Abstract
Anthracnose caused by Colletotrichum spp. is an important disease of blueberries and results in large economic losses for blueberry growers. Samples of anthracnose were collected from six main blueberry cultivation areas in Sichuan Province. In total, 74 Colletotrichum isolates were obtained through a single-spore purification method and identified to the species through morphological characteristics and phylogenetic analyses based on partial DNA sequences of glyceraldehyde-3-phosphate dehydrogenase (GAPDH), internal transcribed spacer (ITS) regions, and the β-tubulin (TUB2), actin (ACT) and calmodulin (CAL) genes. Among all species, Colletotrichum fructicola was the most dominant species, with an isolation percentage of up to 66.2% in Sichuan, followed by Colletotrichum siamense (17.6%), C. kahawae (5.4%), C. karstii (5.4%), C. nymphaeae (2.7%) and C. sichuaninese (2.7%). Pathogenicity tests showed all species were able to cause typical anthracnose symptoms on blueberry leaves and stems. Colletotrichum fructicola was the predominant species with strong aggressiveness. Moreover, C. fructicola, C. kahawae, C. sichuaninese and C. nymphaeae are first reported here to cause blueberry anthracnose. This study provides a comprehensive reference for the association of different Colletotrichum spp., which may support the sustainable management of blueberry anthracnose.
Keywords: blueberry, anthracnose, Colletotrichum spp., multilocus phylogeny, pathogenicity
1. Introduction
Blueberry (Vaccinium corymbosum) belongs to the family Ericaceae, subfamily Vaccinoideae [1]. Blueberries are rich in antioxidants, anthocyanins, tannins and folic acid, which help prevent and delay aging [2]. Therefore, the market demand is expanding, global blueberry production is increasing, and the price of blueberries continues to increase [3]. Because of their high nutrient and business prospects, blueberries have been widely cultivated worldwide [4,5]. The increased cultivation of blueberries drastically promotes severe infectious diseases that cause significant economic losses to the blueberry industry. Among these diseases, blueberry anthracnose has been considered one of the most serious diseases causing economic damage in North America [6], Japan [7], Spain [8], South Korea [9], China [10], and other countries worldwide. Due to the high humidity conditions in Sichuan Province, the incidence of anthracnose in major blueberry regions usually reaches 30%, and the disease worsens with tree age, resulting in severe deciduous leaves or dead trees, with yields decreasing by 10~15% in some severe regions.
Previous reports have shown that blueberry anthracnose is mainly caused by C. acutatum J.H. Simmonds [8,9,11] and C. gloeosporioides Penz., as based on morphological identification [9,12,13]. In 2015, C. karstii was also recognized as a pathogen causing blueberry anthracnose in Brazil [14]. In China, this disease was first reported in 2012 in Liaoning Province and was caused by C. gloeosporioides and C. acutatum [15,16]. Since then, the identification of blueberry anthracnose has rarely been reported.
Three classification systems mainly based on morphological characteristics have been used to identify Colletotrichum species [17]. The first traditional classification system was presented by Saccardo in 1882. Later, in 1957, the Von Arx classification system was introduced, which established the first taxonomic system based on the morphology of the Colletotrichum genus. In 1980, Sutton improved Von Arx’s system and advocated different criteria for species identification with respect to morphological characteristics, host range and pathogenicity. However, it is still very difficult to distinguish the majority of similar species merely on the basis of morphological characteristics [18,19,20,21]. With the molecular characterization of the pathogen and the development of DNA sequencing over past decades, a combination of morphological characteristics and multilocus phylogeny has recently been used to identify Colletotrichum species. To date, many anthracnose diseases have been diagnosed in various crops, such as apple [22], grapevine [23], pepper [24], jute [25], persimmon [26] and citrus [27].
Sichuan Province is the main blueberry cultivation area in China and marked blueberry anthracnose has occurred in recent years. However, the pathogens causing this disease, have not yet been reported. Therefore, the objective of the current study was to clarify the composition of Colletotrichum species on blueberries in China using a combination of morphological characteristics and multiple locus phylogenetic analysis. Our study reveals the associations of Colletotrichum species with blueberries and may help growers to sustainably manage blueberry anthracnose.
2. Results
2.1. Symptomatology and Fungal Isolation
Based on field observations, anthracnose symptoms on leaves and stems were recorded. The disease started at the leaf margin, evidenced by a red halo at the junction of diseased and healthy portions, and the center of the diseased spot was dark gray and irregularly expanded. Then, leaf blight developed, and a yellow color was observed (Figure 1a,b). The diseased stems showed irregular black spots that started from the base or middle, and then the whole branches withered (Figure 1c,d). From 2016 to 2018, 74 isolates of Colletotrichum were isolated from 85 samples (Table 1). Among 48 isolates obtaind from leaves, 30 isolates were identified as C. fructicola, 10 as C. siamense, and the remaining 8 as other Colletotrichum species. In total, 26 isolates were isolated from stems; 19 isolates were identified as C. fructicola, and the remaining 7 isolates belonged to other Colletotrichum species. Other fungi, such as Alternaria and Pestalotiopsis, were also isolated, but they were not considered in this study.
Figure 1.
Symptoms of anthracnose on blueberries under field conditions. (a) Spots on blueberry leaves; (b) a close-up image of diseased leaves; (c) stems blackened from the base; (d) stems blackened from the middle.
Table 1.
List of different isolates with their geographical information isolated from blueberry anthracnose in Sichuan Province.
