Abstract
BACKGROUND
Ovarian undifferentiated carcinomas are significantly rare and have an aggressive clinical course. Surgical resection is the only curative treatment in early-stage ovarian undifferentiated carcinomas that has a favorable prognosis. In case of recurrent and metastatic disease, palliative chemotherapy is the only available treatment. However, the effectiveness of standard chemotherapy regimen is not well-known, specifically in the case of extra-ovarian spread. We report an ovarian undifferentiated carcinoma of recurrent and inoperable advanced stage that was successfully treated with high-dose combination chemotherapy.
CASE SUMMARY
A 52-year-old woman presented with a 1-mo history of right lower quadrant and epigastric pain. A computed tomography (CT) scan of the abdomen revealed a multicystic mass with extensive internal necrosis of the right ovary without evidence of metastatic disease. A total hysterectomy with bilateral salpingo-oophorectomy and omentectomy was performed, but the surgery had a positive resection margin. Pathologically, it was diagnosed as ovarian undifferentiated carcinoma with sarcomatoid change. Although adjuvant chemotherapy was planned, it was delayed for 6 wk because of postoperative recovery, and the patient complained of abdominal pain. A CT scan and positron emission tomography-CT revealed a huge mass with multiple nodules in the pelvic cavity and para-aortic lymph node metastasis. Instead of standard therapy such as paclitaxel and platinum, combined chemotherapy with etoposide, ifosfamide, and cisplatin was administered. The patient experienced no recurrence for 5 years.
CONCLUSION
This is a case of metastatic ovarian undifferentiated carcinoma with sarcomatoid change that was successfully treated with high-dose combination cytotoxic chemotherapy.
Keywords: Ovarian undifferentiated carcinoma; Sarcomatoid change; Chemotherapy; Etoposide, ifosfamide, cisplatin; Case report
Core Tip: Palliative chemotherapy is the only available treatment for metastatic ovarian undifferentiated carcinoma. However, we used a high-dose combination chemotherapy of etoposide, ifosfamide, and cisplatin instead of the widely used paclitaxel and platinum and successfully treated an extremely rare case of inoperable metastatic ovarian undifferentiated carcinoma.
INTRODUCTION
Ovarian undifferentiated carcinomas do not classify into any type of carcinoma of the ovarian surface epithelium[1,2].
Ovarian undifferentiated carcinomas account for approximately 5% of primary epithelial ovarian cancers and are clinically and significantly aggressive[1,3]. In most cases, this subtype spreads widely beyond the ovary at diagnosis; hence, complete tumor resection is considered difficult[4]. As in most other cancers, the detection of ovarian undifferentiated carcinomas in advanced stages is associated with poor prognosis[4,5]. A reported case of significantly rapid and fatal prognosis has been reported[6]. Furthermore, the efficacy of paclitaxel and platinum combination regimen that is generally used in epithelial ovarian cancer is unknown in the case of ovarian undifferentiated carcinoma[6]. Thus, there are no standard chemotherapy regimens available.
We report an interesting and rare case of metastatic ovarian undifferentiated carcinoma that was successfully treated with a high-dose combination cytotoxic chemotherapy.
CASE PRESENTATION
Chief complaints
Right lower quadrant and epigastric pain.
History of present illness
A 52-year-old woman presented with a 1-mo history of right lower quadrant pain and epigastric pain.
History of past illness
Her medical history and that of her family were otherwise unremarkable.
Physical examination
Her abdomen was smooth and soft without any tenderness or palpable mass.
Laboratory examinations
Complete blood count results were as follows (normal ranges in parentheses): White blood cell count, 26.6 × 103/μL (4.0-10.0 × 103/μL); hemoglobin, 10.8 g/dL (12-16 g/dL); and platelet count, 674 × 103/μL (150-400 × 103/μL). The results of blood biochemistry tests and urine analysis were normal. The serum C-reactive protein level was 4.26 mg/dL (0.0-0.5 mg/dL). The erythrocyte sedimentation rate was 41 mm/h (0–30 mm/h). The level of cancer antigen 125 was 58.5 U/mL (< 35 U/mL), and both carcinoembryonic antigen (1.1 ng/mL; normal range 0.0–5.0 ng/mL) and carbohydrate antigen 19-9 (7.48 U/mL; normal range 0-33 U/mL) levels were normal.
