Abstract
The coronavirus disease 2019 (COVID-19) pandemic has instigated severe global turmoil both medically and socioeconomically. Research continues to rapidly develop in order to fully comprehend the new severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This study focuses on the rare ophthalmologic manifestations of the SARS-CoV-2 disease process in both adults and children. There is evidence to suggest that viral transmission can occur via tears and conjunctival secretions, although it is not a predominant finding. This review considers all the published studies describing ocular findings and SARS-CoV-2 viral transmission through the eye. The review addresses the ongoing debate over the importance of ocular manifestations during this pandemic. The most updated safety guidelines, protocols, timelines of ocular manifestations during the disease course, and treatment recommendations are discussed. The majority of patients with COVID-19 with eye symptoms presented with them initially. It is possible that the virus becomes inoculated at the site of the eye and spreads via the nasolacrimal duct to the respiratory system. There are also some reports which show that ocular findings present later in the disease course, suggestive of a correlation between ocular manifestation and increased disease severity as the infection becomes systemic. We highlight the importance of recognizing conjunctivitis as an early finding of COVID-19, and that testing or appropriate follow-up could be beneficial in both the pediatric and adult populations.
Keywords: Conjunctivitis, Coronavirus, COVID-19, Multi-system inflammatory syndrome, Ocular manifestation, Ophthalmology, SARS-CoV-2
Key Summary Points
| This study aims to summarize various ocular manifestations of COVID-19, in both children and adults, and how ocular manifestation may allow for early testing and better outcomes. |
| Ocular manifestations of COVID-19 remain rare; however, the majority present in the form of a viral conjunctivitis. |
| There is controversy over ocular findings of COVID-19 being congruent with disease severity or rather being an early disease finding. |
| Evidence suggests that if conjunctivitis is present, it occurs as an initial finding, suggesting that transmission could occur via entry through receptors in the eye. This can be confirmed by the presence of SARS-CoV-2 RNA in conjunctival swabs. |
| There is a benefit to using eye protection for healthcare workers and the general public and studies suggest that the lack of eye protection when other precautions are taken can result in contraction of the viral disease. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.13013984.
Introduction
The coronavirus disease 2019 (COVID-19) epidemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) rapidly progressed from a rising concern stemming from China in early January 2020 to the declaration of a pandemic on March 11, 2020. The clinical disease caused by SARS-CoV-2, now termed COVID-19, originated in the city of Wuhan, China in December of 2019 [1]. To date, there have been over 30,000,000 confirmed COVID-19 cases globally with over 900,000 deaths reported. Approximately 25% of these deaths have occurred in the USA alone, accounting for the majority of the deaths globally [2]. The pandemic continues, and while new symptoms and clinical manifestations of infection caused by this virus are constantly being identified, what is now becoming apparent is that the post-viral infection has severe complications on organ systems such as the heart, liver, and brain. While respiratory compromise is reported to be the predominant cause of death, case by case reports of the infection caused by this virus are seemingly dissimilar to one another. Learning and recognizing early signs and symptoms may allow for reduced transmission and can possibly mitigate the progression to severe complications of the disease. Early symptom recognition will allow for early testing and potentially minimize spread of the disease.
Angiotensin converting enzyme 2 (ACE2) serves as the receptor for the virus and is found in the eye, suggesting that the virus may be transmittable via tears [3]. The molecular mechanism of ACE2-driven infection and the time course of eye involvement, among children and adults, were the focus of this review with the intent that informed clinicians will not miss the diagnoses of COVID-19. While the ocular manifestations of COVID-19 have been considered minimal, there exist considerable data to support the importance of ocular findings [4]. Some studies support that ocular changes manifest once the disease becomes systemic, while other studies suggests that the ocular findings can be an initial finding and should raise suspicion for COVID-19 [4]. It should be addressed that the research and case reports on this topic are being rapidly distributed during this pandemic and interpretation of the studies should be done with caution since it is too early to verify the accuracy and quality of the published data.
Infectious conjunctivitis is commonly a prodrome or complication of a viral illness, most commonly adenovirus, as viruses cause approximately 80% of acute conjunctivitis cases. The conjunctiva becomes inflamed with resultant hyperemia in the eye. The consequences of this infection are intensified by the severely contagious nature of adenovirus infections [5]. In contrast, the rate of transmission for SARS-CoV-2 infection via eye secretions is not yet known and is an area under active investigation. General recommendations include eye protection and minimizing eye-touching, especially when risk for exposure is high, such as for healthcare workers.
Younger individuals with more robust immune systems were previously thought to present asymptomatically, or with less severe disease; however, recent case studies have shown a “Kawasaki disease”-like presentation in children, known as multisystem inflammatory syndrome (MIS-C) [6, 7]. Kawasaki disease is an acute febrile multisystem vasculitis that mostly affects children. It is clinically diagnosed by a complex set of at least four or five symptoms, which can include bilateral conjunctival hyperemia [8]. The bilateral conjunctival hyperemia typically presents within 2–4 days of disease onset and diminishes within 1–2 weeks, or it improves quickly following onset of intravenous immunoglobulin therapy (IVIG). In some instances, conjunctival hyperemia persists for more than a few weeks [8].
Previous SARS Outbreak and Ocular Findings
A wide spectrum of ocular manifestations are possible, from mild conjunctivitis or anterior uveitis, to vision-threatening manifestations including retinitis and optic neuritis [9]. The severe acute respiratory syndrome coronavirus (SARS-CoV), which emerged in 2002, is phylogenetically similar to the new SARS-CoV-2 virus [1, 10]. The Middle Eastern respiratory syndrome coronavirus (MERS) caused another similar outbreak in 2012 [1]. The routes of transmission via respiratory droplets and direct contact seem to be similar for all three forms of the virus [10]. Both SARS and MERS also demonstrated a wide range of symptoms from asymptomatic to a rapidly progressive and fulminant disease. Patients affected by SARS and MERS had persistent fevers, myalgias, dry cough, and dyspnea similar to COVID-19 [11]. Although mortality continues to rise for illness caused by SARS-CoV-2, the overall case fatality rate appears to be lower than for SARS and MERS [10]. One study suggested that SARS-CoV and MERS-CoV had lower potential for community transmission and were unable to maintain long-term stability [1]. However, patients affected by COVID-19 are more likely to be afebrile than those with SARS and MERS, which affects surveillance and potentiates spread [10].
SARS-CoV has been detected in tears [12]. Seah et al. discuss the following case studies in which tear samples were tested for presence of this virus [9]. In 2004, a case series of 36 patients with suspected SARS-CoV demonstrated that viral RNA was detected in the tears of three patients. This suggested that transmission was possible through ocular secretions [9]. In contrast, a study of 17 patients with confirmed SARS-CoV infection detected no RNA in tears or conjunctival swabs. A retrospective analysis in 2005 during the SARS-CoV crisis in France discovered that three out of 18 patients developed conjunctivitis [9]. While there was some evidence to suggest that SARS-CoV has ocular transmission, no studies detailed its pathogenic mechanism in ocular tissue [9]. These findings support the need to integrate viral transmission in tears and ocular tissue for SARS-CoV-2.