| Isolate No. | Parts | Cultivars | Location | Latitude and Longitude | Species |
|---|---|---|---|---|---|
| LMT1 | Leaf | Sharpblue | Daguang, Dujiangyan | N 30°51′4″ E 103°34′44″ | C. sichuaninese |
| LMT2 | Leaf | Sharpblue | Daguang, Dujiangyan | N 30°51′4″ E 103°34′44″ | C. fructicola |
| LMT3 | Stem | Misty | Tuqiao, Jintang | N 30°31′17″ E 104°50′5″ | C. fructicola |
| LMT4 | Stem | Misty | Tuqiao, Jintang | N 30°31′17″ E 104°50′5″ | C. kahawae |
| LMT5 | Leaf | Misty | Taiping, Shuangliu | N 30°25′53″ E 104°11′54″ | C. fructicola |
| LMT6 | Leaf | O’Neal | Taiping, Shuangliu | N 30°25′53″ E 104°11′54″ | C. fructicola |
| LMT7 | Leaf | Sharpblue | Daguang, Dujiangyan | N 30°51′4″ E 103°34′44″ | C. karstii |
| LMT8 | Leaf | Sharpblue | Daguang, Dujiangyan | N 30°51′4″ E 103°34′44″ | C. fructicola |
| LMT9 | Stem | O’Neal | Caichang, Dayi | N 30°30′8″ E 103°41′57″ | C. fructicola |
| LMT10 | Stem | O’Neal | Caichang, Dayi | N 30°30′8″ E 103°41′57″ | C. fructicola |
| LMT11 | Leaf | Misty | Tuqiao, Jintang | N 30°31′17″ E 104°50′5″ | C. kahawae |
| LMT12 | Leaf | Misty | Daguang, Dujiangyan | N 30°51′4″ E 103°34′44″ | C. fructicola |
| LMT13 | Leaf | Sharpblue | Caichang, Dayi | N 30°30′8″ E 103°41′57″ | C. nymphaeae |
| LMT14 | Leaf | Sharpblue | Caichang, Dayi | N 30°30′8″ E 103°41′57″ | C. fructicola |
| LMT15 | Leaf | Misty | Taiping, Shuangliu | N 30°25′53″ E 104°11′54″ | C. siamense |
| LMT16 | Leaf | O’Neal | Taiping, Shuangliu | N 30°25′53″ E 104°11′54″ | C. fructicola |
| LMT17 | Leaf | Misty | Tuqiao, Jintang | N 30°31′17″ E 104°50′5″ | C. fructicola |
| LMT18 | Leaf | Misty | Tuqiao, Jintang | N 30°31′17″ E 104°50′5″ | C. fructicola |
| LMT19 | Leaf | Sharpblue | Daguang, Dujiangyan | N 30°51′4″ E 103°34′44″ | C. siamense |
| LMT20 | Leaf | Sharpblue | Taiping, Shuangliu | N 30°25′53″ E 104°11′54″ | C. fructicola |
| LMT21 | Leaf | O’Neal | Taiping, Shuangliu | N 30°25′53″ E 104°11′54″ | C. fructicola |
| LMT22 | Stem | Sharpblue | Caichang, Dayi | N 30°30′8″ E 103°41′57″ | C. fructicola |
| LMT23 | Leaf | Sharpblue | Caichang, Dayi | N 30°30′8″ E 103°41′57″ | C. fructicola |
| LMT24 | Leaf | Sharpblue | Caichang, Dayi | N 30°30′8″ E 103°41′57″ | C. siamense |
| LMT25 | Stem | O’Neal | Wuyang, Pengshan | N 30°11′44″ E 103°52′8″ | C. karstii |
| LMT26 | Stem | O’Neal | Wuyang, Pengshan | N 30°11′44″ E 103°52′8″ | C. fructicola |
| LMT27 | Stem | O’Neal | Wuyang, Pengshan | N 30°11′44″ E 103°52′8″ | C. fructicola |
| LMT28 | Leaf | O’Neal | Taiping, Shuangliu | N 30°25′53″ E 104°11′54″ | C. fructicola |
| LMT29 | Leaf | O’Neal | Taiping, Shuangliu | N 30°25′53″ E 104°11′54″ | C. fructicola |
| LMT30 | Leaf | Sharpblue | Caichang, Dayi | N 30°30′8″ E 103°41′57″ | C. fructicola |
| LMT31 | Leaf | Sharpblue | Caichang, Dayi | N 30°30′8″ E 103°41′57″ | C. fructicola |
| LMT32 | Leaf | Sharpblue | Caichang, Dayi | N 30°30′8″ E 103°41′57″ | C. fructicola |
| LMT33 | Leaf | Sharpblue | Caichang, Dayi | N 30°30′8″ E 103°41′57″ | C. siamense |
| LMT34 | Leaf | O’Neal | Taiping, Shuangliu | N 30°25′53″ E 104°11′54″ | C. siamense |
| LMT35 | Stem | O’Neal | Gongping, Wenjiang | N 30°43′22″ E 103°49′28″ | C. siamense |
| LMT36 | Stem | O’Neal | Gongping, Wenjiang | N 30°43′22″ E 103°49′28″ | C. siamense |
| LMT37 | Stem | O’Neal | Gongping, Wenjiang | N 30°43′22″ E 103°49′28″ | C. fructicola |
| LMT38 | Stem | O’Neal | Gongping, Wenjiang | N 30°43′22″ E 103°49′28″ | C. fructicola |
| LMT39 | Stem | O’Neal | Gongping, Wenjiang | N 30°43′22″ E 103°49′28″ | C. fructicola |
| LMT40 | Stem | O’Neal | Gongping, Wenjiang | N 30°43′22″ E 103°49′28″ | C. fructicola |
| LMT41 | Stem | O’Neal | Gongping, Wenjiang | N 30°43′22″ E 103°49′28″ | C. fructicola |
| LMT42 | Stem | Misty | Tuqiao, Jintang | N 30°31′17″ E 104°50′5″ | C. fructicola |
| LMT43 | Stem | Misty | Tuqiao, Jintang | N 30°31′17″ E 104°50′5″ | C. fructicola |
| LMT44 | Leaf | O’Neal | Taiping, Shuangliu | N 30°25′53″ E 104°11′54″ | C. siamense |
| LMT45 | Leaf | O’Neal | Taiping, Shuangliu | N 30°25′53″ E 104°11′54″ | C. fructicola |
| LMT46 | Leaf | Sharpblue | Daguang, Dujiangyan | N 30°51′4″ E 103°34′44″ | C. fructicola |
| LMT47 | Leaf | Sharpblue | Daguang, Dujiangyan | N 30°51′4″ E 103°34′44″ | C. fructicola |
| LMT48 | Stem | O’Neal | Caichang, Dayi | N 30°30′8″ E 103°41′57″ | C. fructicola |
| LMT49 | Stem | O’Neal | Caichang, Dayi | N 30°30′8″ E 103°41′57″ | C. fructicola |
| LMT50 | Leaf | Misty | Tuqiao, Jintang | N 30°31′17″ E 104°50′5″ | C. siamense |
| LMT51 | Leaf | Sharpblue | Daguang, Dujiangyan | N 30°51′4″ E 103°34′44″ | C. sichuaninese |
| LMT52 | Leaf | Sharpblue | Caichang, Dayi | N 30°30′8″ E 103°41′57″ | C. fructicola |
| LMT53 | Leaf | Sharpblue | Caichang, Dayi | N 30°30′8″ E 103°41′57″ | C. fructicola |
| LMT54 | Leaf | O’Neal | Taiping, Shuangliu | N 30°25′53″ E 104°11′54″ | C. fructicola |
| LMT55 | Leaf | O’Neal | Taiping, Shuangliu | N 30°25′53″ E 104°11′54″ | C. fructicola |
| LMT56 | Leaf | Misty | Tuqiao, Jintang | N 30°31′17″ E 104°50′5″ | C. siamense |
| LMT57 | Leaf | Misty | Tuqiao, Jintang | N 30°31′17″ E 104°50′5″ | C. fructicola |
| LMT58 | Leaf | Sharpblue | Daguang, Dujiangyan | N 30°51′4″ E 103°34′44″ | C. siamense |
| LMT59 | Leaf | Misty | Taiping, Shuangliu | N 30°25′53″ E 104°11′54″ | C. fructicola |
| LMT60 | Leaf | Misty | Taiping, Shuangliu | N 30°25′53″ E 104°11′54″ | C. fructicola |
| LMT61 | Stem | O’Neal | Caichang, Dayi | N 30°30′8″ E 103°41′57″ | C. nymphaeae |
