Abstract
Two species of microsporidia, Nosema apis and Nosema ceranae, are obligate intracellular parasites that are widespread in the world and cause the infectious disease (Nosemosis) of the Western honey bee Apis mellifera. Information on the prevalence and distribution of Nosema species in North Asia conditions is scarce. The main aim of the present study is to determine the prevalence of Nosema spp. (Nosemosis) in honey bees inhabiting some inland regions of North Asia (Western and Eastern Siberia, Altai Territory, Russia, and northeastern part of Kazakhstan). The objective of the paper is also to assess the influence of climatic factors on the spread of N. ceranae. Eighty apiaries in four ecological regions of North Asia (southern taiga, sub-taiga zone, forest steppe, and mountain taiga forests) were investigated with regard to distribution, prevalence, and diversity of Nosema infection in honey bees using duplex-PCR. Nosema infected bees were found in 65% apiaries of ecoregions studied, and coinfection was predominant (36.3% of Nosema-positive apiaries). Both N. apis and N. ceranae occur across subarctic and warm summer continental climates, but while N. apis predominates in the former, N. ceranae is more predominant in the latter. No statistically significant differences in Nosema distribution were identified in various climatic zones. In the sub-taiga zone (subarctic climate), low presence of colonies with pure N. ceranae and a significantly higher proportion of coinfection apiaries were revealed. Long-term epidemiological study of Nosema spp. prevalence in the sub-taiga zone showed a surprising percentage increase of Nosema-positive apiaries from 46.2% to 74.1% during 2012–2017. From 2012 to 2015, N. apis became a predominant species, but in 2016–2017, the coinfection was mainly detected. In conclusion, the results of this investigation showed that N. ceranae is widespread in all study ecoregions of North Asia where it exists in combination with the N. apis, but there is no replacement of N. apis by N. ceranae in the studied bee populations.
Keywords: honey bee, Apis mellifera, Nosema ceranae, Nosema apis, epidemiology, replacement, ecoregions, North Asia
1. Introduction
In Western honey bees, two species of Microsporidia causing nosemosis have been described: Nosema apis Zander, 1909 [1] and Nosema ceranae Fries et al., 1996 [2]. The microsporidian N. apis, a relatively benign pathogen, was considered to be the only causative agent of nosemosis in Apis mellifera for a long time [3]. Since 2006, a second Nosema species, N. ceranae, was identified in A. mellifera populations worldwide [4,5,6,7,8,9,10,11,12,13]. It should be noted that N. ceranae had been described as a microsporidian parasite of the Eastern honey bee Apis cerana in 1996 [2]. The parasite N. apis, responsible for nosemosis type A, is characterized by moderate virulence (mainly nosemosis is accompanied by dysentery), and bee colonies can often cure themselves under favorable environmental conditions [3,6]. N. ceranae, responsible for nosemosis type C, has been associated with reduced honey production, weakness, and increased colony mortality not preceded by any visible symptoms [14,15,16]. Nosema species also differ morphologically, genetically, in their ability to adapt to temperature, survival, and effects on the host [17,18,19,20,21,22,23,24]. For N. ceranae, a relatively new parasite for the A. mellifera, data on the virulence, the prevalence in different climates, as well as the role and effect on the viability and survival of bee colonies are contradictory [14,19,25,26,27,28,29,30].
Conflicting results from different studies can be attributed to many factors, such as the biological characteristics of honey bees (caste, age of the bees, commercially and traditionally managed bees) [31,32], the genetic diversity of honey bees, bee subspecies and lineages [24,33,34,35], climatic and environmental differences [7,18,36], beekeeping practices [10], as well as diagnostic methods [37] and research conditions (number of analyzed bees, time and method of sampling, natural population research or experiment) [31,38,39]. Nosema species can only be confirmed using molecular methods [39], which can have different levels of resolution, for example, the single-copy Hsp70 gene method qPCR detects a lower amount of N. ceranae copies compared to the multicopy 16S rRNA gene method [37]. When conducting experimental infection studies, the laboratory results may be affected by specific conditions such as the duration of the experiment, the temperature and humidity, the method of infection, number of bees in the cages, the type of their diet, etc. [31,39].
To understand the mechanisms of Nosema disease and the effect of N. ceranae infection on the host it is necessary to study the prevalence and distribution of Nosema species, primarily N. ceranae, in different regions and climatic zones and the long-term dynamics of infection of honey bees with various Nosema species.
The prevalence of Nosema species in A. mellifera populations in Eurasia is currently studied in most European countries [6,25,40,41,42] and some countries of Southwest Asia as well [29,43,44,45,46,47]. As the sole causative agent of nosemosis, N. ceranae was detected in Croatia [48], Central Italy [49,50], Iran [43,47], and Saudi Arabia [45]. In South-European countries, such as Italy [6,49,50] and Greece [25], N. ceranae had indeed practically replaced N. apis while this was not observed in Northern Europe (Ireland, Sweden, Norway, and Germany) [30,38]. For example, in Sweden, in the spring of 2007, 89.0% of Nosema-positive bee colonies had N. apis and 10.0% colonies had mixed N. apis/N. ceranae infections [38]. On the contrary, in Scotland, 86.2% of Nosema-positive bee colonies had mixed infections [40]. In Germany, in 2009, three infection categories were widespread: 48.5% Nosema-positive bee colonies were with N. apis, 33.8% were infected with N. ceranae, and 17.6% had mixed infection [27].
Climate is considered to be one of the main factors in the spread of Nosema species [26,30,49]. In warmer climates, N. ceranae is more competitive than N. apis; in contrast, in cold climates, N. ceranae spores appear to be much more vulnerable than the N. apis spores [26]. Laboratory data also suggest that the spread of N. ceranae across the globe is reduced in colder climates as N. ceranae spores are capable of surviving high temperatures and desiccation but are intolerant of cold [17,18,27,36,51]. However, the impact of weather conditions on the distribution of microsporidian Nosema, primarily N. ceranae, in the field is poorly understood [26].
The different sensitivity to temperatures in the Nosema species may be a potential explanation for the wider N. ceranae prevalence in warmer (subtropical) climate (Southern Europe, for example, the Mediterranean countries) compared to N. apis, which is more prevalent in temperate climates (Northern countries) [26]. Today, bees are threatened, and the cause of the problem is still unknown, which is why it is being described as Colony Collapse Disorder (CCD). Researchers suspect this may be due to a combination of various diseases including N. ceranae, environmental pollution, and farming practices, mainly due to large monoculture cropping and toxic phytosanitary products [52,53,54]. For the first time in Spain, bee colony mortality was clearly attributed to N. ceranae infection [7,14,15,16,55,56]. In 2004–2006, the prevalence of N. ceranae in dead bee colonies was about 90% [7]. It is assumed that colony collapse caused by N. ceranae is not restricted to Spain but is a global problem, at least it is regarded as a Europe-wide phenomenon. For example, in some Mediterranean countries, such as Greece [25], Israel [29], and Turkey [44,46], bee colony losses are also associated with N. ceranae infections. However, in Northern Europe, colony collapse could not be associated with N. ceranae [27,57]. These data pointed to climatic factors differentially influencing the prevalence and the virulence of N. ceranae in Europe [26,27,28] and/or differences in N. ceranae susceptibility between regionally predominating A. mellifera subspecies [24,34,39].
The prevalence of N. ceranae in more severe conditions in the continental regions of North Asia has not been adequately studied yet [13,58,59]. The purpose of this study was to determine the prevalence of Nosema infection in honey bees inhabiting some ecological regions of North Asia (southern taiga, sub-taiga zone, forest steppe taiga, and mountain taiga forests) and assess climatic factors that influence prevalence.