Imaging examinations
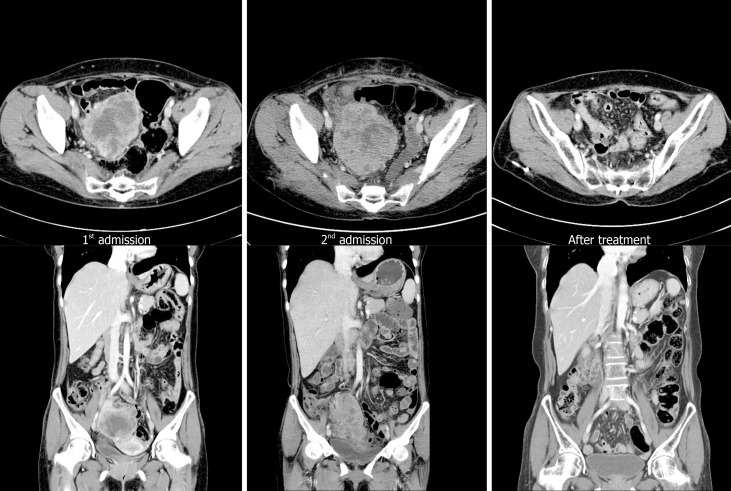
An abdominopelvic computed tomography (CT) scan revealed an 8 cm × 7 cm multicystic mass with extensive internal necrosis of the right ovary without evidence of metastatic lymphadenopathy and other organs (Figure 1). Our patient was clinically diagnosed with early-stage (less than the International Federation of Gynecology and Obstetrics Stage IC) ovarian cancer.
Figure 1.
Computed tomography at admission and follow-up. Computed tomography (CT) scan performed at first admission showing an 8 cm × 7 cm size mass only in the pelvic cavity. However, 6 wk after surgery (second admission), CT scan shows a mass that was larger than the initial mass in the pelvic cavity with peritoneal seeding and para-aortic lymphadenopathy (arrow). These lesions are almost no longer observed after chemotherapy.
Further diagnostic work-up
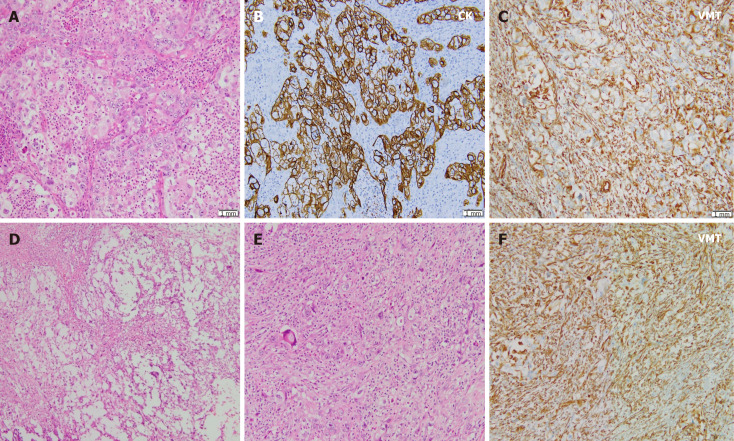
We performed debulking surgery with total hysterectomy, bilateral salpingo-oophorectomy, and omentectomy. At the time of surgery, the gross tumor measured 9 cm × 8 cm × 4 cm, and comprised solid and cystic areas. The tumor was significantly and severely adhesive to the pelvic wall and the intestine around the tumor. After surgery, macroscopic finding of the debulking tissue showed a multinodular cystic lesion filled with a yellowish liquid. Microscopic observation revealed tumor cell nests showing pleomorphic round-shaped nuclei with prominent nucleoli and high mitotic activity in the neutrophilic background. Areas of geographic necrosis are common, often with myxoid matrix. Sarcomatoid change of tumor cells was also identified, with areas showing immunohistochemically reactive for vimentin (Figure 2). The resection margin was positive because the mass was severely adhesive to the pelvic wall.
