Abstract
Introduction
The thalamus is a key hub for regulating cortical connectivity. Dysmaturation of thalamocortical networks that accompany white matter injury has been hypothesized as neuroanatomical correlate of late life neurocognitive impairment following preterm birth. Our objective was to find a link between thalamocortical connectivity measures at term equivalent age and two‐year neurodevelopmental outcome in preterm infants.
Methods
Diffusion tensor MRI data of 58 preterm infants (postmenstrual age at birth, mean (SD), 29.71 (1.47) weeks) were used in the study. We utilized probabilistic diffusion tractography to trace connections between the cortex and thalami. Possible associations between connectivity strength, the length of the probabilistic fiber pathways, and developmental scores (Bayley Scales of Infant Development, Second Edition) were analyzed using multivariate linear regression models.
Results
We found strong correlation between mental developmental index and two complementary measures of thalamocortical networks: Connectivity strength projected to a cortical skeleton and pathway length emerging from thalamic voxels (partial correlation, R = .552 and R = .535, respectively, threshold‐free cluster enhancement, corrected p‐value < .05), while psychomotor development was not associated with thalamocortical connectivity. Post hoc stepwise linear regression analysis revealed that parental socioeconomic scale, postmenstrual age, and the duration of mechanical ventilation at the intensive care unit contribute to the variability of outcome.
Conclusions
Our findings independently validated previous observations in preterm infants, providing additional evidence injury or dysmaturation of tracts emerging from ventral‐specific and various nonspecific thalamus projecting to late‐maturing cortical regions are predictive of mental, but not psychomotor developmental outcomes.
Keywords: mental development, neurodevelopment, newborn, nonspecific thalamus, preterm, thalamocortical tracts, thalamus, ventral thalamus
Our purpose was to find a link between thalamocortical connectivity measures at term equivalent age and two‐year neurodevelopmental outcome in 58 infants following preterm birth. Using probabilistic diffusion tractography, we found that injury or dysmaturation of tracts emerging from ventral‐specific and various nonspecific thalamus projecting to late‐maturing cortical regions is predictive of mental, but not psychomotor developmental outcomes
![]()
1. INTRODUCTION
Preterm birth is a risk factor for a variety of neurodevelopmental impairments. Many children face impairments across multiple neurodevelopmental domains such as cognition, and language, and motor skills (Woodward et al., 2009). Prominent problems are seen in executive functions, language development, and behavior (Van't Hooft et al., 2015) persisting into adolescence (Wehrle et al., 2016) and adulthood (Nosarti et al., 2009). More recently, a preterm behavioral phenotype has been described that exhibits inattention, anxiety, and social communication deficits (Johnson & Marlow, 2011). Early brain alterations associated with preterm birth have been described as a complex amalgam of destructive and developmental disturbances and may lead to atypical brain development and ultimately to such impairments. Indeed, there is convincing evidence that very preterm infants are at substantial risk of brain injury in the perinatal period (Volpe, 2009a). The periventricular white matter (WM) seems to suffer the primary injury, and this WM injury is frequently accompanied by neuronal and axonal disease affecting the cerebral WM, thalamus, basal ganglia, cerebral cortex, brain stem, and cerebellum (Volpe, 2009a, 2009b).
The thalamus acts as a key hub for cortical networks and thalamocortical connections, and it is commonly affected, especially in preterm infants, by WM injury, either directly or through maturational disturbance (Boardman et al., 2006; Nosarti et al., 2008). The development of thalamocortical connections during the mid‐gestational and late gestational periods plays a critical role in shaping brain connectivity during prenatal and early postnatal life (Ghosh, Antonini, McConnell, & Shatz, 1990; Kostovi & Juda, 2010; McQuillen & Ferriero, 2005). The ingrowth of thalamocortical connectivity is a crucial milestone for the sensory‐expectant organization and functional specialization of the cerebral cortex (Kostovic & Judas, 2002; Molliver, Kostovic, & van der Loos, 1973; Molnar, Adams, & Blakemore, 1998). The topographical organization of thalamic connections is established early after side branches reach the cortex radially from the subplate (Catalano, Robertson, & Killackey, 1996; Ghosh & Shatz, 1992), and their organization is further shaped by activity‐dependent synaptic interaction (Molnar et al., 2002). Thalamocortical fibers begin to relocate to the cortical plate around the 24th week of gestation in sensory and later in association cortices (Kostovic & Judas, 2006, 2007). Crucially, this is the period of biological vulnerability due to prematurity. Together, these developments provide strong biological evidence that the maturation of thalamocortical connectivity is affected by premature birth and that injury to the thalamocortical circuitry may indirectly affect cortical functional specialization.
Evidence is emerging for alterations in thalamocortical connectivity after preterm birth at term equivalent age (Ball, Boardman, et al., 2013; Ball, Srinivasan, et al., 2013; Fischi‐Gomez et al., 2016; Kelly et al., 2016; Pandit et al., 2014; van den Heuvel et al., 2015) and that such alterations are associated with later neurodevelopment (Ball et al., 2015; Fischi‐Gomez et al., 2016). Hence, both injury to the thalamus and alterations to the thalamocortical connections might impact the cognitive abilities of preterm infants. For example, the role of the mediodorsal thalamus in distinct cognitive behavior that relies on various prefrontal regions has been described in patients with schizophrenia (Woodward, Karbasforoushan, & Heckers, 2012), and anatomical variability of the connections of the mediodorsal nucleus has been linked to the variation of executive functions in adults (Jakab, Blanc, & Berenyi, 2012).
A better understanding of the pathomechanism of injury to the thalamocortical circuitry after preterm birth is essential to elucidate how its alteration may contribute to cognitive impairment. The present study was designed to provide further evidence and independent validation for the theory that the dysmaturation of thalamocortical connectivity in preterm infants is predictive of later neurodevelopmental outcomes. We hypothesize that the neurodevelopmental sequelae mirror the emergence of thalamocortical connectivity topography and that the thalamocortical connectivity of late‐maturing regions is therefore predictive of cognitive outcomes. Testing this hypothesis required us to identify the subthalamic anatomical locations with the strongest putative correlation between thalamocortical connectivity and later life cognition.
2. METHODS
The preterm infants in this study represent a subgroup of infants enrolled in a randomized, double‐blind, placebo‐controlled, prospective multicenter study titled “Does erythropoietin improve outcome in preterm infants?” (NCT00413946) that were examined by means of a cranial MR at term equivalent age. This subgroup of infants has been described previously (Jakab et al., 2019; O'Gorman et al., 2015): The main criterion for subjects to be enrolled in this subgroup was the availability of good quality cerebral diffusion tensor imaging (DTI) data. Fifty‐eight preterm infants with mean (SD) gestational age at birth of 29.75 (1.44) weeks and at scanning of 41.09 (2.09) weeks) were included in this analysis. The characteristics of the infants are described in Table 1. Socioeconomic status was estimated by a validated 12‐point socioeconomic score based on maternal education and paternal occupation, and the infants were classified into higher class (score 2–5), middle class (6–8), and lower class (9–12) ((Largo et al., 1989). The local ethical committee approved the project (KEK StV‐36/04), and the Swiss drug surveillance unit (Swissmedic, 2005DR3179) approved the study. The trial was registered at ClinicalTrials.gov (number NCT00413946). The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Table 1.
Study population
| Characteristics | Value |
|---|---|
| Mean (SD) GA at births (weeks) | 29.71 (1.47) |
| Mean (SD) birthweight (g) | 1252 (314) |
| Mean (SD) age at MRI | 41.09 (2.1) |
| Female, n (%) | 39 (67%) |
| Chronic lung disease, n (%) | 9 (15.5%) |
| ROP > 3 | 3 (5.1%) |
| Necrotizing enterocolitis, n (%) | 1 (5.2%) |
| Median (range) Socioeconomic Status, SES | 5 (1–10) |
Abbreviation: ROP, Retinopathy of prematurity.
2.1. Outcome assessments
Two‐year neurodevelopmental outcomes of the whole study population have previously been published (Natalucci et al., 2016). A developmental assessment using the Bayley Scales of Infant Development, second edition (BSID‐II, Bayley, 1993), was performed at a mean age of 23.4 (2.33) months by experienced developmental specialists. Two outcome measures were calculated: mental development index (MDI) and psychomotor development index (PDI). The developmental specialists were blinded to the MRI findings.
2.2. MRI acquisition
Neonatal cerebral MRI was performed at term equivalent age with a 3.0 T GE scanner (GE Medical Systems), using an eight‐channel receive‐only head coil. All infants were scanned during natural sleep using a vacuum mattress. Ear plugs and miniMuffs were applied for noise protection. During the scanning, oxygen saturation was monitored, and a neonatologist and a neonatal nurse were present.
