Abstract
Background
Accurate, scalable and sensitive diagnostic tools are crucial in determining prevalence of soil-transmitted helminths (STH), assessing infection intensities and monitoring treatment efficacy. However, assessments on treatment efficacy comparing traditional microscopic to newly emerging molecular approaches such as quantitative Polymerase Chain Reaction (qPCR) are scarce and hampered partly by lack of an established diagnostic gold standard.
Methods
We compared the performance of the copromicroscopic Kato-Katz method to qPCR in the framework of a randomized controlled trial on Pemba Island, Tanzania, evaluating treatment efficacy based on cure rates of albendazole monotherapy versus ivermectin-albendazole against Trichuris trichiura and concomitant STH infections. Day-to-day variability of both diagnostic methods was assessed to elucidate reproducibility of test results by analysing two stool samples before and two stool samples after treatment of 160 T. trichiura Kato-Katz positive participants, partially co-infected with Ascaris lumbricoides and hookworm, per treatment arm (n = 320). As negative controls, two faecal samples of 180 Kato-Katz helminth negative participants were analysed.
Results
Fair to moderate correlation between microscopic egg count and DNA copy number for the different STH species was observed at baseline and follow-up. Results indicated higher sensitivity of qPCR for all three STH species across all time points; however, we found lower test result reproducibility compared to Kato-Katz. When assessed with two samples from consecutive days by qPCR, cure rates were significantly lower for T. trichiura (23.2 vs 46.8%), A. lumbricoides (75.3 vs 100%) and hookworm (52.4 vs 78.3%) in the ivermectin-albendazole treatment arm, when compared to Kato-Katz.
Conclusions
qPCR diagnosis showed lower reproducibility of test results compared to Kato-Katz, hence multiple samples per participant should be analysed to achieve a reliable diagnosis of STH infection. Our study confirms that cure rates are overestimated using Kato-Katz alone. Our findings emphasize that standardized and accurate molecular diagnostic tools are urgently needed for future monitoring within STH control and/or elimination programmes.
Keywords: Trichuris trichiura, qPCR, Kato-Katz, Drug efficacy, Molecular diagnosis, Ivermectin, Soil-transmitted helminths, Diagnostic performance, Albendazole
Background
With an estimated 1.5 billion infections, the soil-transmitted helminths (STHs), namely Ascaris lumbricoides, Trichuris trichiura and the hookworms Necator americanus and Ancylostoma duodenale, are of enormous public health importance in subtropical and tropical regions, particularly amongst the most marginalized populations [1]. Diseases accompanying these infections can cause considerable burden manifested as malnutrition [2, 3], impairment in physical and cognitive development in children [4], reduction in work performance in adulthood [5] and adverse pregnancy outcomes [3, 6]. Preventive chemotherapy, the periodic large-scale administration of anthelminthic medicines to at-risk populations without prior diagnosis is the cornerstone of helminth control recommended by the World Health Organization (WHO). It is considered simple and cost-effective in its implementation and to have a strong impact on morbidity by decreasing the worm burden [7]. Accurate, scalable and sensitive diagnostic tools are crucial to assess and monitor treatment efficacy, prevalence and intensity of infection to guide future interventions, including the early detection of possible resistance development [8–13]. Cost-effective, sensitive techniques are paramount especially in areas of low endemicity, where a robust surveillance system is needed to approach and monitor elimination [13].
The microscopic Kato-Katz technique is a relatively simple and low-cost method recommended by the WHO for the detection of STH and other helminth eggs in faecal samples [14–16]. Consequently, it is widely used in randomised controlled trials (RCTs), epidemiological surveys and surveillance studies to determine the impact of STH interventions. Yet, the technique has considerable shortcomings. There is substantial variation in the readings, resulting from uneven distribution of eggs within a single stool sample (within sample variation), day-to-day fluctuations of egg excretion (between sample variations) and ultimately results depend on the readers’ skills and experience [17–20]. Most importantly, the Kato-Katz method may particularly miss low-intensity infections leading to underestimation of the actual prevalence, but in the case of efficacy trials artificially inflate cure rates (CRs) from undetected residual low-egg count infections post-treatment [21]. Moreover, expertise in microscopy is increasingly rare [22, 23].
Over the past few decades, molecular diagnostic methods have been developed for the use in human parasitology in order to increase sensitivity and specificity of the diagnosis of intestinal helminths. qPCR-based assays for the detection of helminth DNA or ribosomal RNA on faecal samples are the most widely used molecular methods [11, 23–25]. In recent years, further improvements of the DNA isolation step were made, and multiplex approaches have been developed to detect different parasite targets in a single procedure [17, 26]. Higher specificity and sensitivity of molecular diagnostics are generally observed in studies comparing the Kato-Katz thick smear stool examination to molecular methods (primarily qPCR), with rare exceptions [11, 20, 27–29]. The semi-quantitative output of PCR also reflects the amount of parasite DNA present, which could be of further interest as parasite burden rather than absence or presence of a STH infection is a key determinant of morbidity [17, 30]. Moreover, nucleic acid amplification may improve the detection in infections with low parasitic burden and has the ability to differentiate between morphologically identical species [31].
Evaluations on drug efficacy using molecular approaches are scarce, even though monitoring drug efficacy is of utmost importance for making treatment recommendations for novel therapies and in the light of possible upcoming anthelminthic resistance [32, 33]. Given the higher sensitivity and specificity of qPCR, the few available studies showed that treatment efficacy based on CRs is lower using qPRC detection compared to the microscopic Kato-Katz method. It is worth highlighting that STHs do not release eggs at a constant rate [34–36] and therefore, we hypothesize multiple collection of faecal samples might increase the sensitivity of qPCR.
The aim of the present study was to compare the performance of the microscopic Kato-Katz method and the molecular qPCR method for the diagnosis of soil-transmitted helminthiasis and its impact on treatment efficacy and day-to-day variation analysing two stool samples before and after treatment respectively. Stool samples were collected within the framework of a phase III, parallel group, double blind RCT assessing the safety and efficacy of the current standard treatment (albendazole) versus combination therapy (ivermectin-albendazole).
Methods
Trial design
Trial details are summarized in the published trial protocol [37] and in the trial registration (clinicaltrials.gov, reference: NCT03527732, date assigned: 17 May 2018). Participants were invited for clinical examination and treatment if found positive for T. trichiura infection in at least two slides of quadruple Kato-Katz thick smears with an infection intensity of at least 100 eggs per gram (EPG) of stool. The samples analysed in this work were collected at baseline and 14–21 days post-treatment between September 2018 and December 2018 in one of the three study settings, on Pemba Island, United Republic of Tanzania.
Laboratory procedures
Two fresh morning stool samples were obtained from each participant within a maximum of 5 days using a door-to-door approach. Collected stool samples were kept in a cool box containing ice packs while being transported to the laboratory. Samples were examined with quadruplicate Kato-Katz microscopy within 24 h after collection for the detection of STH ova by experienced laboratory technicians following the WHO standard procedures [15]. An independent quality control of the Kato-Katz readings for T. trichiura and A. lumbricoides was conducted for 10% of the slides.
Stool samples of participants fulfilling eligibility criteria (minimal egg count for T. trichiura ≥ 100 EPG, 2 or more out of 4 Kato-Katz slides positive) and all identified STH egg negative participants (negative controls without any co-infection) were further processed. In total, 160 randomly selected T. trichiura Kato-Katz positive participants with complete aliquot pairs per treatment arm (n = 320) and 180 identified Kato-Katz helminth negative participants with two baseline aliquots were analysed. An aliquot of stool (~ 1 g) was mixed with 80% ethanol and preserved at 4 °C and shipped at room temperature to the Swiss Tropical and Public Health Institute (Swiss TPH) in Basel, Switzerland for subsequent qPCR analyses.
