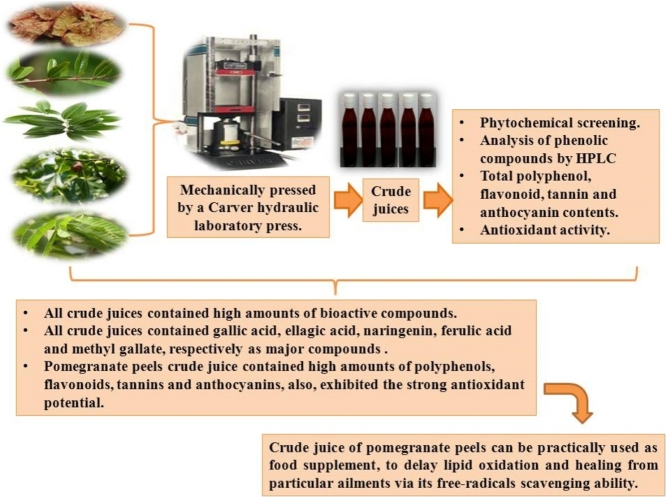
Graphical abstract
Keywords: Crude juices, Phytochemical screening, Polyphenol constituents, HPLC, Antioxidant activity
Highlights
-
•
Agricultural and food industrial byproducts can be used as source of health-promoting compounds.
-
•
Pomegranate leaves and peels, fig leaves, guava leaves and olive leaves was evaluated.
-
•
No solvent was used in the extraction to avoid the bad effects of many solvents on human health.
-
•
Pomegranate peels crude juice exhibited the strong antioxidant activity.
-
•
Pomegranate peels crude juice can be used as food supplement and to retard lipid oxidation.
Abstract
Leaves of fig, guava, olive and pomegranate and peels of ripe pomegranate fruits were mechanically pressed to obtain the crude juices. The resultant crude juices were subjected to the estimation of certain phytochemicals, i.e. total phenols, flavonoids, tannins and anthocyanins by HPLC. The assessment of their antioxidant activities were performed by three methods, i.e. DPPH, reducing power and metal chelating assays. The results indicated that the amounts of polyphenols, flavonoids, tannins and anthocyanins in crude pomegranate peels juices were markedly higher than those of other medicinal plants crude juices. The polyphenolic constituents in fig leaves, pomegranate leaves and peels, guava leaves and olive leaves were distinguished using HPLC. The major compounds found in all crude juices were gallic acid, ellagic acid, naringenin, ferulic acid and methyl gallate, respectively. Pomegranate peels crude juice exhibited the highest antioxidant activity assessed by the aforementioned methods in comparison with other medicinal plants crude juices.
1. Introduction
Plants have been widely used for healing diverse diseases, since ancient times. Plants produce an important provenance of efficient natural products which vary vastly in chemical structures, mechanism of actions and biological properties. It is recognized that free radicals result in oxidative stress and thus they able to induce deterioration of DNA molecules, lipids and proteins in biological systems, causing various ailments such as rheumatism, inflammatory bowel and coronary artery diseases. Antioxidants are highly able to retard or prevent oxidation of main substances through free radical scavenging [1,2]. Several phytochemicals particularly polyphenols like phenolic acids, flavonoids, tannins, anthocyanins, are familiar to be liable for the free radical scavenging and antioxidant activities. The agricultural and food industries remnants constitute a critical from an issue environmental and economic perspective, and hence utilization of these by-products, i.e., pomegranate leaves and peels, fig leaves, guava leaves and olive leaves could lead to high value-added products. These plants are often cheaper, locally available and easily consumable, as simple medicinal preparations for healing from various diseases. Therefore, such plants can be examined to understand their medicinal properties, safety and efficiency.
Several scientists were conducted researches on various botanical origin extracts using solvents of different polarities. To the best of our knowledge, very little researches were conducted on the internal sap of plants without recourse to solvents. One has to recall that several solvents have harmful effect on mankind health [3,4]. It was of interest to investigate the antioxidant and polyphenol profile of pomegranate leaves, fig leaves, guava leaves and olive leaves crude juices.
2. Materials and methods
2.1. Plant samples
Leaves of ripe pomegranate fruits, fig, guava and olive were collected in September while the fruits of pomegranate where collected in October, 2017 from the Research Farm of Faculty of Agriculture, Cairo University, Giza, Egypt. Samples were handpicked from different trees and verified by Dr. Abdalatif, A. M. Assistant Professor of Horticulture Department, Faculty of Agriculture, Cairo University as shown in Table 1.
Table 1.
Medicinal plants under study.
| Family name | Cultivar name | Scientific name | English name |
|---|---|---|---|
| Moraceae | Conadria | Ficus carica L. | Edible fig |
| Lythraceae | Wonderful | Punica granatum L. | Pomegranate |
| Myrtaceae | Seedling trees | Psidium guajava L. | Guava |
| Oleaceae | Coratina | Olea europaea L. | Olive |
2.2. Preparation of pomegranate leaves and peels, fig leaves, guava leaves and olive leaves crude juices
Leaves of fig, guava, olive and pomegranate and peels of ripe pomegranate fruits were manually peeled and washed to remove the unwanted materials to ensure that the peels and leaves were cleaned before proceeding to the next step. The seeds were removed. The peels and leaves of botanical parts (1 kg) were mechanically pressed by a Carver hydraulic laboratory press (Carver model C S/N 37000- 156; Fred S. Carver Inc, Menomonee Falls, WI, USA, raise force 10 tons/inch2, capacity 1 kg) to obtain the crude juices (amounts of resultant crude juices were varied according to the botanical parts under study). Freeze- dryer (Labconco Corporation, Kansas City, M.O. USA) was used to concentrate the resultant crude juices then preserved in brown bottles at −5 °C till use.
2.3. Chemicals
Authentic phenolic compounds: catechin, methyl gallate, gallic acid, chlorogenic acid, caffeic acid, vanillin, ferulic acid, syringic acid, procatechol, rutin, ellagic acid, naringenin, cinnamic acid, taxifolin, kaempferol, coumaric acid, quercetin, tannic acid, vitamin C, 2,2-diphenyl-1-picryl-hydrazyl (DPPH), ethylenediaminetetraacetic acid (EDTA), butylated hydroxytoluene (BHT) and Folin-Ciocalteau phenol reagent were purchased from Sigma-Aldrich Company for Chemicals (St Louis, MO, USA). HPLC was used to check the purity of these compounds and one peak was given by each compound. All solvents were of analytical reagent degree grade and redistilled before use.
