Abstract
Background
Cervical cancer is the second-most common gynecological cancer, early screening plays a key role in the diagnosis and treatment of cervical intraepithelial neoplasia (CIN). Sustained E7 protein expression is the pathological basis for CIN and cervical cancer.
Methods
We collected the cervical cell samples of women who visited the gynecological clinic of Peking Union Medical College Hospital between September 2018 and September 2019 and submitted them to the high-risk human papillomavirus (Hr-HPV) test. We performed a magnetic particle–based chemiluminescence enzyme immunoassay to analyze the HPV16/18 E7 protein level in CIN of different severities and compared the results with those of cervical pathology (gold standard) and the HPV test.
Results
The positive rate of HPV16/18 E7 protein increased with the severity of CIN: 26.6% in normal tissue, 58.3% in CIN1, and 70.6% in CIN2 or higher (CIN2+). For CIN2+, the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of the E7 protein were 70.6, 67.9, 52.2, and 82.3%, respectively. These values of the HPV test were 86.8, 44.5, 43.7, and 87.1%, respectively. With the combination of the E7 protein assay and HPV test, the specificity for diagnosing CIN2+ was 78.1%, which was significantly higher than that of the HPV test alone.
Conclusions
HPV16/18 E7 protein level is correlated with the severity of CIN and has a high concordance rate with the pathological result. For cervical cancer screening, the combination of HPV16/18 E7 protein assay and HPV test improves the CIN diagnostic specificity, detection rate, and detection accuracy.
Keywords: Cervical intraepithelial neoplasia (CIN), E7 oncoprotein, High-risk human papillomavirus (Hr-HPV)
Background
Cervical cancer is the second-most common gynecological cancer [1]. Early screening plays a key role in the diagnosis and treatment of cervical intraepithelial neoplasia (CIN). Cervical cytology is the first screening technique and is widely used. The sensitivity and specificity of cytology are 53.0 and 96.5%, respectively [2]. To further improve the diagnostic sensitivity and address limitations such as the shortage and varying skill levels of technicians, in 2014 the World Health Organization (WHO) recommended the use of a high-risk human papillomavirus (Hr-HPV) test for cervical cancer screening in developing countries [3, 4]. However, approximately 90% of HPV infections are transient [3]. Different studies have shown great variability in the specificity of HPV testing for high-grade CIN, from 24.8 to 56.1% [5, 6]. Researchers agree that the high sensitivity and low specificity of the HPV test contribute to a high colposcopy referral rate [7]. Improving the diagnostic specificity would prevent unnecessary panic and reduce colposcopy referral rates and healthcare costs. To achieve this, it is important to search for more effective biomarkers. HPV E7 protein inhibits retinoblastoma protein, thereby promoting the uncontrolled proliferation and malignant transformation of infected cells [8, 9]. Sustained E7 protein expression is the pathological basis for CIN and cervical cancer [10–13]. Therefore, E7 protein may be a marker for CIN [14, 15]. In this study, we analyzed HPV 16/18 E7 protein level in CIN of different severities in order to investigate the relationship between the results of the HPV 16/18 E7 protein assay and the severity of CIN, as well as to investigate the value of the HPV16/18 E7 protein assay in CIN screening and evaluated its role as an auxiliary diagnostic biomarker.
