Abstract
D-dimer levels are reported to relate with tumor stage, prognosis, and lymph node involvement, as well as overall survival (OS) in patients with solid tumors. The purpose of this study was to investigate association between the value of D-dimer and the prognosis of oral cancer (OC). We designed a retrospective cohort study and enrolled a sample of patients who were diagnosed with OC and treated with surgery and/or radiotherapy. The predictor was the D-dimer and outcome variable was OS. Other variables included age, neutrocyte count, neutrophil lymphocyte ratio (NLR), C-reactive protein (CRP), and management. Differences in OS rate were analyzed by log-rank test. A Cox proportional hazards model was used to adjust for the effects of potential confounders. Differences with a P value less than 0.05 were considered statistically significant. In 88 patients with OC, D-dimer median value for the predicting OS was 0.7 µg/mL. There was a significant difference in OS when patients were stratified according to D-dimer, with an OS rate of 77.8% for patients with low D-dimer (<0.7), and 57.3% with high D-dimer (≥0.7) (p = 0.035). Univariate analyses revealed close correlations between OS and age, neutrocyte count, NLR, CRP, and D-dimer (<0.7 and ≥0.7). Cox multivariate analysis identified management (mainly surgery vs. radiotherapy) (HR 3.274, 95% CI 1.397–7.676; p = 0.006) as independent predictive factors for OS. There was a significant difference in OS when patients were stratified according to D-dimer with low (<0.7) and high D-dimer (≥0.7) (p = 0.035). Though, as a predictive factor, management was associated with OS.
Keywords: oral cancer (OC), D-dimer, overall survival (OS), management, neutrophil lymphocyte ratio (NLR), C-reactive protein (CRP)
1. Introduction
D-dimer is a stable end product of the degradation of cross-linked fibrin, which results from enhanced fibrin formation and fibrinolysis. Recently, it has been reported that increases in D-dimer levels have a correlation between malignant diseases. Moreover, some studies report that D-dimer levels are related to tumor stage, tumor prognosis, and lymph node metastasis, as well as overall survival (OS) in patients with lung cancer, breast cancer, gastric cancer, colon cancer, and gynecological cancer. Elevated D-dimer levels may influence multifactorial interactions between carcinoma growth and the hemostatic fibrinolytic system in malignancy [1,2,3,4].
The 8th edition of the American Joint Committee on Cancer (AJCC) staging in oral squamous cell carcinoma (OSCC) was published and two new parameters, namely depth of invasion (DOI) and extranodal extension (ENE), were included. The use of ENE in pN classification was reported as identifying patients with a worse prognosis. Furthermore, the use of lymph node ratio (LNR) improve the predictive capacity of the AJCC staging manual [5]. The ranges of D-dimer levels in head and neck (H&N) cancer patients differed from other malignancies. D-dimer levels in patients with the H&N cancer were much lower than those in patients with gynecological, esophageal, stomach, colorectal, or lung cancer because H&N cancers have lower metastatic rates and a more favorable prognosis than these malignancies [6]. To the best of our knowledges, there was only one report that high D-dimer levels were associated with OS and an increased risk of mortality in nasopharyngeal cancer patients [7]. The purpose of this study was to investigate the association between the value of D-dimer and the prognosis in patients with OC.
2. Materials and Methods
2.1. Study Design and Sample
To address the research purpose, we designed and implemented a retrospective cohort study. The study population was composed of all patients presenting for evaluation and management subjects from patients who were diagnosed with OC and received treatment with surgery and/or radiotherapy for 3 years between 2015 to 2018 at the Department of Oral and Maxillofacial Surgery, University of Tsukuba Hospital (Ibaraki, Japan). Of about 155 oral cancer patients, excluding no surgical and/or radiotherapy and no d-dimer evaluation at pretreatment, 88 patients were included in this study which took place more than 1 year after treatment. Serum D-dimer concentration was evaluated pretreatment and during treatment, while venous ultrasonography (US) of the lower extremities was performed by an experienced sonographer to establish the incidence and location of deep vein thrombosis (DVT) for the patients D-dimer > 1.0 μg/mL according to rules of our hospital. The iliac, femoral, great saphenous, popliteal, peroneal, post-tibial, and soleal veins were evaluated bilaterally [8]. Surgical patients with surgical time more than 2 h received mechanical thromboprophylaxis, such as compression stockings and intermittent pneumatic compression during surgery. Cancer was staged according to the 2017 Union for International Cancer Control (UICC) categories. This study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of University of Tsukuba Hospital (No. R01-270, 31 January 2020). Informed consent was waived due to the retrospective nature of the study.