| LMT62 | Stem | Sharpblue | Wuyang, Pengshan | N 30°11′44″ E 103°52′8″ | C. fructicola |
| LMT63 | Stem | Sharpblue | Wuyang, Pengshan | N 30°11′44″ E 103°52′8″ | C. siamense |
| LMT64 | Stem | Sharpblue | Wuyang, Pengshan | N 30°11′44″ E 103°52′8″ | C. fructicola |
| LMT65 | Leaf | Misty | Taiping, Shuangliu | N 30°25′53″ E 104°11′54″ | C. karstii |
| LMT66 | Leaf | Misty | Taiping, Shuangliu | N 30°25′53″ E 104°11′54″ | C. siamense |
| LMT67 | Leaf | O’Neal | Caichang, Dayi | N 30°30′8″ E 103°41′57″ | C. fructicola |
| LMT68 | Leaf | O’Neal | Caichang, Dayi | N 30°30′8″ E 103°41′57″ | C. fructicola |
| LMT69 | Leaf | O’Neal | Caichang, Dayi | N 30°30′8″ E 103°41′57″ | C. fructicola |
| LMT70 | Leaf | O’Neal | Caichang, Dayi | N 30°30′8″ E 103°41′57″ | C. karstii |
| LMT71 | Leaf | Misty | Taiping, Shuangliu | N 30°25′53″ E 104°11′54″ | C. kahawae |
| LMT72 | Stem | Misty | Gongping, Wenjiang | N 30°43′22″ E 103°49′28″ | C. fructicola |
| LMT73 | Stem | Misty | Gongping, Wenjiang | N 30°43′22″ E 103°49′28″ | C. fructicola |
| LMT74 | Stem | Misty | Gongping, Wenjiang | N 30°43′22″ E 103°49′28″ | C. kahawae |
2.2. Morphological Characterization
After a comprehensive morphological analysis, all isolates (n = 74) were classified into six morphological groups (Figure 2). The morphological characteristics of six Colletotrichum spp. (C. fructicola, C. siamense, C. kahawae, C. karstii, C. nymphaeae and C. sichuaninese) were obviously different, and are provided below in detail.
Figure 2.
Illustration of the morphological and cultural characteristics of Colletotrichum spp. associated with blueberry anthracnose in Sichuan. Each row from left to right: views of the surface and the bottom of potato dextrose agar (PDA) plate of representative isolates, conidia, conidial appressoria and mycelial appressoria.
Colletotrichum fructicola, C. kahawae, C. sichuaninese and C. nymphaeae are reported as novel pathogens of blueberries. In this study, C. fructicola developed a black-colored colony with a thin aerial hyphal growth, and developed gray, orange-red uredinia after approximately 7 days of incubation (Figure 2A-1). However, the colonies of the other three species were gray and fluffy aerial hyphae after incubation (Figure 2). Colletotrichum kahawae had the slowest average mycelial growth rate (3.4 ± 0.09 mm/d) (Table 2). The conidia of C. nymphaeae were fusiform (Figure 2D-3), but C. fructicola, C. kahawae and C. sichuaninese conidia were cylindrical in shape (Figure 2A-3,C-3,E-3). The growth rate (6.2 ± 0.08 mm/day) of C. nymphaeae was higher than C. siamense, C. kahawae and C. sichuaninese (Table 2). The spore appressorium of C. nymphaeae was gray and round (Figure 2D-4), but that of C. fructicola, C. kahawae and C. sichuaninese was brown to dark black (Figure 2A-4,C-4,E-4).
Table 2.
Morphological characteristics of Colletotrichum species from blueberry in Sichuan, China.
| Group | Species | Colony | Growth Rate (mm/d) | Conidia | Appressoria | Mycelial Appressoria | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Length (μm) | Width (μm) | Shape | Length (μm) | Width (μm) | Shape and Color | |||||
| 1 | Colletotrichum fructicola | black and gray, reverse black | 6.0 ± 0.04 a | 13.3 ± 0.21 b | 5.4 ± 0.24 a | cylindrical | 6.7 ± 0.16 a | 5.0 ± 0.17 a | brown to dark black, ovoid to slightly irregular | oval to irregular, light brown to dark brown, margin lobed or deeply lobed |
| 2 | Colletotrichum siamense | white, reverse pale yellowish | 4.3 ± 0.12 c | 11.7 ± 0.41 c | 5.4 ± 0.21 a | cylindrical | 5.7 ± 0.39 b | 4.7 ± 0.22 b | brown to dark black, irregular with a crenate edge | irregularly shaped and brown, with a deep, marginal, lobed edge |
| 3 | Colletotrichum kahawae | pale gray, reverse pale gray | 3.4 ± 0.09 d | 14.4 ± 0.60 a | 5.0 ± 0.94 b | cylindrical | 6.7 ± 0.40 a | 5.4 ± 0.17 c | brown to dark black, round | ovoid or irregularly shaped, light brown, with a deep, marginal, lobed, or smooth edge |
| 4 | Colletotrichum nymphaeae | gray, reverse dark gray | 6.2 ± 0.08 a | 10.5 ± 0.08 d | 4.6 ± 0.43 c | fusiform | 7.4 ± 0.33 c | 5.9 ± 011 d | gray, round | oval, light brown, smooth edge |
| 5 | Colletotrichum sichuaninese | gray, reverse pale gray | 5.5 ± 0.39 b | 14.5 ± 0.32 a | 5.2 ± 0.30 ab | long cylindrical | 12.0 ± 0.56 d | 8.8 ± 0.21 e | brown to dark black, ovoid to slightly irregular | oval or round, brown to dark brown, with a smooth border |
| 6 | Colletotrichum karstii | white, reverse white | 6.4 ± 0.05 a | 15.7 ± 0.12 e | 7.0 ± 0.26 d | fusiform | 8.9 ± 0.32 e | 7.4 ± 0.46 f | brown to dark black, round | oval to round, light brown, and smooth edges |
Note: In a column, averages followed by different small letters indicate statistically significant differences (p < 0.05); a–f: the values with the same letter in a column do not significantly differ according to Duncan’s multiple range test.
C. siamense and C. karstii have been reported to cause blueberry anthracnose in other areas [14,28]. The colonies of C. siamense were white and developed sparse and white aerial hyphae with orange-red spores after approximately 10 days of incubation (Figure 2B-1). The mycelial appressoria were irregularly shaped and brown, with a deep, marginal, lobed edge (Figure 2B-5). C. karstii developed white colonies with thin aerial hyphae after approximately 6 days of incubation (Figure 2F-1). The spore appressorium and mycelial appressoria were round (Figure 2F-4,F-5), but the spore appressorium of C. siamense was irregular (Figure 2B-4).