Method
Clinical data
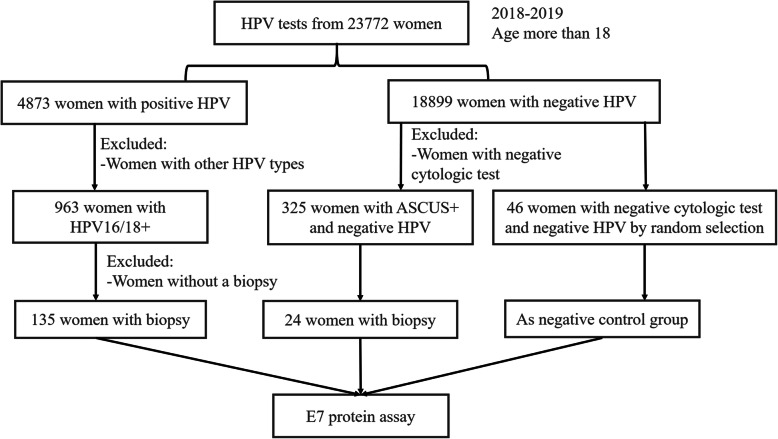
The study was approved by the Ethics Committee of Peking Union Medical College Hospital (approval number HS1624) and was conducted after obtaining the patient’s informed consent. Cervical cell samples of 23,772 women aged 18 or above who visited the gynecological clinic of Peking Union Medical College Hospital between September 2018 and September 2019 were collected and submitted to the HPV test. A total of 963 samples with HPV16/18 positive and 325 HPV negative patients with remarkable cytological abnormalities (Atypical Squamous Cells of Undetermined Significance and more serious, ASC-US+) were included in the study. Moreover, a total of 159 patients underwent colposcopy and biopsy for pathological examination, among which, 135 patients were positive for HPV16/18 and 24 samples were ASC-US+ with HPV negative. Besides, 46 randomly selected samples who did not undergo colposcopy or biopsy due to negative HPV test and cytologic test were included in the negative control group for statistical analysis (Fig. 1). The patients were aged from 24 to 79 (42.57 ± 11.27) years.
Fig. 1.
The flow chart of the study
The aim
To investigate the relationship between the results of the HPV 16/18 E7 protein assay and the severity of CIN, as well as to investigate the value of the HPV16/18 E7 protein assay in CIN screening.
The design
We collected the cervical cell samples of 23,772 women who visited the gynecological clinic and submitted them to the HPV test. A total of 135 samples with HPV16/18 positive and histological results and 24 samples with ASC-US+, HPV negative, and histological results were included in the study. Moreover, 46 randomly selected samples that were negative for both HPV and cytologic test were included in the negative control group for statistical analysis. We performed a magnetic particle–based chemiluminescence enzyme immunoassay to analyze the HPV16/18 E7 protein level in CIN of different severities and compared the results with those of cervical pathology (gold standard) and the HPV test.
Cytologic test
Cytologic specimens were processed with the ThinPrep liquid-based cytology systems (Hologic, CA, USA). Cytology results were classified with the Bethesda System for Reporting Cervical Cytology as revised in 2014 [16].
Hr-HPV DNA test
The Cobas 4800 HPV test (Roche Diagnostics, GmbH, Mannheim, Germany), which is a qualitative test for detection of HPV DNA, was used to analyze the samples. This test amplifies target DNA in cervical epithelial cells by PCR and nucleic acid hybridization (Cobas PCR collection media, Roche Molecular Systems, Inc.) to detect 14 Hr-HPV types, of which HPV 16 and HPV 18 are of the greatest importance. This analysis also enables the detection of Hr-HPV types 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68 at clinically significant levels of infectivity.
E7 protein assay
The HPV16/18 E7 magnetic particle–based chemiluminescence enzyme immunoassay kit from AMID Biotech (Tianjin) Co., Ltd. was used to analyze the E7 protein level according to the instructions. The patients were instructed to refrain from intercourse and vaginal medication. The supernatant was collected for analysis within 2 h. The assay used the double-antibody sandwich method, where magnetic particles were used as the solid phase of the immune response, and highly specific antibodies were used to detect high-risk E7 protein in cervical cell samples.
Pathological examination
The pathological result was used as the gold standard. CIN was pathologically classified as mild (CIN1), moderate (CIN2), or severe (CIN3).
Statistical analysis
SPSS v25.0 was used for statistical analysis. Count data are expressed as a percentage or n (%). The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were evaluated with the receiver operating characteristic (ROC) curve and area under the curve (AUC). P < 0.05 was considered statistically significant.