DNA extraction was performed using the QIAamp DNA Mini kit (Qiagen; Hilden, Germany) with slight modifications from the standard protocol validated and described by Kaisar et al. [17]. A multiplex real-time qPCR was used for simultaneous detection of A. lumbricoides, T. trichiura, N. americanus, A. duodenale and Strongyloides stercoralis. However, the latter parasite was not expected in these samples [38] but was placed together with the hookworm species in the same color channel, in case further specification would be of interest in a second round. Amplification consisted of 2 min at 50 °C, 10 min at 95 °C followed by 45 cycles of 15 s at 95 °C and 1 min at 58 °C. Testing was performed using CFX Maestro™ (Bio-Rad Laboratories, Inc, Hercules, CA, USA). qPCR plate planes were generated with a random distribution and balance; however, all samples of one participant were distributed within one plate to reduce between-plate variability and twelve worm-negative controls were placed in between. Four negative controls containing double-distilled water were randomly placed on each plate to ensure detection of confounding factors. For subsequent standardization of each plate, nine positive controls with rising plasmid concentrations (101, 103 and 105 plasmids/µl) containing an insert with the sequence of the STH qPCR product were included in each amplification run. Standard curves were generated by plotting cycle threshold (Ct) values against the logarithm of starting DNA quantities.
The DNA amplification results of a serial 10-fold dilution series of the plasmids from each specimen were compared in separate reactions. Each dilution series was tested both with and without the other target DNAs to assess the assay’s ability to detect mixed infections. The details of all primers and detection probes (Eurofin Genomics, Ebersberg, Germany) and the concentrations of the qPCR using TaqMan GeneExpression MasterMix (ThermoFischer, Switzerland) are presented in the supplementary data (Additional file 1: Tables S1, Additional file 2: Table S2). Extraction of DNA, preparation of the master mix and handling of qPCR products were all performed in different rooms to prevent contamination.
Data preparation
All qPCR assays with an observed copy number above zero were considered positive. All qPCR assays for which no amplification curves were obtained, were considered negative (equalling zero copy numbers). Kato-Katz results were calculated as mean egg counts of the two slides of each time point assessment (baseline day 1, baseline day 2, follow-up day 1 and follow-up day 2) and samples considered positive if at least 0.5 eggs per sample were identified. Data of the amplification curves were cleaned and standardised according to the standard curves with CFX Maestro™ Software and then uploaded to ELIMU-MDx, an open-source platform for storage, management and analysis of diagnostic qPCR data [39]. Subsequent statistical analyses were conducted using Stata IC15 (StataCorp., College Station, TX).
The Ct value is defined as the number of qPCR cycles needed for the detection of fluorescence signal of the amplified products to pass the fixed threshold value. Accordingly, exceeding that threshold can be interpreted as the earliest qPCR cycle at which point a sample’s amplification product is statistically different from the background fluorescence [40]. Consequently, higher quantities of helminth DNA are inversely proportional and thus, result in lower Ct values and vice versa [23]. Amplification curves not following a sigmoidal shape were considered as negative results interpreting these signals as unspecific background noise. Samples below Ct value of 15 were excluded for that species, as the range of standards tested and detected was above Ct 15. Data of the amplification curves were then translated into copies/µl DNA by inserting the average slopes and y-intercepts for each quantified target of the standard curves into a linear equation. Thus, the cycle cut-off points vary for each quencher, depending on the calibration curves obtained. This procedure was done to avoid choosing an arbitrary Ct cutoff which is known to not be ideal, by either being too low (eliminating valid results) or being too high (increasing false-positive results) [40].
Statistical analysis
Based on available summarised efficacy measures from a recent review [41] and the published literature, the CR of albendazole against T. trichiura was assumed to be 30% compared to 50% in the ivermectin-albendazole treatment regimen according to Kato-Katz. Moreover, the correlation between the two diagnostic test results was assumed to be 0.6. A sample size of 320 Kato-Katz T. trichiura positives (160/treatment arm) was chosen to detect a 10% difference in CRs against T. trichiura between Kato Katz and qPCR with a power of 80% assuming a two-sided type 1 error of 5%. An additional subsample of 320 Kato-Katz negatives (1:1 ratio to the positives) was aimed for to determine the sensitivity of qPCR versus Kato-Katz. Since STH infections are staggeringly prevalent on Pemba Island, we only found 180 helminth negative individuals within the screening phase.
Correlation between microscopic egg count and DNA copy number
Correlation between copy numbers/µl DNA according to qPCR and egg count numbers derived by the Kato-Katz thick smear method were assessed as a base for sensitivity and specificity estimates. Spearman’s rank correlation coefficients rS were calculated for each species and each time point among the samples, which were positive according to both diagnostic methods to assess potential correlation. The degree of agreement was categorised as “poor” (rS < 0.2), “fair” (0.2 ≤ rS < 0.4), moderate (0.4 ≤ rS < 0.6) good (0.6 ≤ rS < 0.8) and very good (rS ≥ 0.8) agreement [42].
Diagnostic method variability between samples
To assess the agreement of test results between baseline day 1 and day 2 and follow-up day 1 and day 2, for qPCR and Kato-Katz, Spearman’s rank correlation of copy numbers and egg counts, respectively, was performed among all samples, which were found positive according to both techniques. An alternative assessment was based on Cohen’s Kappa, comparing positivity of qPCR and Kato-Katz between baseline day 1 and day 2 and between follow-up day 1 and 2, including negative and positive test results. The κ-statistics categorised in the same way as the rank correlation coefficients rS.
Overall sensitivity of Kato-Katz and qPCR
The sensitivity was determined assuming a 100% sensitivity and specificity of each diagnostic method, as disclosed by the morphology of the eggs or by the species-specific qPCR assays. Sensitivities of qPCR relative to Kato-Katz and vice versa were calculated for baseline and follow-up separately and for both time points combined. A qPCR test at baseline or follow-up was considered positive if at least one of the two samples taken on the respective consecutive days provided a positive result. The 95% confidence intervals for sensitivities across both time points were computed using a logistic regression model with robust standard errors adjusting for longitudinal correlations of test results within individuals.
Cure rates according to Kato-Katz and qPCR
CRs were calculated as the proportion of participants negative for infection (EPG or transformed DNA copy equalling zero) in both follow-up stool samples among those who were positive at baseline in any sample. Moreover, CRs assessed by qPCR were also calculated considering only the first follow-up sample to assess if test result reliability affects CRs. Logistic regression models were used to compare CRs between different treatment arms. Comparisons of CRs between the qPCR and the Kato-Katz method also required the use of robust standard errors adjusting for correlations of outcomes within subjects. Statistical significance of observed differences or associations was defined as a two-sided P-value smaller than 0.05.