2.2. Study Variables
The primary predictor variable of this study was pre-treatment D-dimer value. The patients were divided into binary subgroups using the median value as the cut-off point, which determined that the best cut-off value for D-dimer was 0.7 µg/mL. The D-dimer level of cancer patients which served as an indicator of prognosis was reported as 0.7 μg/mL in lung cancer [6]. The primary outcome variable was OS, and the other variables were age, management, and blood laboratory results, namely neutrophil count, neutrophil lymphocyte ratio (NLR), and C-reactive protein (CRP).
2.3. Data Analyses
Patients were divided into high- or low-risk subgroups using the median values for the D-dimer, and differences between the subgroups were analyzed for significance. Survival curves were calculated by the Kaplan–Meier method, and differences in OS rate was analyzed by log-rank test. OS was calculated from the date of first diagnosis to death from any cause. The cut-off date for surviving patients was December 2019 and median (IQR) time point of OS analyzed was 28.2 (16.9–37.1) months. Subgroups were compared by the Mann–Whitney U test and χ2 test. A Cox proportional hazards model was used to adjust for the effects of potential confounders. All statistical analyses were performed with the SPSS software version 25 for Macintosh (SPSS, Chicago, IL, USA). Differences with a p value less than 0.05 were considered statistically significant.
3. Results
3.1. Patient Characteristics
We retrospectively reviewed 88 patients who were diagnosed with OC. Based on D-dimer median value, there were 28 patients with low D-dimer (<0.7) and 60 with high D-dimer (≥0.7). In terms of gender, there were 56 men and 32 women with a median age of 72.5 years (range 34–93). The most common primary tumor sites were tongue (n = 27) and mandibular gingiva (n = 25). The association with study variables versus D-dimer are presented in Table 1 and Table 2. The median D-dimer was 0.7 μg/mL (range 0.4–12.2 μg/mL). The variables associated with D-dimer were smoking, age, and CRP. There were 22(78.6%) patients with low D-dimer (<0.7) and 34 (56.7%) with high D-dimer (≥0.7) absent smoking. There was a significant difference regarding the absence of smoking, pre and present (p = 0.019). The D-dimer was associated with a median age of 65.5 years of D-dimer (<0.7) and 76.0 years D-dimer (≥0.7) (p < 0.0001), while CRP 0.40 mg/µL of D-dimer (<0.7) and 0.15 mg/µL of D-dimer (≥0.7) (p = 0.002).
Table 1.
Study variables versus D-dimer.