2. Materials and Methods
2.1. Ecological and Geographical Characteristics of the Region
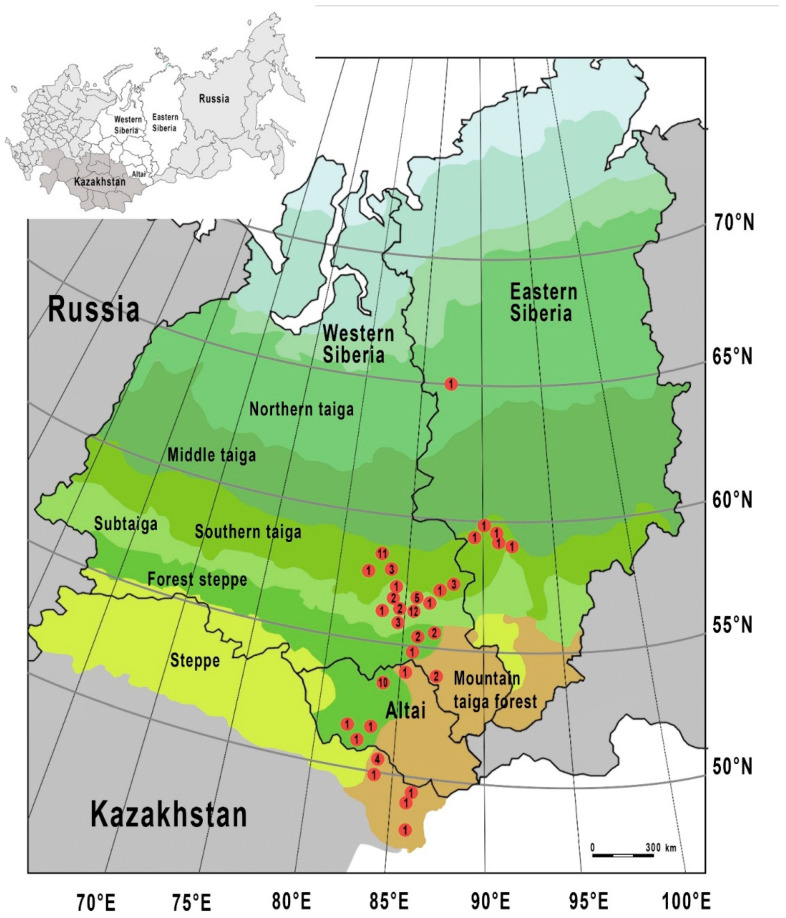
We explored the Nosema infestation of honey bees in several regions of North Asia (Western and Eastern Siberia, Altai Territory, Russia, and northeastern part of Kazakhstan). The study apiaries were located in an area extending from 48° N to 65° N in the meridional direction and from 81° E to 92° E in the zonal direction (Figure 1).
Figure 1.
Distribution of monitored apiaries within the study ecoregions of North Asia (southern taiga, sub-taiga zone, forest steppe taiga, and mountain taiga forests). The number of the examined apiaries in the locality is indicated by numbers.
In this region, located at a significant distance from the ocean, the climate is regarded as a continental one (Köppen climate classification). The characteristic features of the continental climate are cold winters and hot summers. Air masses are usually cold in winter, warm in summer, but always relatively dry continental. The average temperature is above 10 °C in the warmest months, and during the coldest month, it is on the average below 0 °C. The annual temperature amplitudes can be 70 °C. The vegetation period in the regions of North Asia is short, wintering of honey bees is long (it usually lasts for 6 months, but sometimes it is up seven months).
In North Asia, various ecological regions are represented (Figure 1). We investigated apiaries located in 4 ecoregions, namely southern taiga, sub-taiga zone, forest steppe taiga, and mountain taiga forests (Table 1). In these regions, beekeeping is well-developed, especially there are lots of apiaries in the sub-taiga zone, forest steppe taiga, and mountain taiga forests. In the southern taiga, there are many apiaries, but they are located in far-away areas. In addition, we also investigated one apiary (near Turukhansk, 65°47′35″ N, 87°57′44″ E) located in the Northern taiga (the north of Eastern Siberia; Figure 1). Beekeeping is not well-developed in this ecoregion due to a very cold climate, but there exist single apiaries in that area [59].
Table 1.
Geographic and climatic characteristics of the study ecoregions of North Asia.
| Ecological Region | Area Coordinates | Climate (Group D) # | Altitude, m | Average Temperature (°C) | Average Annual Precipitation, mm |
Frost-Free Period (Days) | Σt, Degree Days * |
||
|---|---|---|---|---|---|---|---|---|---|
| Annual | In January | In July | |||||||
| Southern taiga (boreal forest) | from 57°00′00″–59°50′00″ to 82°39′00″–92°08′00″ |
Subarctic climate | 60–130 | −0.9–(−2.0) | −19.8–(−21.6) | 17.9–18.3 | 250–500 | 90–120 | 1472–1820 |
| Sub-taiga zone | from 56°15′00″–56°48′21″ to 83°58′00″–86°42′00″ |
Subarctic climates | 90–120 | −0.6 | −19.2 | 18.1 | 350–550 | 100–120 | 1650–1800 |
| Forest steppe | from 51°00′00″–55°19′59″ to 81°28′00″–85°30′00″ |
Warm summer continental climate | 200–250 | 2.1–2.6 | −16–(−19) | 18–20 | 350–450 | 120–123 | 1900–2100 |
| Mountain taiga forests | from 48°34′03″–53°45′00″ to 82°18′25″–87°07′00″ |
Warm summer continental climate | 290–1685 | 0.3–2.4 | −12.6–(−24.0) | 17–22 | 500–900 | 123–135 | 2200–2400 |
# Köppen climate classification was used. * Degree days are a specialist kind of weather data, calculated from readings of outside air temperature.
Selected apiaries were situated in both flatland (southern taiga, sub-taiga zone, forest steppe taiga) and mountainous (mountain taiga forests) parts under different climatic conditions. The southern taiga and sub-taiga are characterized by a subarctic climate, while the forest steppe taiga and mountain taiga forests are distinguished by a warm summer continental climate (Table 1). Although the southern taiga and sub-taiga are characterized by a subarctic climate, there are some differences in the average monthly and average annual temperatures, sum of active temperatures above +10.0 °C (Σt). For the southern taiga zone, significant variability of these indicators is observed. Similarly, for zones with a warm summer continental climate (forest steppe taiga and mountain taiga forests), a significant range of the main climatic indicators is surveyed in the zone of mountain taiga forests (Table 1).
2.2. Historical Background
In Siberia and Altai Territory, the honey bee was introduced about 230 years ago. It was a dark forest bee Apis mellifera mellifera that well-adapted to the local climate and plant communities as well. The honey bee population is an artificial population whose wintering is controlled by people [60]. The first apiaries were formed in the mountainous regions of Western Siberia and Altai Territory. Subsequently, the development of beekeeping took place in Eastern Siberia, namely in the southern taiga, sub-taiga, and forest steppe.
2.3. Research Algorithm
It should be noted that we have conducted our research according to two lines. The first line of research is the study of Nosema infection of honey bees in apiaries in North Asia. A total of 80 distant apiaries located in four ecoregions (southern taiga, sub-taiga zone, forest steppe taiga, and mountain taiga forests) were monitored for Nosema infection between spring 2016 and autumn 2017 (for most apiaries, the material was mainly collected in the early summer due to the long cold spring; usually after the first flight of bees). We examined 24 apiaries in the southern taiga, 27 apiaries in sub-taiga, 18 apiaries in forest steppe taiga, and 11 apiaries in the mountain taiga forests (Figure 1). In addition, in the northern taiga, one apiary near Turukhansk was also investigated. Within each apiary, a minimum of three colonies (10% of the total colonies) were randomly selected with regard to Nosema detection.
The second line of research is connected with a retrospective analysis (a long-term dynamics) of Nosema infestation of apiaries in the sub-taiga zone in 2012–2017. A total of 79 distant apiaries were analyzed. For a retrospective study, we used bee samples collected in sub-taiga apiaries from 2012 to 2017 and stored them in a biobank at a temperature of −20 °C.
It is worth pointing out that the apiaries were visited only once. In each sampling, forager bees were collected at the entrance of each sampled hive. For each bee sample, the beekeeper provided information, including the location, history and other characteristics of the apiary, the origin of bees, the incidence of bee colonies, etc. No hives of the apiaries sampled had a history of external signs referable to nosemosis and no signs of the disease were present at the time of sampling. Mass death of bees after wintering in the study apiaries was not observed. Between 60 to 70 workers from each bee colony were pooled and used for DNA isolation. The presence of nosemosis in the bee colony was examined using a polymerase chain reaction (PCR).
2.4. Experimental Procedures
DNA was extracted from the midgut of bees (a pool of 60–70 individuals was formed) using a DNA purification kit, PureLink™ Mini (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. After the DNA extraction, the samples were submitted to duplex-PCR [7]. For the diagnosis of two Nosema species, we used two types of primers. The primer sequences used to amplify the 321 bp fragment corresponding to the 16S ribosomal gene of N. apis were 321APIS-FOR 5′-GGGGGCATGTCTTTGACGTACTATGTA-3′ and 321APIS-REV 5′- GGGGGGCGTTTAAAATGTGAAACAACTATG-3′. The primer sequences utilized to amplify the 218 bp fragment corresponding to the 16S ribosomal gene of N. ceranae were 218MITOC-FOR 5′-CGGCGACGATGTGATATGAAAATATTAA-3′ and 218MITOC-REV 5′-CCCGGTCATTCTCAAACAAAAAACCG-3′ [7].