Results
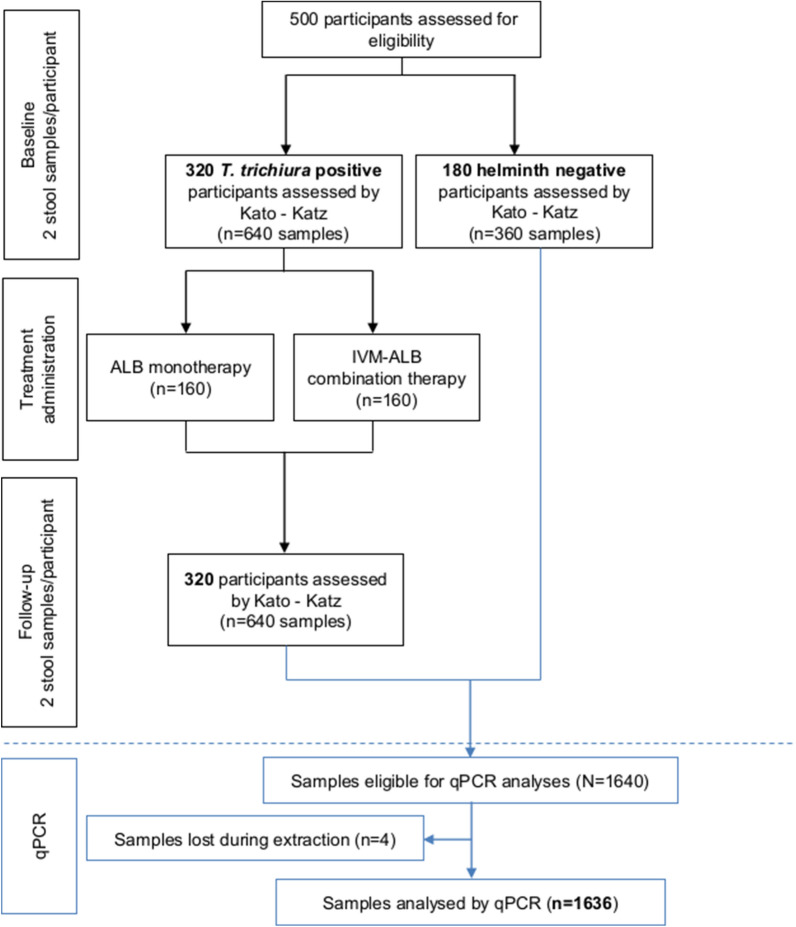
Two stool samples of 320 T. trichiura positive participants, partially co-infected with A. lumbricoides and hookworm at baseline and two stool samples 14–21 days post-treatment were processed by both, Kato-Katz and qPCR method. As negative controls, two faecal samples of 180 individuals negative for STH eggs as assessed by Kato-Katz were analysed (Fig. 1).
Fig. 1.
Study design. Abbreviations: T. trichiura, Trichuris trichiura; ALB, albendazole; IVM-ALB, ivermectin-albendazole; qPCR, quantitative polymerase chain reaction
Overall positivity agreement according to Kato-Katz and qPCR for all four examination time points pooled
In total, 1020 samples were positive for T. trichiura according to Kato-Katz and 1134 were positive for T. trichiura according to qPCR. There were 394 samples with discordant results, 254 where only the qPCR-result was positive and 140 where only the Kato-Katz result was positive. Results for A. lumbricoides and hookworm showed even more pronounced differences, with most discordant tests being positive for qPCR and negative for Kato-Katz (Table 1).
Table 1.
Positivity agreement according to Kato-Katz and qPCR for all four-examination time points pooled (n = 1636) for T. trichiura, A. lumbricoides and hookworm
| No. of qPCR negatives (%) | No. of qPCR positives (%) | Total (%) | ||
|---|---|---|---|---|
| T. trichiura | Kato-Katz negative | 362 (58.8) | 254 (41.2) | 616 (100) |
| Kato-Katz positive | 140 (13.7) | 880 (86.3) | 1020 (100) | |
| Total | 502 (30.7) | 1134 (69.3) | ||
| A. lumbricoides | Kato-Katz negative | 1236 (84.1) | 233 (15.9) | 1469 (100) |
| Kato-Katz positive | 28 (16.8) | 139 (83.2) | 167 (100) | |
| Total | 1264 (77.3) | 372 (22.7) | ||
| Hookworm | Kato-Katz negative | 1369 (89.5) | 160 (10.5) | 1529 (100) |
| Kato-Katz positive | 18 (16.8) | 89 (83.2) | 107 (100) | |
| Total | 1387 (84.8) | 249 (15.2) |
Abbreviations: T. trichiura, Trichuris trichiura; A. lumbricoides, Ascaris lumbricoides
Correlation between microscopic egg count and DNA copy number
For T. trichiura, correlation of positive parasite loads assessed by the two diagnostic methods was moderate (rS = 0.47, 0.45, 0.47, 0.51) for all four time points (baseline day 1, baseline day 2, follow-up day 1, follow-up day 2). For A. lumbricoides, correlation was moderate (rS = 0.55) for the first and fair (rS = 0.36) for the second baseline examination time point. It was not possible to apply the Spearman’s rank correlation test for the follow-up examination time points, due to only a few Kato-Katz positive samples. For hookworm, correlation was moderate (rS = 0.48, 0.47) for both baseline examination time points and the first follow-up examination time point (rS = 0.49), whereas the second follow-up time point showed fair agreement (rS = 0.26) (Table 2). Both correlations had P-values > 0.2 and them being chance results can therefore not be ruled out.
Table 2.
Spearman’s rank correlations between EPG and DNA copy numbers for each time point among positive tests for each STH species
| No. of positive test resultsa | ρ | P-value | ||
|---|---|---|---|---|
| T. trichiura | Baseline day 1 | 272 | 0.47 | < 0.001 |
| Baseline day 2 | 267 | 0.45 | < 0.001 | |
| Follow-up day 1 | 175 | 0.47 | < 0.001 | |
| Follow-up day 2 | 166 | 0.51 | < 0.001 | |
| A. lumbricoides | Baseline day 1 | 68 | 0.55 | < 0.001 |
| Baseline day 2 | 69 | 0.36 | < 0.001 | |
| Follow-up day 1 | 2 | b– | b– | |
| Follow-up day 2 | 0 | b– | b– | |
| Hookworm | Baseline day 1 | 35 | 0.48 | < 0.001 |
| Baseline day 2 | 34 | 0.47 | < 0.001 | |
| Follow-up day 1 | 7 | 0.49 | 0. 2682 | |
| Follow-up day 2 | 13 | 0.26 | 0.3833 |
aPositive according to both diagnostic methods
b–, Sample size is not sufficient for Spearman’s rank correlation analysis
Abbreviations: ρ, Spearman’s rank correlation coefficient; T. trichiura, Trichuris trichiura; A. lumbricoides, Ascaris lumbricoides
Diagnostic method variability between samples
To assess the variability within one diagnostic method, the agreements between baseline day 1 and day 2 as well as between follow-up day 1 and day 2 were calculated using Spearman’s rank correlation, including all positive test results according to both techniques. qPCR showed moderate agreement for T. trichiura (rS = 0.51) and good agreement for A. lumbricoides (rS = 0.62) and hookworm (rS = 0.64) at baseline. At follow-up, moderate agreement for T. trichiura (rS = 0. 45) and hookworm (rS = 0.49) and good agreement for A. lumbricoides (rS = 0.71) was found. Kato-Katz results showed moderate agreement for T. trichiura (rS = 0.49) and good agreement for A. lumbricoides (rS = 0.65) and hookworm (rS = 0.70) between the two baseline samples. Moderate agreement between the follow-up samples was shown for T. trichiura (rS = 0.60) and poor agreement for hookworm (rS = − 0.55) was observed. Agreement could not be assessed for A. lumbricoides at follow-up as no samples were positive by both methods (Table 3).
Table 3.