| Variable | D-Dimer (<0.7) (n = 28) | D-Dimer (≥0.7) (n = 60) | p Value |
|---|---|---|---|
| Gender | 0.574 | ||
| Male | 19 (67.9%) | 37 (61.7%) | |
| Female | 9 (32.1%) | 23 (38.3%) | |
| Site | 0.962 | ||
| Tongue | 9 (32.1%) | 18 (30.0%) | |
| Lower gingiva | 8 (28.6%) | 17 (28.3%) | |
| Buccal mucosa | 3 (10.7%) | 9 (15.0%) | |
| Upper gingiva | 2 (7.1%) | 6 (10.0%) | |
| Floor of mouth | 3 (10.7%) | 3 (5.0%) | |
| Maxillary sinus | 1 (3.6%) | 3 (5.0%) | |
| Others | 2 (7.1%) | 4 (6.7%) | |
| T stage | 0.501 | ||
| T1 | 7 (25%) | 7 (11.7%) | |
| T2 | 7 (25%) | 20 (30.0%) | |
| T3 | 1 (3.6%) | 5 (8.3%) | |
| T4a | 9 (32.1%) | 21 (35.0%) | |
| T4b | 4 (14.3%) | 7 (11.6%) | |
| N stage | 0.402 | ||
| N0 | 20 (71.4%) | 31 (51.7%) | |
| N1 | 3 (10.7%) | 9 (15.0%) | |
| N2b | 5 (17.9%) | 16 (26.7%) | |
| N2c | 0 (0%) | 3 (5.0%) | |
| N3 | 0 (0%) | 1 (1.7%) | |
| Stage | 0.456 | ||
| I | 7 (25%) | 6 (10.0%) | |
| II | 6 (21.4%) | 14 (23.3%) | |
| III | 1 (3.6%) | 3 (5.0%) | |
| IVA | 11 (39.3%) | 31 (51.7%) | |
| IVB | 3 (10.7%) | 6 (10.0%) | |
| Management a | 0.157 | ||
| S | 16 (57.1%) | 34 (56.7%) | |
| S+RT | 4 (14.3%) | 4 (6.7%) | |
| S+CRT | 6 (21.4%) | 6 (10.0%) | |
| RT | 1 (3.6%) | 9(15.0%) | |
| RT+C | 1 (3.6%) | 7 (11.7%) | |
| Pathological diagnosis b | 0.093 | ||
| SCC | 25 (89.3%) | 59 (98.3%) | |
| ACC | 2 (7.1%) | 0 (0%) | |
| Others | 1 (3.6%) | 1 (1.7%) | |
| VTE c | 0.094 | ||
| Absent | 27 (96.4%) | 49 (81.7%) | |
| Present | 1 (3.6%) | 11 (18.3%) | |
| Smoking | 0.019 * | ||
| Absent | 22 (78.6%) | 34 (56.7%) | |
| Pre | 1 (3.6%) | 18 (30.0%) | |
| Present | 5 (17.9%) | 8 (13.3%) | |
| Alcohol | 0.299 | ||
| Absent | 20 (71.4%) | 36 (60.0%) | |
| Present | 8 (28.6%) | 24 (40.0%) |
a Management: S, surgery; RT, radiotherapy; C, chemotherapy; CRT, chemoradiotherapy. b Pathological diagnosis: SCC, squamous cell carcinoma; ACC, adenoidocystic carcinoma. c VTE, venous thromboembolism. * p < 0.05. N stage: N0 vs. N2b, 2c, 3 (p = 0.080). Management: S, S + RT, S + CRT vs. others (p = 0.046 *).
Table 2.
Study variables versus D-dimer.
| Variable Median (Range) | D-Dimer (<0.7) (n = 28) | D-Dimer (≥0.7) (n = 60) | p Value |
|---|---|---|---|
| Age (Years) | 65.5 (34~84) | 76.0 (39~93) | <0.0001 ** |
| BMI (Kg/m2) | 21.52 (16.81~29.48) | 22.26 (14.29~32.65) | 0.610 |
| Caprini Score | 6 (4~8) | 6 (4~8) | 0.064 |
| Neutrocyte count (×103/µL) | 4.129 (1.995~6.845) | 4.513 (1.666~8.880) | 0.182 |
| CRP (mg/µL) | 0.40 (0~0.60) | 0.15 (0.03~10.92) | 0.002 ** |
| PLT count (×103/µL) | 216.50 (164.0~384.0) | 226.5 (68.0~454.0) | 0.750 |
| Lymphocyte count (×103/µL) | 1.653 (0.630~2.843) | 1.570 (0.529~3.081) | 0.979 |
| Monocyte count (×103/µL) | 0.358 (0.168~0.820) | 0.384 (0.122~0.792) | 0.232 |
| LMR | 4.72 (1.66~9.85) | 3.98 (1.461~19.263) | 0.216 |
| NLR | 2.34 (1.38~6.99) | 2.91 (0.96~12.22) | 0.173 |
| PLR | 150.8 (82.7~344.5) | 148.5 (70.6~319.6) | 0.795 |
BMI: body mass index, CRP: C-reactive protein, PLT: platelet, LMR: lymphocyte monocyte ratio, NLR: neutrocyte lymphocyte ratio, PLR: platelet lymphocyte ratio, ** p < 0.01.
