Abstract
Objective
The melanocortin 4 receptor (MC4R) is a G protein-coupled receptor that plays major roles in the central control of energy balance. Loss-of-function mutations of MC4R constitute the most common monogenic cause of early-onset extreme obesity in humans, whereas gain-of-function mutations appear to be protective. In particular, two relatively frequent alleles carrying the non-synonymous coding mutations V103I or I251L are associated with lower risks of obesity and type-2 diabetes. Although V103I and I251L MC4Rs showed more efficient signalling in transfected cells, their specific effects in live animals remain unexplored. Here, we investigated whether the introduction of V103I and I251L mutations into the mouse MC4R leads to a lean phenotype and provides protection against an obesogenic diet.
Methods
Using CRISPR/Cas9, we generated two novel strains of mice carrying single-nucleotide mutations into the mouse Mc4r which are identical to those present in V103I and I251L MCR4 human alleles, and studied their phenotypic outcomes in mice fed with normal chow or a high-fat diet. In particular, we measured body weight progression, food intake and adiposity. In addition, we analysed glucose homeostasis through glucose and insulin tolerance tests.
Results
We found that homozygous V103I females displayed shorter longitudinal length and decreased abdominal white fat, whereas homozygous I251L females were also shorter and leaner due to decreased weight in all white fat pads examined. Homozygous Mc4rV103I/V103I and Mc4rI251L/I251L mice of both sexes showed improved glucose homeostasis when challenged in a glucose tolerance test, whereas Mc4rI251L/I251L females showed improved responses to insulin. Despite being leaner and metabolically more efficient, V103I and I251L mutants fed with a hypercaloric diet increased their fasting glucose levels and adiposity similar to their wild-type littermates.
Conclusions
Our results demonstrate that mice carrying V103I and I251L MC4R mutations displayed gain-of-function phenotypes that were more evident in females. However, hypermorphic MC4R mutants were as susceptible as their control littermates to the obesogenic and diabetogenic effects elicited by a long-term hypercaloric diet, highlighting the importance of healthy feeding habits even under favourable genetic conditions.
Keywords: Melaconocortins, Obesity, Type 2 diabetes, CRISPR/Cas9, Mouse models
Highlights
-
•
Single nucleotide gain-of-function mutations of human MC4R were introduced into the genome of inbred mice using CRISPR/Cas9.
-
•
Females carrying V103I Mc4r alleles are shorter and display reduced adiposity, and improved glucose homeostasis in both sexes.
-
•
I251L Mc4r mutations induce shorter longitudinal length, decreased abdominal fat and improved glucose metabolism in females.
-
•
Mc4r V103I and I251L gain-of-function mutations do not protect against diabetogenic and adipogenic effects of a high-fat diet.
1. Introduction
Over the last three decades, there has been a dramatic global increase in the prevalence of overweight and obesity in children, teenagers and adults with an associated increase in chronic conditions, such as type 2 diabetes (T2D), hypertension and cardiovascular disease [1,2]. Although mainly driven by the massive intake of ultra-processed edibles of poor nutritional value, the obesogenic contemporary human lifestyle does not affect all individuals equally, suggesting some level of genetic predisposition to develop or resist overweight and increased adiposity. Several genome-wide association studies performed during the last 15 years have identified nearly 100 different loci associated with high body mass index (BMI), increased adiposity, high leptin levels or T2D [[3], [4], [5], [6]]. However, most familial cases appear to be driven by several coexisting low-frequency polymorphic alleles, each of them contributing to minor effects and a small percentage to the overall phenotype. Notwithstanding, there are a few exceptions of human monogenic obesity syndromes, including variants of genes involved in the central melanocortin system [7,8].
The melanocortin 4 receptor (MC4R) is a G protein-coupled receptor mainly expressed in the brain and autonomic sympathetic ganglia that is involved in the regulation of food intake and energy expenditure [9,10]. Loss-of-function mutations in the MC4R constitute the most common monogenic cases of early-onset extreme obesity in humans worldwide [11,12]. Individuals carrying non-synonymous MC4R variants display different levels of overweight, while those carrying total loss of function mutations display voracious hyperphagia and early-onset extreme obesity, such as Mc4r null-allele mice that become extremely hyperphagic and obese after weaning [13].
In addition to the existence of several hypomorphic alleles predisposing to obesity, some individuals carry non-synonymous MC4R variants that appear to protect against overweight and increased adiposity. The most frequent MC4R polymorphism reported to have a protective effect on obesity is V103I, with a carrier frequency of 2–4% [14,15]. In vitro studies performed in HEK293 cells stably transfected with a human MC4R clone carrying the V103I mutation showed a 2-fold decrease in the potency of the MC4R antagonist hAGRP (87–132) [16], suggesting diminished activity of endogenous AGRP in the blockade of MC4R necessary to promote food intake. Several case–control studies performed with individuals of European origin [17,18] and subsequent meta-analysis [14,19] showed that individuals carrying the V103I polymorphism have 2–18% lower risk for obesity. Another large meta-analysis that included six East Asian studies and 31 studies of other ethnic groups, involving 19,822 obese cases and 35,373 non-obese controls, showed that individuals with the V103I allele have 21% lower risk for obesity [20]. However, other studies performed with people of Chinese or Japanese descent showed no difference in obesity rates between MC4R 103V and 103I carriers [21,22]. Another non-synonymous polymorphism of MC4R, I251L, has also been reported to confer a 48% reduced risk for obesity in a meta-analysis of Europeans [19], in agreement with a previous study showing that this I251L substitution confers elevated MC4R-dependent cyclic adenosine monophosphate (cAMP) levels in transfected cells [16]. In eight out of nine case–control studies performed in populations of European descent, the MC4R I251L polymorphism has been reproducibly associated with protection against obesity. A more recent study based on genetic and clinical data obtained from nearly half a million people of European-ancestry identified 61 non-synonymous MC4R variants including 4 gain of function alleles (V103I, I251L, I289L, I317V) characterised by enhanced β-arrestin recruitment rather than increased cAMP production when tested in transfected HEK293 cells [15]. Interestingly, carriers of these 4 gain-of-function variants showed significantly lower body mass index and lower risk of obesity, T2D and coronary artery disease [15].
Although these studies suggest that gain of function variants of MC4R provide protection for increased adiposity and associated disorders, the multigenic nature underlying food intake and energy expenditure together with the great variability of the human genetic pool and people's habits regarding food intake and physical activity also reveal the difficulty to rigorously test the functional association of a polymorphic allele with a particular behaviour, physiological parameter or risk of disease. To directly challenge the hypothesis that some MC4R polymorphic variants protect against obesity, we introduced the most common human gain of function mutations V103I and I251L into identical Mc4r coding positions of inbred laboratory mice, which, together with a homogeneous environmental setting, provide an ideal experimental framework to functionally test the effects of these variants in energy balance.
2. Materials and methods
2.1. Multiple sequence alignment
Mouse and human MC4R amino acid sequences were aligned using ClustalW2 [23] with default parameters. UniProt accession numbers for the MCR proteins are: mMC4R, P56450; mMC1R, Q01727; mMC2R, Q64326; mMC3R, P33033; mMC5R, P41149; hMC4R, P32245; hMC1R, Q01726; hMC2R, Q01718; hMC3R, P41968; hMC5R, P33032.
2.2. Imaging and patch clamp experiments in HEK293T cells
Mouse Mc4r sequences and the mutant versions V103I and I251L were amplified by polymerase chain reaction (PCR) from mouse genomic DNA (see primers sequences in Table S1), inserted into the EcoRI and BamHI sites of PRK6-YFP vector (IGF, Montpellier, France) and Sanger-sequenced (Macrogen). Imaging and patch clamp experiments were performed in HEK293T cells transfected with wild-type Mc4r, Mc4rV103I or Mc4rI251L tagged with YFP coding sequences (yellow fluorescent protein) and treated with Hoechst fluorescent dye to stain nuclei. Forty-eight hours after transfection, 1 mg/ml of CellMask™ Orange plasma membrane stain (ThermoFisher Scientific) was added to the culture medium for 1 min at 37 °C, and cells were then washed three times with phosphate-buffered saline (PBS). Fluorescence photomicrographs were obtained with a Zeiss epifluorescence microscope using a pseudoconfocal Apotome module. Analyses of photomicrographs were performed with FIJI free software. Wild-type and mutant MC4R functionality was tested in HEK293T cells by means of MC4R agonist-evoked activation of inhibitory currents on type 2.2 voltage-gated calcium channels (CaV2.2) after bath application of the MC4R/MC3R agonist melanotan II (MTII, Phoenix Pharmaceutical) as previously described [24]. Constitutive activity of the MCRs was assessed in HEK293T cells by means of their inhibitory effect on type 2.1 voltage-gated calcium channels (CaV2.1) as previously described [25].
