Abstract
Introduction
Cerebral small vessel disease (cSVD) accounts for 20%–25% of strokes and is the most common cause of vascular cognitive impairment (VCI). In an animal VCI model, inducing brief periods of limb ischaemia-reperfusion reduces subsequent ischaemic brain injury with remote and local protective effects, with hindlimb remote ischaemic conditioning (RIC) improving cerebral blood flow, decreasing white-matter injury and improving cognition. Small human trials suggest RIC is safe and may prevent recurrent strokes. It remains unclear what doses of chronic daily RIC are tolerable and safe, whether effects persist after treatment cessation, and what parameters are optimal for treatment response.
Methods and analysis
This prospective, open-label, randomised controlled trial (RCT) with blinded end point assessment and run-in period, will recruit 24 participants, randomised to one of two RIC intensity groups: one arm treated once daily or one arm twice daily for 30 consecutive days. RIC will consistent of 4 cycles of blood pressure cuff inflation to 200 mm Hg for 5 min followed by 5 min deflation (total 35 min). Selection criteria include: age 60–85 years, evidence of cSVD on brain CT/MRI, Montreal Cognitive Assessment (MoCA) score 13–24 and preserved basic activities of living. Outcomes will be assessed at 30 days and 90 days (60 days after ceasing treatment). The primary outcome is adherence (completing ≥80% of sessions). Secondary safety/tolerability outcomes include the per cent of sessions completed and pain/discomfort scores from patient diaries. Efficacy outcomes include changes in cerebral blood flow (per arterial spin-label MRI), white-matter hyperintensity volume, diffusion tensor imaging, MoCA and Trail-Making tests.
Ethics and dissemination
Research Ethics Board approval has been obtained. The results will provide information on feasibility, dose, adherence, tolerability and outcome measures that will help design a phase IIb RCT of RIC, with the potential to prevent VCI. Results will be disseminated through peer-reviewed publications, organisations and meetings.
Trial registration number
Keywords: clinical trials, dementia, stroke
Strengths and limitations of this study.
This trial will enrol patients using established neuroimaging criteria for the diagnosis of cerebral small vessel disease (cSVD), ensuring a valid sample of the target condition.
Patients will be enrolled into two active comparator groups of remote ischaemic preconditioning (RIC), with the primary goal of comparing the tolerability of different doses.
The use of intent-to-treat analysis, prespecified primary and secondary outcomes and candidate biomarkers for monitoring treatment response will improve on previous small studies of remote ischaemic preconditioning in cSVD; however, the lack of a non-treated or sham control group means that only within-patient changes can be analysed.
The use of a 60-day washout period after 30 days of treatment will help clarify the persistence of any RIC-related treatment effects.
Participants and healthcare providers will not be blinded to the intervention, but end point assessment will be blinded to treatment allocation.
Introduction
Cerebral small vessel disease (cSVD) is the most common cause of vascular cognitive impairment (VCI), accounting for about 30% of all cases of dementia in community-based neuropathological studies.1–3 cSVD can be identified on MRI using markers like small subcortical infarcts, lacunes and white matter hyperintensities (WMHs).2 Patients with cSVD have frequent, small brain infarcts, making this an ideal condition to study an intervention to condition the brain to resist ischaemia.4 5 Although each new infarct is insidious and may not have an easily identified acute presentation, over time the cumulative burden leads to accelerated cognitive decline.6 7 There are no proven therapies for preventing cSVD progression.8 Strategies that can be safely applied early in the disease course would be particularly desirable.9
Experimentally inducing brief periods of ischaemia-reperfusion that do not result in tissue injury before an ischaemic event can reduce subsequent injury.10 This process, known as ischaemic preconditioning, is thought to induce an endogenous protective environment, consisting of humoral and neuronal-mediated responses that promote cell survival/repair and dampen apoptotic/inflammatory pathways, mitigating ischaemic injury.11 These protective mechanisms do not seem organ-specific, exerting systemic and remote protective effects; thus, remote ischaemic preconditioning (RIC) applied to a limb can promote tolerance to cerebral ischaemia.10 The RIC stimulus appears to precipitate an early phase of short-term metabolic, energy utilisation, and blood flow changes lasting a few hours, and a late phase of longer-lasting changes in gene expression, inflammatory and oxidative pathways (16–96 hours post-RIC).12 The exact mechanisms for signal transmission from the periphery to the brain to protect against ischaemia remain unclear, so there is uncertainty regarding the optimal biomarkers of RIC. Candidate biomarkers include circulating nitrite, heat shock protein 27, microRNA-144 and interleukin-10.13–16
In a bilateral carotid occlusion model of VCI in mice, chronic daily RIC demonstrated increased angiogenesis (capillary density), cerebral flood flow and preservation of white matter myelination at 1 month and 4 months.17 In humans, RIC has been trialled for percutaneous coronary intervention (PCI) in the setting of acute myocardial infarction (MI),18 19 elective PCI19 and cardiac surgery.20 RIC has also been studied in the past few years in cerebrovascular disease, mostly applied to the upper limb but some in the lower limb,21–26 and in several studies of periconditioning/postconditioning (happening after ischaemic/haemorrhagic injury).27–29 Bilateral upper-limb RIC protects against recurrent stroke in intracranial arterial stenosis.22 A systematic review of RIC included three trials (371 participants) for ischaemic stroke prevention and four trials (364 participants) for ischaemic stroke treatment, and found low-quality evidence that RIC reduces recurrent stroke risk in patients with intracerebral artery stenosis and reduces stroke severity in patients undergoing carotid stenting.30 There is also preliminary evidence of efficacy for this therapy in cSVD. A trial of 17 patients with cSVD randomised to RIC or sham-RIC reported improved mean flow velocity of the middle cerebral artery, lower dizziness handicap inventory score and lower post-treatment WMH volume in the RIC group.23 A trial in 36 patients with cSVD reported a significant reduction in WMH volume at 1 year compared with sham-RIC and a significant difference on visuospatial and executive function sections of the Montreal Cognitive Assessment (MoCA), although there was no significant change in the number of lacunes.24
Prior studies of RIC in cSVD have been small and essentially hypothesis-generating, and several uncertainties remain. First, the required ‘dose’ of RIC sessions to observe a favourable effect is uncertain: a number of published studies have used bilateral upper-arm RIC twice daily,22 24 but if similar results are obtained with once-daily and/or single upper-arm sessions, this would be especially appealing for patients and facilitate treatment adoption. Importantly, human31 and animal model32 33 studies show that single-limb RIC with only 3–4 cycles can reduce end-organ ischaemic damage. As there are few human data to guide dose choices, the most comprehensive dose-finding data come from an animal study,33 which found that more than one limb, more than four cycles and >5 min of ischaemia conferred no additional reductions in infarct size in a mouse model of acute MI. Second, most published studies have reported exceptionally high patient compliance (>80%), even with bilateral upper-arm, twice-daily sessions—requiring at least 100 min daily, during which they can do little meaningful activity. It is uncertain whether similarly high compliance can be expected in the trial target population of persons with cognitive impairment. Third, the persistence of treatment effects beyond RIC cessation—as suggested by the ‘late phase’ of RIC-related physiological changes per laboratory studies17—remains to be demonstrated. The aforementioned mouse model of bilateral carotid occlusion showed similar efficacy of RIC in mice receiving 1 month or 4 months of therapy,17 but it is unclear if such persistence can be seen in humans. Fifth, prior cSVD trials (including of non-RIC treatments) have suffered from common methodological problems including lack of neuroimaging for diagnosis/classification, low-quality trial design (lack of intent-to-treat analysis or prespecified primary outcomes, failure to account for multiple comparisons) and lack of biomarkers for monitoring and treatment response.34
Therefore, we propose an early phase trial to lay the foundation for a research programme to further investigate the effect of RIC on prevention of cognitive decline caused by brain infarction from cSVD. We will examine whether different doses of daily RIC performed for 1 month are tolerable and safe, whether they result in improved cerebral blood flow (CBF) and whether the biomarker effects of 1 month of treatment are sustained at 3 months.