Diffusion tensor imaging (DTI) was acquired using a pulsed gradient spin echo planar imaging sequence with TE/TR: 77/9,000 ms, field of view = 18 cm, matrix = 128 × 128, slice thickness = 3 mm. For each infant, 21 noncollinear gradient encoding directions with b = 700 s/mm2 and four interleaved b = 0 images were acquired. The DTI data (n = 58) used in our study are part of a previously reported data set (n = 140; Natalucci et al., 2016).
2.3. Image postprocessing
An overview of the image postprocessing steps is provided in Figure 1. DTI data were visually controlled for artifacts. Image frames and the corresponding entries in the b‐matrix and b‐value descriptor files were removed from further analysis if head movement of the infant caused extensive signal dropout throughout the brain in the given frame in more than one slice along the superoinferior axis. We discarded these data if the newborn woke up or moved excessively during the DTI scan. The entire data set was excluded if more than three diffusion‐weighting gradient volumes were corrupted by motion artifacts. We experienced high dropout rate because DTI was acquired toward the end of the examination, and many infants woke up or moved excessively (excluded data sets based on qualitative visual assessment: n = 78). Four infants were excluded because of cystic lesions (n = 4). In the remaining cases, the number of removed image frames (due to excessive patient motion) was recorded as a confounder.
Figure 1.
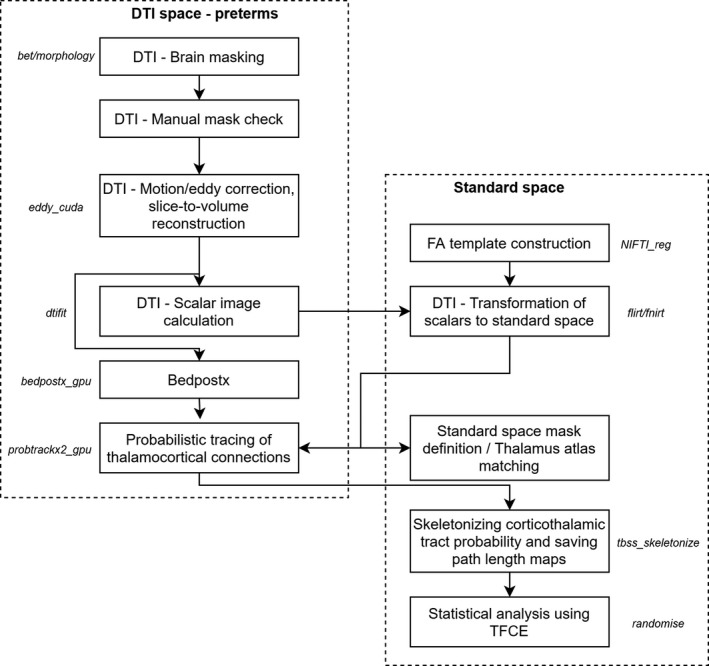
Overview of the diffusion tensor imaging (DTI) processing steps
We used a custom script written in Bash language for Linux to process the neonatal DTI images, which embedded the following image processing libraries and software. During the first step of postprocessing, spurious image shifts originating from eddy currents were corrected with the eddy command in the Functional Magnetic Resonance Imaging of the Brain Software Library (FSL) software (volume‐to‐volume reconstruction, GPU implementation). Afterward, Rician noise filtering of DTI was performed using the command line module JointRicianLMMSEImageFilter in Slicer 3D (Aja‐Fernandez, Tristan‐Vega, & Alberola‐Lopez, 2009). Diffusion tensors and scalar maps were estimated in each voxel using the dtifit program in FSL with weighted least squares estimation.
Next, a standard space fractional anisotropy (FA) template image was created by the three‐step coregistration of 40 FA images to the 42‐week template of the ALBERTs neonatal atlas (Gousias et al., 2012). The source of the DTI data was an independent study group from the same institution and consisted of normally developing newborns imaged at term equivalent age (mean, SD (range) postmenstrual age (PMA) PMA of the subjects: 42.5 ± 1.9 (39–48.7) weeks, 20 females and 20 males). The data of the template cohort were acquired on the same MRI scanner, however, with different scanner software version and using a DTI sequence with 25 diffusion encoding directions. First, the S0 (equivalent to T2‐weighting) images were aligned to the T2‐weighted template from the ALBERTs atlas using a 12‐degrees‐of‐freedom linear registration in FSL. The resulting transformation matrix was used to coarsely align the FA image of each individual to the template, and a mean linear FA template was saved by averaging the resulting images. Then, each normal subjects’ FA image was linearly and then nonlinearly coregistered with the mean linear FA template to achieve a sharper, nonlinear FA template. For the nonlinear registration step, the reg_f3d command was used in the NIFTIREG software package. The deformation grid vertex distance was 9 mm; the regularization criterion was the weight of the bending energy penalty term set to 0.05; and the smoothness of the deformation fields was achieved by a Gaussian smoothing with a spherical kernel of 4 mm diameter. The FA image of each individual was used to guide the nonlinear transformation that achieved overlap between the DTI space and the standard space FA template. This transformation was used to propagate three binary masks from the ALBERTs atlas to the subject space: whole cortex map, whole thalamus map, and cerebrospinal fluid spaces.
2.4. Anatomical subdivisions of the newborn thalamus
As there are currently no cyto‐ or myeloarchitecture‐based atlases for the human newborn brain, we utilized the statistical shape model version of the adult Morel atlas to localize thalamic subdivisions in the newborn brain (Krauth et al., 2010). This atlas incorporates histological definitions of nuclei based on seven postmortem samples. We delineated the visible macroscopic borders of the thalamus on the T2‐weighted, gestational age‐specific template of the ALBERT atlas. First, each training sample was aligned to the template by minimizing the surface distance between the MRI and histology‐based thalamus outlines with a thin‐plate registration algorithm. The statistical shape model stores anatomical correspondences between the outer borders of the whole thalamus and each thalamic nucleus in each training sample, and this function was used to predict the unobservable geometry (within‐thalamus borders) for the rest of the thalamus. Confidence region maps for nuclei were generated by projecting the matching uncertainty of each vertex into a separate grayscale image for each nucleus. A label value in each voxel identified the most probable thalamic nucleus based on the highest confidence (using the find_the_biggest command in FSL). The delineation and statistical shape‐matching procedure was performed in the NeuroShape extension of the Slicer 3D software, version 4.8.10. The NeuroShape software is based on the method described in our previous work (Jakab, Blanc, Berenyi, & Szekely, 2012). The resulting masks (Figure 2) were used to localize the thalamic specificity of our findings.
Figure 2.
