Abstract
Fragaria viridis Weston or creamy strawberry is one of the less-known species of the Fragaria genus (Rosaceae family) with a wide distribution in Eurasia and is still in the shadow of more popular relatives F. ananassa (garden strawberry) or F. vesca (wild strawberry). Importantly, there is a lack of scientific knowledge on F. viridis compounds, their stability in the postharvest period, and bioactivity. In this study, metabolites of F. viridis fruits in three ripening stages were characterized with high-performance liquid chromatography with photodiode array and electrospray ionization triple quadrupole mass spectrometric detection (HPLC-PAD-ESI-tQ-MS). In total, 95 compounds of various groups including carbohydrates, organic acids, phenolics, and triterpenes, were identified for the first time. The quantitative content of the compounds varied differently during the ripening progress; some of them increased (anthocyanins, organic acids, and carbohydrates), while others demonstrated a decrease (ellagitannins, flavonols, etc.). The most abundant secondary metabolites of F. viridis fruits were ellagitannins (5.97–7.54 mg/g of fresh weight), with agrimoniin (1.41–2.63 mg/g) and lambertianin C (1.20–1.86 mg/g) as major components. Antioxidant properties estimated by in vitro assays (2,2-diphenyl-1-picrylhydrazyl radical (DPPH), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) cation radical (ABTS), ferric reducing antioxidant power (FRAP), and oxygen radical absorbance capacity (ORAC)) showed good antioxidant potential in all ripening stages of F. viridis fruits. The pilot human experiment on the effect of F. viridis fruit consumption on the serum total antioxidant capacity confirmed the effectiveness of this kind of strawberry. Postharvest storage of ripe fruits at 4 °C and 20 °C lead to declining content in the majority of compounds particularly ascorbic acid, ellagitannins, and flavonols, with the most significant loss at room temperature storage. These results suggest that F. viridis fruits are a prospective source of numerous metabolites that have potential health benefits.
Keywords: Fragaria viridis, creamy strawberry, ellagitannins, HPLC, mass spectrometry, fruit ripening, antioxidant potential
1. Introduction
Genus Fragaria (strawberry) of the Rosaceous family is a well-known source of dietary fruits that are popular all over the world and is widely consumed due to its unique taste and fragrance. The global production of strawberries has reached 9 million tons per year with maximal production levels in China, USA, and Mexico [1]. Such high consumption makes it necessary to examine the various aspects of biology, chemistry, cultivation, and technology of strawberry manufacturing. The most common studies devoted to the metabolic diversity of the Fragaria genus include many groups of compounds such as carbohydrates, organic acids, vitamins, anthocyanins, ellagitannins, flavonoids, and minerals [2]. Special attention is also paid to understanding the nature of postharvest changes in the chemical profile and physical properties of strawberry fruits in different storage conditions [3]. Despite the significance of strawberry as a food, pharmaceutical interest is also given due to the presence of various bioactive compounds including antioxidative [4], anti-inflammatory [5], antibacterial [6], anti-allergic [7], antidiabetic [8], and cancer preventive [9] properties. The antioxidative properties of various strawberries were discussed previously, and high effectiveness was revealed for Fragaria extracts and unprocessed fruits [10], thereby strengthening the interest to study strawberries. An age-old tradition of strawberry use resulted in the cultivation of many Fragaria species, not only popular species such as F. ananassa (garden strawberry), F. vesca (wild strawberry), and F. moschata (musk strawberry) but also exotic species like F. chiloensis (Chilean strawberry), F. × bifera, and F. viridis (creamy strawberry) [2]. In this regard, significant attention also needs to be focused on little-known strawberries, specifically F. viridis (Figure 1).
Figure 1.
Fragaria viridis Weston (creamy strawberry).
Botanically, F. viridis is a green perennial herbaceous rhizomatous plant that can grow up to 25 cm tall with numerous adventitious roots. The stems are erect, and the length of the leaves are slightly longer, densely dressed with protruding trichomes. The stipules are narrow and brown, and the leaves have shaggy petioles from prominent trichomes that densely cover them. The inflorescences are small, corymbose, loose, and few-flowered and are dressed at the base with a solid or tripartite apical leaf. The pedicels are short and dressed with appressed or occasionally horizontally protruding trichomes. The flowers are relatively large, up to 2.5 cm in diameter, usually bisexual with triangular and lanceolate sepals. The petals are rounded, overlapping each other, short-clawed, and yellowish-white. The fragrant fruits are spherical, narrowed at the base, mostly yellowish-white, only reddish at the top, and rarely entirely pink or pale red, with achenes slightly immersed in the pulp, which are difficult to separate from the receptacle [11]. The fruits are separated from the stem together with sepals at inconsist density, and they are distinguished by good transportability, better that F. vesca and F. ananassa. The species is ecologically plastic. It grows in aspen-birch groves, on open grassy mountain slopes, on edges and glades of mountain forests, in meadows, and in meadow steppes in Europe, Russia, the Caucasus, and Western and Eastern Siberia. Sensory evaluation of F. viridis fruits demonstrated good taste and extraordinary fresh-fruity flavour [12].
The literature data about F. viridis are meagre and demonstrated that the essential oil of leaves consists of major components β-linalool, n-nonanal, tetradecanal, nerolidol, α-bisabolol, and phytol, which distinguishes it from fruit essential oil with the dominant m/p-xylene, isoledene, methyleugenol, α-cedrene, α-muurolene, and α-cedrol [13]. The known phenolics of F. viridis fruits are catechin, epicatechin, epigallocatechin gallate, cyanidin 3-O-glucoside, pelargonidin 3-O-glucoside, quercetin 3-O-glucoside, ellagic acid [14], quercetin 3-O-galactoside, quercetin 3-O-rutinoside, and chlorogenic acid [15], equalling the phenolic profile of F. viridis leaves. Both fruits and leaf extracts showed good radical-scavenging and ferric-reducing ability [14]. Despite easy cultivation, high breeding potential, and good sensory parameters, F. viridis remains one of the underutilized strawberry species [12]. The growing interest in new strawberries as perspective food sources obliges us to do more in-depth research, particularly in the area of Fragaria metabolomics using high-performance liquid chromatography-mass spectrometric techniques (nothing has been done previously with these techniques). It is, of course, of great interest in revealing specificities of metabolite changes in F. viridis fruits during the ripening progress as well as metabolite transformation during fruit storage.
In the present report, we realized the first detailed metabolomic profiling of F. viridis fruits in three stages of ripening (unripe, intermediate ripe, and fully ripe) using high-performance liquid chromatography with photodiode array and electrospray ionization triple quadrupole mass spectrometric detection (HPLC-PAD-ESI-tQ-MS), and antioxidant properties of F. viridis fruits were also studied in four in vitro models (scavenging capacity against 2,2-diphenyl-1-picrylhydrazyl radical and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) cation radical, ferric reducing antioxidant power, and oxygen radical absorbance capacity) during ripening progress and one pilot human experiment (serum total antioxidant capacity). Finally, the variation of selected compounds and antioxidant potential of ripe F. viridis fruits was investigated in response to cool and room temperature storage. To our knowledge, this is the first comprehensive study of F. viridis fruits.
2. Results and Discussion
2.1. Metabolites of F. viridis Fruits: LS-MS Profile
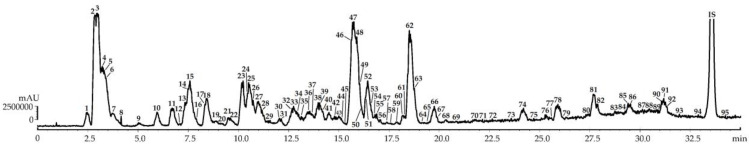
Chromatographic profiling of F. viridis fruit metabolites was completed by high-performance liquid chromatography with photodiode array and electrospray ionization mass spectrometric detection (HPLC-PAD-ESI-tQ-MS). The identification of components found in F. viridis was done after a precise interpretation of chromatographic (retention times) and spectral data (ultraviolet-visible spectra and mass spectral patterns) in comparison with reference standards and literature data. The extraction procedures of fresh fruits were preliminarily tested with various solvents (methanol, ethanol, isopropanol, water, and acetone), solvent–material ratios, temperatures (20–90 °C), and methods of extraction (ultrasound-, microwave-, and water-bath-assisted). The resultant protocol used was 100% methanol with a solvent–material ratio of 1:1 followed by 5 min homogenization and sonification (30 min, 45 °C). After comparison of F. viridis fruit extracts in three ripening stages (unripe, intermediate ripe, and fully ripe), the HPLC-ESI-tQ-MS chromatogram of fully ripe fruit extract showed the presence of the maximal amount of compounds (95) with interpretable data (Figure 2), details of which are provided in Table 1.
Figure 2.
High-Performance Liquid Chromatography with Electrospray Ionization Triple Quadrupole Mass Spectrometric Detection (HPLC-ESI-tQ-MS) chromatogram (Total Ion Chromatogram (TIC) mode, negative ionization) of extract of F. viridis ripe fruits: compounds are numbered as listed in Table 1. IS—internal standard (genkwanin).
Table 1.
Chromatographic (tR) and mass-spectrometric data of compounds 1–95 found in F. viridis fruits.
| No. | tR, min | [M-H]− I, [M-2H]− II, [M-2H]2− III, m/z | MS/MS, m/z | Group a | Compound [ref.] b | Presence in Ripening Stages c |
|---|---|---|---|---|---|---|
| 1 | 2.51 | 341 I | CR | Hexosyl-hexose L [16] | +/+/+ | |
| 2 | 2.94 | 179 I | CR | Hexose L [16] | +/+/+ | |
| 3 | 3.08 | 191 I | OA | Citric acid S | +/+/+ | |
| 4 | 3.33 | 133 I | OA | Malic acid S | +/+/+ | |
| 5 | 3.41 | 149 I | OA | Tartaric acid S | +/+/+ | |
| 6 | 3.50 | 115 I | OA | Fumaric acid S | +/+/+ | |
| 7 | 3.82 | 175 I | OA | Ascorbic acid S | +/+/+ | |
| 8 | 4.22 | 89 I | OA | Oxalic acid S | +/+/+ | |
| 9 | 5.03 | 331 I | 169, 125 | GA | 1-O-Galloyl glucose S [17] | +/+/+ |
| 10 | 5.98 | 169 I | GA | Gallic acid S [17] | +/+/+ | |
| 11 | 6.71 | 783 I; 391 III | 633, 481, 301 | ET | Pedunculagin S [18] | +/+/+ |
| 12 | 7.03 | 161 I | CO | Umbelliferone S [19] | +/+/+ | |
| 13 | 7.43 | 353 I | 191, 179, 173, 135 | HC | 4-O-Caffeoylquinic acid S [20] | +/+/+ |
| 14 | 7.50 | 633 I | 481, 331, 301 | ET | Strictinin S [18] | +/+/+ |
| 15 | 7.58 | 353 I | 191, 165 | HC | 5-O-Caffeoylquinic acid S [20] | +/+/+ [15] |
| 16 | 7.44 | 633 I | 481, 331, 301 | ET | Strictinin isomer L [18] | +/+/+ |
| 17 | 7.83 | 353 I | 191, 179, 135 | HC | 3-O-Caffeoylquinic acid S [20] | +/+/+ |
| 18 | 8.01 | 783 I; 391 III | 633, 481, 301 | ET | Pedunculagin isomer L [18] | +/+/+ |
| 19 | 8.85 | 577 I | 289 | PC | Procyanidin B2 (catechin dimer) S [17] | +/+/+ |
| 20 | 9.15 | 609 II | 447, 285 | CY | Cyanidin 3-O-sophoroside S [21] | −/+/+ |
| 21 | 9.48 | 577 I | 289 | PC | Procyanidin B4 (catechin-epicatechin dimer) S [17] | +/+/+ |
| 22 | 9.67 | 593 II | 431, 269 | CY | Pelargonidin di-O-hexoside L [21] | −/+/+ |
| 23 | 10.14 | 289 I | CT | Catechin S [21] | +/+/+ [14] | |
| 24 | 10.49 | 325 I | 163, 119 | HC | p-Coumaric acid O-hexoside L [22] | +/+/+ |
| 25 | 10.58 | 325 I | 163, 119 | HC | p-Coumaric acid 4-O-glucoside S [22] | +/+/+ |
| 26 | 10.79 | 593 II | 447, 285 | CY | Cyanidin 3-O-rutinoside S [21] | −/+/+ |
| 27 | 11.02 | 447 II | 285 | CY | Cyanidin 3-O-glucoside S [21] | −/+/+ [14] |
