res. 2012;14:776–779. doi: 10.1080/10286020.2012.693078. [DOI] [PubMed] [Google Scholar]
- 132.Mei W.L., Zheng B., Zhao Y.X., Zhong H.M., Chen X.L.W., Zeng Y.B., Dong W.H., Huang J.L., Proksch P., Dai H.F. Meroterpenes from endophytic fungus A1 of Mangrove plant Scyphiphora hydrophyllacea. Mar. Drugs. 2012;10:1993–2001. doi: 10.3390/md10091993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zeng Y.B., Wang H., Zuo W.J., Zheng B., Yang T., Dai H., Mei W.L. A fatty acid glycoside from a marine-derived fungus isolated from Mangrove plant Scyphiphora hydrophyllacea. Mar. Drugs. 2012;10:598–603. doi: 10.3390/md10030598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zeng Y.B., Dai H.F., Huang J.L., Mei W.L. Separation, purification and structural elucidation of the bio-active components from fermentation broth of endophytic fungus C22 from Scyphiphora hydrophyllacea. Chin. J. Antibiot. 2011;36:276–279. [Google Scholar]
- 135.Martinez-Luis S., Cherigo L., Higginbotham S., Arnold E., Spadafora C., Ibañez A., Gerwick W.H., Cubilla-Rios L. Screnning and evaluation of antiparasitic and in vitro anticancer activities of Panamanian endophytic fungi. Int. Microbiol. 2011;14:95–102. doi: 10.2436/20.1501.01.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Moreno E., Varughese T., Spadafora C., Arnold E., Coley P.D., Gerwick W.H., Cubilla-Rios L. Chemical constituints of the new endophytic fungus Mycosphaerella sp. nov. and their anti-parasitic activity. Nat. Prod. Commun. 2011;6:835–840. [PMC free article] [PubMed] [Google Scholar]
- 137.da Silva B.F., Rodrigues-Filho E. Production of a benzylated flavonoid from 5,7,3′,4′,5′-pentamethoxyflavanone by Penicillium griseoroseum. J. Mol. Catal. B Enzym. 2010;67:184–188. doi: 10.1016/j.molcatb.2010.07.022. [DOI] [Google Scholar]
- 138.da Silva J.V., Fill T.P., da Silva B.F., Rodrigues-Filho E. Diclavatol and tetronic acids from Penicillium griseoroseum. Nat. Prod. Res. 2013;27:9–16. doi: 10.1080/14786419.2011.647021. [DOI] [PubMed] [Google Scholar]
- 139.Vega F.E., Posada F., Peterson S.W., Gianfagna T.J., Chaves F. Penicillium species endophytic in coffee plants and ochratoxin A production. Mycologia. 2006;98:31–42. doi: 10.1080/15572536.2006.11832710. [DOI] [PubMed] [Google Scholar]
- 140.Valente A.M.M.P., Ferreira A.G., Daolio C., Rodrigues Filho E., Boffo E.F., Souza A.Q.L., Sebastianes F.L.S., Melo I.S. Production of 5-hydroxy-7-methoxy-4-methylphthalide in a culture of Penicillium crustosum. An. Acad. Bras. Cienc. 2013;85:487–496. doi: 10.1590/S0001-37652013005000024. [DOI] [PubMed] [Google Scholar]
- 141.da Silva J.V., Fill T.P., Lotufo L.V., Pessoa C.O., Rodrigues-Filho E. Biosynthesis of bromoroquefortines in a high saline medium by Penicillium chrysogenum, a terrestrial endophyte collected from Coffea arabica. Helv. Chim. Acta. 2014;97:1345–1353. doi: 10.1002/hlca.201300447. [DOI] [Google Scholar]