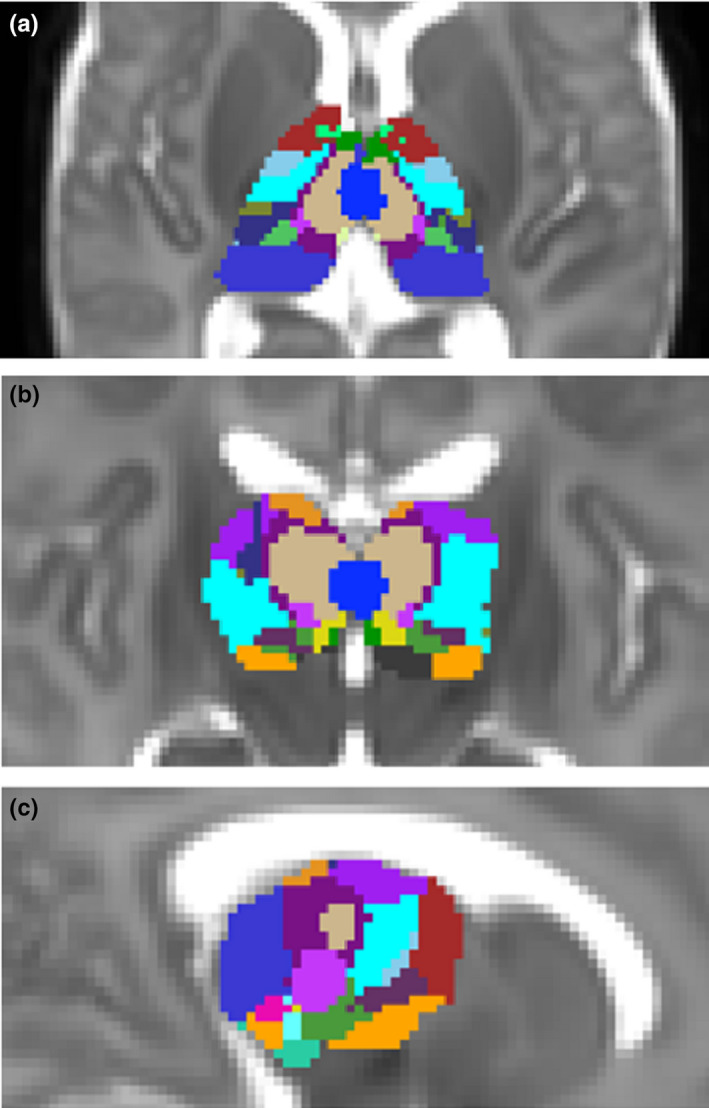
Neonatal thalamus atlas derived from the statistical shape model‐based adaptation of an adult thalamus atlas to a T2‐weighted neonatal atlas template corresponding to the 42nd postmenstrual week
2.5. Thalamocortical connectivity mapping
The association between cognitive and thalamocortical development was tested with four complementary analysis approaches. The first analysis (step 1) was based on a method previously reported (Ball et al., 2015). In this step, thalamocortical connectivity strength (TC) was projected to a cortical skeleton. In step 2, we analyzed the thalamic origins of the fiber pathways of the first step by measuring the apparent length of thalamocortical fiber pathways reaching the thalamus (TC length). In step 3, we quantified how strongly each thalamic nucleus is connected to the cortical regions in which thalamocortical connectivity was found to be predictive of 2‐year cognitive development. As diffusion tractography is unable to differentiate between afferent and efferent connectivity, we use the expression “thalamocortical” for both corticothalamic and thalamocortical connections throughout the manuscript. In step 4, we performed a confirmatory analysis by seeding the tractography from the thalamus and terminating at the cortex. This was carried out in order to investigate whether our findings have biological basis and to rule out if associations with cognitive development emerged as a result of technical difficulties in resolving the fiber pathways adjacent to the cortex,
Thalamocortical anatomical connectivity was mapped with probabilistic diffusion tractography in the FSL software (Probtrackx2 command). The possible orientation distribution priors were estimated using a Bayesian method (bedpostx script), allowing for two fiber populations per voxel. Probabilistic tractography was seeded from a region of interest covering the supratentorial cortical gray matter (cortex label of the ALBERTs atlas; Gousias et al., 2012). In steps 1–3, from each cortical voxel, 5,000 probabilistic tracing particles were emitted and a mask of cerebrospinal fluid spaces was set to terminate any tracing samples reaching the cerebrospinal fluid. The thalamus mask was used as target for tractography; during this step, only those pathways were kept that passed through the thalamus. A counter in each cortical voxel increased whenever the tracing particle from that voxel reached the thalamus, resulting in seed‐to‐target probability maps (step 1). Whole‐brain maps depicting the connectivity strength (fdt_paths) and the mean length of probabilistic fiber pathways in each voxel, determined from the length of tractography tracking steps taken in diffusion space, were exported for further analysis (step 2). While probabilistic tractography was calculated is diffusion space, the results were saved and interpolated and rounded in standard space, which is the standard functioning of Probtrackx in the presence of standard space transformations.
We used a modified version of the tract‐based statistics (TBSS; Smith et al., 2006) for the analysis of thalamocortical connectivity across subjects. TBSS offers a way to overcome the limitation of compromised cross‐subject registration during group analysis by providing an alignment‐invariant representation of fiber tracts. Instead of the standard method, which consists of generating a representation of the fiber tracts based on fractional anisotropy images, we calculated the centerline of the cortical mantle based on a groupwise cortex probability map of the 42‐week template in the ALBERT atlas. The strength of thalamocortical connectivity at each cortical voxel was projected to the nearest cortical centerline voxel using the approach described in the TBSS literature (Smith et al., 2006). Next, in order to provide reference when evaluating any regional specificity in the results, we calculated a map of the mean thalamocortical connectivity in the study population, which depicts which regions of the thalamus are intrinsically more connected to the cortex. In step 3, the average connectivity in the thalamic nuclei was calculated, for which the regions of interests were determined from the standard space neonatal thalamus atlas described in the previous section. In step 4, probabilistic diffusion tractography (Probtrackx2 command) was seeded from the standard space thalamus mask. The same exclusion masks were used. The cortex mask was used as a target in the tractography process.
2.6. Statistical analysis
First, we used multivariate linear regression to test whether MDI and PDI correlated with the TC values that were projected onto the cortical skeleton. Statistical analyses were completed using the threshold‐free cluster enhancement method (TFCE; Smith & Nichols, 2009) with randomized nonparametric permutation testing implemented in the randomise program (Winkler, Ridgway, Webster, Smith, & Nichols, 2014), version 2.9, part of the FSL software package, build 509 (Jenkinson, Beckmann, Behrens, Woolrich, & Smith, 2012). The default parameter settings of TFCE were used with 5,000 permutations. For the initial analysis, familywise error‐corrected p ≤ .05 was accepted as significant. The same statistical test was then performed for the TC length of all voxels inside the thalamus. Next, post hoc analysis was performed using stepwise linear regression in IBM SPSS V22 (IBM) to select any further demographic or clinical parameters that could influence the correlation between TC, TC length, and the cognitive scores. During the stepwise selection of variables from a pool of demographic and neonatal clinical parameters (Table S1), the probability of F was set to .05 for a variable to enter and .1 for removal. If clinical variables were selected, we performed the first test again with and without these variables to evaluate whether they are mediators or confounders of the effect.
3. RESULTS
3.1. Cognitive scores
The mean (SD) age at the neurodevelopmental assessment was 23.4 (2.33) months. The mean (SD) mental developmental index of the BSID‐II was 94.2 (14.3), and the mean (SD) psychomotor index was 92.1 (13.3). There was a moderate, negative correlation of SES with MDI (PMCC, −0.463) but not with PDI (PMCC, 0.0306).
3.2. Correlation between thalamocortical connectivity strength (TC) and outcome at 2 years of age
Our statistical models were corrected for PMA, as diffusion anisotropy and TC might change rapidly during early development, as was also confirmed by our data (Figure S1). The variable that described the assignment of infants to EPO‐treated or placebo groups was not used as a confounder, as neither did EPO treatment predict outcome in this subgroup nor correlated with thalamocortical connectivity in the same cluster‐level analysis (TFCE, corrected p > .05).
We found that the 2‐year psychomotor development index (PDI) was not correlated with TC in a model adjusted for PMA. The cortically projected TC was significantly correlated (p < .05) with MDI in predominantly frontal lobe areas, extending from the precentral gyrus to the dorsolateral prefrontal cortex anteriorly and the middle frontal gyrus ventrally (Figure 3a,b). Additionally, clusters of correlation were observed in the medial orbitofrontal cortex and the right inferior parietal lobule (Figure 3a,c). To capture the effect size and the contribution of the explanatory variables (PMA and thalamocortical connectivity strength) to the variance of MDI, the average thalamocortical connectivity strength over the voxels which were correlated with MDI was entered into a multivariate linear regression model. We found that TC was strongly, positively correlated with MDI (partial correlation = 0.55), while PMA was weakly, negatively correlated with MDI; this second correlation did not reach significance in the model (Table 2). Thalamocortical connectivity strength was not found to be correlated with MDI when tractography was seeded from the thalamus and terminated in the cortical mask.
Figure 3.
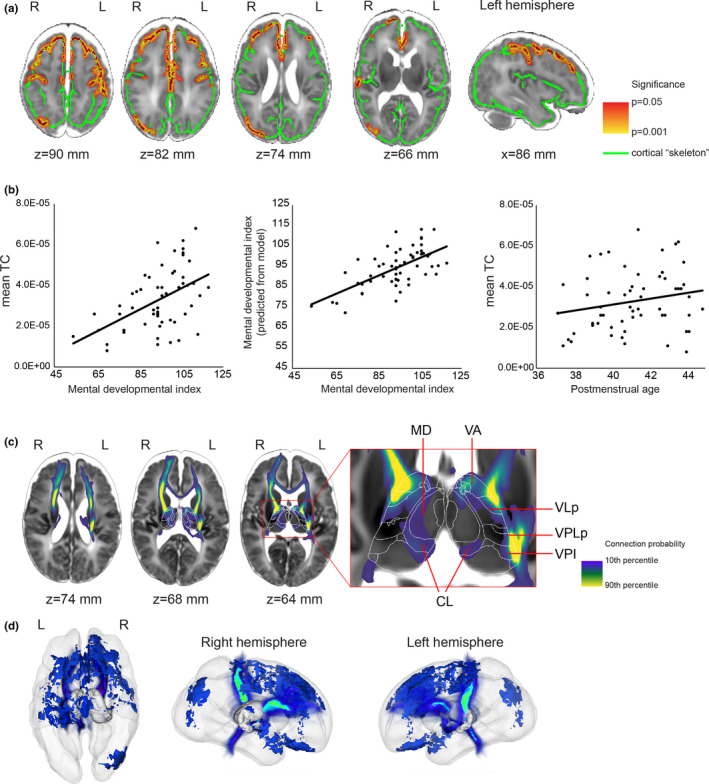
Thalamocortical (TC) connections at term equivalent age significantly correlated with 2‐year mental development index (MDI) scores in preterm born infants. (a) Parts of the cortex where TC and MDI were significantly correlated were marked with red. The significant voxels are dilated to increase visibility, and the dilated voxels are displayed as an overlay colored yellow and red. The cortical skeleton is displayed as green overlay on a T2‐weighted MRI template of the 42‐week newborn brain. (b) Linear regression plots of the TC–MDI correlation and gestational age dependence of TC averaged over the significant voxels. (c) 2D cross‐sectional images showing the projection of fiber pathways from the cortical skeleton voxels, where TC was correlated with 2‐year mental development. (d) 3D representation of the population mean in a glass brain. Thalamic nuclei abbreviations are given in Table S2
Table 2.