PCR was performed using a thermal MyCycler T100 (BioRad, Foster City, CA, USA) in a reaction volume of 20 μL containing 1 μL of template DNA, 1× PCR buffer, 1.5 mM MgCl2, 200 μM of each dNTP, 0.2 μM of each forward and reverse primer and 1U Taq polymerase (Fermentas, Thermo Fisher Scientific, Chelmsford, MA, USA). The routine consisted of an initial denaturation step at 94 °C for 2 min, followed by 35 cycles of 94 °C for 30 s, 58 °C for 30 s and 72 °C for 1 min, and a final extension step at 72 °C for 5 min. PCR products were analyzed on 1.5% (m/v) agarose gels. Gels were stained with ethidium bromide and visualized using UV illumination (Gel Doc XR+, BioRad, Foster City, CA, USA). All analyses were carried out in duplicate and identical results were obtained. For each PCR, positive control (reference N. apis and N. ceranae DNA extracts as template) was used. Negative control (ddH2O) was also included in each run of PCR amplification to detect possible contamination.
If only N. ceranae or only N. apis were detected in all examined bees, the apiary infection category was pure N. ceranae or pure N. apis, respectively. An apiary was considered coinfected if both Nosema species were detected in honey bees.
2.5. Meteorological Data
To study the climatic characteristics of ecoregions (Table 1), we used reference material [61,62,63] including the average indicators of meteorological observations over a long-term period (for 30 years), namely, the average annual temperature and average annual precipitation, the average temperature in January and July, the duration of the frost-free period and the sum of active temperatures (Σt).
To assess long-term temporal trends in the prevalence of two Nosema species in the sub-taiga zone and analyze the associations of the prevalence of Nosema infection with climatic factors, we used the meteorological data obtained by the Tomsk weather station, 56°29′ N, 84°56′ E [62]. The following data were employed: average annual temperatures, average daily temperatures, duration of the period with active temperatures, sum of precipitation just for the same period of the year. Reference data were used to calculate two parameters: sums of active temperatures (Σt) and hydrothermal coefficient (HTC).
The sum of active temperatures is one of the main indicators of the territory’s thermal resources. Active temperatures designate daily average temperatures above +10.0 °C. The sum of the active temperatures (Σt) is calculated for a period with an average daily air temperature above +10.0 °C.
To characterize the moisture supply (humification conditions) in the area concerned, we used the hydrothermal coefficient suggested by Selyaninov (HTC) [64]. The hydrothermal coefficient (K) is calculated as the ratio of precipitation (R) to the sum of temperatures (Σt) for a period with temperatures above +10.0 °C: K = R*10/Σt. At HTC values 1.1–1.4, moisture supply is regarded as optimal; at values 0.76–1.0 it is insufficient, and at values 1.41–1.5 it is an elevated one.
2.6. Statistical Analysis
The results of the research are presented using numbers and percentages. Statistical significance of qualitative data was determined using the Chi-square test or the Z-test for proportions. To compare apiaries infected and uninfected with Nosema between different ecoregions, we used a two-sample proportion Z-test. The Chi-square test was used to compare the incidence of Nosema infection in the sub-taiga zone between three sampling years. In the case of a small number of one of the comparison classes, we used the chi-square test with Yates’ correction. Nosema-positive, N. apis-positive, N. ceranae-positive, and coinfected apiaries were subgroups to be compared. Statistical analysis was performed using the Statistica program.
3. Results
3.1. Infestation of Apiaries with Nosema Species in Four Ecoregions of North Asia in 2016–2017
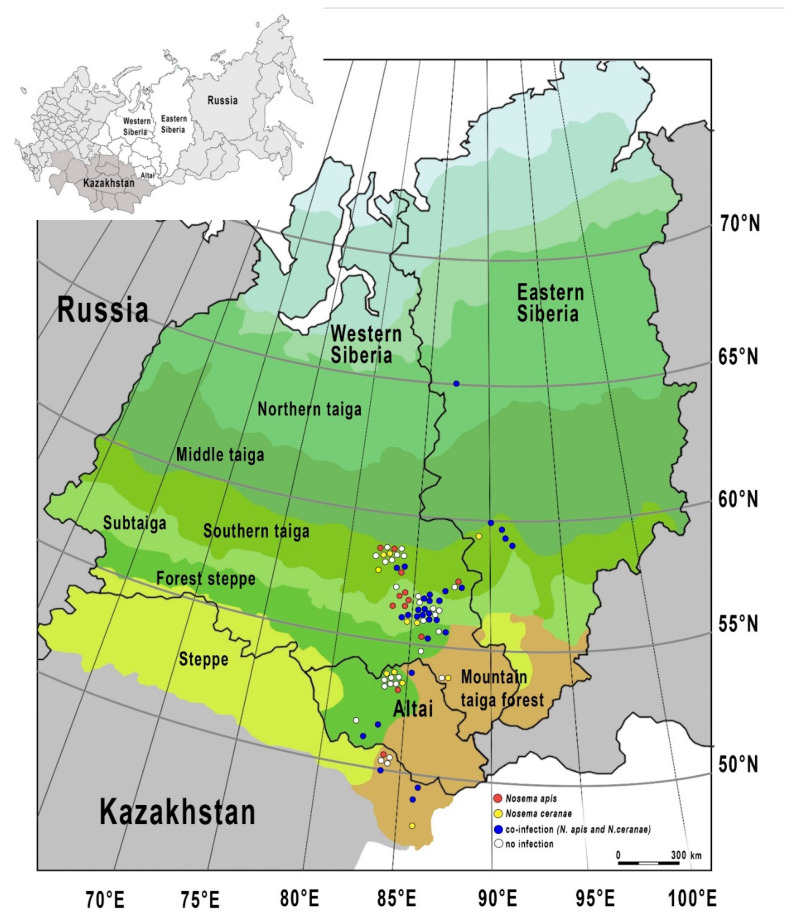
Honey bee samples collected in 2016–2017 from 80 apiaries of several ecological regions of North Asia were examined for the presence of Nosema infections (Table 2). In all the ecoregions of North Asia studied, two species of Microsporidia were registered in honey bees: N. apis and N. ceranae. In total, among 80 apiaries which had been analyzed, Nosema spp. were detected in 52 honey bee apiaries (65.0%): 15.0% of Nosema-positive apiaries were infected with only N. apis; 13.75%—only N. ceranae; in most apiaries (36.25%), both Nosema species were detected. In general, in North Asia, the infestation of apiaries with both types of Nosema (coinfection) was higher compared to the infestation of apiaries with only N. apis or only N. ceranae, but statistically significant differences were not shown (proportion Z-test; Z = 1.31, p > 0.05).
Table 2.
Prevalence of colonies infected with only N. apis (N. apis) or only N. ceranae (N. ceranae) or coinfection (N. apis and N. ceranae) in apiaries in several ecoregions of North Asia from spring 2016 to autumn 2017.
| Ecoregion | Total Number of Analyzed Apiaries | Nosema Infection not Detected | Infected Apiaries (Infection Categories) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N. apis | N. ceranae | N. apis and N. ceranae | |||||||
| N | % | N | % | N | % | N | % | ||
| Southern taiga | 24 | 8 | 33.33 | 4 | 16.67 | 4 | 16.67 | 8 | 33.33 |
| Sub-taiga zone | 27 | 7 | 25.93 | 5 | 18.52 | 2 | 7.41 | 13 | 48.15 |
| Forest steppe | 18 | 9 | 50.00 | 2 | 11.11 | 3 | 16.67 | 4 | 22.22 |
| Mountain taiga forests | 11 | 4 | 36.36 | 1 | 9.10 | 2 | 18.18 | 4 | 36.36 |
| Total | 80 | 28 | 35.00 | 12 | 15.00 | 11 | 13.75 | 29 | 36.25 |
The total number of analyzed apiaries along with the numbers (n) and proportions (%) of apiaries within each infection category are presented.
In all study ecoregions of North Asia (southern taiga, sub-taiga, forest steppe, and mountain taiga forests), a significant number of apiaries infected with Nosema were detected: ranging from 50.0% of apiaries in the forest steppe zone to 74.1% in the sub-taiga zone (Figure 2).
Figure 2.
Prevalence of Nosema infections in several ecoregions of North Asia (southern taiga, sub-taiga, forest steppe, and mountain taiga forests).