| 28 | 11.14 | 577 II | 431, 269 | CY | Pelargonidin 3-O-rutinoside S [21] | −/+/+ |
| 29 | 11.52 | 431 II | 269 | CY | Pelargonidin 3-O-glucoside S [21] | +/+/+ [14] |
| 30 | 12.01 | 933 I; 466 III | 301 | ET | Castalagin S [18,23] | +/+/+ |
| 31 | 12.11 | 865 I | 577, 289 | PC | Procyanidin C2 (catechin trimer) S [17] | +/+/+ |
| 32 | 12.63 | 593 II | 447, 285 | CY | Cyanidin O-p-coumaroyl-O-hexoside L [21] | −/+/+ |
| 33 | 12.78 | 935 I; 467 III | 633, 463, 301 | ET | Casuarictin isomer [18] | +/+/+ |
| 34 | 12.88 | 933 I; 466 III | 301 | ET | Castalagin isomer L [18,23] | +/+/+ |
| 35 | 13.09 | 1103 I; 551 III | 951, 933, 783, 633, 481, 301 | ET | Sanguiin H2 S [18,24] | +/+/+ |
| 36 | 13.41 | 865 I | 577, 289 | PC | Procyanidin trimer (catechin/epicatechin trimer) L [17] | +/+/+ |
| 37 | 13.70 | 577 II | 431, 269 | CY | Pelargonidin O-p-coumaroyl-O-hexoside L [21] | −/+/+ |
| 38 | 13.98 | 489 II | 447, 285 | CY | Cyanidin O-acetyl-O-hexoside L [21] | −/+/+ |
| 39 | 14.03 | 625 I | 463, 301 | FG | Quercetin 3-O-sophoroside S [25] | +/+/+ |
| 40 | 14.11 | 935 I; 467 III | 633, 463, 301 | ET | Casuarictin isomer [18] | +/+/+ |
| 41 | 14.51 | 433 I | 301 | ET | Ellagic acid O-pentoside L [26,27] | +/+/+ |
| 42 | 14.88 | 1567 I; 783 III | 933, 633, 301 | ET | Sanguiin H10 S [18,24] | +/+/+ |
| 43 | 15.01 | 447 I | 301 | ET | Ellagic acid O-desoxyhexoside L [26,27] | +/+/+ |
| 44 | 15.11 | 1103 I; 551 III | 933, 783, 633, 481, 301 | ET | Sanguiin H2 isomer L [18,24] | +/+/+ |
| 45 | 15.34 | 609 I | 463, 301 | FG | Quercetin 3-O-rutinoside S [25] | +/+/+ [15] |
| 46 | 15.53 | 1869 I; 934 III | 1567, 1265, 935, 783, 633, 481, 301 | ET | Sanguiin H6 isomer L [18,24] | +/+/+ |
| 47 | 15.72 | 1401 III | 1235, 933, 783, 633, 301 | ET | Lambertianin C S [18,24] | +/+/+ |
| 48 | 15.81 | 473 II | 431, 269 | CY | Pelargonidin O-acetyl-O-hexoside L [21] | −/+/+ |
| 49 | 15.94 | 463 I | 301 | FG | Quercetin 3-O-glucoside S [25] | +/+/+ [14] |
| 50 | 16.02 | 477 I | 301 | FG | Quercetin 3-O-glucuronide S [25] | +/+/+ |
| 51 | 16.33 | 1103 I; 551 III | 801, 783, 499, 481, 319, 301 | ET | Agrimonic acid A S [28] | +/+/+ |
| 52 | 16.50 | 1869 I; 934 III | 1701, 1567, 1265, 1085, 935, 783, 633, 481, 301 | ET | Sanguiin H6 S [18,24] | +/+/+ |
| 53 | 16.71 | 301 I | 229 | ET | Ellagic acid S [18] | +/+/+ [14] |
| 54 | 16.81 | 1103 I; 551 III | 801, 783, 499, 481, 319, 301 | ET | Agrimonic acid B S [28] | +/+/+ |
| 55 | 16.95 | 433 | 301 | FG | Quercetin 3-O-xyloside S [25] | +/+/+ |
| 56 | 17.07 | 433 | 301 | FG | Quercetin 3-O-arabinoside S [25] | +/+/+ |
| 57 | 17.41 | 593 I | 447, 285 | FG | Kaempferol 3-O-rutinoside S [25] | +/+/+ |
| 58 | 17.56 | 447 I | 285 | FG | Kaempferol 3-O-glucoside S [25] | +/+/+ |
| 59 | 17.92 | 461 I | 285 | FG | Kaempferol 3-O-glucuronide S [25] | +/+/+ |
| 60 | 18.21 | 609 I | 463, 301 | FG | Quercetin 3-O-(6″-O-p-coumaroyl)-glucoside S [25] | +/+/+ |
| 61 | 18.29 | 609 I | 463, 301 | FG | Quercetin O-p-coumaroyl-O-hexoside L [25] | +/+/+ |
| 62 | 18.50 | 1869 I; 934 III | 1567, 1265, 1085, 935, 783, 633, 481, 301 | ET | Agrimoniin S [23,29] | +/+/+ |
| 63 | 18.68 | 1018 III | 1691, 1567, 1265, 1209, 935, 783, 633, 481, 301 | ET | Fragariin A L [23,29] | +/+/+ |
| 64 | 19.06 | 549 I | 463, 301 | FG | Quercetin O-malonyl-O-hexoside L [30,31] | +/+/+ |
| 65 | 19.42 | 549 I | 463, 301 | FG | Quercetin 3-O-(6″-O-malonyl)-glucoside S [30,31] | +/+/+ |
| 66 | 19.75 | 593 I | 447, 285 | FG | Kaempferol 3-O-(6″-O-p-coumaroyl)-glucoside S [25] | +/+/+ |
| 67 | 19.83 | 939 I | 787, 635, 483, 331, 169 | GA | 1,2,3,4,6-Penta-O-galloylglucose S [17] | +/+/+ |
| 68 | 20.39 | 533 I | 447, 285 | FG | Kaempferol O-malonyl-O-hexoside L [30,31] | +/+/+ |
| 69 | 20.82 | 533 I | 447, 285 | FG | Kaempferol 3-O-(6″-O-malonyl)-glucoside S [30,31] | +/+/+ |
| 70 | 21.83 | 505 I | 463, 301 | FG | Quercetin 3-O-(2″-O-acetyl)-glucoside S [32] | +/+/+ |
| 71 | 22.14 | 505 I | 463, 301 | FG | Quercetin 3-O-(6″-O-acetyl)-glucoside S [32] | +/+/+ |
| 72 | 22.67 | 489 I | 447, 285 | FG | Kaempferol O-acetyl-O-hexoside L [30,31,32] | +/+/+ |
| 73 | 23.52 | 489 I | 447, 285 | FG | Kaempferol O-acetyl-O-hexoside L [30,31,32] | +/+/+ |
| 74 | 24.21 | 301 I | FG | Quercetin S [25] | +/+/+ [14] | |
| 75 | 24.78 | 811 I | 649, 487 | TR | Tormentic acid di-O-hexoside L [16] | +/+/+ |
| 76 | 25.34 | 285 I | FG | Kaempferol S [25] | +/+/+ | |
| 77 | 25.54 | 795 I | 633, 471 | TR | Pomolic acid di-O-hexoside L [16] | +/+/+ |
| 78 | 25.87 | 649 I | 487 | TR | Tormentic acid O-hexoside L [16] | +/+/+ |
| 79 | 26.41 | 547 I | 505, 463, 301 | FG | Quercetin 3-O-(2″,6″-di-O-acetyl)-glucoside S [32] | +/+/+ |
| 80 | 27.52 | 591 I | 549, 505, 463, 301 | FG | Quercetin O-acetyl-O-malonyl-O-hexoside L [30,31,32] | +/+/+ |
| 81 | 27.73 | 487 I | TR | Tormentic acid S [16] | +/+/+ | |
| 82 | 27.89 | 461 I | 315, 301 | ET | Ellagic acid O-methyl ester-O-desoxyhexoside L [26,27] | +/+/+ |
| 83 | 28.78 | 633 I | 471 | TR | Pomolic acid O-hexoside L [16] | +/+/+ |
| 84 | 29.14 | 695 I | 609, 463, 301 | FG | Quercetin O-malonyl-O-p-coumaroyl-O-hexoside L [30,31,32] | +/+/+ |
| 85 | 29.49 | 695 I | 609, 463, 301 | FG | Quercetin O-malonyl-O-p-coumaroyl-O-hexoside L [30,31,32] | +/+/+ |
| 86 | 29.57 | 475 I | 329, 301 | ET | Ellagic acid di-O-methyl ester-O-desoxyhexoside L [26,27] | +/+/+ |
| 87 | 30.08 | 531 I | 489, 447, 285 | FG | Kaempferol di-O-acetyl-O-hexoside L [30,31,32] | +/+/+ |
| 88 | 30.41 | 651 I | 609, 463, 301 | FG | Quercetin O-acetyl-O-p-coumaroyl-O-hexoside L [30,31,32] | +/+/+ |
| 89 | 30.92 | 575 I | 533, 489, 447, 285 | FG | Kaempferol O-acetyl-O-malonyl-O-hexoside L [30,31,32] | +/+/+ |
| 90 | 31.02 | 679 I | 593, 447, 285 | FG | Kaempferol O-malonyl-O-p-coumaroyl-O-hexoside L [30,31,32] | +/+/+ |
| 91 | 31.22 | 471 I | TR | Pomolic acid S [16] | +/+/+ | |
| 92 | 31.38 | 679 I | 593, 447, 285 | FG | Kaempferol O-malonyl-O-p-coumaroyl-O-hexoside L [30,31,32] | +/+/+ |
| 93 | 31.98 | 635 I | 593, 447, 285 | FG | Kaempferol O-acetyl-O-p-coumaroyl-O-hexoside L [30,31,32] | +/+/+ |
| 94 | 32.86 | 693 I | 651, 609, 463, 301 | FG | Quercetin di-O-acetyl-O-p-coumaroyl-O-hexoside L [30,31,32] | +/+/+ |
| 95 | 34.26 | 737 I | 695, 651, 609, 463, 301 | FG | Quercetin O-acetyl-O-malonyl-O-p-coumaroyl-O-hexoside L [30,31,32] | +/+/+ |
a Groups of compounds: CO—coumarins; CR—carbohydrates; CY—anthocyanins; ET—ellagitannins; FG—flavonols and flavonol glycosides; GA—gallic acid derivatives; HC—hydroxycinnamates; OA—organic acids; PC—procyanidins; and TR—triterpenes. b Compound identification was based on comparison of retention time, UV and MS spectral data with reference standard (S), or interpretation of UV and MS spectral data and comparison with literature data (L). c Compounds were detected (+) or not (−) in unripe/intermediate ripe/fully ripe F. viridis fruits; if compound was previously reported in F. viridis fruits, the reference no. is mentioned.
2.1.1. Carbohydrates
Two types of carbohydrates were discovered in F. viridis fruits including hexosyl-hexose (m/z 341; 1) and hexose (m/z 179; 2). The HPLC-MS method used does not allow for identification of the nature of carbohydrates, so we used the HPLC-DAD procedure demonstrating the presence of three compounds identified as glucose, fructose, and saccharose (Figure S1), usual mono- and disaccharides of strawberry fruits [2].
2.1.2. Organic Acids
Citric (3), malic (4), tartaric (5), fumaric (6), ascorbic (7), and oxalic acids (8) were the organic acids found in F. viridis fruits. All mentioned compounds were shown previously in F. ananassa [33] and F. vesca [34].
2.1.3. Gallic Acid Derivatives
Gallic acid (10), 1-O-glucoside (9), and 1,2,3,4,6-penta-O-galloylglucose (67) were identified by comparison with reference standards. Gallic acid derivatives and gallotannins are not typical phenolics of Fragaria genus, but gallic acid is a metabolite found in F. ananassa [2] and compound 67 is detected in F. ananassa fruits [35] and F. vesca leaves [36].
2.1.4. Ellagic Acid Derivatives and Ellagitannins
Ellagic acid (53), four ellagic acid glycosides (41, 43, 82, and 86), and eighteen ellagitannins (11, 14, 16, 18, 30, 33–35, 40, 42, 44, 46, 47, 51, 52, 54, 62, and 63) were found in F. viridis fruits. The ellagic acid glycosides were ellagic acid O-pentoside (41), ellagic acid O-desoxyhexoside (43), ellagic acid O-methyl ester-O-desoxyhexoside (82), and ellagic acid di-O-methyl ester-O-desoxyhexoside (86) due to the presence of ions with m/z 301 typical for ellagic acid derivatives [18,23] and the size of loss particles with m/z 132 (O-pentose) or 146 (O-desoxyhexose) [16].
Known strawberry ellagitannins, lambertianin C (47), sanguiin H10 (42), sanguiin H6 (52; 46 as isomer), sanguiin H2 (35; 44 as isomer), and pedunculagin (11; 18 as isomer), were identified using reference standards [18]. The literature data gave additional identification of three Fragaria ellagitannin structures: strictinin (14 and 16), castalagin (30 and 34), and casuarictin (33 and 40) [18]. Agrimoniin (62) gave typical ions of deprotonated fragment [M-H]− (m/z 1869) and double-charged particle [M-2H]2− (m/z 934). The further MS/MS fragmentation of molecule 62 demonstrated the loss of fragments of hexahydroxydiphenoyl (HHDP; 302 Da), bis-HHDP-glucose (bis-HHDP-Glc; 784 Da), and galloyl-bis-HHDP-glucose (Gall-bis-HHDP-Glc; 934 Da), creating a cascade of ions with m/z 1567 [(M-H)-HHDP]−, 1265 [(M-H)-2×HHDP]−, 1085 [(M-H)-(bis-HHDP-Glc)]−, 935 [(M-H)-(Gall-bis-HHDP-Glc)]−, 633 [(M-H)-(Gall-bis-HHDP-Glc)-HHDP]−, and 481 [((bis-HHDP-Glc)-H)-HHDP]− [23,29]. Agrimoniin was previously identified as the main ellagitannin of strawberry fruits from F. vesca and F. ananassa [24] and were found in F. viridis for the first time in this study. Compound 63 with a [M-2H]2− ion with m/z 1018 gave the weak deprotonated ion [M-H]− (m/z 2037); after decarboxylation (−44 Da) and loss of HHDP, gave the fragment with m/z 1691; and then degraded to fragments with m/z 1567 (loss of trihydroxy benzene) and 1265 (loss of HHDP). The alternative pathway of fragmentation of the particle with m/z 1691 led to the formation of de-HHDP-glucosylated ion with m/z 1209 and the fragments with m/z 935 [Gall-bis-HHDP-Glc–H]−, 783 [(bis-HHDP-Glc)-H]−, 633 [((Gall-bis-HHDP-Glc)-H)-HHDP]−, and 481 [((bis-HHDP-Glc)-H)-HHDP]−. The close mass spectrometric pattern gave the known strawberry ellagitannin fragariin A found in F. ananassa and has the structure of galloylated derivative of agrimoniin [29]. Two isomeric ellagitannins, 51 and 54, were identified as biogenetic relatives to agrimoniin compounds, agrimonic acids A and B, respectively [28].
After all, only ellagic acid was mentioned previously as a component of F. viridis [14], indicating that this is the first report describing the profile of ellagic acid derivatives from F. viridis fruits. Traditionally used strawberries, such as F. ananassa and F. vesca, are a good source of ellagitannins and ellagic acid glycosides [2,18]. Lambertianin C, saguiins, and agrimoniin were also found in many varieties of cultivated strawberries, demonstrating their obligate position in the Fragaria metabolome [2,18,26,27].
2.1.5. Hydroxycinnamates and Coumarins
Four known hydroxycinnamates were identified by comparison with reference standards, including 4-O-caffeoylquinic acid (13), 5-O-caffeoylquinic acid (15), 3-O-caffeoylquinic acid (17), and p-coumaric acid 4-O-glucoside (25). Only 5-O-caffeoylquinic acid was previously found in F. viridis [15]. Component 24 produced a deprotonated ion with m/z 325 and dehexosylated fragment with m/z 163 characteristic for p-coumaric acid O-hexoside [22]. One coumarin umbelliferone (12) was also identified by comparison with a reference standard.
2.1.6. Catechins and Procyanidins
Catechin (23) and procyanidins B2 (19), B4 (21), and C2 (31) as well as an isomer to 31 catechin/epicatechin trimer 36 were detected in F. viridis fruits. Monomer 23 was already found in whole plant F. viridis [14].