2.3. Molecular Characterization and Phylogeny of Isolates
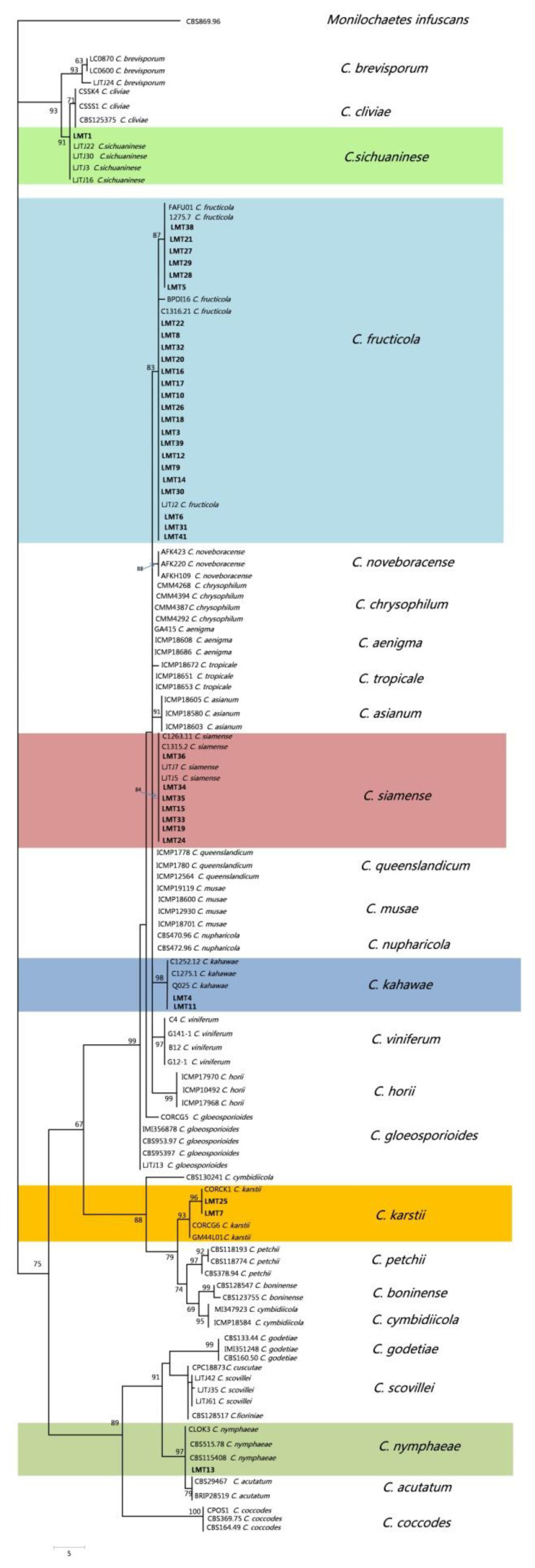
A maximum-parsimony tree and maximum likelihood tree were constructed based on the GAPDH gene region using 74 isolates obtained in this study (Figures S1 and S2). Based on the 260 conserved characteristics used in the phylogenetic analysis, the tree contained six primary clades. After a comprehensive analysis of morphological characteristics and the GAPDH gene tree, all isolates were divided into the 6 different groups as explained above, and 37 representative isolates were further selected from different clades for molecular identification. A multilocus phylogenetic tree, based on 2335 characters consisting of ITS, TUB2, ACT, CAL and GAPDH, was constructed using 121 isolates of Colletotrichum (37 from this study) and Monilochaetes infuscans (CBS869.96) as the outgroup; the other 84 isolates of Colletotrichum species reported on different hosts were obtained from GenBank (Table S1).
Both maximum parsimony (MP) and maximum likelihood (ML) analyses revealed that the 37 representative isolates selected in this study clustered with C. fructicola, C. siamense, C. kahawae, C. karstii, C. nymphaeae and C. sichuaninese. Phylogenetic analyses performed using the maximum parsimony and maximum likelihood method produced similar topologies; only the combined dataset based on maximum parsimony is shown in Figure 3 (CI = 0.615, RI = 0.927, RC= 0.570), and the maximum likelihood tree in Figure S3. We found that the isolates from Group 1 clustered with C. fructicola, including the majority of isolates in this study; those from Group 2 clustered with C. siamense, and those from Group 3 clustered with C. kahawae. The isolates composed a highly supported clade (98% bootstrap support) with C1275.1 in Arabian coffee from Angola, C1252.12 in Kunzea ericoides from New Zealand and Q025 in blackberry from Colombia. The isolates from Group 4 clustered within C. nymphaeae, those from Group 5 clustered with C. sichuaninese, and those from Group 6 clustered with C. karstii (CORCG6, CORCK1, GM44L01), with strong bootstrap support of 96%.
Figure 3.
Phylogenetic tree generated from maximum parsimony analysis based on GAPDH, ITS, TUB2, ACT and CAL gene sequences. Parsimony bootstrap values of more than 50% are shown at the nodes. Isolates from this study are shown in bold. The tree is rooted with Monilochaetes infuscans. Detailed information is provided in Table S1.
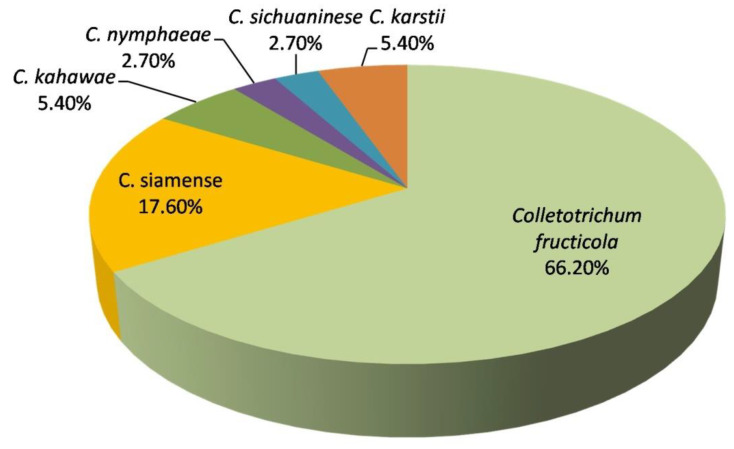
According to morphological characteristics and molecular analysis, the identification of the 74 isolates showed that C. fructicola was the most dominant species (66.2%), followed by C. siamense (17.6%), C. kahawae (5.4%), C. karstii (5.4%), C. nymphaeae (2.7%) and C. sichuaninese (2.7%) (Figure 4). The sequences of all the representative isolates in the current study have been deposited in the NCBI database GenBank (Table S1).
Figure 4.
The proportion of Colletotrichum species associated with blueberry anthracnose in Sichuan.