At-Risk Populations for SARS-COV-2 Outbreak
The COVID-19 pandemic has caused widespread panic because of asymptomatic carriers and their potential to infect vulnerable populations, such as those with pre-existing comorbidities or immunosuppressed individuals. These populations tend to have more severe manifestations and a poorer prognosis [13]. Animal studies were performed to obtain preliminary evidence for ocular manifestation since research thus far has been minimal. The studies suggest that more severe disease associated with a coronavirus (CoV) was more likely to present with conjunctival findings and other ocular manifestation such as anterior uveitis, choroiditis with retinal detachment, and retinal vasculitis [9]. Specifically, in cats, the feline CoV virus (FCoV) initially transmitted via the fecal–oral route, but later mutated within monocytes and macrophages and dramatically altered the course of the disease. The mechanism for ocular manifestation likely involved an underlying systemic vasculitis that also caused inflammation in the eye. That study showed that symptoms can begin with gastrointestinal manifestations and later become systemic [9].
Ocular manifestations are associated with a poorer prognosis [9]. This hypothesis seems to align with other case reports during this pandemic. For example, a small retrospective study which included 38 patients in China found 12 patients to have ocular findings. These 12 patients had higher white blood cell counts, neutrophil counts, procalcitonin, C-reactive protein (CRP), and lactate dehydrogenase, which are biomarkers for severe disease [14]. The elevated levels of procalcitonin in one patient with COVID may not be a common finding among all positive patients and likely represents bacterial coinfection during the severe form of the illness [15]. This suggests the possible correlation between ocular symptoms and disease severity [4]. Similarly, a meta-analysis study of 1167 patients evaluated the incidence of conjunctivitis in patients affected by severe versus non-severe COVID-19. While the overall rate of conjunctivitis was only 1.1%, the rates in the severe and non-severe subgroups were 3% and 0.7%, respectively, showing that severe disease has an increased incidence of conjunctivitis [16]. Importantly, patients with more critical illness will often be treated in intensive care units (ICU), and ocular findings may be under-reported in this setting [14]. Close monitoring of ocular findings in moderate to severe cases of disease is needed moving forward. This is evidenced by a large cross-sectional study conducted in Iran by Abrishami et al. which included 142 patients in both ICU and non-ICU settings. A total of 69 of these patients had visible ocular findings and there were significantly more ocular findings in the ICU group than the non-ICU group. None of the patients developed ocular symptoms prior to systemic involvement [17]. Similarly, Poddar et al. described the case of a 65-year-old man who presented to the hospital after having 3 weeks of rhinorrhea, cough, and dyspnea. Seven days after beginning ventilation, he developed acute follicular reaction and hyperemia of the conjunctiva. The symptoms were self-limiting; however, the follicles persisted for 2 weeks after hyperemia resolved [18].
Pathophysiology
Viral binding occurs in the eyes and nose with a variety of receptors [19]. Ocular tropism is well documented specifically by species D adenoviruses and the subtype H7 of influenza virus [20]. Other commonly known respiratory viruses, including coronavirus, have shown rare ocular associations, but evidence supports that numerous human and zoonotic respiratory viruses have the ability to use the eye as both a site of replication and a portal for viral entry [20]. There are lectin-binding sites, specifically α2–3-linked sialic acids, that are found in the epithelium of the human lacrimal sac and nasolacrimal duct, which are used by both adenovirus and influenza virus as forms of entry [20].
ACE2 has recently been highlighted as a binding protein for SARS-CoV-2 in the respiratory tract. ACE2 was previously thought to only be located in the aqueous humor and the retina of the eye, but not in the conjunctiva or cornea [9, 14]. Some preliminary studies showed evidence for ACE2 expression in the corneal and conjunctival cells but suggested that fibroblasts and dendritic cells, which are found under the epithelial layers of the eye, express more ACE2 [3]. Other studies have stated that ocular manifestations in the form of local, transient vasculitis cannot be excluded because of the high vascularity of the conjunctiva and the expression of ACE2 on the surface of endothelial cells [21]. Transmission of the virus through the ocular tissue has been controversial for a while, and it was thought that the virus may migrate from the upper respiratory tract to the eye via the nasolacrimal duct [22]. This potential route of transmission of virus through tears was studied in a group of patients with COVID-19 (n = 17) in Singapore. This study attempted to isolate SARS-CoV-2 in both nasopharyngeal and tear swabs for each individual. Only one of the patients clinically presented with conjunctival hyperemia and chemosis; however, none of the patients had SARS-CoV-2 RNA detectable in their tears even though nasopharyngeal swabs were positive. The results of this study suggested that viral shedding in tears may be less likely [22]. Furthermore, Willcox et al. discusses how viral entry is facilitated by initial binding to a viral receptor known as heparan sulfate on the ocular surface; however, they suggest that coronavirus cannot enter via this mechanism. This entry path can be further inhibited by the presence of lactoferrin in tears or decoy receptors, such as 9-O-acetylated sialic acid in tear glycoproteins, both of which can block coronavirus binding at receptor sites [3]. On the contrary, Zhou et al. showed ACE2 expression on the ocular surface, predominantly on the superficial conjunctival and corneal epithelial surfaces [23]. With this evidence that the eye can serve as a portal for viral entry, the authors highlighted the importance of proper protection for preventing disease transmission [23].
Ocular Manifestations Reported in COVID-19
There have been several individual case reports and small-scale studies performed which documented ophthalmic involvement of COVID-19. Many of the reported ocular symptoms are summarized in Table 1, which highlights adult cases. It includes the study design, number of subjects, age of subjects, and reverse transcription polymerase chain reaction (RT-PCR) results for presence of SARS-CoV-2 in nasal and lacrimal secretions. It also has symptom presentation and day of onset if available, along with treatment regimen used for that case. If the patient presented to the provider with an ocular manifestation, this was noted as an initial finding, unless it was otherwise specified. Geographic location, age distribution, or general COVID-19 presentation may be contributing factors and there are inadequate numbers of studies at this time to suggest any definitive findings.
Table 1.