2.1.7. Anthocyanins
Derivatives of cyanidin (20, 26, 27, 32, and 38) and pelargonidin (22, 28, 29, 37, and 48) were found in F. viridis fruits based on UV-Vis patterns (525–535 nm for cyanidins and 498–505 nm for pelargonidins) and mass spectral behaviour of aglycone fragments (m/z 285 for cyanidins and 269 for pelargonidins). Five phenolics were identified after comparing spectra with reference standards: cyanidin-3-O-sophoroside (20), cyanidin-3-O-rutinoside (26), cyanidin-3-O-glucoside (27), pelargonidin-3-O-rutinoside (28), and pelargonidin-3-O-glucoside (29). Anthocyanin O-glucosides 27 and 29 are the most frequent phenolic pigments of Fragaria fruits [2,14,26], and O-rutinoside 28 was found in F. ananassa [26]. Compound 22 gave an [M-2H]− ion with m/z 593 and two dehexosylated fragments with m/z 431 [(M-2H)-hexose]− and 269 [(M-2H)-2×hexose]− and was determined as pelargonidin di-O-hexoside. The closest phenolic to 22 is pelargonidin-3-O-sophoroside detected in F. ananassa [27]. Two compounds, 32 and 37, have additional maxima in UV spectra at approximately 312 nm characteristic to acylic anthocyanins with p-coumaroyl fragments [37], and they were preliminary determined as cyanidin O-p-coumaroyl-O-hexoside (32) and pelargonidin O-p-coumaroyl-O-hexoside (37). Two mono-acetylated O-hexosides, 38 and 48, gave mass spectral ion fragments with m/z 42 and 162 and were identified as cyanidin O-acetyl-O-hexoside (38) and pelargonidin O-acetyl-O-hexoside (48). The known acylated anthocyanins of Fragaria species traditionally have moieties of acetic and malonic acids [2,26,27,34] so the p-coumaroyl esters of anthocyanins were found in Fragaria fruits for the first time.
2.1.8. Flavonols
Flavonols were defined by their specific UV spectral patterns with absorption at 256/268/360 nm for quercetin derivatives and 265/343 nm for kaempferol derivatives [17]. Thirty-four compounds were flavonols found in F. viridis fruits including two aglycones—quercetin (74) and kaempferol (76)—and 32 glycosides with non-acylated and acylated fragments linked with carbohydrate moieties.
Quercetin glycosides were the most diverse group of F. viridis phenolics with 19 members, some of which were identified using standard references. There are non-acylated compounds, such as quercetin-3-O-sophoroside (39), quercetin-3-O-rutinoside (45; rutin), quercetin-3-O-glucoside (49; isoquercitrin), quercetin-3-O-glucuronide (50; miquelianin), quercetin-3-O-xyloside (55; reynoutrin), and quercetin-3-O-arabinoside (56; avicularin) as well as acylated derivatives quercetin-3-O-(6″-O-p-coumaroyl)-glucoside (60; helichrysoside), quercetin-3-O-(6″-O-malonyl)-glucoside (65), quercetin-3-O-(2″-O-acetyl)-glucoside (70), quercetin-3-O-(6″-O-acetyl)-glucoside (71), and quercetin-3-O-(2″,6″-di-O-acetyl)-glucoside (79). Compounds 45, 49, and 74 were reported in F. viridis by Raudonis et al. [14] and in F. ananassa by many authors [26,27,35]. The remaining quercetin glycosides were acylated derivatives of quercetin O-hexoside giving the same MS/MS fragments with m/z 463 (quercetin O-hexoside) and 301 (aglycone). Compound 61 had [M-H]− at m/z 609 and MS/MS fragmentation close to 60, making it an isomer with the most likely structure of quercetin O-p-coumaroyl-O-hexoside. Glycoside 64 gave deprotonated ions with m/z 549 and is an isomer of quercetin 3-O-(6″-O-malonyl)-glucoside (65) or quercetin O-malonyl-O-hexoside.
A series of mixed O-acylated quercetin O-hexosides had higher retention times than quercetin. Their specific MS patterns showed the loss of fragments with m/z 42 (acetyl), 86 (malonyl), and/or 146 (p-coumaroyl). Five combinations of acylated quercetin O-hexosides were found, such as acetyl/malonyl (80), malonyl/p-coumaroyl (84 and 85), acetyl/p-coumaroyl (88), di-acetyl/p-coumaroyl (94), and acetyl/malonyl/p-coumaroyl (95). To date, there are no known analogues of compounds 80, 84, 85, 88, 94, and 95, but most likely, structures are quercetin-3-O-glucosides with a substituted glucose moiety at positions 2″, 3″, 4″, and 6″.
Thirteen kaempferol glycosides were found in F. viridis fruits, and four were partially identified using a comparison of tR, UV, and mass spectrometric data with reference standards. There were kaempferol-3-O-rutinoside (57; nicotiflorin), kaempferol-3-O-glucoside (58; astragalin), kaempferol-3-O-glucuronide (59), kaempferol-3-O-(6″-O-p-coumaroyl)-glucoside (66; tiliroside), and kaempferol-3-O-(6″-O-malonyl)-glucoside (69). No kaempferol glycosides were previously found in F. viridis, but other Fragaria species were reported to contain compounds 57, 58, 59, and 66 (F. ananassa, F. vesca, and F. chiloensis) [2]. Non-mixed acylated kaempferol O-hexosides were defined as kaempferol O-malonyl-O-hexosides (68), kaempferol O-acetyl-O-hexosides (72,73), and kaempferol di-O-acetyl-O-hexoside (87). Among the possible known analogs of observed flavonols, kaempferol-3-O-(2″-O-malonyl)-glucoside (for 68), kaempferol-3-O-(6″-O-acetyl)-glucoside (for 72,73), and kaempferol-3-O-(3″,4″-di-O-acetyl)-glucoside (for 87) should be mentioned [38]. Mixed acylated kaempferol O-hexosides were also found in F. viridis extract and identified using the same principle as quercetin O-hexosides; these compounds include kaempferol O-acetyl-O-malonyl-O-hexoside (89), kaempferol O-malonyl-O-p-coumaroyl-O-hexosides (90 and 92), and kaempferol O-acetyl-O-p-coumaroyl-O-hexoside (93). Contrary to mixed quercetin O-hexosides, there are some known variants of mixed kaempferol O-hexosides like kaempferol-O-3-(3″/4″-O-acetyl-6″-O-p-coumaroyl)-glucosides as an alternative for 93 [38].
2.1.9. Triterpenes
Six compounds were triterpenes, and two reference standard defined compounds were tormentic acid (81) and pomolic acid (91). Both compounds are the usual Rosaceous metabolites [39,40] but were not found in Fragaria fruits early studies. Glycosidic derivatives of 81 and 91 were described as two O-hexosides, 78 and 83, and two di-O-hexosides, 75 and 77, and are unusual components of strawberry fruits.
2.2. Quantitative Content and LS-MS Profile Variation of F. viridis Fruits during Ripening
We studied F. viridis fruits at three different stages of ripening, including unripe fruits, the stage of technological ripeness (intermediate stage), and the stage of full ripeness (Table 2). The total simple carbohydrate (mono- and disaccharides) content in F. viridis fruits varied from 41.14 mg/g in the unripe stage to 45.17 mg/g in ripe fruits. The main components were monosaccharides, glucose, and fructose, with a concentration of 41.10–45.16 mg/g responsible for the sweet taste of F. viridis fruits. Monosaccharides are the dominant sugars of F. ananassa [33,41,42] and F. vesca [43], but in some strawberry varieties, it happens that saccharose shows the highest content [34,44]. The sugar content of F. ananassa fruits demonstrated the same trend during ripening, with the lowest content in unripe fruits (3.61–4.45 mg/g) rising to the ripe stage with 4.82–8.20 mg/g [33], indicating the close character of carbohydrates changing in strawberries.
Table 2.
Content of compounds in unripe, intermediate ripe, and fully ripe fruits of F. viridis, mg/g of fresh fruit weight ± S.D.
| Compound | Stage of Ripeness | ||
|---|---|---|---|
| Unripe | Intermediate | Ripe | |
| Carbohydrates | |||
| Hexose (glucose+fructose) | 41.10 ± 0.82 | 43.26 ± 0.90 | 45.16 ± 0.92 |
| Hexosyl-hexose (saccharose) | 0.04 ± 0.00 | 0.06 ± 0.00 | 0.11 ± 0.00 |
| Total carbohydrates | 41.14 | 43.32 | 45.27 |
| Organic acids | |||
| Ascorbic acid | 0.62 ± 0.02 | 0.86 ± 0.02 | 1.12 ± 0.02 |
| Citric acid | 2.83 ± 0.06 | 3.18 ± 0.07 | 5.63 ± 0.11 |
| Malic acid | 0.42 ± 0.01 | 0.45 ± 0.01 | 0.59 ± 0.02 |
| Tartaric acid | 0.37 ± 0.01 | 0.40 ± 0.01 | 0.42 ± 0.01 |
| Fumaric acid | 0.01 ± 0.00 | 0.03 ± 0.00 | 0.07 ± 0.00 |
| Oxalic acid | traces | traces | 0.05 ± 0.00 |
| Total organic acids | 4.25 | 4.92 | 7.88 |
| Gallic acid derivatives | |||
| Gallic acid | traces | 0.01 ± 0.00 | 0.01 ± 0.00 |
| 1-O-Galloyl glucose | 0.05 ± 0.00 | 0.03 ± 0.00 | 0.03 ± 0.00 |
| 1,2,3,4,6-Penta-O-galloylglucose | traces | traces | traces |
| Total gallic acid derivatives | 0.05 | 0.04 | 0.04 |
| Hydroxycinnamates and coumarins | |||
| p-Coumaric acid 4-O-glucoside | 0.35 ± 0.01 | 0.33 ± 0.01 | 0.29 ± 0.00 |
| p-Coumaric acid O-hexoside 24 | 0.14 ± 0.00 | 0.11 ± 0.00 | 0.08 ± 0.00 |
| 3-O-Caffeoylquinic acid | 0.12 ± 0.00 | 0.08 ± 0.00 | 0.04 ± 0.00 |
| 4-O-Caffeoylquinic acid | 0.08 ± 0.00 | 0.07 ± 0.00 | 0.05 ± 0.00 |
| 5-O-Caffeoylquinic acid | 0.28 ± 0.00 | 0.21 ± 0.00 | 0.14 ± 0.00 |
| Umbelliferone | traces | traces | traces |
| Total hydroxycinnamates and coumarins | 0.97 | 0.80 | 0.60 |
| Ellagic acid derivatives and ellagitannins | |||
| Ellagic acid | 0.10 ± 0.00 | 0.10 ± 0.00 | 0.12 ± 0.00 |
| Ellagic acid O-pentoside 41 | 0.05 ± 0.00 | 0.09 ± 0.00 | 0.11 ± 0.00 |
| Ellagic acid O-desoxyhexoside 43 | 0.01 ± 0.00 | 0.04 ± 0.00 | 0.07 ± 0.00 |
| Ellagic acid O-methyl ester-O-desoxyhexoside 82 | 0.14 ± 0.00 | 0.16 ± 0.00 | 0.24 ± 0.00 |
| Ellagic acid di-O-methyl ester-O-desoxyhexoside 86 | 0.10 ± 0.00 | 0.18 ± 0.00 | 0.30 ± 0.00 |
| Pedunculagin | 0.26 ± 0.00 | 0.30 ± 0.01 | 0.32 ± 0.01 |
| Pedunculagin isomer 18 | 0.05 ± 0.00 | 0.07 ± 0.00 | 0.11 ± 0.00 |
| Strictinin isomer 14 | 0.10 ± 0.00 | 0.10 ± 0.00 | 0.12 ± 0.00 |
| Strictinin isomer 16 | 0.11 ± 0.00 | 0.12 ± 0.00 | 0.18 ± 0.00 |
| Castalagin isomer 30 | traces | 0.02 ± 0.00 | 0.04 ± 0.00 |
| Castalagin isomer 34 | traces | traces | 0.01 ± 0.00 |
| Casuarictin isomer 33 | traces | traces | 0.02 ± 0.00 |
| Casuarictin isomer 40 | 0.06 ± 0.00 | 0.08 ± 0.00 | 0.14 ± 0.00 |
| Sanguiin H2 | traces | 0.01 ± 0.00 | 0.05 ± 0.00 |
| Sanguiin H2 isomer 44 | 0.09 ± 0.00 | 0.05 ± 0.00 | 0.02 ± 0.00 |
| Sanguiin H6 | 0.36 ± 0.01 | 0.25 ± 0.00 | 0.22 ± 0.00 |
| Sanguiin H6 isomer 46 | 0.45 ± 0.01 | 0.43 ± 0.01 | 0.40 ± 0.01 |
| Sanguiin H10 | 0.21 ± 0.00 | 0.15 ± 0.00 | 0.08 ± 0.00 |
| Lambertianin C | 1.86 ± 0.04 | 1.42 ± 0.03 | 1.20 ± 0.02 |
| Agrimonic acid A | 0.02 ± 0.00 | 0.05 ± 0.00 | 0.08 ± 0.00 |
| Agrimonic acid B | 0.01 ± 0.00 | 0.03 ± 0.00 | 0.10 ± 0.00 |
| Agrimoniin | 2.63 ± 0.05 | 2.03 ± 0.04 | 1.41 ± 0.03 |
| Fragariin A | 0.93 ± 0.02 | 0.69 ± 0.02 | 0.63 ± 0.01 |
| Total ellagic acid derivatives and ellagitannins | 7.54 | 6.37 | 5.97 |
| Catechins and procyanidins | |||
| Catechin | 0.11 ± 0.00 | 0.05 ± 0.00 | 0.05 ± 0.00 |
| Procyanidin B2 | 0.09 ± 0.00 | 0.05 ± 0.00 | 0.02 ± 0.00 |
| Procyanidin B4 | 0.02 ± 0.00 | 0.01 ± 0.00 | traces |
| Procyanidin C2 | 0.05 ± 0.00 | 0.03 ± 0.00 | 0.01 ± 0.00 |
| Procyanidin trimer 36 | 0.02 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 |
| Total catechins and procyanidins | 0.29 | 0.15 | 0.09 |
| Anthocyanins | |||
| Pelargonidin 3-O-glucoside | traces | 0.02 ± 0.00 | 0.06 ± 0.00 |
| Pelargonidin 3-O-rutinoside | n.d. | n.d. | 0.03 ± 0.00 |
| Pelargonidin di-O-hexoside 22 | n.d. | n.d. | traces |
| Pelargonidin O-acetyl-O-hexoside 48 | n.d. | n.d. | traces |
| Pelargonidin O-p-coumaroyl-O-hexoside 37 | n.d. | n.d. | traces |
| Cyanidin 3-O-glucoside | n.d. | 0.01 ± 0.00 | 0.05 ± 0.00 |
| Cyanidin 3-O-rutinoside | n.d. | n.d. | traces |
| Cyanidin 3-O-sophoroside | n.d. | n.d. | traces |
| Cyanidin O-acetyl-O-hexoside 38 | n.d. | n.d. | traces |
| Cyanidin O-p-coumaroyl-O-hexoside 32 | n.d. | n.d. | traces |
| Total anthocyanins | traces | 0.03 | 0.14 |
| Flavonols and flavonol glycosides | |||
| Kaempferol | traces | traces | 0.01 ± 0.00 |
| Kaempferol 3-O-glucoside | traces | 0.05 ± 0.00 | 0.09 ± 0.00 |
| Kaempferol 3-O-glucuronide | traces | traces | 0.08 ± 0.00 |