2.4. Qualitative phytochemical screening of pomegranate, fig, guava and olive crude juices
Olive leaves, guava leaves, fig leaves and pomegranate leaves and peels crude juices were checked for the existence of main families of phytochemicals as stated by the methods of Harborne [5] Trease and Evans [6] and Sofowora [7]. Wagner's test was used to confirm the occurrence of alkaloids in the crude juices. The flavonoids were revealed by lead acetate test. Ferric chloride test was used to evaluate the presence of phenolic compounds and tannins. The existence of glycosides, sterols and triterpenoids were assessed by Keller- Kiliani test for glycosides and Salkowski test for sterols and triterpenoids. Froth test was used to detect saponins. The occurrence of anthocyanins and coumarins in the crude juices were detected by HCl/NH4OH and NaOH tests, respectively.
2.5. Quantitative analysis of phenolic compounds by high performance liquid chromatography (HPLC)
Polyphenolic constituents of pomegranate peels and leaves, fig leaves, guava leaves and olive leaves crude juices were distinguished by HPLC-UV system with a reversed phase column Eclipse Plus-C18 (250 × 4.6 mm i.d., 5 μm particle size (Agilent 1260 series, USA) and UV detector set at 280 nm (Hewlett- Packard, Pale Alto, A). Elution was carried out by mobile phase composed of water (solvent A) and trifluoroacetic acid in acetonitrile (0.1 %, v⁄v as solvent B), at a flow rate of 1.0 ml/min. The mobile phase was programmed consecutively in a linear gradient as follows: 0–5 min (80 % A); 5−8 min (40 % A); 8−12 min (50 % A); 12−14 min (80 % A) and 14−16 min (80 % A). The column temperature was maintained at 35 °C and polyphenols were assessed quantitatively at a wavelength of 280 nm by the authentic substances, i.e., ellagic acid, gallic acid, caffeic acid, vanillin, chlorogenic acid, ferulic acid, syringic acid, rutin, catechin, naringenin, cinnamic acid, methyl gallate, kaempferol, quercetin, taxifolin, procatechol and coumaric acid.
Retention times and peak areas (%) were utilized to calculate the concentrations of polyphenolic compounds by Hewlett Packard data system. Every crude juice of medicinal plants under study was analyzed in triplicate and the mean values are expressed in the text.
2.6. Total phenols (TP)
The phenols content in the crude juices were calorimetrically estimated using the Folin- Ciocalteau assay [8]. The absorbance was performed at 760 nm by a UV–vis spectrophotometer (Shimadzu, UVmini-1240, Japan). TP content in the crude juices were calculated and presented as milligrams equivalent of gallic acid per gram dry weight (mg GAE/g DW) by refer to regression equation of standard curve (Y = 0.0122x – 0.0066, R2 = 0.9873).
2.7. Total flavonoid content (TF)
The colorimetric aluminum chloride method as described by Karthikeyan and Vidya [9] was carried out to quantify the total flavonoids of the crude juices. The absorbance was performed at a wavelength of 415 nm using a UV–vis spectrophotometer (Shimadzu, UVmini-1240, Japan). The flavonoid content in the crude juices was calculated from the regression equation of calibration plot (Y = 0.0125x-0.0447, R2 = 0.9857) and displayed as milligrams quercetin equivalent /gram dry weight sample (mg QE/g DW).
2.8. Condensed tannins (CT)
The condensed tannins of pomegranate leaves and peels, fig leaves, guava leaves and olive leaves crude juices was evaluated by the vanillin assay as described by Bikoro Bi Athom et al. [10]. The absorbance was read at 500 nm utilizing a UV–vis spectrophotometer (Shimadzu, UVmini-1240, Japan) against the reagent blank. Total tannins were expressed as equivalent to tannic acid (mg TAE/g DW) by refer to regression equation of standard curve (Y = 0.0005x + 0.0018, R² = 0.9814).
2.9. Total anthocyanins (TA)
The TA was estimated by pH differential technique utilizing duo buffer systems, i.e., potassium chloride buffer (pH 1.0, 0.025 M) and sodium acetate buffer (pH 4.5, 0.4 M) as described by Rios-Corripio and Guerrero-Beltran [11]. Absorbance (A) was calculated as follows:
| A= [(A 510 nm –A 700 nm) of pH 1.0 - (A 510 nm – A 700 nm) of pH 4.5]. |
The TA of samples was calculated by the following equation:
| TA = (A × MW × DF × 100) ×1/MA |
Where:
A: absorbance, MW: molecular weight of cyanidin-3-glucoside (449.2 g/mol), DF: dilution factor (10), MA: molar absorptivity coefficient of cyanidin-3-glucoside (26,900).
The results were displayed as milligrams cyanidin-3-glucoside (CGE) equivalent per 100 g dry weight (mg CGE/ 100 g DW). Triplicate measurements were performed and mean values were calculated.
2.10. Assessment of antioxidant activity
2.10.1. 2,2-Diphenyl-1-picryl-hydrazyl (DPPH) assay
The scavenging potency of 2,2-diphenyl-1-picryl-hydrazyl (DPPH) radical of pomegranate leaves and peels, fig leaves, guava leaves and olive leaves crude juices was determined [8]. The absorbance at 517 nm was measured to assess the remaining amount of DPPH. Butylated hydroxytoluene (BHT) was applied as a standard. The ability to scavenge DPPH radicals was calculated using the following equation:
| Inhibition (%) = (A control – A test) / A control × 100. |
Where;
A control = The absorbance of the control reaction.
A test = The absorbance of the pomegranate leaves and peels, fig leaves, guava leaves and olive leaves crude juices.
The results were expressed as the half maximal inhibitory concentration (IC50) and compared with standard. All measurements were fulfilled in triplicate and mean values were calculated.
2.10.2. Reducing power assay
The reducing powers of pomegranate leaves and peels, fig leaves, guava leaves and olive leaves crude juices were accomplished as described by Ayoub et al. [12]. The absorbance was recorded at 700 nm in a UV–vis spectrophotometer (Shimadzu, UVmini-1240, Japan). Ascorbic acid was used as a standard and phosphate buffer as a blank solution. The antioxidant activity of the crude juice was expressed as IC50 and compared with standard. All measurements were accomplished in triplicate.
