Abstract
Objective:
To estimate the effectiveness of Atosiban in improving the outcome after embryo transfer. The effectiveness of embryo transfer per cycle is still relatively low. One possible explanation might be uterine contractility that expels the transferred embryos. Atosiban improved the outcome of embryo transfer by reducing uterine contractility.
Methods:
Data sources: A systematic review of papers in English using MEDLINE and EMBASE (1990-2019). Search terms included Atosiban, embryo transfer. Study selection: We included studies that compared the outcomes of embryo transfer with Atosiban and a control group. Data Extracting: Independent extraction of papers by two authors, using predefined data fields, including study quality indicators.
Results:
All pooled analyses were based on a fixed-effect model. Four randomised controlled trials, including 1,025 women, and two non-randomised trials, including 686 patients, met our inclusion criteria. In both studies, the heterogeneity was moderate. Atosiban increased clinical pregnancy rates regardless of the indication for ART or type of embryo transferred. Pooled OR in randomized controlled trials reached 1.47 (1.18-1.82), and in non-randomised controlled trials it reached 1.50 (95% CI 1.10-2.05)
Conclusion:
Atosiban appears to increase the clinical pregnancy rates in women undergoing embryo transfer.
Keywords: atosiban, in vitro, pregnancy rate
INTRODUCTION
In spite of advanced progress in assisted reproduction technology (ART) over the past 20 years, the effectiveness of embryo transfer (ET) per cycle is still relatively low. In 2015, the delivery rate (DR) per ET in Latin America reached 25.6% in fresh autologous ET, and 36.8% when using donated eggs (Zegers-Hochschild et al., 2017a;b).
After ET the effectiveness of embryo implantation depends on embryo quality, endometrial receptivity and adequate dialogue between them (Achache & Revel, 2006). Traditionally, an abnormal chromosomal complement has been considered as the main cause for implantation failure and, in clinical practice, considerably little effort has been devoted to improve uterine receptivity. Generally, appropriate endometrial status, sufficient endometrial perfusion and absence of excessive uterine contractions are necessary for ideal endometrial receptivity and to facilitate embryo implantation (Pierzynski & Reinheimer, 2007). Although increased contractions have been found in approximately 30% of patients undergoing ET, to date uterine contractility is not included in any diagnostic measures, and the therapies to reduce uterine contractions before ET such as beta agonists, non-steroid anti-inflammatory drugs (NSAIDs) or progesterone had not shown definite benefits (Bernabeu et al., 2006; Fanchin et al., 2001).
Theoretically, uterine contractions can expel the embryos after transfer, as per indicated by a study of mock embryo transfer processes (Fanchin et al., 1998a). As such, a stepwise decrease in implantation rates and clinical ongoing pregnancy rates occurred from the lowest to the highest uterine contraction frequencies (Fanchin et al., 1998b).
Atosiban was administered to inhibit uterine contractions (He et al., 2016a; Hebisha et al., 2016). Atosiban is a uterine-specific, mixed vasopressin V1-a and oxytocin-receptor antagonist, that is registered for tocolysis in imminent premature birth. It also inhibits uterine contractility in nonpregnant women. Thus, Atosiban may decrease uterine contractions and promote uterine receptivity in patients undergoing embryo transfer.
We conducted this systematic review and meta-analysis to investigate whether Atosiban improves pregnancy outcomes in the women undergoing ET.
MATERIALS AND METHODS
Literature search and study selection
We searched the computerised databases Medline and Embase from January 1990 to July 2019. We explored the following terms as free text terms and MeSH terms (shown in italics): (embryo transfer; atosiban) and (fertilization in vitro; atosiban). Additionally, the citation lists of all relevant publications and review papers were hand-searched.
Selection criteria, data extraction and quality assessment
We established the criteria for inclusion/exclusion of studies prior to the literature search. We selected randomised controlled trials and observational studies that compared Atosiban at the time of ET with placebo or no treatment. Trials that included intracytoplasmic injection of sperm as well as in vitro fertilization were eligible, as were studies using fresh and frozen/thawed ET. We excluded trials that evaluated other intervention in conjunction with Atosiban. We imposed no restrictions on publication type (that is, either full article or abstract), and restricted language to English. Two authors (JES and JC) independently selected articles and extracted data, with disagreements resolved by discussion.