Correlation between cortically projected thalamocortical connectivity (TC) in the prefrontal cluster and mental development index at two years
| Predictors a | Standardized Beta | t | p | Zero‐order correlation | Partial correlation | Part correlation |
|---|---|---|---|---|---|---|
| Mean TC b | 0.565 | 4.682 | .000022 | 0.517 | 0.552 | 0.550 |
| Postmenstrual age | −0.210 | −1.742 | .088 | −0.082 | −0.239 | −0.205 |
Overall model fit: F = 11.2, adjusted R 2 = .282, p < .001.
Mean TC calculated from the threshold‐free cluster enhancement (TFCE)‐adjusted significant clusters in the cortical skeleton (p < .05)
Next, we used a post hoc, stepwise, linear regression analysis on the mean TC values from the previous experiments to search for possible confounders or mediators of the effect from clinical and demographic variables. In this test, four variables were selected in the model that best explained the variance of MDI: mean TC, days of mechanical ventilation, SES, and PMA. Interestingly, gestational age (GA) at birth was not found to explain the variance of MDI. The association between mean TC and MDI remained strong (partial R = .608, p < .001) after adjusting for these variables (Table 3). The total time of mechanical ventilation in days was found to be a partial mediator of the effect of mean TC on MDI: After removing this variable from the model, the overall fit was considerably reduced (F = 12.91, no adjustment, vs. F = 17.01—adjusted for mechanical ventilation), and the adjusted R 2 was lower than with the variable in the model (.407 vs. .552). The test statistics of clinical and demographic variables that were either entered or excluded from the final statistical model are given in Table S1.
Table 3.
Correlation between cortically projected thalamocortical connectivity (TC) and mental development index at 2 years after adjusting for clinical and demographic variables
| Predictors a | Standardized Beta | t | p | Zero‐order correlation | Partial correlation | Part correlation |
|---|---|---|---|---|---|---|
| Mean TC b | 0.518 | 5.309 | .000003 | 0.517 | 0.608 | 0.493 |
| Time of mechanical ventilation | −0.390 | −4.100 | .00016 | −0.402 | −0.509 | −0.381 |
| SES | −0.304 | −3.162 | .0027 | −0.463 | −0.415 | −0.294 |
| Postmenstrual age | −0.259 | −2.684 | .01 | −0.082 | −0.361 | −0.249 |
Overall model fit: F = 17.011, adjusted R 2 = .552, p < .001.
Mean TC calculated from the threshold‐free cluster enhancement (TFCE)‐adjusted significant clusters in the cortical skeleton (p < .05).
3.3. Thalamic specificity of probabilistic diffusion tractography results
In each subject, we performed probabilistic diffusion tractography in standard atlas space from the voxels on the cortical skeleton where TC was significantly associated with MDI, and we then analyzed the trajectory and thalamic projection of these connections (Figure 3c). By using the standard space neonatal thalamus atlas, we quantified the probability of each nucleus being connected to the significant voxels (Table 4) by dividing the number of probabilistic streamlines entering all the voxels of a given nucleus by the total number of streamlines. The TC fibers most correlated with MDI were localized in the ventral and anterior nuclei groups, namely ventral anterior, ventrolateral, ventromedial, and ventral posteromedial nuclei. Lower connection probability was found for the laterodorsal, anteroventral, and centrolateral nuclei. We observed a moderate left–right asymmetry in the thalamic projections: On the right side, nonmotor or sensory‐related nuclei, such as lateral pulvinar or medial geniculate nucleus (MGN), were found to be connected to the significant cortical voxels.
Table 4.
Intrathalamic specificity of our findings: localization of fibers emerging from the cortically projected thalamocortical significantly correlated with mental development index (details and abbreviations of the thalamic nuclei are given in Table S2)
| Left | Right | |||
|---|---|---|---|---|
| Rank | Relative strength (%) of mean thalamocortical connectivity | Relative strength (%) of mean thalamocortical connectivity | ||
| 1 | 427 | VLa | 371 | VPI |
| 2 | 330 | VM | 253 | VLa |
| 3 | 199 | VApc | 250 | VPLp |
| 4 | 108 | VAmc | 212 | VM |
| 5 | 75 | VLpv | 204 | VPM |
| 6 | 75 | VLpd | 177 | VApc |
| 7 | 65 | VPM | 159 | PUL |
| 8 | 52 | LD | 144 | VPLa |
| 9 | 47 | AV | 133 | VAmc |
| 10 | 46 | CL | 120 | MGN |
3.4. Correlation of thalamocortical fiber length and mental developmental index
We tested whether the length of probabilistically traced thalamocortical fibers (=TC length) was correlated with 2‐year cognitive scores. We found that TC length was significantly correlated with (p < .05) MDI (Figure 4a). The post hoc multivariate linear regression analysis revealed that TC length was strongly correlated with MDI, while PMA was weakly, negatively correlated with MDI (Table 5).
Figure 4.
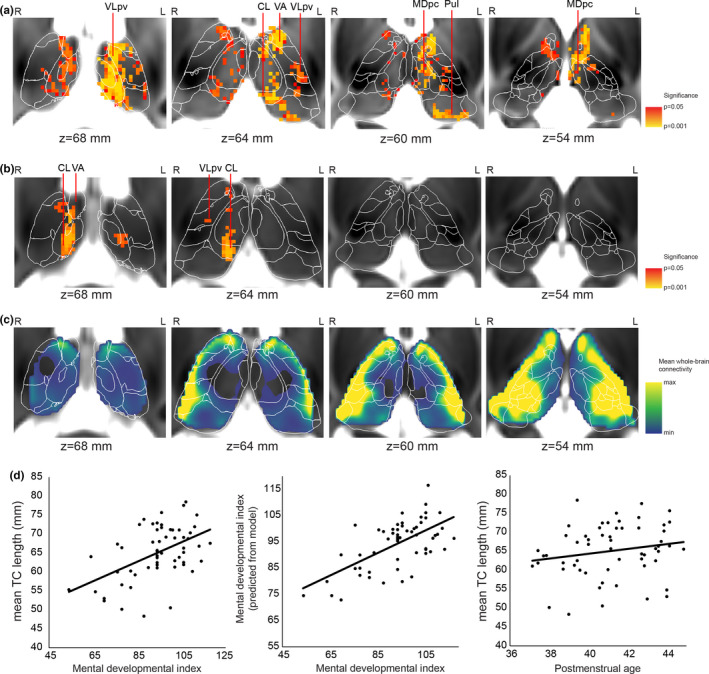
Localization of thalamocortical (TC) connections associated with 2‐year outcome: thalamic specificity of our findings. (a) Thalamic voxels where TC length was significantly associated with 2‐year outcome are displayed as red and yellow overlay (less to more significant) on a T2‐weighted MRI template of the 42‐week newborn brain. Results are based on the tractography seeded from the cortical masks. (b) Confirmatory analysis of the correlation between TC length and 2‐year mental development index (MDI): tractography seeded from the thalamus masks and terminated at the cortex. (c) Population mean of the whole‐brain TC connectivity strength. (d) Regression plots of the TC length–MDI correlation and gestational age dependence of TC length averaged over the significant voxels. Thalamic nuclei abbreviations are given in Table S2
Table 5.
Multivariate linear regression analysis of the correlation between mean thalamocortical fiber length (TC length) in the ventroanterior thalamus and mental development index
| Predictors a | Standardized Beta | t | p | Zero‐order correlation | Partial correlation | Part correlation |
|---|---|---|---|---|---|---|
| Mean TC length b | 0.543 | 4.478 | .000044 | 0.508 | 0.535 | 0.533 |
| Postmenstrual age | −0.184 | −1.519 | .135 | −0.082 | −0.210 | −0.181 |
Overall model fit: F = 10.26, adjusted R 2 = .263, p < .001.
Mean TC length calculated from the threshold‐free cluster enhancement (TFCE)‐adjusted significant clusters in the thalamus (p < .05).
The effect was mostly localized to the ventral anterior and ventrolateral thalamic nuclei, with smaller clusters found in the centrolateral (CL) and pulvinar nuclei. Interestingly, more regions were correlated with MDI in the left thalamus than in the right, and the significance levels were also higher (Figure 4a). The psychomotor development index (PDI) was not correlated with TC length in a model adjusted for PMA. Further analysis of the clinical and demographic variables found a similar relationship between the TC length and MDI: SES, PMA at scanning, and the total time of mechanical ventilation were entered into the model in stepwise linear regression (Table 6, results for all variables including the ones not entered into the model: Table S3). The findings on the intrathalamic distribution of voxels in which the TC length was correlated with MDI are summarized in Table 7.