2.10.3. Metal chelating activity
The chelating activities of pomegranate leaves and peels, fig leaves, guava leaves and olive leaves crude juices were determined following the method described by Oche et al. [13]. The absorbance at 510 nm was recorded using a UV–vis spectrophotometer (Shimadzu, UVmini-1240, Japan). Ethylenediaminetetraacetic acid (EDTA) was utilized as a standard. The chelating ability was calculated as % chelation using the next equation:
| Chelation % = A control – A sample / A control × 100. |
Where;
A control = The absorbance of the control reaction.
A sample = The absorbance of the sample.
The chelation ability was displayed as IC50 and compared with the standard.
2.11. Statistical analysis
The least significant difference (L.S.D) test was employed to compare the difference between treatments. All analyses were accomplished in triplicates and data stated as ± standard error (SE). Data were submitted to analysis of variance (ANOVA). The confidence limits in the present study were based on (P < 0.01). The LSD and ANOVA tests were applied to indicate the mean values of the examined parameters using ASSISTAT Version 7.7 beta (2014).
3. Results and discussion
Several pharmacological researches in vitro in addition to in vivo have been widely used to show the prospects of the plant extracts for the co- therapy of diverse global disseminated ailments, supporting the traditional medicine in cases like cardiovascular diseases, cancer, diabetes mellitus, and parasitic infections due to the presence of natural active compounds which diverge widely in terms of chemical structures, biological characteristic and mechanisms of actions.
Many scientists were investigated the characteristics and constituents of interior sap of botanical origin via extraction with varied solvents of different polarities [14,15]. In the current study, the interior plant sap was acquired by mechanical pressing in absence of solvents. One has to mention that the plant parts are secure naturalistic organs and gained from yearly pruning of the plants under study. It is quite renowned that several solvents may lead to mischievous impacts on the mankind health [3,4]. Accordingly, the main aim of the present study was to get the inner sap of the plant in its native form. Further step is concentrated on the components of pomegranate leaves and peels, fig leaves, guava leaves and olive leaves crude juices accountable for the free-radical scavenging ability. Therefore, HPLC was used to study the phenolic constituents qualitatively and quantitatively.
3.1. Qualitative phytochemical screening of pomegranate, fig, guava and olive crude juices
The identification of phytochemicals in pomegranate leaves and peels, fig leaves, guava leaves and olive leaves crude juices is a pivotal onset point for evaluating their biological, nutritional and technological facets. Each crude juice was inspected for the occurrence of main families of phytochemicals, i.e., phenolic compounds, saponins, glycosides, alkaloids, flavonoids, anthocyanins, coumarins, tannins, triterpenoids and sterols. Generally, there is big disparity of among the phytochemicals and the botanical parts of the plants under study.
Table 2 presents the qualitative phytochemical screening of olive leaves, guava leaves, fig leaves pomegranate leaves and peels crude juices. It is of concern to notice that the pomegranate peels crude juice included high quantities of phenols, flavonoids, anthocyanins, alkaloids, coumarins and triterpenoids than that of pomegranate leaves, fig leaves, guava leaves and olive leaves crude juices. On the other hand, the leaves of pomegranate, fig, guava and olive contained nearly similar quantities of phenolic compounds, flavonoids and alkaloids. In addition, glycosides quantity of pomegranate leaves was higher than that of pomegranate peels, fig leaves, guava leaves and olive leaves whilst, coumarins quantity of pomegranate peels and olive leaves was nearly equal.
Table 2.
Qualitative phytochemical screening of olive leaves, guava leaves, fig leaves pomegranate leaves and peels crude juices.
| Compound detected | Inference |
||||
|---|---|---|---|---|---|
| PP | PL | OL | FL | GL | |
| Phenolic compounds | +++ | ++ | ++ | ++ | ++ |
| Tannins | ++ | + | ++ | ++ | + |
| Flavonoids | +++ | ++ | ++ | ++ | ++ |
| Coumarins | +++ | ++ | +++ | ++ | + |
| Anthocyanins | +++ | + | ++ | + | + |
| Alkaloids | +++ | + | + | + | + |
| Glycosides | ++ | +++ | + | ++ | ++ |
| Saponins | + | + | ++ | ++ | + |
| Triterpenoids | +++ | + | ++ | + | ++ |
| Sterols | ++ | + | ++ | + | ++ |
PP, PL, OL, FL and GL refer to pomegranate peel, pomegranate leaves, olive leaves, fig leaves and guava leaves, respectively.
The symbols: +++, ++, + and – refer to appreciable amounts, moderate, trace and absent amounts, respectively.
Masoko and Mamabolo [16] reported that the leaves of olive contain tannins, terpenoids, steroids and flavonoids. Preliminary phytochemical screening of Sharma et al. [17] revealed the presence of various chemical compounds like alkaloids, glycosides, flavonoids, tannins, phenols of fig leaves extracts. In this respect, Ali et al. [18] showed that leaf extracts (aqueous and ethanol) of guava contained alkaloids, tannins, terpenoid, anthraquinone, flavonoids, saponins, glycosides and phenols.
The work of Sharma et al. [19] illustrated that pomegranate peels contained alkaloids, flavonoids, phenolic compounds, glycosides and saponins. The preliminary phytochemical screening tests can be useful in the investigation of the bioactive principles and afterward may drive to the development and drug invention [20].
3.2. Analysis of phenolic compounds by high performance liquid chromatography (HPLC)
The characterization and quantification of phenolic components of pomegranate peels and leaves, fig leaves, guava leaves and olive leaves crude juices was carried out using HPLC. Table 3 presents the phenolic components of juice of the botanical parts under study. Due to The lack of certain equipments, i.e., mass spectrometer, and some of authentic substances prevented the complete identification the components of botanical parts' crude juices.
Table 3.
