Highlights
-
•
Separation surgery is a new concept for metastatic spinal cord compression treatment.
-
•
Stereotactic radiosurgery increased local control, overcoming radio-resistance’s idea.
-
•
The surgery goal shifted towards creating targets for radiations avoiding cord damages.
-
•
Minimal invasive strategies could allow quick return to systemic therapies.
Abbreviations: MESCC, Metastatic Epidural Spinal Cord Compression; SRS, Stereotactic Radiosurgery; SBRT, Stereotactic Body Radiation Therapy; LC, Local Control; cEBRT, conventional External Beam Radiation Therapy; SS, Separation Surgery; KPS, Karnofsky Performance Status; ECOG PS, Eastern Cooperative Oncology Group Performance Status Scale; ESCC, Epidural Spinal Cord Compression; SINS, Spinal Instability Neoplastic Score; NSCLC, Non-Small Cell Lung Cancer; NSE, Neurologic Stability Epidural compression; PEEK, Polyetheretherketone; PLL, Posterior Longitudinal Ligament; PMMA, Poly-Methyl-Methacrylate; MAS, Minimal Access Spine; MIS, Minimally Invasive Surgical; LITT, Laser Interstitial Thermal Therapy; GTV, Gross tumor volume; CTV, Clinical tumor volume; PTV, Planning target volume; PRV, Spinal cord planning risk volume
Keywords: Separation surgery, Spinal metastases, Epidural spinal cord compression, Stereotactic body radiation therapy, MIS techniques, Carbon fiber/PEEK cement
Abstract
Introduction
The new concept of separation surgery has changed the surgical paradigms for the treatment of metastatic epidural spinal cord compression (MESCC), shifting from aggressive cytoreductive surgery towards less invasive surgery with the aim to achieve circumferential separation of the spinal cord and create a safe target for high dose Stereotactic Body Radiation Therapy (SBRT), which turned out to be the real game-changer for disease’s local control.
Discussion
In this review a qualitative analysis of the English literature has been performed according to the rating of evidence, with the aim to underline the increasingly role of the concept of separation surgery in MESCC treatment. A review of the main steps in the evolution of both radiotherapy and surgery fields have been described, highlighting the important results deriving from their integration.
Conclusion
Compared with more aggressive surgical approaches, the concept of separation surgery together with the advancements of radiotherapy and the use of SBRT for the treatment of MESCC showed promising results in order to achieve a valuable local control while reducing surgical related morbidities and complications.
1. Introduction
Spinal metastases are considered one of the most relevant health burdens in oncological care, with a prevalence of approximately 30–40% among patient suffering from cancer [1], [2], [3], [4], [5], [6]. Metastatic Epidural Spinal Cord Compression (MESCC) occurs in 10% of cases, with its related high risk of neurological impairments and disability [7], [8].
In recent years, significant development in radiological diagnostic tools and new oncological treatments have radically changed life expectancy in metastatic patients [9], [10], [11] and consequentially the management of spinal cord metastases. Specifically, a longer survival of metastatic patients supported the possibility to prescribe ablative treatments as an emerging oncological strategy, also in spine metastases. Moreover, technological improvements in Radiation Oncology field allowed a dose painting to the target and a sparing of normal tissues. In this scenario, Stereotactic Radiosurgery (SRS) or Stereotactic Body Radiation Therapy (SBRT) are emerging treatment for spinal metastases in order to obtain greater local control (LC) than conventional External Beam Radiation Therapy (cEBRT) [12], [13], [14], [15], [16], [17], [18], [19].
Considering the systemic nature of MESCC, surgical treatment plays a functional role in preserving or restoring neurological status and spinal stability [6], [16], [20]. With the introduction of ablative radiation treatment (SRS or SBRT) in the new paradigms of treatment [16], surgery for decompression in MESCC has evolved, shifting from aggressive cytoreduction, in order to obtain neurological outcomes improvement and better LC, towards the modern idea of “separation surgery”(SS) [7].
Separation surgery represents an innovative and promising way to improve tumor resection and therefore offer the possibility of performing adequate radiation therapy. The circumferential decompression of the spinal cord/nerve roots is useful not only in order to preserve or restore neurological functions, but also to create - above all - an ablative target for SRS. This technique creates a safe distance between the tumor and the spinal cord, allowing the delivery of ablative doses on target lesion [13], [21], [22], [23]. The achievement of a proper decompression of the cord, even with Minimally Invasive Surgical (MIS) techniques, in order to ensure safe high-dose SRS or SBRT, eventually combined with systemic treatment, has become the new target in high volume centers with availability of SRS [2], [7], [21], [22], [24], [25].
According to these new concepts, MESCC treatment should need could not ignore anymore the need for a multidisciplinary management involving spine surgeons, radiation oncologists and oncologists [16], [26], [27], [28], [29]. The aim of this review is to describe the State of the Art about the concept of SS in its surgical and clinical aspects, while examining the medical evidence published on this topic.