Figure 2.
Pathologic findings. A: Tumor cell nests such as round to oval nuclei, prominent nucleoli, and many atypical mitotic figures are observed in the neutrophilic background; B and C: These cells are immunoreactive to pan-cytokeratin, but negative for vimentin (VMT) (B) and focally to VMT (C); D: Geographic necrosis is common with myxoid matrix; E: In some areas, sarcomatoid features are frequently observed; F: Tumor cells showing no organoid feature directly admixed with stromal components and were immunoreactive to VMT. CK: Cytokeratin; VMT: Vimentin.
FINAL DIAGNOSIS
The patient was diagnosed with ovarian undifferentiated carcinoma with sarcomatoid change and underwent incomplete surgical tumor resection.
TREATMENT
We planned to administer adjuvant chemotherapy immediately because surgical resection margin was positive, and according to the literature review, this rare entity was known to have an aggressive course.
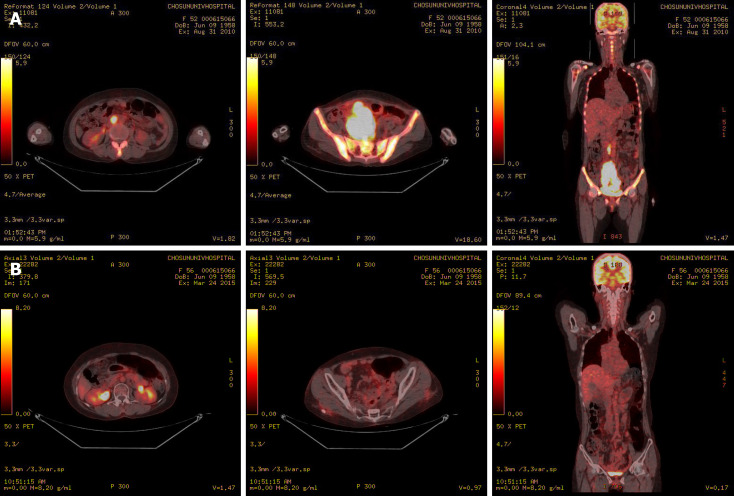
However, chemotherapy was delayed due to patient’s general weakness and poor oral intake during the postoperative recovery period. Six weeks later, the patient suddenly complained of abdominal discomfort. A CT scan revealed an 11 cm × 7 cm mass with multiple nodules in the pelvic cavity, multiple peritoneal nodules, and enlargement of the para-aortic lymph nodes (Figure 1). Positron emission tomography-CT was performed for staging of the recurrent ovarian cancer, and it showed hypermetabolism in the lesions observed on CT scan (Figure 3). We strongly suspected metastatic and inoperable ovarian undifferentiated carcinoma due to the presence of multiple pelvic nodules and diffuse peritoneal seeding.
Figure 3.
Positron emission tomography–computed tomography. A: Positron emission tomography–computed tomography revealing hypermetabolism of the huge mass in the pelvic cavity, multiple peritoneal nodules, and para-aortic lymph nodes; B: Hypermetabolism of the huge mass in the pelvic cavity, multiple peritoneal nodules, and para-aortic lymph nodes showing improvement after chemotherapy.
There is no standard chemotherapy regimen for ovarian undifferentiated carcinoma.