Table 6.
Correlation between mean thalamocortical fiber length (TC length) and mental development index at 2 years after adjusting for clinical and demographic variables
| Predictors a | Standardized Beta | t | p | Zero‐order correlation | Partial correlation | Part correlation |
|---|---|---|---|---|---|---|
| Mean TC length b | 0.460 | 4.481 | .000 | 0.508 | 0.543 | 0.440 |
| Time of mechanical ventilation | −0.354 | −3.529 | .001 | −0.402 | −0.454 | −0.347 |
| SES | −0.308 | −3.023 | .004 | −0.463 | −0.400 | −0.297 |
| Postmenstrual age | −0.223 | −2.212 | .032 | −0.082 | −0.304 | −0.217 |
Overall model fit: F = 17.011, adjusted R 2 = .552, p < .001.
Mean TC calculated from the threshold‐free cluster enhancement (TFCE)‐adjusted significant clusters (p < .05).
Table 7.
Intrathalamic specificity of our findings: thalamocortical length significantly correlated with mental development index
| Left | Right | |||
|---|---|---|---|---|
| Rank | % volume of nucleus | % volume of nucleus | ||
| 1 | 69.8 | VAmc | 49.0 | LD |
| 2 | 68.4 | VLpd | 43.1 | AV |
| 3 | 62.1 | LD | 35.7 | VAmc |
| 4 | 57.4 | VApc | 30.9 | VM |
| 5 | 56.0 | CL | 30.8 | Hb |
| 6 | 45.2 | MDpc | 25.7 | CL |
| 7 | 41.8 | VM | 24.5 | mtt |
| 8 | 36.8 | CeM | 21.9 | MDmc |
| 9 | 36.8 | mtt | 18.9 | MDpc |
| 10 | 33.8 | Pf | 13.3 | VApc |
Abbreviations of the thalamic nuclei are given in Table S2.
In a validation experiment, we found that TC length is correlated with MDI when tractography was seeded from the thalamus and terminated in the cortex mask (Figure 4b). This finding showed smaller clusters where TC length is associated with MDI, predominantly in the left and right CL, right VA and right VLpv.
4. DISCUSSION
Our analysis revealed strong correlations between cognitive performance at 2 years, weaker TC strength, and shorter TC length in infants born preterm. Our findings imply that injury or dysmaturation of thalamocortical fiber pathways that may emerge from ventral and nonspecific parts of the thalamus is associated with mental development (MDI), but not psychomotor development (PDI). The main results of our work were replicated in an additional experiment with different tractography settings only in case of the TC length, which implies a mix of technically and biologically driven findings and warrants further investigation. This provides further evidence for alterations in thalamocortical connectivity after preterm birth (Ball, Boardman, et al., 2013; Ball, Srinivasan, et al., 2013; Fischi‐Gomez et al., 2016; Kelly et al., 2016; Pandit et al., 2014; van den Heuvel et al., 2015). Our work is an independent validation of a previous study describing a correlation between TC strength and 2‐year mental development (Ball et al., 2015). Our findings may indicate a predilection for such association with the frontal and parietal cortical regions. The significant correlations were mostly seen in thalamic structures that are intrinsically more weakly connected to the cortex when using tractography, most likely due to the higher uncertainty in estimation of the fiber orientations in deeper structures in the thalamus. The cortical regions that correlated with cognition at two years of age were more confined to the frontal cortex, and the TC length was significantly associated with MDI in the ventrolateral, ventral anterior, laterodorsal, and numerous nonspecific thalamic nuclei. However, due to the technical difficulties of tractography to reach deeper thalamic structures, the generalizability of these findings is limited and may necessitate further investigation using more advanced image acquisition techniques.
This relationship appears to be affected by parents’ SES and duration of mechanical ventilation during the NICU stay. SES has previously been confirmed to be an important mediator of cognitive and language development in newborns (Benavente‐Fernandez et al., 2019). Duration of mechanical ventilation has been associated with more brain abnormalities (Brouwer et al., 2017), with white matter abnormalities (Anjari et al., 2009; Ball et al., 2010) and, more recently, with alterations in fiber density (Pecheva et al., 2019). Such comorbidities contribute to impaired WM development and, as confirmed by our post hoc statistical analysis, might be regarded as partial mediators of the main effect found in our analysis. The duration of mechanical ventilation could be also a surrogate marker of the overall level of sickness and cardiorespiratory instability of the study subjects during neonatal course, which also may affect brain integrity.
The thalamus actively participates in cognitive processes by relaying pathways to association cortices, and hence, injury to nonspecific or association thalamic nuclei impacts cognitive capabilities. This is reflected by the observation that prematurity affects the thalamocortical connectome (Ball, Boardman, et al., 2013; Ball, Srinivasan, et al., 2013) and cortical microstructure (Ball, Boardman, et al., 2013; Ball, Srinivasan, et al., 2013; Ball et al., 2012), and the degree of brain injury predicts later cognitive development (Berman et al., 2005; Gui et al., 2018; Little et al., 2010; Woodward, Anderson, Austin, Howard, & Inder, 2006; Woodward, Clark, Pritchard, Anderson, & Inder, 2011). The volume of the thalamus was reported to be associated with later life academic outcomes, motor skills, and IQ (Loh et al., 2017). There is evidence for a close relationship between thalamus injury and impaired cognition in prematurity (Ball et al., 2015). Lower cognitive performance at 2 years of age was not only found to be linked to a global reduction of thalamocortical connectivity (Ball et al., 2015) but also to lower neuronal metabolite concentrations in the thalamus (Hyodo et al., 2018). Reduced thalamus volume is associated with decreased structural integrity of posterior fiber pathways in the brain of school‐age children born prematurely. In severe cases with periventricular leukomalacia, thalamic volume reduction was found to have an effect on general intelligence and working memory measures (Zubiaurre‐Elorza et al., 2012). The thalamus is an important relay station to cortical regions involved in higher cognition (Parnaudeau, Bolkan, & Kellendonk, 2018). This is confirmed by studies that found association between the circuitry of the mediodorsal nucleus (MD) and executive functions (Ardila, 2019; Jakab, Blanc, & Berenyi, 2012).
While there is no strong support for premature birth selectively affecting neuronal circuits, our findings may shed light on a way in which cognitive development becomes disrupted due to increased vulnerability of its neural correlates to injury after premature birth. Our study population included infants born between the 26th and 31st weeks of gestation. During this period, the exposure of the immature brain to various toxic events in the neonatal intensive care unit and critical developmental processes such as myelination and the emergence of thalamocortical connections occur. By this time, the first TC fibers have already reached the cortex (Kostovic & Jovanov‐Milosevic, 2006; Kostovic & Judas, 2002), and the brain has progressed to a stage of rapid development, characterized by nonlinear increase in brain volume and an increase in synaptic density. Human thalamocortical axons show prolonged growth (4 months), and somatosensory fibers precede the ingrowth of fibers destined for frontal and occipital areas (Krsnik, Majic, Vasung, Huang, & Kostovic, 2017). At term equivalent age, the topography of thalamocortical connections largely resembles that of an adult (Ferradal et al., 2019). Thus, there is overwhelming neuroanatomical evidence for the rapid and late development of the frontal lobe in the human brain (Hodel, 2018), which confers increased vulnerability to adverse environments. Fetal studies have characterized a posterior‐to‐frontal (sensory‐to‐higher association) gradient in functional brain development (Jakab et al., 2014; Thomason et al., 2013), meaning that regional differences in TC development between the 27th and 31st weeks of gestation may open a window of selective vulnerability of frontal and parietal circuitry. During the early postnatal period in preterm infants, frontal lobe myelination differs from that in other brain regions by the longer persistence of premyelinating oligodendrocytes (Back et al., 2001); these are more vulnerable than mature oligodendrocytes to perinatal insults (Liu, Shen, Plane, & Deng, 2013). This observation allows us to speculate that impaired frontal lobe myelination, presumably also affecting the anterior thalamic radiation, would result in lower structural connectivity indices, as tractography becomes more uncertain at lower anisotropy values.