2. Methods
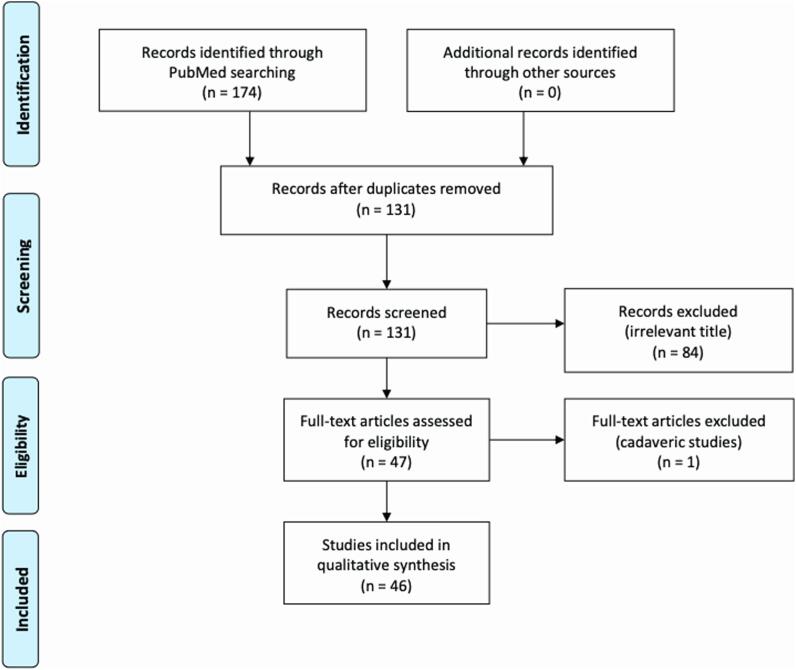
Selection criteria and references for this review were identified by searching PubMed using the terms “separation surgery”, “metastatic epidural compression”, “separation surgery AND spinal metastases”, “separation surgery AND epidural spinal compression” and “separation surgery AND metastatic epidural compression”. Only articles published in English, until May 10, 2020 were reviewed. Inclusion criteria of the references were based on the scope of this review, according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analysis) guidelines [30]. A total number of 174 articles were found, but only 48 of them were considered to be relevant after thorough evaluation (Fig. 1). Hence, each article was classified according to its evidence rate using the Sacket Grading System [31]. Some studies did not satisfy the grading system, which resulted not applicable in those cases (Table 1).
Fig. 1.
PRISMA Flow Chart.
Table 1.
Reviewed articles and evidence rate according to Sacket Grading System [31].
| Study Number | Reference | Type of Study | Evidence Rate [31] |
|---|---|---|---|
| 1 | Moussazadeh et al, 2014 [6] | Review | 3 |
| 2 | Spratt et al, 2017 [7] | Review | 3 |
| 3 | Katsoulakis et al, 2017 [15] | Review | 3 |
| 4 | Amankulor et al, 2013 [88] | Retrospective cohort study | 2 |
| 5 | Barzilai et al, 2018 [86] | Prospective cohort study | 2 |
| 6 | Joaquim et al, 2015 [3] | Review | 3 |
| 7 | Tatsui et al, 2016 [100] | Prospective cohort study | 2 |
| 8 | Tseng et al, 2017 [13] | Review | 3 |
| 9 | Thind et al, 2017 [89] | Case series | 4 |
| 10 | Zhou et al, 2019 [67] | Retrospective cohort study | 3 |
| 11 | Husain et al, 2017 [29] | Review | 3 |
| 12 | Di Martino et al, 2016 [69] | Review | 3 |
| 13 | Laufer et al, 2013 [12] | Retrospective outcome study | 2 |
| 14 | Caruso et al, 2015 [11] | Review | 3 |
| 15 | Bate et al, 2015 [66] | Retrospective cohort study | 3 |
| 16 | Bilsky et al, 2014 [73] | Review | 3 |
| 17 | Komagata et al, 2004 [74] | Case report | N/A |
| 18 | Tatsui et al, 2015 [99] | Case series | 4 |
| 19 | Xiaozhou et al, 2019 [67] | Prospective outcome study | 1 |
| 20 | Cofano et al, 2019 [81] | Case series | 4 |
| 21 | Conti et al, 2019 [36] | Review | 3 |
| 22 | Barzilai et al, 2017 [39] | Prospective cohort study | 2 |
| 23 | Barzilai et al, 2018 [2] | Expert opinion | 5 |
| 24 | Rothrock et al, 2020 [22] | Expert opinion | 5 |
| 25 | Drakhshandeh et al, 2018 [87] | Pro/Retrospecitve cohort study | 2/3 |
| 26 | Zuckerman et al, 2016 [76] | Review | 3 |
| 27 | Hadzipasic et al, 2020 [101] | Case report | N/A |
| 28 | Hu et al, 2020 [8] | Retrospective outcome/cohort study | 2/3 |
| 29 | Meleis et al, 2019 [63] | Retrospective outcome study | 2 |
| 30 | Alghamdi et al, 2019 [14] | Retrospective cohort study | 3 |
| 31 | Kelly et al, 2019 [62] | Review | 2/3 |
| 32 | Vega et al, 2019 [75] | Expert opinion | 5 |
| 33 | Turel et al, 2017 [104 [ | Case series | 4 |
| 34 | Fanous et al, 2017 [49] | Review | 3 |
| 35 | Greenwood et al, 2015 [93] | Retrospective cohort study | 3 |
| 36 | Jandial et al, 2013 [85] | Retrospective cohort study | 3 |
| 37 | Fridley et al, 2017 [48] | Review | 3 |
| 38 | Delgado-Lopez et al, 2019 [25] | Review | 3 |
| 39 | De Almeida Bastos et al, 2020 [98] | Prospective cohort study | 2 |
| 40 | Davarski et al 2013 [84] | Retrospective cohort study | 3 |
| 41 | Ghogawala et al, 2001 [54] | Retrospective cohort study | 3 |
| 42 | Rades et al, 2011 [46] | Retrospective outcome/cohort study | 2/3 |
| 43 | Anand et al, 2015 [65] | Prospective cohort study | 2 |
| 44 | Miller et al, 200083 | Retrospective outcome study | 2 |
| 45 | Vega et al, 2020 [94] | Review | 3 |
| 46 | Ho et al, 2016 [55] | Retrospective outcome study | 2 |
3. Background
The first step of in the management of patients with MESCC should be represented by a comprehensive assessment in order to evaluate of the performance status, patient’s eligibility for surgery and/or radiotherapy, and a global prognostic estimation when achievable [3], [13], [22], [29]. Karnofsky Performance Status (KPS) and Eastern Cooperative Oncology Group Performance Status Scale (ECOG PS) are the most useful scores to assess patient’s functional status [32], [33]. Throughout the years, many prognostic scoring systems have been developed in order to helping decision making. Tomita and Tokuhashi scores [34], [35] have represented real cornerstones for surgeons, but have become progressively out of date because the stability assessment and the impact of new advancements in from radiotherapy/radiosurgery and systemic treatment targeted therapy/immunotherapy were not considered [3], [22], [36]. Furthermore, the decision making was based only on survival prognostic factors. Gasbarrini et al. proposed a multidisciplinary algorithm for the treatment of spinal metastases focusing on functional status (neurology and stability of the spine), considering the ASA score for the surgical eligibility and oncological (chemotherapy and radiotherapy) treatments available [26].