Methods and analysis
Study design
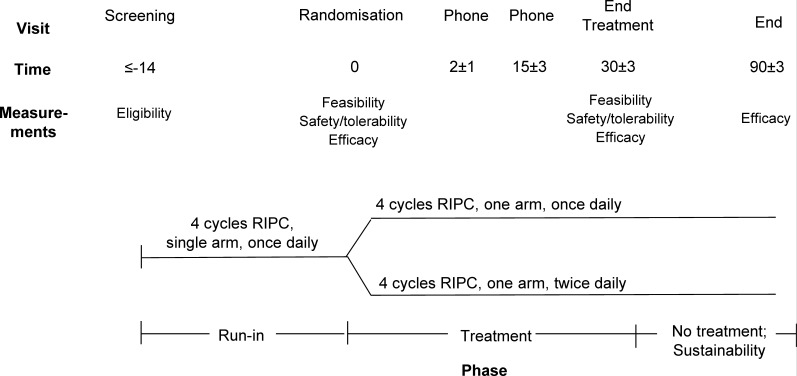
TRIC-VCI will be a prospective, open-label RCT with blinded end point assessment (PROBE)35 and a run-in period, testing two regimens of RIC. The trial scheme is shown in figure 1. This manuscript described protocol V.2.0.
Figure 1.
Trial design for the TRIC-VCI study. RIPC, Remote Ischemic Pre-Conditioning.
The trial will begin with a ‘run-in’ period of 14 days in which all patients will be asked to perform once-daily single-arm RIC. Participants demonstrating >80% completion of treatment sessions (ie, at least 12 of 14 sessions based on review of device records) will then be randomised to either: (1) RIC performed once-daily on one arm or (2) RIC performed twice-daily on one arm.
Intervention
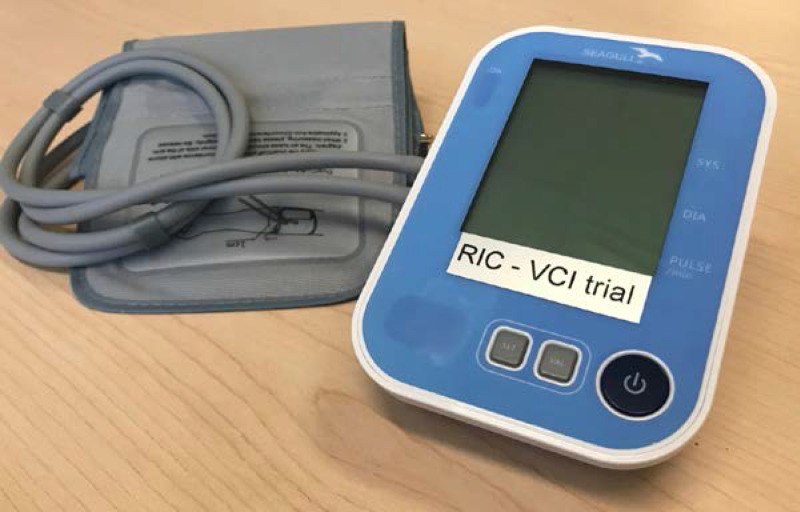
Each RIC session will consist of 4 cycles of unilateral upper arm ischaemia for 5 min followed by reperfusion for another 5 min. The procedure will be performed by using an electric auto-control device (manufactured by Seagull Apps, Denmark) with cuffs that inflate to a pressure of 200 .mm Hg during the ischaemic period (figure 2). This will first be demonstrated by a clinic-based nurse and will subsequently be performed by the patient at home, once daily or twice daily according to the randomised treatment assignment. The device records and documents each RIC cycle. The RIC process can be stopped at any time by the subject, if the subject experiences any major discomfort. Whereas the target inflation pressure of 200 mm Hg is likely higher than what is needed to achieve occlusion in many patients, the same device with the same pressure settings was well tolerated by patients in a Danish study of acute stroke.27
Figure 2.
Device for applying remote ischaemic conditioning (Seagull Aps, Denmark). The device applies four cycles of remote ischaemic conditioning on pressing the button. Device activations are recording, including the number of cycles. Systolic blood pressure, diastolic blood pressure and pulse are displayed.
The device will document each RIC cycle. Recordings will be obtained from the device at the in-person randomisation visit (to determine whether the participant is eligible to be randomised based on adherence during the run-in period) and 30-day visits. The proportion that complete the run-in period will be a secondary end point.
Discontinuation from study treatment
If any of the following criteria are met at any time, treatment will be discontinued:
Patient declares unwillingness to proceed with the intervention.