Quantitative analysis (ppm) of polyphenolic compounds of pomegranate leaves and peels, fig leaves, guava leaves and olive leaves crude juices.
| Phenolic compound | Concentration (ppm) |
||||
|---|---|---|---|---|---|
| PP | PL | OL | FL | GL | |
| Gallic acid | 12622.08 | 256.69 | 97.19 | 20.57 | 142.74 |
| Chlorogenic acid | 333.41 | NP | 89.62 | 25.87 | 9.01 |
| Catechin | 633.81 | 226.90 | 143.21 | 58.36 | NP |
| Methyl gallate | 30.09 | 18.47 | 6.97 | 2.44 | 3.90 |
| Caffeic acid | 53.72 | NP | 7.07 | 19.54 | 5.03 |
| Syringic acid | 37.51 | 7.80 | 6.65 | 10.60 | NP |
| Pyrocatechol | NP | 14.24 | 28.43 | 16.77 | 112.41 |
| Rutin | NP | 11.16 | NP | 18.38 | NP |
| Ellagic acid | 496.25 | 82.95 | 22.43 | 38.54 | 20.90 |
| Coumaric acid | 17.59 | NP | 11.38 | 47.46 | 1.77 |
| Vanillin | 44.92 | 5.32 | NP | 8.99 | 5.77 |
| Ferulic acid | 47.25 | 19.15 | 21.62 | 23.65 | 16.39 |
| Naringenin | 90.12 | 23.20 | 113.45 | 31.20 | 5.85 |
| Taxifolin | NP | 5.16 | 151.39 | 115.03 | 28.05 |
| Cinnamic acid | NP | NP | 0.99 | 1.36 | 0.70 |
| Kaempferol | NP | NP | NP | 4.55 | NP |
NP refers to not present.
PP, PL, OL, FL and GL refer to pomegranate peels, pomegranate leaves, olive leaves, fig leaves and guava leaves, respectively.
Dealing with the pomegranate crude peel juice, it contained gallic acid, catechin, ellagic acid and chlorogenic acid as major substances. While, in pomegranate leaves crude juice contained gallic acid, catechin and ellagic as major substances. In addition, olive leaves crude juice contained catechin, taxifolin, naringenin and gallic acid as major materials. Fig leaves crude juice was distinguished by high contents of taxifolin, coumaric acid and catechin but the crude juice of guava leaves included gallic acid and pyrocatechol as main components.
The phenolic substances, i.e., pyrocatechol and taxifolin were present in pomegranate leaves, fig leaves, guava leaves and olive leaves crude juices and not in pomegranate peels crude juice. Kaempferol was present only in fig leaves crude juice.
The following compounds: chlorogenic, caffeic and coumaric acids were present in all crude juices under study except in pomegranate leaves crude juice. These results demonstrated that there were great divergence between the phenolic ingredient of pomegranate peels and leaves, fig leaves, guava leaves and olive leaves crude juices.
Several authors studied the polyphenols of pomegranate, olive, fig and guava botanical parts in different growing regions using HPLC. For instance, Mushtaq et al. [21] reported that vanillic, syringic, ferulic, p-coumaric, sinapic and caffeic acids were the main phenols in pomegranate peels of Pakistan cultivars. Du et al. [22] found that punicalagin, was identified to be the victorious phenolic compound of pomegranate peels polyphenols, followed by ellagic acid, catechin, gallic acid, epicatechin and chlorogenic acid.
It is worth mentioning that the HPLC results under study agreed quite well with the data of Farag et al. [23] who illustrated that pomegranate crude peels juice contained protocatechuic and gallic acid as major constituents while the polyphenol compounds: chlorogenic, caffeic and ferulic acids, catechin, coumarins, vanillic, caffeine and catechol were found as minor substances.
Cittan and Çelik [24] found 31 phenolic compounds in olive leaves of which 8 were phenolic compounds which are taxifolin, gallic acid, chlorogenic acid, caffeic acid, pyrocatechol, syringic acid, ferulic acid and p-coumaric acid were consistent with the results under study.
The present study are consistent with those of Abdel-Aziz et al. [25] who mentioned that phenolic compound, i.e., gallic acid, chlorogenic acid, caffeic acid, syringic acid, acid, ferulic acid, ellagic acid, kampherol and p-coumaric were found in olive and fig leaves.
The findings of Afzal et al. [26] revealed that quercetin, vanillic acid, syringic acid, coumeric acid and cinnamic acid were polyphenols of guava leaves.
3.3. Total polyphenolic, flavonoid, tannin and anthocyanin contents of pomegranate leaves and peels, fig leaves, guava leaves and olive leaves crude juices
Phenolic components such as phenolic acids, tannins flavonoids, etc. are considered the most substantial phytochemical components produced by plants. In fact, these compounds are existing in various parts of the plant and their quantities significantly depends on the kind of the plant organ, climate, variety, location, etc. [27].
The values of phenols, flavonoids, tannins and anthocyanins contents in the present study slightly different in comparison with the literature. This could be due to the methods of extraction, the geographical variation or duration which may affect the amounts of phenolics [28].
Table 4 displays the amounts of phenols, flavonoids, tannins and anthocyanins of pomegranate leaves and peels, fig leaves, guava leaves and olive leaves crude juices. The data elucidated that the amounts of phenols, flavonoids, condensed tannins and anthocyanins varied according to the botanical part. According to the data in Table 4, pomegranate peels crude juice contained the highest total phenolic, flavonoids, condensed tannins and anthocyanins contents while they were the lowest in fig leaves (the lowest in polyphenols and flavonoids), pomegranate leaves and olive leaves crude juices, respectively.
Table 4.
Total polyphenolic, flavonoid, condensed tannin and anthocyanin contents of pomegranate leaves and peels, fig leaves, guava leaves and olive leaves crude juices.
| Parameter | PP | PL | OL | FL | GL |
|---|---|---|---|---|---|
|
Total polyphenols (mg GAE /g dry weight) |
144.37 ± 1.99a | 84.612 ± 0.334b | 67.045 ± 1.024c | 35.8 ± 0.356e | 59.267 ± 0.348d |
|
Total flavonoids (mg QE /g dry weight) |
40.597 ± 0.780a | 22.967 ± 0.298c | 17.107 ± 0.470d | 14.334 ± 0.076e | 25.796 ± 0.701b |
|
Tannins (mg TAE /g dry weight) |
30.336 ± 0.647a | 6.726 ± 0.172e | 9.625 ± 0.071d | 12.45 ± 0.215c | 17.796 ± 0.193b |
|
Total anthocyanins (mg CGE/ 100 g dry weight) |
54.103 ± 0.536a | 29.956 ± 0.809b | 10.807 ± 1.409e | 14.962 ± 0.8d | 26.282 ± 0.44c |
Values are means of three replicates of each parameter ± standard error.
GAE, QE, TAE and CGE refer to gallic acid, quercetin, tannic acid and cyanidin-3-glycoside, respectively.