In 2013 Laufer et al. proposed the Neurologic, Oncologic, Mechanical Instability and Systemic Disease (NOMS) framework that introduced a dynamic and updated model for decision making and patients selection. Moreover, oncological assessment is predicted taking into account the tumor histotype and its known responses obtained with the current treatment modalities (chemotherapy, immunotherapy, biologic therapy, hormones, cEBRT, SBRT and SRS) thus, overcoming the technological related bias that affected the previous scores [16]. Systemic assessment was mainly influenced by patient’s co-morbidities and performance status. Neurological and spinal stability evaluations were considered the most important aspects in order to define surgical indications [3], [16].
Neurological assessment reflected the degree of Epidural Spinal Cord Compression (ESCC), evaluated with the ESCC scale (Bilsky Scale) (Table2), and the presence or absence of myelopathy and / or radiculopathy due to neural foramen collapse [16], [37]. The ESCC scale is used to discern the absence (Grade 0) or minimal epidural compression (Grade 1a, 1b, 1c) from high grade epidural compression (Grade 2 or 3) [3], [37]. Mechanical instability assessment was performed using the Spinal Instability Neoplastic Score (SINS) system by Fisher et al. (Table 3), in order to recognize patients with potential or overt spinal instability (values >6) and to provide the best treatment [38]. Mechanical pain, indeed, typically related to the patient’s movements and resulting from an unstable spine, usually requires surgical treatment since it could not be treated with radiotherapy or chemotherapy [2], [3], [7], [22], [39].
Table 2.
Bilsky’s Epidural Spinal Cord Compression (ESCC) grading system37.
| Grade | Description | |
|---|---|---|
| Low Grade | 0 | Bone only disease |
| 1 a | Epidural impingement, without deformation of thecal sac | |
| 1 b | Deformation of the thecal sac, without spinal cord abutment | |
| 1 c | Deformation of the thecal sac, with spinal cord abutment, without cord compression | |
| High Grade | 2 | Spinal cord compression, with cerebrospinal fluid (CSF) visible around the cord |
| 3 | Spinal cord compression, no CSF leak visible around the cord | |
Table 3.
SINS score. Recommendation TS >= 7: Consider surgical intervention.
| Component | Score |
|---|---|
| Location | |
| Junctional (O-C2; C7-T2; T11-L1; L5-S1) | 3 |
| Mobile spine (C3-6; L2-4) | 2 |
| Semirigid (T3-T10) | 1 |
| Rigid (S2-S5) | 0 |
| Mechanical pain* | |
| Yes | 3 |
| No | 2 |
| Pain free lesion | 1 |
| Bone Lesion | |
| Lytic | 2 |
| Mixed (lytic/blastic) | 1 |
| Blastic | 0 |
| Radiographic spinal alignment | |
| Subluxation/translation present | 4 |
| Deformity (kyphosis/scoliosis) | 2 |
| Normal | 0 |
| Vertebral body collapse | |
| >50% collapse | 3 |
| <50% collapse | 2 |
| No collapse with >50% body involved | 1 |
| None of the above | 0 |
| Posterolateral involvement± | |
| Bilateral | 3 |
| Unilateral | 1 |
| None of the above | 0 |
| Criteria of instability | |
| Total score (TS) 0–6 | Stable |
| Total score (TS) 7–11 | Potential unstable |
| Total score (TS) 12–18 | Unstable |
Pain improvement with recumbency and/or pain with movement/loading of the spine.
Facet, pedicle, joint fracture or replacement with tumor [38].
Hence, once assessed neurology and spinal instability, another essential issue is represented by primitive tumor radio-sensitiveness [3]. Myeloma, lymphoma, and seminoma in particular, but also breast tumor [40], prostate tumor and ovarian tumor have been traditionally considered radiosensitive tumors: in these cases, cEBRT showed to be effective in inducing a local cytoreduction [3], [16], [41], [42], [43], [44]. On the other hand, renal, thyroid, hepatocellular, colon, sarcoma, melanoma and non small cell lung cancer (NSCLC) represented a group of neoplasms less radiosensitive. characterized by high resistance to cERBT [45], [46]. During the last decade, the concept of radio-resistance in oncological spine has been overcome with the introduction of SRS and SBRT. In 2008 Yamada et al. published the first series of patients treated with single fraction SRS (dose range 18–24 Gy), showing a recurrence rate and local control consistently improved in comparison with cERBT, regardless of tumor histology and volume [17], [19]. The only limit of SRS is represented by the need to create a safe distance between the tumor and the spinal cord, which is indeed the aim of SS in high grade ESCC [16], [22], [47], [48], [49], [50]. Recently, Cofano et al. proposed the Neurologic Stability Epidural compression (NSE) score with the aim to translate all these concepts in a practical score evaluating the need for surgery in patients with MESCC, according to the latest evidence and assessing ASA, performance status, neurological status, spine stability and ESCC [28]. In the setting of a more comprehensive approach with SRS as a new and relevant treatment option, SS has carved out a key role in MESCC treatment, creating the adequate conditions for radiations [2], [6], [7], [12], [20], [22], [26].