Results
In this study, 46 patients were negative on both the cytologic test and HPV test. Pathologically, 67 patients were normal, 24 patients were CIN1, and 68 patients were CIN2/CIN3. E7 protein level was analyzed in 205 cell samples.
Diagnostic efficacy of ROC curve
When the ROC curve of E7 protein concentration was drawn, the AUC was 0.718 (95% CI 0.628–0.808) for the HPV16/18 E7 protein assay, and 8.270 was the optimal cut-off value (Fig. 2).
Fig. 2.
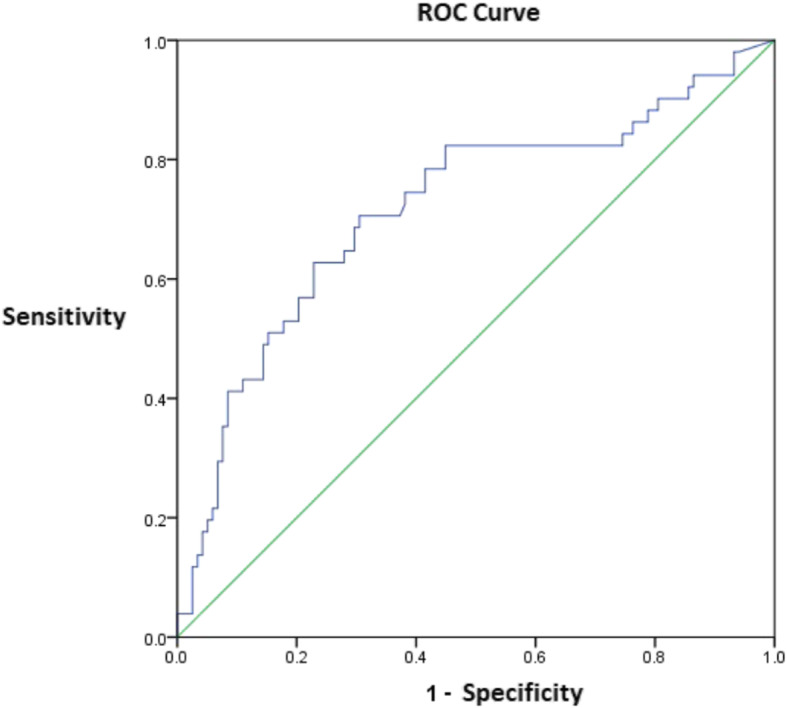
The ROC curve of E7 protein
The positive rate of E7 protein expression in CIN of different severities
E7 protein expression was considered positive if the test value was 8.27 or above. The positive rate was 26.6% (30/113) in pathologically normal/inflammation cases, including 18 cases of positive HPV16/18 test results and 7 cases with both negative cytologic test and HPV test results. The positive rate was 58.3% (14/24) in CIN1 cases, 70.6% (48/68) in CIN2+ cases and 67.4% (31/46) in CIN3+ cases. The results indicated that the positive rate of E7 protein expression increased with the severity of CIN: it was 32.1% in normal and CIN1 patients (the negative control group) and 70.6% in CIN2+ patients (the pathological positive group) (P < 0.001). (Table 1).
Table 1.