2.3. Animal care
Mice were housed in ventilated cages under controlled temperature and photoperiod (12-h light/12-h dark cycle, lights on from 7:00 AM to 7:00 PM), with tap water and laboratory chow containing 28.8% protein, 5.33% fat and 65.87% carbohydrate available ad libitum. High caloric diet containing 23% protein, 23% fat and 56% carbohydrate was prepared with a mixture of laboratory chow and peanut butter fudge. All mouse procedures followed the Guide for the Care and Use of Laboratory Animals [26] and in agreement with the Institutional Animal Care and Use Committee of the Instituto de Investigaciones en Ingeniería Genética y Biología Molecular (INGEBI, CONICET).
2.4. Mutant mouse generation
Single point nonsynonymous coding mutations in Mc4r were introduced following a homology-directed repair strategy based on the CRISPR/Cas9 system and a single-stranded oligodeoxynucleotide (ssODN) microinjected in FVB/NJ mouse zygotes. Single guides were selected using the crispr.mit.edu website of the Zhang Lab and CasOFFinder algorithm [27] according to proximity to the codons of interest and low off-targets (Table S2). Guides were generated by oligonucleotide hybridization (IDT, sequences available in Table S1) and subcloned in plasmid DR274 (Addgene, Plasmid 42250). sgRNAs were synthesized using a MEGAshortscript T7 Transcription Kit (Ambion, AM1354). Cas9 mRNA was synthesized from plasmid MLM3613 (Addgene, Plasmid #42251) using mMESSAGEmMACHINE™ T7 Transcription Kit (Ambion, AM1344) and Poly(A) Tailing Kit (Ambion, AM1350). Ninety-three nt ssODNs were purchased (Sigma, sequences available in Table S1). Cas9 mRNA (50 ng/μl for V103I mice and 80 ng/μl for I251L mice), sgRNA (50 ng/μl for V103I and 30 ng/μl for I251L) and ssODN (50 ng/μl for V103I and 30 ng/μl for I251L) were injected into the cytoplasm and pronucleus of one-cell FVB/NJ embryos (microinjection details in Table S3). Founder mice were analyzed by PCR using primers described in Table S1, and PCR products were subcloned in pGEM.T Easy Vector for sequencing (Promega). Genotypes of F1 and F2 mice were confirmed by PCR and PCR product sequencing (primers available in Table S1). Strains carrying V130I or I251L MC4R mutations were selected and maintained in a FVB/NJ background.
2.5. Mc4r mRNA quantification by reverse transcription quantitative PCR (RT-qPCR)
Whole mouse adult hypothalami were dissected, collected in ice-cold TriPure Isolation Reagent (Sigma, 11667165001) and stored at −80 °C until RNA extraction, which was performed following the manufacturer's instructions. RNA integrity was assessed by gel electrophoresis with clear 28S and 18s rRNA observed in an approximate 2:1 ratio. Quantification was performed using a Nanodrop, and 260/280 and 260/230 nm ratios were checked to assess purity. One microgram of RNA was treated with DNAse I (Ambion, AM2222) and used for first-strand cDNA synthesis, using a High-Capacity Reverse Transcription Kit with random primers (Applied Biosystems, 4368814). Primers were designed using the Primer 3 programme (sequences are listed in Table S1). Mc4r mRNA was quantified using primers spanning the single Mc4r exon relative to internal control transcript β-actin. Samples were run in triplicate in an Applied Biosystems 7500 Real-Time PCR System machine using Power Up SYBR Green Master Mix (Applied Biosystems, A25472). Melt curves were analysed to confirm the specificity of the PCR product. Relative quantification was performed by interpolating Ct values in standard curves or by 2-ΔΔCT.
2.6. Body weight, length and fat composition
Body weight was monitored weekly from weaning until 16 weeks of age. After that, mice were euthanised by cervical dislocation. After measuring the body longitudinal axial length from the nose to the base of the tail, mice were dissected, and the inguinal, retroperitoneal and gonadal white fads removed and weighed, followed by the interscapular brown adipose tissue and the livers.
2.7. Food intake
Thirteen-week-old mice were individually housed with ad libitum access to chow food pellets. A week later, daily food intake was determined by subtracting the weight of food pellets placed on each cage at 6 PM by that obtained the following mornings at 10 AM.
2.8. Glucose and insulin tolerance tests
Fifteen-week-old mice were fasted for 18 h (6 PM–12 PM) previous to a glucose tolerance test or for 4 h (8 AM–12 PM) for the insulin tolerance test. For basal fasting levels, blood samples were taken from the tail tip and glucose concentration measured with a One Touch R glucometer (LifeScan, Johnson & Johnson). Following a d-glucose (1 g/kg; Sigma, G5767) intraperitoneal (i.p.) injection, blood samples were taken at 15, 30, 60 and 120 min. For the insulin tolerance test, insulin (1 IU/kg, Humulin R; Lilly; i.p.) was administered and blood samples taken at 15, 30, 60 and 120 min. The total area under the curve (AUC) was calculated using the trapezoidal rule.
2.9. Statistics
All data presented are the mean ± SEM and were analysed using GraphPad Prism Software (version 5.01, 2007, GraphPad Software) or R Studio (version 1.1.456) by analysis of variance (ANOVA). Post hoc pairwise comparisons between groups were performed by Holm–Sidak test unless otherwise stated. Normality of the distributions was assessed by Shapiro–Wilk test (p > 0.05), and the equality of the variance was measured using Bartlett's or F test (p > 0.05). P values < 0.05 were considered to be significant.
3. Results
3.1. Generation of mutant mice carrying V103I and I251L MC4R variants
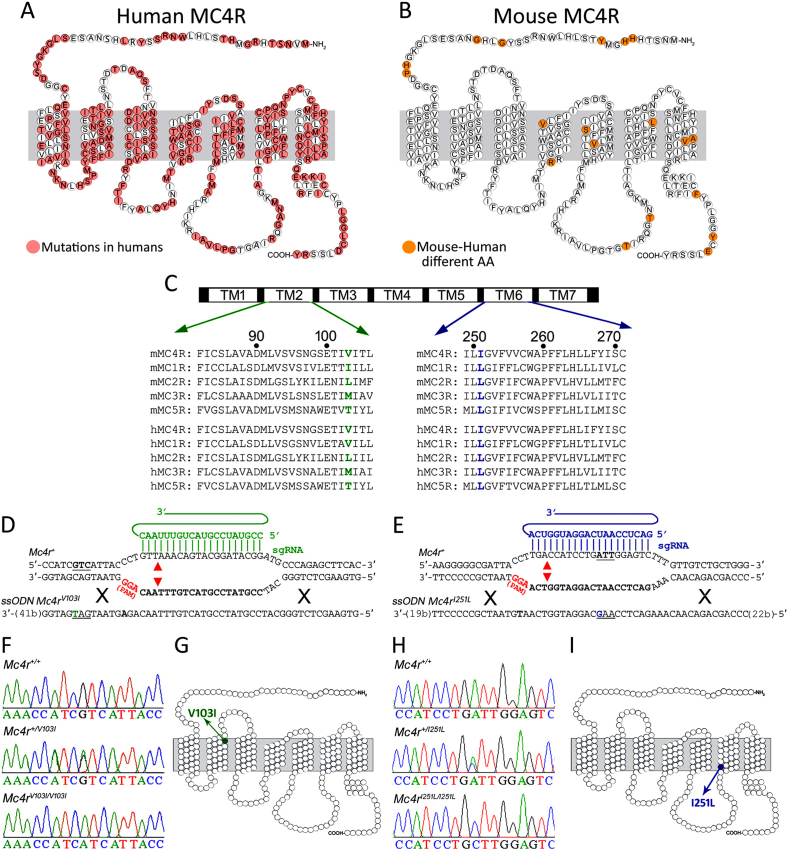
The human MC4R is highly polymorphic in its coding region with up to 220 non-synonymous mutations identified within its 322 amino acids (Figure 1A). Despite the great number of human variants, MC4R is a highly conserved vertebrate gene with a remarkable identity at the amino acid sequence level among mammals. In particular, human and mouse MC4Rs are 94.3% identical (Figure 1B). The MC4R 103-Val residue is highly conserved not only between humans and mice (Figure 1B) but also in all vertebrate Classes. The 251-Ile position, however, is an amniote (reptiles, birds and mammals) novelty that evolved from a leucine still present in extant fish and amphibians. Thus, the I251L gain of function mutation found in humans represents a regression to the vertebrate ancestral amino acid. Interestingly, all other human and mouse melanocortin receptors (MC1R, MC2R, MC3R and MC5R) also carry a highly conserved leucine in this position (Figure 1C) as all vertebrates do, with the only exception being the teleost fish MC2R.