Outcome measures
The pre-specified primary outcomes were clinical pregnancy (that is, presence of at least one gestational sac or foetal heartbeat, confirmed by transvaginal ultrasound) and live birth.
Risk of publication bias
For each trial, we plotted the effect by the inverse of its standard error. The symmetry of such ‘funnel plots’ was assessed visually and formally analyzed to help understand whether the results of their review are robust, all of which should be reported. Such analyses include sensitivity analysis, subgroup analysis, and meta-regression.
Risk of bias assessment
We evaluated the methodological quality of trials using the Cochrane risk of bias tool (Sterne et al., 2016; Higgins et al., 2011). The items evaluated in randomized trials were: concealment of randomisation sequence allocation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias) and other biases (Higgins et al., 2011). In the case of non-randomized trials, the items evaluated were: confounding, participant selection, intervention classification, deviation from intended intervention, missing data, outcomes measurement, results report (Sterne et al., 2016).
Statistical analysis
The measure of treatment effect was the pooled odds ratio of achieving a clinical pregnancy or live birth per ET for women in the Atosiban group, compared with women in the control group. For pooled data, we calculated summary test statistics using the Mantel-Haenzel method, using Rev-Man software, version 5.1. We based our meta-analyses on the number of women randomized, not on the number of women undergoing treatment.
We evaluated heterogeneity using the I2 test (Higgins et al., 2003) which indicates the proportion of variability across trials not explained by chance alone, and the p-value of X2 test of heterogeneity. Although interpreting the importance of inconsistency depends on other factors, the I2 values (e.g. p-value from X2 test, magnitude and direction of effects), the Cochrane Handbook suggests the following rough guide to interpreting the I2 values: low, moderate, and high to I2 values of 25%, 50%, and 75% test (Higgins et al., 2003).
A fixed effects model was used where no statistically significant heterogeneity was present, whereas in the presence of statistically significant heterogeneity, a random effects model was applied. Statistical significance was set at a p level of 0.05. The presence of publication bias was tested by using the Harbord-Egger’s test (Harbord et al., 2006).
Subgroup analysis
If the overall I2 value for all trials was reduced when we separated the trials into subgroups according to source of bias, we used the subgroup results as primary. Otherwise, the pooled results from all trials would be used for our primary analysis, but with the results from the two subgroups also presented.
RESULTS
Search results
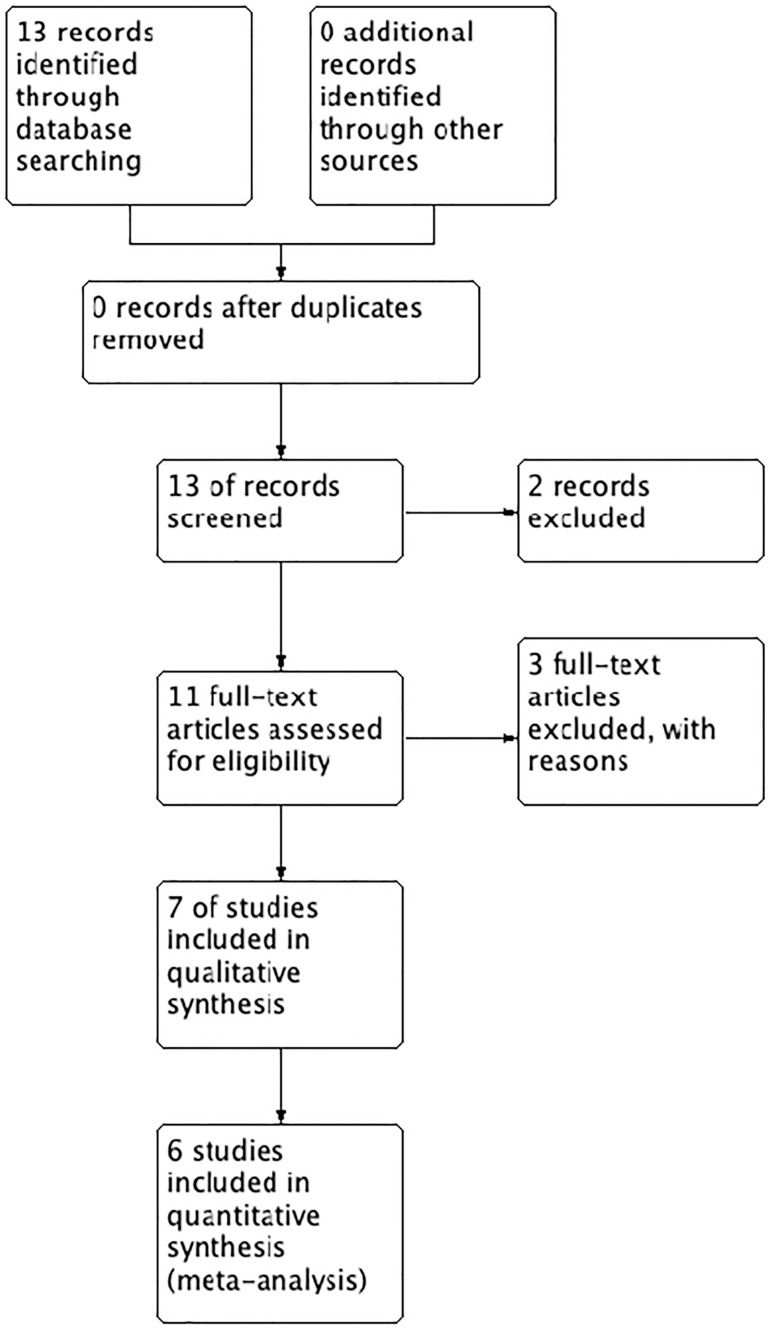
The extensive literature search performed between the years 1990-2018 on Medline, EMBASE, yielded 13 publications. Of these, two were excluded based on the title and abstract. We then obtained the full text of the remaining 11 papers. See flow diagram in Figure 1.
Figure 1.
Flow diagram
Included studies
