Abstract
Chikungunya is a rapidly emerging infectious disease worldwide caused by a virus that belongs to the Togaviridae family. It can have varied presentations, but vesiculobullous lesions are commonly described. A widespread dissemination of such lesions, however, is extremely rare. Person-to-person transmission has not been documented, but rare reports have described maternal-fetal vertical transmission. We herein describe a unique case of congenital chikungunya resulting in a staphylococcal scalded skin syndrome-like presentation and discuss the clinical presentation, underlying pathophysiology, and how to differentiate this condition from true Stevens Johnson Syndrome-Toxic epidermal Necrolysis (SJS-TEN).
Keywords: Chikungunya, Stevens-Johnson syndrome, Toxic epidermal necrolysis, Perinatal infection, Neonatal infection
Introduction
Chikungunya (CK) infection typically results in a febrile illness characterized by a maculopapular rash with painful and potentially debilitating symmetric joints. This infection is transmitted by the bite of an infected Aedes aegypti or Aedes albopictus mosquito, a vector that also transmits dengue, zika, and yellow fever [1]. Although person-to-person transmission has not been documented, rare reports have described maternal-fetal vertical transmission [2]. Following an incubation period of about 1–12 days, patients experience cutaneous manifestations that typically include a maculopapular rash that lasts for 2–3 days [1]. This rash can sometimes recur with recrudescence of fever. The trunk and limbs are commonly involved, but the face, palms, and soles may also show lesions. Pruritus and irritation can be present, and this rash may desquamate or may simply fade. Other skin manifestations include: hyperpigmentation (especially centrofacial freckle‐like macules, diffuse pigmentation of the face, pinna and extremities and flagellate pigmentation of the face and extremities); multiple aphthous‐like ulcers over the scrotum, penis, groin, perianal region, axillae, and oral mucosa; transient nasal erythema; vesiculobullous lesions; ecchymosis; subungual hemorrhage; generalized erythema; tenderness and edema of the hands and feet; vasculitic