3.2. Clinical Factors and Survival
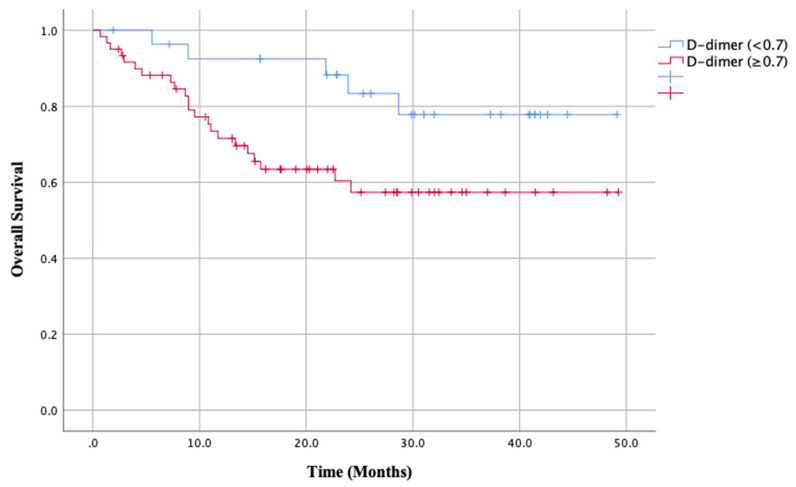
The association with study variables versus overall survival were presented in Table 3. The variables associated with OS was only management (p < 0.0001). Surgical treatment with only surgery, surgery and radiotherapy, surgery and chemoradiotherapy were performed in 70 patients with OS 72.6% and radiotherapy and chemoradiotherapy in 18 patients with OS 22.3%. There was a significant difference in OS between main treatment of surgery and radiotherapy (p < 0.0001). There were no significant differences in OS when patients were stratified by stage classification (OS rates: I 75%, II 72.6%, III 50.0%, IVA 62.4% and IVB 41.7%). In contrast, there were significant differences in the primary outcome variable of OS when patients were stratified according to the primary predictor variable (D-dimer (<0.7 vs. ≥0.7)), with a rate of 77.8% for patients with low D-dimer (<0.7) and 57.3% for patients with high D-dimer (≥0.7) (p = 0.035; Figure 1).
Table 3.
Study variables versus overall survival.