Figure 1.
Generation of mutant mice carrying V103I and I251L MC4R variants. (A) Schematic of the human MC4R with all identified substitutions in red (mc4r.org.uk). (B) Schematic of the mouse MC4R. Non-conserved amino acids between humans and mice are highlighted in orange. (C) Sequence alignment of the second and sixth transmembrane (TM) domains of mouse (top) and human (bottom) MC4R with MC1R, MC2R, MC3R and MC5R sequences. The 103-Val residue at TM2 is shown in green and the 251-Ile residue at TM6 is shown in blue. (D–E) Schematic of the targeting regions of the mouse Mc4r locus. sgRNAs driving Cas9-mediated cuts are shown in green for the V103I mutation and blue for the I251L mutation. Target sequences are shown in bold, the protospacer adjacent motifs (PAM) in red and the theoretical cut sites indicated with arrows. (D) A ssODN donor containing a point mutation in codon 103 (green T), and a mutation in the PAM (bold A) was co-injected with a sgRNA and Cas9 mRNA into mouse zygotes to replace a coding Val with Ile (GTC to ATC) in the 103 residue. (E) Another ssODN donor was used to replace a coding Ile with Leu (ATT to CTT, shown in blue) in position 251. (F) Representative Mc4r PCR products confirming the c.307G > A point mutation in Mc4rV103I alleles that predict a V103I mutation (G). (H) PCR products showing the c.751A > C point mutation in Mc4rI251Lalleles that predict a I251L substitution (I).
After assessing that mouse MC4R variants V103L and I251L are functional in transfected cells (Figure S1), we sought to investigate the in vivo functional effects of these two mutations. To this end, we generated two knock-in mutant mouse strains carrying the identical mutations found in the human population by means of CRISPR/Cas9-mediated gene-editing. For each mutation, we microinjected FVB/NJ mouse zygotes with one sgRNA targeting Mc4r sequences close to the mutation together with a 93-nt ssODN donor carrying the identical nonsynonymous point mutations found in humans flanked by mouse Mc4r sequences (Tables S1 and S2) to promote homologous-directed repair (Figure 1D,E). DNA sequences at the target sites from all mutant F0 mice generated are shown in Table S4. Mc4r DNA sequences taken from F2 mice confirmed the c.307 G > A point mutation that converts the amino acid V103 into a coding isoleucine (Figure 1F,G), whereas a c.751 A > C point mutation changes amino acid I251 into a coding leucine (Figure 1H,I).
3.2. Mc4rV103I/V103I mice exhibit gain of function phenotypes
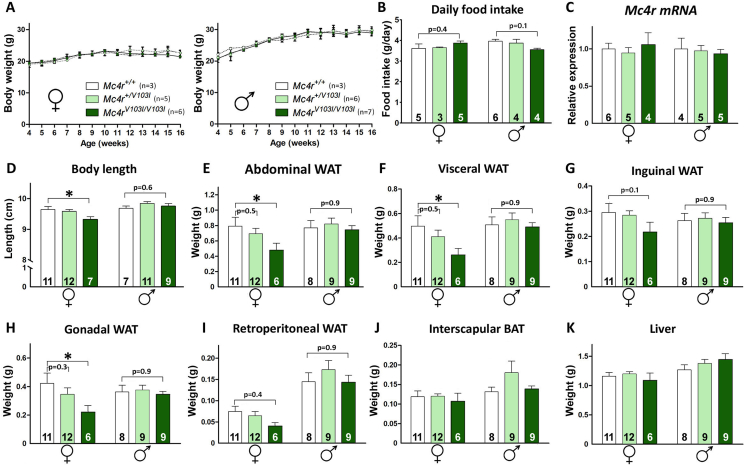
Heterozygous Mc4r+/V103I breeding pairs displayed normal fertility rates and produced mice of the 3 genotypes at Mendelian ratios. Mc4rV103I/V103I mice of both sexes and their wild-type (WT) littermates displayed identical body weight curves up to 16 weeks of age when fed ad libitum with normal chow (Figure 2A). At this age, daily food intake in Mc4rV103I/V103I females and males was not different from their WT siblings (p = 0.4 and 0.1, respectively; Figure 2B). In addition, mice of all genotypes and sexes displayed normal hypothalamic Mc4r mRNA levels (Figure 2C). However, 16-week-old Mc4rV103I/V103I females showed shorter body length than their WT and heterozygous littermates (Figure 2D, left). This difference was not observed in the mutant males (Figure 2D, right). The calculated BMI (g/cm2) of 16-week-old mice showed no statistical differences for all genotypes and sexes. Despite their normal body weight, the abdominal white adipose tissue (WAT) of 16-week-old Mc4rV103I/V103I females weighed 40% less than that of WT littermates (Figure 2E, left), a difference that was not observed between WT and mutant males (Figure 2E, right). The lower abdominal WAT weight found in Mc4rV103I/V103I females was better explained by a reduction in visceral WAT (Figure 2F) rather than inguinal WAT (Figure 2G), with a major effect found in the visceral gonadal fat pad (Figure 2H) and a non-significant reduction in retroperitoneal WAT (Figure 2I). The weight differences observed between the fat pads of Mc4rV103I/V103I and WT littermates were female-specific and not found in males (Figure 2E–I). The weight of the interscapular brown adipose tissue (BAT) and the liver were normal in males and females of all genotypes (Figure 2J,K).
Figure 2.
Gain of function phenotypes in Mc4rV103I/V103Imice. (A) Body weight curves of Mc4r+/V103I and Mc4rV103I/V103I females and males and their respective controls (n = 3–7 per group). (B) Average daily food intake measured during 2 consecutive weeks at 14 weeks of age. (C) Hypothalamic Mc4r mRNA levels in females and males of the 3 genotypes at 16 weeks of age assessed by qRT-PCR and expressed in arbitrary units. (D) Body length of 16-week-old females and males of the 3 genotypes. (E–K) Determination of abdominal, visceral, inguinal, gonadal and retroperitoneal white fat pad, interscapular brown fat pad and liver weights in 16-week-old female and male Mc4r+/V103I and Mc4rV103I/V103I mice and their respective controls. Values represent the mean ± SEM of the number of mice indicated at the base of eac bar. ∗P < 0.05 (two-way ANOVA followed by Holm–Sidak test).