Treatment is interrupted for >48 hours for any reason.
Diagnosis of deep venous thrombosis (DVT) or pulmonary embolism (PE).
Surgery on the upper extremity is performed or clinically indicated prior to cessation of the 30-day active treatment period.
Initiation of anticoagulation is clinically indicated.
Patient develops any other serious adverse event deemed by the attending physician to merit cessation of RIC.
The time-point of discontinuation will be recorded as accurately as possible (using device data) to determine the total number of actual treatment days for each patient. All patients will be followed to the end of the study period and analysed in their assigned treatment arm.
Randomisation scheme
All subjects will be enrolled in this study consecutively and randomised into the two treatment groups in a 1:1 ratio. Randomisation will use a web‐based algorithm with treatment assignment allocated by web‐based real‐time interaction with the site. Treatment assignments will be made using the Permuted Blocks method with randomly selected block sizes of 2, 4 or 6.
Methods for protecting against bias (blinding)
Participant assignments will not be concealed from treating physicians or subjects.
Investigators and assessors responsible for evaluating the results of cognitive testing, activities of daily living (ADLs), neuroimaging and plasma testing will be blinded to treatment assignment. After enrolment of each subject, the site will designate a blinded evaluator (declared in the randomisation form) to perform 30-day and 90-day follow-up evaluations. This individual cannot be involved in the participant’s care and must remain blinded to treatment assignment. Participants will be instructed not to disclose their treatment group to evaluators. Neuroimaging end points will be determined by the core imaging laboratory blinded to treatment allocation.
Inclusion and exclusion criteria
Full details of the inclusion and exclusion criteria are listed in table 1. Briefly, we will enrol patients with mild vascular neurocognitive disorder, or the earlier stages of major vascular neurocognitive disorder. This will include patients with neuroimaging evidence of significant cSVD burden (as defined in table 1), objective evidence of cognitive impairment (MoCA ≤24) but independent in basic ADLs and for whom concerns regarding cognition are expressed by the patient, caregiver or referring clinician. To target patients in the milder range of cognitive impairment, we will exclude patients with MoCA <13.
Table 1.
Inclusion and exclusion criteria for the TRIC-VCI study
| Inclusion criteria | Operationalised as: |
| 1. Evidence of cerebral small vessel disease on CT or MRI | Evidence of either: 1. Beginning confluent WMH (ARWMC46 grade 2) on any slice on CT or MRI OR 2. Two or more supratentorial subcortical infarcts |
| 2. Objective evidence of cognitive impairment | MoCA36 score ≤24 |
| 3. Concern on the part of the patient, caregiver or clinician that there has been a decline from previous level of cognitive functioning | AD8 questionnaire47 (administered to informant) with two or more positive responses, or clinical judgement based on self report of participant or observations by examiner |
| 4. Independent with basic daily activities of living | BADLS48 response (a) for questions 2, 4, 5, 6, 7, 8, 9 and 14. |
| 5. Age 60–85 years | |
| Exclusion criteria | |
| 1. Cortical infarcts >0 mm axial diameter | Based on site review of clinical CT or MRI |
| 2. Symptomatic ischaemic or haemorrhagic stroke occurring within the last 90 days | |
| 3. Neuroimaging evidence of mass lesion, intracerebral haemorrhage, vascular malformation or evidence of non-vascular disease such as hydrocephalus | Based on site review of clinical CT or MRI. Microbleeds are allowed. |
| 4. Residence in long-term care facility | |
| 5. Other significant neurological or psychiatric disease (eg, multiple sclerosis) | |
| 6. Subject does not have a study partner who can provide corroborative information | Partner is required to complete the BADLS and MBI checklist.49 |
| 7. English or French is not sufficiently proficient for clinical assessment and neuropsychological testing | |
| 8. Total score on the MoCA <13 | |
| 9. Unable to undergo MRI due to medical contraindications or inability to tolerate the procedure | |
| 10. Comorbid medical illness that in the judgement of the study investigator makes it unlikely that the participant will be able to complete 3 months of study follow-up | |
| 11. On therapeutic anticoagulation with doses used for treatment of deep venous thrombosis, pulmonary embolism or for stroke prevention in atrial fibrillation | Lower dose anticoagulation for prevention of coronary artery disease, eg, rivaroxaban 2.5 mg twice daily orally, will be allowed |
| 12. Significant bleeding diathesis | Including but not limited to haemostatic disorder, platelet count <100×109/L, INR >1.7, history of liver cirrhosis |
| 13. Any symptomatic or previously known arm soft-tissue disease, vascular injury or peripheral vascular disease | Defined as patients with symptoms of vascular claudication or prior arterial thromboembolism in limbs |
| 14. Hypertension with systolic blood pressure ≥180 mm Hg despite medical treatment at the time of enrolment | |
| 15. Planned revascularisation (any angioplasty or vascular surgery) within the next 3 months | |
| 16. Planned surgical procedure within the next 3 months | |
| 17. Currently receiving an investigational drug or device by other studies |
ARWMC, age-related white matter changes; BADLS, Bristol Activities of Daily Living Scale; MBI checklist, mild behavioural impairment checklist; INR, international normalised ratio; MoCA, Montreal Cognitive Assessment; WMH, white matter hyperintensitie.
Participants with small cortical infarcts will be allowed but patients with larger (>10 mm axial diameter) cortical infarcts will be excluded. This is because large destructive lesions may confound study assessments of the impact of progressive cSVD by independently causing clinical disabilities (aphasia, anosognosia, etc) or by confounding neuroimaging processing pipelines. For similar reasons, we exclude patients with a prior history of stroke-related disability, who by definition will not meet our inclusion criterion of being independent for basic ADLs. Whereas all patients will meet inclusion criteria for demonstrating evidence of cSVD on CT/MRI, we will not require testing for biomarkers of Alzheimer’s disease in our study, with the understanding that some patients will have mixed dementia.
Frequency and duration of follow-up
After their initial recruitment into the study (screening visit), all patients will receive instruction on how to use the RIC device. They will be asked to perform RIC therapy once daily, in one arm, for a total of ≥14 days (‘run-in’ period). This will be followed by a telephone follow-up visit intended to assess and address tolerability and compliance issues at 1–3 days after beginning the run-in period, and to provide further education on how to use the device. Another in-person clinic visit may be scheduled, at the discretion of the site investigator, if further training and education are needed.