The positive rate of E7 protein in CIN of different severities (n/N, %)
| Pathology | N | Positive E7 cases (n) | Positive E7 rate (%) |
|---|---|---|---|
| Normal | 113 | 30 | 26.6% |
| CIN1 | 24 | 14 | 58.3% |
| CIN2+ | 68 | 48 | 70.6% |
| CIN3+ | 46 | 31 | 67.4% |
| Total | 205 | 92 | 44.9% |
Diagnostic efficacy of the E7 protein assay versus the HPV test
For CIN2+ detection, the E7 protein assay had a sensitivity, specificity, PPV, and NPV of 70.6, 67.9, 52.2, and 82.3%, respectively. For the HPV test, the values were 86.8, 44.5, 43.7, and 87.1%, respectively. The E7 protein assay was significantly less sensitive (P = 0.021) but was significantly more specific (P < 0.001). The combination of E7 protein assay and HPV test had a specificity and PPV of 78.1 and 58.3%, respectively, which were significantly higher than those of the HPV test alone (P < 0.001 and P = 0.045, respectively) (Table 2). For CIN3+ detection, the E7 protein assay had a sensitivity, specificity, PPV, and NPV of 67.4, 61.6, 33.7, and 86.7%, respectively. The combination of E7 protein assay and HPV test had a specificity of 72.3%, which was significantly higher than that of the HPV test alone (P < 0.001).
Table 2.
Diagnostic efficacy of the E7 protein assay versus the HPV test (n/N, %)
| Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |
|---|---|---|---|---|
| CIN2+ | ||||
| E7 protein | 70.6% (48/68) | 67.9% (93/137) | 52.2% (48/92) | 82.3% (93/113) |
| HPV | 86.8% (59/68) | 44.5% (61/137) | 43.7% (59/135) | 87.1% (61/70) |
| E7 protein + HPV | 61.8% (42/68) | 78.1% (107/137) | 58.3% (42/72) | 80.4% (107/133) |
| CIN3+ | ||||
| E7 protein | 67.4% (31/46) | 61.6% (98/159) | 33.7% (31/92) | 86.7% (98/113) |
| HPV | 89.1% (41/46) | 40.9% (65/159) | 30.4% (41/135) | 92.9% (65/70) |
| E7 protein + HPV | 60.9% (28/46) | 72.3% (115/159) | 38.9% (28/72) | 86.5% (115/133) |
Note: CIN2 and CIN3 cases were considered as pathological positive; CIN1, normal, and TCT/Hr-HPV-negative cases were considered as negative, PPV positive predicative value, NPV negative predictive value
Discussion
Hr-HPV is the causative pathogen of cervical cancer [17], The 2012 American Society for Colposcopy and Cervical Pathology guidelines state that patients positive for HPV16/18 should undergo colposcopy and biopsy [18], However, 90% of HPV infections are transient [3], so the positive predictive value of CIN2+ is only 11.4% in women with HPV16/18 and normal cytology [19]. This means that many patients without CIN are undergoing colposcopy, which increases the caseload of unnecessary colposcopies. Therefore, it is important to search for better auxiliary diagnostic markers in order to improve the accuracy rate.
As a protein product of Hr-HPV mRNA, E7 affects cell proliferation [10]. In this study, ROC analysis showed that the HPV16/18 E7 protein assay had an AUC of 0.718 for the diagnosis of CIN2+. For CIN2 and CIN3 cases, the sensitivity and the specificity were 70.6 and 67.9%, respectively. The specificity level was ideal for the diagnosis of CIN2 + .
This study showed that E7 protein level was correlated with the severity of CIN. With the cut-off expression value of 8.27, the positive rate was 26.6% in pathologically normal/inflamed cases, 58.3% in CIN1 cases, and 70.6% in CIN2/CIN3 cases, indicating that the positive rate increased with the severity of CIN. In CIN2 and CIN3 cases, the sensitivity was 70.6%, which was lower than that of the HPV test (86.8%), but the specificity was 67.9%, which was significantly higher than that of the HPV test (44.5%). With the combination of the E7 protein assay and the HPV test, the specificity was 78.1%, which was significantly higher than that of the HPV test alone (P < 0.001); the PPV was 58.3%, which was also higher than that of the HPV test alone. Besides, similar with CIN2+ cases, when combined the E7 protein assay with the HPV test in CIN3+ cases detection, the specificity was 72.3%, which was significantly higher than that of the HPV test alone (40.9%) (P < 0.001). If the HPV test is used alone for CIN screening, many patients with transient HPV infection (rather than precancerous lesions) will undergo colposcopy and biopsy, which causes anxiety and panic and increases healthcare costs. The combination of E7 protein assay and HPV test improves the specificity of CIN detection and reduces the colposcopy referral rate and invasive examination rate.