3.3. Mc4rI251L/I251L mice also exhibit gain of function phenotypes
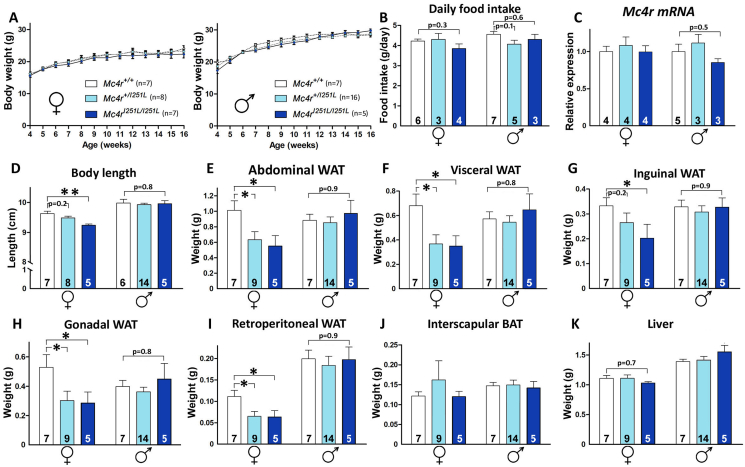
Similar to Mc4r+/V103I mice, heterozygous carriers of the I251L mutation were healthy and fertile. We raised a colony of Mc4rI251L/I251L mice and WT littermates by mating heterozygote Mc4r+/I251L mice and found that body weight curves up to 16 weeks of age in mutant mice of both sexes were normal (Figure 3A). Daily food intake was indistinguishable between WT and homozygous mutant females (p = 0.3) or males (p = 0.6) (Figure 3B). In addition, hypothalamic levels of Mc4r mRNA were normal in all genotypes and sexes (Figure 3C). Mc4rI251L/I251L females exhibited a shorter body length than their WT littermates (Figure 3D, left), a difference that was not found in males (Figure 3D, right). BMI of Mc4rI251L/I251L mice was also normal in both sexes. The abdominal and visceral WAT were remarkably lighter in 16-week-old Mc4rI251L/I251L females in comparison to WT female littermates (Figure 3E,F). Different from the V103I heterozygous mutants, Mc4r+/I251L females also showed reduced abdominal and visceral WAT weight compared to their WT female littermates (Figure 3E,F). Again, these differences were not found in males. The reduced fat accumulation was observed in the three white fat pads studied (inguinal, gonadal and retroperitoneal) of Mc4rI251L/I251L females, which displayed lower weights than those observed in their WT female littermates (Figure 3G,H and 3I). The visceral fat fads (gonadal and retroperitoneal) of heterozygous Mc4r+/I251L females were also lighter than those in WT females (Figure 3H,I). As found with the V103I mutants, the weight of interscapular BAT and livers of Mc4rI251L/I251L mice were normal in both sexes (Figure 3J,K).
Figure 3.
Mc4rI251L/I251Lmice also exhibit gain of function phenotypes. (A) Body weight curves of Mc4r+/I251L and Mc4rI251L/I251L females and males and their respective controls (n = 5–16 per group). (B) Average daily food intake measured during 2 consecutive weeks at 14 weeks of age. (C) Hypothalamic Mc4r mRNA levels in Mc4r+/I251L and Mc4rI251L/I251L females and males at 16 weeks of age relative to controls assessed by qRT-PCR and expressed in arbitrary units. (D) Body length of 16-week-old females and males of the 3 genotypes. (E–K) Determination of abdominal, visceral, inguinal, gonadal and retroperitoneal white fat pad, interscapular brown fat pad and liver weights in 16-week-old female and male Mc4r+/I251L and Mc4rI251L/I251L mice and their respective controls. Values represent the mean ± SEM of a number of mice per group indicated at the base of each bar. ∗P < 0.05, ∗∗P < 0.01 (two-way ANOVA followed by Holm–Sidak test).
3.4. Mice carrying MC4R V103I and I251L mutations display more efficient glucose homeostasis
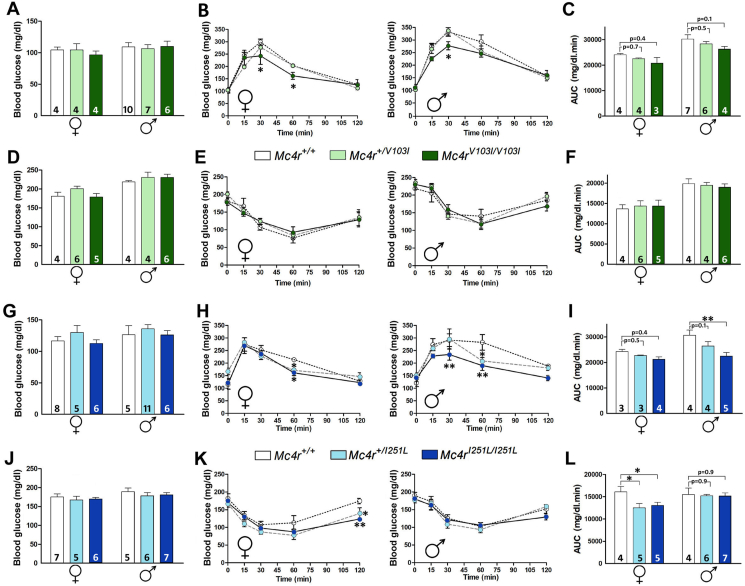
Since V103I and I251L human variants have been associated with lower odds of T2D [15], we sought to determine whether the mutant mice carrying either of these two mutations have improved glucose homeostasis. Mc4rV103I/V103I males and females showed normal fasting blood glucose levels (Figure 4A). Interestingly, female and male homozygous mutants showed improved glucose homeostasis evidenced by smaller increases in blood glucose concentrations 30 and 60 min after receiving an intraperitoneal injection of d-glucose (1 g/kg) than those found in their Mc4r+/V103I and WT littermates (Figure 4B,C). An insulin tolerance test performed in WT and V103I mice showed that insulin sensitivity is normal in all genotypes and sexes (Figure 4E,F).
Figure 4.
Mice carrying MC4R V103I and I251L mutations display more efficient glucose homeostasis. (A) Blood glucose concentration in 15-week-old WT, Mc4r+/V103I and Mc4rV103I/V103I female and male littermates after an 18-h fast, (B) glucose tolerance test (GTT) in mice receiving a 1 g/kg (i.p.) injection of d-glucose (n = 3–7 per group; P: genotype effect of RMA ∗P < 0.05; Holm–Sidak) and (C) the area under the curve (AUC). (D) Blood glucose concentration in 15-week-old WT,Mc4r+/V103I and Mc4rV103I/V103I female and male siblings after a 4-h fast, (E) insulin tolerance test (ITT) in mice receiving a 1 IU/kg (i.p.) injection of insulin (n = 4–6 per group) and (F) AUC of E. (G) Blood glucose concentration in 15-week-old WT, Mc4r+/I251L and Mc4rI251L/I251L female and male littermates after an 18-h fast, (H) GTT (n = 3–5 per group; P: genotype effect of RMA ∗p < 0.05; ∗∗p < 0.01; Holm–Sidak) and (I) AUC of H, ∗∗p < 0.01; two-way ANOVA, Holm–Sidak. (J) Blood glucose concentration in 15-week-old WT,Mc4r+/I251L and Mc4rI251L/I251L female and male siblings after a 4-h fast, (K) ITT (n = 4–7 per group; p: genotype effect of RMA ∗p < 0.05; ∗∗p < 0.01; Holm–Sidak) and (L) AUC of K, ∗P < 0.05; two-way ANOVA, Holm–Sidak. Values represent the mean ± SEM of a number of mice per group indicated at the base of each bar.
Similar to V103I mutants, Mc4rI251L/I251L females and males also showed normal fasting glycaemia and improved glucose clearing when challenged in a glucose tolerance test (GTT; Figure 4H,I). Interestingly, Mc4rI251L/I251L females, but not males, showed improved insulin response when challenged in an insulin tolerance test (Figure 4K,L). Altogether, these results show that MC4R V103I and I251L mutations lead to improved glucose homeostasis suggesting a more efficient insulin response.