| Variable | No of Patients (%) | Overall Survival (%) | p Value |
|---|---|---|---|
| Gender | 0.842 | ||
| Male | 56 (63.6) | 61.8 | |
| Female | 32 (36.4) | 67.3 | |
| Site | 0.588 | ||
| Tongue | 27 (30.7) | 59.6 | |
| Lower gingiva | 25 (28.4) | 76.1 | |
| Buccal mucosa | 12 (13.6) | 46.9 | |
| Upper gingiva | 8 (9.1) | 75.0 | |
| Floor of mouth | 6 (6.8) | 62.5 | |
| Maxillary sinus | 4 (4.5) | 50.0 | |
| Others | 6 (6.8) | 80.0 | |
| T stage | 0.270 | ||
| T1 | 14 (15.9) | 68.2 | |
| T2 | 27 (30.7) | 77.2 | |
| T3 | 6 (6.8) | 55.6 | |
| T4a | 30 (34.1) | 58.3 | |
| T4b | 11 (12.5) | 39.8 | |
| N stage | 0.899 | ||
| N0 | 51 (58.0) | 65.2 | |
| N1 | 12 (13.6) | 73.3 | |
| N2b | 21 (23.9) | 55.1 | |
| N2c | 3 (3.4) | 66.7 | |
| N3 | 1 (1.1) | 100 | |
| Stage | 0.364 | ||
| I | 13 (14.8) | 75.0 | |
| II | 20 (22.7) | 72.6 | |
| III | 5 (5.7) | 50.0 | |
| IVA | 42 (4.8) | 62.4 | |
| IVB | 9 (10.2) | 41.7 | |
| Management a | <0.0001 ** | ||
| S | 50 (56.8) | 90.0 | |
| S+RT | 8 (9.1) | 37.5 | |
| S+CRT | 12 (13.6) | 40.0 | |
| R | 10 (11.4) | 28.1 | |
| R+C | 8 (9.1) | 21.9 | |
| Pathological diagnosis b | 0.719 | ||
| SCC | 84 (95.5) | 63.3 | |
| ACC | 2 (2.3) | 100 | |
| Others | 2 (2.3) | 50.0 | |
| VTE c | 0.387 | ||
| Absent | 76 (86.4) | 71.1 | |
| Present | 12 (13.6) | 58.3 | |
| Smoking | 0.922 | ||
| Absent | 56 (63.6) | 63.1 | |
| Pre | 19 (21.6) | 73.0 | |
| Present | 13 (14.8) | 61.5 | |
| Alcohol | 0.345 | ||
| Absent | 56 (63.6) | 65.9 | |
| Present | 32 (36.4) | 59.6 |
a Management: S, surgery; RT, radiotherapy; C, chemotherapy; CRT, chemoradiotherapy.b Pathological diagnosis: SCC, squamous cell carcinoma; ACC, adenoidocystic carcinoma.c VTE, venous thromboembolism, ** p < 0.01. Management: S, S + RT, S + CRT vs. R, R+C; OS 72.6% vs. 22.3% (p < 0.0001 **).
Figure 1.
Kaplan–Meier analysis according to D-dimer with a median of 0.7. When patients were stratified by D-dimer, the overall survival rate was 77.8% for patients with low D-dimer (<0.7) and 57.3% for patients with high D-dimer (≥0.7). This difference was significant (p = 0.035).
3.3. Cox Multivariate Regression Analysis
Univariate analyses showed that OS was significantly associated with D-dimer (<0.7 vs. ≥0.7), with a hazards ratio (HR) of 2.744 and 95% confidence interval (CI) of 1.034–7.281 (p = 0.043). We also found significant associations between OS and age (HR 1.035, 95% CI 1.001-1.069; p = 0.042), neutrocyte count (HR 1.297, 95% CI 1.061–1.585; p = 0.011), NLR (HR 1.172, 95% CI 1.007–1.364: p = 0.041), CRP (HR 1.174, 95% CI 1.036–1.329: p = 0.012), and management (mainly surgery vs. radiotherapy; HR 4.972, 95% CI 2.282–10.834; p = 0.0002). Details are shown in Table 4.
Table 4.
Prognostic factors for overall survival a.