Patients demonstrating the required >80% completion of run-in period treatment sessions will proceed to randomisation. At the randomisation visit (occurring as soon as possible, but not sooner, than 14 days into the run-in period), patients who meet adherence targets will be randomly allocated to one of the two treatment groups. A telephone follow-up visit will be done 1–3 days after randomisation, to assess and address tolerability and compliance issues. A similar telephone visit will be performed at 15±3 days to further encourage compliance.
Patients will stop their assigned treatments on day 30±3 days postrandomisation, at which point they have an in-person follow-up visit. A final follow-up in-person visit will occur at 90±3 days postrandomisation (approximately 2 months free of RIC).
Near study close-out, participants and their care partners at the Calgary study site will be invited to participate in an exit interview in a group setting regarding their experiences in the trial. We will aim to include four to six participants with their care partners.
Primary and secondary outcome measures
The primary feasibility/compliance outcomes will be adherence rate at 30 days, defined as the percentage of sessions completed. Secondary safety/tolerability and efficacy end points are specified in table 2. The main efficacy end points include change in cognitive test scores on the MoCA,36 Trail-Making A and B,37 Controlled Oral Word Association Test (COWAT)38 39 and CERAD 10-item word list learning40 at 30 days and 90 days, change in MRI peak skeletonised mean diffusivity of the white matter41 and change in WMH volume.
Table 2.
Secondary end points for the trial and the statistical test to be used for each
| Secondary safety/tolerability end points | Statistical test of choice |
| 1. Discontinuation prior to 30 days | Fisher’s exact test |
| 2. Proportion completing the run-in period and entering the randomisation phase | Fisher’s exact test |
| 3. Physical examination signs of tissue or neurovascular injury resulting from RIC treatment at 30 days | Fisher’s exact test |
| 4. Development of symptomatic upper extremity deep vein thrombosis at 30 days and 90 days | Fisher’s exact test |
| 5. Peak and end-cycle pain levels reported by the participant using the Visual Analogue Scale during the 30-day treatment period | Repeated measures analysis with linear mixed models will be used to estimate the mean VAS per session, using all VAS data and including the subject as a random effects term to account for within-subject correlation. Peak and end VAS will be analysed in separate models. The proportion with intolerable pain, defined as estimated mean VAS >8, will be compared by Fisher’s exact test. Subjects with insufficient VAS data, defined as <3 recorded VAS peak or <3 recorded VAS end levels, will be excluded from these analyses |
| Secondary efficacy end points | |
| 1. Change in MRI WMH volume at 30 days and 90 days | Volumes at baseline and follow-up will be logarithmically transformed (natural log) to give a more normal distribution. Then differences between each group will be compared using a linear mixed model |
| 2. Change in MRI diffusion tensor imaging peak skeletonised mean diffusivity41 at 30 days and 90 days | Linear mixed model, testing difference at 30 days and 90 days |
| 3. Number of new MRI infarcts at 30 days and 90 days | Fisher’s exact test |
| 4. Number of new MRI DWI-positive lesions at 30 days and 90 days | Fisher’s exact test |
| 5. Change in MRI ASL grey matter cerebral blood flow at 30 days and 90 days | Linear mixed model, testing difference at 30 days and 90 days |
| 6. Change in MoCA36 score at 30 days and 90 days | Linear mixed model, testing difference at 30 days and 90 days |
| 7. Change in Trail-Making A and B37 at 30 days and 90 days | Volumes at baseline and follow-up will be logarithmically transformed (natural log) to give a more normal distribution. Linear mixed model, testing difference at 30 days and 90 days |
| 8. Change in Controlled Oral Word Association38 39 score at 30 days and 90 days | Linear mixed model, testing difference at 30 days and 90 days |
| 9. Change in CERAD 10-item word list learning40 score at 30 days and 90 days | Linear mixed model, testing difference at 30 days and 90 days |
| 10. Change in total score on MBI Tracking Tool, adapted from the MBI checklist,49 at 30 days and 90 days | Linear mixed model, testing difference at 30 days and 90 days |
| 11. Change in BADLS48 at 30 days and 90 days | Linear mixed model, testing difference at 30 days and 90 days |
| 12. Difference in candidate blood biomarkers at 30 days and 90 days | Linear mixed model, testing difference at 30 days and 90 days |
ASL, arterial spin labelling; BADLS, Bristol Activities of Daily Living Scale; DWI, diffusion-weighted imaging; MBI checklist, mild behavioural impairment checklist; MoCA, Montreal Cognitive Assessment; RIC, remote ischaemic conditioning; VAS, Visual Analog Scale; WMH, white matter hyperintensity.
The specifications of how these outcome measures will be measured are presented in online supplemental file 1.
bmjopen-2020-040466supp001.pdf (58.5KB, pdf)
Procedures and variables
The schedule of procedures and variable collection for the trial is presented in table 3.
Table 3.
Overview of the schedule of procedures and variable collection
| Visit | ||||||
| Screening | Randomisation | Phone Follow-up | Phone Follow-up | Follow-up | End | |
| Activity | 0 | Within 30 days | 1–3 days | 15±3 | 30±3 | 90±3 |
| Written consent | ✔ | |||||
| Demographics | ✔ | |||||
| Medical history | ✔ | ✔ | ✔ | ✔ | ||
| Medications | ✔ | ✔ | ✔ | ✔ | ||
| Physical exam | ✔ | ✔ | ✔ | |||
| NIH Stroke Scale | ✔ | ✔ | ✔ | ✔ | ||
| Hachinski ischaemic score | ✔ | |||||
| MoCA | ✔ | ✔ | ✔ | ✔ | ||
| Bristol Activities of Daily Living Scale | ✔ | ✔ | ✔ | ✔ | ||
| AD8 Informant Questionnaire | ✔ | |||||
| IQCODE | ✔ | |||||
| Inclusion/Exclusion criteria | ✔ | |||||
| RIC device provision | ✔ | |||||
| RIC device training | ✔ | ✔ | ✔ | ✔ | ||
| Subject diary provision | ✔ | |||||
| Subject diary review | ✔ | ✔ | ||||
| Adherence (device print out) | ✔ | ✔ | ||||
| Randomisation | ✔ | |||||
| Cognitive tests | ✔ | ✔ | ✔ | |||
| MBI checklist | ✔ | ✔ | ✔ | |||
| Blood draw | ✔ | ✔ | ✔ | ✔ | ||
| MRI | ✔ | ✔ | ✔ | |||
MBI checklist, mild behavioural impairment checklist; MoCA, Montreal Cognitive Assessment; NIH, National Institutes of Health; RIC, remote ischaemic conditioning.