lesions; purpuric macules; vesicles arising over purpuric macules; perianal erosions; and resolution leaving post‐inflammatory hypopigmentation. Vesiculobullous lesions have been reported in infants. Joint pain is symmetric and usually involves the ankles, fingers, and wrists. Typical acute symptoms resolve in 7–10 days; however, arthralgias can persist for months to years and are a key distinguishing feature from a dengue infection. Deaths following infection typically occur in the very young and very old. The time from disease onset to clinical presentation of CK ranges from 1 to 9 days.
Case
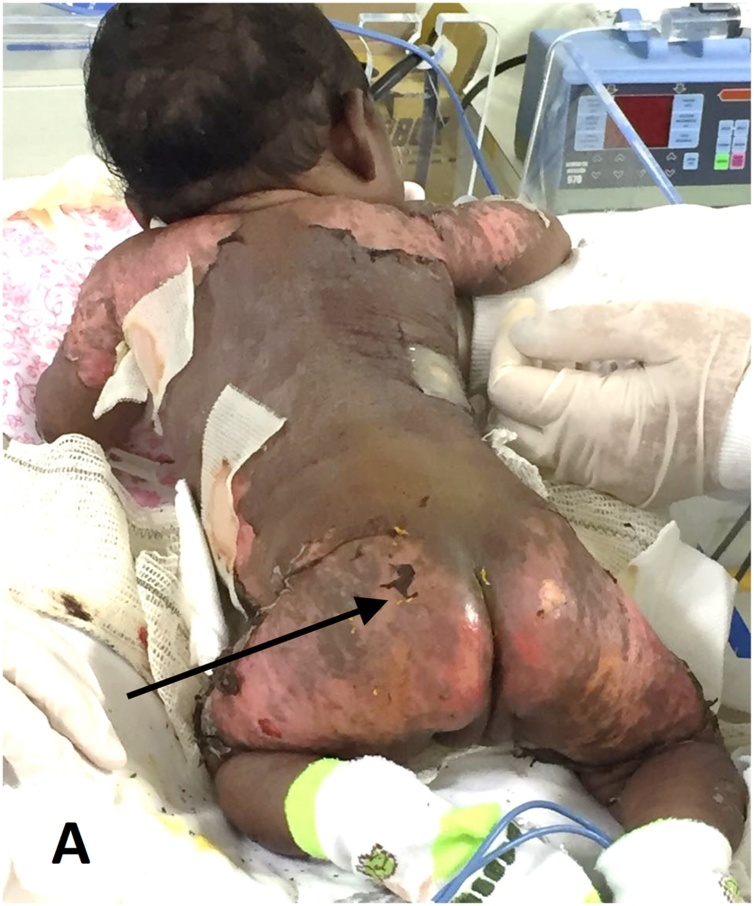
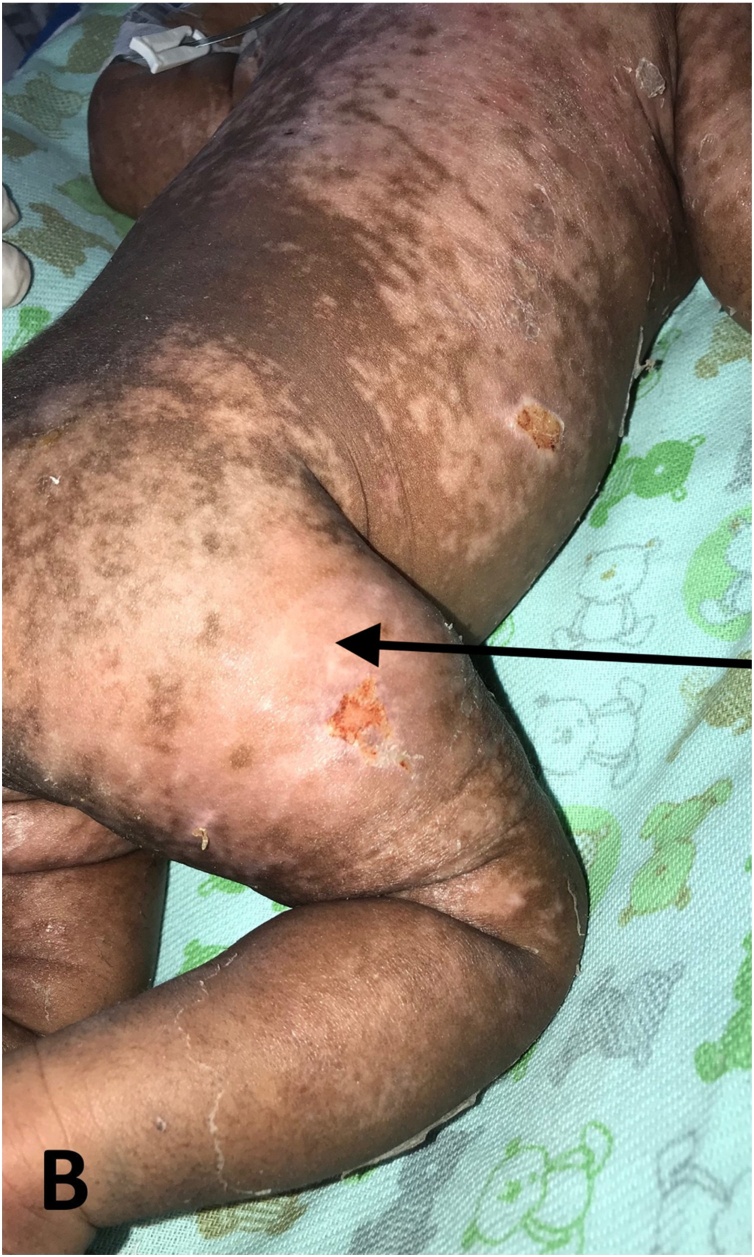
A neonate presented to the local hospital in Rio de Janeiro, Brazil for evaluation of a severe infection. Although the immediate postnatal period was uneventful, on the 3rd day of life, the mother had fever and arthralgia, and was suspected to have CK infection. The neonate developed high-grade fever and was admitted to the neonatal intensive care unit on the 7th day. There were no other specific clinical findings at the time of admission. Presuming sepsis, the neonate was started on gentamicin, ampicillin, vancomycin, and other supportive measures. By the 9th day, the fever had decreased in intensity. On the 10th day, the neonate developed a generalized morbilliform eruption, followed by the onset of widespread, symmetrically distributed vesicles and bulla over the legs, trunk, abdomen, and extremities, excluding the palms and soles, preceding large erosions some days later (Fig. 1). Necrosis and desquamation were also seen. Nikolsky’s sign was positive. Erythema and exfoliation were present, and resolving lesions left hypopigmentation (Fig. 2). Joint swelling was not present. The neonate’s serum, which was collected 10 days after birth, tested positive for both chikungunya virus RNA via reverse transcriptase polymerase chain reaction and IgM antibodies to chikungunya virus. The mother’s serum, which was collected two days prior to the neonate’s serum, also tested positive for both of these tests. Both the mother’s serum and the neonate’s serum tested negative for IgG antibodies to chikungunya virus.