Ocular findings of COVID-19 reported in the adult population
| Reference | Type of study; location | Number of subjects | Age | Number of patients with visible ocular findings | RT-PCR results for SARS-CoV-2 | Day of symptom presentation (if noted) | Symptomology and physical exam findings | Treatments (if noted) | Other important findings |
|---|---|---|---|---|---|---|---|---|---|
| Wu et al. [4] | Retrospective case series; Hubei province, China | 38 (28 confirmed COVID+) | Mean 65.8 | 12 out of 38 (31.6%) | 2 positive for both C and NP swabs | Not noted | Conjunctival hyperemia, chemosis, epiphora, or increased secretions | ||
| Guan et al. [10] | 30 provinces in China | 1099 | Median 47 | 9 (0.8%) | All confirmed positive on NP swab | Not noted | Conjunctival congestion | ||
| Loffredo et al. [16] | Meta-analysis of 3 studies; China | 1167 | 47–68 | 1.1% | In 2 studies, 3 out 68 had positive C swab | Not noted | Conjunctivitis | Patients with severe COVID-19 had increased incidence of conjunctivitis (P = 0.030) | |
| Abrishami et al. [17] | Cross-sectional, observational study; Northeastern Iran | 142 | Mean 62.6 (range 23–96) | 69 | 77 positive on NP swab | None detected as presenting complaint |
Complaints included tearing, erythema, irritation, itching, foreign body sensation, periorbital pain, photophobia, and blurred vision On external exam, erythema and conjunctival swelling noted On SLE, conjunctival hyperemia and chemosis were noted |
||
| Poddar et al. [18] | Case report | 1 | 65 | 1 | Positive on NP swab | Day 7 after admission, day 30 after symptom onset | Unilateral conjunctival congestion with severe follicular reaction on lower palpebral conjunctiva with conjunctival prolapse | Lubricants and prophylactic preservative-free moxifloxacin 0.5% | Hyperemia resolved in 5 days; follicles resolved after 2 weeks |
| Seah et al. [22] | Prospective case study; Singapore | 17 | Median 37 (range 20–75) | 1 | All confirmed positive on NP swab, no positive C swabs | Day 17 | Conjunctival hyperemia and chemosis | ||
| Colavita et al. [24] | Case report; traveler from Wuhan to Italy | 1 | 65 | 1 | Positive on NP swab until day 20, positive on C swab on day 3 and day 27 | Initial | Bilateral conjunctivitis | ||
| Cheema et al. [25] | Case report | 1 | 29 | 1 | Positive NP swab, weakly positive on conjunctival swab with retrospective testing | Initial | Unilateral conjunctivitis, photophobia, watery discharge progressively worsened to sore, swollen lid, mucoid discharge, and eventually vision impairment; SLE showed follicular conjunctivitis, 2+ hyperemia, and positive for lymphadenopathy | Oral valacyclovir and moxifloxacin drops in right eye | |
| Daruich et al. [26] | Case study; Argentina | 1 | 27 | 1 | Positive NP swab | Day 1 | Foreign body sensation and redness of left eye; on exam unilateral eyelid edema, and moderate conjunctival hyperemia | Topical antihistamine and steroid | |
| Khavandi et al. [27] | Case study | 1 | 65 | 1 | Positive NP swab twice | Initial | Burning eye and discharge; SLE showed mucoid discharge and follicular conjunctivitis | Oseltamivir and hydroxychloroquine | |
| Salducci et al. [28] | Case study; Diamond cruise in Italy | 1 | 72 | 1 | Positive NP swab | Day 1 | Bilateral conjunctivitis and photophobia with serous secretions, chemosis, pseudo membranes of fibrin on tarsal conjunctiva; this was his only symptom | Cold compress, artificial tears, local antiviral ganciclovir gel | |
| Zhou et al. [29] | Retrospective cohort; Wuhan, China | 67 patients |
Mean 35.7 (range 22–78) Patient with conjunctivitis: 48 |
1 | 63 confirmed positive on NP swab. Of these, 1 positive and 2 probable positive C swabs | Initial | Conjunctivitis | Resolved without treatment | The patient with conjunctivitis did not have positive RT-PCR result on C swab |
| Sun et al. [30] | Cross-sectional; Tongji Hospital, China | 102 |
Mean 58.68 Patient with positive conjunctival swab: 29 |
2 | 72 positive on NP swab, 1 positive C swab | Initial | Conjunctivitis; on exam conjunctival congestion and watery discharges in both eyes with normal visual acuity, normal corneal epithelium, quiescent anterior chamber, and no tenderness or enlargement of the preauricular lymph node | ||
| Xia et al. [31] | Prospective interventional case series study; Zhejiang University Hospital | 30 | 53 | 1 | 30 confirmed positive on NP swab, 1 patient positive for tear swab, C swab, and sputum sample | Within 3 days, which was total disease course length | Viral conjunctivitis with conjunctival congestion and aqueous secretion | ||
| Atum et al. [32] | Prospective interventional case series; Sakarya University Education and Research Hospital | 40 | Mean 41.3 | 10 | All positive on NP swab, 3 positives on C swab | Not noted | Conjunctivitis | Of the 10 with conjunctivitis, only 1 had positive C swab | |
| Lomi et al. [33] | Retrospective cross-sectional observational study; North India | 127 | Median 38.8 (range 5–73) | 8 with visible manifestation, 11 total with ocular complaints | All confirmed positive on NP swab |
Initial: 5 out of 11 Late onset: 6 out of 11 |
Conjunctival congestion: 7 bilateral, 1 unilateral Other complaints: burning sensation and tearing |
||
| Chen et al. [34] | Case study; Shenzhen, China | 1 | 30 | 1 | Positive on C swab | Day 13 | Bilateral conjunctivitis; SLE showed bilateral acute follicular conjunctivitis | Ribavirin eye drop | |
| Guo et al. [35] | Case report; First Affiliated Hospital of Zhejiang University | 1 | 53 | 1 | Positive C swab during unilateral presentation of L eye. Negative C swab during bilateral presentation | Day 10 | Unilateral L eye conjunctivitis lasted 6 days with complete resolution. Then, 5 days later the patient developed bilateral keratoconjunctivitis |
First time, the L eye was treated with levofloxacin hydrochloride drops and 0.1% sodium hyaluronate Second time, bilateral eyes treated with ganciclovir ophthalmic gel, levofloxacin hydrochloride drops, and 0.1% sodium hyaluronate drops with no resolution 0.1% fluorometholone drops resulted in complete resolution |
|
| Marinho et al. [36] | Case series on retinal findings | 12 | Range 25–69 | 12 | 9 positive on NP swab, 2 positive for serum antibody | 11–33 days after symptom onset |
B-scan OCT: 12 patients with hyperreflective lesions at level of inner plexiform and ganglion cell layers Retinal fundoscopy: 4 patients with subtle CWS and microhemorrhages |
||
| Landecho et al. [37] | Case series on retinal findings | 27 | Median 56 (with CWS) Median 61 (without CWS) | 6 | Post-COVID infection: All negative NP swab, positive serum antibody | 2 weeks following hospital discharge (mean 43 days after first symptom) | Retinal fundoscopy: CWS 5 unilateral, 1 bilateral | All patients were treated with prophylactic low molecular weight heparin during hospitalization |
SARS-CoV-2 severe acute respiratory syndrome coronavirus 2, RT-PCR reverse transcription polymerase chain reaction test for RNA of virus, NP nasopharyngeal, C conjunctival, SLE slit lamp exam, CWS cotton wool spots, OCT ocular coherence tomography
In larger-scale studies, ocular findings may not be documented because the focus of most studies has been on the lung, cardiac, or gastroenterological effects of the virus. Therefore, the majority of the ocular findings have come from individual case reports. In the following paragraphs, we highlight several case reports that describe ocular findings as part of the early presentation of COVID-19.