Details of study assessments at each visit are presented in online supplemental file 2. Cognitive testing and MRI will be done at randomisation, 30 days and 90 days. Each study participant will also have an informant, ideally one who lives with them or is a caregiver, who will provide important collateral data about their cognitive and behavioural status (via the AD8 informant questionnaire, IQCODE and the MBI checklist) and daily activities (via the Bristol ADL Scale longitudinally).
bmjopen-2020-040466supp002.pdf (1.1MB, pdf)
Sample size justification
The selected sample size is based on the precision for measurement of the primary outcome (adherence rate), feasibility based on recruitment rate and funding and the desire to avoid exposing an unnecessarily large number of trial participants to an intolerable treatment arm. With 12 subjects per study arm, if 83% adhere to the treatment arm (meeting our prespecified outcome of ≥80% adherence) then we can predict with 95% confidence that the true adherence rate is 52%–98%. This would provide enough confidence to proceed to a subsequent phase II study with a randomised sham control.
Sample size calculations for biomarker efficacy are based on the ability to restore more normal grey matter CBF in patients with VCI due to cSVD. Prior literature on CBF measurements in cSVD has recently been systematically reviewed.42 Based on a prior study of patients with VCI due to cSVD,43 we estimate grey matter CBF will be 37.8±12.4 mL/100 g brain tissue/min in cSVD and 55.8±12.4 mL/100 g/min in age-matched healthy controls. We estimate that RIC will restore 52% of normal CBF (ie, an increase to 46.8 mL/100 g/min), as seen in an animal model of VCI.17 CBF can be measured with good precision using MRI PCASL (estimated within-subject coefficient of variation 4.1% based on two studies).44 45 Based on these assumptions and two-tailed alpha=0.05, the current trial will provide >99% power to detect a mean increase of 9 mL/100 g/min CBF from baseline within each arm. For a future phase IIb study, a sample size of 32 in each arm would provide 80% power and a sample size of 42 in each arm would provide 90% power to determine whether RIC increases CBF by 9 mL/100 g/min compared with a sham control. Since this is a relatively novel use of arterial spin labelling (ASL), and our estimate for CBF increase are based on a small study sample with mild dementia,43 our sample size estimation for the biomarker component must be interpreted with caution and the CBF measure is best interpreted as an exploratory outcome.
Recruitment strategy and projected recruitment rate
Patients will be screened at specialty stroke/transient ischaemic attack clinics and cognitive clinics (generally staffed by neurologists, geriatricians or psychiatrists) at each of the study sites. The initial screening can be done by clinicians as part of usual care, since a number of the evaluations needed to determine study eligibility (clinical history of cognitive symptoms, MoCA and neuroimaging) are commonly used clinical tests recommended by Canadian clinical guidelines. We aim for a recruitment rate of 1 patient per month per site (5/month across all sites), aiming to achieve our target sample size of 24 in 7–8 months.
Number of centres
There are five participating sites across Canada: University of Calgary (lead site), University of British Columbia, McMaster University, University of Toronto, Western University.
Proposed analysis
Primary and secondary outcomes will be compared between the two study groups (or in all subjects at the end of the run-in phase, as specified), with intent-to-treat analysis. To investigate the sustainability of changes at 90 days (60 days after ceasing RIC) and 30 days for relevant secondary outcomes, tests will compare the two treatment groups at 30 days and then the two treatment groups at 90 days. Given the relatively small sample size, normality assumptions will be based on prior literature and not testing within the trial data set.
The primary outcome, adherence rate at 30 days, will be calculated as: number of sessions completed/(number of sessions per day×number of scheduled days of therapy). Subjects are expected to complete 27–33 days of therapy, per protocol. Fisher’s exact test will be used to compare proportions completing ≥80% of assigned sessions. The mean number of sessions completed will be compared by analysis of variance.
The statistical test for each secondary outcome is specified in table 2. If the linear mixed models planned for some of the variables do not converge, we will compare the difference from baseline to 30 days/90 days in the two arms using the t-test or Wilcoxon rank-sum test. Since our main motivation for implementing ASL in this study is to determine its suitability as an outcome measure for a larger subsequent trial, we will also examine the variation in ASL measurements across sites and within-person variation at each site.
For the qualitative exit interview with participants, an audio recording of the group session will be transcribed and analysed for emerging themes regarding the ease of use of the RIC device, the quality of the user manual and other patient instructions, the tolerability of the treatment and advice for conduct of future trials.
Handling of missing data
Baseline characteristics and treatment assignments of patients with and without missing data will be compared with identify significant differences that might affect the interpretation of results. Given the relatively small sample size, we will not perform multiple imputation on missing data.
Subgroup analyses
A priori subgroup analyses will include assessing tolerability and treatment effects by age, sex and baseline burden of SVD. For secondary clinical outcomes of interest—MoCA, Trail-Making, COWAT, CERAD 10-item word list learning score, MBI checklist and BADLS scores—analyses will be adjusted for the participants’ respective baseline score on that measure, since these outcomes may be especially influenced by the baseline level of cognitive impairment.
Patient and public involvement
Patients and the public were not directly involved in the study design. However, the primary and secondary outcomes are focused on assessing the burden and tolerability of the intervention for patients, in preparation for larger-scale trials. As noted above, we will also conduct a qualitative interview near study close-out to obtain feedback from patients based on their experience, thereby giving them a voice in subsequent trial designs. Results will be disseminated through patients and study participants through our institution’s social media platform and the website of the Canadian Consortium on Neurodegeneration (www.ccna-ccnv.ca).