Seven studies were considered in the synthesis, including 3 observational studies (Chou et al., 2011; He et al., 2016b; Lan et al., 2012) and 4 randomized controlled trials (He et al., 2016a; Hebisha et al., 2016; Moraloglu et al., 2010; Ng et al., 2014). The characteristics of the included trials are shown in Table 1.
Table 1.
Characteristics of the studies included
| Study, year | Design | Inclusion Criteria | Outcomes | Atosiban dose |
|---|---|---|---|---|
| Moraloglu et al., 2010 | Prospective, randomized, placebo-controlled clinical study | Women undergoing intracytoplasmic sperm injection who had top-quality embryos | Clinical pregnancy rate per cycle and implantation rate | Intravenous atosiban 30 min before the embryo transfer with a bolus dose of 6.75 mg, and the infusion was continued with an infusion rate of 18 mg/h. After performing embryo transfer, the dose of atosiban was reduced to 6 mg/h and the infusion was continued for 2 h (total administered dose: 37.5 mg). |
| Chou et al., 2011 | Retrospective cohort study | Repeated implantation failure (RIF) | Implantation rate, clinical pregnancy rate, live birth rate | Forty patients received a single bolus dose (6.75 mg, 0.9 mL/vial) of atosiban before ET (Group 2), and 30 patients received a bolus dose of 6.75 mg atosiban followed by infusion at 18 mg/hr. for 3 hours immediately after ET (Group 3). |
| Lan et al., 2012 | Prospective cohort study | Women with repeated implantation failure | Uterine contraction, implantation rate (IR) and clinical pregnancy rate (CPR) | I.V. bolus of 6.75 mg at 30 min prior to embryo transfer followed by i.v. infusion at a rate of 18 mg/h for 1h and 6 mg/h for the subsequent 2h. The total dose administered was 36.75 mg. |
| Ng et al., 2014 | Multi-center randomized double blind study | Consecutive subfertile women undergoing IVF treatment | The primary outcome measure was the live birth rate and the secondary outcome measures including positive pregnancy test, clinical pregnancy, ongoing pregnancy, miscarriage, multiple pregnancy and ectopic pregnancy rates. | I.V. Atosiban 30 min before the transfer with a bolus dose of 6.75 mg, and the infusion was continued at a rate of 18 mg/h for 1h. The dose of Atosiban was then reduced to 6 mg/h after embryo transfer and the infusion was continued for another 2h. Therefore, the total administered dose was 37.5 mg. |
| He et al., 2016a | Randomized, controlled clinical trial. | Women with endometriosis undergoing frozen–thawed embryo transfer | Implantation rate and pregnancy rate. | IV bolus of 6.75 mg at approximately 30 min before ET. |
| He et al., 2016b | Prospective cohort study | Patients undergoing IVF/ET using cryopreserved embryos | Uterine contraction, clinical pregnancy rate | I.V. bolus of 6.75 mg at about 30 min prior before ET. |
| Hebisha et al., 2016 | Randomized controlled trial. | One hundred and eighty two women, prepared for intracytoplasmic sperm injection for male or tubal factor infertility, using long agonist protocol | Pregnancy rate, implantation rate. | 7.5 mg Atosiban by slow IV injection |
Methods in the included studies
The study population included patients undergoing ICSI with the transfer of top-quality embryos (Moraloglu et al., 2010), regular IVF (Ng et al., 2014), women with repeated implantation failure (Chou et al., 2011; Lan et al., 2012), transfer of frozen/thawed embryos (He et al., 2016b), and transfer of frozen/thawed embryos in women with endometriosis (He et al., 2016a).