- 142.Forcina G.C., Castro A., Bokesch H.R., Spakowicz D.J., Legaspi M.E., Kucera K., Villota S., Narváez-Trujillo A., McMahon J.B., Gustafson K.R., et al. Stelliosphaerols A and B, sesquiterpene-polyol conjugates from an Ecuadorian fungal endophyte. J. Nat. Prod. 2015;78:3005–3010. doi: 10.1021/acs.jnatprod.5b00749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Abreu L.M., Costa S.S., Pfenning L.H., Takahashi J.A., Larsen T.O., Andersen B. Chemical and molecular characterization of Phomopsis and Cytospora-like endophytes from different host plants in Brazil. Fungal Biol. 2012;116:249–260. doi: 10.1016/j.funbio.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 144.Shylaja G., Sasikumar K., Sathiavelu A. Antimycobacterial potential of resorcinol type lipid isolated from Chaetomium cupreum, an endophytic fungus from Mussaenda luteola. Bangladesh J. Pharmacol. 2018;13:114–119. doi: 10.3329/bjp.v13i2.34860. [DOI] [Google Scholar]
- 145.Shylaja G., Sathiavelu A. Evaluation of bioactive metabolites isolated from endophytic fungus Chaetomium cupreum of the plant Mussaenda luteola. Indian J. Pharm. Educ. Res. 2019;53:s255–s263. doi: 10.5530/ijper.53.3s.95. [DOI] [Google Scholar]
- 146.Andrioli W.J., Conti R., Araujo M.J., Zanasi R., Cavalcanti B.C., Manfrim V., Toledo J.S., Tedesco D., De Moraes M.O., Pessoa C. Mycoleptones A-C and polyketides from the endophyte mycoleptodiscus indicus. J. Nat. Prod. 2014;77:70–78. doi: 10.1021/np4006822. [DOI] [PubMed] [Google Scholar]
- 147.Teles A.P.C., Takahashi J.A. Paecilomide, a new acetylcholinesterase inhibitor from Paecilomyces lilacinus. Microbiol. Res. 2013;168:204–210. doi: 10.1016/j.micres.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 148.Wang Z., Ma Z., Wang L., Tang C., Hu Z., Chou G.X., Li W. Active anti-acetylcholinesterase component of secondary metabolites produced by the endophytic fungi of Huperzia serrata. Electron. J. Biotechnol. 2015;18:399–405. doi: 10.1016/j.ejbt.2015.08.005. [DOI] [Google Scholar]
- 149.Li Z., Ma N., Zhao P.J. Acetylcholinesterase inhibitory active metabolites from the endophytic fungus Colletotrichum sp. YMF432. Nat. Prod. Res. 2019;33:1794–1797. doi: 10.1080/14786419.2018.1434648. [DOI] [PubMed] [Google Scholar]
- 150.Lee H.W., Kim Y.J., Nam S.J., Kim H. Potent selective inhibition of monoamine oxidase A by alternariol monomethyl ether isolated from Alternaria brassicae. J. Microbiol. Biotechnol. 2017;27:316–320. doi: 10.4014/jmb.1610.10053. [DOI] [PubMed] [Google Scholar]
- 151.Youdim M.B.H., Edmondson D., Tipton K.F. The therapeutic potential of monoamine oxidase inhibitors. Nat. Rev. Neurosci. 2006;7:295–309. doi: 10.1038/nrn1883. [DOI] [PubMed] [Google Scholar]
- 152.Herraiz T., González D., Ancin-Azpilicueta C., Aran V.J., Guillen H. β-Carboline alkaloids in Peganum harmala and inhibition of human monoamine oxidase (MAO) Food Chem. Toxicol. 2010;48:839–845. doi: 10.1016/j.fct.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 153.Oliveira C.M., Silva G.H., Regasini L.O., Flausino O., Lopez S.N., Abissi B.M., Berlinck R.G.S., Sette L.D., Bonugli-Santos R.C., Rodrigues A. Xylarenones C−E from an endophytic fungus isolated from Alibertia macrophylla. J. Nat. Prod. 2011;74:1353–1357. doi: 10.1021/np1005983. [DOI] [PubMed] [Google Scholar]
- 154.Liu P., Cheng H., Roberts T.M., Zhao J.J. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat. Rev. Drug Discov. 2009;8:627–644. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tomasselli A.G., Heinrikson R.L. Targeting the HIV-protease in AIDS therapy: A current clinical perspective. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 2000;1477:189–214. doi: 10.1016/S0167-4838(99)00273-3. [DOI] [PubMed] [Google Scholar]