The following limitations of our study merit mentioning. The generalizability of our results is limited by the relatively low case number (n = 58), which reduces the statistical power of multivariate linear regression analysis with multiple covariates in the model. Furthermore, long‐term follow‐up results are necessary to confirm whether the association revealed between TC, TC length, and mental developmental index is sustained in later stages of development. Probabilistic thalamocortical tractography faces inherent technical limitations. First, afferent and efferent thalamic pathways are indistinguishable because diffusion direction does not respect tract polarity. Probabilistic tractography becomes increasingly uncertain at the WM/gray matter interface and in regions with lower diffusion anisotropy, such as the thalamus. While numerous reports have confirmed the value of this method for describing the connectional topography of the human thalamus (Behrens et al., 2003; Ferradal et al., 2019), it is likely that the current spatial and angular resolution is insufficient to identify some of the nonspecific nuclei of the thalamus, as fibers emerging from these must cross adjacent specific thalamic nuclei encompassing highly ordered, white matter‐rich regions. Furthermore, the small size of the neonatal thalamus may give rise to partial volume effects, in particular when estimating the diffusion orientations and fiber distributions close to the ventricle borders. Despite these limitations, we rely confidently on the ability of probabilistic tractography for mapping thalamus‐frontal and prefrontal connectivity based on previous neuroanatomical and imaging studies (Jakab, Blanc, & Berenyi, 2012; Klein et al., 2010; Le Reste, Haegelen, Gibaud, Moreau, & Morandi, 2016).
The use of TC length as indicator of thalamocortical connectivity strength is not straightforward because it may not only reflect physical distance. Shorter length may be caused by the shorter distance that probabilistic tracing particles on average traverse if the connection probability is lower, and by the same token, pathways are terminated earlier if FA is lower. TC and TC length should therefore be interpreted as variables that do not directly reflect the number of mono‐ or polysynaptic pathways reaching the cortex, which would be the simplest definition of anatomical connectivity strength, but a variety of diverse effects. These include the cumulative effect of diffusion anisotropy and fiber uncertainty along the pathways, which in turn may also reflect myelination and the actual number of axons in a voxel. Importantly, the order of how the seed and target maps are used (i.e. thalamus to cortex or vice versa) results in different tract distributions, which likely contributed to why we could only replicate the TC length–MDI correlation in our confirmatory experiment.
The complete lack of histologically defined, human newborn thalamus atlases increases the difficulty of interpreting our results on thalamic specificity. While structural and functional connectivity at birth appears to largely overlap with adult patterns, the precise variability of this in preterm infants remains to be characterized. Our previous report using the shape model‐based alignment of the thalamus atlas was found to be flexible enough to tackle large shape variability, as proven by matching the atlas to high‐resolution MRI of postmortem, fixed thalamus samples (Jakab, Blanc, Berenyi, & Szekely, 2012).
5. CONCLUSIONS
Our study confirmed the value of TC circuitry in predicting cognitive development in preterm born infants. Cognitive abilities are important for school and academic success and ultimately for quality of life. Hence, early identification of aberrant brain development that underlies later cognitive deficits is essential to start early interventions and to monitor the effects and efficacy of intervention on brain development.
AUTHOR CONTRIBUTION
AJ, GN, BK, CR, and CH designed and conceptualized the study. AJ and CH created the research hypothesis. GN, BK, CR, and CH recruited the subjects and coordinated the study. AJ, RT, GN, and CH contributed the design and implemented the research. AJ and RT acquired and analyzed the neuroimaging data. AJ, CH, and RT were involved in the statistical analysis. AJ, GN, RT, CR, and CH prepared and edited the manuscript.
Peer Review
The peer review history for this article is available at https://publons.com/publon/10.1002/brb3.1786.
Supporting information
Fig S1
Table S1‐S3
ACKNOWLEDGMENTS
A.J. was supported by the Prof. Dr. Max Cloetta Foundation, Uniscientia Foundation, OPO Foundation, the Forschungszentrum für das Kind (FZK) Grant, the Foundation for Research in Science and the Humanities at the University of Zurich, the Anna Müller Grocholski Foundation and the EMDO Foundation, and R.T. was supported by the EMDO Foundation. The study was supported by a grant received from the Swiss National Science Foundation (3200B0‐108176) and was registered at clinicaltrials.gov (identifier: NCT 00313946). We would like to thank Simon Milligan for his help in language editing the manuscript. The authors declare that they do not have conflict of interest.
Jakab A, Natalucci G, Koller B, Tuura R, Rüegger C, Hagmann C. Mental development is associated with cortical connectivity of the ventral and nonspecific thalamus of preterm newborns. Brain Behav. 2020;10:e01786 10.1002/brb3.1786
DATA AVAILABILITY STATEMENT
Supporting neuroimaging data are available upon request from the corresponding author.
REFERENCES
- Aja‐Fernandez, S. , Tristan‐Vega, A. , & Alberola‐Lopez, C. (2009). Noise estimation in single‐ and multiple‐coil magnetic resonance data based on statistical models. Magnetic Resonance Imaging, 27, 1397–1409. 10.1016/j.mri.2009.05.025 [DOI] [PubMed] [Google Scholar]
- Anjari, M. , Counsell, S. J. , Srinivasan, L. , Allsop, J. M. , Hajnal, J. V. , Rutherford, M. A. , & Edwards, A. D. (2009). The association of lung disease with cerebral white matter abnormalities in preterm infants. Pediatrics, 124, 268–276. 10.1542/peds.2008-1294 [DOI] [PubMed] [Google Scholar]
- Ardila, A. (2019). Executive dysfunction in subcortical diseases In Ardila A., Fatima S. & Rosselli M., et al (Eds.), Dysexecutive syndromes: Clinical and experimental perspectives (pp. 143–153). Cham, Switzerland: Springer International Publishing. [Google Scholar]
- Back, S. A. , Luo, N. L. , Borenstein, N. S. , Levine, J. M. , Volpe, J. J. , & Kinney, H. C. (2001). Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. Journal of Neuroscience, 21, 1302–1312. 10.1523/JNEUROSCI.21-04-01302.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball, G. , Boardman, J. P. , Aljabar, P. , Pandit, A. , Arichi, T. , Merchant, N. , … Counsell, S. J. (2013). The influence of preterm birth on the developing thalamocortical connectome. Cortex, 49, 1711–1721. 10.1016/j.cortex.2012.07.006 [DOI] [PubMed] [Google Scholar]
- Ball, G. , Boardman, J. P. , Rueckert, D. , Aljabar, P. , Arichi, T. , Merchant, N. , … Counsell, S. J. (2012). The effect of preterm birth on thalamic and cortical development. Cerebral Cortex, 22, 1016–1024. 10.1093/cercor/bhr176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball, G. , Counsell, S. J. , Anjari, M. , Merchant, N. , Arichi, T. , Doria, V. , … Boardman, J. P. (2010). An optimised tract‐based spatial statistics protocol for neonates: Applications to prematurity and chronic lung disease. NeuroImage, 53, 94–102. 10.1016/j.neuroimage.2010.05.055 [DOI] [PubMed] [Google Scholar]
- Ball, G. , Pazderova, L. , Chew, A. , Tusor, N. , Merchant, N. , Arichi, T. , … Counsell, S. J. (2015). Thalamocortical connectivity predicts cognition in children born preterm. Cerebral Cortex, 25, 4310–4318. 10.1093/cercor/bhu331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball, G. , Srinivasan, L. , Aljabar, P. , Counsell, S. J. , Durighel, G. , Hajnal, J. V. , … Edwards, A. D. (2013). Development of cortical microstructure in the preterm human brain. Proceedings of the National Academy of Sciences USA, 110, 9541–9546. 10.1073/pnas.1301652110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayley, N. (1993). Bayley scales of infant development: Manual. Psychological Corporation. [Google Scholar]
- Behrens, T. E. J. , Johansen‐Berg, H. , Woolrich, M. W. , Smith, S. M. , Wheeler‐Kingshott, C. , Boulby, P. A. , … Matthews, P. M. (2003). Non‐invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nature Neuroscience, 6, 750–757. 10.1038/nn1075 [DOI] [PubMed] [Google Scholar]