Spearman’s rank correlations of copy numbers (qPCR) resp. egg counts (Kato-Katz) between baseline day 1 and day 2 and between follow-up day 1 and day 2 for qPCR and Kato-Katz (restricted to positive test results)
| Baseline (day 1 vs day 2) | Follow-up (day 1 vs day 2) | |||||
|---|---|---|---|---|---|---|
| No. of positive test results | ρ | P-value | No. of positive test results | ρ | P-value | |
| qPCR | ||||||
| T. trichiura | 293 | 0.51 | < 0.001 | 190 | 0.45 | < 0.001 |
| A. lumbricoides | 114 | 0.62 | < 0.001 | 6 | 0.71 | 0.1108 |
| Hookworm | 57 | 0.64 | < 0.001 | 22 | 0.49 | 0.0214 |
| Kato-Katz | ||||||
| T. trichiura | 302 | 0.49 | < 0.001 | 166 | 0.6 | < 0.001 |
| A. lumbricoides | 77 | 0.65 | < 0.001 | 0 | – | – |
| Hookworm | 32 | 0.7 | < 0.001 | 6 | − 0.55 | 0.2574 |
Abbreviations: ρ, Spearmanʼs rank correlation coefficient; T. trichiura, Trichuris trichiura; A. lumbricoides, Ascaris lumbricoides
Additionally, Cohen’s Kappa was used to assess reproducibility including positive and negative test results between baseline day 1 and day 2 and between follow-up day 1 and day 2. qPCR test results between baseline and between follow-up samples showed moderate agreement for T. trichiura (κ = 0.57, 0.42) and hookworm (κ = 0.58, 0.52). For A. lumbricoides, qPCR showed good agreement between baseline (κ = 0.63), but only poor agreement (κ = 0.1) between follow-up samples. Kato-Katz showed very good agreement for T. trichiura (κ = 0.92) and A. lumbricoides (κ = 0.93) and good agreement for hookworm (κ = 0.72) between baseline samples. The agreement between follow-up samples was moderate for T. trichiura (κ = 0.56) and hookworm (κ = 0.56). However, there was poor agreement (κ = − 0.004) for A. lumbricoides, possibly because only a few positive samples were found by the Kato-Katz technique (Table 4).
Table 4.
Cohens Kappa for positivity of qPCR resp. Kato-Katz between baseline day 1 and day 2 and between follow-up day 1 and day 2
| Baseline (day 1 vs day 2) | Follow-up (day 1 vs day 2) | |||||||
|---|---|---|---|---|---|---|---|---|
| Observed agreement (%) | Expected agreementa (%) | κ | P-value | Observed agreement (%) | Expected agreement (%) | κ | P-value | |
| qPCR | ||||||||
| T. trichiura | 81.4 | 56.4 | 0.57 | < 0.001 | 76.4 | 59.3 | 0.42 | < 0.001 |
| A. lumbricoides | 84.4 | 57.5 | 0.63 | < 0.001 | 83.5 | 81.8 | 0.1 | 0.043 |
| Hookworm | 88.2 | 71.8 | 0. 58 | < 0.001 | 89.9 | 78.9 | 0.52 | < 0.001 |
| Kato-Katz | ||||||||
| T. trichiura | 96.4 | 53 | 0.92 | < 0.001 | 79.4 | 53.2 | 0.57 | < 0.001 |
| A. lumbricoides | 98 | 72.6 | 0.93 | < 0.001 | 99.1 | 99.1 | 0 | 0.5319 |
| Hookworm | 95.6 | 84.3 | 0.72 | < 0.001 | 97.2 | 93.6 | 0.56 | < 0.001 |
aPercentage of agreement expected based on chance alone (i.e. if the two results compared were completely uncorrelated)
Abbreviations: κ, Cohen’s kappa coefficient; T. trichiura, Trichuris trichiura; A. lumbricoides, Ascaris lumbricoides
Overall, qPCR results showed greater variability between both baseline and follow-up samples compared to Kato-Katz, especially when looking only at the positivity and negativity of samples and using Cohen’s kappa coefficient.
Overall sensitivity of Kato-Katz and qPCR
Across all comparisons between the two methods, the sensitivity of qPCR in detecting positive samples according to Kato-Katz was higher than the respective sensitivity of Kato-Katz in detecting positive samples according to qPCR (Table 5). When pooling baseline and follow-up results, the pooled sensitivity of qPCR relative to Kato-Katz was 93.7% for T. trichiura, 84.4% for A. lumbricoides and 88.4% for hookworm, while the sensitivity of Kato-Katz relative to qPCR was 79.5%, 30.4% and 35.9% for T. trichiura, A. lumbricoides and hookworm, respectively. Interestingly, the sensitivity of Kato-Katz relative to qPCR significantly dropped from 38.5% (31.6–45.8) at baseline to 3.4% (0.4–11.9) at follow-up in the case of A. lumbricoides.
Table 5.
Mutual sensitivities calculated for combined Kato-Katz and combined qPCR for T. trichiura, A. lumbricoides and hookworm at baseline and 14–21 days follow-up
| Sensitivity (%) (95% CI) | Sensitivity (%) (95% CI) | P-value* | Pooled analysis (%) (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| n | Baseline | n | Follow-up | n | Baseline + Follow-up | ||
| T. trichiura | |||||||
| Kato-Katz vs PCR (= Ref) | 386 | 78.2 (73.8–82.2) | 264 | 81.4 (76.2–85.9) | 0.35 | 650 | 79.7 (76.4–82.6) |
| PCR vs Kato-Katz (= Ref) | 320 | 94.4 (91.3–96.6) | 232 | 92.7 (88.5–95.7) | 0.42 | 552 | 93.7 (91.3–95.4) |
| A. lumbricoides | |||||||
| Kato-Katz vs PCR (=Ref) | 192 | 38.5 (31.6–45.8) | 58 | 3.4 (0.4–11.9) | < 0.001 | 250 | 30.4 (25.0–36.4) |
| PCR vs Kato-Katz (=Ref) | 87 | 85.1 (75.8–91.8) | 3 | 66.7 (9.4–99.2) | 0.41 | 90 | 84.4 (75.4–90.6) |
| Hookworm | |||||||
| Kato-Katz vs PCR (=Ref) | 116 | 39.7 (30.7–49.2) | 54 | 27.8 (16.5–41.6) | 0.13 | 170 | 35.9 (29.0–43.4) |
| PCR vs Kato-Katz (=Ref) | 54 | 85.2 (72.9–93.4) | 15 | 100 (78.2–100) | 0.19 | 69 | 88.4 (78.5–94.1) |
*P-values were calculated for the difference in the respective sensitivity measure between the baseline and follow-up examination time point using logistic regression models with robust standard errors adjusting for longitudinal correlations of test results within individuals
Note: The number of observations (n) is determined by samples found positive by the method considered as reference. The reference method was assumed to be the gold standard, i.e. with a 100% sensitivity and specificity, when assessing sensitivities of the other method
Abbreviations: Ref, reference; T. trichiura, Trichuris trichiura; A. lumbricoides, Ascaris lumbricoides
Cure rates according to Kato-Katz and qPCR
Cure rates for T. trichiura were significantly lower with albendazole monotherapy than with combination therapy (ivermectin-albendazole) with both diagnostic methods, while CRs were comparable for A. lumbricoides and hookworm. CRs of the combination therapy according to Kato-Katz were slightly higher (46.8 vs 36.4%, 100 vs 85.7%, 78.3 vs 71.4%) for T. trichiura, A. lumbricoides and hookworm, respectively, when only the first qPCR stool sample was considered. However, CRs according to Kato-Katz were significantly higher than CRs based on qPCR (46.8 vs 23.2%, 100 vs 75.3%, 78.3 vs 52.4%) for T. trichiura, A. lumbricoides and hookworm, respectively, when considering an additional second qPCR stool sample for the combination therapy. This was also the case for A. lumbricoides (95.0 vs 77.5%) with albendazole, while the CRs were comparable for T. trichiura (6.3 vs 5.3%) and hookworm (73.3 vs 66.7%).