E7 protein expression was positive in 30 pathologically normal samples (26.6%), including 12 cases with negative HPV tests. This may be due to technical limitations. Moreover, 18 pathologically negative cases were positive on the HPV16/18 test. E7 protein level may increase before positive pathological findings or may be more sensitive than a pathological examination. In a previous study, E7 protein expression was positive in 14.3% of pathologically normal cases [20]. The 18 patients with positive HPV16/18 test should be followed up to further observe the relationship between E7 protein level and disease progression.
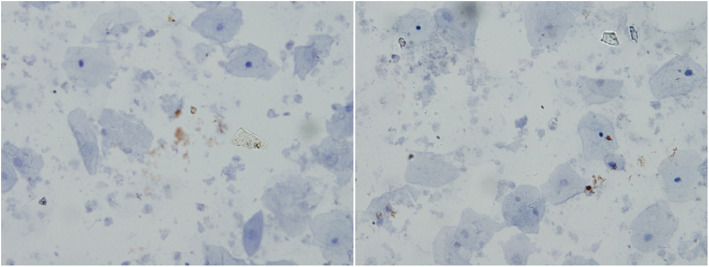
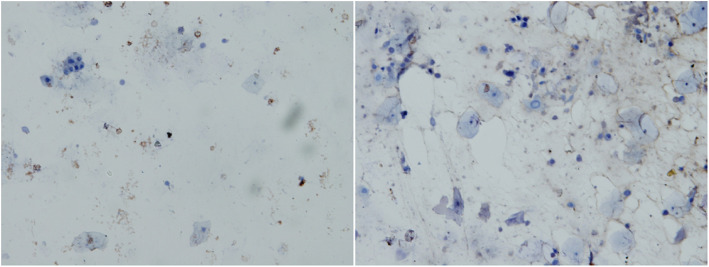
In this study, E7 protein expression was negative in 20 CIN2/CIN3 cases (20/68, 29.4%). The false-negative results may be related to sampling, laboratory procedures, or technical limitations. In our preliminary experiment, we analyzed the false-negative results of E7 protein in seven CIN2+ samples. The samples were tested using the BD Accuri C6 flow cytometer, and the results showed that each of the seven samples contained 16,000 to 760,000 cells. Moreover, streptavidin-peroxidase immunocytochemical staining detected E7 protein expression in two samples, however, the expression was detected in only a few cells. Specifically, one of the seven samples (CIN3) showed substantially intact cells, with low E7 protein expression in a few cells (brown particles in the nucleus and cytoplasm), as well as some cell fragmentation and protein release (Fig. 3). Another sample (cervical cancer) showed significant cell fragmentation with few normal cells and very low E7 protein expression due to cell fragmentation (Fig. 4). These data indicate that sampling has a significant impact on the E7 protein assay, suggesting that it is important to standardize the sampling procedures to ensure the optimal cell number and integrity for the E7 protein assay.
Fig. 3.
The immunocytochemistry staining of E7 protein in one of CIN3 samples
Fig. 4.
The immunocytochemistry staining of E7 protein in a cervical cancer sample
E6 and E7 are the most important oncogenes of HPV [21]. Studies have confirmed the role and value of the HPV E6 protein assay. In 2014, Qiao et al. [22] found that when diagnosing high-grade CIN, the sensitivity and specificity of the E6 protein assay were 42.8 and 94.3%, respectively, and in the study of Zhang et al. (2017) [23], the values were 44.8 and 93.5%, respectively. This study showed that the E7 protein assay has its own strength and limitations relative to the E6 assay, which was consistent with previous reports [20]. E7 protein is a promising auxiliary marker for the screening of CIN.