PP, PL, OL, FL and GL refer to pomegranate peels, pomegranate leaves, olive leaves, fig leaves and guava leaves, respectively.
From the aforementioned results it appears that pomegranate peels crude juice contained high quantities of phenols and flavonoids, condensed tannins and anthocyanins than of pomegranate leaves, fig leaves, guava leaves and olive leaves crude juices. These findings are consistent with and confirm the results obtained from the qualitative phytochemical screening of botanical parts crude juices under study which indicated that pomegranate peels crude juice included high quantities of phenols, flavonoids, anthocyanins in comparison with the other crude juices under study.
The findings of Farag et al. [23] agreed quite well with the present data where pomegranate peels crude juice contained remarkable contents of total polyphenols and flavonoids, tannins and total anthocyanins being about 1.22, 1.43, 1.16 and 1.29 times as high as that in leaves juice, respectively. Russo et al. [29] demonstrated that total phenolic content of pomegranate peels (Wonderful variety) was 137.28 ± 1.19 mg GAE/g DW.
Luo et al. [30] indicated that polyphenols content of olive leaves extracts was being 1.39 times as that of flavonoids.
Mopuri et al. [31] mentioned that the aqueous extract of fig leaves contained polyphenols content higher total phenolic content being 1.35 times as that of flavonoids. Petruccelli et al. [32] reported that total phenolic content of fig leaves was found in the range of 38.91−16.22 mg GAE/g DW.
Akila et al. [33] indicated that the total phenolic content of fresh guava leaf was 99.25 mg GAE/g while total flavonoid content was 13.292 mg QE/g and tannin content was 2.962 mg CE/g. Simamora et al. [34] investigated the total phenolic and flavonoid content of guava leaves aqueous extract were 114.81 mg GAE/g DW and 152.17 mg RE/g DW, respectively.
Biological, genetic variation, environmental, year-to-year divergence and seasonal strongly influenced the polyphenol constituent [35].
3.4. Antioxidant activity of pomegranate leaves and peels, fig leaves, guava leaves and olive leaves crude juices
Antioxidants play a vital role in preventing pathogenic processes associated with cancer, macular degeneration, cardiovascular disease, respiratory disorder and cataracts, and able to enhance immune system. Antioxidants conserve the body from the injurious action of free radicals produced as byproducts of normal metabolism [36].
The naturalistic antioxidants like phenolics, flavonoids, tannins, terpenoids, coumarins, curcuminoids, xanthons, and lignans are found in different plant products [37,38] and they are renowned to preserve components of food which are able to oxidize easily from oxidation. This effect differ vastly relying on the growing conditions, extraction process, and a multitude sides of the chemical structure of the active constituents, i.e., the amount, position of hydroxyl groups, molecular weight, particle size, concentration of solvent, time of contact, temperature, and mass-solvent ratio, between others aspects [39].
In the present work, pomegranate peels and leaves, fig leaves, guava leaves and olive leaves crude juices as a source of naturalistic antioxidants were evaluated. The contents of total phenols and flavonoids, tannins and anthocyanins were estimated since various antioxidant components have several modes of action. Therefore, assorted methods were used to evaluate the crude juices antioxidant efficiency.
As stated by multiple reports, phenolic constituents possess free radical repression, peroxide degradation, metal inhibition or oxygen suppression in biological systems besides blocking oxidative ailment [40]. Thus, the recent research was intended to assess the antioxidant activity of pomegranate peels and leaves, fig leaves, guava leaves and olive leaves crude juices. As pointed out by Huang et al. [41], no individual technique is sufficient for assessing the antioxidant capability of foods, since varied ways able to yield quite diversing findings. Diversified assays, on base various mechanisms must be utilized. Therefore, the 2,2-diphenyl-1-picryl-hydrazyl (DPPH), reducing power assays and metal chelating activity were applied.
The antioxidant ability of pomegranate leaves and peels, fig leaves, guava leaves and olive leaves crude juices were evaluated by the aforementioned methods. Considering the data in Table 5, pomegranate peels crude juice exhibited the highest antioxidant activity assessed by the aforementioned methods in comparison with pomegranate leaves, fig leaves, guava leaves and olive leaves crude juices.
Table 5.
The antioxidant activity of pomegranate leaves and peels, fig leaves, guava leaves and olive leaves crude juices.
| Method | Antioxidant activity |
|||||
|---|---|---|---|---|---|---|
| PP | PL | OL | FL | GL | Standard | |
| DPPH (IC50, μg/mL) | 28.843 ± 0.521c | 29.327 ± 0.578c | 42.504 ± 0.059b | 45.928 ± 0.547a | 28.006 ± 0.003c | 21.283 ± 0.526d |
|
Reducing power (IC50, μmol Fe2+ /g) |
148.329 ± 1.786e | 259.315 ± 1.753d | 327.969 ± 4.052b | 285.725 ± 1.251c | 449.2 ± 4.687a | 58.8 ± 0.519f |
| Metal chelating activity (IC50,μg/mL) | 142.651 ± 0.817e | 204.898 ± 1.443a | 155.559 ± 0.554d | 182.623 ± 1.145b | 160.174 ± 0.61c | 37.95 ± 0.583f |
Values are means of three replicates of each parameter ± standard error.
PP, PL, OL, FL and GL refer to pomegranate peels, pomegranate leaves, olive leaves, fig leaves and guava leaves, respectively.
The highest antioxidant activity by DPPH assay was recorded in pomegranate peels crude juice followed by guava leaves, pomegranate leaves, olive leaves crude juices while it was the lowest in fig leaves crude juice. Comparable results were gained from the reducing power assay, pomegranate peels crude juice exhibited a better reducing power activity. Instead, a lower activity was observed for guava leaves crude juice. Pomegranate peels crude juice exhibited strong potential to act as a metal chelator while pomegranate leaves showed lower capacity for metal ion chelation.
Bustamante et al. [42] reported that pomegranate peels extract of Wonderful cultivar from Chile exhibited antioxidant activity of 99.4 μg trolox equivalent (TE)/g under ideal conditions of extraction. Kaur et al. [43] mentioned that the highest antioxidant activity assessed by DPPH method was recorded in red fruits (IC50 = 70.33 μg/mL) while the lowest in leaves (IC50 = 120.78 μg/mL). Also, the maximum antioxidant activity by ferric reducing power assay was in red fruits while the lowest antioxidant phenomenon was recorded in leaves (310.99 ± 0.98 μmol Fe2+/g and 69.99 ± 0.45 μmol Fe2+/g dry matter, respectively).