Odds ratios (ORs) were calculated for the combination therapy (ivermectin-albendazole) compared to albendazole. The odds of being cured was significantly higher under combination therapy as compared to monotherapy in T. trichiura positives irrespective of the diagnostic approach and the amount of qPCR follow-up stool samples. CRs and ORs according to Kato-Katz and qPCR are listed in Table 6.
Table 6.
Comparison of efficacy in terms of Cure Rates (CRs) and Odds Ratio (OR) for being cured between treatment arms (albendazole vs ivermectin-albendazole), by diagnostic approach (Kato-Katz on samples of day 1 and 2 vs qPCR on first day sample only and qPCR on samples of day 1 and 2)
| Kato-Katz | qPCR 1st Follow-up sample |
qPCR 1st and 2nd Follow-up samples |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| ALB | IVM-ALB | P-valuea | ALB | IVM-ALB | P-valuea | ALB | IVM-ALB | P-valuea | |
| T. trichiura | |||||||||
| No. cured/No. positive BL | 10/160 | 75/160 | 8/150 | 55/151 | 8/150 | 35/151 | |||
| Cure rates, % (95% CI) | 6.3 (3.0–11.2) | 46.8 (39.7–55.9) | 13.3 (8.3–19.8) | 36.4 (28.8–44.6) | 5.3 (2.3–10.2) | 23.2 (16.7–30.7) | |||
| ORcure (95% CI) | 1 | 13.7 (6.7–28.0) | < 0.001 | 1 | 3.7 (2.1–6.6) | < 0.001 | 1 | 5.4 (2.4–12.0) | < 0.001 |
| A. lumbricoides | |||||||||
| No. cured/No. positive BL | 38/40 | 47/47 | 64/71 | 66/77 | 55/71 | 58/77 | |||
| Cure rates, % (95% CI) | 95 (83.1–99.4) | 100 (92.5–100) | 90.1 (80.7–95.9) | 85.7 (75.9–92.6) | 77.5 (66.0–86.5) | 75.3 (64.2–84.4) | |||
| ORcure (95% CI) | 1 | NA (0.62, –>) | 0.21 | 1 | 0.6 (0.2–1.8) | 0.41 | 1 | 0.6 (0.2–1.3) | 0.76 |
| Hookworm | |||||||||
| No. cured/No. positive BL | 22/30 | 18/23 | 32/42 | 30/42 | 28/42 | 22/42 | |||
| Cure rates, % (95% CI) | 73.3 (54.1–87.7) | 78.3 (56.3–92.5) | 76.2 (60.5–87.9) | 71.4 (55.4–84.3) | 66.7 (50.5–80.4) | 52.4 (36.4–68.0) | |||
| ORcure (95% CI) | 1 | 1.3 (0.4–4.7) | 0.68 | 1 | 0.8 (0.3–2.0) | 0.62 | 1 | 0.9 (0.4–1.9) | 0.184 |
aP-values of the odds ratio (OR) for being cured between albendazole monotherapy (ALB) and ivermectin-albendazole (IVM-ALB) derived from logistic regression models
Note: CRs in bold highlight significant differences (P < 0.05) between CRs assessed by Kato-Katz and two qPCR samples in the respective treatment arm (i.e. ALB or IVM-ALB)
Abbreviations: BL, baseline; NA, not applicable; T. trichiura, Trichuris trichiura; A. lumbricoides, Ascaris lumbricoides
Discussion
We evaluated the diagnostic performance of qPCR compared to standard Kato-Katz microscopy for the diagnosis of STHs and the resulting treatment efficacies by both methods. This study was done within the framework of a phase III, parallel group, double blind RCT assessing the efficacy and safety of the current standard treatment (albendazole) versus a combination therapy of ivermectin-albendazole. This was the first study analysing two stool samples before and two stool samples after treatment of each participant to assess diagnostic method variability between samples to elucidate reproducibility of test results of both diagnostic methods. Moreover, we present data on the efficacy of the most promising therapy to date for treating STH infections, ivermectin-albendazole, based on molecular diagnosis.
An interesting finding from the comparison of these two diagnostic methods is that an additional 41.2% of microscopy-negative samples were found T. trichiura-positive when assessed by qPCR. We hypothesise that lower DNA loads found in Kato-Katz-negative samples reflect higher detection rates by qPCR due to a higher sensitivity rather than lower specificity of the qPCR assays as remaining DNA of already dead worms or eggs can still act as a template DNA during qPCR [43].
We found that qPCR results indicate higher sensitivity for all species across all examination days compared to Kato-Katz, which substantiates previous findings [17, 27, 30, 34, 44–46]. Interestingly, follow-up Kato-Katz results differ significantly compared to the baseline results in the case of A. lumbricoides, indicating a time-dependent difference. The very low number of Kato-Katz positive test results post-treatment show the difficulty of detecting low A. lumbricoides worm burden by use of Kato-Katz, while qPCR was able to detect a considerable number of treatment failures.
Of note, the eligibility criteria (minimal T. trichiura egg count ≥ 100 EPG, 2 or more out of 4 Kato-Katz slides positive) for trial inclusion did not consider low T. trichiura infection intensities. Interestingly we observed that qPCR positivity of Kato-Katz-negative samples (EPG = 0) at baseline was lower compared to follow-up, implying higher sensitivity for qPCR when assessing low infection intensities. However, interpretation requires caution, as it is unclear how long residual DNA persists after parasite clearance leading to false-positive qPCR results [18, 47].
Our results highlight, that the combination therapy (ivermectin-albendazole) shows a significantly better efficacy compared to the monotherapy for T. trichiura with both diagnostic methods, while CRs were comparable for A. lumbricoides and hookworm. However, the observed low to moderate CRs for ivermectin-albendazole with qPCR (23.2% for T. trichiura, 75.3% for A. lumbricoides and 52.4% for hookworm) are far from benchmark target product profiles for anthelminthic drug candidates and combinations and highlight the need to develop novel efficacious treatments. A particularly striking difference in CRs of the combination chemotherapy (and for A. lumbricoides after albendazole treatment) between Kato-Katz and qPCR was observed when two qPCR samples were considered post-treatment. These results also stress to analyse two qPCR samples post-treatment in clinical trials to elucidate the true efficacy of treatments.
Although hypothesised, we only observed a fair to moderate agreement between microscopic egg count and DNA copy, which is in agreement with findings from Barda et al. [33]. Our results do not corroborate the observation of Mejia et al. [18], who found a significant good correlation (r = 0.7) between egg counts measured by the coprological Kato-Katz method and the DNA quantified by qPCR for A. lumbricoides and T. trichiura. The reason for this discrepancy is not entirely clear, but one partial explanation could be that only a few A. lumbricoides- or hookworm-positive samples were found post-treatment according to Kato-Katz in our study and that infection intensities were relatively low in these participants. As there is no strong correlation between egg counts and DNA copy number, finding a real gold standard for practical use remains a profound challenge, hampering the comparison of STH diagnostic tools.