| Factor | HR | 95% CI | p Value |
|---|---|---|---|
| Univariate analysis | |||
| Age | 1.035 | 1.001–1.069 | 0.042 * |
| BMI | 0.968 | 0.860–1.089 | 0.377 |
| Smoking (Present vs. Pre, Absent) | 1.161 | 0.438–3.076 | 0.764 |
| Caprini Score | 0.877 | 0.630–1.222 | 0.439 |
| Stage (I, II vs. II, IV) | 0.443 | 0.179–1.098 | 0.079 |
| PLT | 1.001 | 0.996–1.006 | 0.767 |
| Neutrocyte count | 1.297 | 1.061–1.585 | 0.011 * |
| Lymphocyte count | 0.625 | 0.313–1.413 | 0.289 |
| Monocyte count | 6.610 | 0.597–73.151 | 0.124 |
| NLR | 1.172 | 1.007–1.364 | 0.041 * |
| LMR | 0.855 | 0.693–1.055 | 0.144 |
| PLR | 1.002 | 0.996–1.007 | 0.583 |
| CRP | 1.174 | 1.036–1.329 | 0.012 * |
| D-dimer (<0.7 vs. ≥0.7) | 2.744 | 1.034–7.281 | 0.043 * |
| Management (Mainly S vs. R) | 4.972 | 2.282–10.834 | 0.0002 * |
| Multivariate survival analysis | |||
| Age | 1.027 | 0.984–1.072 | 0.222 |
| Neutrocyte count | 1.247 | 0.957–1.625 | 0.101 |
| CRP | 0.969 | 0.821–1.1441 | 0.713 |
| D-dimer (<0.7 vs. ≥0.7) | 1.332 | 0.440–4.032 | 0.612 |
| Management (Mainly S vs. R) | 3.274 | 1.397–7.676 | 0.006 * |
HR, hazards ratio; 95% CI, 95% confidence interval; BMI, body mass index; PLT, platelet; NLR, neutrocyte lymphocyte ratio; LMR, lymphocyte monocyte ratio; PLR, platelet lymphocyte ratio; CRP, C reactive protein. a Evaluated by Cox proportional hazards regression, * p < 0.05.
Cox multivariate analysis of the parameters selected by univariate analysis identified one independent predictive factor for OS, namely management (surgery vs. radiotherapy; HR 3.274, 95% CI 1.397–7.676: p = 0.006). This result indicates that the management (surgery vs. radiotherapy) is a better prognostic marker than the D-dimer (<0.7 vs. ≥0.7).
4. Discussion
D-dimer level is associated with risk of venous thromboembolism (VTE) in cancer patients. Guidelines for the management of VTE provided by the American Society of Hematology in 2018 recommended, for patients at low VTE risk, using D-dimer as the initial test reduces the need for diagnostic imaging. For pulmonary embolism diagnosis, ventilation-perfusion scanning and computed tomography (CT) pulmonary angiography are the most validated tests, whereas lower or upper extremity DVT diagnosis uses US [9]. D-dimer, a stable end product of the degradation of cross-linked fibrin, results from enhanced fibrin formation and fibrinolysis. In the H&N cancer surgery, D-dimer is used in the diagnosis of VTE and reported association of free flap venous thrombosis. For patients with preoperative D-dimer < 0.4 μg/mL, the likelihood of venous thrombosis was greater than for patients with D-dimer ≥ 0.4 μg/mL. The anticoagulation and coagulation systems are always in a dynamic balance. Therefore, the preoperative higher D-dimer values represented a more effective anticoagulation system in patients with free flap success [10]. On the other hand, D-dimer level was independent of gender but was affected by the age of the patient [6]. In our study, there was no significant difference in gender but in age between D-dimer (<0.7 and ≥0.7). Age adjustment is desired to decide the optimal cut off value with large samples in a future study. Moreover, there was a significant difference in CRP between D-dimer (<0.7 and ≥0.7). A previous study reported that the correlation between CRP and D-dimer, and a significant elevation among patients with DVT [11].
Recently, it has been reported that increases in D-dimer levels are correlated with malignant diseases. D-dimer level is associated with risk of VTE and DVT in cancer patients. Most cancer patients have abnormal D-dimer levels based on the normal reference range. The D-dimer range of H&N cancer was 0.22, 0.44, and 2.19 (median, 5th, 95th). Various cancer patients with high initial D-dimer were shown a tendency of poor prognosis in survival rate [6]. Some studies report that D-dimer levels have a relation to tumor stage, prognosis, and lymph node metastasis, as well as OS in patients with solid tumors, such as lung, breast, gastric, colon cancers [1,2,3,4]. Although there was a significant difference in OS when patients were stratified according to D-dimer with low D-dimer (<0.7) and high D-dimer (≥0.7) (p = 0.035) in our study, not selected as a predictive factor in multivariate survival analysis. It was reported that H&N cancers have lower metastatic rates and a more favorable prognosis than other malignancies and the D-dimer level was lower than other ones [6]. Our OC cases may correlate with these previous results of lower metastatic rates and a favorable prognosis than other cancers.