Ethics and dissemination
Ethical considerations
This protocol and the informed consent form (ICF) have been reviewed and approved by the Conjoint Health Research Ethics Board at the University of Calgary. A signed ICF must be obtained from the subject at the screening visit prior to the ‘run-in’ period or any other study procedures (online supplemental file 3). The ICF describes the purpose of the study, the procedures to be followed, and the risks and benefits of participation. Consent will be obtained by a physician investigator or coinvestigator. Ethics approval, including for protocol and consent changes, is required by separate review boards at each study site. Declarations of competing interests are provided to the ethics boards and will be included with manuscript submissions.
bmjopen-2020-040466supp003.pdf (206.9KB, pdf)
Data management
De-identified data will be housed and managed in a password-protected custom database at the University of Calgary Clinical Research Unit. The data will be supported by a Food and Drug Administration-compliant commercial database (iDATAFAX), which will allow electronic data capture or fax-back data capture on a site-by-site basis. Sites will maintain patient identifiable source data in a secure location. The principal investigator (PI) and co-investigators will have access to the data.
Data recording
The Sponsor‐Investigator (and any Participating Site Investigators) will maintain adequate and accurate records to enable the conduct of the study to be fully documented and the study data to be subsequently verified. These documents are classified into two different separate categories: investigator’s study file and subject clinical source documents.
The investigator’s study file will contain the protocol/amendments, case report forms (CRFs) and query forms, institutional review board and governmental approval with correspondence, all versions of ethics-approved ICFs, staff curriculum vitae and authorisation forms and other appropriate documents/correspondence.
Subject clinical source documents would include subject hospital/clinic records, physician’s and nurse’s notes, appointment book, original laboratory reports, imaging reports, completed CRFs (online supplemental file 4), any relevant pathology and special assessment reports, signed ICFs, consultant letters and subject screening and enrolment logs.
bmjopen-2020-040466supp004.pdf (27.3MB, pdf)
For each subject enrolled, a CRF will be completed and signed by the Sponsor-Investigator (and any Participating Site Investigator) or authorised delegate from the study staff. This also applies to records for those patients who fail to complete the study (even during a pre-randomisation screening period if a CRF was initiated). If a subject withdraws from the study, the reason must be noted on a CRF. If a subject is withdrawn from the study because of a treatment-limiting AE, thorough efforts will be made to clearly document the outcome.
Monitoring
All data will be monitored centrally by the coordinating centre at the University of Calgary for accuracy and completeness. The initial performance‐monitoring assessment will take place after the initial subject is enrolled, and the next assessment will take place at close-out. The close‐out monitoring assessment will take place at completion of subject enrolment and protocol required follow‐up visits at the performance site. Monitoring visits will be done remotely by teleconference, but the coordinating centre reserves the right to conduct on-site monitoring at its discretion. The monitor will verify the adequacy of site facilities and staff, site recruitment, subject randomisation, ICFs and the presence of regulatory documents. During the visit, any omissions/corrections to data submitted to the database are noted and queries are generated by the monitor. At close-out, sites are instructed in the record retention of all trial documents. PIs will issue a final report to the ethics board.
Details on study coordination, the steering committee, data processing, audit and inspection and archiving protocols are presented in online supplemental file 5.
bmjopen-2020-040466supp005.pdf (49.8KB, pdf)
Safety and adverse events
Adverse events should be reported as they occur on the CRF. Documentation must be supported by an entry in the subject’s file. Each event should be described in detail along with start and stop dates, severity, relationship to the therapy as judged by the investigator, action taken and outcome. Serious adverse events (SAEs) must be reported within 1 business day of the local investigator or outcome assessor’s first awareness of its occurrence. SAEs will be reviewed by the trial medical monitor. Based on the device risk classification, SAEs do not require reporting to Health Canada, the regulatory authority. Because the adverse event profile of RIC has been quite benign in previous trials, we do not predict that there will be unexpected SAEs.
Safety outcomes of DVT/PE, arm neurovascular injury and SAEs will be adjudicated by a medical monitor, an independent neurologist with experience in clinical trials, who will report these events to the steering committee.
Data dissemination
Results will be disseminated through peer-reviewed publications, professional organisations and conferences. The de-identified study dataset and analysis code will be posted to the University of Calgary section of the PRISM dataverse at the time of publication of the main study results. The data will complement work by our basic/translational science collaborators who will be conducting parallel animal studies to explore dose response relationships with various additional RIC regimens in greater granularity—which we are unable to do in our trial for practical reasons of cost and the available patient population.
The data from this trial will be used to inform decisions on study design for a subsequent phase IIb trial including: (1) the frequency (once or twice daily) of RIC, based on adherence and safety data, (2) the choice of clinical cognitive and functional tests and assessment scales, based on feasibility and reliability and (3) the choice of biomarkers, based on feasibility, reliability and sensitivity to change over time.
Supplementary Material
Footnotes
Twitter: @draravindganesh
Contributors: AG assisted with the design of the study protocol, drafted the first version of the manuscript and prepared subsequent revisions. PB, DC, SEB, TSF, RF, VH, ZI, LMM, CRM, DS, MS and RHS participated in the revisions of the study protocol, read and reviewed the manuscript and approved the final version of the manuscript. EES conceived, designed and supervised the study protocol, read and reviewed the manuscript and approved the final version of the manuscript.
Funding: This work was supported by the Canadian Institutes of Health Research Canadian Consortium on Neurodegeneration in Ageing (CNA-163902) and the Katthy Taylor Chair in Vascular Dementia (University of Calgary).