As egg excretion is highly variable over time, there is considerable variation in EPG of faecal samples collected on consecutive days [34–36]. It is well known that Kato-Katz shows improved sensitivity when performed on several samples on different days [48, 49]. We observed that Kato-Katz showed very good agreement for T. trichiura and A. lumbricoides and good agreement for hookworm at baseline between day 1 and day 2, whereas qPCR only showed good agreement between day 1 and day 2 for A. lumbricoides, but not for T. trichiura and hookworm, indicating lower test result reproducibility of the qPCR method. This apparent high correlation of Kato-Katz test results might be explained by the laboratory technician’s skills, as the same well-trained microscopists were reading the stool samples every day, in addition to rather high T. trichiura parasite load (EPG ≥ 100 as inclusion criterion). The reason for the surprisingly low qPCR test reproducibility is not entirely clear; however, Pilotte et al. [50] found that common qPCR assays make use of sub-optimal target sequences limiting detection and species-specificity. Another explanation could be that, in contrast to bacteria and viruses, isolation of parasite DNA out of faecal samples is a challenging process as the wall of helminth eggs is difficult to lyse and thus several additional steps are needed to achieve release of nucleic acids [12, 51, 52]. Moreover, we based our analyses on DNA copies/µl as qPCR parameter for infection intensity, while consensus has not been reached on the optimal qPCR parameter with regard to reliability and reproducibility assessment.
We are aware that a number of limitations might have influenced the results obtained. The sample input volumes of the Kato-Katz assays are considerably larger than those of the qPCR assays, which might substantially increase sensitivity for Kato-Katz given the stochastic distribution of eggs in stool samples. On the other hand, increasing the stool volume for DNA extraction would not be possible, as faecal specimens contain various substances acting in a qPCR inhibitory manner [53]. Another limitation is that, in contrast to Kato-Katz, qPCR samples were not analysed in duplicates. Further research needs to be performed to elucidate if and for how long residual DNA may persist after parasite clearance as this might lead to false-positive qPCR results post-treatment [18, 29, 47]. Furthermore, we fully agree with Levecke et al. [54], that an agreement on an absolute universal unit for qPCR is needed to establish best comparison parameters for these two diagnostic methods. Lastly, it is important to note, that the standardisation and adherence to one approved protocol would help to achieve more readily comparable results between different research laboratories.
Conclusions
The sensitive and scalable nature of qPCR makes its usage in large-scale diagnosis of intestinal helminths appealing over the rather operator-dependent microscopic method. DNA samples can be stored for further use, such as genetic characterisation and molecular typing, which might be of interest in surveillance studies to detect sporadic and focal infections or to monitor disease recrudescence. The evidence from this study implies statistically lower CRs (the primary outcome of this trial) for the combination therapy (ivermectin-albendazole) for all three species when assessed with two qPCR samples compared to Kato-Katz. Thus, it underlines the importance of the need for standardised and accurate molecular diagnostic tools, which are applicable in peripheral field settings, for future monitoring within STH control and/or elimination programmes and for developing novel efficacious treatments. This study has revealed for the first time, that qPCR test results show greater between day variability for baseline as well as post-treatment samples compared to Kato-Katz calling for a multi-sample analysis approach in order to improve qPCR-based diagnosis. This is of particular importance for studies aiming at assessing accurate disease prevalence as well as treatment efficacy. However, it needs to be carefully evaluated if the obtained higher sensitivity comes at the cost of the lower test reproducibility and how important this finding is in the context of preventive chemotherapy and the surveillance of low prevalence settings. Daily stool sample analyses to monitor dynamics of DNA copy numbers over a longer period post-treatment using the qPCR method might be one way forward to answer this question.
Supplementary information
Additional file 1: Table S1. Primers and probes used to identify the different helminth species.
Additional file 2: Table S2. GeneExpression MasterMix.
Acknowledgements
The authors sincerely acknowledge all participants for their contribution; the village heads for their support and commitment; and the team of the Public Health Laboratory–Ivo de Carneri for all their collaborating work. We would like to deeply acknowledge Dr Christian Schindler for his very helpful assistance with the statistics used in this manuscript.
Abbreviations
- CI
Confidence intervals
- CR
Cure rates
- Ct
Cycle threshold
- EKNZ
Ethikkommission Nordwest-und Zentralschweiz
- EPG
Eggs per gram of stool
- OR
Odds ratio
- qPCR
Quantitative polymerase chain reaction
- RCT
Randomized controlled trial
- STH
Soil-transmitted helminths
- Swiss TPH
Swiss Tropical and Public Health Institute
- WHO
World Health Organization
- ZAMREC
Zanzibar Medical Resarch and Ethics Committee
Authors’ contributions
EH and JK did the conceptualization of the trial, whereas LK, CP and SW did the conceptualization and data curation of the laboratory work. LK and JK drafted the original manuscript. TS helped with the methodology and the software. CP, SW, EH, TS and CS assisted with reviewing and editing. All authors read and approved the final manuscript.
Funding
This study was funded by the Bill and Melinda Gates foundation [OPP1153928]. The funders of the study had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
Data supporting the conclusions of this article are included within the article and its additional files. The datasets used and analysed during the present study are available from the corresponding author upon reasonable request.
Ethics approval and consent to participate
Ethical clearance was granted by the Ethics Committee of Northwestern and Central Switzerland (EKNZ; reference no: BASEC Nr Req-2018-00494; date of approval 5 July 2018). On Pemba Island, ethical clearance was obtained from the Zanzibar Medical Research and Ethics Committee (ZAMREC, reference no.: ZAMREC/0003/Feb/2018; date of approval 23 May 2018). The trial was registered with the number NCT03527732 on clinicaltrials.gov. Written informed consent was sought from adult participants and parents or legal guardians of children below the age of adulthood prior to study enrolment.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ladina Keller, Email: ladina.keller@swisstph.ch.
Chandni Patel, Email: chandni.patel@swisstph.ch.
Sophie Welsche, Email: sophie.welsche@swisstph.ch.
Tobias Schindler, Email: tobias.schindler@swisstp.ch.
Eveline Hürlimann, Email: eveline.huerlimann@swisstph.ch.
Jennifer Keiser, Email: jennifer.keiser@swisstph.ch.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13071-020-04401-x.
References
- 1.Pullan RL, Smith JL, Jasrasaria R, Brooker SJ. Global numbers of infection and disease burden of soil transmitted helminth infections in 2010. Parasit Vectors. 2014;7:37. doi: 10.1186/1756-3305-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crompton DW, Nesheim MC. Nutritional impact of intestinal helminthiasis during the human life cycle. Annu Rev Nutr. 2002;22:35–59. doi: 10.1146/annurev.nutr.22.120501.134539. [DOI] [PubMed] [Google Scholar]
- 3.Hall A, Hewitt G, Tuffrey V, De Silva N. A review and meta-analysis of the impact of intestinal worms on child growth and nutrition. Matern Child Nutr. 2008;4:118–236. doi: 10.1111/j.1740-8709.2007.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bethony J, Brooker S, Albonico M, Geiger SM, Loukas A, Diemert D, et al. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet. 2006;367:1521–1532. doi: 10.1016/S0140-6736(06)68653-4. [DOI] [PubMed] [Google Scholar]
- 5.Haas JD, Brownlie T. Iron deficiency and reduced work capacity: a critical review of the research to determine a causal relationship. J Nutr. 2001;131:676S–688S. doi: 10.1093/jn/131.2.676S. [DOI] [PubMed] [Google Scholar]
- 6.Mpairwe H, Tweyongyere R, Elliott A. Pregnancy and helminth infections. Parasite Immunol. 2014;36:328–337. doi: 10.1111/pim.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO. Preventive chemotherapy in human helminthiasis - Coordinated use of anthelminthic drugs in control interventions: a manual for health professionals and programme managers. Geneva, Switzerland: World Health Organization; 2006. https://apps.who.int/iris/handle/10665/43545.