At the same time, the distribution of Nosema species differed in the study ecoregions (Table 2). We detected three possible infection categories of apiaries: only N. apis infection, only N. ceranae infection, and coinfection of two Nosema species. coinfection prevailed in apiaries of all the ecoregions and it was detected in 65.0% of Nosema-positive apiaries (Figure 2).
The spread of Nosema infection in apiaries of various ecoregions was as follows:
-
(i)
In the southern taiga, the number of apiaries infected with either N. apis or N. ceranae was the same (16.7%).
-
(ii)
In the forest steppe and mountain taiga forests (similar climatic conditions—warm summer continental climate), the spread of Nosema species is similar: 9.1% and 11.1% of apiaries with only N. apis; 16.7% and 18.2% of apiaries with only N. ceranae; 22.2% and 36.4% of apiaries with coinfection (N. apis and N. ceranae), respectively (proportion Z-test; Z < 0.39, p > 0.05).
-
(iii)
In the sub-taiga zone, the spread of Nosema infection in apiaries is different from that in the other ecoregions. The smallest number of apiaries with only N. ceranae (7.4%) and the highest number of apiaries with mixed infection (48.2%) were revealed. In addition, about a quarter of apiaries (18.5% of Nosema-positive apiaries) are infected only with N. apis (similar to the southern taiga zone; subarctic climate).
However, no statistically significant differences in the distribution of both common Nosema infections and individual Nosema infection (pure N. apis, pure N. ceranae, coinfection) were found between the ecological regions (Z < 1.76, p > 0.05). Additionally, no statistically significant differences in Nosema distribution were identified in various climatic zones (subarctic climate and warm summer continental climate; Z < 1.52, p > 0.05).
3.2. Dynamics of Infection of Apiaries with Different Nosema Species in Sub-Taiga from 2012 to 2017
Despite the fact that no significant differences were found in the incidence of Nosema infection both between ecological regions and climates (probably due to the small number of study apiaries), there exist some trends in research. For example, a difference in Nosema prevalence characteristic of the two groups of apiaries with coinfection between the sub-taiga and forest steppe zones approached the level of statistical significance (proportion Z-test; Z = 1.76, p < 0.10).
To gain a more complete understanding of the spread of Nosema infections in the sub-taiga zone, we examined long-term temporal trends in the prevalence of two Nosema species from 2012 to 2017 (Table 3).
Table 3.
Spreading of Nosema species in apiaries of the sub-taiga zone from 2012 to 2017.
| Study Period | Total Number of Analyzed Apiaries | Nosema Infection not Detected | Infected Apiaries (Infection Categories) | ||
|---|---|---|---|---|---|
| N. apis | N. ceranae | Coinfection | |||
| 2012–2013 | 26 | 53.85 | 42.31 | 3.85 | 0 |
| 2014–2015 | 26 | 42.31 | 42.31 | 3.85 | 11.54 |
| 2016–2017 | 27 | 25.93 | 18.52 | 7.41 | 48.15 |
| Total | 79 | 40.51 | 34.18 | 5.06 | 20.25 |
In the course of six years from 2012 to 2017, there was a surprising increase in Nosema infection in the sub-taiga zone (Table 3). The percentage of Nosema-positive apiaries increased from 46.1 to 57.7% over a period of the first four years to 74.1% during the last two years (χ2 = 4.316, p = 0.038). If in 2012–2015, N. apis was predominant among the other infectious categories compared to 2016–2017 (χ2 = 10.800, p = 0.002) whereas, in 2016–2017, coinfection (N. apis and N. ceranae) was mainly detected among Nosema-positive groups (χ2 = 5.298, p = 0.022; Table 3).
Three various periods with regard to the frequency of coinfection apiaries were established in the following order: from 2012 to 2013, from 2014 to 2015, and from 2016 to 2017. During the first period (2012–2013), no apiary with coinfection was detected. In the course of the second period (2014–2015), a small number of apiaries with coinfection was found (11.5%). Interestingly, the number of apiaries, either with only N. apis (42.3%), or with only N. ceranae (3.9%), was the same during these study periods (2012–2013, and 2014–2015). Finally, in the course of the third period (2016–2017), coinfection was identified in about half of the study apiaries (48.2%), which is statistically different from the 2014–2015 period (χ2 = 6.776, p = 0.01). In addition, the number of apiaries where only N. apis was diagnosed decreased by half (from 42.3 to 18.5%; χ2 = 3.557, p = 0.06). In 2016–2017, the smallest number of apiaries was detected with only N. ceranae (7.4%) but it doubled during the observation period, from 3.9% to 7.4% (χ2 = 0.001, p = 0.97).
We tried to assess the influence of both climatic factors separately (temperature, humidity) and the integrated indicator “hydrothermal coefficient” (HTC) on the spread of Nosema infection in the sub-taiga zone in 2012–2017.
An increase in the Nosema infection of honey bees and the spread of N. ceranae in the sub-taiga zone may be associated with an increase in the duration and heat supply of the active life period of biological objects from 2012 to 2017 (Table 4). On the whole, the indicators of the temperature regime in the sub-taiga zone from 2012 to 2017 reflect a general tendency of warming of a landing air layer in the Russian territory. So, average annual temperature deviations from average long-term values toward an increase by 1.2–3.1 °C were observed in the study area. The biggest deviations were identified in 2013 and from 2015 to 2017. What is more, abnormally high indicators of the vegetation period heat supply were observed in 2012 and in 2015–2017. For example, in 2016, the sum of active temperatures (Σt) comprised 2217 degree days in view of a basic value of 1650–1800 degree days.
Table 4.
Temperature regime and humification conditions in the sub-taiga zone according to observations made at the Tomsk weather station, 56°29’ N, 84°56’ E.
| Year of Study | Average Annual Temperature (°C) |
Σt, Degree Days * |
Period with Active Temperatures, Days | Amount of Precipitation for the Period with Active Temperatures, mm | Hydrothermal Coefficient (HTC) |
|---|---|---|---|---|---|
| 2012 | 0.6 | 2064 | 121 | 199 | 0.96 |
| 2013 | 1.7 | 1587 | 86 | 200 | 1.26 |
| 2014 | 0.8 | 1590 | 90 | 162 | 1.01 |
| 2015 | 2.5 | 2107 | 128 | 277 | 1.31 |
| 2016 | 1.7 | 2217 | 128 | 203 | 0.92 |
| 2017 | 2.2 | 1912 | 124 | 285 | 1.49 |
* Degree days are a specialist kind of weather data, calculated from readings of outside air temperature. Σt—the sum of the temperatures for a period with an average daily air temperature above +10.0 °C. Active temperatures are average daily temperatures above +10.0 °C.
We also observe significant variations in such climatic indicators as the period with active temperatures and the hydrothermal coefficient (HTC) in the study area (Table 4). For example, in 2012–2017, the hydrothermal coefficient (HTC) ranged from 0.92 to 1.49. An alternating number of years with an insufficient period of moisture supply (2012, 2014, 2016) was followed by a period of optimal moisture supply. It is interesting to note that the moisture period of 2017 is characterized as an excessive one.
The year 2012 along presents a certain interest because it was characterized by high summer temperatures and a low moisture supply. During the active growing season, 86 cases of the absolute maximum air temperature exceeding in the course of one day were recorded. Most cases were reported in the south of Western Siberia, where abnormally hot weather was observed. For example, in the Tomsk Region (Western Siberia), a significant deviation temperature from the required standard (from 1.3 °C to 7.2 °C) was first observed in June–July over the past 60 years. In Tomsk in particular (56°29′ N, 84°56′ E), the temperature deviation from the average long-term standard came to 5.7 °C in June and 5.3 °C in July. According to the data of the Tomsk weather station, in June, the amount of precipitation was 54% according to the required standard, it comprised only 33% of the standard data. In Tomsk, in July, the HTC value accounted for 0.35, which was indicative of severe drought. That is the reason for the extreme meteorological conditions of 2012 which were quite different from those during a long-term observation period in this area [65].
Under these conditions, we expected a widespread occurrence of Microsporidia, primarily N. ceranae, in this region. However, in the 2012 beekeeping season, we did not find a single bee colony infected with Nosema spores, and the bees were distinguished by a high flying activity and a high honey productivity as well. Therefore, there is no need for making any assumptions relating to the impact of climatic conditions on the Nosema distribution in 2012. It is probably not the temperature, but the good resistance of the examined bee colonies to Microsporidia which is the determining factor.
In 2013–2014, the heat supply to the growing season was very low (86 and 90, respectively), whereas, in 2015–2017, the period with active temperatures was the longest (from 124 to 128 days). If in 2013–2015, in apiaries of the sub-taiga, N. apis was the predominant species, then in 2016–2017, coinfection was most common. None of the studied climatic indicators can be associated with the spread of Nosema, coinfection in particular, in the sub-taiga zone.