3.5. V1O3I and I251L MC4R mutations do not protect against a high-fat diet
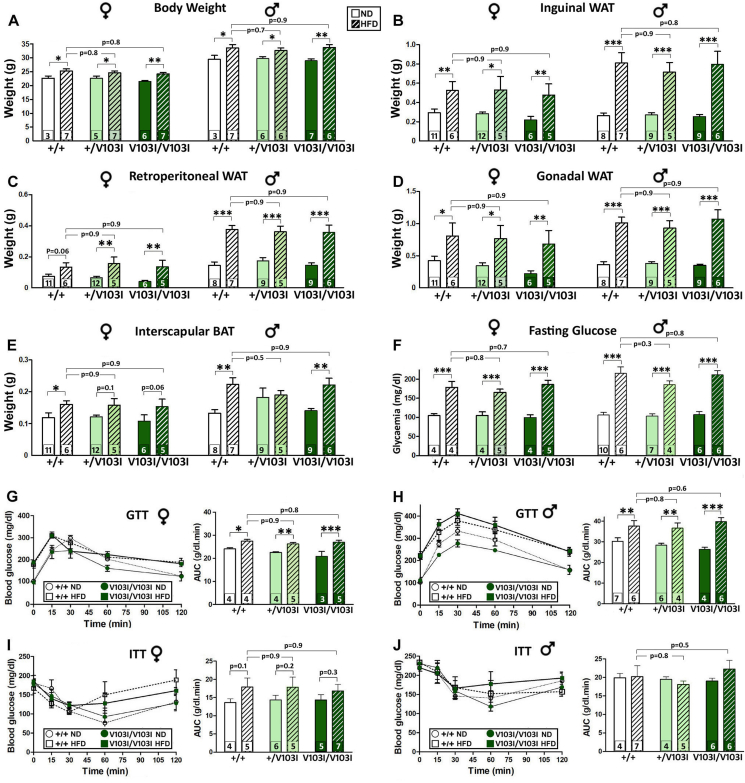
Given that humans carrying gain of function MC4R variants were associated with lower BMI, obesity and T2D [15], and that Mc4rV103I/V103I and Mc4rI251L/I251L female mice showed reduced WAT adiposity (Figure 2, Figure 3), we decided to determine whether the V103I and I251L alleles would protect from the hyperglycaemic and obesogenic effects normally elicited in FVB/N mice after a long-term high fat diet (HFD) [28,29]. After weaning, mice were fed ad libitum with a normal diet (ND) for two weeks and then switched to a HFD for 11 more weeks. At 16 weeks of age, Mc4rV103I/V103I females and their WT littermates fed on a HFD were heavier than those receiving a ND (Figure 5A, left). However, no differences were found among genotypes receiving either ND or HFD (Figure 5A). Similar results were found in males (Figure 5A, right). The increased body weight is largely due to an exaggerated fat deposition in subcutaneous (inguinal; Figure 5B) and visceral (retroperitoneal and gonadal) white fat pads (Figure 5C,D) as well as in interscapular BAT (Figure 5E). The increased adiposity elicited by a long-term HFD was not different in any mouse genotype or gender. In contrast to what we observed in all fat pads analyzed, the weights of the livers of mice receiving HFD were not statistically different from those measured in mice fed with a ND across all genotypes and genders. We also found that mice fed on a HFD exhibited a remarkable increase in fasting blood glucose levels that was similar across all genotypes and in both sexes (Figure 5F). In agreement with increased adiposity and higher fasting glucose levels, mice receiving a HFD showed impaired glucose clearing when challenged in a GTT. This HFD-induced deficit was similarly found in mice of all genotypes and sexes (Figure 5G,H). Although being hyperglycaemic, mice of all genotypes and sexes fed on a HFD displayed normal responses to an insulin challenge that were no different from those observed in mice receiving a ND (Figure 5I,J).
Figure 5.
The V1O3I MC4R mutation does not protect against a high-fat diet. Metabolic phenotype of Mc4r+/V103I and Mc4rV103I/V103I females and males and their respective WT controls under the effect of a 3-month high-fat diet (HFD) compared to a normal diet (ND). (A) Body weight of 16-week-old mice. (B–E) Determination of inguinal (B), retroperitoneal (C) and gonadal (D) white fat pads and interscapular brown fat pad (E) of 16-week-old mice. (F) Blood glucose concentration after 18 h of fasting in 15-week-old mice. (G–H) GTT using 1 g/kg d-glucose i.p. injection in 15-week-old mice fasted for 18 h. Bars represent the area under the curve (n = 3–7 per group). (I–J) ITT using 1 IU/kg human recombinant insulin (i.p.) in 15-week-old mice of both sexes fasted for 4 h. Bars represent the area under the curve. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001; two-way ANOVA, Holm–Sidak. Values represent the mean ± SEM of a number of mice per group indicated at the base of each bar.
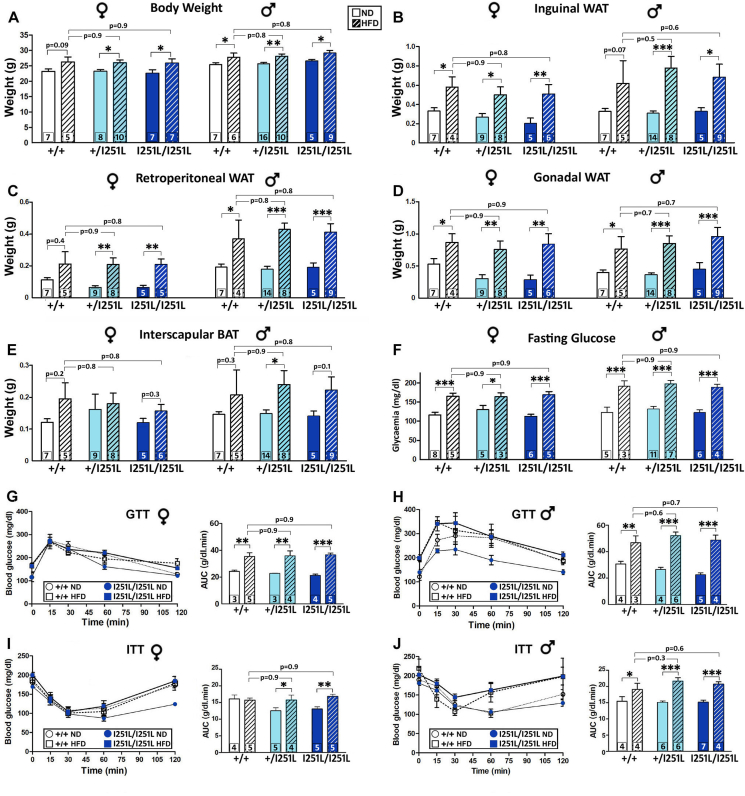
Analysis of mice carrying the I251L mutation in MC4R and fed on a HFD yielded very similar results to those described above for carriers of V103I alleles, including higher total body weight at 16 weeks of age (Figure 6A), increased weight in white fat pads (Figure 6B,D), elevated fasting blood glucose levels (Figure 6F) and impaired glucose clearing when challenged in a GTT (Figure 6G,H). Thus, in all these parameters, we did not observe any difference between WT and Mc4rI251L/I251L females or males fed with a HFD. We have also detected a few differences in the I251L group relative to the V103I cohort that included an impaired response to insulin in females carrying one or two I251L alleles fed with a HFD relative to those receiving a ND (Figure 6I) and in males of all genotypes (Figure 6J). These results indicate that although homozygous carriers of V103I or I251L mutations in the MC4R exhibited reduced adiposity and improved glucose metabolism, they were not protected against the obesogenic and diabetogenic effects elicited by a HFD.
Figure 6.
The I251L MC4R mutation does not protect against a high-fat diet. Metabolic phenotype of Mc4r+/I251L and Mc4rI251L/I251L females and males and their respective WT control littermates under the effect of a 3 month-high fat diet (HFD) compared to a normal diet (ND). (A) Body weight of 16-week-old mice (n = 5–16 per group). (B–E) Determination of inguinal (B), retroperitoneal (C) and gonadal (D) white fat pads and interscapular brown fat pad (E) of 16-week-old mice. (F) Blood glucose concentration after 18 h of fasting in 15-week-old mice. (G–H) GTT using 1 g/kg d-glucose i.p. injection in 15-week-old females and males fasted 18 h. Bars represent the area under the curve. (I–J) ITT using 1 IU/kg human recombinant insulin (i.p.) in 15-week-old females and males fasted for 4 h. Bars represent the area under the curve. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001; two-way ANOVA, Holm–Sidak. Values represent the mean ± SEM of a number of mice per group indicated at the base of each bar.
4. Discussion
The search for the genetic bases underlying a higher predisposition for obesity and T2D has been boosted in the last two decades by several genome-wide studies involving a large number of case–control groups in different countries [3,4]. Although the statistical power of these studies allowed the association of more than 100 loci with obesity-related phenotypes, each identified gene showed a relatively small effect on disease risk. Given the incalculable loss of years and quality of life of the patients and the enormous economic consequences of the obesity pandemic, the low predictive value of the vast majority of these loci for assessing an individual's risk is disappointing. One of the few exceptions, however, is MC4R, since its non-synonymous coding mutations are responsible for severe early-onset obesity [8,30], and single-nucleotide polymorphisms (SNPs) in its 3' flanking region have been associated with elevated BMI [31]. Recent population studies have also found non-synonymous coding mutations in MC4R that appear to protect from obesity and T2D [15]. Although these human mutations displayed hypermorphic (gain of function) outcomes when tested in vitro, they have never been experimentally tested in vivo. Because the genetic programmes, neuroanatomical circuits and physiological regulation of the human and mouse central melanocortin systems are highly similar, mouse models designed to study the effects of orthologous human mutations have high construct and predictive value, providing a useful platform to analyze the functional effects of such mutations in a live mammal.