- 8.Speich B, Utzinger J, Marti H, Ame SM, Ali SM, Albonico M, et al. Comparison of the Kato-Katz method and ether-concentration technique for the diagnosis of soil-transmitted helminth infections in the framework of a randomised controlled trial. Eur J Clin Microbiol. 2014;33:815–822. doi: 10.1007/s10096-013-2019-1. [DOI] [PubMed] [Google Scholar]
- 9.McCarthy JS, Lustigman S, Yang G-J, Barakat RM, García HH, Sripa B, et al. A research agenda for helminth diseases of humans: diagnostics for control and elimination programmes. PLoS Negl Trop Dis. 2012;6:1601. doi: 10.1371/journal.pntd.0001601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bergquist R, Johansen MV, Utzinger J. Diagnostic dilemmas in helminthology: what tools to use and when? Trends Parasitol. 2009;25:151–156. doi: 10.1016/j.pt.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 11.Knopp S, Salim N, Schindler T, Karagiannis Voules DA, Rothen J, Lweno O, et al. Diagnostic accuracy of Kato-Katz, FLOTAC, Baermann, and PCR methods for the detection of light-intensity hookworm and Strongyloides stercoralis infections in Tanzania. Am J Trop Med Hyg. 2014;90:535–545. doi: 10.4269/ajtmh.13-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Basuni M, Mohamed Z, Ahmad M, Zakaria NZ, Noordin R. Detection of selected intestinal helminths and protozoa at Hospital Universiti Sains Malaysia using multiplex real-time PCR. Trop Biomed. 2012;29:434–442. [PubMed] [Google Scholar]
- 13.Becker SL, Liwanag HJ, Snyder JS, Akogun O, Belizario V, Jr, Freeman MC, et al. Toward the 2020 goal of soil-transmitted helminthiasis control and elimination. PLoS Negl Trop Dis. 2018;12:e0006606. doi: 10.1371/journal.pntd.0006606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barda BD, Keiser J, Albonico M. Human trichuriasis: diagnostics update. Curr Trop Med Rep. 2015;2:201–208. doi: 10.1007/s40475-015-0063-x. [DOI] [Google Scholar]
- 15.Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick-smear technique in schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo. 1972;14:397–400. [PubMed] [Google Scholar]
- 16.WHO. Bench aids for the diagnosis of intestinal parasites, second edition. Geneva, Switzerland: World Health Organization; 2019. https://www.who.int/intestinal_worms/resources/9789241515344/en/.
- 17.Kaisar MMM, Brienen EAT, Djuardi Y, Sartono E, Yazdanbakhsh M, Verweij JJ, et al. Improved diagnosis of Trichuris trichiura by using a bead-beating procedure on ethanol preserved stool samples prior to DNA isolation and the performance of multiplex real-time PCR for intestinal parasites. Parasitology. 2017;144:965–974. doi: 10.1017/S0031182017000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mejia R, Vicuña Y, Broncano N, Sandoval C, Vaca M, Chico M, et al. A novel, multi-parallel, real-time polymerase chain reaction approach for eight gastrointestinal parasites provides improved diagnostic capabilities to resource-limited at-risk populations. Am J Trop Med Hyg. 2013;88:1041–1047. doi: 10.4269/ajtmh.12-0726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gordon CA, McManus DP, Acosta LP, Olveda RM, Williams GM, Ross AG, et al. Multiplex real-time PCR monitoring of intestinal helminths in humans reveals widespread polyparasitism in Northern Samar, the Philippines. Int J Parasitol. 2015;45:477–483. doi: 10.1016/j.ijpara.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 20.Schär F, Odermatt P, Khieu V, Panning M, Duong S, Muth S, et al. Evaluation of real-time PCR for Strongyloides stercoralis and hookworm as diagnostic tool in asymptomatic schoolchildren in Cambodia. Acta Trop. 2013;126:89–92. doi: 10.1016/j.actatropica.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 21.Gandasegui J, Martínez-Valladares M, Grau-Pujol B, Krolewiecki AJ, Balaña-Fouce R, Gelaye W, et al. on behalf of the stopping transmission of intestinal parasites project c. Role of DNA-detection-based tools for monitoring the soil-transmitted helminth treatment response in drug-efficacy trials. PLoS Negl Trop Dis. 2020;14:e0007931. doi: 10.1371/journal.pntd.0007931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Lieshout L, Roestenberg M. Clinical consequences of new diagnostic tools for intestinal parasites. Clin Microbiol Infect. 2015;21:520–528. doi: 10.1016/j.cmi.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 23.Basuni M, Muhi J, Othman N, Verweij JJ, Ahmad M, Miswan N, et al. A pentaplex real-time polymerase chain reaction assay for detection of four species of soil-transmitted helminths. Am J Trop Med Hyg. 2011;84:338–343. doi: 10.4269/ajtmh.2011.10-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taniuchi M, Verweij JJ, Noor Z, Sobuz SU, Lieshout L, Petri WA, et al. High throughput multiplex PCR and probe-based detection with Luminex beads for seven intestinal parasites. Am J Trop Med Hyg. 2011;84:332–337. doi: 10.4269/ajtmh.2011.10-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verweij JJ, Stensvold CR. Molecular testing for clinical diagnosis and epidemiological investigations of intestinal parasitic infections. Clin Microbiol Rev. 2014;27:371–418. doi: 10.1128/CMR.00122-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verweij JJ, Brienen EA, Ziem J, Yelifari L, Polderman AM, Van Lieshout L. Simultaneous detection and quantification of Ancylostoma duodenale, Necator americanus, and Oesophagostomum bifurcum in fecal samples using multiplex real-time PCR. Am J Trop Med Hyg. 2007;77:685–690. doi: 10.4269/ajtmh.2007.77.685. [DOI] [PubMed] [Google Scholar]
- 27.Easton AV, Oliveira RG, O’Connell EM, Kepha S, Mwandawiro CS, Njenga SM, et al. Multi-parallel qPCR provides increased sensitivity and diagnostic breadth for gastrointestinal parasites of humans: field-based inferences on the impact of mass deworming. Parasit Vectors. 2016;9:38. doi: 10.1186/s13071-016-1314-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cimino RO, Jeun R, Juarez M, Cajal PS, Vargas P, Echazú A, et al. Identification of human intestinal parasites affecting an asymptomatic peri-urban Argentinian population using multi-parallel quantitative real-time polymerase chain reaction. Parasit Vectors. 2015;8:380. doi: 10.1186/s13071-015-0994-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verweij JJ. Application of PCR-based methods for diagnosis of intestinal parasitic infections in the clinical laboratory. Parasitology. 2014;141:1863–1872. doi: 10.1017/S0031182014000419. [DOI] [PubMed] [Google Scholar]
- 30.Llewellyn S, Inpankaew T, Nery SV, Gray DJ, Verweij JJ, Clements ACA, et al. Application of a multiplex quantitative PCR to assess prevalence and intensity of intestinal parasite infections in a controlled clinical trial. PLoS Negl Trop Dis. 2016;10:e0004380. doi: 10.1371/journal.pntd.0004380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong SS, Fung KS, Chau S, Poon RW, Wong SC, Yuen KY. Molecular diagnosis in clinical parasitology: when and why? Exp Biol Med. 2014;239:1443–1460. doi: 10.1177/1535370214523880. [DOI] [PubMed] [Google Scholar]