- Benavente‐Fernandez, I. , Synnes, A. , Grunau, R. E. , Chau, V. , Ramraj, C. , Glass, T. , … Miller, S. P. (2019). Association of socioeconomic status and brain injury with neurodevelopmental outcomes of very preterm children. JAMA Network Open, 2, e192914 10.1001/jamanetworkopen.2019.2914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman, J. I. , Mukherjee, P. , Partridge, S. C. , Miller, S. P. , Ferriero, D. M. , Barkovich, A. J. , … Henry, R. G. (2005). Quantitative diffusion tensor MRI fiber tractography of sensorimotor white matter development in premature infants. NeuroImage, 27, 862–871. 10.1016/j.neuroimage.2005.05.018 [DOI] [PubMed] [Google Scholar]
- Boardman, J. P. , Counsell, S. J. , Rueckert, D. , Kapellou, O. , Bhatia, K. K. , Aljabar, P. , … Edwards, A. D. (2006). Abnormal deep grey matter development following preterm birth detected using deformation‐based morphometry. NeuroImage, 32, 70–78. 10.1016/j.neuroimage.2006.03.029 [DOI] [PubMed] [Google Scholar]
- Brouwer, M. J. , Kersbergen, K. J. , van Kooij, B. J. M. , Benders, M. J. N. L. , van Haastert, I. C. , Koopman‐Esseboom, C. , … Groenendaal, F. (2017). Preterm brain injury on term‐equivalent age MRI in relation to perinatal factors and neurodevelopmental outcome at two years. PLoS One, 12, e0177128 10.1371/journal.pone.0177128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalano, S. M. , Robertson, R. T. , & Killackey, H. P. (1996). Individual axon morphology and thalamocortical topography in developing rat somatosensory cortex. The Journal of Comparative Neurology, 367, 36–53. [DOI] [PubMed] [Google Scholar]
- Ferradal, S. L. , Gagoski, B. , Jaimes, C. , Yi, F. , Carruthers, C. , Vu, C. , … Zollei, L. (2019). System‐specific patterns of thalamocortical connectivity in early brain development as revealed by structural and functional MRI. Cerebral Cortex, 29, 1218–1229. 10.1093/cercor/bhy028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischi‐Gomez, E. , Munoz‐Moreno, E. , Vasung, L. , Griffa, A. , Borradori‐Tolsa, C. , Monnier, M. , … Huppi, P. S. (2016). Brain network characterization of high‐risk preterm‐born school‐age children. NeuroImage Clinical, 11, 195–209. 10.1016/j.nicl.2016.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh, A. , Antonini, A. , McConnell, S. K. , & Shatz, C. J. (1990). Requirement for subplate neurons in the formation of thalamocortical connections. Nature, 347, 179–181. 10.1038/347179a0 [DOI] [PubMed] [Google Scholar]
- Ghosh, A. , & Shatz, C. J. (1992). Pathfinding and target selection by developing geniculocortical axons. Journal of Neuroscience, 12, 39–55. 10.1523/JNEUROSCI.12-01-00039.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gousias, I. S. , Edwards, A. D. , Rutherford, M. A. , Counsell, S. J. , Hajnal, J. V. , Rueckert, D. , & Hammers, A. (2012). Magnetic resonance imaging of the newborn brain: Manual segmentation of labelled atlases in term‐born and preterm infants. NeuroImage, 62, 1499–1509. 10.1016/j.neuroimage.2012.05.083 [DOI] [PubMed] [Google Scholar]
- Gui, L. , Loukas, S. , Lazeyras, F. , Huppi, P. S. , Meskaldji, D. E. , & Borradori Tolsa, C. (2018). Longitudinal study of neonatal brain tissue volumes in preterm infants and their ability to predict neurodevelopmental outcome. NeuroImage. 10.1016/j.neuroimage.2018.06.034 [DOI] [PubMed] [Google Scholar]
- Hodel, A. S. (2018). Rapid infant prefrontal cortex development and sensitivity to early environmental experience. Developmental Review, 48, 113–144. 10.1016/j.dr.2018.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyodo, R. , Sato, Y. , Ito, M. , Sugiyama, Y. , Ogawa, C. , Kawai, H. , … Hayakawa, M. (2018). Magnetic resonance spectroscopy in preterm infants: Association with neurodevelopmental outcomes. Archives of Disease in Childhood ‐ Fetal and Neonatal Edition, 103, F238–F244. 10.1136/archdischild-2016-311403 [DOI] [PubMed] [Google Scholar]
- Jakab, A. , Blanc, R. , & Berenyi, E. L. (2012). Mapping changes of in vivo connectivity patterns in the human mediodorsal thalamus: Correlations with higher cognitive and executive functions. Brain Imaging and Behavior, 6, 472–483. 10.1007/s11682-012-9172-5 [DOI] [PubMed] [Google Scholar]
- Jakab, A. , Blanc, R. , Berenyi, E. L. , & Szekely, G. (2012). Generation of individualized thalamus target maps by using statistical shape models and thalamocortical tractography. AJNR. American Journal of Neuroradiology, 33, 2110–2116. 10.3174/ajnr.A3140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakab, A. , Ruegger, C. , Bucher, H. , Huppi, P. , Makki, M. , Tuura, R. , & Hagmann, C. (2019). Network based statistics reveals trophic and neuroprotective effect of early high dose erythropoetin on brain connectivity in very preterm infants. NeuroImage: Clinical, 22, 101806 10.1016/j.nicl.2019.101806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakab, A. , Schwartz, E. , Kasprian, G. , Gruber, G. M. , Prayer, D. , Schöpf, V. , & Langs, G. (2014). Fetal functional imaging portrays heterogeneous development of emerging human brain networks. Frontiers in Human Neuroscience, 8, 852 10.3389/fnhum.2014.0085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson, M. , Beckmann, C. F. , Behrens, T. E. , Woolrich, M. W. , & Smith, S. M. (2012). Fsl. NeuroImage, 62, 782–790. 10.1016/j.neuroimage.2011.09.015 [DOI] [PubMed] [Google Scholar]
- Johnson, S. , & Marlow, N. (2011). Preterm birth and childhood psychiatric disorders. Pediatric Research, 69, 11R–18R. 10.1203/PDR.0b013e318212faa0 [DOI] [PubMed] [Google Scholar]
- Kelly, C. E. , Cheong, J. L. , Gabra Fam, L. , Leemans, A. , Seal, M. L. , Doyle, L. W. , … Thompson, D. K. (2016). Moderate and late preterm infants exhibit widespread brain white matter microstructure alterations at term‐equivalent age relative to term‐born controls. Brain Imaging and Behavior, 10, 41–49. 10.1007/s11682-015-9361-0 [DOI] [PubMed] [Google Scholar]
- Klein, J. C. , Rushworth, M. F. S. , Behrens, T. E. J. , Mackay, C. E. , de Crespigny, A. J. , D'Arceuil, H. , & Johansen‐Berg, H. (2010). Topography of connections between human prefrontal cortex and mediodorsal thalamus studied with diffusion tractography. NeuroImage, 51, 555–564. 10.1016/j.neuroimage.2010.02.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostovic, I. , & Jovanov‐Milosevic, N. (2006). The development of cerebral connections during the first 20–45 weeks' gestation. Seminars in Fetal & Neonatal Medicine, 11, 415–422. 10.1016/j.siny.2006.07.001 [DOI] [PubMed] [Google Scholar]
- Kostovic, I. , & Judas, M. (2002). Correlation between the sequential ingrowth of afferents and transient patterns of cortical lamination in preterm infants. Anatomical Record, 267, 1–6. 10.1002/ar.10069 [DOI] [PubMed] [Google Scholar]
- Kostovic, I. , & Judas, M. (2006). Prolonged coexistence of transient and permanent circuitry elements in the developing cerebral cortex of fetuses and preterm infants. Developmental Medicine and Child Neurology, 48, 388–393. 10.1017/S0012162206000831 [DOI] [PubMed] [Google Scholar]
- Kostovic, I. , & Judas, M. (2007). Transient patterns of cortical lamination during prenatal life: Do they have implications for treatment? Neuroscience and Biobehavioral Reviews, 31, 1157–1168. 10.1016/j.neubiorev.2007.04.018 [DOI] [PubMed] [Google Scholar]
- Kostović, I. , & Judaš, M. (2010). The development of the subplate and thalamocortical connections in the human foetal brain. Acta Pædiatrica, 99, 1119–1127. 10.1111/j.1651-2227.2010.01811.x [DOI] [PubMed] [Google Scholar]
- Krauth, A. , Blanc, R. , Poveda, A. , Jeanmonod, D. , Morel, A. , & Székely, G. (2010). A mean three‐dimensional atlas of the human thalamus: Generation from multiple histological data. NeuroImage, 49, 2053–2062. 10.1016/j.neuroimage.2009.10.042 [DOI] [PubMed] [Google Scholar]
- Krsnik, Z. , Majic, V. , Vasung, L. , Huang, H. , & Kostovic, I. (2017). Growth of thalamocortical fibers to the somatosensory cortex in the human fetal brain. Frontiers in Neuroscience, 11, 233 10.3389/fnins.2017.00233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Largo, R. H. , Pfister, D. , Molinari, L. , Kundu, S. , Lipp, A. , & Duc, G. (1989). Significance of prenatal, perinatal and postnatal factors in the development of AGA preterm infants at five to seven years. Developmental Medicine and Child Neurology, 31, 440–456. 10.1111/j.1469-8749.1989.tb04022.x [DOI] [PubMed] [Google Scholar]