4. Evolutions in radiotherapy
4.1. Conventional external beam radiation therapy
Many studies analyzed effectiveness of cEBRT both as primary and adjuvant treatment for MESCC. Historically, cERBT with palliative intent (30 Gy in 10 fractions, 20 Gy in 5 fractions or 8 Gy in one fraction) have been considered the main treatment options for spinal metastases [20]. In a recent review, Barzilai et al. underlined the wide range of response duration and recurrence rate after adjuvant cERBT and its strong dependency from tumor histology of primary tumor, with poorly responses in radioresistant tumors [36], [42], [51]. Pain relief, neurological status and LC were the outcomes analyzed in different series [42]. Maranzano et al. reported approximately 60% of pain relief with median duration of less than 4 months (LC at 2 years: 30%) in radioresistant tumors, with better results among patients suffering from radiosensitive tumors (LC at 2 years: 86%) [51]. In a meta-analysis of 25 RCTs, Chow et al. reported lower recurrence pain rates (8% vs 20%) and lower complications rate for patients receiving fractionated treatment compared to single fraction group [52]. In a subset analysis of Radiation Therapy Oncology Group (RTOG) trial 97–14 by Howell et al, reported less acute toxicity for 8 Gy/1 fraction, but with a higher rate of re-treatment than 30 Gy/10 fractions. The two schedules of treatment resulted in comparable pain relief and narcotic use at 3 months.
Lastly, a randomized study by Rades et al. compared administration of 20 Gy/5 fractions and 30 Gy/10 fractions in a cohort of 203 patients with poor life expectancy and motor deficits of the lower extremities, reporting no differences in 1-month overall motor response between the two groups; therefore, short-course fractionation schedule could be considered in these selected cases [52].
4.2. SBRT and SRS
SRS and SBRT could be considered the real “game changer” in the paradigm of MESCC treatment [20], [29], [53], [54], [55].
These techniques allow the delivery of high total dose in a single or few fraction (s) to small target volumes, minimizing the dose exposure of normal tissue [13]. As Katsulakis et al. reported in their review, the possibilities to create steep dose gradients around the spinal cord, minimizing dose to the nearby structures (e.g. spine, nerve roots, esophagus), while delivering ablative radiation doses to the tumor, represented the real introduced novelty [15]. Typically, SRS is delivered in a single treatment, whereas SBRT is delivered as two to five treatments [7]. The high doses of radiations induce tumour cell killing through a direct tumoricidal effect and promote different cellular apoptosis pathways activation, resulting in a strong ablative effect [7].
Many studies have analyzed SBRT or SRS with or without surgery, but the retrospective analysis by Laufer et al. is still considered the landmark study in this field [7], [12], [13], [15], [22], [23], [56], [57]. They reported results from 186 patients who underwent surgery and SRS treatment with different regimens [12]. Firstly, the treatment efficacy was independent from the tumor histotype, as already observed in the aforementioned study by Yamada et al.[17], [18]. Then, they found that radiation dose was the only factor significantly associated with tumor progression; lower recurrence rates were reported in patients treated with high-dose hypo-fractionated SRS (median total dose 27 Gy in 3 fractions) or single fraction SRS (24 Gy) compared to patients who underwent low-dose hypo-fractionated SRS (median total dose 30 Gy in 5 or 6 fractions) (Recurrence rate: 4.1%, 9%, 22% respectively) [12].
Al-Omair et al. emphasized the importance of epidural decompression, reporting outcomes in 80 patients undergoing post-surgical SBRT with 1-year LC of 84%. Interestingly, epidural space was the most common site of recurrence (66% of recurrences) and multivariate analysis showed that epidural residual was the only factor significantly related to LC rate [58]. Recently, Redmond et al. reported results from the first prospective study analyzing post-operative SBRT (30 Gy in 5 fractions), showing 1-year LC of 91.4% [59]. Notably, Ito et al. analyzed a retrospective series of 28 patients previously treated with cEBRT, who underwent surgery and subsequent re-irradiation with SBRT (24 Gy in 2 fractions), reporting 1-year LC of 70%, showing SBRT feasibility even for re-irradiation [60].
Nevertheless, although many subsequent studies have reported comparable outcomes, the first prospective trial with the aim to compare outcomes of cEBRT (8 Gy in a single fraction) versus radiosurgery (16 Gy or 18 Gy in a single fraction) is still ongoing (ClinicalTrials.gov NLM Identifier: NCT02512965) [13], [61].
However, contouring guidelines, treatment schedules and complications represented the most interesting debatable issues [8], [56], [62], [63], [64], [65]. Bate et al. reported a series of 57 patients showing the efficacy of SBRT and SRS both with and without surgery. Remarkably, they found higher LC rate in single fraction RT group, but it was not statistically significant [66]. Moreover, recent series reported cumulative 1-year local failure related to epidural grade and association between LC and epidural disease downgrading, pain relief rate of 88.5% and association between SRS and overall survival [14], [67]. Associations with overall survival could be related to the synergistic effects between ablative radiotherapy treatment and systemic treatment, also known as abscopal effect, recently investigated by Caruso at al. in patients with metastatic spinal melanoma [11].
Vertebral compressive fractures (VCF) and radiation myelopathy were considered the main complications of SRS/SBRT [7], [13], [22], [56]. A systematic review by Chang et al. reported the incidence of VCF ranging from 0.7% to 40.5%, variable with different schedules [56]. Although radiation myelopathy was reported especially in the first series, maybe due to the initial lack of understanding the tolerance of the spinal cord (now assessed to 14–16 Gy) [64], it should be considered a rare complication and should not be a contraindication for treatment [56]. Consensus guidelines by Redmond et al., provided the best indications to obtain the higher ablative effect with the lesser risk of complications [64] (Table 4).
Table 4.