Akila et al. [33] mentioned that total antioxidant capability of fresh guava leaves determined by DPPH method was 71.16 % radical scavenging activity. Olatunde et al. [44] found that guava leaf powder extract from dechlorophyllized chloroform was the highest antioxidant activity (240.78, 2598.41 μmol TE/g and 2327.42, for DPPH, FRAP and ABTS methods, respectively). Simamora et al. [34] mentioned that guava aqueous leaf extract exhibited more effective scavenging activity than fruit extract with IC50 of 843.84 and 74.77 μg/mL for fruit and leaves aqueous extracts, respectively.
It appears from the results of the present study that there is a correlation between the antioxidant adequacy and the chemical structure of phenolic compounds. The evidence for this compositional requirement is supported by Bendary et al. [45] who mentioned that phenolic components are perfect electron donors due to the ability of their hydroxyl groups to participate to antioxidant process. In this context, Benjakul et al. [46] reported that the differences in chemical structures and numeral of the hydroxyl groups in phenolic components contribute to the diversity in their antioxidant activity. It is worth mentioning that Derakhshan et al. [47] mentioned that there is a significant positive relation between antioxidant ability and total phenols and that confirms the findings of the present study which indicated that pomegranate peels crude juice contained high quantities of total phenols possessed the highest antioxidant activity assessed by the aforementioned methods in comparison with other crude juices under study.
Phenols have several mechanisms of action, like bio-membrane transmission or interaction, metal chelation, free radical scavenging, restrain of oxidative enzymes, and put out reactive oxygen species. Hence, controlling their antioxidant abilities variedly [46]. One would also has to mention that flavonoids are phytochemicals with antioxidant activity, the potency of which relays on the number and position of free OH groups [48].
Al-Rawahi et al. [49] suggested that ellagic acid, ellagi-tannins and gallic acid are the most responsible for the antioxidant activity of pomegranate peels. Furthermore, Amjad and Shafighi [50] mentioned that ellagic acid, as a member of phenolics, is deemed to play a substantial part in antioxidant activity. This acid can react with free radicals due to its ability to chelate with metal cations, a potent oxidant against lipid peroxidation in mitochondrion and microsome.
The finding of the current study proposes that the crude juice of pomegranate peels fit to be practically employed as food complements, and to delay lipid oxidization.
4. Conclusion
The present work was focused on evaluating the resultant crude juices from the mechanical pressing of some agricultural and food industries by-products i.e., pomegranate leaves and peels, fig leaves, guava leaves and olive leaves as a source of naturalistic antioxidants. In general, HPLC, data showed that the crude juices of pomegranate leaves and peels, fig leaves, guava leaves and olive leaves under study contained high amounts of bioactive compounds. Pomegranate peels crude juice contained high amounts of phenols, flavonoids, tannins and anthocyanins, also, exhibited the strong antioxidant potential. In this context, pomegranate peels crude juice is a valuable source of health-promoting compounds, fulfilling concurrently the promising antioxidant activity that can be utilized virtually as food complements, to tardiness lipid oxidization and healing from particular ailments via its free-radicals scavenging ability. It would be interesting to conduct more researches to inspect the role of bioactive components which responsible for these activities. Hence, more studies are necessary to estimate the antioxidant, anticancer and antimicrobial efficiencies of their individual purified fractions.
Author contributions
Authors contributed to conception or design, contributed to acquisition, analysis, and/or interpretation of data, drafted the manuscript, and conclusively revised the manuscript for important intellectual content. The mentioned authors gave final approval and agree to be responsible for all sides of the work.
Ethical statement
This research does not include any studies with human participants, animal studies, clinical trial, or biosecurity that require ethical approval.
Author statement
Authors contributed to conception or design, contributed to acquisition, analysis, and/or interpretation of data, drafted the manuscript, and conclusively revised the manuscript for important intellectual content. The mentioned authors gave final approval and agree to be responsible for all sides of the work.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgments
Grateful appreciation is extended to the Faculty of Agriculture, Cairo University for supporting and providing funds and necessary facilities throughout this work.
References
- 1.Sanchez-Moreno C. Review: methods used to evaluate the free radical scavenging activity in foods and biological systems. Food Sci. Technol. Int. 2002;8(3):121–137. doi: 10.1177/1082013202008003770. [DOI] [Google Scholar]
- 2.Chang H.Y., Ho Y.L., Sheu M.J., Lin Y.H., Tseng M.C., Wu S.H., Huang G.J., Chang Y.S. Antioxidant and free radical scavenging activities of Phellinus merrillii extracts. Bot. Stud. 2007;48:407–417. [Google Scholar]
- 3.Capello C., Fischer U., Hungerbühler K. What is a green solvent? A comprehensive framework for the environmental assessment of solvents. Green Chem. 2007;9:927–934. doi: 10.1039/B617536H. [DOI] [Google Scholar]
- 4.Ghoreishi S.M., Shahrestani R.G. Subcritical water extraction of mannitol from olive leaves. J. Food Eng. 2009;93:474–481. doi: 10.1016/j.jfoodeng.2009.02.015. [DOI] [Google Scholar]