Thus, in this study, we challenged the hypothesis that the V103I and I251L mutations in the mouse MC4R protect against increased adiposity and T2D, as has been suggested in association studies of human carriers of either one of these orthologous gain-of-function mutations [14,15,19]. To this end, we generated and studied mutant mice carrying the same nucleotide substitutions yielding V103I and I251L MCR4 alleles. In particular, we show that: 1) homozygous females carrying the V103I mutation displayed shorter longitudinal length and decreased abdominal white fat mainly due to a large reduction in gonadal fat; 2) homozygous females carrying the I251L mutation also displayed shorter longitudinal length and decreased weight of all abdominal white fat pads examined: gonadal, retroperitoneal and inguinal; 3) homozygous Mc4rV103I/V103I and Mc4rI251L/I251L mice of both sexes showed improved glucose homeostasis when challenged with a GTT; 4) Mc4rI251L/I251L females, but not males, showed improved insulin response when challenged in an insulin tolerance test; 5) the MC4R V103I and I251L mutations did not protect against the diabetogenic and adipogenic effects of a HFD as evidenced by a similar increase in fasting glucose levels and augmented adiposity in Mc4rV103I/V103I and Mc4rI251L/I251L mice of both sexes in comparison to their WT littermates. Altogether, the phenotypes observed in mice carrying Mc4r alleles with homozygous V103I or I251L substitutions are compatible with gain-of-function mutations, in contrast with loss-of-function MC4R mutants that display increased adiposity, longer stature and T2D [[11], [12], [13]]. Further studies are needed to determine potential differences in respiratory exchange ratio, energy expenditure and distribution of fat deposition in the mutant mice.
Homozygous mutant females carrying V103I or I251L Mc4r alleles display shorter longitudinal growth, a phenotype also found in a recent association study performed in children carrying V103I MC4R variants [32]. Animal longitudinal length and human height are highly heritable polygenic traits, and although many genes contribute to a small percentage of the overall variation of human height, there are some monogenic examples that produce relatively large effects, including MC4R. In fact, MC4R loss-of-function mutations in the human population have been linked to increased stature [33,34], and a similar phenotype was described in MC4R null-allele mutant mice [13]. Given the importance of insulin in body growth [35] and that MC4R stimulation diminishes insulin release in mice [36], it is tempting to speculate that hypermorphic V103I and I251L MC4Rs lead to a reduction in insulin levels and, consequently, to shorter longitudinal body length.
A noticeable outcome of our study is that the reduced adiposity, shorter axial length and improved glucose metabolism observed in V103I and I251L MC4R mutants were much more pronounced in females than in males. Body fat distribution and energy balance are sexually dimorphic, probably because most metabolic traits are highly influenced by sexual hormones. For example, male mice display higher food intake and energy expenditure even when normalised by their larger body mass and also gain more body weight than females when fed a hypercaloric diet. Females, in turn, have more pro-opiomelanocortin (POMC) neurons in the arcuate nucleus of the hypothalamus [37] and are more sensitive to the anorexigenic effects of central leptin [38,39]. In agreement, sexually dimorphic phenotypes are also commonly found in animal models used to study the effects of gene mutations in the control of energy balance and metabolism. For example, we previously showed that extremely obese mice lacking hypothalamic Pomc considerably improved their body weight and adiposity only in females once Pomc expression was re-established, whereas males were more resistant to regaining their normal weight [40]. In addition, Pomc rescue restricted to 5-HT2C positive arcuate neurons re-established normal levels of physical activity, energy expenditure and body weight only in male mice [41]. Regarding MC4R, female mice lacking this receptor exhibit an even greater increase in body weight than males [13]. In humans, SNP rs17782313 has also been linked to obesity and increased T2D risk only in women [[42], [43], [44]], whereas several loss of function human variants showed a greater increase in BMI in females than in males [45]. Another study showed that women carrying the gain of function mutation V103I displayed a more pronounced reduction in BMI than men [46]. Altogether, these studies suggest that females are more responsive to loss or gain of function mutations of this receptor.
MC4R exhibits a great level of genetic variation in the human population (Figure 1A). For example, a recent association study performed in 452,300 individuals of European ancestry taken from the UK Biobank detected 61 non-synonymous polymorphic variants, including 46 missense mutations that yielded loss and/or gain of function phenotypes when tested in transfected HEK293 cells for cAMP production or the recruitment of β-arrestin-2 [15]. While loss-of-function variants identified in this study were associated with higher odds of obesity, T2D and coronary artery disease, individuals carrying gain of function variants showed lower odds to develop these conditions. In particular, the gain of function variants V103I and I251L are present in the UK Biobank population at relatively high frequencies (2.0% and 1.3%, respectively), in quite contrast to all other variants that showed an allele frequency lower than 0.07%. It may be speculated that these two alleles are maintained at relatively higher frequencies because they provide some level of protection against environmental conditions of excessive overconsumption of hypercaloric edibles.
Interestingly, the melanocortin 1 receptor gene (MC1R), paralog and ancestor of MC4R, is also polymorphic in its coding region and produces heritable gain of function variation with functional adaptive consequences [47,48], as found in several wild mouse populations that underwent an evolutionary darkening of their dorsal pelage after sandy rock terrains transitioned to black cooled lava flow lands following activity of nearby volcanoes [49]. The newly established darker landscape was likely to exert selective pressure for the fixation of novel Mc1r mutations that promoted the production of dark eumelanin-stained hairs restoring camouflage adaptation against visual predators, such as hunting owls. Thus, MC4R and MC1R are likely to share high levels of evolvability, defined as an ability to produce heritable and adaptive phenotypic variation driven by environmental changes [50]. While the thrifty gene theory suggests that genetic programs controlling energy balance evolved to adapt to extensive periods of food scarcity [51], such conditions are no longer present in vast sectors of modern human societies, allowing hypermorphic variants of MC4R to coexist in relatively high frequencies within the current genetic pool and even provide some protection against the deleterious consequences of current obesogenic diets. Although the V103I and I251L mutant mice studied here were found to be leaner and metabolically more efficient when eating normal chow, they failed to overcome the negative consequences of a hypercaloric diet, highlighting the importance of healthy feeding habits even under favourable genetic conditions.
Author contributions
D.R. and M.R. designed this study; D.R., C.M. and J.R. conducted the research; J.R. and M.R. contributed new reagents and analytic tools; D.R., C.M., J.R. and M.R. analyzed the data and prepared the figures; M.R. wrote the paper; and D.R., C.M., J.R. and M.R. revised the paper.
Acknowledgements
The authors thank Marta Treimun and Carolina Álvarez for excellent technical work. This work was supported by the Agencia Nacional de Promoción Científica y Tecnológica, PICT2016-1742. (M.R.). D.R. received a doctoral fellowship from CONICET.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2020.101077.
Conflict of interest
The authors declare no conflict of interest.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Mouse mutant MC4Rs display normal membrane expression and are fully functional in vitro. (A) Representative microphotographs of HEK293T cells transfected with WT MC4R, V103 MC4R or I251L MC4R tagged with YFP (yellow fluorescent protein) and treated with Hoechst fluorescent dye to stain nuclei. Scale bars indicate 5 μm. Imaging experiments confirmed that YFP-tagged V103 MC4R and I251L MC4R expression levels were equal to the MC4R WT control. (B) Averaged ICa and representative current traces registered in tsA201 cells transfected with CaV2.1, auxiliary subunits, WT MC4R, V103 MC4R or I251L MC4R (1:1 M proportion compared to CaV2.1). Kruskal–Wallis test of one-way ANOVA. Dunn's post-test (CaV2.1). ∗p < 0.005 vs Cav2.1 control. (C) MC4R agonist-evoked activation inhibitory effect on voltage-mediated calcium channels type 2.2 (CaV2.2). CaV2.2 whole-cell currents were registered in transfected HEK293T cells and bath application of the agonist MTII was able to inhibit calcium currents in WT MC4R (max inhibition = 30.43 ± 3.84%, EC50 = 135.5 nM, r2 = 0.61), V103I MC4R (max inhibition = 32.12 ± 2.88%, EC50 = 170.3 nM, r2 = 0.84) and I251L MC4R (max inhibition = 32.03 ± 2.38%, EC50 = 162.9 nM, r2 = 0.86). Values represent the mean ± SEM.