After reviewing various literature, we administered the etoposide, ifosfamide, and cisplatin (VIP) chemotherapy regimen with dose modification (cisplatin: 80 mg/m2 on day 1, etoposide: 100 mg/m2 on days 2-4, and ifosfamide: 1500 mg/m2 on days 2-4) that targeted the sarcoma. We did not select the regimen commonly used for ovarian epithelial cancer (paclitaxel and platinum) since it was reported to have a poor response that led to rapid growth of recurrent tumor. After 2 cycles of aggressive combination chemotherapy, tumor size markedly decreased (from 10 cm × 7 cm to 4 cm × 3 cm). Furthermore, after 4 cycles of VIP regimen, almost the entire mass was no longer observed (Figures 1 and 3).
Therefore, we strongly recommend reoperation or definitive pelvic irradiation as a curative treatment option. Because the patient experienced the side effects of high-dose combination chemotherapy such as grade III febrile neutropenia, thrombocytopenia, and mucositis, she strongly refused to perform additional chemotherapy and further definitive treatment. We recommended a short-term follow-up.
OUTCOME AND FOLLOW-UP
Fortunately, the tumor did not recur, and the patient is in complete remission. Five years after chemotherapy, this remission has been maintained, and the patient is alive without any tumor recurrence. Therefore, we had successfully treated the metastatic inoperable ovarian undifferentiated carcinoma.
DISCUSSION
Ovarian undifferentiated carcinomas are rare. The World Health Organization classification describes ovarian undifferentiated carcinomas as a tumor showing solid growth of pleomorphic cells with no differentiation or only small foci of differentiation[7]. Similar to other epithelial ovarian cancers, it has a relatively favorable prognosis in its early stage[5,8].
Traditionally, the prognosis of ovarian undifferentiated carcinoma is poor, and significantly rapid growth and fatal prognosis have been reported in advanced stage cases, such as this case. Furthermore, the cases with significantly rapid growth and fatal prognosis do not respond to paclitaxel and platinum treatment[6].
In this case, instead of the standard regimen for the treatment of epithelial ovarian carcinoma (paclitaxel and platinum), we targeted the sarcomatous component of the ovarian undifferentiated carcinoma with the combined chemotherapy VIP regimen that is commonly used in sarcoma[9]. In sarcoma, anthracycline-based combination regimens (doxorubicin or epirubicin with ifosfamide and/or dacarbazine) have been generally used and have similar or severe side effects to VIP regimen[10]. Whole abdominal radiotherapy is rarely used for ovarian cancer. Palliative localized radiotherapy is an option for symptom control in patients with recurrent disease[11]. In our case, radiotherapy alone was insufficient to obtain therapeutic effect due to high tumor burden, and the patients at age of 52 years was relatively young to be able to tolerate the VIP chemotherapy. The patient was followed up at our outpatient department for 5 years after chemotherapy (4 cycles in 3 wk). After receiving an aggressive chemotherapy, the patient was free of the disease.
CONCLUSION
We have successfully treated a metastatic inoperable case of ovarian undifferentiated carcinoma using high-dose combination chemotherapy. The effectiveness of the widely used chemotherapy regimen (paclitaxel and platinum) in treating inoperable ovarian undifferentiated carcinoma is uncertain. Therefore, aggressive combination chemotherapy can be used as an alternative treatment option. Moreover, ovarian undifferentiated carcinoma is a significantly rare disease with low clinical information. Thus, this case is an educational report that is considered beneficial in treating future patients.
Footnotes
Informed consent statement: Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Conflict-of-interest statement: The authors declare no conflict-of-interest.
CARE Checklist (2016) statement: The manuscript was prepared and revised according to the CARE Checklist (2016).
Manuscript source: Unsolicited manuscript
Peer-review started: March 3, 2020
First decision: July 25, 2020
Article in press: September 2, 2020
Specialty type: Oncology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kai K, Kung WM, Sergi C S-Editor: Wang JL L-Editor: A P-Editor: Xing YX
Contributor Information
Hong Beum Kim, Department of Premedical Course, Chosun University School of Medicine, Gwngju 505717, South Korea.