To the best of our knowledges, there was no report about indicator of poor prognosis with optimal cut-off value in OC. Yu et al. reported tumor specific D-dimer concentration according to various cancers. Cancer patients with high initial D-dimer reported to be shown a tendency of poor prognosis in survival rate. The initial D-dimer level of lung cancer patients which served as an indicator of prognosis was reported as 0.7 μg/mL [6]. Altiay et al. reported a cut-off value (≤0.65 and >0.65) for the optimal prediction of survival for lung cancer [12]. Moreover, the median value of D-dimer was 0.7 μg/mL in our study. Therefore, we defined the cut-off value ≥ 0.7 μg/mL with predicting poor prognosis from lung cancer reports and our median value. On the other hand, 0.8 μg/mL which was 3rd quartile values as the optimal cut-offs, high D-dimer levels were associated with poor OS in nasopharyngeal cancer patients [7]. The D-dimer levels predicting poor prognosis in gastrointestinal cancer of a meta-analysis reported 0.6 μg/mL [2]. Our initial D-dimer level of median with 0.7 μg/mL nearly corresponded with these previous reports. However, our sample size is small, and a further study will be desired in a large cohort to make a conclusion regarding the defined D-dimer value which can serve as an indicator of poor prognosis in OC.
In univariate analysis, there were significant differences in neutrocyte count, NLR and CRP with OS. The systemic inflammatory response has been shown to play an important role in cancer progression. The CRP as a sensitive measure of the systemic inflammatory response reported an independent prognostic value [13]. Recently, CRP and albumin with the Glasgow prognostic score (GPS) reported an independent prognostic value in patients with cancer [14]. Moreover, NLR was indicated which reduced the survival rate and associates with cancer prognosis [15]. Our results are correlated with these reports about the association with the systemic inflammatory response markers and cancer prognosis.
The OS of management with surgery was 72.6% and radiotherapy was 22.3%. Although the stage had no significant difference with OS, only management had a significant difference in multivariate analysis. This may be a selection bias according to unresectable cancer and/or non-adaptation for surgery because of bad general conditions in favor of surgery or radiotherapy. A limitation of this study is the retrospective nature, the small sample, and short observation periods of patients with OC which warrants further validation in a large cohort. Therefore, prospective and multicentral studies are needed to find an accurate relation between D-dimer and the outcome of patients with OC.
5. Conclusions
There was a significant difference in OS when patients were stratified according to D-dimer value with low D-dimer (<0.7) and high D-dimer (≥0.7) (p = 0.035). Though, as a predictive factor, only management was associated with OS.