References
- 1.Preventing and managing the global epidemic of obesity. World Health Organization; 2015. [PubMed] [Google Scholar]
- 2.James W.P.T. Obesity: a global public health challenge. Clinical Chemistry. 2018;64(1):24–29. doi: 10.1373/clinchem.2017.273052. [DOI] [PubMed] [Google Scholar]
- 3.Thorleifsson G., Walters G.B., Gudbjartsson D.F., Steinthorsdottir V., Sulem P., Helgadottir A. Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity. Nature Genetics. 2009;41(1):18–24. doi: 10.1038/ng.274. [DOI] [PubMed] [Google Scholar]
- 4.Wheeler E., Huang N., Bochukova E.G., Keogh J.M., Lindsay S., Garg S. Genome-wide SNP and CNV analysis identifies common and low-frequency variants associated with severe early-onset obesity. Nature Genetics. 2013;45(5):513–517. doi: 10.1038/ng.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaulton K.J., Ferreira T., Lee Y., Raimondo A., Mägi R., Reschen M.E. Genetic fine mapping and genomic annotation defines causal mechanisms at type 2 diabetes susceptibility loci. Nature Genetics. 2015;47(12):1415–1425. doi: 10.1038/ng.3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loos R.J.F. Genetic determinants of common obesity and their value in prediction. Best Practice & Research Clinical Endocrinology & Metabolism. 2012:211–226. doi: 10.1016/j.beem.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Krude H., Biebermann H., Luck W., Horn R., Brabant G., Grüters A. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nature Genetics. 1998;19(2):155–157. doi: 10.1038/509. [DOI] [PubMed] [Google Scholar]
- 8.Vaisse C., Clement K., Guy-Grand B., Froguel P. A frameshift mutation in human MC4R is associated with a dominant form of obesity [2] Nature Genetics. 1998;20(2):113–114. doi: 10.1038/2407. [DOI] [PubMed] [Google Scholar]
- 9.Balthasar N., Dalgaard L.T., Lee C.E., Yu J., Funahashi H., Williams T. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123(3):493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 10.Krashes M.J., Lowell B.B., Garfield A.S. Melanocortin-4 receptor-regulated energy homeostasis. Nature Neuroscience. 2016;19(2):206–219. doi: 10.1038/nn.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vaisse C., Clement K., Durand E., Hercberg S., Guy-Grand B., Froguel P. Melanocortin-4 receptor mutations are a frequent and heterogeneous cause of morbid obesity. Journal of Clinical Investigation. 2000;106(2):253–262. doi: 10.1172/JCI9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farooqi I.S., Keogh J.M., Yeo G.S.H., Lank E.J., Cheetham T., O'Rahilly S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. New England Journal of Medicine. 2003;348(12):1085–1095. doi: 10.1056/NEJMoa022050. [DOI] [PubMed] [Google Scholar]
- 13.Huszar D., Lynch C.A., Fairchild-Huntress V., Dunmore J.H., Fang Q., Berkemeier L.R. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997 doi: 10.1016/S0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 14.Young E.H., Wareham N.J., Farooqi S., Hinney A., Hebebrand J., Scherag A. The V103I polymorphism of the MC4R gene and obesity: population based studies and meta-analysis of 29 563 individuals. International Journal of Obesity. 2007 doi: 10.1038/sj.ijo.0803609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lotta L.A., Mokrosiński J., Mendes de Oliveira E., Li C., Sharp S.J., Luan J. Human gain-of-function MC4R variants show signaling bias and protect against obesity. Cell. 2019;177(3):597–607. doi: 10.1016/j.cell.2019.03.044. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiang Z., Litherland S.a., Sorensen N.B., Proneth B., Wood M.S., Shaw A.M. Pharmacological characterization of 40 human melanocortin-4 receptor polymorphisms with the endogenous proopiomelanocortin-derived agonists and the agouti-related protein (AGRP) antagonist. Biochemistry. 2006;45(23):7277–7288. doi: 10.1021/bi0600300. [DOI] [PubMed] [Google Scholar]
- 17.Gotoda T., Scott J., Aitman T.J. 1997. Molecular screening of the human melanocortin-4 receptor gene : identification of a missense variant showing no association with obesity, plasma glucose, or insulin 4; pp. 976–979. [DOI] [PubMed] [Google Scholar]
- 18.Geller F., Reichwald K., Dempfle A., Illig T., Vollmert C., Herpertz S. Melanocortin-4 receptor gene variant I103 is negatively associated with obesity. The American Journal of Human Genetics. 2004;74(3):572–581. doi: 10.1086/382490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stutzmann F., Vatin V., Cauchi S., Morandi A., Jouret B., Landt O. Non-synonymous polymorphisms in melanocortin-4 receptor protect against obesity: the two facets of a Janus obesity gene. Human Molecular Genetics. 2007 doi: 10.1093/hmg/ddm132. [DOI] [PubMed] [Google Scholar]
- 20.Wang D., Ma J., Zhang S., Hinney A., Hebebrand J., Wang Y. Association of the MC4R V103I polymorphism with obesity: a Chinese case-control study and meta-analysis in 55,195 individuals. Obesity. 2010 doi: 10.1038/oby.2009.268. [DOI] [PubMed] [Google Scholar]
- 21.Ohshiro Y., Sanke T., Ueda K., Shimajiri Y., Nakagawa T., Tsunoda K. Molecular scanning for mutations in the melanocortin-4 receptor gene in obese/diabetic Japanese. Annals of Human Genetics. 1999;63(6):483–487. doi: 10.1046/j.1469-1809.1999.6360483.x. [DOI] [PubMed] [Google Scholar]
- 22.Wang C.L., Liang L., Wang H.J., Fu J.F., Hebebrand J., Hinney A. Several mutations in the melanocortin 4 receptor gene are associated with obesity in Chinese children and adolescents. Journal of Endocrinological Investigation. 2006;29(10):894–898. doi: 10.1007/BF03349193. [DOI] [PubMed] [Google Scholar]
- 23.Larkin M.A., Blackshields G., Brown N.P., Chenna R., Mcgettigan P.A., McWilliam H. Clustal W and clustal X version 2.0. Bioinformatics. 2007;23(21):2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 24.Agosti F., López Soto E.J., Cabral A., Castrogiovanni D., Schioth H.B., Perelló M. Melanocortin 4 receptor activation inhibits presynaptic N-type calcium channels in amygdaloid complex neurons. European Journal of Neuroscience. 2014;40(5):2755–2765. doi: 10.1111/ejn.12650. [DOI] [PubMed] [Google Scholar]
- 25.Agosti F., Cordisco Gonzalez S., Martinez Damonte V., Tolosa M.J., Di Siervi N., Schioth H.B. Melanocortin 4 receptor constitutive activity inhibits L-type voltage-gated calcium channels in neurons. Neuroscience. 2017;346(January):102–112. doi: 10.1016/j.neuroscience.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 26.Nih Od., Oer, Olaw . 2011. Guide laboratory animals for the care and use OF eighth edition committee for the update of the guide for the Care and use of laboratory animals institute for laboratory animal research division on earth and life studies. [Google Scholar]
- 27.Bae S., Park J., Kim J.S. Cas-OFFinder: a fast and versatile algorithm that searches for potential off-target sites of Cas9 RNA-guided endonucleases. Bioinformatics. 2014;30(10):1473–1475. doi: 10.1093/bioinformatics/btu048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montgomery M.K., Hallahan N.L., Brown S.H., Liu M., Mitchell T.W., Cooney G.J. Mouse strain-dependent variation in obesity and glucose homeostasis in response to high-fat feeding. Diabetologia. 2013;56(5):1129–1139. doi: 10.1007/s00125-013-2846-8. [DOI] [PubMed] [Google Scholar]