One traveler from Wuhan to Italy developed bilateral conjunctivitis initially as part of her viral prodrome and ocular swabs were collected daily beginning on day 3. The swabs were positive for SARS-CoV-2 RNA throughout the entire disease course. By day 20, the conjunctivitis disappeared, but positive swabs were collected at day 27, even after nasopharyngeal swabs had become negative [24].
Another traveler returning from the Philippines presented with unilateral keratoconjunctivitis and photophobia that was presumed to be herpetic in nature, given the presence of one small pseudodendrite on exam. Despite antibacterial and antiviral treatment, the erythema and irritation worsened, and vision began to decline. An ocular swab taken for gonorrhea and chlamydia was retrospectively tested for SARS-CoV-2 and was weakly positive [25].
A telemedicine report from Argentina identified a patient with a chief complaint of foreign body sensation and redness of the left eye with no other systemic symptoms. On exam, unilateral eyelid edema and moderate conjunctival hyperemia were found. Three hours later, this patient developed a high fever with severe headache, and 12 h later developed cough and severe dyspnea. The patient tested positive for SARS-CoV-2 on nasopharyngeal swab. Ocular symptoms resolved at day 11 of his disease course [26].
In a similar case, a patient presented with burning eye pain with a discharge for 2 days. On slit lamp exam, mucoid discharge and follicular conjunctivitis were seen. The individual was diagnosed with viral conjunctivitis and treated with artificial tears. Two days later, he went to the emergency department with sudden onset of fever, cough, and shortness of breath and was found to be COVID-19 positive [27].
Another case report described a confirmed positive COVID-19 cruise ship traveler who had bilateral conjunctivitis, characterized by irritation, photophobia, and watery secretions, as his only symptom during the entire disease course [28].
These case studies support the importance of conjunctivitis as an early presenting symptom and COVID-19 should be considered in the differential diagnosis of conjunctivitis [19]. A few larger-scale studies have also been reported.
Siedlecki et al. reviewed 21 articles on ocular findings in COVID-19 and discussed findings from each study. Ocular findings included conjunctival hyperemia, chemosis, inferior palpebral conjunctival follicles, lid swelling, and keratitis with subepithelial infiltrates and overlying epithelial defects. The timing of presentation was also variable, some with conjunctivitis as an initial presentation and some developing it later in the disease course [14].
Zhou et al. performed a retrospective cohort study which had 63 confirmed COVID-positive patients. Only one of the patients had conjunctivitis and this individual was not positive by RT-PCR for SARS-CoV-2 on conjunctival swab. However, three others who did not have ocular symptoms showed positive viral detection on conjunctival swabs [29].
Sun et al. performed a cross-sectional study at Tongji Hospital in China which had 72 confirmed COVID-positive individuals. Of these, two individuals presented with conjunctivitis. Interestingly, only one out of the two individuals with conjunctivitis had positive RT-PCR on a conjunctival swab [30].
Xia et al. presented a prospective interventional case series on 30 coronavirus patients with pneumonia in which two ocular swabs were tested for SARS-CoV-2 on RT-PCR for each patient. One individual had conjunctivitis as a symptom along with positive viral detection on both swabs, while the swabs from the remaining 29 patients were all negative [31].
Finally, Atum et al. presented a study which tested RT-PCR positivity on nasopharyngeal and conjunctival samples for 40 patients. Ten of the subjects had conjunctivitis on exam, and one of these 10 subjects had a positive conjunctival swab for SARS-CoV-2. A total of three patients had positive conjunctival swabs [32].
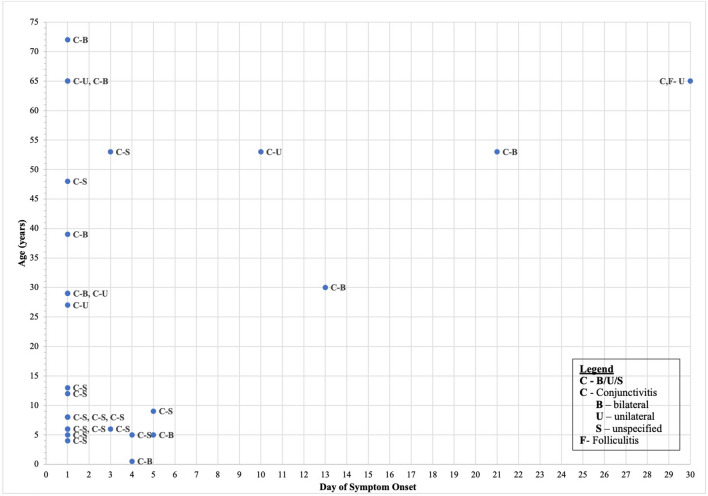
In Table 1, we emphasize when in the course of COVID-19 the ocular symptoms were experienced, and indeed there are cases in which it does not present initially. For example, Lomi et al. conducted a retrospective study in India which included 127 subjects. Eleven patients reported ocular symptoms. Of these, six developed symptoms later in the course with other systemic findings, while five had ocular manifestation as the primary COVID-related finding [33]. Furthermore, Chen et al. described a 30-year-old individual in Shenzhen, China who developed foreign body sensation, along with bilateral erythema and tearing 13 days following the onset of systemic COVID-19 symptoms. His conjunctival swab remained positive for SARS-CoV-2 on RT-PCR testing from day 13 to 17. Slit lamp exam of this patient showed findings that were consistent with acute viral conjunctivitis including bilateral moderate conjunctival hyperemia, watery discharge, inferior palpebral conjunctival follicles, and tender palpable preauricular lymph nodes [34]. Guo et al. described a 53-year-old-man who developed unilateral conjunctivitis 10 days after the onset of symptoms at which time RT-PCR testing of conjunctival secretion was positive for the virus. Interestingly, the patient’s symptoms resolved with levofloxacin drops, but returned as bilateral keratoconjunctivitis 5 days later, this time with negative PCR testing [35]. However, the vast majority of studies placed ocular manifestation as early in the disease course, apparent on Fig. 1 which organizes all ocular findings by age and day of symptom onset.
Fig. 1.