2.4. Pathogenicity
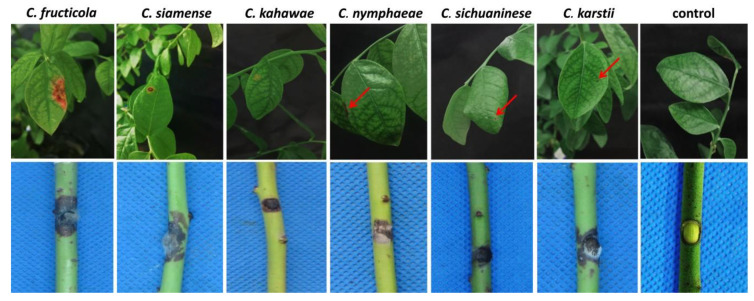
The representative isolates (n = 37) of the six different species isolated, that grew on PDA medium at 28 °C for 5 days, were inoculated onto one-year-old healthy potted blueberry plants cv. O’Neal. The plants displayed disease symptoms at 7 days of inoculation, showing that all these isolates were able to infect blueberry leaves and detached stems; however, inoculation of the attached blueberry stems did not result in any symptoms (Figure 5). The typical symptoms produced by artificial inoculation conformed with the original symptoms under natural conditions, and Koch’s postulate was verified by identifying reisolated strains based on morphological characteristics and ITS sequences. Moreover, different species induced variable pathogenic effects on blueberry tissues and showed distinct aggressiveness. However, C. fructicola was the species with the highest aggressiveness on attached leaves and detached stems. At 3 days after inoculation, symptoms began to appear on leaves and stems; 5 days after inoculation, the symptoms on the leaves were significantly expanded, and abundant mycelia and orange spores appeared on the detached stems. In contrast, C. nymphaeae and C. karstii were weakly aggressiveness to leaves, and at 7 days after inoculation, the symptoms on leaves did not markedly spread. C. sichuaninese, C. kahawae and C. nymphaeae were weakly pathogenic on detached stems, no orange spores were seen on the stems, and the symptoms did not markedly spread.
Figure 5.
Symptoms of blueberry leaves and detached stems inoculated with different Colletotrichum species. Note: Representative isolates of C. fructicola, C. siamense, C. kahawae, C. nymphaeae, C. sichuaninese and C. karstii are LMT12, LMT19, LMT4, LMT13, LMT1 and LMT25, respectively; the control was inoculated with sterile water or only PDA plugs. The red arrows indicate the site of the lesions.
We also evaluated the pathogenicity of six Colletotrichum species via the diameter of lesions. Different species showed differences in pathogenicity (Table 3); the lesion diameters of leaves were 5.8–14.5 mm, and the lesion diameters of stems ranged from 1.4 mm to 15.2 mm. In terms of each species, C. fructicola has strong aggressiveness, and at 5 days after inoculation, both leaves and stems had significantly larger lesions (14.2 ± 0.35 mm, 15.0 ± 0.20 mm). Conversely, C. sichuaninese showed weak pathogenicity, with a small range of lesions on stems and leaves after inoculation (6.0 ± 0.22 mm, 1.6 ± 0.10 mm).
Table 3.
Aggressiveness differentiation of Colletotrichum species.
| Species | Average Lesion Diameter (mm) | |
|---|---|---|
| Detached Stems | Leaves on Seedings | |
| C. fructicola | 14.2 ± 0.35 a | 15.0 ± 0.21 a |
| C. siamense | 12.1 ± 0.30 b | 2.9 ± 0.21 c |
| C. kahawae | 6.2 ± 0.15 e | 2.8 ± 0.15 c |
| C. nymphaeae | 7.0 ± 0.22 d | 5.1 ± 0.21 b |
| C. sichuaninese | 6.0 ± 0.22 e | 1.6 ± 0.10 d |
| C. karstii | 10.1 ± 0.26 c | 1.6 ± 0.20 d |
Note: In a column, averages followed by different small letters indicate statistically significant differences (p < 0.05). a–e: the values with the same letter in a column do not significantly differ according to Duncan’s multiple range test.
3. Discussion
In our study, 74 isolates were obtained from 85 blueberry anthracnose samples (leaves and stems), mainly collected from major blueberry cultivation areas in Sichuan Province, China. Six species of Colletotrichum associated with blueberry anthracnose were characterized using a phylogenetic approach. Colletotrichum fructicola, C. siamense and C. kahawae belong to the C. gloeosporioides species complex, and C. nymphaeae and C. karstii belong to the C. acutatum species complex and the C. boninense species complex, respectively. C. sichuaninese belongs to the C. orchidarium species complex. Colletotrichum fructicola, C. kahawae, C. sichuaninese and C. nymphaeae were first recorded in this study to cause blueberry anthracnose.
Although C. gloeosporioides and C. acutatum have previously been identified as the main causal agents of blueberry anthracnose in different countries worldwide [8,9,15,16,29,30], we found that none of the isolates from blueberry in the current study belong to C. gloeosporioides species sensu stricto and the C. acutatum species sensu stricto. Since the early identifications were mainly based on morphological characterization and ITS sequence, which have been shown to be unable to distinguish among certain species of Colletotrichum, we speculate that the early reported species were probably species complexes of C. gloeosporioides and C. acutatum, respectively. Our results appear to be quite different from previous studies, with most isolates mainly belonging to the C. gloeosporioides species complex and the C. acutatum species complex, and accounting for 92% of all the isolates in this study. From the perspective of the species complex, the results are basically consistent with previous studies. Six species were found through the use of multiple representative genes (GAPDH, BAT, CAL, ACT and ITS) in phylogenetic analyses in this study, indicating the very rich diversity of the Colletotrichum species in blueberries in Sichuan. It is speculated that the diversity of Colletotrichum may be detected through polygenic phylogenetic analysis in other regions worldwide, for example, C. karstii was first found on blueberry anthracnose in Brazil [14].
According to the result of our study, C. fructicola was the dominant and highly aggressive pathogen causing the disease. Colletotrichum fructicola is a nonhost-specific pathogen that has been reported on certain hosts, such as apple [22], Citrus spp. [31,32], Pyrus spp. [33], Camellia sinensis [34], Capsicum sp. [24,35], Mangifera indica [36,37] and Malus sp. [38]. Although C. sichuaninese was first observed on pepper in Sichuan Province [24], to the best of our knowledge, this is the first recorded species to cause anthracnose disease in blueberries worldwide. As the wide host range of C. sichuaninese may pose a serious threat to the blueberry industry, further work will focus on the diversity of the Colletotrichum species, the nonhost specificity of most species and the characteristics of the newly recorded species associated with blueberries in Sichuan.
In conclusion, this study is the first detailed report of blueberry anthracnose in China and provides a basis for disease management. Nonetheless, the pathogenic mechanisms of different Colletotrichum spp. in blueberry need to be further studied.