Conclusions
In summary, this study shows that the combination of E7 protein assay and HPV test improves the diagnostic specificity and PPV for diagnosing CIN, which reduces the colposcopy referral rate and maybe one of promising strategies for minimizing regional variation in screening for CIN, and reduces healthcare costs. Large multicenter studies are needed to validate the results and continue the search for more effective screening tools for CIN.
Acknowledgements
Not applicable.
Abbreviations
- CIN
Cervical intraepithelial neoplasia
- Hr-HPV
High-risk human papillomavirus
- PPV
Positive predictive value
- NPV
Negative predictive value
- WHO
World Health Organization
- ASC-US
Atypical Squamous Cells of Undetermined Significance
- ROC
Receiver operating characteristic
- AUC
Area under the curve
Authors’ contributions
HS, LK and LZ conceived of the original idea for the study, interpreted results, obtained ethical approval, edited the paper and was overall guarantor. HS, LK, XX, SS, ML and TX carried out the experimental studies and statistical analysis, contributed to the preparation of the data set, interpreted results and contributed to drafts of the paper. HL, PL, SS, YZ, CL, RG, YL, XY, HL and QF contributed to the study design and sample collection. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Peking Union Medical College Hospital (approval number HS1624) and written informed consent was obtained from individual or guardian participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Honghui Shi, Email: honghuishi@sina.com.
Lan Zhu, Email: zhu_julie@vip.sina.com.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Katki HA, Kinney WK, Fetterman B, Lorey T, Poitras NE, Cheung L, Demuth F, Schiffman M, Wacholder S, Castle PE. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol. 2011;12(7):663–672. doi: 10.1016/S1470-2045(11)70145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370(9590):890–907. doi: 10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- 4.WHO Guidelines for Screening and Treatment of Precancerous Lesions for Cervical Cancer Prevention. Geneva: World Health Organization; 2013. PMID: 24716265. [PubMed]
- 5.Wright TC, Jr, Stoler MH, Behrens CM, Sharma A, Sharma K, Apple R. Interlaboratory variation in the performance of liquid-based cytology: insights from the ATHENA trial. Int J Cancer. 2014;134(8):1835–1843. doi: 10.1002/ijc.28514. [DOI] [PubMed] [Google Scholar]
- 6.Arbyn M, Roelens J, Simoens C, Buntinx F, Paraskevaidis E, Martin-Hirsch PP, Prendiville WJ. Human papillomavirus testing versus repeat cytology for triage of minor cytological cervical lesions. Cochrane Database Syst Rev. 2013;2013(3):CD008054. [DOI] [PMC free article] [PubMed]
- 7.Koliopoulos G, Nyaga VN, Santesso N, Bryant A, Martin-Hirsch PP, Mustafa RA, Schunemann H, Paraskevaidis E, Arbyn M. Cytology versus HPV testing for cervical cancer screening in the general population. Cochrane Database Syst Rev. 2017;8:CD008587. doi: 10.1002/14651858.CD008587.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seedorf K, Oltersdorf T, Krammer G, Rowekamp W. Identification of early proteins of the human papilloma viruses type 16 (HPV 16) and type 18 (HPV 18) in cervical carcinoma cells. EMBO J. 1987;6(1):139–144. doi: 10.1002/j.1460-2075.1987.tb04731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trope A, Sjoborg K, Eskild A, Cuschieri K, Eriksen T, Thoresen S, Steinbakk M, Laurak V, Jonassen CM, Westerhagen U, et al. Performance of human papillomavirus DNA and mRNA testing strategies for women with and without cervical neoplasia. J Clin Microbiol. 2009;47(8):2458–2464. doi: 10.1128/JCM.01863-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghittoni R, Accardi R, Hasan U, Gheit T, Sylla B, Tommasino M. The biological properties of E6 and E7 oncoproteins from human papillomaviruses. Virus Genes. 2010;40(1):1–13. doi: 10.1007/s11262-009-0412-8. [DOI] [PubMed] [Google Scholar]