Ocular findings of COVID-19 reported by timing of symptom onset
Retinal Findings Associated with COVID-19
Optical coherence tomography (OCT) is a non-invasive imaging modality used to detect retinal changes that manifest during systemic disease [36]. COVID-positive individuals typically manifest conjunctivitis and ocular surface pathology; however, OCT and retinal fundus imaging have demonstrated the presence of cotton wool spots (CWS) [37]. CWS are retinal nerve fiber layer infarcts that can be visualized as lesions on imaging [38]. While viral retinitis often presents with signs of vitreoretinal inflammation, such as vasculitis or vitritis, these findings were not noted in a cohort of 27 patients who were previously hospitalized with COVID-19. Rather, 6 out of 27 patients (22%) were found to have CWS. It is unclear if the vascular pathology of COVID-19 is due to hypercoagulation, ischemia (in individuals with diabetes), or a direct virus-induced vasculitis. Landecho et al. suggest that a fundoscopic exam on admission may help identify individuals who may be more prone to acute vascular disease, because despite all patients being on heparin, 22% of individuals still developed CWS [37]. Similarly, Marinho et al. presented a study which included 12 COVID-positive individuals examined by OCT and fundus images at least 10 days after symptom onset. They identified that all 12 individuals had hyperreflective lesions at the level of inner plexiform and ganglion cell layers by OCT and four individuals had subtle CWS and microhemorrhages; however, no signs of intraocular inflammation were detected [36]. It should be mentioned that Vavvas et al. commented on the pathology described by Marinho et al., stating that the CWS lesions may demonstrate areas of nerve myelination, which are actually considered normal. The normal OCT angiography obtained also made the pathological findings less likely. The authors argue that if the lesions are proven to be CWS, they cannot be noted as definite ocular manifestation in COVID-19 because they can also be a consequence of other comorbidities, such as diabetes. Thus, it is unclear if these other disease processes may have confounded the interpretation of the Marinho study. They also commented that the hyperreflective lesions at the level of inner plexiform and ganglion cell layers displayed on imaging resemble normal retinal vessels. In order to confirm, they suggested following with near-infrared reflectance (NIR) registration [38]. NIR is another non-invasive imaging modality which allows for identification of subretinal pathology, such as neovascularization, without needing contrast dye or pupil dilation [39].
Review of Treatments for COVID-19
Systemic treatment of COVID-19 infection has been shown to improve ocular symptoms. Since SARS-CoV-2 is an RNA virus, a broad-spectrum antiviral that targets RNA synthesis, such as the nucleoside analogue ribavirin, has been successfully used. Ahn et al. used orally administered ribavirin in combination with pegylated interferon and demonstrated resolution of all symptoms, including ocular pathology. They also reported other antiviral agents as potential treatments for COVID-19; however, to date no definitive regimen has been established [40]. Khavandi et al. reported an individual presenting with ocular findings who was successfully treated with combination of oseltamivir and hydroxychloroquine. This regimen improved ocular and systemic symptoms [27]. Although initially it was thought to have promising results, the antimalarial drug hydroxychloroquine has not been reported to be an effective treatment since benefits do not outweigh potential risks [41]. Chloroquine eye drops (0.03%) used for dry eye disease have no reported adverse effects unlike oral therapy and may be considered [42]. The UK released a potential coronavirus breakthrough with a commonly used steroid known as dexamethasone. The large-scale randomized control trial proved that low dose dexamethasone for adults was effective at reducing mortality in the most severe cases of COVID-19, especially those who require oxygen supplementation with or without ventilator support [43]. While evidence is minimal and there is no perfect regimen to effectively reduce mortality, we can feel reassured that systemic treatment should resolve all coexisting complications, including ocular findings.
Local treatments for ocular symptoms have also been reported. In one study, ribavirin eye drops were used and resulted in resolution of ocular symptoms following 6 days of treatment. Ribavirin, which has previously been used in the treatment of SARS and MERS, is a guanine analogue that is commonly used against hepatitis C and respiratory syncytial virus (RSV) infections [40]. Case reports indicated that ocular symptoms can be effectively treated using combination therapy of a topical antibiotic and corticosteroid, a regimen of cold compresses, artificial tears, and topically administered ganciclovir [26, 28]. Although topical antivirals and antibiotics can be used, they are typically not considered as first-line treatment of viral conjunctivitis because of the risk of cross-contamination between eyes with improper dropper hygiene and development of drug resistance [5]. Thus, symptom control is reported as the mainstay of therapy with artificial tears, topical antihistamines, and cold compresses to alleviate symptoms. However, when considering the use of topical steroids, the risk of inadequately monitored intraocular pressures should be considered during this pandemic [42]. Unresolving conjunctivitis, severe ocular pain, photophobia, constant blurred vision, or hyper-purulent discharge require immediate attention in subjects with suspected or confirmed COVID-19 [5].
Topical versions of systemic antiviral agents found to be effective against COVID-19 will be developed once efficacy is confirmed and the success of these agents on ocular complications is established [20]. However, as with any viral conjunctivitis, it has been recommended that COVID-19-associated conjunctivitis at minimum requires symptomatic treatment, decreased contact with others, and monitoring for delayed COVID-like symptoms [19].
Conjunctivitis in Pediatric Patients with COVID-19
The acute febrile multisystem vasculitis known as Kawasaki disease which presents in young children can present similarly when affected by COVID-19. This atypical Kawasaki disease presentation, known as multisystem inflammatory syndrome in children (MIS-C), oftentimes presents with conjunctivitis [8]. COVID-19 has proven to be less severe and has shown lower rates of mortality in the pediatric population, compared with adults, even though the rate of transmission is apparently the same. The viruses SARS-CoV and MERS-CoV also showed lower case fatality rates in children compared to adults [1]. However, since late April, reports of MIS-C have increased worldwide and are appearing to be associated with severe illness, including shock and myocardial dysfunction, in addition to fever, diarrhea, conjunctivitis, rash and anasarca [6]. Just like in Kawasaki disease, COVID-19 can cause excessive inflammation and cytokine storm during the active phase of the infection [7]. It remains unclear if the presence of conjunctivitis is attributed to the coronavirus itself or if it is a manifestation of the vasculitis [20]. Studies and case report findings in children are shown in Table 2, which highlights pediatric cases.
Table 2.