| Kaempferol 3-O-rutinoside | 0.28 ± 0.00 | 0.23 ± 0.00 | 0.11 ± 0.00 |
| Kaempferol O-acetyl-O-hexoside 72 | traces | traces | traces |
| Kaempferol O-acetyl-O-hexoside 73 | traces | traces | traces |
| Kaempferol di-O-acetyl-O-hexoside 87 | traces | traces | traces |
| Kaempferol O-malonyl-O-hexoside 68 | 0.01 ± 0.00 | traces | traces |
| Kaempferol O-malonyl-O-hexoside 69 | 0.02 ± 0.00 | 0.01 ± 0.00 | traces |
| Kaempferol 3-O-(6″-O-p-coumaroyl)-glucoside | 0.08 ± 0.00 | 0.04 ± 0.00 | 0.04 ± 0.00 |
| Kaempferol O-acetyl-O-malonyl-O-hexoside 89 | 0.02 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 |
| Kaempferol O-malonyl-O-p-coumaroyl-O-hexoside 90 | traces | traces | traces |
| Kaempferol O-malonyl-O-p-coumaroyl-O-hexoside 92 | traces | traces | traces |
| Kaempferol O-acetyl-O-p-coumaroyl-O-hexoside 93 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 |
| Total kaempferol derivatives | 0.42 | 0.35 | 0.35 |
| Quercetin | traces | traces | 0.02 ± 0.00 |
| Quercetin 3-O-xyloside | traces | traces | 0.03 ± 0.00 |
| Quercetin 3-O-arabinoside | traces | traces | 0.01 ± 0.00 |
| Quercetin 3-O-glucoside | traces | 0.04 ± 0.00 | 0.08 ± 0.00 |
| Quercetin 3-O-glucuronide | traces | 0.05 ± 0.00 | 0.11 ± 0.00 |
| Quercetin 3-O-rutinoside | 0.32 ± 0.00 | 0.28 ± 0.00 | 0.25 ± 0.00 |
| Quercetin 3-O-sophoroside | 0.11 ± 0.00 | 0.08 ± 0.00 | 0.03 ± 0.00 |
| Quercetin 3-O-(2″-O-acetyl)-glucoside | 0.06 ± 0.00 | 0.03 ± 0.00 | 0.01 ± 0.00 |
| Quercetin 3-O-(6″-O-acetyl)-glucoside | 0.03 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 |
| Quercetin 3-O-(2″,6″-di-O-acetyl)-glucoside | 0.03 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 |
| Quercetin 3-O-(6″-O-malonyl)-glucoside | 0.04 ± 0.00 | 0.04 ± 0.00 | 0.02 ± 0.00 |
| Quercetin O-malonyl-O-hexoside 64 | 0.01 ± 0.00 | traces | traces |
| Quercetin 3-O-(6″-O-p-coumaroyl)-glucoside | 0.11 ± 0.00 | 0.06 ± 0.00 | 0.05 ± 0.00 |
| Quercetin O-p-coumaroyl-O-hexoside 61 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.01 ± 0.00 |
| Quercetin O-acetyl-O-malonyl-O-hexoside 80 | 0.01 ± 0.00 | 0.01 ± 0.00 | traces |
| Quercetin O-malonyl-O-p-coumaroyl-O-hexoside 84 | 0.02 ± 0.00 | 0.01 ± 0.00 | traces |
| Quercetin O-malonyl-O-p-coumaroyl-O-hexoside 85 | 0.01 ± 0.00 | 0.01 ± 0.00 | traces |
| Quercetin O-acetyl-O-p-coumaroyl-O-hexoside 88 | 0.01 ± 0.00 | traces | traces |
| Quercetin di-O-acetyl-O-p-coumaroyl-O-hexoside 94 | traces | traces | traces |
| Quercetin O-acetyl-O-malonyl-O-p-coumaroyl-O-hexoside 95 | 0.04 ± 0.00 | 0.02 ± 0.00 | 0.01 ± 0.00 |
| Total quercetin derivatives | 0.82 | 0.68 | 0.66 |
| Total flavonols and flavonol glycosides | 1.24 | 1.03 | 1.01 |
| Triterpenes | |||
| Pomolic acid | traces | traces | 0.01 ± 0.00 |
| Pomolic acid O-hexoside 83 | traces | traces | traces |
| Pomolic acid di-O-hexoside 77 | traces | traces | traces |
| Tormentic acid | traces | traces | 0.02 ± 0.00 |
| Tormentic acid O-hexoside 78 | traces | traces | 0.01 ± 0.00 |
| Tormentic acid di-O-hexoside 75 | traces | traces | traces |
| Total triterpenes | traces | traces | 0.04 |
The highest total content of organic acids was found in ripe fruits of F. viridis (7.88 mg/g) and the lowest was found in the unripe stage (4.25 mg/g) including the greatest share of citric acid in all stages of ripening (2.83–5.63 mg/g). The remaining organic acids were minor components with concentration values 0.42–0.59 mg/g for malic acid, 0.37–0.42 mg/g for tartaric acid, 0.01–0.07 mg/g for fumaric acid, and trace–0.05 mg/g for oxalic acid. This results in an increase in the acidity of F. viridis fruits during ripening, which had a positive impact on strawberry taste. The domination of citric acid was demonstrated previously in many F. ananassa cultivars grown in Slovenia (4.4–10.5 mg/g) [42], Pakistan (12.0–14.3 mg/g) [33], and Turkey (5–10 mg/g) [45] as well as in F. vesca (5.6 mg/g) [34]. Moreover, the fruit development resulted in an increase in organic acids in strawberries [33], and malic, tartaric, fumaric, and oxalic acids were the minor acids in other Fragaria fruits [33,34,45]. Particularly noteworthy was the presence of a high level of ascorbic acid, up to 1.12 mg/g in ripe F. viridis fruits, that was significantly more than found in F. vesca (0.4 mg/g) and F. ananassa (0.25–0.9 mg/g) [33,45].
Galic acid derivatives showed trace (gallic acid, 1,2,3,4,6-penta-O-galloylglucose) or low-level content (1-O-galloyl glucose) without significant variation during ripening (0.04–0.05 mg/g). In contrast to the gallic acid derivatives, hydroxycinnamates were important compounds of F. viridis fruits with medium content although the amount decreased during ripening from 0.97 mg/g to 0.60 mg/g. The highest level was found for the p-coumaric acid 4-O-glucoside (0.29–0.35 mg/g), its isomer 24 (0.08–0.14 mg/g), and 5-O-caffeoylquinic acid (0.14–0.28 mg/g). The early study of 5-O-caffeoylquinic acid content variation in dry strawberries showed it ranging from 1.8 to 2.9 mg/g for F. vesca, from 1.2 to 1.7 mg/g for F. viridis, and from 0.7 to 1.8 mg/g for F. moschata [15]. The level of coumaroyl glycosides in Norway F. ananassa cultivars varied from 0.02 mg/g to 0.14 mg/g with maximal content in fully ripe fruits [25], which is different from our findings.
The most significant groups of phenolics with the highest content in all stages of maturity of F. viridis fruits were ellagitannins and ellagic acid derivatives. The general rule of ellagitannin variation in F. viridis fruits (with few exceptions) was decreasing content during ripening. This was particularly manifested in dominant compounds of agrimoniin (2.63→1.41 mg/g), lambertianin C (1.86→1.20 mg/g), fragariin A (0.93→0.63 mg/g), and sanguiin H6 (0.36→0.22 mg/g), where values were maximal in unripe fruits. The decrease of ellagitannin level in strawberry fruits during development was previously shown in some Norway cultivars of F. ananassa; the variation of agrimoniin content in Blink, Polka, and Senga cultivars was 0.72→0.57, 0.66→0.58, and 0.68→0.55 mg/g, respectively [25]. The most drastic fall in ellagitannin content, from 1.14 to 0.30 mg/g, was found in Italian cultivars of F. ananassa [18]. The most likely reason for the ellagitannin changes is due to increasing activity of specific enzymes, such as tannases, reaching the highest values in ripening fruits [46].
This is further illustrated by the slight rising content of ellagic acid, some ellagic acid O-glycosides, and low molecular weight ellagitannins (as pedunculagin, strictinin, castalagin, and casuarictin) that can be considered the breakdown products of ellagitannin molecules, but of course, this issue needs to be discussed additionally.
Catechins and procyanidins are compounds with medium levels in F. viridis fruits, for which the total concentration decreased from unripe to the ripe stage (0.29→0.09 mg/g). The content of major components showed the same behaviour, including catechin (0.11→0.05 mg/g), procyanidin B2 (0.09→0.02 mg/g), and procyanidin C2 (0.05→0.01 mg/g). Aaby et al. [25] reported similar data for 27 cultivars of F. ananassa for the content of catechin (0.02–0.08 mg/g), procyanidin dimers (0.05–0.16 mg/g), and trimers (0.05–0.19 mg/g).
Anthocyanins, which are important phenolics of strawberries, were at a low level in F. viridis characterized by slight red pigmentation of the outer layer of fruits and depigmented pulp. It is for this reason that the unripe and pre-ripe fruits had trace anthocyanin content. In the ripe stage, the domination of pelargonidin 3-O-glucoside (0.06 mg/g), cyanidin 3-O-glucoside (0.05 mg/g), and pelargonidin 3-O-rutinoside (0.03 mg/g) was observed. The remaining pigments were found in trace levels. Pelargonidin and cyanidin glycosides were also found as components of phenolic pigments of all studied strawberries including F. ananassa [47], F. vesca, and F. moschata [14]. It is to be expected that the ripening of strawberry fruits resulted in intense pigmentation caused by the accumulation of anthocyanins, as in the case of Norway cultivars from 0.2 mg/g in the pre-ripe stage to 0.8 mg/g in fully ripe fruits of F. ananassa [25].
The total concentration of flavonols in F. viridis demonstrated decreasing levels during fruit development from 1.24 mg/g in unripened fruits to 1.01 mg/g in ripened fruits. Quercetin derivatives (0.66–0.82 mg/g) prevailed over kaempferol derivatives (0.35–0.42 mg/g) in all stages of ripening; this was also found in F. ananassa (0.01–0.05 mg/g for quercetin derivatives vs. 0.01–0.02 mg/g for kaempferol derivatives) [9]. The main components of flavonol complex of F. viridis were quercetin 3-O-rutinoside (0.25–0.32 mg/g) and kaempferol 3-O-rutinoside (0.11–0.28 mg/g), followed by two acylated compounds: quercetin 3-O-(6″-O-p-coumaroyl)-glucoside (0.05–0.11 mg/g) and kaempferol 3-O-(6″-O-p-coumaroyl)-glucoside (0.04–0.08 mg/g). The gradual decline of flavonoid concentration was found for flavonol di-O-glycosides and acylated flavonol O-glycosides in contrast to aglycones and flavonol mono-O-glycosides accumulated in ripe fruits. Again, the variation of enzymatic activity of hydrolases may be relevant in the progress of F. viridis fruits ripening. This phenomenon has not been mentioned previously in any strawberries and needs additional experimental data to confirm this finding.
Triterpenes found in F. viridis fruits were trace compounds, but despite this, the quantifiable levels of tormentic acid, its O-hexoside, and pomolic acid were found in the stage of full ripening. The variation of triterpenoids in any Fragaria species was not discussed previously, but it is known that the accumulation of triterpenoids reaches the maximal level in the fully ripe stage of fruits; this was also declared for Chardonnay grape [48], olive fruits [49], and tomato [50].
2.3. Antioxidant Potential of F. viridis Fruits: Comparision with Other Strawberries
The activity of fruit total extracts of F. viridis in three stages of ripening was studied in four antioxidant assays including the scavenging capacity against 2,2-diphenyl-1-picrylhydrazyl radical (DPPH), and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) cation radical (ABTS), ferric reducing antioxidant power (FRAP), and oxygen radical absorbance capacity (ORAC) (Table 3). In comparison, the activity of two extracts from commercially available ripe fruits of F. vesca (wild strawberry, Regina cultivar) and F. ananassa (garden strawberry, Senga Sengana cultivar) was also studied. Both species are much more common strawberries, and their antioxidant potential has been analysed many times [2].
Table 3.
Antioxidant activity of Fragaria extracts in four assays, μM trolox-eq./g of dry weight ± S.D.
| Assay a | F. viridis | F. vesca (Ripe) | F. ananassa (Ripe) | ||
|---|---|---|---|---|---|
| Unripe | Intermediate | Ripe | |||
| DPPH | 29.2 ± 0.6 d,e | 28.4 ± 0.5 c | 27.5 ± 0.5 c,d | 15.2 ± 0.3 b | 9.3 ± 0.2 a |
| ABTS | 35.1 ± 0.8 h,i | 35.3 ± 0.8 h | 36.2 ± 0.9 i | 19.7 ± 0.4 f,g | 14.7 ± 0.3 f |
| FRAP | 42.6 ± 1.0 l | 45.4 ± 1.0 l,m | 47.1 ± 1.0 m | 27.1 ± 0.5 k | 21.1 ± 0.4 j |
| ORAC | 33.6 ± 0.8 p,q | 32.8 ± 0.7 o,p | 33.0 ± 0.8 p | 25.1 ± 0.5 n,o | 18.9 ± 0.4 n |
a DPPH—scavenging capacity against 2,2-diphenyl-1-picrylhydrazyl radical; ABTS—scavenging capacity against 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) cation radical; FRAP—ferric reducing antioxidant power; and ORAC—oxygen radical absorbance capacity. Averages ± standard deviation (S.D.) were obtained from five different experiments. Values with different letters (a–q) indicate statistically significant differences among groups at p < 0.05 by one-way ANOVA.