- 11.McLaughlin-Drubin ME, Munger K. The human papillomavirus E7 oncoprotein. Virology. 2009;384(2):335–344. doi: 10.1016/j.virol.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sen P, Ganguly P, Ganguly N. Modulation of DNA methylation by human papillomavirus E6 and E7 oncoproteins in cervical cancer. Oncol Lett. 2018;15(1):11–22. doi: 10.3892/ol.2017.7292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sorbye SW, Arbyn M, Fismen S, Gutteberg TJ, Mortensen ES. HPV E6/E7 mRNA testing is more specific than cytology in post-colposcopy follow-up of women with negative cervical biopsy. PLoS One. 2011;6(10):e26022. doi: 10.1371/journal.pone.0026022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Honegger A, Schilling D, Bastian S, Sponagel J, Kuryshev V, Sultmann H, Scheffner M, Hoppe-Seyler K, Hoppe-Seyler F. Dependence of intracellular and exosomal microRNAs on viral E6/E7 oncogene expression in HPV-positive tumor cells. PLoS Pathog. 2015;11(3):e1004712. doi: 10.1371/journal.ppat.1004712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang R, Lu H, Lyu YY, Yang XM, Zhu LY, Yang GD, Jiang PC, Re Y, Song WW, Wang JH, et al. E6/E7-P53-POU2F1-CTHRC1 axis promotes cervical cancer metastasis and activates Wnt/PCP pathway. Sci Rep. 2017;7:44744. doi: 10.1038/srep44744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nayar R, Wilbur DC. The pap test and Bethesda 2014. Cancer Cytopathol. 2015;123(5):271–281. doi: 10.1002/cncy.21521. [DOI] [PubMed] [Google Scholar]
- 17.zur Hausen H. Papillomaviruses in human cancer. Appl Pathol. 1987;5(1):19–24. [PubMed] [Google Scholar]
- 18.Saslow D, Solomon D, Lawson HW, Killackey M, Kulasingam SL, Cain J, Garcia FA, Moriarty AT, Waxman AG, Wilbur DC, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62(3):147–172. doi: 10.3322/caac.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sorbye SW, Arbyn M, Fismen S, Gutteberg TJ, Mortensen ES. Triage of women with low-grade cervical lesions--HPV mRNA testing versus repeat cytology. PLoS One. 2011;6(8):e24083. doi: 10.1371/journal.pone.0024083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yali Yuan GH, Li M, et al. Expression level and clinical value of HPV-16/18 E6 and E7 proteins in cervical intraepithelial lesion. J China-Japan Friendship Hosp. 2018;32(6):352–355. [Google Scholar]
- 21.Zhang L, Yang B, Zhang A, Zhou A, Yuan J, Wang Y, Sun L, Cao H, Wang J, Zheng W. Association between human papillomavirus type 16 E6 and E7 variants with subsequent persistent infection and recurrence of cervical high-grade squamous intraepithelial lesion after conization. J Med Virol. 2016;88(11):1982–1988. doi: 10.1002/jmv.24541. [DOI] [PubMed] [Google Scholar]
- 22.Qiao YL, Jeronimo J, Zhao FH, Schweizer J, Chen W, Valdez M, Lu P, Zhang X, Kang LN, Bansil P, et al. Lower cost strategies for triage of human papillomavirus DNA-positive women. Int J Cancer. 2014;134(12):2891–2901. doi: 10.1002/ijc.28616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Q, Dong L, Hu S, Feng R, Zhang X, Pan Q, Ma J, Zhang L, Zhao X, Sankaranarayanan R, et al. Risk stratification and long-term risk prediction of E6 oncoprotein in a prospective screening cohort in China. Int J Cancer. 2017;141(6):1110–1119. doi: 10.1002/ijc.30807. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.