Ocular findings of COVID -19 reported in the pediatric population
| Reference | Type of study; location | Number of subjects | Age | Number of patients with visible ocular findings | RT-PCR results for SARS-CoV-2 | Day of symptom presentation | Symptomology and physical exam findings | Treatments | Other important findings |
|---|---|---|---|---|---|---|---|---|---|
| Chiotos et al. [6] | Case series; Philadelphia, USA | 6 |
Age 5–14 Patients with conjunctivitis: 5 and 9 |
2 out of 6 (33.3%) | 9-year-old on HD5, 5-year-old on HD1 | Conjunctivitis | |||
| Rauf et al. [7] | Case report; Kerala, India | 1 | 5 | 1 | Negative twice on NP swab | Presented with conjunctivitis to this center after symptoms persisted more than 5 days | Non-purulent bulbar conjunctivitis | IVIG, aspirin, steroids, IV ceftriaxone administration, and diuretics | |
| Jones et al. [45] | Case report | 1 | 6 months | 1 | Positive testing | Presented with conjunctivitis after 4 days of symptoms | Limbic sparing conjunctivitis | IVIG and high dose aspirin | |
| Blondiaux et al. [46] | Case series | 4 |
Mean 9 (range 6–12) Patients with conjunctivitis: 8 and 12 |
2 | Positive on serology; all negative on NP swab | Both present to ER with symptoms but onset unknown | Conjunctivitis | IVIG | |
| Riphagen et al. [47] | Case series | 8 |
Age 4–14 Patients with conjunctivitis 4, 8, 6, 6, and 13 |
5 | All negative on BAL or NP swab | On presentation | Conjunctivitis | ||
| Cheung et al. [48] | Cross-sectional; New York City | 17 | Median 8 (range 1.8–16) | 11 | 8 positive on NP swab and 9 positive on serology | Initial | Conjunctivitis | IVIG and steroids | |
| Heidemann et al. [49] | 3 case reports | 3 | Ages 5, 6 and 7 | 2 |
7-year-old—negative NP swab; positive IgG antibody 6-year-old—positive NP swab and IgG antibody 5-year-old—negative NP swab |
6-year-old on HD3 5-year-old on HD4 |
Conjunctival hyperemia | IVIG and aspirin | |
| Godfred-Cato et al. [50] | Literature analysis; USA | 570 | Median 8 (range 2 weeks–20 years) | 276 | 565 positive on NP swab | Not noted | Conjunctival hyperemia | Majority given IVIG and steroids |
SARS-CoV-2 severe acute respiratory syndrome coronavirus 2, RT-PCR reverse transcription polymerase chain reaction test for RNA of virus, NP nasopharyngeal, BAL bronchoalveolar lavage, SLE slit lamp exam, HD hospital day
In order to diagnose MIS-C, the Centers for Disease Control and Prevention (CDC) requires the individual to be less than 21 years of age, have at least a 24-h history of fever, a necessity for hospitalization, and involvement of two or more organ systems. Interestingly, the majority of cases are more commonly showing positive testing for SARS-CoV-2 on serology rather than on nasopharyngeal swab, which suggested that the syndrome is a more delayed immunologic response that follows a COVID-19 infection and is characterized by a hyperinflammatory condition [44]. Treatments are aimed to suppress systemic inflammation and improve organ function and consist of immunomodulatory agents. If Kawasaki disease criteria are met, IVIG and aspirin can be used. Corticosteroids can also be considered; however, precaution should be taken if the subject is positive by RT-PCR for SARS-CoV-2. The use of anakinra and tocilizumab as refractory treatments are also under investigation [44].
Ocular manifestations in COVID-positive children have been reported globally. In one study performed at the Children’s Hospital of Philadelphia, two out of six children with MIS-C were reported to have conjunctivitis as one of their Kawasaki-like symptoms. One of them had conjunctivitis on presentation along with altered mental status and irritability. Another child developed conjunctivitis on hospital day 5 [6]. In one hotspot in Kerala, India, a 5-year-old boy with known COVID infection presented with multi-organ dysfunction, along with non-purulent bulbar conjunctivitis. The child also had an elevated erythrocyte sedimentation rate (ESR) and CRP, hypoalbuminemia, sterile pyuria, and myocarditis, which suggested an atypical presentation of Kawasaki disease [7]. A COVID-positive 6-month-old full-term infant who was fully immunized and previously healthy presented with irritability and limbic sparing conjunctivitis [45]. Blondiaux et al. described that in a cohort of four COVID-positive children between the ages of 6 and 12 years, two had conjunctivitis [46]. Riphagen et al. presented a case series of eight COVID-positive children, ages 4–14, in which five presented with conjunctivitis on presentation [47]. Cheung et al. performed a cross-sectional study in New York of 17 COVID-positive children ages 1–16 with MIS-C and revealed that 11 had conjunctivitis which was present at the beginning of disease course [48]. In a smaller study, Heidemann et al. described three individual case reports of children ages 5–7 who were hospitalized with MIS-C associated with COVID. Two developed conjunctivitis 3–4 days after admission [49]. In a large-scale analysis of COVID-related MIS-C patients in the USA described by Godfred-Cato et al., 570 children between ages 2 weeks of age and 20 years old were included. Two hundred and seventy-six patients had conjunctival hyperemia as part of their symptoms [50] and the majority of these cases were treated with a combination of aspirin, IVIG, and/or steroids.
The majority of children affected by COVID-19 had a household contact with the disease who presented with symptoms before the child. Although this suggests that children may not be the primary reservoir, adults with known COVID-positive disease, even if asymptomatic, are recommended to quarantine from family members [1].
Eye Protection
Given that conjunctivitis can be the presenting symptom of COVID-19, individuals with conjunctivitis are being treated with extra caution as early detection may result in an improved prognosis. Early detection can also mitigate spread since the risk of transmission in tears, although apparently low, remains a possibility [42]. Any individual that has a diagnosis of a viral disease should be considered highly contagious, even if COVID status is negative or unknown [27]. The 2012 MERS crisis followed the protocols set during the SARS outbreak in 2003, which included contact and droplet precautions such as gowns, gloves, masks, and eye protection [11]. Belser et al. described that during the SARS outbreak, viral transmission between infected patients and healthcare workers occurred when eye protection was not worn, suggesting consideration of these precautions during the current pandemic, although details about what protection was used, along with overall health status, were not addressed [20]. The following recently published algorithm was suggested by Shetty et al. [42]. While obtaining a history, COVID symptoms and travel history were included. Necessary examinations were completed following strict disinfection and contact minimization guidelines. Ready-made packages containing a combination of topical antibiotic (ciprofloxacin hydrochloride 0.3% or moxifloxacin hydrochloride 0.5%) and topical lubricant were quickly distributed to patients who were diagnosed with bacterial or viral conjunctivitis. Every patient was called within 2 weeks of their appointment and guidelines were provided for follow-up recommendations in the event that symptoms were worse or persisted [42].
The American Academy of Ophthalmology (AAO) also published recommendations for ophthalmologists during this pandemic, which included using frequent and meticulous disinfection of all areas of clinics, proper screening at entrances, and the wearing of face coverings by both patients and healthcare workers. They recommend eye protection for ophthalmologists. However, this is based on a theoretical risk and they recognize that this may be impractical at times. While it is suggested that patients with an urgent ophthalmic problem and no fever or other COVID-19 risk factor be seen in clinic, confirmed COVID-19-positive patients should be seen with strict protocols, including isolation from other patients and staff and N95 being worn by the clinician if available. A COVID-19-positive patient with worsening symptoms should be seen in a hospital setting where all appropriate measures can be taken [51]. Fieldstadt describes a previously healthy virologist and epidemiologist who developed COVID-19 requiring hospitalization following a flight, at that time packed, with unmasked passengers. Interestingly, he wore gloves, a mask, and used disinfecting wipes throughout his journey, but did not wear any glasses to protect his eyes, suggesting that he contracted the virus through ocular contact, thus supporting the use of eye protection in such situations [52]. The World Health Organization (WHO)-sponsored study that was used to establish current guidelines on physical distancing also suggested that eye protection can be effective in preventing transmission in the community setting and may provide additional benefits [53].