- 29.Nascimento-Sales M., Fredo-da-Costa I., Borges Mendes A.C.B., Melo S., Ravache T.T., Gomez T.G.B. Is the FVB/N mouse strain truly resistant to diet-induced obesity? Physiological Reports. 2017;5(9) doi: 10.14814/phy2.13271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yeo G.S.H., Farooqi I.S., Aminian S., Halsall D.J., Stanhope R.G., O'Rahilly S. A frameshift mutation in MC4R associated with dominantly inherited human obesity. Nature Genetics. 1998;20(2):111–112. doi: 10.1038/2404. [DOI] [PubMed] [Google Scholar]
- 31.Loos R.J.F., Loos R.J.F., Lindgren C.M., Lindgren C.M., Li S., Li S. Common variants near MC4R are associated with fat mass, weight and risk of obesity. Nature Genetics. 2008;40(6):768–775. doi: 10.1038/ng.140.Common. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herrfurth N., Volckmar A.L., Peters T., Kleinau G., Müller A., Cetindag C. Relevance of polymorphisms in MC4R and BDNF in short normal stature. BMC Pediatrics. 2018;18(1):5–9. doi: 10.1186/s12887-018-1245-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martinelli C.E., Keogh J.M., Greenfield J.R., Henning E., Van Der Klaauw A.A., Blackwood A. Obesity due to melanocortin 4 receptor (MC4R) deficiency is associated with increased linear growth and final height, fasting hyperinsulinemia, and incompletely suppressed growth hormone secretion. Journal of Clinical Endocrinology & Metabolism. 2011;96(1):181–188. doi: 10.1210/jc.2010-1369. [DOI] [PubMed] [Google Scholar]
- 34.Vollbach H., Brandt S., Lahr G., Denzer C., Von Schnurbein J., Debatin K.M. Prevalence and phenotypic characterization of MC4R variants in a large pediatric cohort. International Journal of Obesity. 2017;41(1):13–22. doi: 10.1038/ijo.2016.161. [DOI] [PubMed] [Google Scholar]
- 35.Hill D.J., Milner R.D.G. vol. 19. 1985. (Insulin as a growth factor). [DOI] [PubMed] [Google Scholar]
- 36.Fan W., Dinulescu D.M., Butler A.A., Zhou J., Marks D.L., Cone R.D. The central melanocortin system can directly regulate serum insulin levels. Endocrinology. 2000;141(9):3072–3079. doi: 10.1210/endo.141.9.7665. [DOI] [PubMed] [Google Scholar]
- 37.Wang C., He Y., Xu P., Yang Y., Saito K., Xia Y. TAp63 contributes to sexual dimorphism in POMC neuron functions and energy homeostasis. Nature Communications. 2018;9(1):1–11. doi: 10.1038/s41467-018-03796-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clegg D.J., Riedy C.A., Smith K.A.B., Benoit S.C., Woods S.C. Differential sensitivity to central leptin and insulin in male and female rats. Diabetes. 2003;52(3):682–687. doi: 10.2337/diabetes.52.3.682. [DOI] [PubMed] [Google Scholar]
- 39.Clegg D.J., Brown L.M., Woods S.C., Benoit S.C. Erratum: gonadal hormones determine sensitivity to central leptin and insulin (Diabetes (2006) 55 (978-987)) Diabetes. 2007;56(10):2649. doi: 10.2337/db07-er10. [DOI] [PubMed] [Google Scholar]
- 40.Bumaschny V.F., Yamashita M., Casas-Cordero R., Otero-Corchón V., de Souza F.S.J., Rubinstein M. Obesity-programmed mice are rescued by early genetic intervention. Journal of Clinical Investigation. 2012;122(11):4203–4212. doi: 10.1172/JCI62543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burke L.K., Doslikova B., D'Agostino G., Greenwald-Yarnell M., Georgescu T., Chianese R. Sex difference in physical activity, energy expenditure and obesity driven by a subpopulation of hypothalamic POMC neurons. Molecular Metabolism. 2016;5(3):245–252. doi: 10.1016/j.molmet.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Horstmann A., Kovacs P., Kabisch S., Boettcher Y., Schloegl H., Tönjes A. Common genetic variation near MC4R has a sex-specific impact on human brain structure and eating behavior. PloS One. 2013;8(9):14–16. doi: 10.1371/journal.pone.0074362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu G., Zhu H., Lagou V., Gutin B., Barbeau P., Treiber F.A. Common variants near MC4R are associated with general and visceral adiposity in European- and African-American youth. Journal of Pediatrics. 2014;156(4):598–605. doi: 10.1016/j.jpeds.2009.10.037.Common. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Renström F., Payne F., Nordström A., Brito E.C., Rolandsson O., Hallmans G. Replication and extension of genome-wide association study results for obesity in 4923 adults from northern Sweden. Human Molecular Genetics. 2009;18(8):1489–1496. doi: 10.1093/hmg/ddp041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dempfle A., Hinney A., Heinzel-Gutenbrunner M., Raab M., Geller F., Gudermann T. Large quantitative effect of melanocortin-4 receptor gene mutations on body mass index. Journal of Medical Genetics. 2004;41(10):795–800. doi: 10.1136/jmg.2004.018614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heid I.M., Vollmert C., Hinney A., Döring A., Geller F., Löwel H. Association of the 103I MC4R allele with decreased body mass in 7937 participants of two population based surveys. Journal of Medical Genetics. 2005:e21. doi: 10.1136/jmg.2004.027011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoekstra H.E., Hirschmann R.J., Bundey R.A., Insel P.A., Crossland J.P. A single amino acid mutation contributes to adaptive beach mouse color pattern. Science. 2006;313(5783):101–104. doi: 10.1126/science.1126121. [DOI] [PubMed] [Google Scholar]
- 48.Gonçalves G.L., Paixão-Côrtes V.R., Freitas T.R.O. Molecular evolution of the melanocortin 1-receptor pigmentation gene in rodents. Genetics and Molecular Research. 2013;12(3):3230–3245. doi: 10.4238/2013.February.28.24. [DOI] [PubMed] [Google Scholar]
- 49.Nachman M.W., Hoekstra H.E., D'Agostino S.L. The genetic basis of adaptive melanism in pocket mice. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(9):5268–5273. doi: 10.1073/pnas.0431157100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Payne J.L., Wagner A. The causes of evolvability and their evolution. Nature Reviews Genetics. 2019:24–38. doi: 10.1038/s41576-018-0069-z. [DOI] [PubMed] [Google Scholar]
- 51.Zimmet P., Thomas C.R. Genotype, obesity and cardiovascular disease - has technical and social advancement outstripped evolution? Journal of Internal Medicine. 2003:114–125. doi: 10.1046/j.1365-2796.2003.01170.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mouse mutant MC4Rs display normal membrane expression and are fully functional in vitro. (A) Representative microphotographs of HEK293T cells transfected with WT MC4R, V103 MC4R or I251L MC4R tagged with YFP (yellow fluorescent protein) and treated with Hoechst fluorescent dye to stain nuclei. Scale bars indicate 5 μm. Imaging experiments confirmed that YFP-tagged V103 MC4R and I251L MC4R expression levels were equal to the MC4R WT control. (B) Averaged ICa and representative current traces registered in tsA201 cells transfected with CaV2.1, auxiliary subunits, WT MC4R, V103 MC4R or I251L MC4R (1:1 M proportion compared to CaV2.1). Kruskal–Wallis test of one-way ANOVA. Dunn's post-test (CaV2.1). ∗p < 0.005 vs Cav2.1 control. (C) MC4R agonist-evoked activation inhibitory effect on voltage-mediated calcium channels type 2.2 (CaV2.2). CaV2.2 whole-cell currents were registered in transfected HEK293T cells and bath application of the agonist MTII was able to inhibit calcium currents in WT MC4R (max inhibition = 30.43 ± 3.84%, EC50 = 135.5 nM, r2 = 0.61), V103I MC4R (max inhibition = 32.12 ± 2.88%, EC50 = 170.3 nM, r2 = 0.84) and I251L MC4R (max inhibition = 32.03 ± 2.38%, EC50 = 162.9 nM, r2 = 0.86). Values represent the mean ± SEM.