Author Contributions
Conceptualization, K.Y. and S.F.; data curation, F.U.; writing—original draft preparation, K.Y.; writing—review and editing, N.I.-K. and T.Y.; supervision, H.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Shiina Y., Nakajima T., Yamamoto T., Tanaka K., Sakairi Y., Wada H., Yoshino I. The D-dimer level predicts the postoperative prognosis in patients with non-small cell lung cancer. PLoS ONE. 2019;14:e0222050. doi: 10.1371/journal.pone.0222050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rong G., Fan W., Shen J. High pretreatment plasma D-dimer levels predict poor prognosis in gastrointestinal cancers. Medicine. 2019;98:e16520. doi: 10.1097/MD.0000000000016520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diao D., Zhu K., Wang Z., Cheng Y., Li K., Pei L., Dang C. Prognostic Value of the D-Dimer Test in Oesophageal Cancer During the Perioperative Period. J. Surg. Oncol. 2013;108:34–41. doi: 10.1002/jso.23339. [DOI] [PubMed] [Google Scholar]
- 4.Ay C., Dunkler D., Pirker R., Thaler J., Quehenberger P., Wagner O., Zielinski C., Pabinger I. High D-dimer levels are associated with poor prognosis in cancer patients. Haematologica. 2012;97:1158–1164. doi: 10.3324/haematol.2011.054718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mascitti M., Rubini C., De Michele F., Balercia P., Girotto R., Troiano G., Muzio L.L., Santarelli A. American Joint Committee on Cancer staging system 7th edition versus 8th edition: Any improvement for patients with squamous cell carcinoma of the tongue? Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018;126:415–423. doi: 10.1016/j.oooo.2018.07.052. [DOI] [PubMed] [Google Scholar]
- 6.Yu J., Li D., Lei D., Yuan F., Pei F., Zhang H., Yu A., Wang K., Chen H., Chen L., et al. Tumor-Specific D-Dimer Concentration Ranges and Influencing Factors: A Cross-Sectional Study. PLoS ONE. 2016;11:e0165390. doi: 10.1371/journal.pone.0165390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen W., Tang L., Wang F., Li C., Tian X., Huang X., Mai S.J., Liao Y.J., Deng H.X., Chen Q.Y., et al. Elevated levels of plasma D-dimer predict a worse outcome in patients with nasopharyngeal carcinoma. BMC Cancer. 2014;14:583. doi: 10.1186/1471-2407-14-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Satoh T., Matsumoto K., Tanaka Y.O., Akiyama A., Nakao S., Sakurai M., Ochi H., Onuki M., Minaguchi T., Sakurai H., et al. Incidence of venous thromboembolism before treatment in cervical cancer and the impact of management on venous thromboembolism after commencement of treatment. Thromb. Res. 2013;131:e127–e132. doi: 10.1016/j.thromres.2013.01.027. [DOI] [PubMed] [Google Scholar]
- 9.Lim W., Gal L., Bates S.M., Righini M., Haramati L.B., Lang E., Kline J.A., Chasteen S., Snyder M., Patel P., et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: Diagnosis of venous thromboembolism. Blood Adv. 2018;2:3226–3256. doi: 10.1182/bloodadvances.2018024828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu K., Lei J.S., Mao Y.Y., Cao W., Wu H.J., Ren Z.H. Prediction of Flap Compromise by Preoperative Coagulation Parameters in Head and Neck Cancer Patients. J. Oral Maxillofac. Surg. 2018;76:2453.e1–2453.e7. doi: 10.1016/j.joms.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Bucek R.A., Reiter M., Quehhenberger P., Minar E. C-reactive protein in the diagnosis of deep vein thrombosis. Br. J. Haematol. 2002;119:385–389. doi: 10.1046/j.1365-2141.2002.03886.x. [DOI] [PubMed] [Google Scholar]
- 12.Altiay G., Ciftci A., Demir M., Kocak Z., Sut N., Tabakoglu E., Hatipoglu O.N., Caglar T. High Plasma D-dimer Level Is Associated With Decreased Survival in Patients With Lung Cancer. Clin. Oncol. 2007;19:494–498. doi: 10.1016/j.clon.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 13.McMillan D.C., Canna K., McArdle C.S. Systemic inflammatory response predicts survival following curative resection of colorectal cancer. Br. J. Surg. 2003;90:215–219. doi: 10.1002/bjs.4038. [DOI] [PubMed] [Google Scholar]
- 14.McMillan D.C. The systemic inflammation-based Glasgow Prognostic Score: A decade of experience in patients with cancer. Cancer Treat. Rev. 2013;39:534–540. doi: 10.1016/j.ctrv.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Wu C.N., Chuang H.C., Lin Y.T., Fang F.M., Li S.H., Chien C.Y. Prognosis of neutrophil-to-lymphocyte ratio in clinical early-stage tongue (cT1/T2N0) cancer. OncoTargets Ther. 2017;10:3917–3924. doi: 10.2147/OTT.S140800. [DOI] [PMC free article] [PubMed] [Google Scholar]