Conclusion
This literature review aims to address the importance of ocular findings as an early manifestation of COVID-19 for the purpose of promoting isolation from others to mitigate spread of the disease and for optimization of clinical management of the patient. Our literature review supports that conjunctivitis is a clinical symptom associated with COVID-19 and, when present, most often occurs at disease onset. If eye pathology emerges later on in the clinical course, it appears to be associated with increasing severity of COVID-19. Regardless, early recognition of COVID-related ocular findings should reduce disease transmission overall, though direct transmission via eye secretions is still unproven. The use of proper eye protection in both the hospital setting and by the general public could be effective in minimizing SARS-CoV-2 transmission.
Acknowledgements
Funding
Unrestricted Grant from Research to Prevent Blindness and NIH grants R01EY025383, R01EY012601, R01EY028858, R01EY028037, to MBG. The Rapid Service Fee was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
Veena Danthuluri wrote the manuscript. Maria B. Grant wrote the manuscript and is the guarantor of this work.
Disclosures
Veena Danthuluri and Maria B. Grant have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1.Zimmermann P, Curtis N. Coronavirus infections in children including COVID-19: an overview of the epidemiology, clinical features, diagnosis, treatment and prevention options in children. Pediatr Infect Dis J. 2020;39(5):355–368. doi: 10.1097/INF.0000000000002660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.COVID-19 Map. https://coronavirus.jhu.edu/map.html. Accessed 23 Sep 2020.
- 3.Willcox MD, Walsh K, Nichols JJ, Morgan PB, Jones LW. The ocular surface, coronaviruses and COVID-19. Clin Exp Optomet. 2020 doi: 10.1111/cxo.13088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu P, Duan F, Luo C, et al. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol. 2020;138(5):575–578. doi: 10.1001/jamaophthalmol.2020.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azari AA, Barney NP. Conjunctivitis a systematic review of diagnosis and treatment. JAMA. 2013;310(16):1721. doi: 10.1001/jama.2013.280318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiotos K, Bassiri H, Behrens EM, et al. Multisystem inflammatory syndrome in children during the COVID-19 pandemic: a case series. J Pediatric Infect Dis. 2020 doi: 10.1093/jpids/piaa069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rauf A, Vijayan A, John ST, et al. Multisystem inflammatory syndrome with features of atypical Kawasaki disease during COVID-19 pandemic. Indian J Pediatr. 2020 doi: 10.1007/s12098-020-03357-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawasaki T. Kawasaki disease. Proc Jpn Acad Ser B Phys Biol Sci. 2006;82:59–71. doi: 10.2183/pjab.82.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seah I, Agrawal R. Can the coronavirus disease 2019 (COVID-19) affect the eyes? A review of coronaviruses and ocular implications in humans and animals. Ocular Immunol Inflamm. 2020;28(3):391–395. doi: 10.1080/09273948.2020.1738501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hui DS, Azhar EI, Memish ZA, Zumla A. Human coronavirus infections—severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS), and SARS-CoV-2. Ref Module Biomed Sci. 2020 doi: 10.1016/b978-0-12-801238-3.11634-4. [DOI] [Google Scholar]
- 12.Li JO, Lam DS, Chen Y, Ting DS. Novel coronavirus disease 2019 (COVID-19): the importance of recognising possible early ocular manifestation and using protective eyewear. Br J Ophthalmol. 2020;104(3):297–298. doi: 10.1136/bjophthalmol-2020-315994. [DOI] [PubMed] [Google Scholar]
- 13.Ortiz-Prado E, Simbaña-Rivera K, Gómez-Barreno L, et al. Clinical, molecular, and epidemiological characterization of the SARS-CoV-2 virus and the coronavirus disease 2019 (COVID-19), a comprehensive literature review. Diagn Microbiol Infect Dis. 2020;98(1):115094. doi: 10.1016/j.diagmicrobio.2020.115094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siedlecki J, Brantl V, Schworm B, et al. COVID-19: ophthalmological aspects of the SARS-CoV 2 global pandemic. COVID-19: ophthalmologische Aspekte der globalen SARS-CoV-2-Pandemie. Klin Monatsbl Augenheilkd. 2020;237(5):675–680. doi: 10.1055/a-1164-9381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lippi G, Plebani M. Procalcitonin in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chim Acta. 2020;505:190–191. doi: 10.1016/j.cca.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loffredo L, Pacella F, Pacella E, Tiscione G, Oliva A, Violi F. Conjunctivitis and COVID-19: a meta-analysis. J Med Virol. 2020 doi: 10.1002/jmv.25938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abrishami M, Tohidinezhad F, Daneshvar R, et al. Ocular manifestations of hospitalized patients with COVID-19 in Northeast of Iran. Ocular Immunol Inflamm. 2020;28(5):739–744. doi: 10.1080/09273948.2020.1773868. [DOI] [PubMed] [Google Scholar]
- 18.Poddar C, Nayak B, Panigrahi M, Tripathy S, Mishra B. Late manifestation of follicular conjunctivitis in ventilated patient following COVID-19 positive severe pneumonia. Indian J Ophthalmol. 2020;68(8):1675. doi: 10.4103/ijo.ijo_1682_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dockery DM, Rowe SG, Murphy MA, Krzystolik MG. The ocular manifestations and transmission of COVID-19: recommendations for prevention. J Emerg Med. 2020 doi: 10.1016/j.jemermed.2020.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Belser JA, Rota PA, Tumpey TM. Ocular tropism of respiratory viruses. Microbiol Mol Biol Rev. 2013;77(1):144–156. doi: 10.1128/MMBR.00058-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aiello F, Gallo Afflitto G, Mancino R, et al. Coronavirus disease 2019 (SARS-CoV-2) and colonization of ocular tissues and secretions: a systematic review. Eye (Lond) 2020 doi: 10.1038/s41433-020-0926-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seah I, Anderson DE, Kang A, et al. Assessing viral shedding and infectivity of tears in coronavirus disease 2019 (COVID-19) patients. Ophthalmology. 2020;127(7):977–979. doi: 10.1016/j.ophtha.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou L, Xu Z, Castiglione GM, Soiberman US, Eberhart CG, Duh EJ. ACE2 and TMPRSS2 are expressed on the human ocular surface, suggesting susceptibility to SARS-CoV-2 infection. Ocular Surf. 2020;18(4):537–544. doi: 10.1016/j.jtos.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colavita F, Lapa D, Carletti F, et al. SARS-CoV-2 isolation from ocular secretions of a patient with COVID-19 in Italy with prolonged viral RNA detection. Ann Intern Medi. 2020 doi: 10.7326/M20-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheema M, Aghazadeh H, Nazarali S, et al. Keratoconjunctivitis as the initial medical presentation of the novel coronavirus disease 2019 (COVID-19) Can J Ophthalmol. 2020 doi: 10.1016/j.jcjo.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daruich A, Martin D, Bremond-Gignac D. Unilateral conjunctivitis as first presentation of coronavirus disease 2019 (COVID-19): a telemedicine diagnosis. J Fr Ophtalmol. 2020 doi: 10.1016/j.jfo.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khavandi S, Tabibzadeh E, Naderan M, Shoar S. Corona virus disease-19 (COVID-19) presenting as conjunctivitis: atypically high-risk during a pandemic. Contact Lens Anterior Eye. 2020;43(3):211–212. doi: 10.1016/j.clae.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salducci M, La Torre G. COVID-19 emergency in the cruise's ship: a case report of conjunctivitis. Clin Ter. 2020;171(3):e189–e191. doi: 10.7417/CT.2020.2212. [DOI] [PubMed] [Google Scholar]
- 29.Zhou Y, Zeng Y, Tong Y, Chen C. Ophthalmologic evidence against the interpersonal transmission of 2019 novel coronavirus through conjunctiva. medRxiv. 2020. 10.1101/2020.02.11.20021956.