The extracts of F. viridis fruits in all stages of ripening were effective radical scavengers against both radicals DPPH and ABTS. Variations of the antioxidant potential values were 27.53–29.18 μM trolox-eq./g in the DPPH assay and 35.07–36.22 μM trolox-eq./g in the ABTS assay, while the more active scavenger in the DPPH assay was the extract of unripe fruits and the ABTS assay gave the extract of ripe fruits as more active. The extracts of F. vesca and F. ananassa were less effective in DPPH/ABTS scavenging assays with values of trolox-equivalent content 15.21/19.73 and 9.33/14.67 μM/g, respectively. An early research of F. ananassa extracts in the DPPH assay showed a wide range of fluctuation of antiradical activity from 9.75–12.83 μM BHT-eq./g for Brazil cultivars [4] to 3.00–13.15 μM trolox-eq./g for Polish cultivars [10]. Sikmilar characteristics were found for ABTS assay data varying from 1.50–2.27 μM trolox-eq./g for Japan varieties [51] to 7.06–29.73 μM trolox-eq./g for Polish cultivars [10]. Raudonis et al. [14] analysed the activity of F. vesca and F. moschata extracts using HPLC-assisted ABTS assay, which gave the values of protection 25.11 and 8.24 μM trolox-eq./g, respectively.
The FRAP assay is one of the most popular assays to estimate antioxidant activity for analysis of edible fruits, and strawberries are not an exception. The level of ferric reducing antioxidant power of F. viridis extracts was high and increased during fruit ripening from 42.63 μM trolox-eq./g in the unripe stage to 47.11 μM trolox-eq./g in ripe fruit extract. The parameters of F. vesca (27.14 μM trolox-eq./g) and F. ananassa (21.06 μM trolox-eq./g) extracts were lower but close to known data (24.84 μM trolox-eq./g for F. vesca [14]). The level of antioxidant activity in the ORAC assay for F. viridis fruit extracts was similar to ABTS data and slightly decreased in ripening progress from 33.62 μM trolox-eq./g (unripe fruits) to 32.98 μM trolox-eq./g (ripe fruits). The ORAC data of F. vesca extract 25.05 μM trolox-eq./g was higher than for F. ananassa extract (18.87 μM trolox-eq./g) but at a level lower than F. viridis. The known information about ORAC potential of strawberries demonstrated high effectiveness of anthocyanins fraction of F. ananassa (2.7–24.46 mM trolox-eq./g) [52], opposite the activity of the total fruit extract (8.90–16.63 μM trolox-eq./g) [53].
Applying the DPPH-radical scavenging-assisted HPLC-PDA-ESI-tQ-MS assay, we identified the compounds responsible for the antioxidant defence of F. viridis extracts. For that to happen, an aliquot of the total extract was separated with chromatography and portions of the eluates were collected. A part of the eluates was used for the DPPH decolouration assay, and the remainder were used for HPLC-PDA-ESI-tQ-MS assay for the qualitative confirmation of compounds (Figure S2). The results showed that the majority of compounds found in F. viridis were involved in the process of radical scavenging (most likely because of their phenolic nature), but 12 sites of elution gave more pronounced decolouration of the DPPH solution. There were ascorbic acid, ellagic acid, five ellagitannins (pedunculagin, sanguiin H6, lambertianin C, agrimoniin, and fragariin A), two anthocyanins (pelargonidin 3-O-glucoside, and cyanidin 3-O-glucoside), and three flavonols (quercetin 3-O-glucoside, quercetin 3-O-glucuronide, and quercetin 3-O-rutinoside). Three compounds, ascorbic acid, lambertianin C, and agrimoniin, were the most active due to their high content so they were the principal antioxidants of F. viridis fruits.
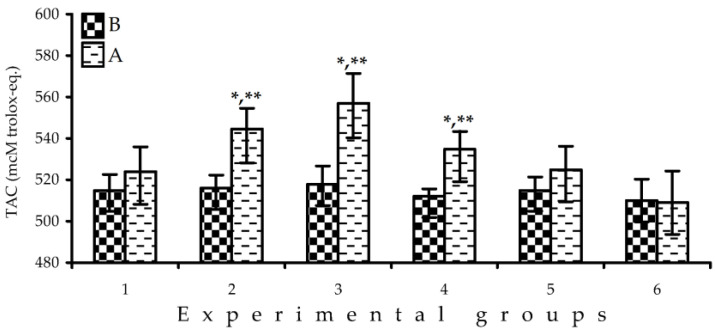
In brief, the information obtained in four in vitro assays demonstrates the high effectiveness of F. vesca fruit extracts as antioxidant agents, exceeding the activity of two well-known strawberries: F. vesca and F. ananassa. In light of the obtained results about the activity of F. vesca fruits, checking its usefulness in antioxidant protection of the human organisms was considered. To this end, this was done by pilot experiment by analysing total antioxidant capacity (TAC) of blood serum of healthy male volunteers after a 1-week intake of F. viridis fresh ripe fruits at doses of 100, 250, and 400 g/day. The level of TAC before F. vesca fruit intake was 510–516 μM trolox-eq./L. The week-long consumption of F. viridis fresh fruits gave a statistically significant increase of serum TAC level in all dose groups up to 524, 544, and 557 μM trolox-eq./L, respectively, for groups with 100, 250, and 400 g/day consumption (Figure 3).
Figure 3.
Changes in serum total antioxidant capacity (TAC) before (B) and after (A) 1-week intake of Fragaria fresh ripe fruits (group 1—F. viridis, 100 g/day, n = 5; group 2—F. viridis, 250 g/day, n = 5; group 3—F. viridis, 400 g/day, n = 5; group 4—F. vesca, 250 g/day, n = 4; and group 5—F. ananassa, 250 g/day, n = 6) and 10 g/day fructose (group 6, control group; n = 3). * p < 0.05 vs. control group after intake; ** p < 0.05 vs. same group before intake.
By comparison, the results of F. vesca and F. ananassa fruit groups (both 250 g/day) were also positive—the consumption of both kinds of strawberries resulted in increases in serum TAC levels, but to a lesser degree (535 μM trolox-eq./L for F. vesca, 525 μM trolox-eq./L for F. ananassa).
This illustrates the good antioxidant potential of F. viridis fruits in any dose applied. To date, it is known that the consumption of F. ananassa resulted in increases in serum TAC by 7–25% [54]. The possible reasons for that phenomenon may be an increase in human serum of strawberry-related metabolites such as pelargonidin-glucuronide, urolithin A-glucuronide [55] and p-hydroxybenzoic acid [56] possessing high antioxidant potential and also the rising of serum antioxidants (glutathione) and serum level of antioxidant enzyme activity (catalase, glutathione peroxidase, and glutathione reductase) [57]. The metabolite profile of F. viridis is qualitatively and quantitatively close to F. ananassa; therefore, it is logical to assume that the increase of serum TAC after F. viridis consumption is caused by enhancement of the serum level of antioxidant metabolites of phenolic nature, serum antioxidants, and antioxidant enzymes.
2.4. Storage Stability of Antioxidants and Antioxidant Potential of F. viridis Ripe Fruits
Twenty compounds with the most pronounced antioxidant effects were quantified in F. viridis fruits in two series of storage experiments (Table 4). Primarily, we studied the change in concentration of ripe fruits stored at two temperatures, 4 °C (cool temperature) and 20 °C (room temperature), to define the stability of antioxidants in fresh fruits for a short period.
Table 4.
Content of selected antioxidants in ripe fruits of F. viridis (mg/g of fresh fruit weight ± S.D.) and its total antioxidant potential (coulometric titration assay; μmol trolox-eq./g of fresh fruit weight ± S.D.) after storage at 4 °C (1 week) and 20 °C (3 days).
| Compound | T, °C | Day of Storage | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||
| Ascorbic acid | 4 | 1.14 ± 0.02 | 1.02 ± 0.02 | 0.95 ± 0.02 | 0.89 ± 0.02 | 0.86 ± 0.02 | 0.73 ± 0.02 | 0.55 ± 0.02 | 0.51 ± 0.02 |
| 20 | 0.85 ± 0.02 | 0.54 ± 0.01 | 0.36 ± 0.01 | n.a. | n.a. | n.a. | n.a. | ||
| Ellagic acid | 4 | 0.10 ± 0.00 | 0.10 ± 0.00 | 0.10 ± 0.00 | 0.12 ± 0.00 | 0.14 ± 0.00 | 0.15 ± 0.00 | 0.17 ± 0.00 | 0.19 ± 0.00 |
| 20 | 0.10 ± 0.00 | 0.12 ± 0.00 | 0.25 ± 0.00 | n.a. | n.a. | n.a. | n.a. | ||
| Pedunculagin | 4 | 0.33 ± 0.01 | 0.34 ± 0.01 | 0.34 ± 0.01 | 0.35 ± 0.01 | 0.35 ± 0.01 | 0.38 ± 0.01 | 0.38 ± 0.01 | 0.40 ± 0.01 |
| 20 | 0.35 ± 0.01 | 0.38 ± 0.01 | 0.49 ± 0.01 | n.a. | n.a. | n.a. | n.a. | ||
| Sanguiin H6 | 4 | 0.20 ± 0.00 | 0.20 ± 0.00 | 0.20 ± 0.00 | 0.21 ± 0.00 | 0.22 ± 0.00 | 0.24 ± 0.00 | 0.25 ± 0.00 | 0.25 ± 0.00 |
| 20 | 0.20 ± 0.00 | 0.21 ± 0.00 | 0.30 ± 0.00 | n.a. | n.a. | n.a. | n.a. | ||
| Lambertianin C | 4 | 1.26 ± 0.02 | 1.24 ± 0.02 | 1.20 ± 0.02 | 1.15 ± 0.02 | 1.11 ± 0.02 | 0.99 ± 0.02 | 0.97 ± 0.02 | 0.93 ± 0.02 |
| 20 | 1.04 ± 0.02 | 0.90 ± 0.02 | 0.72 ± 0.02 | n.a. | n.a. | n.a. | n.a. | ||
| Agrimoniin | 4 | 1.47 ± 0.03 | 1.45 ± 0.03 | 1.40 ± 0.03 | 1.37 ± 0.03 | 1.35 ± 0.03 | 1.25 ± 0.02 | 1.22 ± 0.02 | 1.17 ± 0.02 |
| 20 | 1.33 ± 0.03 | 1.08 ± 0.02 | 0.93 ± 0.02 | n.a. | n.a. | n.a. | n.a. | ||
| Fragariin A | 4 | 0.65 ± 0.02 | 0.65 ± 0.02 | 0.62 ± 0.02 | 0.60 ± 0.02 | 0.59 ± 0.01 | 0.55 ± 0.01 | 0.53 ± 0.02 | 0.51 ± 0.01 |
| 20 | 0.42 ± 0.01 | 0.34 ± 0.01 | 0.22 ± 0.00 | n.a. | n.a. | n.a. | n.a. | ||
| Pelargonidin 3-O-glucoside | 4 | 0.07 ± 0.00 | 0.07 ± 0.00 | 0.07 ± 0.00 | 0.07 ± 0.00 | 0.06 ± 0.00 | 0.06 ± 0.00 | 0.05 ± 0.00 | 0.05 ± 0.00 |
| 20 | 0.04 ± 0.00 | 0.02 ± 0.00 | traces | n.a. | n.a. | n.a. | n.a. | ||
| Cyanidin 3-O-glucoside | 4 | 0.05 ± 0.00 | 0.05 ± 0.00 | 0.05 ± 0.00 | 0.04 ± 0.00 | 0.04 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 |
| 20 | 0.02 ± 0.00 | traces | traces | n.a. | n.a. | n.a. | n.a. | ||
| Quercetin 3-O-glucoside | 4 | 0.10 ± 0.00 | 0.10 ± 0.00 | 0.10 ± 0.00 | 0.10 ± 0.00 | 0.10 ± 0.00 | 0.10 ± 0.00 | 0.11 ± 0.00 | 0.11 ± 0.00 |
| 20 | 0.10 ± 0.00 | 0.11 ± 0.00 | 0.12 ± 0.00 | n.a. | n.a. | n.a. | n.a. | ||
| Quercetin 3-O-glucuronide | 4 | 0.15 ± 0.00 | 0.15 ± 0.00 | 0.15 ± 0.00 | 0.16 ± 0.00 | 0.17 ± 0.00 | 0.17 ± 0.00 | 0.17 ± 0.00 | 0.17 ± 0.00 |
| 20 | 0.15 ± 0.00 | 0.15 ± 0.00 | 0.17 ± 0.00 | n.a. | n.a. | n.a. | n.a. | ||
| Quercetin 3-O-rutinoside | 4 | 0.24 ± 0.00 | 0.24 ± 0.00 | 0.24 ± 0.00 | 0.24 ± 0.00 | 0.24 ± 0.00 | 0.22 ± 0.00 | 0.21 ± 0.00 | 0.20 ± 0.00 |
| 20 | 0.24 ± 0.00 | 0.20 ± 0.00 | 0.18 ± 0.00 | n.a. | n.a. | n.a. | n.a. | ||
| Total antioxidant potential | 4 | 4.12 ± 0.09 | 4.10 ± 0.08 | 4.07 ± 0.08 | 4.02 ± 0.08 | 3.97 ± 0.08 | 3.86 ± 0.08 | 3.59 ± 0.07 | 3.27 ± 0.07 |
| 20 | 2.88 ± 0.05 | 1.72 ± 0.04 | 0.52 ± 0.02 | n.a. | n.a. | n.a. | n.a. | ||
n.a.—not analyzed.
The seven-day storage of ripe F. viridis fruits at 4 °C and three-day storage at 20 °C were the maximal periods of storage without external damage (browning, rotting, untypical smell, and taste) [58].
Results from HPLC data show that the storage of ripe F. viridis fruits at 4 °C caused a decrease of ascorbic acid content by 55.2% (1.14→0.51 mg/g), anthocyanin content by 28.6–60.0% (0.07→0.05 mg/g for pelargonidin 3-O-glucoside and 0.05→0.02 mg/g for cyanidin-3-O-glucoside), ellagitannin polymers content by 20.4–26.2% (1.26→0.93 mg/g for lambertianin C, 1.47→1.17 mg/g for agrimoniin, and 0.65→0.51 mg/g for fragariin A), and quercetin 3-O-rutinoside content by 16.7% (0.24→0.20 mg/g). There has also been an increase in the concentration of ellagic acid by 90% (0.10→0.19 mg/g), ellagitannin monomers and dimers by 17.5–25.0% (0.33→0.40 mg/g for pedunculagin and 0.20→0.25 mg/g for sanguiin H6), and flavonol monoglucosides by 10.0–13.3% (0.10→0.11 mg/g for quercetin 3-O-glucoside and 0.15→0.17 mg/g for quercetin 3-O-glucuronide). As a result of chemical changes, the reduction of bioactivity of fruits was also observed, and the loss of total antioxidant potential was 20.6% (4.12→3.27 μmol trolox-eq./g).