- Le Reste, P. J. , Haegelen, C. , Gibaud, B. , Moreau, T. , & Morandi, X. (2016). Connections of the dorsolateral prefrontal cortex with the thalamus: A probabilistic tractography study. Surgical and Radiologic Anatomy, 38, 705–710. 10.1007/s00276-015-1603-8 [DOI] [PubMed] [Google Scholar]
- Little, D. M. , Kraus, M. F. , Joseph, J. , Geary, E. K. , Susmaras, T. , Zhou, X. J. , … Gorelick, P. B. (2010). Thalamic integrity underlies executive dysfunction in traumatic brain injury. Neurology, 74, 558–564. 10.1212/WNL.0b013e3181cff5d5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X. B. , Shen, Y. , Plane, J. M. , & Deng, W. (2013). Vulnerability of premyelinating oligodendrocytes to white‐matter damage in neonatal brain injury. Neuroscience Bulletin, 29, 229–238. 10.1007/s12264-013-1311-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh, W. Y. , Anderson, P. J. , Cheong, J. L. Y. , Spittle, A. J. , Chen, J. , Lee, K. J. , … Thompson, D. K. (2017). Neonatal basal ganglia and thalamic volumes: Very preterm birth and 7‐year neurodevelopmental outcomes. Pediatric Research, 82, 970–978. 10.1038/pr.2017.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuillen, P. S. , & Ferriero, D. M. (2005). Perinatal subplate neuron injury: Implications for cortical development and plasticity. Brain Pathology, 15, 250–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molliver, M. E. , Kostovic, I. , & van der Loos, H. (1973). The development of synapses in cerebral cortex of the human fetus. Brain Research, 50, 403–407. 10.1016/0006-8993(73)90741-5 [DOI] [PubMed] [Google Scholar]
- Molnar, Z. , Adams, R. , & Blakemore, C. (1998). Mechanisms underlying the early establishment of thalamocortical connections in the rat. Journal of Neuroscience, 18, 5723–5745. 10.1523/JNEUROSCI.18-15-05723.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar, Z. , Lopez‐Bendito, G. , Small, J. , Partridge, L. D. , Blakemore, C. , & Wilson, M. C. (2002). Normal development of embryonic thalamocortical connectivity in the absence of evoked synaptic activity. Journal of Neuroscience, 22, 10313–10323. 10.1523/JNEUROSCI.22-23-10313.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natalucci, G. , Latal, B. , Koller, B. , Ruegger, C. , Sick, B. , Held, L. , … Swiss EPO Neuroprotection Trial Group (2016). Effect of early prophylactic high‐dose recombinant human erythropoietin in very preterm infants on neurodevelopmental outcome at 2 years: a randomized clinical trial. JAMA, 315, 2079–2085. 10.1001/jama.2016.5504 [DOI] [PubMed] [Google Scholar]
- Nosarti, C. , Giouroukou, E. , Healy, E. , Rifkin, L. , Walshe, M. , Reichenberg, A. , … Murray, R. M. (2008). Grey and white matter distribution in very preterm adolescents mediates neurodevelopmental outcome. Brain, 131, 205–217. 10.1093/brain/awm282 [DOI] [PubMed] [Google Scholar]
- Nosarti, C. , Shergill, S. S. , Allin, M. P. , Walshe, M. , Rifkin, L. , Murray, R. M. , & McGuire, P. K. (2009). Neural substrates of letter fluency processing in young adults who were born very preterm: Alterations in frontal and striatal regions. NeuroImage, 47, 1904–1913. 10.1016/j.neuroimage.2009.04.041 [DOI] [PubMed] [Google Scholar]
- O'Gorman, R. L. , Bucher, H. U. , Held, U. , Koller, B. M. , Huppi, P. S. , Hagmann, C. F. , & Swiss EPO Neuroprotection Trial Group (2015). Tract‐based spatial statistics to assess the neuroprotective effect of early erythropoietin on white matter development in preterm infants. Brain, 138, 388–397. 10.1093/brain/awu363 [DOI] [PubMed] [Google Scholar]
- Pandit, A. S. , Robinson, E. , Aljabar, P. , Ball, G. , Gousias, I. S. , Wang, Z. , … Edwards, A. D. (2014). Whole‐brain mapping of structural connectivity in infants reveals altered connection strength associated with growth and preterm birth. Cerebral Cortex, 24, 2324–2333. 10.1093/cercor/bht086 [DOI] [PubMed] [Google Scholar]
- Parnaudeau, S. , Bolkan, S. S. , & Kellendonk, C. (2018). The mediodorsal thalamus: an essential partner of the prefrontal cortex for cognition. Biological Psychiatry, 83, 648–656. 10.1016/j.biopsych.2017.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecheva, D. , Tournier, J. D. , Pietsch, M. , Christiaens, D. , Batalle, D. , Alexander, D. C. , … Counsell, S. J. (2019). Fixel‐based analysis of the preterm brain: Disentangling bundle‐specific white matter microstructural and macrostructural changes in relation to clinical risk factors. NeuroImage Clinical, 23, 101820 10.1016/j.nicl.2019.101820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, S. M. , Jenkinson, M. , Johansen‐Berg, H. , Rueckert, D. , Nichols, T. E. , Mackay, C. E. , … Behrens, T. E. (2006). Tract‐based spatial statistics: Voxelwise analysis of multi‐subject diffusion data. NeuroImage, 31, 1487–1505. 10.1016/j.neuroimage.2006.02.024 [DOI] [PubMed] [Google Scholar]
- Smith, S. M. , & Nichols, T. E. (2009). Threshold‐free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage, 44, 83–98. 10.1016/j.neuroimage.2008.03.061 [DOI] [PubMed] [Google Scholar]
- Thomason, M. E. , Dassanayake, M. T. , Shen, S. , Katkuri, Y. , Alexis, M. , Anderson, A. L. , … Romero, R. (2013). Cross‐hemispheric functional connectivity in the human fetal brain. Science Translational Medicine, 5, 173ra24 10.1126/scitranslmed.3004978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel, M. P. , Kersbergen, K. J. , de Reus, M. A. , Keunen, K. , Kahn, R. S. , Groenendaal, F. , … Benders, M. J. (2015). The neonatal connectome during preterm brain development. Cerebral Cortex, 25, 3000–3013. 10.1093/cercor/bhu095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van't Hooft, J. , van der Lee, J. H. , Opmeer, B. C. , Aarnoudse‐Moens, C. S. , Leenders, A. G. , Mol, B. W. , & de Haan, T. R. (2015). Predicting developmental outcomes in premature infants by term equivalent MRI: Systematic review and meta‐analysis. Systematic Reviews, 4, 71–015‐0058‐7 10.1186/s13643-015-0058-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe, J. J. (2009a). Brain injury in premature infants: A complex amalgam of destructive and developmental disturbances. The Lancet Neurology, 8, 110–124. 10.1016/S1474-4422(08)70294-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe, J. J. (2009b). The encephalopathy of prematurity–brain injury and impaired brain development inextricably intertwined. Seminars in Pediatric Neurology, 16, 167–178. 10.1016/j.spen.2009.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrle, F. M. , Kaufmann, L. , Benz, L. D. , Huber, R. , O'Gorman, R. L. , Latal, B. , & Hagmann, C. F. (2016). Very preterm adolescents show impaired performance with increasing demands in executive function tasks. Early Human Development, 92, 37–43. 10.1016/j.earlhumdev.2015.10.021 [DOI] [PubMed] [Google Scholar]
- Winkler, A. M. , Ridgway, G. R. , Webster, M. A. , Smith, S. M. , & Nichols, T. E. (2014). Permutation inference for the general linear model. NeuroImage, 92, 381–397. 10.1016/j.neuroimage.2014.01.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward, L. J. , Anderson, P. J. , Austin, N. C. , Howard, K. , & Inder, T. E. (2006). Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. New England Journal of Medicine, 355, 685–694. 10.1056/NEJMoa053792 [DOI] [PubMed] [Google Scholar]
- Woodward, L. J. , Clark, C. A. , Pritchard, V. E. , Anderson, P. J. , & Inder, T. E. (2011). Neonatal white matter abnormalities predict global executive function impairment in children born very preterm. Developmental Neuropsychology, 36, 22–41. 10.1080/87565641.2011.540530 [DOI] [PubMed] [Google Scholar]
- Woodward, L. J. , Moor, S. , Hood, K. M. , Champion, P. R. , Foster‐Cohen, S. , Inder, T. E. , & Austin, N. C. (2009). Very preterm children show impairments across multiple neurodevelopmental domains by age 4 years. Archives of Disease in Childhood ‐ Fetal and Neonatal Edition, 94, F339–F344. 10.1136/adc.2008.146282 [DOI] [PubMed] [Google Scholar]
- Woodward, N. D. , Karbasforoushan, H. , & Heckers, S. (2012). Thalamocortical dysconnectivity in schizophrenia. American Journal of Psychiatry, 169, 1092–1099. 10.1176/appi.ajp.2012.12010056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubiaurre‐Elorza, L. , Soria‐Pastor, S. , Junque, C. , Fernandez‐Espejo, D. , Segarra, D. , Bargallo, N. , … Macaya, A. (2012). Thalamic changes in a preterm sample with periventricular leukomalacia: Correlation with white‐matter integrity and cognitive outcome at school age. Pediatric Research, 71, 354–360. 10.1038/pr.2011.70 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Table S1‐S3
Data Availability Statement
Supporting neuroimaging data are available upon request from the corresponding author.