- 30.Sun X, Zhang X, Chen X, et al. The infection evidence of SARS-COV-2 in ocular surface: a single-center cross-sectional study. medRxiv. 2020. 10.1101/2020.02.26.20027938.
- 31.Xia J, Tong J, Liu M, Shen Y, Guo D. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. J Med Virol. 2020;92(6):589–594. doi: 10.1002/jmv.25725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Atum M, Boz AA, Çakır B, et al. Evaluation of conjunctival swab PCR results in patients with SARS-CoV-2 infection. Ocular Immunol Inflamm. 2020;28(5):745–748. doi: 10.1080/09273948.2020.1775261. [DOI] [PubMed] [Google Scholar]
- 33.Lomi N, Sindhuja K, Asif M, Tandon R. Clinical profile and prevalence of conjunctivitis in mild COVID-19 patients in a tertiary care COVID-19 hospital: a retrospective cross-sectional study. Indian J Ophthalmol. 2020;68(8):1546. doi: 10.4103/ijo.ijo_1319_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen L, Liu M, Zhang Z, et al. Ocular manifestations of a hospitalised patient with confirmed 2019 novel coronavirus disease. Br J Ophthalmol. 2020;104(6):748–751. doi: 10.1136/bjophthalmol-2020-316304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo D, Xia J, Wang Y, Zhang X, Shen Y, Tong J. Relapsing viral keratoconjunctivitis in COVID-19: a case report. Virol J. 2020 doi: 10.1186/s12985-020-01370-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marinho PM, Marcos AA, Romano AC, Nascimento H, Belfort R. Retinal findings in patients with COVID-19. Lancet. 2020;395(10237):1610. doi: 10.1016/s0140-6736(20)31014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Landecho MF, Yuste JR, Gándara E, et al. COVID-19 retinal microangiopathy as an in vivo biomarker of systemic vascular disease? J Intern Med. 2020 doi: 10.1111/joim.13156. [DOI] [PubMed] [Google Scholar]
- 38.Vavvas DG, Sarraf D, Sadda SR, et al. Concerns about the interpretation of OCT and fundus findings in COVID-19 patients in recent Lancet publication. Eye. 2020 doi: 10.1038/s41433-020-1084-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Theelen T, Berendschot TT, Hoyng CB, Boon CJ, Klevering BJ. Near-infrared reflectance imaging of neovascular age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2009;247(12):1625–1633. doi: 10.1007/s00417-009-1148-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahn DG, Shin HJ, Kim MH, et al. Current status of epidemiology, diagnosis, therapeutics, and vaccines for novel coronavirus disease 2019 (COVID-19) J Microbiol Biotechnol. 2020;30(3):313–324. doi: 10.4014/jmb.2003.03011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meyerowitz EA, Vannier AGL, Friesen MGN, et al. Rethinking the role of hydroxychloroquine in the treatment of COVID-19. FASEB J. 2020;34:6027–6037. doi: 10.1096/fj.202000919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shetty R, D'Souza S, Lalgudi VG. What ophthalmologists should know about conjunctivitis in the COVID-19 pandemic? Indian J Ophthalmol. 2020;68(5):683–687. doi: 10.4103/ijo.IJO_869_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ledford H. Coronavirus breakthrough: dexamethasone is first drug shown to save lives. Nature. 2020. https://www.nature.com/articles/d41586-020-01824-5. Accessed 8 Aug 2020. [DOI] [PubMed]
- 44.Nakra NA, Blumberg DA, Herrera-Guerra A, Lakshminrusimha S. Multi-system inflammatory syndrome in children (MIS-C) following SARS-CoV-2 infection: review of clinical presentation, hypothetical pathogenesis, and proposed management. Children (Basel) 2020;7(7):69. doi: 10.3390/children7070069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jones VG, Mills M, Suarez D, et al. COVID-19 and Kawasaki disease: novel virus and novel case. Hosp Pediatrics. 2020;10(6):537–540. doi: 10.1542/hpeds.2020-0123. [DOI] [PubMed] [Google Scholar]
- 46.Blondiaux E, Parisot P, Redheuil A, et al. Cardiac MRI of children with multisystem inflammatory syndrome (MIS-C) associated with COVID-19: case series. Radiology. 2020 doi: 10.1148/radiol.2020202288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395(10237):1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheung EW, Zachariah P, Gorelik M, et al. Multisystem inflammatory syndrome related to COVID-19 in previously healthy children and adolescents in New York City. JAMA. 2020;324(3):294. doi: 10.1001/jama.2020.10374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heidemann SM, Tilford B, Bauerfeld C, et al. Three cases of pediatric multisystem inflammatory syndrome associated with COVID-19 due to SARS-CoV-2. Am J Case Rep. 2020 doi: 10.12659/ajcr.925779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Godfred-Cato S, Bryant B, Leung J, et al. COVID-19–associated multisystem inflammatory syndrome in children—United States, March–July 2020. MMWR Morb Mortal Wkly Rep. 2020;69(32):1074–1080. doi: 10.15585/mmwr.mm6932e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chodosh J, Holland, GN, Yeh S. Important coronavirus updates for ophthalmologists. American Academy of Ophthalmology. 2020. https://www.aao.org/headline/d6e1ca3c-0c30-4b20-87e0-7668fa5bf906. Accessed 23 Sep 2020.
- 52.Fieldstadt E. Virologist hospitalized with coronavirus believes he got it through his eyes. NBC News. 2020. https://www.nbcnews.com/news/us-news/virologist-hospitalized-coronavirus-believes-he-got-it-through-his-eyes-n1206956. Accessed 15 June 2020.
- 53.Chu DK, Akl EA, Duda S, et al. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: A systematic review and meta-analysis. Lancet. 2020 doi: 10.1016/s0140-6736(20)31142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.