Competing interests: AG has a patent pending for a system to deliver remote ischaemic conditioning, not related to the device (or manufacturer) being used in this trial. EES reports consulting fees from Alnylam Pharmaceuticals and Biogen; and royalties from UpToDate.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol 2010;9:689–701. 10.1016/S1474-4422(10)70104-6 [DOI] [PubMed] [Google Scholar]
- 2.Wardlaw JM, Smith EE, Biessels GJ, et al. . Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013;12:822–38. 10.1016/S1474-4422(13)70124-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schneider JA, Arvanitakis Z, Bang W, et al. . Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 2007;69:2197–204. 10.1212/01.wnl.0000271090.28148.24 [DOI] [PubMed] [Google Scholar]
- 4.Conklin J, Silver FL, Mikulis DJ, et al. . Are acute infarcts the cause of leukoaraiosis? brain mapping for 16 consecutive weeks. Ann Neurol 2014;76:899–904. 10.1002/ana.24285 [DOI] [PubMed] [Google Scholar]
- 5.Kimberly WT, Gilson A, Rost NS, et al. . Silent ischemic infarcts are associated with hemorrhage burden in cerebral amyloid angiopathy. Neurology 2009;72:1230–5. 10.1212/01.wnl.0000345666.83318.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pavlovic AM, Pekmezovic T, Tomic G, et al. . Baseline predictors of cognitive decline in patients with cerebral small vessel disease. J Alzheimers Dis 2014;42 Suppl 3:S37–43. 10.3233/JAD-132606 [DOI] [PubMed] [Google Scholar]
- 7.van der Flier WM, van Straaten ECW, Barkhof F, et al. . Small vessel disease and general cognitive function in nondisabled elderly: the LADIS study. Stroke 2005;36:2116–20. 10.1161/01.STR.0000179092.59909.42 [DOI] [PubMed] [Google Scholar]
- 8.Smith EE, Saposnik G, Biessels GJ, et al. . Prevention of stroke in patients with silent cerebrovascular disease: a scientific statement for healthcare professionals from the American heart Association/American stroke association. Stroke 2017;48:e44–71. 10.1161/STR.0000000000000116 [DOI] [PubMed] [Google Scholar]
- 9.Jia J, Wei C, Liang J, et al. . The effects of DL-3-n-butylphthalide in patients with vascular cognitive impairment without dementia caused by subcortical ischemic small vessel disease: a multicentre, randomized, double-blind, placebo-controlled trial. Alzheimers Dement 2016;12:89–99. 10.1016/j.jalz.2015.04.010 [DOI] [PubMed] [Google Scholar]
- 10.Stokfisz K, Ledakowicz-Polak A, Zagorski M, et al. . Ischaemic preconditioning - Current knowledge and potential future applications after 30 years of experience. Adv Med Sci 2017;62:307–16. 10.1016/j.advms.2016.11.006 [DOI] [PubMed] [Google Scholar]
- 11.Pan J, Li X, Peng Y. Remote ischemic conditioning for acute ischemic stroke: dawn in the darkness. Rev Neurosci 2016;27:501–10. 10.1515/revneuro-2015-0043 [DOI] [PubMed] [Google Scholar]
- 12.Berger MM, Macholz F, Mairbäurl H, et al. . Remote ischemic preconditioning for prevention of high-altitude diseases: fact or fiction? J Appl Physiol 2015;119:1143–51. 10.1152/japplphysiol.00156.2015 [DOI] [PubMed] [Google Scholar]
- 13.Hess DC, Blauenfeldt RA, Andersen G, et al. . Remote ischaemic conditioning-a new paradigm of self-protection in the brain. Nat Rev Neurol 2015;11:698–710. 10.1038/nrneurol.2015.223 [DOI] [PubMed] [Google Scholar]
- 14.Rassaf T, Totzeck M, Hendgen-Cotta UB, et al. . Circulating nitrite contributes to cardioprotection by remote ischemic preconditioning. Circ Res 2014;114:1601–10. 10.1161/CIRCRESAHA.114.303822 [DOI] [PubMed] [Google Scholar]
- 15.Li J, Rohailla S, Gelber N, et al. . MicroRNA-144 is a circulating effector of remote ischemic preconditioning. Basic Res Cardiol 2014;109:423. 10.1007/s00395-014-0423-z [DOI] [PubMed] [Google Scholar]
- 16.Cai ZP, Parajuli N, Zheng X, et al. . Remote ischemic preconditioning confers late protection against myocardial ischemia-reperfusion injury in mice by upregulating interleukin-10. Basic Res Cardiol 2012;107:277. 10.1007/s00395-012-0277-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khan MB, Hafez S, Hoda MN, et al. . Chronic remote ischemic conditioning is cerebroprotective and induces vascular remodeling in a VCID model. Transl Stroke Res 2018;9:51–63. 10.1007/s12975-017-0555-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McLeod SL, Iansavichene A, Cheskes S. Remote ischemic perconditioning to reduce reperfusion injury during acute ST-segment-elevation myocardial infarction: a systematic review and meta-analysis. J Am Heart Assoc 2017;6. 10.1161/JAHA.117.005522. [Epub ahead of print: 17 May 2017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blusztein DI, Brooks MJ, Andrews DT. A systematic review and meta-analysis evaluating ischemic conditioning during percutaneous coronary intervention. Future Cardiol 2017;13:579–92. 10.2217/fca-2017-0042 [DOI] [PubMed] [Google Scholar]
- 20.Pierce B, Bole I, Patel V, et al. . Clinical outcomes of remote ischemic preconditioning prior to cardiac surgery: a meta-analysis of randomized controlled trials. J Am Heart Assoc 2017;6. 10.1161/JAHA.116.004666. [Epub ahead of print: 20 Feb 2017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meng R, Ding Y, Asmaro K, et al. . Ischemic conditioning is safe and effective for Octo- and nonagenarians in stroke prevention and treatment. Neurotherapeutics 2015;12:667–77. 10.1007/s13311-015-0358-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meng R, Asmaro K, Meng L, et al. . Upper limb ischemic preconditioning prevents recurrent stroke in intracranial arterial stenosis. Neurology 2012;79:1853–61. 10.1212/WNL.0b013e318271f76a [DOI] [PubMed] [Google Scholar]
- 23.Mi T, Yu F, Ji X, et al. . The interventional effect of remote ischemic preconditioning on cerebral small vessel disease: a pilot randomized clinical trial. Eur Neurol 2016;76:28–34. 10.1159/000447536 [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Meng R, Song H, et al. . Remote ischemic conditioning may improve outcomes of patients with cerebral small-vessel disease. Stroke 2017;48:3064–72. 10.1161/STROKEAHA.117.017691 [DOI] [PubMed] [Google Scholar]