The intervention included the administration of a single bolus dose (Chou et al., 2011; Hebisha et al., 2016; He et al., 2016a;b) prior to the embryo transfer, or the administration of a bolus doses plus maintaining a continues dose (Lan et al., 2012; Moraloglu et al., 2010; Ng et al., 2014).
The outcomes evaluated included implantation rate (Chou et al., 2011; He et al., 2016a; Lan et al., 2012; Moraloglu et al., 2010), clinical pregnancy rate (He et al., 2016;b; Lan et al., 2012; Moraloglu et al., 2010; Chou et al., 2011; Hebisha et al., 2016) and delivery rate (Chou et al., 2011; Ng et al., 2014).
Methodological quality of included studies
According to the guidelines suggested by the Cochrane Collaboration, the quality of most of the included studies was low to moderate due to unclear selection, performance and detection bias. Table 2 depicts the quality assessment of the included trials.
Table 2.
Bias risks of the included RCT
| Study, year | Domain 1 | Domain 2 | Domain 3 | Domain 4 | Domain 5 | Domain 6 | Domain 7 |
|---|---|---|---|---|---|---|---|
| Moraloglu et al., 2010 | High | High | Low | unknown | Low | Low | unknown |
| Ng et al., 2014 | Unknown | Low | Low | unknown | Low | Low | Low |
| He et al., 2016a | Low | Low | unknown | unknown | Low | Low | Low |
| Hebisha et al., 2016 | Unknown | unknown | unknown | unknown | Low | Low | unknown |
Random sequence generation (selection bias)
allocation concealment (selection bias)
blinding of participants and personnel (performance bias)
blinding of outcome assessment (detection bias) (patient-reported outcome)
blinding of outcome assessment (detection bias) (all-cause main outcome)
incomplete outcome data (attrition data)
selective reporting (reporting bias)
Result of the outcome measures
In total 1,025 women were allocated to Atosiban and 953 were allocated to a control group. Overall, we analysed four randomized controlled trials, including 1,292 patients, and two observational studies, including 686 patients.
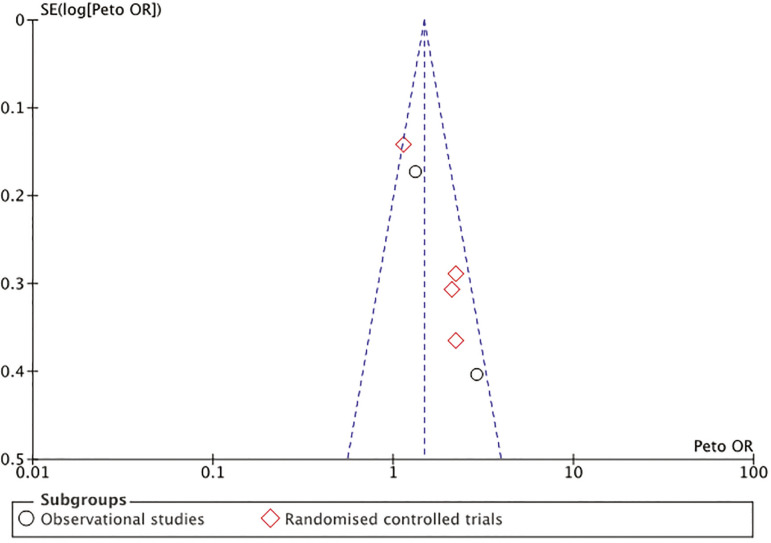
In both, observational and randomized controlled studies, Atosiban was associated with an increased risk of clinical pregnancy. In the case of observational studies, the OR (95% CI) was 1.50 (1.10-2.05), with a moderate level of heterogeneity (I2 68%, p=0.08). In the case of randomized controlled trials, the OR (95% CI) of clinical pregnancy was 1.47 (1.18-1.82), with moderate heterogeneity (I2= 62%, p=0.05). Figure 2 shows a forest plot with subgroup analyses for randomized and non-randomised controlled trials. To explore the heterogeneity, a funnel plot was drawn. The funnel plot (Figure 3) shows evidence of considerable symmetry.