- 5.Harborne J.B. 2nd ed. Chapman and Hall; New York: 1973. Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis; pp. 88–185. [DOI] [Google Scholar]
- 6.Trease G.E., Evans W.C. Textbook of Pharmacognosy. 12th ed. Balliese, Tindall and Co Publishers; London: 1989. Phenols and phenolic glycosides; pp. 343–383. [Google Scholar]
- 7.Sofowora A. Spectrum Books Ltd; Nigeria: 1993. Phytochemical Screening of Medicinal Plants and Traditional Medicine in Africa; pp. 150–156. [Google Scholar]
- 8.Cano-Lamadrid M., Lech K., Calin-Sanchez A., Rosas-Burgos E.C., Figiel A., Wojdylo A., Wasilewska M., Carbonell-Barrachina A.A. Quality of pomegranate pomace as affected by drying method. J. Food Sci. Technol. 2018;55(3):1074–1082. doi: 10.1007/s13197-017-3022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karthikeyan G., Vidya A.K. Phytochemical analysis, antioxidant and antimicrobial activity of pomegranate peel. Res. J. Life Sci. Bioinform. Pharm. Chem. Sci. 2019;5(1):218–231. doi: 10.26479/2019.0501.22. [DOI] [Google Scholar]
- 10.Bikoro Bi Athom A., Engozogho Anris S.P., Safou-Tchiama R., Santiago-Medina F.J., Cabaret T., Pizzi A., Charrier B. Chemical composision of African mahagony (K. ivorensis A. Chev) extractive and tannin structures of the bark by MALDI-TOF. Ind. Crops Prod. 2018;113:167–178. doi: 10.1016/j.indcrop.2018.01.013. [DOI] [Google Scholar]
- 11.Rios-Corripio G., Guerrero-Beltran J.A. Antioxidant and Physicochemical characteristics of unfermented and fermented pomegranate (Punica granatum L.) beverages. J. Food Sci. Technol. 2019;56(1):132–139. doi: 10.1007/s13197-018-3466-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ayoub L., Hassan F., Hamid S., Abdelhamid Z., Souad A. Phytochemical screening, antioxidant activity and inhibitory potential of Ficus carica and Olea europaea leaves. Bioinformation. 2019;15(3):226–232. doi: 10.6026/97320630015226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oche J.I., Johnson T.O., Akinsanmi A.O., Jaryum K.H., Francies T. In vitro Antioxidant activity and inhibition of Fe 2+ and SNP lipid peroxidation of African mistletoes (Tapinanthus globiferus) from three selected host plants in Jos Plateau state Nigeria. J. App. Life Sci. int. 2019;20(4):1–10. doi: 10.9734/jalsi/2019/v20i430090. [DOI] [Google Scholar]
- 14.Tiwari P., Kumar B., Kaur M., Kaur G., Kaur H. Phytochemical screening and extraction. A review. Intr. Pharm. Sci. 2011;1(1):98–106. [Google Scholar]
- 15.Miguel G., Dandlen S., Antunes D., Neves A., Martins D. The effect of two methods of pomegranate (Punica granatum L.) juice extraction on quality during storage at 4 °C. J. Biomed. Biotech. 2004;5:332–337. doi: 10.1155/S1110724304403064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masoko P., Mamabolo S.K. Phytochemical and biological investigations of Olea europaea subspecies africana leaves. J. Pharm. Sci. Res. 2019;11(8):3020–3029. [Google Scholar]
- 17.Sharma M., Abid R., Sajgotra M. Phytochemical screening and thin layer chromatography of Ficus carica leaves extract. UK J. Pharm. Biosci. 2017;5(1):18–23. doi: 10.20510/ukjpb/5/i1/147022. [DOI] [Google Scholar]
- 18.Ali M., Yahaya A., Zage A.U., Yusuf Z.M. In-vitro antibacterial activity and phytochemical screening of Psidium guajava on some enteric bacterial isolates of public health importance. J. Adv. Med. Pharm. Sci. 2017;12(3):1–7. doi: 10.9734/JAMPS/2017/31126. [DOI] [Google Scholar]
- 19.Sharma K., Akansha, Chauhan E.S. Comparative studies of proximate, mineral and phytochemical compositions of pomegranate (Punica granatum) in peel, seed and whole fruit powder. Int. J. Food Sci. Nutr. 2018;3(2):192–196. [Google Scholar]
- 20.Varadarajan P., Rathinaswamy G., Asirvatahm D. Antimicrobial properties and phytochemical constituents of Rhoeo discolor. Ethnobotanical. Leaflet. 2008;12:841–845. [Google Scholar]
- 21.Mushtaq M., Sultana B., Anwar F., Adnan A., Rizvi S.S. Enzyme-assisted supercritical fluid extraction of phenolic antioxidants from pomegranate peel. J. Supercrit. Fluids. 2015;104:122–131. doi: 10.1016/j.supflu.2015.05.020. [DOI] [Google Scholar]
- 22.Du L., Li J., Zhang X., Wang L., Zhang W. Pomegranate peel polyphenols inhibits inflammation in LPS-induced RAW264.7 macrophages via the suppression of MAPKs activation. J. Funct. Foods. 2018;43:62–69. doi: 10.1016/j.jff.2018.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farag R.S., Abdel-Latif M.S., Emam S.S., Tawfeek L.S. Phytochemical screening and polyphenol constituents of pomegranate peels and leave juices. Landmark Res. J. Agric. Soil Sci. 2014;1(6):86–93. [Google Scholar]
- 24.Cittan M., Çelik A. Development and validation of an analytical methodology based on liquid chromatography-electrospray tandem mass spectrometry for the simultaneous determination of phenolic compounds in olive leaf extract. J. Chromatogr. Sci. 2018;56(4):336–343. doi: 10.1093/chromsci/bmy003. [DOI] [PubMed] [Google Scholar]
- 25.Abdel-Aziz M.E., Darwish M.S., Mohamed A.H., El-Khateeb A.Y., Hamed S.E. Potential activity of aqueous fig leaves extract, olive leaves extract and their mixture as natural preservatives to extend the shelf life of pasteurized buffalo milk. Foods. 2020;9(5):615–636. doi: 10.3390/foods9050615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Afzal M., Iqbal R., Mahmood Z., Zeshan B., Wattoo J.I. Study of GC-MS and HPLC characterized metabolic compounds in guava (Psidium guajava L.) leaves. Pak. J. Agri. Sci. 2019;56(3):709–713. doi: 10.21162/PAKJAS/19.7131. [DOI] [Google Scholar]