The storage of fresh F. viridis fruits at room temperature (20 °C) resulted in more drastic changes within a shorter period. The level of ascorbic acid declined from 1.14 mg/g to 0.36 mg/g (68.4%) for three days; additionally, anthocyanins became a trace compound. The strong reduction of content was detected for the polymeric ellagitannins, lambertianin C (42.6%), agrimoniin (36.7%), and fragariin A (66.2%) in opposition to ellagic acid, pedunculagin, and sanguiin H6, which increased at 150.0, 48.5, and 50.0%, respectively. The flavonoid biocide quercetin 3-O-rutinoside showed a statistically significant decrease of content from 0.24 mg/g to 0.18 mg/g (25%) coupled with a rising level of quercetin 3-O-glucoside and quercetin 3-O-glucuronide.
The parameter of total antioxidant potential decreased from 4.12 to 0.52 μmol trolox-eq./g or 87.4% less antioxidant potential. Postharvest storage of ripe fruits is inextricably linked to senescence causing changes in biochemical profiles, biomolecules and polymers degradation, cell dysfunction and disintegration, and the leaking of enzymes [58]. Not long after, the fruits begin rotting, which reduces its alimentary value. In our study, the ripe F. viridis fruits after storage showed negative changes in content of ascorbic acid and anthocyanins, which are environmentally unstable plant compounds diminished in light and high humidity [59,60], just like polymeric ellagitannins and rutin typically degrading after contact with oxygen and esterase-like enzymes [61,62]. The preservative value of cool temperature (4 °C) was higher than room temperature (20 °C), saving antioxidants and the antioxidant potential of fruits longer. The decrease in phenolic compounds and ascorbic acid content in strawberries was shown in the number of papers. Anthocyanin content decreased in F. ananassa fruits during refrigerated storage at 4 °C in cultivars Camarosa (385→46 mg/kg) [63,64] and Elsanta (40→20 mg/g) [65]. The ascorbic acid level was also unstable at 0–20 °C with a loss of about 40% (cultivars Dover, Campineiro, and Mazi) [3] or more (cultivar Camarosa) [63]. The content of ellagic acid and flavonol monoglucosides in cool storage (5 °C) of F. ananassa fruits tends to rise as in the Selva cultivar from 19.9 to 26.8 μg/g for ellagic acid, from 40.1 to 44.1 μg/g for quercetin derivatives, and from 13.7 to 15.8 μg/g for kaempferol derivatives [66]. Our findings revealed that various strawberries (F. viridis and F. ananassa) have the same response during storage at cool and room temperature conditions.
3. Materials and Methods
3.1. Plant Materials and Chemicals
Samples of Fragaria viridis fruits were collected in Sakha (Yakutia) Republic (Aldanskii ulus, 58°37′27.1″ N, 125°17′17.5″ E, 15–25 July 2019) in three ripening stages (unripe—green fruits, intermediate ripe—half red fruits, and fully ripe—red fruits). The species was authenticated by N.I. Kashchenko (IGEB SB RAS, Ulan-Ude, Russia). The fruits were conditioned in plastic boxes and transported to the laboratory at 4 °C within 2–3 h. The ripe fruits of F. vesca (Regina cultivar) and F. ananassa (Senga Sengana cultivar) were purchased via a local market. The reference compounds were purchased from BioCrick (Chengdu, PRC), BOC Sciences (Shirley, NY, USA), Carbosynth Ltd. (Compton, UK), ChemFaces (Wuhan, PRC), Extrasynthese (Lyon, France), Funakoshi Co. Ltd. (Tokyo, Japan), Sigma-Aldrich (St. Louis, MO, USA), Toronto Research Chemicals (North York, ON, Canada), and TransMIT GmbH (Gießen, Germany) (Table S1). Ellagitannins sanguiins H2, H6, and H10; agrimonic acids A and B; and agrimoniin were isolated previously in our laboratory from Rosaceous species (purity 90–95%) [28,67,68], and flavonols quercetin 3-O-(2″-O-acetyl)-glucoside, quercetin 3-O-(2″-O-acetyl)-glucoside, and quercetin 3-O-(2″,6″-di-O-acetyl)-glucoside were isolated from Calendula officinalis [32]. Selected chemicals were from Sigma-Aldrich—acetonitrile for HPLC (Cat. No 34851, ≥99.9%), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (Cat. No A1888, ≥98%), 2,2′azobis(2-methylpropionamidine) dihydrochloride (Cat. No 440914, ≥97%), 2,2-diphenyl-1-picrylhydrazyl radical (Cat. No 281689, ≥97%), formic acid (Cat. No 33015, ≥98%), fructose (Cat. No 47739, ≥99%), hydrogen peroxide (Cat. No H1009, ≥30%), methanol (Cat. No. 322415, ≥99.8%), myoglobin (Cat. No M0630, ≥95%), potassium bromide (Cat. No 243418, ≥99%), sulphuric acid (Cat. No 339741, ≥99%), 2,4,6-tri(2-pyridyl)-1,3,5-triazine (Cat. No 93285, ≥99%), and trolox (Cat. No 238813, ≥97%).
3.2. Total Extract Preparation from Fragaria Fruits
For preparation of the total extract of Fragaria fruits, the fresh material was homogenized in a Grindomix GM 200 grinder (Retsch GmbH, Haan, Germany) and 100 g was extracted twice with stirring in a glass flask (0.5 L) with methanol (100 mL) using an ultrasonic bath Sapphire 2.8 (Sapphire Ltd., Moscow, Russia) for 30 min and at 50 °C (ultrasound power 100 W and frequency 35 kHz). The extracts were filtered through cellulose, concentrated in vacuo until dryness, and stored at 4 °C before use for chemical analysis and biological activity study. The yields of total extracts of Fragaria fruits were 10.63 g (F. viridis unripe fruits), 11.02 g (F. viridis intermediate ripe fruits), 12.43 g (F. viridis fully ripe fruits), 15.63 g (F. vesca fully ripe fruits), and 17.33 g (F. ananassa fully ripe fruits).
3.3. High-Performance Liquid Chromatography with Photodiode Array Detection and Electrospray Ionization Triple Quadrupole Mass Spectrometric Detection (HPLC-PDA-ESI-tQ-MS): Metabolite Profiling
Metabolite profiling of F. viridis extracts was realized using high-performance liquid chromatography with photodiode array detection and electrospray ionization triple quadrupole mass spectrometric detection (HPLC-PDA-ESI-tQ-MS) performed on a liquid chromatograph LC-20 Prominence coupled photodiode array detector SPD-M30A (wavelength range 200–600 nm), triple-quadrupole mass spectrometer LCMS 8050 (all Shimadzu, Columbia, MD, USA) and C18 column (GLC Mastro; 150 × 2.1 mm, Ø 3 μm; Shimadzu, Kyoto, Japan) at the column temperature 30 °C. Gradient elution was implemented with two eluents A (0.5% HCOOH in water) and B (0.5% HCOOH in MeCN) and the following gradient program: 0–5 min 5–7% B, 5–7 min 7–8% B, 7–10 min 8–19% B, 10–14 min 19–29% B, 14–20 min 29–52% B, 20–25 min 52–73% B, 25–35 min 73–90% B, and 35–45 min 90–5% B. The values of injection volume and elution flow were 1 μL and 100 μL/min, respectively. The UV-Vis spectra were obtained in the spectral range of 200–600 nm. MS detection was performed in negative ESI mode using the parameters as follows: temperature levels of ESI interface, desolvation line, and heat block were 300 °C, 250 °C, and 400 °C, respectively. The flow levels of nebulizing gas (N2), heating gas (air), and collision-induced dissociation gas (Ar) were 3 L/min, 10 L/min, and 0.3 mL/min, respectively. The MS spectra were recorded in the negative mode (−3–−5 kV source voltage) by scanning in the range of m/z 50–2000 at the collision energy of 5–40 eV. The system was managed under LabSolution’s workstation software with the inner LC-MS library. The identification of compounds was done by the analysis of their retention time, ultraviolet, and mass-spectrometric data, comparing the same parameters with the reference samples and/or literature data. Before analysis, the sample of F. viridis fruits dry extract (10 mg) was dissolved in 50% MeCN (25 mL), filtered (0.22-μm PTFE syringe filter), and injected (1 μL) into the HPLC-DAD-ESI-tQ-MS system for analysis.
3.4. High-Performance Liquid Chromatography with Diode Array Detection (HPLC-DAD): Carbohydrate Analysis
The composition of free carbohydrates was analyzed by high-performance liquid chromatography with diode array detection (HPLC-DAD) using the procedure described previously [69]. To prepare the sample, dry extracts of F. viridis fruits (5 mg) were dissolved in 20 mL of deionized water and passed sequentially through a series of two cartridges Dowex® 50WX8 (H+-form; 10 mL) and Dowex® 1 × 8 (Cl−-form; 10 mL) eluted with water (20 mL). The final eluates were reduced in vacuo (20 mL) and filtered using 0.22-μm PTFE syringe filter before injection into the HPLC-DAD system for analysis.
3.5. HPLC-ESI-tQ-MS: Metabolite Quantification
To quantify compounds 1–95 in F. viridis fruits, we used HPLC-MS data (MS peak area) obtained in early conditions (Section 3.3). The reference standards (48 compounds; Table S2) were accurately weighed (10 mg) and individually dissolved in DMSO-50% methanol mixture (1:10) in a volumetric flask (10 mL). The stock solutions were used to build external standard calibration curves generated using six data points, 100, 50, 25, 10, 5, and 1 µg/mL followed by plotting the MS peak area vs. the concentration levels. The validation criteria (correlation coefficients, r2; standard deviation, SYX; limits of detection, LOD; limits of quantification, LOQ; and linear ranges) were calculated using the previous recommendations [70] (Table S2). All analyses were carried out in triplicate, and the data were expressed as mean value ± standard deviation (S.D.). The sample solution was prepared from homogenized F. viridis fruits (50 mg) and 5 mL of methanol in an Eppendorf tube. The mixture was sonicated for 30 min at 50 °C (ultrasound power 100 W, frequency 35 kHz), centrifuged (6000× g), filtered (using 0.22-μm PTFE syringe filter), and transferred to the volumetric flask (10 mL), and the final volume was reduced to 10 mL by 50% MeOH before HPLC-ESI-tQ-MS analysis. Genkwanin was used as the internal standard (final concentration 25 μg/mL in acetonitrile).
3.6. Antioxidant Activity: In Vitro Assays
Radical scavenging activity of Fragaria extracts against the 2,2-diphenyl-1-picrylhydrazyl radical (DPPH) and the 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) cation radical (ABTS) was studied using microplate spectrophotometric decoloration assays as described previously [71,72]. The value of the ferric reducing antioxidant power (FRAP) was measured by spectrophotometric assay based on the reduction of the Fe3+-2,4,6-tri(2-pyridyl)-1,3,5-triazine complex to the ferrous form at low pH [73]. To determine the level of oxygen radical absorbance capacity (ORAC), we used an assay based on peroxyl radical generation by thermal decomposition of 2,2′-azobis(2-amidino-propane) dihydrochloride followed by fluorimetric detection [74]. All assays used trolox as a reference standard (methanolic solution 0.5–100 μg/mL), and the calibration curve was created by plotting the trolox concentration (μg/mL) vs. the absorbance (or fluorescence). The values of antioxidant parameters were expressed as μmol trolox-equivalents/g of dry weight. All the analyses were carried out five times and the data were expressed as mean value ± standard deviation (SD).
3.7. DPPH Radical Scavenging Assisted HPLC-PDA-ESI-tQ-MS Assay
High-performance liquid chromatography with photodiode array detection and electrospray ionization triple quadrupole mass spectrometric detection (HPLC-PDA-ESI-tQ-MS) assisted with spectrophotometric DPPH radical scavenging assay was realized in the chromatographic conditions described in Section 3.3 with enlarged injection volume at 30 μL. The eluates (50 µL) were collected every 30 s using an automated fraction collector (Econova, Novosibirsk, Russia) in 96-well microplates, then dried under a N2-stream, and redissolved in 50 µL of 50% methanol. An aliquot (25 µL) of the methanolic solution was mixed with DPPH solution (50 µg/mL in methanol) and absorbance was measured at 520 nm fifteen minutes later by a Bio-Rad microplate reader Model 3550 UV (Bio-Rad Labs, Richmond, CA, USA). The most active antioxidants gave strong decoloration of the DPPH solution, and corresponding eluates were separated in known HPLC-PDA-ESI-tQ-MS conditions again in order to confirm the presence of separate compounds.
3.8. Serum Total Antioxidant Capacity
Twenty-eight men, aged 20–25 years, were recruited. All were free from hypertension, cardiovascular disorders, and alcohol abuse; none smoked or took any other drug and oral medication. We had the guarantee that all subjects had a similar diet and lifestyle because they were recruited from the same community with a refectory service. All subjects gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Institute of General and Experimental Biology (protocol No. LM-0324, 27 January 2012). The volunteers were divided on six experimental groups: group 1—F. viridis fruits, 100 g/day (n = 5); group 2—F. viridis fruits, 250 g/day (n = 5); group 3—F. viridis fruits, 400 g/day (n = 5); group 4—F. vesca fruits, 250 g/day (n = 4); group 5—F. ananassa fruits, 250 g/day (n = 6); and group 6—fructose, 10 g/day (n = 3). Then, they took Fragaria fruits or fructose for 1 week (3 times a day in equal portions). Before and after the test, blood was drawn from the antecubital vein into a heparinized syringe, and immediately after blood drawing, serum was prepared by centrifugation (6000× g) and the serum total antioxidant capacity was estimated. Phosphate buffer (10 mM, pH 7.2; 100 μL), myoglobin solution (5 μM; 50 μL), ABTS solution (3 mM; 20 μL), and serum sample (20 μL) were mixed in 96-well microplate and incubated 3 min at 25 °C. Then H2O2 solution (250 μM; 20 μL) was added and immediately measured at 600 nm for 5 min at 25 °C. A lag time (in sec) was estimated as the suppression period of ABTS oxidation (or absorbance increasing). The reference compound (trolox; 1, 2.5, 5, and 10 μM) was analyzed using the same protocol, and the calibration curve was created by plotting the lag time (in s) vs. the absorbance at 600 nm. The value of the serum total antioxidant capacity was expressed as μmol trolox-equivalents/L. All the analyses were carried out in triplicate, and the data were expressed as mean value ± standard deviation (SD).