Figure 2.
Forest plot
Figure 3.
Funnel plot
DISCUSSION
Atosiban was associated with an increase in the chance of clinical pregnancy. This increased probability was seen in both observational and randomized controlled studies, using different doses. ET is one of the crucial steps in ART, and the use of high-quality embryos together with the presence of an optimal intrauterine environment are the basic determinants of ET success (Dessolle et al., 2009). Fanchin et al. (1998a) demonstrated that uterine contractions occur during the course of ET. They reported that excessive uterine contractions can expel embryos from the uterus and that the frequency of uterine contractions was negatively correlated with implantation and clinical pregnancy rates. Since Atosiban is a combined oxytocin/vasopressin V1A antagonist, it works mainly by blocking oxytocin and vasopressin V1a receptors to decrease the frequency and amplitude of uterine contractions, which may enhance implantation and pregnancy rates (Pierzynski, 2011).
This is the most up-to-date review on this subject. The main strengths are the large sample of patients included, and an increased risk of pregnancy is supported by both, observational and randomized controlled studies. Furthermore, there was a positive effect of Atosiban regardless of the dose used. On the other hand, the main weakness of our study is that only six studies were found (since 2016, no new studies have been published) and the quality of the studies was relatively low.
In summary, we found that Atosiban was associated to an improvement in ART cycle outcomes, which might be of clinical significance, although, its administration requires a peripheral venous catheterization, longer hospitalization, and makes ET more expensive. Perhaps, the development of Nolasibam, an oral oxytocin receptor antagonist with the potential to decrease uterine contractions, will overrule these disadvantages in the near future.
Footnotes
CONFLICT OF INTERESTS
There is no conflict of interest.
REFERENCES
- Achache H, Revel A. Endometrial receptivity markers, the journey to successful embryo implantation. Hum Reprod Update. 2006;12:731–746. doi: 10.1093/humupd/dml004. [DOI] [PubMed] [Google Scholar]
- Bernabeu R, Roca M, Torres A, Ten J. Indomethacin effect on implantation rates in oocyte recipients. Hum Reprod. 2006;21:364–369. doi: 10.1093/humrep/dei343. [DOI] [PubMed] [Google Scholar]
- Chou PY, Wu MH, Pan HA, Hung KH, Chang FM. Use of an oxytocin antagonist in in vitro fertilization-embryo transfer for women with repeated implantation failure: a retrospective study. Taiwan J Obstet Gynecol. 2011;50:136–140. doi: 10.1016/j.tjog.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Dessolle L, Daraï E, Cornet D, Rouzier R, Coutant C, Mandelbaum J, Antoine JM. Determinants of pregnancy rate in the donor oocyte model: a multivariate analysis of 450 frozen-thawed embryo transfers. Hum Reprod. 2009;24:3082–3089. doi: 10.1093/humrep/dep303. [DOI] [PubMed] [Google Scholar]
- Fanchin R, Righini C, Ayoubi JM, Olivennes F, de Ziegler D, Frydman R. Uterine contractions at the time of embryo transfer: a hindrance to implantation? Contracept Fertil Sex. 1998a;26:498–505. [PubMed] [Google Scholar]
- Fanchin R, Righini C, Olivennes F, Taylor S, de Ziegler D, Frydman R. Uterine contractions at the time of embryo transfer alter pregnancy rates after in-vitro fertilization. Hum Reprod. 1998b;13:1968–1974. doi: 10.1093/humrep/13.7.1968. [DOI] [PubMed] [Google Scholar]