Probably, both biotic and abiotic factors are involved to a greater or lesser extent individually or synergistically. Environmental conditions can exert a direct impact on the parasite or indirectly influence them by altering host physiology, behavior, and immunity [36].
4. Discussion
We have investigated the spread of Nosema species in several ecological regions of North Asia (southern taiga, sub-taiga, forest steppe, and mountain taiga forests) and the influence of climatic conditions (subarctic and warm summer continental climates) on the prevalence of N. ceranae.
The geographic distribution of Nosema spp. in several ecoregions of North Asia can be represented as follows:
-
(i)
The presence of bee colonies infected with pure N. ceranae in all the study ecoregions;
-
(ii)
No significant differences were identified in the incidence of Nosema infection between subarctic and warm summer continental climates. There is only a trend towards a high proportion of apiaries with only N. apis infection in the subarctic climate, and vice versa, towards a higher proportion of apiaries with only N. ceranae infection in warm summer continental climate;
-
(iii)
Coinfection detected in most study apiaries of North Asia;
-
(iv)
A higher proportion of coinfection apiaries and a lower presence of colonies with pure N. ceranae in the sub-taiga zone (subarctic climate);
-
(v)
There is no replacement of N. apis by N. ceranae in the study honey bee populations of North Asia, but their coexistence is registered.
Our results show the widespread use of N. ceranae in all study ecoregions of North Asia, as has been documented in many regions of the world [66]. In addition, N. ceranae was found in the apiary in very cold climates in Northern taiga (near Turukhansk). Our epidemiology data clearly show that N. ceranae became successfully established and expanded its presence in honey bee populations in both the southern and northern regions of North Asia. It seems likely that changes in the spread of two Nosema species should be considered in the context of stable changes to the heat supply regime of the study area. Moreover, the spread of the parasite during a certain period is determined by the weather conditions of the previous period. For example, unfavorable conditions in 2013–2014 could have been the reason for a decrease in bee resistance to diseases and, consequently, for the spread of infection in subsequent years (2015–2017), characterized by an increase in heat supply during the growing season.
The increasing worldwide prevalence of N. ceranae in the past decade, and, conversely, a decrease in the N. apis prevalence (even the absence of this parasite) in some regions suggests that N. ceranae might be displacing N. apis [6,26,67]. However, while in Southern Europe, especially in the Mediterranean countries like Spain, Italy, Israel, Greece, and Turkey, N. ceranae has been the dominant species for 10 years [6,25,37,49], in Northern Europe (Ireland, Sweden, Norway, and Germany), N. apis is still the predominant species [27,30,38]. For example, in Sweden, the majority of bee colonies (89%) were infected only with N. apis, and in other bee colonies, coinfection was identified [38]. In Europe, there is a South to North gradient in the distribution of Nosema species, which can be determined by the climatic characteristics of the regions [6,27,30]. In European countries with hot summers and moderate winters, N. ceranae is predominant and nearly replaced N. apis over the past decade in Spain and Italy [5,6,7,14]. On the contrary, in countries with rather cold and long winters such as Sweden and Germany, N. apis is a prevailing species [27,30]. For example, in Northeast Germany, despite a significant increase in N. ceranae prevalence during the last 12 years, no replacement of N. apis by N. ceranae took place in the honey bee population. For replacement of N. apis by N. ceranae at the population level, a simple increase in N. ceranae infection prevalence is not sufficient but N. apis infection prevalence should have concomitantly decreased during the study period. However, in Northeast Germany, a significant decrease in N. apis infection prevalence was only observed in autumn, and no significant change in N. apis infection prevalence was found in spring [30].
In this study (for example, sub-taiga zona) and in our earlier studies of various regions of North Asia, for example the Tomsk Region [13], we also showed that starting from a very low level, the prevalence of N. ceranae infections significantly increased continuously in the observed honey bee populations over the last six years. However, unlike Northeast Germany, where values for coinfection prevalence of bee colonies ranged between 0.0% and 10.0% during 2005–2016 [30], in North Asia, coinfection is widespread and was found in more than 30% of apiaries. However, a similar distribution pattern of Nosema infection has been identified in North Asia and Scotland. So, in Scotland, in the 70.4% of the bee colonies, the presence of both N. ceranae and N. apis was detected [40]. Interestingly, an increasing gradient of coinfection from North to South was also observed in some countries of the southern hemisphere, for example, in Argentina: coinfection was more prevalent in regions with temperate (77.9%) as compared to those of subtropical climate (22.1%) [68].
The high prevalence of coinfection (N. ceranae and N. apis) in the studied ecoregions of North Asia may suggest that N. ceranae is moving from warm summer continental to subarctic climate regions. Despite the distribution of N. ceranae infection in more severe climatic conditions (subarctic climate), we revealed some trends in the prevalence of pure Nosema infections in subarctic and warm summer continental climates. In warm summer continental climate, pure N. ceranae infection predominates, while in subarctic climate, pure N. apis infection is widespread.
As recently demonstrated in laboratory studies, mixed infections (N. apis and N. ceranae) negatively affected honey bee survival more than single Nosema infections [69]. However, contrary to this publication, in Siberia, bee colonies living in nature in very cold climates for a long time were relatively healthy, and N. ceranae is not associated with colony depopulation or honey bee collapse.
To determine the possible causes of the observed pattern of Nosema infection in honey bees in North Asia, we studied the history of beekeeping in Siberia and Altai Territory, namely, bee diseases and registered cases of mass death of bee colonies. A characteristic feature of the honeybee populations in Siberia is their long-term habitation in an isolated area. Honey bees have adapted to very cold climates. Under these conditions, certain parasite–host relationships, including Microsporidia, were formed.
Previously, nosemosis in honey bees in Siberia and Altai Territory was attributed exclusively to N. apis. The first description of N. ceranae infection in bees in North Asia using molecular genetic methods refers to 2009: the Tyumen Region, Altai Territory [58], and the Tomsk Region [13,60]. In addition, we identify the N. ceranae in the A. m. mellifera bee colonies from the long-isolated apiaries in the far-away taiga (Yenisei population of Krasnoyarsk Krai, Eastern Siberia), where new bees have not been imported for more than 60 years [59]. Our results provide evidence that N. ceranae infection occurs in bee colonies living in cold climates in Siberia, and this parasite is not associated with a colony depopulation or honey bee collapse. The connection of a long wintering and the nosemosis caused by N. apis was noted, but cases of mass bee mortality in study areas of North Asia are rather rare [70,71].
The first case of mass mortality of bee colonies in Siberia from the southern taiga to the forest steppe and mountain forests was described in the 1880s. However, the reasons for such an occurrence remain unknown. The first documented cases of mass bee mortality from nosemosis date back to 1914–1917 [71]. The presence of the causative agent was confirmed both by the clinical picture and by the microcopy of the pathological material. It is interesting to note that in the following years (1980s), the most noticeable losses of bee colonies in Siberia were associated with the rapid spread of varroosis (Varroa destructor) [70]. In 2017, in Altai Territory, the mass bee mortality was marked at the end of wintering [72]. The most probable reason for the spread of diseases, including nosemosis, and, possibly, the mass bee mortality is bee hybridization because the southern bee subspecies and hybrids characterized by a decrease in immunity did not withstand a particularly prolonged wintering. It can be assumed that diseases, including nosemosis, are probably a consequence of bee hybridization. The intensive importation of honey bees from the southern regions of Russia had been practiced in the Western Altai since the 1940s. For the southern taiga and sub-taiga this process is typical for the last two decades. Probably, both biotic and abiotic factors are involved to a greater or lesser extent individually or synergistically. Environmental conditions can exert a direct impact on the parasite or indirectly influence them by altering host physiology, behavior, and immunity [36]. In addition to the geographic and climatic factors, the genetic features of the host may affect the coadaptation of the honey bee and Nosema parasite, and the Nosema spp. prevalence in honey bee populations [24,34,68]. It is assumed that the variation between bee colonies in susceptibility to infection by N. ceranae is linked to genetic variability in workers from resistance to tolerance [24,34,39].