4. Materials and Methods
4.1. Sample Collection and Isolation
From 2016 to 2018, a total of 85 samples of blueberry leaves (n = 48) and stems (n = 37) showing typical symptoms of anthracnose on three blueberry cultivars were collected from six blueberry growing areas in Sichuan Province, China (Table 1). Some samples came from the same blueberry growing areas, but from different orchards; approximately 20 samples were collected in a 1 hectare orchard. The samples were stored in sterile polythene bags and brought to the laboratory for stereomicroscope (OLYMPUS SZX16, Tokyo, Japan) examination. Then, lesion margin pieces (5 × 5 mm) were cut and sterilized with sodium hypochlorite for 30 s, and mercuric chloride for 25 s, rinsed three times with distilled sterile water, cultured on potato dextrose agar (PDA), and inoculated in a chamber at 28 ± 1 °C with a 12 h photoperiod for 3 days [39]. Single-spore isolates were obtained from cultures on the PDA plates according to Gong et al. [40]. A single spore was inoculated with sterilized acupuncture needles onto a water agar block, and the block was moved to new plates that were incubated at 28 ± 1 °C for 3 days. Later, the hyphal block was transferred to PDA plates and incubated at 28 °C for 5 days. The pure cultures were obtained and stored on PDA slants at 4 °C.
4.2. Morphological Characterization
After culturing on PDA at 28 °C for 5 days, the morphological characteristics of all isolates, including colony, conidia, conidial appressoria and mycelial appressoria, were initially observed. The colony diameter of each isolate from 1 day to 7 days after inoculation was assessed using the cross measurement method [41]. The size of approximately 30 conidia was measured and recorded. Conidial appressoria were induced at 27 °C for 24 h [42], and 30 appressorial sizes from each isolate were randomly measured. Mycelial appressoria were observed by the slide culture technique [43], and the development of mycelia was examined after 7 days of incubation at 28 °C. All developmental stages were observed with a Carl Zeiss Microscope (GmbH37081, Gottingen, Germany). The data on the radial colony growth rate, conidial size and conidial appressoria size were statistically analyzed for variance at the p = 0.05 level (LSD) using SPSS v21.0 software.
4.3. Molecular Characterization
The aerial mycelia of all isolates grown on potato dextrose agar (PDA) for 5 days at 25 °C in the dark were collected for DNA extraction according to the protocol by Than et al. [44]. First, the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene was amplified to select representative isolates [24]. The nuclear rDNA ITS region and the β-tubulin (TUB2), partial actin (ACT) and calmodulin (CAL) genes were amplified for each representative isolate using the primer pairs ITS1/ITS4 [45], Bt2a/Bt2b [46], ACT512F/ACT783R [47] and CL1/CL2A [48], respectively. PCR was performed by following the protocol described by Prihastuti et al. [49]. PCR was conducted on 30 µl: 0.5 µL upstream primers (10 mol/L), 0.5 µL downstream primers (10 mol/L), 1 µL DNA templates (30–50 ng/µL), 15 µL 2 × PCR Mastermix and 13 µL ddH2O. The PCR-amplified product was examined by 1% agarose gel electrophoresis and sequenced by Shanghai Biological Engineering Co. Ltd., P. R. China.
4.4. Phylogenetic Analysis
Phylogenetic analysis was conducted by the method described by Liu et al. [24]. The GAPDH genes of the isolates (n = 74) were blasted to compare the sequence identity with those in the NCBI database and analyzed using Clustal X 1.81. Next, the maximum parsimony and maximum likelihood analyses were used to construct a phylogenetic tree in MEGA 6. Representative isolates (n = 37) were selected according to the morphological characterization and GAPDH gene tree and were combined with their high-similarity isolates retrieved in the NCBI database and type specimens [19,20,21,42,50] (Table S1) to build polygenic trees (ITS, TUB2, ACT, CAL and GAPDH) by the maximum parsimony and maximum likelihood analyses. The consistency index (CI), retention index (RI) and rescaled consistency index (RC) were also calculated.
4.5. Pathogenicity Tests
The pathogenicity of representative isolates (n = 37) for the six species was tested on one-year-old healthy potted blueberry plants (cv. O’Neal). The plants were surface sterilized with 75% alcohol and then rinsed three times with sterile distilled water before being used for the following inoculations. All isolates were grown on PDA medium at 28 °C for 5 days, and then spore suspensions at a concentration of 1 × 105 conidia/mL or 5 mm diameter mycelial plugs were selected for inoculation. Sterile water or only PDA plugs were used as controls. For the inoculation of leaves in vivo, the leaves of potted plants were sprayed with 1 × 105 conidia/mL spore suspensions using a handheld sprayer. For inoculation of stems in vitro, the stems were cut into 8 cm lengths, and 5 mm diameter mycelial plugs were applied to a wound of stems made using a sterile cork borer. The inoculated stems were then placed on a disk and covered with plastic wrap [51]. For the inoculation of stems in vivo, wounds were generated with a sterilized pointed needle in the middle of the potted plant stem and then inoculated with 5 mm diameter mycelial plugs. Five healthy leaves and three stems were inoculated on different plants as one replicate, and three replicates were used for each treatment and control. All treated samples were incubated in a humid atmosphere for 25 °C in a greenhouse until disease symptoms appeared. All experiments were repeated twice. Symptoms were recorded every day, and the diameters of the lesions were measured at 5 days after inoculation. The data were analyzed with SPSS Statistics 21.0 by one-way ANOVA.
The inoculated leaves and stems were reisolated to confirm the pathogen identity based on morphological characteristic and ITS sequence analyses.
Acknowledgments
We are grateful to our team at the Plant Protection Department & Major Crop Disease Laboratory, College of Agronomy, Sichuan Agricultural University for supporting our research. The authors also thank the anonymous reviewers for their constructive suggestions.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-0817/9/9/718/s1, Table S1. GenBank accession numbers and sources of different strains used in the current study. Figure S1. A maximum parsimony tree based on partial GAPDH gene sequences from 74 Colletotrichum isolates. Parsimony bootstrap values of more than 50% are shown at the nodes. Isolates selected for subsequent phylogenetic analyses are highlighted in red. Figure S2. A maximum likelihood tree based on partial GAPDH gene sequences from 74 Colletotrichum isolates. Parsimony bootstrap values of more than 50% are shown at the nodes. Isolates selected for subsequent phylogenetic analyses are highlighted in blue. Figure S3. Phylogenetic tree generated from maximum likelihood analysis based on GAPDH, ITS, TUB2, ACT and CAL gene sequences. Parsimony bootstrap values of more than 50% are shown at the nodes. Isolates from this study are shown in bold, and the species of the same color belong to the same complex species of Colletotrichum. The tree is rooted with Monilochaetes infuscans. Detailed information is provided in Table S1.