Consensus conturing guidelines for SBRT62.
| Volume | Include |
|---|---|
| Gross tumor volume (GTV) | Postoperative residual based on MRI |
| Clinical tumor volume (CTV) | Entire extent of preoperative tumor, anatomic compartment involved, & any postoperative residual Surgical instrumentation & incision not included unless involved Prophylactic circumferential treatment of epidural space controversial Additional expansion up to 5 mm for paraspinal extension controversial Consider an additional expansion of up to 5 mm cranio-caudally beyond known epidural disease extent based on pre- & postoperative imaging |
| Planning target volume (PTV) | 0- to 2-mm expansion from CTV |
| Spinal cord | True spinal cord based on postoperative T2-weighted MRI or CT myelogram in cases of significant hardware artifact |
| Spinal cord planning risk volume (PRV) | 0- to 2-mm expansion of spinal cord volume |
On one hand, SBRT introduction has radically refocused the radiation therapy’s goal, from the simple pain relief towards the more attractive attempt to achieve LC, overcoming the concept of radio-resistance. On the other hand, this new option in the radiation therapy armamentarium has led to the innovative concept of hybrid therapy, shifting the paradigm of MESCC surgery from extended cytoreductive surgery to effective separation surgery in order to create a target avoiding damages to the spinal cord.
5. Evolution in surgery
Different surgical approaches and strategies to treat MESCC were adopted through the years, with the aim to decompress neural structures and to restore spinal stability, in order to obtain palliative pain relief and neurological preservation [24], [29]. Siegal et al. analyzed the ability to walk in 78 patients suffering from MESCC and who underwent different type of surgical decompression (anterior decompression vs laminectomy). In the anterior decompression group 28% of patients were ambulatory before surgery and then, 80% were ambulatory after surgery. Among patients who underwent laminectomy 8% were ambulatory before surgery and 39% after surgery [68]. In a systematic review by Di Martino et al., recommendations in favor to early surgical treatment (within 48 h) of MESCC were reported [69].
The first study that have substantially changed the role of surgery in this field was the randomized prospective trial by Patchell et al. that analyzed the ability to walk among patients undergoing decompressive surgery and radiation and those treated with radiation alone (radiation was 30 Gy / 10 fractions in both groups) [7], [13], [20], [22], [29], [70]. Patients in the surgical arm showed significative higher rate of ability to walk compared to patients treated with cERBT alone (84% vs 57%, Odds Ratio 6.2, p 0.001). Moreover, even secondary endpoints such as urinary continence, pain relief, using of opioid analgesics and steroids, resulted to be improved into the surgical group [70], [71].
More recently, Fehlings et al. showed significant improvements both in clinical outcomes (ability to walk and pain relief) and patient’s quality of life (QoL) among patients that underwent surgery and radiation, compared to patients belonging at radiation only group [72].
Nevertheless, as reported by Rothrock et al., these relevant studies did not consider a longitudinal follow up regarding LC; this could be considered a major limitation, probably due to the fact that at the time LC analysis was not a priority since median survival was often less than a year [22].
Nowadays, indeed, while the role of surgery in achieving neurologic improvement and stability is clearly defined, the possibility of durable symptoms palliation with cERBT is still debatable for MESCC in radio-resistant tumors. Moreover, the newest systemic agents have significantly improved the overall survival for the majority of tumor histotypes, making durable LC a new goal of this combined strategy [22].
5.1. Separation surgery – surgical technique
The term separation surgery was coined by Lyliana Angelov and Edward Benzel to designate a procedure in which the goal of surgical resection was to decompress the spinal cord and provide a safe target for SRS or SBRT [7]. This term has really changed the surgeons’ idea of decompressive surgery in MESCC. The durable and reliable LC rates provided by cERBT in MESCC in radiosensitive tumors and by SRS or SBRT in MESCC in radio resistant tumors, have questioned the need for extensive and aggressive cytoreductive surgery [6], [20]. Nowadays, aggressive tumor resection (en bloc vertebrectomy or extended intralesional removal of the vertebral body) has lost its role and many authors suggest to perform this strategy only in selected cases, such as selected single metastatic spine lesion from radiosensitive tumors, or in cases of MESCC due to radioresistant tumors in contexts where SRS or SBRT are not fully available [20], [26]. Bilsky et al. suggested aggressive surgery for superior sulcus lung tumors involving vertebral bodies since they are usually complicated by the the use of neo-adjuvant chemotherapy and/or radiotherapy, and by related comorbidities, including osteoporosis and history of smoking. Hence, superior sulcus tumors required both a posterior incision over the spine and a posterolateral thoracotomy [73], [74]. The aforementioned papers by Moulding et al. first and Laufer et al. later, reported higher rates of LC among patients treated with SRS or SBRT, creating the basis for the idea of “hybrid therapy” in the treatment of high grade MESCC due to radioresistant tumors, that could not be considered candidates for “up-front” radiation treatment [2], [22], [39]. The tumoricidal high doses of SRS or SBRT cannot be provided sparing the spinal cord if no space from the tumor was detected. Hence, separation surgery should be the first step of the hybrid therapy and has the paramount task to create this space (at least 2–3 mm) providing safe targets for the second step that is represented by SRS or SBRT [2], [6], [7], [12], [16], [20], [21], [22], [26], [75].