- 25.Bilgin-Freiert A, Dusick JR, Stein NR, et al. . Muscle microdialysis to confirm sublethal ischemia in the induction of remote ischemic preconditioning. Transl Stroke Res 2012;3:266–72. 10.1007/s12975-012-0153-1 [DOI] [PubMed] [Google Scholar]
- 26.Zhao W, Meng R, Ma C, et al. . Safety and efficacy of remote ischemic preconditioning in patients with severe carotid artery stenosis before carotid artery stenting: a proof-of-concept, randomized controlled trial. Circulation 2017;135:1325–35. 10.1161/CIRCULATIONAHA.116.024807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hougaard KD, Hjort N, Zeidler D, et al. . Remote ischemic perconditioning as an adjunct therapy to thrombolysis in patients with acute ischemic stroke: a randomized trial. Stroke 2014;45:159–67. 10.1161/STROKEAHA.113.001346 [DOI] [PubMed] [Google Scholar]
- 28.England TJ, Hedstrom A, O'Sullivan S, et al. . RECAST (remote ischemic conditioning after stroke trial): a pilot randomized placebo controlled phase II trial in acute ischemic stroke. Stroke 2017;48:1412–5. 10.1161/STROKEAHA.116.016429 [DOI] [PubMed] [Google Scholar]
- 29.Koch S, Katsnelson M, Dong C, et al. . Remote ischemic limb preconditioning after subarachnoid hemorrhage: a phase Ib study of safety and feasibility. Stroke 2011;42:1387–91. 10.1161/STROKEAHA.110.605840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao W, Zhang J, Sadowsky MG, et al. . Remote ischaemic conditioning for preventing and treating ischaemic stroke. Cochrane Database Syst Rev 2018;7:CD012503. 10.1002/14651858.CD012503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hildebrandt HA, Kreienkamp V, Gent S, et al. . Kinetics and signal activation properties of circulating factor(s) from healthy volunteers undergoing remote ischemic pre-conditioning. JACC Basic Transl Sci 2016;1:3–13. 10.1016/j.jacbts.2016.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pickard JMJ, Davidson SM, Hausenloy DJ, et al. . Co-dependence of the neural and humoral pathways in the mechanism of remote ischemic conditioning. Basic Res Cardiol 2016;111:50. 10.1007/s00395-016-0568-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnsen J, Pryds K, Salman R, et al. . The remote ischemic preconditioning algorithm: effect of number of cycles, cycle duration and effector organ mass on efficacy of protection. Basic Res Cardiol 2016;111:10. 10.1007/s00395-016-0529-6 [DOI] [PubMed] [Google Scholar]
- 34.Smith EE, Cieslak A, Barber P, et al. . Therapeutic strategies and drug development for vascular cognitive impairment. J Am Heart Assoc 2017;6:e005568. 10.1161/JAHA.117.005568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hansson L, Hedner T, Dahlöf B. Prospective randomized open blinded end-point (probe) study. A novel design for intervention trials. Blood Press 1992;1:113–9. 10.3109/08037059209077502 [DOI] [PubMed] [Google Scholar]
- 36.Nasreddine ZS, Phillips NA, Bédirian V, et al. . The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–9. 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- 37.Reitan RM. Validity of the TRAIL making test as an indicator of organic brain damage. Percept Mot Skills 1958;8:271–6. 10.2466/pms.1958.8.3.271 [DOI] [Google Scholar]
- 38.Rosen WG. Verbal fluency in aging and dementia. J Clin Neuropsychol 1980;2:135–46. 10.1080/01688638008403788 [DOI] [Google Scholar]
- 39.Barr A, Brandt J. Word-list generation deficits in dementia. J Clin Exp Neuropsychol 1996;18:810–22. 10.1080/01688639608408304 [DOI] [PubMed] [Google Scholar]
- 40.Mirra SS, Heyman A, McKeel D, et al. . The Consortium to establish a Registry for Alzheimer's disease (CERAD). Part II. standardization of the neuropathologic assessment of Alzheimer's disease. Neurology 1991;41:479–86. 10.1212/WNL.41.4.479 [DOI] [PubMed] [Google Scholar]
- 41.Baykara E, Gesierich B, Adam R, et al. . A novel imaging marker for small vessel disease based on skeletonization of white matter tracts and diffusion histograms. Ann Neurol 2016;80:581–92. 10.1002/ana.24758 [DOI] [PubMed] [Google Scholar]
- 42.Shi Y, Thrippleton MJ, Makin SD, et al. . Cerebral blood flow in small vessel disease: a systematic review and meta-analysis. J Cereb Blood Flow Metab 2016;36:1653–67. 10.1177/0271678X16662891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schuff N, Matsumoto S, Kmiecik J, et al. . Cerebral blood flow in ischemic vascular dementia and Alzheimer's disease, measured by arterial spin-labeling magnetic resonance imaging. Alzheimers Dement 2009;5:454–62. 10.1016/j.jalz.2009.04.1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen Y, Wang DJJ, Detre JA. Test-retest reliability of arterial spin labeling with common labeling strategies. J Magn Reson Imaging 2011;33:940–9. 10.1002/jmri.22345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu B, Lou X, Wu X, et al. . Intra- and interscanner reliability and reproducibility of 3D whole-brain pseudo-continuous arterial spin-labeling Mr perfusion at 3T. J Magn Reson Imaging 2014;39:402–9. 10.1002/jmri.24175 [DOI] [PubMed] [Google Scholar]
- 46.Wahlund LO, Barkhof F, Fazekas F, et al. . A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke 2001;32:1318–22. 10.1161/01.STR.32.6.1318 [DOI] [PubMed] [Google Scholar]
- 47.Galvin JE, Roe CM, Powlishta KK, et al. . The AD8: a brief informant interview to detect dementia. Neurology 2005;65:559–64. 10.1212/01.wnl.0000172958.95282.2a [DOI] [PubMed] [Google Scholar]
- 48.Bucks RS, Ashworth DL, Wilcock GK, et al. . Assessment of activities of daily living in dementia: development of the Bristol activities of daily living scale. Age Ageing 1996;25:113–20. 10.1093/ageing/25.2.113 [DOI] [PubMed] [Google Scholar]
- 49.Ismail Z, Agüera-Ortiz L, Brodaty H, et al. . The mild behavioral impairment checklist (MBI-C): a rating scale for neuropsychiatric symptoms in pre-dementia populations. J Alzheimers Dis 2017;56:929–38. 10.3233/JAD-160979 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-040466supp001.pdf (58.5KB, pdf)
bmjopen-2020-040466supp002.pdf (1.1MB, pdf)
bmjopen-2020-040466supp003.pdf (206.9KB, pdf)
bmjopen-2020-040466supp004.pdf (27.3MB, pdf)
bmjopen-2020-040466supp005.pdf (49.8KB, pdf)