Hee Jeong Lee, Department of Internal Medicine, Hemato-oncology, Chosun University Hospital, Gwangju 505717, South Korea. hjangel21c@hanmail.net.
Ran Hong, Department of Pathology, Chosun University Hospital, Gwangju 505717, South Korea.
Sang-Gon Park, Department of Internal Medicine, Hemato-oncology, Chosun University Hospital, Gwangju 505717, South Korea.
References
- 1.Kurman RJ, Shih IeM. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol. 2010;34:433–443. doi: 10.1097/PAS.0b013e3181cf3d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaku T, Ogawa S, Kawano Y, Ohishi Y, Kobayashi H, Hirakawa T, Nakano H. Histological classification of ovarian cancer. Med Electron Microsc. 2003;36:9–17. doi: 10.1007/s007950300002. [DOI] [PubMed] [Google Scholar]
- 3.Silva EG, Tornos C, Bailey MA, Morris M. Undifferentiated carcinoma of the ovary. Arch Pathol Lab Med. 1991;115:377–381. [PubMed] [Google Scholar]
- 4.Tafe LJ, Garg K, Chew I, Tornos C, Soslow RA. Endometrial and ovarian carcinomas with undifferentiated components: clinically aggressive and frequently underrecognized neoplasms. Mod Pathol. 2010;23:781–789. doi: 10.1038/modpathol.2010.41. [DOI] [PubMed] [Google Scholar]
- 5.Silva EG, Deavers MT, Bodurka DC, Malpica A. Association of low-grade endometrioid carcinoma of the uterus and ovary with undifferentiated carcinoma: a new type of dedifferentiated carcinoma? Int J Gynecol Pathol. 2006;25:52–58. doi: 10.1097/01.pgp.0000183048.22588.18. [DOI] [PubMed] [Google Scholar]
- 6.Chen L, Pang S, Shen Y, Liu Z, Luan J, Shi Y, Liu Y. Low-grade endometrioid carcinoma of the ovary associated with undifferentiated carcinoma: case report and review of the literature. Int J Clin Exp Pathol. 2014;7:4422–4427. [PMC free article] [PubMed] [Google Scholar]
- 7.Kurman RJ, Carcangiu ML, Herrington CS, Young RH. Lyon: IARC;; 2014. WHO classification of tumours of the female reproductive organs; pp. 121–151. [Google Scholar]
- 8.Tabata T, Nishiura K, Tanida K, Kondo E, Okugawa T, Sagawa N. Carboplatin chemotherapy in a pregnant patient with undifferentiated ovarian carcinoma: case report and review of the literature. Int J Gynecol Cancer. 2008;18:181–184. doi: 10.1111/j.1525-1438.2007.00974.x. [DOI] [PubMed] [Google Scholar]
- 9.Pápai Z, Bodoky G, Szántó J, Poller I, Rahóty P, Eckhardt S, Láng I, Szendroi M. The efficacy of a combination of etoposide, ifosfamide, and cisplatin in the treatment of patients with soft tissue sarcoma. Cancer. 2000;89:177–180. [PubMed] [Google Scholar]
- 10.Casali P, Pastorino U, Zucchinelli P, Devizzi L, Azzarelli A, Quagliuolo V, Bignami P, Santoro A, Bonadonna G. Epirubicin plus ifosfamide and dacarbazine (EID) in advanced soft tissue sarcomas. Ann Oncol. 1992;3 Suppl 2:S125–S126. doi: 10.1093/annonc/3.suppl_2.s125. [DOI] [PubMed] [Google Scholar]
- 11.Yan J, Milosevic M, Fyles A, Manchul L, Kelly V, Levin W. A hypofractionated radiotherapy regimen (0-7-21) for advanced gynaecological cancer patients. Clin Oncol (R Coll Radiol) 2011;23:476–481. doi: 10.1016/j.clon.2011.01.001. [DOI] [PubMed] [Google Scholar]