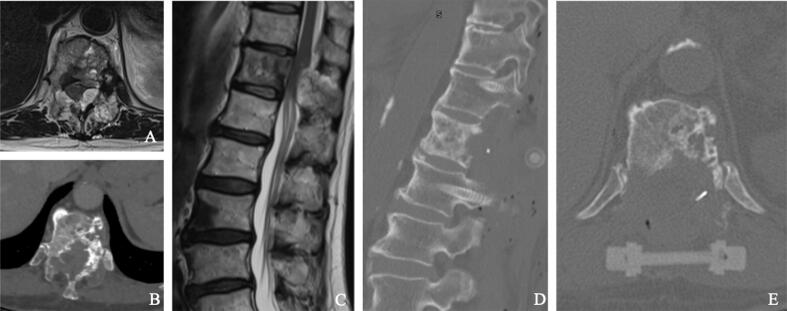
Barzilai et al. described the technique in the thoracic spine in case of ventral compression that consists in a first stabilizing step with the aim to restore/prevent spinal stability and obtain mechanical pain relief. All patients that underwent circumferential separation surgery, indeed, would require spinal instrumentation due to the tumor extension and to the need of lamina and pedicle/joint removal in order to achieve ventral decompression. Spinal instrumentation should be performed as the first step to reduce the risk of spinal cord damage (Fig. 2). Long segment fixation, that means 2 levels above and 2 levels below the tumor while skipping the involved vertebra, was described [2]. Free hand or navigated and open or percutaneous techniques could be adopted [75], [76]. Moreover, considering the known need for subsequent radiation treatment, carbon fiber/polyetheretherketone (PEEK) instrumentation has been considered by other authors, in order to reduce scattering and artifacts related to titanium implants [77], [78], [79], [80]. The second step represents the decompressive phase (Fig. 3). Due to the high grade MESCC, one should avoid to transmit pressure to the spinal cord. In order to obtain this, the bony structures should be thinned using the high-speed drill and then removed with Kerrison rongeurs. A bilateral corridor to the ventral aspect should be performed drilling the facet joint and the pedicles. Thus, the ventral component of the tumor should be carefully dissected away from the dura. About 20% of the involved body should be removed to create a ventral cavity, then Hoffman ligaments and posterior longitudinal ligament (PLL) should be cut by tenotomy scissors to detach the anterior side of the dura. Lastly, the dissected epidural ventral tumor should be depressed anteriorly far from the dura with Woodson dissector, obtaining circumferential separation of the spinal cord [2], [7], [21], [22]. Extended tumor removal inside the vertebral body and/or into the paraspinal tissues should not be required. However, poly-methyl-methacrylate (PMMA) insertion, Steinman pins using and PEEK/carbon fiber or non-expandable titanium mesh cages could be adopted when more than than 50% of vertebral body is removed [22]. Intra-operative neuromonitoring has been largely suggested in order to prevent surgical related spinal cord damage due to the high grade ESCC [2], [81], [82]. The aforementioned principle surgical principles of circumferential separation could also be applied – with different and specific approaches - for tumors involving the cervical or lumbar spine [83], [84], [85].
Fig. 2.
Short posterior carbon fiber instrumentation ad separation surgery (D, E) in a case of high grade ESCC (A), due to lytic lung metastatic lesion of T8 (B, C).
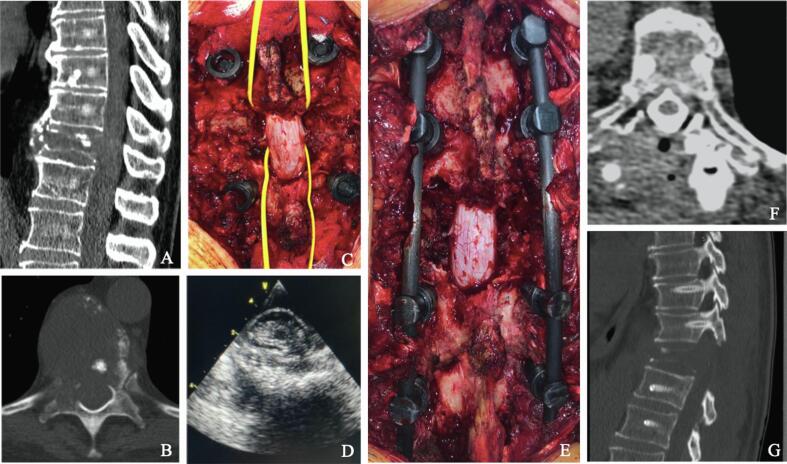
Fig. 3.
Clinical case (A-G): A, B) Pre-operative CT scan showing T9 MESCC (Bilsky grade: 2, SINS score: 12) from lung cancer, C) Circumferential separation surgery was performed and D) intra-operative US assessing ventral separation of the spinal cord from the tumor is shown. E) Posterior carbon fiber instrumentation two level above and below the pathological vertebra. F) Post-operative CT myelography showing restored CSF space around the cord. G) Post-operative CT scan revealing screw instrumentation.
Bate et al. published their results from a retrospective series of 57 patients with high grade MESCC treated with SRS alone or separation surgery followed by adjuvant SRS. Of the 21 patients in separation surgery plus SRS group, 9.5% rate of local failure was reported at 1-year, while the regression analysis did not showed variables resulting to be significant factors [66].
Recently, a prospective study including 111 patients by Barzilai et al., reported results about QoL after “hybrid MESCC therapy”. Spine pain severity at 3 months was significantly reduced and general activity was also improved (p = 0.001); local recurrence rate resulted to be 2.1% and 4.3% at 6 months and 12 months, respectively. Moreover, re-operated patients were associated with diminished patient reported outcome improvement [39], [86].
Many studies have also analyzed post-operative complications in patients that underwent separation surgery. Wound healing and hardware failure were the major concerns, considering both the need for radiation therapy, the lack of anterior column reconstruction and the low possibility to achieve bone fusion in cancer patients due to adjuvant therapies and shorter life expectancy.
Drakhsandeh et al. reported no hardware failure in a retrospective series of 27 patients undergoing posterior instrumentation without fusion, while the more reliable 7-years retrospective experience by Amankulor et al. that analyzing 318 patients treated with posterior instrumentation without anterior column reconstruction reported 2.8% of hardware failure incidence, while instrumentation longer than 6 levels and chest wall resection resulted to be the risk factors for failure [87], [88], [89]. Cofano et al. have recently reported no hardware failure in a retrospective series of patients undergoing posterior carbon fiber / PEEK instrumentation, with a mean follow up of 11 months [77].