3.9. F. viridis Fruit Storage Experiment
Seven and three portions of the fresh F. viridis fruits (200 g) were placed into individual polystyrene bags (300 mL) and incubated at 4 °C (7 days) or 20 °C (3 days), respectively, in a ventilated MK 53 thermostat (BINDER GmbH, Tuttlingen, Germany). Five portions (20 g each) of fresh F. viridis fruits were taken out of storage for analysis every 24 h, extracted as described previously (Section 3.5), and analyzed using HPLC-ESI-tQ-MS quantitative procedure (Section 3.5) or used without pre-extraction for the total antioxidant potential determination by coulometric assay (Section 3.10).
3.10. Total Antioxidant Potential of Fresh F. viridis Fruits: Coulometric Assay
The total antioxidant potential of fresh F. viridis fruits was found using a sightly modified bromine radical scavenging assay based on the coulometric titration method with electrogenerated bromine radicals [17,75]. Potentiostat Expert-006 (Econics Expert Ltd., Moscow, Russia) with a four-electrode two-compartment electrochemical cell was used for measurements. The working electrode was a bare platinum foil (surface area 1 cm2), and the auxiliary electrode was a platinum wire isolated from the anodic cell with a semipermeable diaphragm. To detect the titration end-point (ΔE = 200 mV), a pair of polarized platinum electrodes was used and the electrochemical generation was carried out from the supporting electrolyte (0.25 M KBr in 0.1 M H2SO4) at a current density 5 mA∙cm−2, providing 100% current yield. To start the measurement, the portion fruit of F. viridis (50 g) with various storage periods was homogenized and 10 mg of homogenate was inserted into the coulometric cell (50 mL) containing 20.0 mL of supporting electrolyte. The time of titration was used for the total antioxidant potential calculation expressed in units of the quantity of electricity (Coulombs (C)) spent for titration of the full probe of homogenized fruits. The trolox solutions were used as a reference compound (500, 250, 100, 50, and 10 μg/mL in methanol) titrated coulometrically, and a calibration curve was plotted in coordinates “concentration (μg/mL)—the quantity of electricity (C)”. Finally, the value of the total antioxidant potential was calculated as mg trolox-equivalents per g of fresh fruits. Values are expressed as mean obtained from ten independent experiments.
3.11. Statistical Analysis
Statistical analyses were performed using a one-way analysis of variance (ANOVA), and the significance of the mean difference was determined by Duncan’s multiple range test. Differences at p < 0.05 were considered statistically significant. The results are presented as mean values ± S.D. (standard deviations) of some (3–10) replicates.
4. Conclusions
The current study reported the metabolic profile of fruits of Fragaria viridis in various stages of ripening using the HPLC-DAD-ESI-tQ-MS technique not applied previously to this strawberry species. About a hundred compounds were characterized, and this is many more than the previously reported amount of F. viridis metabolites [14,15]. The largest number of components were phenolics, particularly ellagitannins and flavonol glycosides, forming the basis of F. viridis metabolome in all stages of ripening. In addition, derivatives of gallic acid, ellagic acid, hydroxycinnamates, coumarins, procyanidins, catechins, and anthocyanins were also found. Non-phenolic compounds, such as carbohydrates and organic acids, were quantitatively predominant, opposite triterpenes, with trace levels found. The concentrations of all compounds were affected by the ripening process with increased (anthocyanins and non-phenolics) or decreased (the majority of phenolic compounds) values to a fully ripe stage. This indicates that the ripening of F. viridis fruits is a complex process impacting the quantitative profile of metabolites. The high content of ascorbic acid and selected phenolics in F. viridis fruits were the source of strong antioxidant properties of fruit extracts, in particular free radical scavenging capacity, ferric reducing antioxidant power, and oxygen radical absorbance capacity studied in in vitro models. The same is true for human experiments, which demonstrated that the serum total antioxidant capacity increased significantly after a week’s consumption of F. viridis fruits. Changes in antioxidant content and total antioxidant potential of fresh F. viridis fruits was found during storage at 4 °C and 20 °C, with the safest condition at 4 °C storage used within a week. The information received in our study highlighted the potential of F. viridis fruits as a source of antioxidant metabolites that need more scientific attention and wider implementation in the human diet.
Acknowledgments
The authors acknowledge the Buryat Research Resource Center for the technical support in chromatographic and mass-spectrometric research.
Supplementary Materials
The following are available online at https://www.mdpi.com/1424-8247/13/9/262/s1, Figure S1: High-performance liquid chromatography with diode array detection chromatogram of free sugars in F. viridis ripe fruits, Figure S2: High-performance liquid chromatography with electrospray ionization triple quadrupole mass spectrometric detection chromatogram of F. viridis ripe fruits extract coupled with spectrophotometric DPPH radical scavenging assay, Table S1: Reference standards used for the qualitative and quantitative analysis by HPLC-DAD-ESI-tQ-MS assays, Table S2: Regression equations, correlation coefficients, standard deviation, limits of detection, limits of quantification, and linear ranges for 48 reference standards.
Author Contributions
Conceptualization, D.N.O.; methodology, D.N.O. and N.K.C.; software, D.N.O.; validation, D.N.O. and N.K.C.; formal analysis, A.G.V.; investigation, D.N.O. and N.K.C.; resources, N.K.C., and A.G.V.; data curation, N.K.C. and A.G.V.; writing—original draft preparation, D.N.O.; writing—review and editing, N.K.C.; visualization, D.N.O.; supervision, D.N.O.; project administration, N.K.C.; funding acquisition, D.N.O. and N.K.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ministry of Education and Science of the Russian Federation, grant numbers AAAA-A17-117011810037-0 and FSRG-2020-0019, and by the Russian Foundation for Basic Research, grant number 19-09-00361.
Conflicts of Interest
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- 1.Liston A., Cronn R., Ashman T.L. Fragaria: A genus with deep historical roots and ripe for evolutionary and ecological insights. Am. J. Bot. 2014;101:1686–1699. doi: 10.3732/ajb.1400140. [DOI] [PubMed] [Google Scholar]
- 2.Fierascu R.C., Temocico G., Fierascu I., Ortan A., Babeanu N.E. Fragaria genus: Chemical composition and biological activities. Molecules. 2020;25:498. doi: 10.3390/molecules25030498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cordenunsi B.R., Nascimento J.R.O., Lajolo F.M. Physico-chemical changes related to quality of five strawberry fruit cultivars during cool-storage. Food Chem. 2003;83:167–173. doi: 10.1016/S0308-8146(03)00059-1. [DOI] [Google Scholar]
- 4.Pineli L.L.O., Moretti C.L., dos Santos M.S., Campos A.B., Brasileiro A.V., Cordova A.C., Chiarello M.D. Antioxidants and other chemical and physical characteristics of two strawberry cultivars at different ripeness stages. J. Food Compos. Anal. 2011;24:11–16. doi: 10.1016/j.jfca.2010.05.004. [DOI] [Google Scholar]
- 5.Gasparrini M., Giampieri F., Forbes-Hernandez T.Y., Afrin S., Cianciosi D., Reboredo-Rodriguez P., Varela-Lopez A., Zhang J., Quiles J.L., Mezzetti B., et al. Strawberry extracts effiently counteract inflammatory stress induced by the endotoxin lipopolysaccharide in human dermal fibroblast. Food Chem. Toxicol. 2018;114:128–140. doi: 10.1016/j.fct.2018.02.038. [DOI] [PubMed] [Google Scholar]
- 6.Cardoso O., Donato M.M., Luxo C., Almeida N., Liberal J., Figueirinha A., Batista M.T. Anti-Helicobacter pylori potential of Agrimonia eupatoria L. and Fragaria vesca. J. Funct. Food. 2018;44:299–303. doi: 10.1016/j.jff.2018.03.027. [DOI] [Google Scholar]
- 7.Ninomiya M., Itoh T., Ishikawa S., Saiki M., Narumiya K., Yasuda M., Koshikawa K., Nozawa Y., Koketsu M. Phenolic constituents isolated from Fragaria ananassa Duch. inhibit antigen-stimulated degranulation through direct inhibition of spleen tyrosine kinase activation. Bioorg. Med. Chem. 2010;18:5932–5937. doi: 10.1016/j.bmc.2010.06.083. [DOI] [PubMed] [Google Scholar]
- 8.Abdulazeez S.S. Effects of freeze-dried Fragaria × ananassa powder on alloxan-induced diabetic complications in Wistar rats. J. Taibah Univ. Med. Sci. 2014;9:268–273. doi: 10.1016/j.jtumed.2014.03.007. [DOI] [Google Scholar]
- 9.Somasagara R.R., Hegde M., Chiruvella K.K., Musini A., Choudhary B., Raghavan S.C. Extracts of strawberry fruits induce intrinsic pathway of apoptosis in breast cancer cells and inhibits tumor progression in mice. PLoS ONE. 2012;7:e47021. doi: 10.1371/journal.pone.0047021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nowicka A., Kucharska A.Z., Sokół-Łẹtowska A., Fecka I. Comparison of polyphenol content and antioxidant capacity of strawberry fruit from 90 cultivars of Fragaria × ananassa Duch. Food Chem. 2019;270:32–46. doi: 10.1016/j.foodchem.2018.07.015. [DOI] [PubMed] [Google Scholar]
- 11.Komarov V.L. Flora of USSR. Volume X. AN SSSR; Moscow, Russia: 1941. pp. 58–67. [Google Scholar]
- 12.Gruner P., Ulrich D., Neinhuis C., Olbricht K. Fragaria viridis Weston: Diversity and breeding potential of an underutilised strawberry species. Acta Horticult. 2017;1156:203–208. doi: 10.17660/ActaHortic.2017.1156.31. [DOI] [Google Scholar]
- 13.Kirillov V., Stikhareva T., Atazhanova G., Makubayeva A., Serafimovich M., Kabanova S., Rakhimzhanov A., Adekenov S. Composition of essential oil of leaves and fruits of green strawberry (Fragaria viridis Weston) growing wild in Northern Kazakhstan. J. Appl. Bot. Food Qual. 2019;92:39–48. doi: 10.5073/JABFQ.2019.092.006. [DOI] [Google Scholar]
- 14.Raudonis R., Raudone L., Jakstas V., Janulis V. Comparative evaluation of post-column free radical scavenging and ferric reducing antioxidant power assays for screening of antioxidants in strawberries. J. Chromatogr. A. 2012;1233:8–15. doi: 10.1016/j.chroma.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 15.Bagdonaite E., Jakstas V., Raudonis R., Janulis V. Chlorogenic acid, rutin and hyperoside content in Fragaria vesca, F. viridis and F. moschata in Lithuania. Nat. Prod. Res. 2013;27:181–184. doi: 10.1080/14786419.2012.660634. [DOI] [PubMed] [Google Scholar]
- 16.Olennikov D.N., Gadimli A.I., Isaev J.I., Kashchenko N.I., Prokopyev A.S., Katayeva T.N., Chirikova N.K., Vennos C. Caucasian Gentiana species: Untargeted LC-MS metabolic profiling, antioxidant and digestive enzyme inhibiting activity of six plants. Metabolites. 2019;9:271. doi: 10.3390/metabo9110271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olennikov D.N., Chirikova N.K., Vasilieva A.G., Fedorov I.A. LC-MS profile, gastrointestinal and gut microbiota stability and antioxidant activity of Rhodiola rosea herb metabolites: A comparative study with subterranean organs. Antioxidants. 2020;9:526. doi: 10.3390/antiox9060526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gasperotti M., Masuero D., Guella G., Palmieri L., Martinatti P., Pojer E., Mattivi F., Vrhovsek U. Evolution of ellagitannin content and profile during fruit ripening in Fragaria spp. J. Agric. Food Chem. 2013;61:8597–8607. doi: 10.1021/jf402706h. [DOI] [PubMed] [Google Scholar]
- 19.Olennikov D.N., Chirikova N.K., Kashchenko N.I., Nikolaev V.M., Kim S.-W., Vennos C. Bioactive phenolics of the genus Artemisia (Asteraceae): HPLC-DAD-ESI-TQ-MS/MS profile of the Siberian species and their inhibitory potential against α-amylase and α-glucosidase. Front. Pharmacol. 2018;9:756. doi: 10.3389/fphar.2018.00756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clifford M.N., Wu W., Kuhnert N. The chlorogenic acids of Hemerocallis. Food Chem. 2006;95:574–578. doi: 10.1016/j.foodchem.2005.01.045. [DOI] [Google Scholar]
- 21.Sun J., Lin L., Chen P. Study of the mass spectrometric behaviors of anthocyanins in negative ionization mode and its applications for characterization of anthocyanins and non-anthocyanin polyphenols. Rapid Commun. Mass Spectrom. 2012;26:1123–1133. doi: 10.1002/rcm.6209. [DOI] [PubMed] [Google Scholar]
- 22.Schuster B., Winter M., Herrmann K. 4-O-β-D-Glucosides of hydroxybenzoic and hydroxycinnamic acids—Their synthesis and determination in berry fruit and vegetable. Z. Naturforsch. C. 1986;41:511–520. doi: 10.1515/znc-1986-5-603. [DOI] [Google Scholar]
- 23.Moilanen J., Sinkkonen J., Salminen J.-P. Characterization of bioactive plant ellagitannins by chromatographic, spectroscopic and mass spectrometric methods. Chemoecology. 2013;23:165–179. doi: 10.1007/s00049-013-0132-3. [DOI] [Google Scholar]
- 24.Vrhovsek U., Guella G., Gasperotti M., Pojer E., Zancato M., Mattivi F. Clarifying the identity of the main ellagitannin in the fruit of the strawberry, Fragaria vesca and Fragaria ananassa Duch. J. Agric. Food Chem. 2012;60:2507–2516. doi: 10.1021/jf2052256. [DOI] [PubMed] [Google Scholar]