- 32.Vlaminck J, Cools P, Albonico M, Ame S, Ayana M, Cringoli G, et al. Therapeutic efficacy of albendazole against soil-transmitted helminthiasis in children measured by five diagnostic methods. PLoS Negl Trop Dis. 2019;13:e0007471. doi: 10.1371/journal.pntd.0007471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barda B, Schindler C, Wampfler R, Ame S, Ali SM, Keiser J. Comparison of real-time PCR and the Kato-Katz method for the diagnosis of soil-transmitted helminthiasis and assessment of cure in a randomized controlled trial. BMC Microbiol. 2020;20:298. doi: 10.1186/s12866-020-01963-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meurs L, Brienen E, Mbow M, Ochola EA, Mboup S, Karanja DMS, et al. Is PCR the next reference standard for the diagnosis of Schistosoma in stool? A comparison with microscopy in Senegal and Kenya. PLoS Negl Trop Dis. 2015;9:e0003959. doi: 10.1371/journal.pntd.0003959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderson RM, Schad GA. Hookworm burdens and faecal egg counts: an analysis of the biological basis of variation. Trans R Soc Trop Med Hyg. 1985;79:812–825. doi: 10.1016/0035-9203(85)90128-2. [DOI] [PubMed] [Google Scholar]
- 36.Booth M, Vounatsou P, N’Goran EK, Tanner M, Utzinger J. The influence of sampling effort and the performance of the Kato-Katz technique in diagnosing Schistosoma mansoni and hookworm co-infections in rural d’Côte Ivoire. Parasitology. 2003;127:525–531. doi: 10.1017/S0031182003004128. [DOI] [PubMed] [Google Scholar]
- 37.Patel C, Hürlimann E, Keller L, Hattendorf J, Sayasone S, Ali SM, et al. Efficacy and safety of ivermectin and albendazole co-administration in school-aged children and adults infected with Trichuris trichiura: study protocol for a multi-country randomized controlled double-blind trial. BMC Infect Dis. 2019;19:262. doi: 10.1186/s12879-019-3882-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barda B, Albonico M, Buonfrate D, Ame SM, Ali S, Speich B, et al. Side benefits of mass drug administration for lymphatic filariasis on Strongyloides stercoralis prevalence on Pemba Island, Tanzania. Am J Trop Med Hyg. 2017;97:681–683. doi: 10.4269/ajtmh.17-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krähenbühl S, Studer F, Guirou E, Deal A, Mächler P, Hosch S, et al. ELIMU-MDx: a web-based, open-source platform for storage, management and analysis of diagnostic qPCR data. Biotechniques. 2020;68:22–27. doi: 10.2144/btn-2019-0064. [DOI] [PubMed] [Google Scholar]
- 40.Burns M, Valdivia H. Modelling the limit of detection in real-time quantitative PCR. Eur Food Res Technol. 2008;226:1513–1524. doi: 10.1007/s00217-007-0683-z. [DOI] [Google Scholar]
- 41.Moser W, Schindler C, Keiser J. Efficacy of recommended drugs against soil transmitted helminths: systematic review and network meta-analysis. BMJ. 2017;358:j4307. doi: 10.1136/bmj.j4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cardillo G. Cohenʼs kappa: compute the cohen’s kappa ratio on a 2×2 matrix. MathWorks, Inc. 2007. https://www.mathworks.com/matlabcentral/fileexchange/15365-cohen-s-kappa. Accessed 19 May 2020.
- 43.Christoforou M, Orford M, Tsaltas D. Molecular diagnostic tools for nematodes. In: Shah MM, editor. Nematology-concepts, diagnosis and control. Rijeka: IntechOpen; 2017. [Google Scholar]
- 44.Meurs L, Polderman AM, Vinkeles Melchers NVS, Brienen EAT, Verweij JJ, Groosjohan B, et al. Diagnosing polyparasitism in a high-prevalence setting in Beira, Mozambique: detection of intestinal parasites in fecal samples by microscopy and real-time PCR. PLoS Negl Trop Dis. 2017;11:e0005310. doi: 10.1371/journal.pntd.0005310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dunn JC, Papaiakovou M, Han KT, Chooneea D, Bettis AA, Wyine NY, et al. The increased sensitivity of qPCR in comparison to Kato-Katz is required for the accurate assessment of the prevalence of soil-transmitted helminth infection in settings that have received multiple rounds of mass drug administration. Parasit Vectors. 2020;13:324. doi: 10.1186/s13071-020-04197-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benjamin-Chung J, Pilotte N, Ercumen A, Grant JR, Maasch J, Gonzalez AM, et al. Comparison of multi-parallel qPCR and double-slide Kato-Katz for detection of soil-transmitted helminth infection among children in rural Bangladesh. PLoS Negl Trop Dis. 2020;14:e0008087. doi: 10.1371/journal.pntd.0008087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Frickmann H, Schwarz NG, Rakotozandrindrainy R, May J, Hagen RM. PCR for enteric pathogens in high-prevalence settings. What does a positive signal tell us? Infect Dis. 2015;47:491–498. doi: 10.3109/23744235.2015.1022212. [DOI] [PubMed] [Google Scholar]
- 48.Knopp S, Mgeni AF, Khamis IS, Steinmann P, Stothard JR, Rollinson D, et al. Diagnosis of soil-transmitted helminths in the era of preventive chemotherapy: effect of multiple stool sampling and use of different diagnostic techniques. PLoS Negl Trop Dis. 2008;2:e331. doi: 10.1371/journal.pntd.0000331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marti H, Koella JC. Multiple stool examinations for ova and parasites and rate of false-negative results. J Clin Microbiol. 1993;31:3044–3045. doi: 10.1128/JCM.31.11.3044-3045.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pilotte N, Papaiakovou M, Grant JR, Bierwert LA, Llewellyn S, McCarthy JS, et al. Improved PCR-based detection of soil transmitted helminth infections using a next-generation sequencing approach to assay design. PLoS Negl Trop Dis. 2016;10:e0004578. doi: 10.1371/journal.pntd.0004578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O’Connell EM, Nutman TB. Molecular diagnostics for soil-transmitted helminths. Am J Trop Med Hyg. 2016;95:508–513. doi: 10.4269/ajtmh.16-0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Andersen LO, Röser D, Nejsum P, Nielsen HV, Stensvold CR. Is supplementary bead beating for DNA extraction from nematode eggs by use of the NucliSENS easyMag protocol necessary? J Clin Microbiol. 2013;51:1345–1347. doi: 10.1128/JCM.03353-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schrader C, Schielke A, Ellerbroek L, Johne R. PCR inhibitors – occurrence, properties and removal. J Appl Microbiol. 2012;113:1014–1026. doi: 10.1111/j.1365-2672.2012.05384.x. [DOI] [PubMed] [Google Scholar]
- 54.Levecke B, Cools P, Albonico M, Ame S, Angebault C, Ayana M, et al. Identifying thresholds for classifying moderate-to-heavy soil-transmitted helminth intensity infections for FECPAKG2, McMaster, Mini-FLOTAC and qPCR. PLoS Negl Trop Dis. 2020;14:e0008296. doi: 10.1371/journal.pntd.0008296. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Primers and probes used to identify the different helminth species.
Additional file 2: Table S2. GeneExpression MasterMix.
Data Availability Statement
Data supporting the conclusions of this article are included within the article and its additional files. The datasets used and analysed during the present study are available from the corresponding author upon reasonable request.