About wound complications, a series of 140 patients by Wang et al., reported a wound complication rate of 10.6%, while in a review by Bilsky et al., the different rate of wound infection or dehiscence between patients treated with cEBRT and SRS (17% vs 6%) was underlined [73], [90].
5.2. Separation surgery – which is the role of Minimally Invasive Techniques?
Minimal Access Spine (MAS) or Minimally Invasive Surgical (MIS) techniques for the treatment of MESCC have gained increasingly interest after the introduction of hybrid therapy and separation surgery, according to their reported limited post-operative morbidity and their related possibility for quick recovery and return to radiation and systemic treatment [20], [76], [81], [91]. Mini-open approaches, endoscopic or percutaneous techniques and, lastly, ablative procedures have been described [74], [92], [93], [94]. In a review article by Pennington et al. the authors reported shorter operative times, reduced blood loss, shorter recovery times and lower complications rate among patients treated with MIS techniques. Moreover, other studies in the same review reported similar results than open surgery regarding neurological outcomes [95]. Mini-open transpedicular corpectomy was described by Zhou et al, with a midline facial incision over the corpectomy level of interest (in addition to percutaneous instrumentation above and below that level) [92]. Additionally, Lau et al. compared patients who underwent mini-open accesses to patients treated with standard open approaches reporting better results for the mini-open group as for blood loss and length of hospitalization [96], [97]. The use of tubular retractors for decompression purpose have been also analyzed among MIS techniques as reported by Zuckerman et al [76].
Furthermore, endoscopic procedures have been described as MIS technique. Cofano et al. reported promising results describing a series of 9 patients with thoracic MESCC undergoing 3D endoscopic transpedicular route in order to achieve a safe ventral separation of the spinal cord without the need for costotransversectomy, while thoracoscopy resulted useful in patients with thoracic disease that required direct anterior decompression [76], [81].
Another evolution of focusing surgery to epidural decompression was represented by spine Laser Interstitial Thermal Therapy (LITT) [98], [99], [100], [101]. Tatsui et al. have described an ablative percutaneous procedure able to reduce ESCC heating up the epidural component of the tumor using laser under real time thermal MRI control. In their last series of 19 patients no neurological injuries were reported, a mean reduction of 22% of the median thickness of the epidural tumor was observed at 2-months follow up (pre-procedure: 8 mm; follow up: 6.4 mm; p 0.012) and all patients underwent SRS with a median interval of 3 days [99], [100], [101].
Hence, the need for less aggressive approach to obtain spinal cord separation (e.g. mini open approaches), has led to consider MIS techniques also for screws instrumentation, in order to reduce the fixation related morbidities in these fragile patients [75]. Percutaneous screw fixation could be considered, due to the limited muscles dissection and tension band disruption with consequent lower reported blood loss, decreased post-operative pain, earlier mobilization and more expeditious time to radiation [75], [76], [102], [103], [104]. Moreover, short percutaneous instrumentations (only one level above and below the pathological vertebra) could be performed with the aim to reduce both blood loss and the risk of wound issues, since small incisions and limited sub-fascial exposure were obtained both for fixation and for separation steps [90]. Hence, in case of short instrumentations, cement augmentation of the screws or of the anterior column could be an advantageous tool, since it increase the screws’ purchase, decreasing the risk of hardware failure due to osteoporotic bone or to subsequent radiotherapy [105]. Furthermore, cement augmentation of the anterior column (vertebroplasty or kiphoplasty) was associated with significative pain relief as reported by Moussazadeh et al. [105], [106].
Although MIS techniques have shown promising results, standard open approaches are still the most widely used; hence, a careful selection of patients considered suitable for MIS techniques should be the rule [20], [75].
5.3. Separation surgery – the role of imaging
The increasingly spread of separation surgery idea has led to focus surgeon’s attention on the decompressive phase of surgery for MESCC, shifting goal from the “oldest” extensive cytoreduction to the “newest” separation. On the other hand, although an adequate separation is crucial for safe ablative radiation delivery, there are no strong evidence that define what adequate separation should mean. Barzilai et al, describing their surgical technique, reported that decompressive surgery should guarantee at least 2–3 mm of separation from the spinal cord, and this was confirmed in other paper by the same group, also considering the higher-dose constraints for the spinal cord and/or nerve roots [2], [15], [22], [64], [107]. From this angle, an adequate intra and post-operative separation assessment would be critic [105]. While posterior and postero-lateral decompression could be easily evaluated, ventral separation assessment could be critical. Hence, image guidance such as navigation system or intra-operative ultra sound could be considered the most reliable methods to visualize the ventral epidural space and assess restoring of the ventral CSF space as reported by Vasudeva et al. and Kelly at al. [22], [107], [108].
Post-operative assessment of circumferential free-space around the spinal cord is also crucial for radiation planning. Because MRI-related artifacts from hardware were considered a limit for correct radiation planning, CT myelography has been described as the preferred exams to better assess the separation [2], [22]. Consensus contouring guidelines by Redmond et al., indeed, indicated that the treatment should be planned with co-registration of pre-operative and post-operative T1 weighted MRI and spinal cord delineation on T2 weighted post-operative MRI or CT myelography or in both [64].
6. Conclusion
The increasingly evidence of long-term LC obtained with SBRT and the overcoming of radio-resistance have radically changed the surgical management of MESCC, leading to the new concept of SS. The goal of SS should be to separate the spinal cord from the tumor, providing a safe target for SBRT or SRS. Because of the reduced life expectancy of patients suffering from cancer, decreasing the morbidity of surgical treatment is mandatory. From this perspective, the wide spectrum of MIS techniques could represent a useful tool both for separation surgery and instrumentation, in order to guarantee expeditious recovery and prioritize the systemic treatment.