Thus, the issues relating to the distribution of Nosema spp. and the consequences of infection for bee colonies have not been resolved yet. The virulence of N. ceranae could be influenced by climatic conditions [23,26,28] or might actually depend on honeybee race, honeybee genetic diversity [33,34,73,74,75], and multiple N. ceranae genetic variants resulting in different biological consequences [76]. In this regard, it is of considerable interest to study the long-term and seasonal dynamics of Nosema infections in bee colonies, the relationship between pathogens and honeybees taking into account their genetic features, and the role of nosemosis pathogens in mass mortality of honeybees. Evidently, extended and detailed research is urgently required in order to elucidate a complete effect of N. ceranae infection on A. mellifera colonies in various geographical and climatic areas. The protective mechanisms behind this pattern remain to be studied.
5. Conclusions
The results of the research have shown that coinfection of two Nosema species, N. apis and N. ceranae, is widespread in all the ecological regions of North Asia studied. In the sub-taiga, a significant increase in Nosema infection has been observed for the last 6 years. There is no replacement of N. apis by N. ceranae in the studied bee populations. Despite the distribution of N. ceranae infection in subarctic climate, we have revealed some trends in the prevalence of pure Nosema infections in subarctic climate (pure N. apis predominates) and warm summer continental climate (pure N. ceranae is widespread). Further long-term research will contribute to understanding the interaction between the two Nosema species and the role of various factors, primarily climate, in the spread of these parasites of honey bee populations.
Acknowledgments
The work would have been impossible without the help and assistance of Pilyukova A.V., a senior teacher of the Tomsk State University.
Author Contributions
Conceptualization, N.V.O. and O.L.K.; investigation, T.N.K. and S.A.R.; methodology, N.V.O., O.L.K., A.N.K., T.N.K. and S.A.R.; writing—original draft, N.V.O. and O.L.K.; writing—review and editing, N.V.O. and A.N.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Tomsk State University competitiveness improvement programme.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Zander E. Tierische Parasiten als Krankheitserreger bei der Biene. Münchener Bienenztg. 1909;31:196–204. [Google Scholar]
- 2.Fries I., Feng F., da Silva A., Slemenda S.B., Pieniazek N.J. Nosema ceranae n. sp. (Microspora, Nosematidae), morphological and molecular characterization of a microsporidian parasite of the Asian honey bee Apis cerana (Hymenoptera, Apidae) Eur. J. Protistol. 1996;32:356–365. doi: 10.1016/S0932-4739(96)80059-9. [DOI] [Google Scholar]
- 3.Fries I. Nosema apis—A parasite in the honey bee colony. Bee World. 1993;74:5–19. doi: 10.1080/0005772X.1993.11099149. [DOI] [Google Scholar]
- 4.Fries I., Martin R., Meana A., García-Palencia P., Higes M. Natural infections of Nosema ceranae in European honey bees. J. Apic. Res. 2006;47:230–233. doi: 10.3896/IBRA.1.45.4.13. [DOI] [Google Scholar]
- 5.Higes M., Martin R., Meana A. Nosema ceranae, a new microsporidian parasite in honeybees in Europe. J. Invertebr. Pathol. 2006;92:93–95. doi: 10.1016/j.jip.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Klee J., Besana A.M., Genersch E., Gisder S., Nanetti A., Tam D.Q., Chinh T.X., Puerta F., Ruz J.M., Kryger P., et al. Widespread dispersal of the microsporidian Nosema ceranae, an emergent pathogen of the western honey bee, Apis mellifera. J. Invertebr. Pathol. 2007;96:1–10. doi: 10.1016/j.jip.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 7.Martín-Hernández R., Meana A., Prieto L., Salvador A.M., Garrido-Bailon E., Higes M. Outcome of colonization of Apis mellifera by Nosema ceranae. Appl. Environ. Microbiol. 2007;73:6331–6338. doi: 10.1128/AEM.00270-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y., Evans J.D., Smith I.B., Pettis J.S. Nosema ceranae is a long-present and wide-spread microsporidian infection of the European honey bee (Apis mellifera) in the United States. J. Invertebr. Pathol. 2008;97:186–188. doi: 10.1016/j.jip.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 9.Williams G.R., Shafer A.B., Rogers R.E., Shutler D., Stewart D.T. First detection of Nosema ceranae, a microsporidian parasite of European honey bees (Apis mellifera), in Canada and central USA. J. Invertebr. Pathol. 2008;97:189–192. doi: 10.1016/j.jip.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 10.Giersch T., Berg T., Galea F., Hornitzky M. Nosema ceranae infects honey bees (Apis mellifera) and contaminates honey in Australia. Apidologie. 2009;40:117–123. doi: 10.1051/apido/2008065. [DOI] [Google Scholar]
- 11.Chen Y.P., Huang Z.Y. Nosema ceranae, a newly identified pathogen of Apis mellifera in the USA and Asia. Apidologie. 2010;41:364–374. doi: 10.1051/apido/2010021. [DOI] [Google Scholar]
- 12.Yoshiyama M., Kimura K. Distribution of Nosema ceranae in the European honeybee, Apis mellifera in Japan. J. Invertebr. Pathol. 2011;106:263–267. doi: 10.1016/j.jip.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 13.Ostroverkhova N.V., Kucher A.N., Golubeva E.P., Rosseykina S.A., Konusova O.L. Study of Nosema spp. in the Tomsk region, Siberia: Co-infection is widespread in honeybee colonies. Far East. Entomol. 2019;378:12–22. doi: 10.25221/fee.378.3. [DOI] [Google Scholar]
- 14.Higes M., Martín-Hernández R., Botías C., Bailón G.E., González-Porto A.V., Barrios L., del Nozal M.J., Bernal J.L., Jiménez J.J., García-Palencia P., et al. How natural infection by Nosema ceranae causes honeybee colony collapse. Environ. Microbiol. 2008;10:2659–2669. doi: 10.1111/j.1462-2920.2008.01687.x. [DOI] [PubMed] [Google Scholar]
- 15.Higes M., Martín-Hernández R., Garrido-Bailon E., Gonzalez-Porto A.V., Garcia-Palencia P., Meana A., Del Nozal M.J., Mayo R., Bernal J.L. Honeybee colony collapse due to Nosema ceranae in professional apiaries. Environ. Microbiol. Rep. 2009;1:110–113. doi: 10.1111/j.1758-2229.2009.00014.x. [DOI] [PubMed] [Google Scholar]
- 16.Botías C., Martín-Hernández R., Barrios L., Meana A., Higes M. Nosema spp. infection and its negative effects on honey bees (Apis mellifera iberiensis) at the colony level. Vet. Res. 2013;44:25. doi: 10.1186/1297-9716-44-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higes M., García-Palencia P., Botias C., Meana A., Martín-Hernández R. The differential development of microsporidia infecting worker honey bee (Apis mellifera) at increasing incubation temperature. Environ. Microbiol. Rep. 2010;2:745–748. doi: 10.1111/j.1758-2229.2010.00170.x. [DOI] [PubMed] [Google Scholar]
- 18.Martín-Hernández R., Meana A., Garcia-Palencia P., Marin P., Botías C., Garrido Bailon E., Barrios L., Higes M. Effect of temperature on the biotic potential of honeybee microsporidia. Appl. Environ. Microbiol. 2009;75:2554–2557. doi: 10.1128/AEM.02908-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forsgren E., Fries I. Comparative virulence of Nosema ceranae and Nosema apis in individual European honey bees. Vet. Parasitol. 2010;170:212–217. doi: 10.1016/j.vetpar.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 20.Martín-Hernández R., Botías C., Barrios L., Martinez-Salvador A., Meana A., Mayack C., Higes M. Comparison of the energetic stress associated with experimental Nosema ceranae and Nosema apis infection of honeybees (Apis mellifera) Parasitol. Res. 2011;109:605–612. doi: 10.1007/s00436-011-2292-9. [DOI] [PubMed] [Google Scholar]