- 27.Sifaoui I., LoÂpez-Arencibia A., MartõÂn-Navarro C.M., Reyes-Batlle M., Wagner C., Chiboub O., Mejri M., Valladares B., Abderrabba M., Piñero J.E., Lorenzo-Morales J. Programmed cell death in Acanthamoeba castellanii Neff induced by several molecules present in olive leaf extracts. PLoS One. 2017;12(8):1–12. doi: 10.1371/journal.pone.0183795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burri S.C.M., Ekholm A., Håkansson Å., Tornberg E., Rumpunen K. Antioxidant capacity and major phenol compounds of horticultural plant materials not usually used. J. Funct. Foods. 2017;38:119–127. doi: 10.1016/j.jff.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Russo M., Fanali C., Tripodo G., Dugo P., Muleo R., Dugo L., De Gara L., Mondello L. Analysis of phenolic compounds in different parts of pomegranate (Punica granatum) fruit by HPLC-PDA-ESI/MS and evaluation of their antioxidant activity: application to different Italian varieties. Anal. Bioanal. Chem. 2018;410:3507–3520. doi: 10.1007/s00216-018-0854-8. [DOI] [PubMed] [Google Scholar]
- 30.Luo S., Jiang X., Jia L., Tan C., Li M., Yang Q., Du Y., Ding C. In vivo and in vitro antioxidant activities of methanol extracts from olive leaves on Caenorhabditis elegans. Molecules. 2019;24:1–14. doi: 10.3390/molecules24040704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mopuri R., Ganjayi M., Meriga B., Koorbanally N.A., Islam M.S. The effects of Ficus carica on the activity of enzymes related to metabolic syndrome. J. Food Drug Anal. 2018;26:201–210. doi: 10.1016/j.jfda.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petruccelli R., Ieri F., Ciaccheri L., Bonetti A. Polyphenolic profiling and chemometric analysis of leaves from Italian Ficus carica L. varieties. Polyphenol compounds in common fig. Eur. J. Hortic. Sci. 2018;83(2):94–103. doi: 10.17660/eJHS.2018/83.2.5. [DOI] [Google Scholar]
- 33.Akila B., Vijayalakshmi R., Hemalatha G., Arunkumar R. Development and evaluation of functional property of guava leaf based herbal tea. J. Pharm. Phytochem. 2018;7(3):3036–3039. [Google Scholar]
- 34.Simamora A., Paramita L., Hamid N.A.B.M., Santoso A.W., Timotius K.H. In vitro antidiabetic and antioxidant activities of aqueous extract from the leaf and fruit of Psidium guajava L. Indones. Biomed. J. 2018;10(2):156–164. doi: 10.18585/inabj.v10i2.402. [DOI] [Google Scholar]
- 35.Kumar V., Roy B.K. Population authentication of the traditional medicinal plant Cassia tora L. based on ISSR markers and FTIR analysis. Sci. Rep. 2018;8:10714. doi: 10.1038/s41598-018-29114-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakilcioğlu E., Hışıl Y. Research on the phenolic compounds in Sarilop (Ficus carica L.) fig variety. Gida. 2013;38(5):267–274. [Google Scholar]
- 37.Farag R.S., El-Baroty G.S., Basuny A.M. The influence of phenolic extracts obtained from the olive plant (cvs. Picual and Kronakii), on the stability of sunflower oil. Intr. J. Food Sci. Technol. 2003;38:81–87. doi: 10.1046/j.1365-2621.2003.00665.x. [DOI] [Google Scholar]
- 38.Jeong S.M., Kim S.Y., Kim D.R., Nam K.C., Ahn D.U., Lee S.C. Effect of seed roasting conditions on the antioxidant activity of defatted sesame meal extracts. Food Chem. Toxicol. 2004;69:377–381. doi: 10.1111/j.1365-2621.2004.tb10701.x. [DOI] [Google Scholar]
- 39.Soto-Garcia M., Rosales-Castro M. Effect of solvent and solvent-to-solid ratio on the phenolic extraction and the antioxidant capacity of extracts from Pinus durangensis and Quercus sideroxy la BARK. Maderas Cienc. Tecnol. 2016;18(4):701–714. doi: 10.4067/S0718-221X2016005000061. [DOI] [Google Scholar]
- 40.Babbar N., Oberoi H.S., Sandhu S.K. Therapeutic and nutraceutical potential of bioactive compounds extracted from fruit residues. Crit. Rev. Food Sci. Nutr. 2015;55(3):319–337. doi: 10.1080/10408398.2011.653734. [DOI] [PubMed] [Google Scholar]
- 41.Huang D., Band O., Prior R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005;53:1841–1856. doi: 10.1021/jf030723c. [DOI] [PubMed] [Google Scholar]
- 42.Bustamante A., Hinojosa A., Robert P., Escalona V. Extraction and microencapsulation of bioactive compounds from pomegranate (Punica granatum var. wonderful) residues. Int. J. Food Sci. Technol. 2017;52(6):1452–1462. doi: 10.1111/ijfs.13422. [DOI] [Google Scholar]
- 43.Kaur R., Aslam L., Kapoor N., Mahajan R. Phytochemical analysis and antioxidant activity of wild pomegranate collected from patnitop, Jammu and Kashmir. Biosci. Biotech. Res. Asia. 2018;15(2):335–341. doi: 10.13005/bbra/2637. [DOI] [Google Scholar]
- 44.Olatunde O.O., Benjakul S., Vongkamjan K. Antioxidant and antibacterial properties of guava leaf extracts as affected by solvents used for prior dechlorophyllization. J. Food Biochem. 2018;42:1–11. doi: 10.1111/jfbc.12600. [DOI] [Google Scholar]
- 45.Bendary E., Francis R.R., Ali H.M.G., Sarwat M.I., El Hady S. Antioxidant and structure–activity relationships (SARs) of some phenolic and anilines compounds. Ann. Agric. Sci. 2015;58:173–181. doi: 10.1016/j.aoas.2013.07.002. [DOI] [Google Scholar]
- 46.Benjakul S., Kittiphattanabawon P., Sumpavapol P., Maqsood S. Antioxidant activities of lead (Leucaena leucocephala) seed as affected by extraction solvent, prior dechlorophyllisation and drying methods. J. Food Sci. Technol. 2014;51(11):3026–3037. doi: 10.1007/s13197-012-0846-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Derakhshan Z., Ferrante M., Tadi M., Ansari F., Heydari A., Hosseini M.S., Conti G.O., Sadrabad E.K. Antioxidant activity and total phenolic content of ethanolic extract of pomegranate peels, juice and seeds. Food Chem. Toxicol. 2018;114:108–111. doi: 10.1016/j.fct.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 48.Panche A.N., Diwan A.D., Chandra S.R. Flavonoids: an overview. J. Nutr. Sci. 2016;5:e47. doi: 10.1017/jns.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Al-Rawahi A.S., Edwards G., Al-Sibani M., Al-Thani G., Al-Harrasi A.S., Rahman M.S. Phenolic constituents of pomegranate peels (Punica granatum L.) cultivated in Oman. Eur. J. Med. Plants. 2014;4(3):315–331. doi: 10.9734/EJMP/2014/6417. [DOI] [Google Scholar]
- 50.Amjad L., Shafighi M. Antioxidant activity of leaf different extracts in Punica granatum. Int. J. Biol. Med. Res. 2012;3(3):2065–2067. [Google Scholar]