Fig. 1.
Scalded skin syndrome (SSSS)-like presentation.
(A) Positive Nikolsky’s sign.
Fig. 2.
Post-Chikungunya pigmentary disorder.
(B) Residual hypopigmentation within resolving lesion.
Discussion
Cutaneous involvement in CK can be present in 40 %–75 % of patients [3]. It can have varied presentations, but vesicobullous lesions are commonly described in infants [3]. In very rare cases, a widespread dissemination of such lesions can occur. Although the exact mechanism is not yet established, the pathogenesis of Staphyloccocus scalded skin syndrome (SSSS)-like presentation may be related to a direct cytopathic effect due to the cutaneous dissemination of the CK virus with ballooning degeneration of epidermal cells, or it may be related to capillary endothelial injury followed by a cytotoxic immune response [4]. It has been postulated that melanin dispersion, retention, or destruction by the virus leads to the post-CK pigmentary disorder [5].
The Nelson severity index can help determine severity of illness and hence helps to differentiate between true Stevens Johnson Syndrome-Toxic Epidermal Necrolysis (SJS-TEN) and SJS-TEN-like presentation [6]. The Nelson severity index uses five parameters to assess a child’s general medical condition, and it reached index 7 in our patient.
Neonatologists and dermatologists should be aware of CK infection in the differential diagnosis of SJS-TEN-like presentation, which can alert the surveillance system and policies to the control of this high morbidity arbovirosis.
Funding resource
None
Declaration of Competing Interest
None
References
- 1.Burt F.J., Rolph M.S., Rulli N.E., Mahalingam S., Heise M.T. Chikungunya: a re-emerging virus. Lancet. 2012;379(9816):662–671. doi: 10.1016/S0140-6736(11)60281-X. [DOI] [PubMed] [Google Scholar]
- 2.Ramful D., Carbonnier M., Pasquet M., Bouhmani B., Ghazouani J., Noormahomed T. Mother-to-child transmission of Chikungunya virus infection. Pediatr Infect Dis J. 2007;26(9):811–815. doi: 10.1097/INF.0b013e3180616d4f. [DOI] [PubMed] [Google Scholar]
- 3.Bandyopadhyay D., Ghosh S.K. Mucocutaneous manifestations of Chikungunya fever. Indian J Dermatol. 2010;55(1):64–67. doi: 10.4103/0019-5154.60356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riyaz N., Riyaz A., Rahima, Abdul Latheef E.N., Anitha P.M., Aravindan K.P., Nair A.S. Cutaneous manifestations of chikungunya during a recent epidemic in Calicut, north Kerala, south India. Indian J Dermatol Venereol Leprol. 2010;76(6):671–676. doi: 10.4103/0378-6323.72466. [DOI] [PubMed] [Google Scholar]
- 5.Vashi N.A., Kundu R.V. Facial hyperpigmentation: causes and treatment. Br J Dermatol. 2013;169(Suppl. 3):41–56. doi: 10.1111/bjd.12536. [DOI] [PubMed] [Google Scholar]
- 6.Mathias R.C., Jayaseelan E., Augustine M. Spectrum of pediatric dermatological emergencies at a tertiary care hospital in India: a descriptive study. Int J Dermatol. 2013;52(1):27–31. doi: 10.1111/j.1365-4632.2011.05247.x. [DOI] [PubMed] [Google Scholar]