Author Contributions
Conceptualization, G.G., X.L. and X.Z.; Methodology, X.L., X.Z. and G.G.; Software, X.L., X.Z. and X.S.; Validation, X.L., X.Z. and G.G.; Formal Analysis, X.L. and X.Z.; Investigation, X.L. and X.Z.; Resources, X.L., X.Z. and X.S.; Data Curation, X.L. and X.Z.; Writing—Original Draft Preparation, X.L.; Writing—Review and Editing, G.G., M.I.K. and X.C.; Visualization, X.L. and X.S.; Supervision, G.G. and M.I.K.; Project Administration, G.G.; Funding Acquisition, G.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was founded by [Two-Way Support Project of Sichuan Agricultural University] grant number [03572101].
Conflicts of Interest
The authors declare no conflict of interest in this work. This research did not involve human participants or animals. All authors have offered consent to submission.
References
- 1.Gao L.X., Xiao H.L., Li S., Liu F.M., Mo A.Q. Current development situation and prospects of blueberry in Guangdong Province. Asian J. Agric. Res. 2015;9:23–27. [Google Scholar]
- 2.Sun G.B. The health care effect of blueberry and its development trend in other countries. J. Agric. Mech. Res. 2002;2002:225. [Google Scholar]
- 3.Li Y.D., Liu H.G., Zhang Z.D. The import and export trade and production of blueberry. China Fruits. 2010;2010:72–75. [Google Scholar]
- 4.Lobos G.A., Hancock J.F. Breeding blueberries for a changing global environment. Front. Plant Sci. 2015;6:782. doi: 10.3389/fpls.2015.00782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reque P.M., Steffens R.S., Jablonski A. Cold storage of blueberry (Vaccinium spp.) fruits and juice: Anthocyanin stability and antioxidant activity. J. Food Compos. Anal. 2014;33:111–116. doi: 10.1016/j.jfca.2013.11.007. [DOI] [Google Scholar]
- 6.Polashock J.J., Ehlenfeldt M.K., Stretch A.W. Anthracnose fruit rot resistance in blueberry cultivars. Plant Dis. 2005;89:33–38. doi: 10.1094/PD-89-0033. [DOI] [PubMed] [Google Scholar]
- 7.Yoshida S., Tsukiboshi T. Shoot blight and leaf spot of blueberry anthracnose caused by Colletotrichum acutatum. J. Gen. Plant Pathol. 2002;68:246–248. doi: 10.1007/PL00013083. [DOI] [Google Scholar]
- 8.Barrau C., Santos B.D.L., Romero F. First report of Colletotrichum acutatum in blueberry plants in Spain. Plant Dis. 2001;85:1285. doi: 10.1094/PDIS.2001.85.12.1285A. [DOI] [PubMed] [Google Scholar]
- 9.Kim W.G., Hong S.K., Choi H.W., Lee Y.K. Occurrence of anthracnose on highbush blueberry caused by Colletotrichum species in Korea. Mycobiology. 2009;37:310–312. doi: 10.4489/MYCO.2009.37.4.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu H., Wang C., Gu Y. The present status of blueberry growing in China. J. Plant Resour. Environ. 2005;14:42. [Google Scholar]
- 11.Smith B.J., Magee J.B., Gupton C.L. Susceptibility of rabbiteye blueberry cultivars to postharvest diseases. Plant Dis. 1996;80:215–218. doi: 10.1094/PD-80-0215. [DOI] [Google Scholar]
- 12.Daykin M.E., Milholland R.D. Infection of blueberry fruit by Colletotrichum gloeosporioides. Plant Dis. 1984;68:948–950. doi: 10.1094/PD-68-948. [DOI] [Google Scholar]
- 13.Hartung J.S., Burton C.L., Ramsdell D.C. Epidemiological studies of blueberry anthracnose disease caused by Colletotrichum gloeosporioides. Phytopathology. 1981;71:449–453. doi: 10.1094/Phyto-71-449. [DOI] [Google Scholar]
- 14.Rios J.A., Pinho D.B., Moreira W.R. First report of Colletotrichum karstii causing anthracnose on blueberry leaves in Brazil. Plant Dis. 2015;1:157. doi: 10.1094/PDIS-07-14-0717-PDN. [DOI] [PubMed] [Google Scholar]
- 15.Xu C.N., Zhou Z.S., Wu Y.X. First report of Colletotrichum acutatum associated with stem blight of blueberry plants in China. Plant Dis. 2013;97:422. doi: 10.1094/PDIS-08-12-0738-PDN. [DOI] [PubMed] [Google Scholar]
- 16.Xu C.N., Zhou Z.S., Wu Y.X. First report of stem and leaf anthracnose on blueberry caused by Colletotrichum gloeosporioides in China. Plant Dis. 2013;97:845. doi: 10.1094/PDIS-11-12-1056-PDN. [DOI] [PubMed] [Google Scholar]
- 17.Lin X.M. Advances in the classification of plant anthracnose pathogens. J. Luoyang Agric. Coll. 1992;4:50–53. [Google Scholar]
- 18.Cannon P.F., Damm U., Johnston P.R., Weir B.S. Colletotrichum current status and future directions. Stud. Mycol. 2012;73:181–213. doi: 10.3114/sim0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Damm U., Cannon P.F., Woudenberg J.H.C. The Colletotrichum boninense species complex. Stud. Mycol. 2012;73:1–36. doi: 10.3114/sim0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Damm U., Cannon P.F., Woudenberg J.H.C. The Colletotrichum acutatum species complex. Stud. Mycol. 2012;73:37–113. doi: 10.3114/sim0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weir B.S., Johnston P.R., Damm U. The Colletotrichum gloeosporioides species complex. Stud. Mycol. 2012;73:115–180. doi: 10.3114/sim0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khodadadi F., González J.B., Martin P.L., Giroux E., Aimovi S.G., Bilodeau G.J., Peter K.A., Doyle V.P., Aćimović S.G. Identification and characterization of Colletotrichum species causing apple bitter rot in new york and description of C. noveboracense sp. nov. Sci. Rep. 2020;10:11043. doi: 10.1038/s41598-020-66761-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yan J.Y., Jayawardena M.M.R.S., Goonasekara I.D. Diverse species of Colletotrichum associated with grapevine anthracnose in China. Fungal Divers. 2015;71:233–246. doi: 10.1007/s13225-014-0310-9. [DOI] [Google Scholar]