- 21.Huang W.F., Solter L.F. Comparative development and tissue tropism of Nosema apis and Nosema ceranae. J. Invertebr. Pathol. 2013;113:35–41. doi: 10.1016/j.jip.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Chen Y.P., Pettis J.S., Zhao Y., Liu X., Tallon L.J., Sadzewicz L.D., Li R., Zheng H., Huang S., Zhang X., et al. Genome sequencing and comparative genomics of honey bee microsporidia, Nosema apis reveal novel insights into host-parasite interactions. BMC Genom. 2013;14:451–467. doi: 10.1186/1471-2164-14-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van der Zee R., Gomez-Moracho T., Pisa L., Sagastume S., Garcia-Palencia P., Maside X., Bartolomé C., Martín-Hernández R., Higes M. Virulence and polar tube protein genetic diversity of Nosema ceranae (Microsporidia) field isolates from Northern and Southern Europe in honeybees (Apis mellifera iberiensis) Environ. Microbiol. Rep. 2014;6:401–413. doi: 10.1111/1758-2229.12133. [DOI] [PubMed] [Google Scholar]
- 24.Huang W.-F., Solter L., Aronstein K., Huang Z. Infectivity and virulence of Nosema ceranae and Nosema apis in commercially available North American honey bees. J. Invertebr. Pathol. 2015;124:107–113. doi: 10.1016/j.jip.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 25.Bacandritsos N., Granato A., Budge G., Papanastasiou I., Roinioti E., Caldon M., Falcaro C., Gallina A., Mutinelli F. Sudden deaths and colony population decline in Greek honey bee colonies. J. Invertebr. Pathol. 2010;105:335–340. doi: 10.1016/j.jip.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 26.Fries I. Nosema ceranae in European honey bees (Apis mellifera) J. Invertebr. Pathol. 2010;103:S73–S79. doi: 10.1016/j.jip.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 27.Gisder S., Hedtke K., Möckel N., Frielitz M.C., Linde A., Genersch E. Five-year cohort study of Nosema spp. in Germany: Does climate shape virulence and assertiveness of Nosema ceranae? Appl. Environ. Microbiol. 2010;76:3032–3038. doi: 10.1128/AEM.03097-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Higes M., Martín-Hernández R., Martínez-Salvador A., Garrido Bailon E., González-Porto A.V., Meana A., Bernal J.L., Del Nozal M.J., Bernal J. A preliminary study of the epidemiological factors related to honey bee colony loss in Spain. Environ. Microbiol. Rep. 2010;2:243–250. doi: 10.1111/j.1758-2229.2009.00099.x. [DOI] [PubMed] [Google Scholar]
- 29.Soroker V., Hetzroni A., Yakobson B., David D., David A., Voet H., Slabezki Y., Efrat H., Levski S., Kamer Y., et al. Evaluation of colony losses in Israel in relation to the incidence of pathogens and pests. Apidologie. 2011;42:192–199. doi: 10.1051/apido/2010047. [DOI] [Google Scholar]
- 30.Gisder S., Schüler V., Horchler L.L., Groth D., Genersch E. Long-term temporal trends of Nosema spp. infection prevalence in northeast Germany: Continuous spread of Nosema ceranae, an emerging pathogen of honey bees (Apis mellifera), but no general replacement of Nosema apis. Front. Cell. Infect. Microbiol. 2017;7:301. doi: 10.3389/fcimb.2017.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Urbieta-Magro A., Higes M., Meana A., Barrios L., Martín-Hernández R. Age and method of inoculation influence the infection of worker honey bees (Apis mellifera) by Nosema ceranae. Insects. 2019;10:417. doi: 10.3390/insects10120417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taric E., Glavinic U., Vejnovic B., Stanojkovic A., Aleksic N., Dimitrijevic V., Stanimirovic Z. Oxidative stress, endoparasite prevalence and social immunity in bee colonies kept traditionally vs. those kept for commercial purposes. Insects. 2020;11:266. doi: 10.3390/insects11050266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bourgeois A.L., Rinderer T.E., Sylvester H.A., Holloway B., Oldroyd B.P. Patterns of Apis mellifera infestation by Nosema ceranae support the parasite hypothesis for the evolution of extreme polyandry in eusocial insects. Apidologie. 2012;43:539–548. doi: 10.1007/s13592-012-0121-5. [DOI] [Google Scholar]
- 34.Fontbonne R., Garnery L., Vidau C., Aufauvre J., Texier C., Tchamitchian S., El Alaoui H., Brunet J.L., Delbac F., Biron D.G. Comparative susceptibility of three Western honeybee taxa to the microsporidian parasite Nosema ceranae. Infect. Genet. Evol. 2013;17:188–194. doi: 10.1016/j.meegid.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 35.Mendoza Y., Antúnez K., Branchiccela B., Anido M., Santos E., Invernizzi C. Nosema ceranae and RNA viruses in European and Africanized honeybee colonies (Apis mellifera) in Uruguay. Apidologie. 2014;45:224–234. doi: 10.1007/s13592-013-0241-6. [DOI] [Google Scholar]
- 36.Fenoy S., Rueda C., Higes M., Martín-Hernández R., del Aguila C. High-level resistance of Nosema ceranae, a parasite of the honeybee, to temperature and desiccation. Appl. Environ. Microbiol. 2009;75:6886–6889. doi: 10.1128/AEM.01025-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cilia G., Cabbri R., Maiorana G., Cardaio I., Dall’Olio R., Nanetti A. A novel TaqMan® assay for Nosema ceranae quantification in honey bee, based on the protein coding gene Hsp70. Eur. J. Protistol. 2018;63:44–50. doi: 10.1016/j.ejop.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 38.Forsgren E., Fries I. Temporal study of Nosema spp. in a cold climate. Environ. Microbiol. Rep. 2013;5:78–82. doi: 10.1111/j.1758-2229.2012.00386.x. [DOI] [PubMed] [Google Scholar]
- 39.Martín-Hernández R., Bartolomé C., Chejanovsky N., Le Conte Y., Dalmon A., Dussaubat C., García-Palencia P., Meana A., Pinto M.A., Soroker V., et al. Nosema ceranae in Apis mellifera: A 12 years post-detection perspective. Environ. Microbiol. 2018;20:1302–1329. doi: 10.1111/1462-2920.14103. [DOI] [PubMed] [Google Scholar]
- 40.Bollan K.A., Hothersall J.D., Moffat C., Durkacz J., Saranzewa N., Wright G.A., Raine N.E., Highet F., Connolly C.N. The microsporidian parasites Nosema ceranae and Nosema apis are widespread in honeybee (Apis mellifera) colonies across Scotland. Parasitol. Res. 2013;112:751–759. doi: 10.1007/s00436-012-3195-0. [DOI] [PubMed] [Google Scholar]
- 41.Shumkova R., Georgieva A., Radoslavov G., Sirakova D., Dzhebir G., Neov B., Bouga M., Hristov P. The first report of the prevalence of Nosema ceranae in Bulgaria. PeerJ. 2018;6:e4252. doi: 10.7717/peerj.4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matović K., Vidanović D., Manić M., Stojiljković M., Radojičić S., Debeljak Z., Šekler M., Ćirić J. Twenty-five-year study of Nosema spp. in honey bees (Apis mellifera) in Serbia. Saudi J. Biol. Sci. 2020;27:518–523. doi: 10.1016/j.sjbs.2019.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nabian S., Ahmadi K., Nazem Shirazi M.H., Gerami Sadeghian A. First detection of Nosema ceranae, a microsporidian protozoa of European honeybees (Apis mellifera) in Iran. Iran. J. Parasitol. 2011;6:89–95. [PMC free article] [PubMed] [Google Scholar]
- 44.Tunca R.I., Oskay D., Gosterit A., Tekin O.K. Does Nosema ceranae wipe out Nosema apis in Turkey? Iran. J. Parasitol. 2016;11:259–264. [PMC free article] [PubMed] [Google Scholar]
- 45.Ansari M.J., Al-Ghamdi A., Adgaba N., Khan K.A., Alattal Y. Geographical distribution and molecular detection of Nosema ceranae from indigenous honey bees of Saudi Arabia. Saudi J. Biol. Sci. 2017;24:983–991. doi: 10.1016/j.sjbs.2017.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oğuz B., Karapinar E., Dinçer E., Değer M.S. Molecular detection of Nosema spp. and black queen-cell virus in honeybees in Van Province, Turkey. Turk. J. Vet. Anim. Sci. 2017;41:221–227. doi: 10.3906/vet-1604-92. [DOI] [Google Scholar]
- 47.Khezri M., Moharrami M., Modirrousta H., Torkaman M., Salehi S., Rokhzad B., Khanbabai H. Molecular detection of Nosema ceranae in the apiaries of Kurdistan province, Iran. Vet. Res. Forum. 2018;9:273–278. doi: 10.30466/vrf.2018.32086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tlak Gajger I., Vugrek O., Grilec D., Petrinec Z. Prevalence and distribution of Nosema ceranae in Croatian honeybee colonies. Vet. Med. 2010;55:457–462. doi: 10.17221/2983-VETMED. [DOI] [Google Scholar]