- Fanchin R, Righini C, de Ziegler D, Olivennes F, Ledée N, Frydman R. Effects of vaginal progesterone administration on uterine contractility at the time of embryo transfer. Fertil Steril. 2001;75:1136–1140. doi: 10.1016/s0015-0282(01)01787-3. [DOI] [PubMed] [Google Scholar]
- Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med. 2006;25:3443–3457. doi: 10.1002/sim.2380. [DOI] [PubMed] [Google Scholar]
- Hebisha SA, Aboelazm BA, Adel HM, Ahmed AI. Impact of the oxytocin receptor antagonist (ATOSIBAN) administered shortly before embryo transfer on pregnancy outcome after intracytoplasmic sperm injection (ICSI) Fertil Steril. 2016;106:e88–e89. doi: 10.1016/j.fertnstert.2016.07.260. [DOI] [Google Scholar]
- He Y, Wu H, He X, Xing Q, Zhou P, Cao Y, Wei Z. Administration of atosiban in patients with endometriosis undergoing frozen-thawed embryo transfer: a prospective, randomized study. Fertil Steril. 2016a;106:416–422. doi: 10.1016/j.fertnstert.2016.04.019. [DOI] [PubMed] [Google Scholar]
- He Y, Wu H, He X, Xing Q, Zhou P, Cao Y, Wei Z. Application of atosiban in frozen-thawed cycle patients with different times of embryo transfers. Gynecol Endocrinol. 2016b;32:811–815. doi: 10.1080/09513590.2016.1180680. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, Cochrane Bias Methods Group. Cochrane Statistical Methods Group The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan VT, Khang VN, Nhu GH, Tuong HM. Atosiban improves implantation and pregnancy rates in patients with repeated implantation failure. Reprod Biomed Online. 2012;25:254–260. doi: 10.1016/j.rbmo.2012.05.014. [DOI] [PubMed] [Google Scholar]
- Moraloglu O, Tonguc E, Var T, Zeyrek T, Batioglu S. Treatment with oxytocin antagonists before embryo transfer may increase implantation rates after IVF. Reprod Biomed Online. 2010;21:338–343. doi: 10.1016/j.rbmo.2010.04.009. [DOI] [PubMed] [Google Scholar]
- Ng EH, Li RH, Chen L, Lan VT, Tuong HM, Quan S. A randomized double blind comparison of atosiban in patients undergoing IVF treatment. Hum Reprod. 2014;29:2687–2694. doi: 10.1093/humrep/deu263. [DOI] [PubMed] [Google Scholar]
- Pierzynski P, Reinheimer TM, Kuczynski W. Oxytocin antagonists may improve infertility treatment. Fertil Steril. 2007;88:213.e19–213.e22. doi: 10.1016/j.fertnstert.2006.09.017. [DOI] [PubMed] [Google Scholar]
- Pierzynski P. Oxytocin and vasopressin V(1A) receptors as new therapeutic targets in assisted reproduction. Reprod Biomed Online. 2011;22:9–16. doi: 10.1016/j.rbmo.2010.09.015. [DOI] [PubMed] [Google Scholar]
- Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan AW, Churchill R, Deeks JJ, Hróbjartsson A, Kirkham J, Jüni P, Loke YK, Pigott TD, Ramsay CR. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zegers-Hochschild F, Schwarze JE, Crosby J, Musri C, Urbina MT, Latin American Network of Assisted Reproduction (REDLARA) Assisted reproduction techniques in Latin America: the Latin American Registry, 2014. Reprod Biomed Online. 2017a;35:287–295. doi: 10.5935/1518-0557.20170034. [DOI] [PubMed] [Google Scholar]
- Zegers-Hochschild F, Schwarze JE, Crosby J, Musri C, Urbina MT. Assisted reproductive techniques in Latin America: The Latin American Registry, 2014. JBRA Assist Reprod. 2017b;21:164–175. doi: 10.5935/1518-0557.20170034. [DOI] [PMC free article] [PubMed] [Google Scholar]