- 25.Aaby K., Mazur S., Nes A., Skrede G. Phenolic compounds in strawberry (Fragaria × ananassa Duch.) fruits: Composition in 27 cultivars and changes during ripening. Food Chem. 2012;132:86–97. doi: 10.1016/j.foodchem.2011.10.037. [DOI] [PubMed] [Google Scholar]
- 26.Aaby K., Ekeberg D., Skrede G. Characterization of phenolic compounds in strawberry (Fragaria x ananassa) fruits by different HPLC detectors and contribution of individual compounds to total antioxidant capacity. J. Agric. Food Chem. 2007;55:4395–4406. doi: 10.1021/jf0702592. [DOI] [PubMed] [Google Scholar]
- 27.Kajdžanoska M., Gjamovski V., Stefova M. HPLC-DAD-ESI-MSn identification of phenolic compounds in cultivated strawberries from Macedonia. Macedon. J. Chem. Chem. Eng. 2010;29:181–194. doi: 10.20450/mjcce.2010.165. [DOI] [Google Scholar]
- 28.Olennikov D.N., Kashchenko N.I., Chirikova N.K. Phenolic profile of Potentilla anserina L. (Rosaceae) herb of Siberian origin and development of a rapid method for simultaneous determination of major phenolics in P. anserina pharmaceutical products by microcolumn RP-HPLC-UV. Molecules. 2015;20:224–248. doi: 10.3390/molecules20010224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karlińska E., Pecio Ł., Macierzyński J., Stochmal A., Kosmala M. Structural elucidation of the ellagitannin with a molecular weight of 2038 isolated from strawberry fruit (Fragaria ananassa Duch.) and named fragariin A. Food Chem. 2019;296:109–115. doi: 10.1016/j.foodchem.2019.05.191. [DOI] [PubMed] [Google Scholar]
- 30.Olennikov D.N., Kashchenko N.I. New acylated apigenin glycosides from edge flowers of Matricaria chamomilla. Chem. Nat. Comp. 2016;52:996–999. doi: 10.1007/s10600-016-1845-7. [DOI] [Google Scholar]
- 31.Olennikov D.N., Chirikova N.K., Kim E., Kim S.W., Zul’fugarov I.S. New glycosides of eriodictyol from Dracocephalum palmatum. Chem. Nat. Comp. 2018;54:860–863. doi: 10.1007/s10600-018-2499-4. [DOI] [Google Scholar]
- 32.Olennikov D.N., Kashchenko N.I. New isorhamnetin glycosides and other phenolic compounds from Calendula officinalis. Chem. Nat. Comp. 2013;49:833–840. doi: 10.1007/s10600-013-0759-x. [DOI] [Google Scholar]
- 33.Mahmood T., Anwar F., Abbas M., Boyce M.C., Saari N.S. Compositional variation in sugars and organic acids at different maturity stages in selected small fruits from Pakistan. Int. J. Mol. Sci. 2012;13:1380–1392. doi: 10.3390/ijms13021380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dias M.I., Barros L., Morales P., Cámara M., Alves M.J., Oliveira M.B.P.P., Santos-Buelga C., Ferreira I.C.F.R. Wild Fragaria vesca L. fruits: A rich source of bioactive phytochemicals. Food Funct. 2016;7:4523–4532. doi: 10.1039/C6FO01042C. [DOI] [PubMed] [Google Scholar]
- 35.Kosińska A., Diering S., Prim D., Héritier J., Andlauer W. Phenolic compounds profile of strawberry fruits of Charlotte cultivar. J. Berry Res. 2013;3:15–23. doi: 10.3233/JBR-130043. [DOI] [Google Scholar]
- 36.Liberal J., Costa G., Carmo A., Vitorino R., Marques C., Domingues M.R., Domingues P., Gonçalves A.C., Alves R., Sarmento-Ribeiro A.B., et al. Chemical characterization and cytotoxic potential of an ellagitannin-enriched fraction from Fragaria vesca leaves. Arab. J. Chem. 2019;12:3652–3666. doi: 10.1016/j.arabjc.2015.11.014. [DOI] [Google Scholar]
- 37.Jordheim M., Måge F., Andersen Ø.M. Anthocyanins in berries of Ribes including Gooseberry cultivars with high content of acylated pigments. J. Agricult. Food Chem. 2007;55:5529–5535. doi: 10.1021/jf0709000. [DOI] [PubMed] [Google Scholar]
- 38.Williams C.A. Flavone and flavonol O-glycosides. In: Andersen Ø.M., Markham K.R., editors. Flavonoids: Chemistry, Biochemistry, and Applications. CRC Press; Boca Raton, FL, USA: 2006. pp. 749–856. [Google Scholar]
- 39.Zuo G.-Y., Liu S.-L., Xu G.-L., Wang G.-C., Zhang Y.-L., Zheng D. Triterpenoids from the Roots of Rubus obcordatus (Rosaceae) Plant Divers. 2008;30:381–382. [Google Scholar]
- 40.Yean M.-H., Kim J.-S., Hyun Y.-J., Hyun J.-W., Bae K.-H., Kang S.-S. Terpenoids and phenolics from Geum japonicum. Korean J. Pharmacogn. 2012;43:107–121. [Google Scholar]
- 41.Basson C.E., Groenewald J.H., Kossmann J., Cronje C., Bauer R. Sugar and acid-related quality attributes and enzyme activities in strawberry fruits: Invertase is the main sucrose hydrolysing enzyme. Food Chem. 2010;121:1156–1162. doi: 10.1016/j.foodchem.2010.01.064. [DOI] [Google Scholar]
- 42.Sturm K., Koron D., Stampar F. The composition of fruit of different strawberries varieties depending on maturity stage. Food Chem. 2003;83:417–422. doi: 10.1016/S0308-8146(03)00124-9. [DOI] [Google Scholar]
- 43.Blanch M., Sanchez-Ballesta M.T., Escribano M.I., Merodio C. The relationship between bound water and carbohydrate reserves in association with cellular integrity in Fragaria vesca stored under different conditions. Food Bioprocess. Technol. 2015;8:875–884. doi: 10.1007/s11947-014-1449-9. [DOI] [Google Scholar]
- 44.Castro I., Goncalves O., Teixeira J.A., Vicente A.A. Comparative study of selva and camarosa strawberries for the commercial market. J. Food Sci. 2002;67:2132–2137. doi: 10.1111/j.1365-2621.2002.tb09515.x. [DOI] [Google Scholar]
- 45.Kouyncu M.A., Dilmacunal T. Determination of vitamin C and organic acid changes in strawberry by HPLC during cold storage. Not. Bot. Hort. Agrobot. Cluj. 2010;38:95–98. doi: 10.15835/nbha3834819. [DOI] [Google Scholar]
- 46.Palma J.M., Corpas F.J., Freschi L., Valpuesta V. Fruit ripening: From present knowledge to future development. Front. Plant Sci. 2019;10:545. doi: 10.3389/fpls.2019.00545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mazur S.P., Nes A., Wold A.-B., Remberg S.F., Martinsen B.K., Aaby K. Effects of ripeness and cultivar on chemical composition of strawberry (Fragaria × ananassa Duch.) fruits and their suitability for jam production as a stable product at different storage temperatures. Food Chem. 2014;146:412–422. doi: 10.1016/j.foodchem.2013.09.086. [DOI] [PubMed] [Google Scholar]
- 48.Le Fur Y., Hory C., Bard M.H., Olsson A. Evolution of phytosterols in Chardonnay grape berry skins during last stages of ripening. Vitis. 1994;33:127–131. [Google Scholar]
- 49.Stiti N., Triki S., Hartmann M.A. Formation of triterpenoids throughout Olea europaea fruit ontogeny. Lipids. 2007;42:55–67. doi: 10.1007/s11745-006-3002-8. [DOI] [PubMed] [Google Scholar]
- 50.Kosma D.K., Parsons E.P., Isaacson T., Lü S., Rose J.K.C., Jenks M.A. Fruit cuticle lipid composition during development in tomato ripening mutants. Physiol. Plant. 2010;139:107–117. doi: 10.1111/j.1399-3054.2009.01342.x. [DOI] [PubMed] [Google Scholar]
- 51.Zhu Q., Nakagawa T., Kishikawa A., Ohnuki K., Shimizu K. In vitro bioactivities and phytochemical profile of various parts of the strawberry (Fragaria × ananassa var. Amaou) J. Funct. Food. 2015;13:38–49. doi: 10.1016/j.jff.2014.12.026. [DOI] [Google Scholar]
- 52.Cerezo A.B., Cuevas E., Winterhalter P., Garcia-Parrilla M.C., Troncoso A.M. Isolation, identification, and antioxidant activity of anthocyanin compounds in Camarosa strawberry. Food Chem. 2010;123:574–582. doi: 10.1016/j.foodchem.2010.04.073. [DOI] [Google Scholar]
- 53.Álvarez-Fernández M.A., Hornedo-Ortega R., Cerezo A.B., Troncoso A.M., García-Parrilla M.C. Effects of the strawberry (Fragaria ananassa) purée elaboration process on non-anthocyanin phenolic composition and antioxidant activity. Food Chem. 2014;164:104–112. doi: 10.1016/j.foodchem.2014.04.116. [DOI] [PubMed] [Google Scholar]
- 54.Cao G., Russell R.M., Lischner N., Prior R.L. Serum antioxidant capacity is increased by consumption of strawberries, spinach, red wine or vitamin C in elderly women. J. Nutr. 1998;128:2383–2390. doi: 10.1093/jn/128.12.2383. [DOI] [PubMed] [Google Scholar]
- 55.Henning S.M., Seeram N.P., Zhang Y., Li L., Gao K., Lee R.-P., Wang D.C., Zerlin A., Karp H., Thames G., et al. Strawberry consumption is associated with increased antioxidant capacity in serum. J. Med. Food. 2010;13:116–122. doi: 10.1089/jmf.2009.0048. [DOI] [PubMed] [Google Scholar]
- 56.Azzini E., Vitaglione P., Intorre F., Napolitano A., Durazzo A., Foddai M.S., Fumagalli A., Catasta G., Rossi L., Venneria E., et al. Bioavailability of strawberry antioxidants in human subject. Brit. J. Nutr. 2010;104:1165–1173. doi: 10.1017/S000711451000187X. [DOI] [PubMed] [Google Scholar]
- 57.Basu A., Morris S., Nguyen A., Betts N.M., Fu D., Lyons T.J. Effects of dietary strawberry supplementation on antioxidant biomarkers in obese adults with above optimal serum lipids. J. Nutr. Metabol. 2016;2016:3910630. doi: 10.1155/2016/3910630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pott D.M., Vallarino J.G., Osorio S. Metabolite changes during postharvest storage: Effects on fruit quality traits. Metabolites. 2020;10:187. doi: 10.3390/metabo10050187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gazdik Z., Zitka O., Petrlova J., Adam V., Zehnalek J., Horna A., Reznicek V., Beklova M., Kizek R. Determination of vitamin C (ascorbic acid) using high performance liquid chromatography coupled with electrochemical detection. Sensors. 2008;8:7097–7112. doi: 10.3390/s8117097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rubinskiene M., Jasutiene I., Venskutonis P.R., Viskelis P. HPLC determination of the composition and stability of blackcurrant anthocyanins. J. Chromatogr. Sci. 2005;43:478–482. doi: 10.1093/chromsci/43.9.478. [DOI] [PubMed] [Google Scholar]
- 61.Sójka M., Janowski M., Grzelak-Błaszczyk K. Stability and transformations of raspberry (Rubus idaeus L.) ellagitannins in aqueous solutions. Eur. Food Res. Technol. 2019;245:1113–1122. doi: 10.1007/s00217-018-3212-3. [DOI] [Google Scholar]
- 62.Szőke É., Petroianu G., Tekes K., Benkő B., Szegi P., Laufer R., Veress G. HPLC monitoring of the microsomal stability of rutin and quercetin. Acta Chromatogr. 2009;21:399–410. doi: 10.1556/AChrom.21.2009.3.4. [DOI] [Google Scholar]
- 63.Octavia L., Choo W.S. Folate, ascorbic acid, anthocyanin and colour changes in strawberry (Fragaria × annanasa) during refrigerated storage. LWT. 2017;86:652–659. doi: 10.1016/j.lwt.2017.08.049. [DOI] [Google Scholar]
- 64.Hernández-Herrero J.A., Frutos M.J. Colour and antioxidant capacity stability in grape, strawberry and plum peel model juices at different pHs and temperatures. Food Chem. 2014;154:199–204. doi: 10.1016/j.foodchem.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 65.Gössinger M., Moritz S., Hermes M., Wendelin S., Scherbichler H., Halbwirth H., Stich K., Berghofer E. Effects of processing parameters on colour stability of strawberry nectar from puree. J. Food Eng. 2009;90:171–178. doi: 10.1016/j.jfoodeng.2008.06.018. [DOI] [Google Scholar]
- 66.Gil M.I., Holcroft D.M., Kader A.A. Changes in strawberry anthocyanins and other polyphenols in response to carbon dioxide treatments. J. Agric. Food Chem. 1997;45:1662–1667. doi: 10.1021/jf960675e. [DOI] [Google Scholar]
- 67.Kashchenko N.I., Olennikov D.N., Chirikova N.K. Ellagitannins in Rosaceous plants from the flora of Sakha (Yakutia) Republic. Butl. Commun. 2014;39:127–138. [Google Scholar]
- 68.Olennikov D.N., Kruglova M.Y. New quercetin glucoside and other phenolic compounds from Filipendula genus. Chem. Nat. Comp. 2013;49:524–529. doi: 10.1007/s10600-013-0691-0. [DOI] [Google Scholar]
- 69.Olennikov D.N. Free carbohydrates, glucofructans, and other polysaccharides from Rhaponticum uniflorum. Chem. Nat. Comp. 2018;54:751–754. doi: 10.1007/s10600-018-2462-4. [DOI] [Google Scholar]
- 70.Olennikov D.N., Zilfikarov I.N., Penzina T.A. Use of microcolumn HPLC for analysis of aloenin in Aloe arborescens raw material and related drugs. Pharm. Chem. J. 2013;47:494–497. doi: 10.1007/s11094-013-0988-0. [DOI] [Google Scholar]
- 71.Olennikov D.N., Kashchenko N.I., Chirikova N.K., Gornostai T.G., Selyutina I.Y., Zilfikarov I.N. Effect of low temperature cultivation on the phytochemical profile and bioactivity of Arctic plants: A case of Dracocephalum palmatum. Int. J. Mol. Sci. 2017;18:2579. doi: 10.3390/ijms18122579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Olennikov D.N., Chirikova N.K., Okhlopkova Z.M., Zulfugarov I.S. Chemical composition and antioxidant activity of Tánara Ótó (Dracocephalum palmatum Stephan), a medicinal plant used by the North-Yakutian nomads. Molecules. 2013;18:14105–14121. doi: 10.3390/molecules181114105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pellegrini N., Serafini M., Colombi B., Del Rio D., Salvatore S., Bianchi M., Brighenti F. Total antioxidant capacity of plant foods, beverages and oils consumed in Italy assessed by three different in vitro assays. J. Nutr. 2003;133:2812–2819. doi: 10.1093/jn/133.9.2812. [DOI] [PubMed] [Google Scholar]
- 74.Thaipong K., Boonprakob U., Crosby K., Cisneros-Zevallos L., Byrne D.H. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Comp. Anal. 2006;19:669–675. doi: 10.1016/j.jfca.2006.01.003. [DOI] [Google Scholar]
- 75.Olennikov D.N., Kashchenko N.I., Chirikova N.K. Meadowsweet teas as new functional beverages: Comparative analysis of nutrients, phytochemicals and biological effects of four Filipendula species. Molecules. 2017;22:16. doi: 10.3390/molecules22010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.