- 49.Papini R., Mancianti F., Canovai R., Cosci F., Rocchigiani G., Benelli G., Canale A. Prevalence of the microsporidian Nosema ceranae in honeybee (Apis mellifera) apiaries in Central Italy. Saudi J. Biol. Sci. 2017;24:979–982. doi: 10.1016/j.sjbs.2017.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cilia G., Sagona S., Giusti M., dos Santos P.E.J., Nanetti A., Felicioli A. Nosema ceranae infection in honeybee samples from Tuscanian Archipelago (Central Italy) investigated by two qPCR methods. Saudi J. Biol. Sci. 2019;26:1553–1556. doi: 10.1016/j.sjbs.2018.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sánchez Collado J.G., Higes M., Barrio L., Martín-Hernández R. Flow cytometry analysis of Nosema species to assess spore viability and longevity. Parasitol. Res. 2014;113:1695–1701. doi: 10.1007/s00436-014-3814-z. [DOI] [PubMed] [Google Scholar]
- 52.Chauzat M.P., Jacques A., Laurent M., Bougeard S., Hendrikx P., Ribière-Chabert M., EPILOBEE Consortium Risk indicators affecting honey bee colony survival in Europe: One year of surveillance. Apidologie. 2016;47:348–378. doi: 10.1007/s13592-016-0440-z. [DOI] [Google Scholar]
- 53.Seitz N., Traynor K.S., Steinhauer N., Rennich K., Wilson M.E., Ellis J.D. A national survey of managed honey bee 2014–2015 annual colony losses in the USA. J. Apic. Res. 2016;54:292–304. doi: 10.1080/00218839.2016.1153294. [DOI] [Google Scholar]
- 54.Gomes T., Feás X., Iglesias A., Estevinho L.M. Study of organic honey from the Northeast of Portugal. Molecules. 2011;16:5374–5386. doi: 10.3390/molecules16075374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cepero A., Ravoet J., Gómez-Moracho T., Bernal J.L., del Nozal M.J., Bartolomé C., Maside X., Meana A., González-Porto A.V., de Graaf D.C., et al. Holistic screening of collapsing honey bee colonies in Spain: A case study. BMC Res. Notes. 2014;7:649. doi: 10.1186/1756-0500-7-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meana A., Llorens-Picher M., Euba A., Bernal J.L., Bernal J., García-Chao M., Dagnac T., Castro-Hermida J.A., González-Porto A.V., Higes M., et al. Risk factors associated with honey bee colony loss in apiaries in Galicia, NW Spain. Span. J. Agric. Res. 2017;15:e0501. doi: 10.5424/sjar/2017151-9652. [DOI] [Google Scholar]
- 57.Genersch E., Der Ohe W.V., Kaatz H., Schroeder A., Otten C., Büchler R., Berg S., Ritter W., Mühlen W., Gisder S., et al. The German bee monitoring project: A long term study to understand periodically high winter losses of honey bee colonies. Apidologie. 2010;41:332–352. doi: 10.1051/apido/2010014. [DOI] [Google Scholar]
- 58.Tokarev Y.S., Zinatullina Z.Y., Ignatieva A.N., Zhigileva O.N., Malysh J.M., Sokolova Y.Y. Detection of two Microsporidia pathogens of the European honey bee Apis mellifera (Insecta: Apidae) in Western Siberia. Acta Parasitol. 2018;63:728–732. doi: 10.1515/ap-2018-0086. [DOI] [PubMed] [Google Scholar]
- 59.Ostroverkhova N.V. Prevalence of Nosema ceranae (Microsporidia) in the Apis mellifera mellifera bee colonies from long time isolated apiaries of Siberia. Far East. Entomol. 2020;407:8–20. doi: 10.25221/fee.407.2. [DOI] [Google Scholar]
- 60.Ostroverkhova N.V., Konusova O.L., Kucher A.N., Sharakhov I.V. A comprehensive characterization of the honeybees in Siberia (Russia) In: Dechechi Chambo E., editor. Beekeeping and Bee Conservation—Advances in Research. InTech; Rijeka, Croatia: 2016. pp. 1–37. [DOI] [Google Scholar]
- 61.Shumny V.K., Shokin Y.I., Kolchanov N.A. Biodiversity and Ecosystem Dynamics (Information Technology and Modeling) Siberian Branch of the Russian Academy of Sciences; Novosibirsk, Russia: 2006. [(accessed on 28 July 2020)]. 648p. Available online: https://znanium.com/catalog/product/924641. [Google Scholar]
- 62.North Eurasian Climate Center. [(accessed on 28 July 2020)]; Available online: http://seakc.meteoinfo.ru/
- 63.Federal Service for Hydrometeorology and Environmental Monitoring All-Russian Research Institute of hydrometeorological information—World Data Center. [(accessed on 28 July 2020)]; Available online: http://meteo.ru/
- 64.Selyaninov G.T. About climate agricultural estimation. Proc. Agric. Meteorol. 1928;20:165–177. [Google Scholar]
- 65.Polyakov D.V., Barashkova N.K., Kuzhevskaya I.V. Weather and climate description of anomalous summer 2012 in Tomsk Region. Russ. Meteorol. Hydrol. 2014;39:22–28. doi: 10.3103/S106837391401004X. [DOI] [Google Scholar]
- 66.Martín-Hernández R., Botías C., Bailón E.G., Martínez-Salvador A., Prieto L., Meana A., Higes M. Microsporidia infecting Apis mellifera: Coexistence or competition. Is Nosema ceranae replacing Nosema apis? Environ. Microbiol. 2012;14:2127–2138. doi: 10.1111/j.1462-2920.2011.02645.x. [DOI] [PubMed] [Google Scholar]
- 67.Chen Y.W., Chung W.P., Wang C.H., Solter L.F., Huang W.F. Nosema ceranae infection intensity highly correlates with temperature. J. Invertebr. Pathol. 2012;111:264–267. doi: 10.1016/j.jip.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 68.Pacini A., Mira A., Molineri A., Giacobino A., Cagnolo N.B., Aignasse A., Zago L., Izaguirre M., Merke J., Orellano E., et al. Distribution and prevalence of Nosema apis and N. ceranae in temperate and subtropical eco-regions of Argentina. J. Invertebr. Pathol. 2016;141:34–37. doi: 10.1016/j.jip.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 69.Milbrath M.O., van Tran T., Huang W.F., Solter L.F., Tarpy D.R., Lawrence F., Huang Z.Y. Comparative virulence and competition between Nosema apis and Nosema ceranae in honey bees (Apis mellifera) J. Invertebr. Pathol. 2015;125:9–15. doi: 10.1016/j.jip.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 70.Konusova O.L., Pogorelov Y.L., Ostroverkhova N.V., Nechipurenko A.O., Vorotov A.A., Klimova E.A., Prokopiev A.S. Honey bee and bee-farming in the Tomsk region: Past, present and future. Tomsk State Univ. J. Biol. 2009;4:15–28. (In Russian, English Summary) [Google Scholar]
- 71.Sorokin V.S. A Short Guide to Caring for Bees in Frame Hives for Siberia. 2nd ed. Tomsk Beekeeping Society; Tomsk, USSR: 1924. p. 180. [Google Scholar]
- 72.Smolyakova E.O. The development of beekeeping in the Altai Territory. In: Mishchenko V.V., editor. Actual Issues of the Functioning of the Economy in the Altai Territory. Altai State University; Barnaul, Russia: 2018. pp. 177–186. [Google Scholar]
- 73.Meixner M.D., Büchler R., Costa C., Francis R.M., Hatjina F., Kryger P., Uzunov A., Carreck N.L. Honey bee genotypes and the environment. J. Apic. Res. 2014;53:183–187. doi: 10.3896/IBRA.1.53.2.01. [DOI] [Google Scholar]
- 74.Meixner M.D., Francis R.M., Gajda A., Kryger P., Andonov S., Uzunov A., Topolska G., Costa C., Amiri E., Berg S., et al. Occurrence of parasites and pathogens in honey bee colonies used in a European genotype-environment interactions experiment. J. Apic. Res. 2014;53:215–229. doi: 10.3896/IBRA.1.53.2.04. [DOI] [Google Scholar]
- 75.Büchler R., Costa C., Hatjina F., Andonov S., Meixner M.D., Le Conte Y., Uzunov A., Berg S., Bienkowska M., Bouga M., et al. The influence of genetic origin and its interaction with environmental effects on the survival of Apis mellifera L. colonies in Europe. J. Apic. Res. 2014;53:205–214. doi: 10.3896/IBRA.1.53.2.03. [DOI] [Google Scholar]
- 76.Branchiccela B., Arredondo D., Higes M., Invernizzi C., Martín-Hernández R., Tomasco I., Zunino P., Antúnez K. Characterization of Nosema ceranae genetic variants from different geographic origin. Microb. Ecol. 2017;73:978–987. doi: 10.1007/s00248-016-0880-z. [DOI] [PubMed] [Google Scholar]