- 24.Liu F.L., Tang G.T., Zheng X.J., Li Y. Molecular and phenotypic characterization of Colletotrichum species associated with anthracnose disease in peppers from Sichuan Province, China. Sci. Rep. 2016;6:32761. doi: 10.1038/srep32761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niu X., Gao H., Qi J., Chen M., Tao A., Xu J., Dai Z. Colletotrichum species associated with jute (Corchorus capsularis L.) anthracnose in southeastern China. Sci. Rep. 2016;6:25179. doi: 10.1038/srep25179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oliul H., Jong Y.J., Taehyun C. Molecular and morphological characterization of Colletotrichum species in the Colletotrichum gloeosporioides complex associated with persimmon anthracnose in South Korea. Plant Dis. 2018;102:1015–1024. doi: 10.1094/PDIS-10-17-1564-RE. [DOI] [PubMed] [Google Scholar]
- 27.Hu W.L., Ma Y.Z., Chen J.Z. First report of citrus sinensis anthracnose caused by Colletotrichum fructicola in China. Plant Dis. 2019;103:1018. doi: 10.1094/PDIS-08-18-1466-PDN. [DOI] [Google Scholar]
- 28.Hu M.J., Grabke A., Dowling M.E., Holstein H.J., Schnabel G. Resistance in Colletotrichum siamense from peach and blueberry to thiophanate-methyl and azoxystrobin. Plant Dis. 2015;99:806–814. doi: 10.1094/PDIS-10-14-1077-RE. [DOI] [PubMed] [Google Scholar]
- 29.Caruso F.L., Ramsdell D.C. Compendium of Blueberry and Cranberry Diseases. APS Press/American Phytopathological Society; Saint Paul, MN, USA: 1995. No. 634.73. [Google Scholar]
- 30.Verma N., MacDonald L., Punja Z.K. Inoculum prevalence, host infection and biological control of Colletotrichum acutatum: Causal agent of blueberry anthracnose in British Columbia. Plant Pathol. 2006;55:442–450. doi: 10.1111/j.1365-3059.2006.01401.x. [DOI] [Google Scholar]
- 31.Huang F., Chen G.Q., Hou X., Fu Y.S., Cai L., Hyde K.D. Colletotrichum species associated with cultivated citrus in China. Fungal Divers. 2013;61:61–74. doi: 10.1007/s13225-013-0232-y. [DOI] [Google Scholar]
- 32.Peng L.J., Yang Y.L., Kevin D.H. Colletotrichum species on Citrus leaves in Guizhou and Yunnan provinces, China. Cryptogam. Mycol. 2012;33:267–284. [Google Scholar]
- 33.Li H.N., Jiang J.J., Hong N., Wang G.P., Xu W.X. First report of Colletotrichum fructicola causing bitter rot of pear (Pyrus bretschneideri) in China. Plant Dis. 2013;97:1000. doi: 10.1094/PDIS-01-13-0084-PDN. [DOI] [PubMed] [Google Scholar]
- 34.Liu W., Ye N.X., Chen Y.S., Lian L.L., Jin S., Lai J.D. Identification and phylogenetic analysis of anthracnose pathogen Colletotrichum fructicola isolated from Camellia sinensis. J. Tea Sci. 2014;34:95–104. [Google Scholar]
- 35.Diao Y.Z., Zhang C., Liu F., Wang W.Z., Liu L., Cai L., Liu X.L. Colletotrichum species causing anthracnose disease of chili in China. Persoonia. 2017;38:20–37. doi: 10.3767/003158517X692788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joa J.H., Lim C.K., Choi I.Y., Park M.J., Shin H.D. First report of Colletotrichum fructicola causing anthracnose on mango in Korea. Plant Dis. 2016;100:1793. doi: 10.1094/PDIS-10-15-1225-PDN. [DOI] [Google Scholar]
- 37.Mo J.Y., Zhao G., Li Q.L. Identification and characterization of Colletotrichum species associated with mango anthracnose in Guangxi, China. Plant Dis. 2018;102:1283–1289. doi: 10.1094/PDIS-09-17-1516-RE. [DOI] [PubMed] [Google Scholar]
- 38.Nodet P., Chalopin M., Crété X., Baroncelli R., Floch G.L. First report of Colletotrichum fructicola causing apple bitter rot in Europe. Plant Dis. 2019;103:1767. doi: 10.1094/PDIS-11-18-1915-PDN. [DOI] [Google Scholar]
- 39.Zhou Y., Gong G.S., Cui Y.L. Identification of Botryosphaeriaceae species causing kiwifruit rot in Sichuan Province, China. Plant Dis. 2015;99:699–708. doi: 10.1094/PDIS-07-14-0727-RE. [DOI] [PubMed] [Google Scholar]
- 40.Gong G.S., Xu Q., Zhang M., Yang J., Chen H. A simple method for single fungal spore isolation. J. Maize Sci. 2010;18:126–127. [Google Scholar]
- 41.Sun X., Qi X.B., Wang W., Liu X., Zhao H.N., Wu C.P., Chang X.L. Etiology and Symptoms of Maize Leaf Spot Caused by Bipolaris spp. in Sichuan, China. Pathogens. 2020;9:229. doi: 10.3390/pathogens9030229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang Y.L., Liu Z.Y., Cai L., Hyde K.D. Colletotrichum anthracnose of amaryllidaceae. Fungal Divers. 2009;39:123–146. [Google Scholar]
- 43.Sutton B.C. The Coelomycetes Fungi Imperfecti with Pycnidia Acervuli and Stromata. Commonwealth Mycological Institute; Kew, Britain: 1980. pp. 646–661. [Google Scholar]
- 44.Than P.P., Jeewon R., Hyde K.D., Pongsupasamit S., Mongkolpom O., Taylor P.W.J. Characterization and pathogenicity of associated with anthracnose on chili (Capsicum spp.) in Thailand. Plant Pathol. 2008;57:562–572. doi: 10.1111/j.1365-3059.2007.01782.x. [DOI] [Google Scholar]
- 45.White T.J., Bruns T., Lee S., Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. Guide Methods Appl. 1990;18:315–322. [Google Scholar]
- 46.Glass N.L., Donaldson G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 1995;61:1323–1330. doi: 10.1128/AEM.61.4.1323-1330.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carbone I., Kohn L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia. 1999;91:553–556. doi: 10.1080/00275514.1999.12061051. [DOI] [Google Scholar]
- 48.O’Donnell K., Nirenberg H.I., Aoki T., Cigelnik E. A multigene phylogeny of the Gibberella fujikuroi species complex: Detection of additional phylogenetically distinct species. Mycoscience. 2000;41:61–78. doi: 10.1007/BF02464387. [DOI] [Google Scholar]
- 49.Prihastuti H., Cai L., Chen H. Characterization of Colletotrichum species associated with coffee berries in northern Thailand. Fungal Divers. 2009;39:89–109. [Google Scholar]
- 50.Yang Y.L., Cai L., Yu Z., Liu Z., Hyde K.D. Colletotrichum species on Orchidaceae in southwest China. Cryptogam. Mycol. 2011;32:229–253. [Google Scholar]
- 51.Xu C., Zhang H., Zhou Z., Hu T., Wang S., Wang Y., Cao K. Identification and distribution of Botryosphaeriaceae species associated with blueberry stem blight in China. Eur. J. Plant Pathol. 2015;143:737–752. doi: 10.1007/s10658-015-0724-6. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.