Acknowledgments
Acknowledgments
This study was supported by Ministero dell’Istruzione, dell’Università e della Ricerca—MIUR project ‘‘Dipartimenti di eccellenza 2018-2022”.
Funding
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest
The authors have no conflict of interest to disclose.
References
- 1.Ortiz Gómez J.A. The incidence of vertebral body metastases. Int. Orthop. 1995 doi: 10.1007/BF00181116. [DOI] [PubMed] [Google Scholar]
- 2.Barzilai O., Laufer I., Robin A., Xu R., Yamada Y., Bilsky M.H. Hybrid therapy for metastatic epidural spinal cord compression: Technique for separation surgery and spine radiosurgery. Oper. Neurosurg. 2019 doi: 10.1093/ons/opy137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joaquim A.F., Powers A., Laufer I., Bilsky M.H. An update in the management of spinal metastases. Arq. Neuro-Psiq. 2015 doi: 10.1590/0004-282x20150099. [DOI] [PubMed] [Google Scholar]
- 4.Klimo P., Kestle J.R.W., Schmidt M.H. Clinical trials and evidence-based medicine for metastatic spine disease. Neurosurg. Clin. N. Am. 2004 doi: 10.1016/j.nec.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 5.Wong D.A., Fornasier V.L., Macnab I. Spinal metastases: The obvious, the occult, and the impostors. Spine. 1990 doi: 10.1097/00007632-199001000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Moussazadeh N., Laufer I., Yamada Y., Bilsky M.H. Separation surgery for spinal metastases: Effect of spinal radiosurgery on surgical treatment goals. Cancer Control. 2014 doi: 10.1177/107327481402100210. [DOI] [PubMed] [Google Scholar]
- 7.Spratt D.E., Beeler W.H., de Moraes F.Y. An integrated multidisciplinary algorithm for the management of spinal metastases: an International Spine Oncology Consortium report. Lancet Oncol. 2017 doi: 10.1016/S1470-2045(17)30612-5. [DOI] [PubMed] [Google Scholar]
- 8.Hu J.X., Gong Y.N., Jiang X.D. Local tumor control for metastatic epidural spinal cord compression following separation surgery with adjuvant cyberknife stereotactic radiotherapy or image-guided intensity modulated radiotherapy. World Neurosurg. 2020 doi: 10.1016/j.wneu.2020.04.183. [DOI] [PubMed] [Google Scholar]
- 9.Cofano F., Monticelli M., Ajello M. The targeted therapies era beyond the surgical point of view: what spine surgeons should know before approaching spinal metastases. Cancer Control. 2019;26(1) doi: 10.1177/1073274819870549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodwin C.R., Abu-Bonsrah N., Rhines L.D. Molecular markers and targeted therapeutics in metastatic tumors of the spine: Changing the treatment paradigms. Spine. 2016;41(20):S218–S223. doi: 10.1097/BRS.0000000000001833. [DOI] [PubMed] [Google Scholar]
- 11.Caruso J.P., Cohen-Inbar O., Bilsky M.H., Gerszten P.C., Sheehan J.P. Stereotactic radiosurgery and immunotherapy for metastatic spinal melanoma. Neurosurg. Focus. 2015;38(3):1–10. doi: 10.3171/2014.11.FOCUS14716. [DOI] [PubMed] [Google Scholar]
- 12.Laufer I., Iorgulescu J.B., Chapman T. Local disease control for spinal metastases following “separation surgery” and adjuvant hypofractionated or high-dose single-fraction stereotactic radiosurgery: Outcome analysis in 186 patients. J. Neurosurg. Spine. 2013 doi: 10.3171/2012.11.SPINE12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tseng C.L., Eppinga W., Charest-Morin R. Spine stereotactic body radiotherapy: indications, outcomes, and points of caution. Glob. Spine J. 2017;7(2):179–197. doi: 10.1177/2192568217694016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alghamdi M., Sahgal A., Soliman H. Postoperative stereotactic body radiotherapy for spinal metastases and the impact of epidural disease grade. Clin. Neurosurg. 2019;85(6):E1111–E1118. doi: 10.1093/neuros/nyz349. [DOI] [PubMed] [Google Scholar]
- 15.Katsoulakis E., Kumar K., Laufer I., Yamada Y. Stereotactic body radiotherapy in the treatment of spinal metastases. Semin. Radiat. Oncol. 2017;27(3):209–217. doi: 10.1016/j.semradonc.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Laufer I., Rubin D.G., Lis E. The NOMS Framework: approach to the treatment of spinal metastatic tumors. Oncologist. 2013 doi: 10.1634/theoncologist.2012-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamada Y., Bilsky M.H., Lovelock D.M. High-dose, single-fraction image-guided intensity-modulated radiotherapy for metastatic spinal lesions. Int. J. Radiat. Oncol. Biol. Phys. 2008 doi: 10.1016/j.ijrobp.2007.11.046. [DOI] [PubMed] [Google Scholar]
- 18.Moulding H.D., Elder J.B., Lis E. Local disease control after decompressive surgery and adjuvant high-dose single-fraction radiosurgery for spine metastases: Clinical article. J. Neurosurg. Spine. 2010 doi: 10.3171/2010.3.SPINE09639. [DOI] [PubMed] [Google Scholar]
- 19.Yamada Y., Katsoulakis E., Laufer I. The impact of histology and delivered dose on local control of spinal metastases treated with stereotactic radiosurgery. Neurosurg. Focus. 2017 doi: 10.3171/2016.9.FOCUS16369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barzilai O., Boriani S., Fisher C.G. Essential concepts for the management of metastatic spine disease: what the surgeon should know and practice. Glob Spine J. 2019;9(1_suppl):98S–107S. doi: 10.1177/2192568219830323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laufer I., Bilsky M.H. Advances in the treatment of metastatic spine tumors: the future is not what it used to be. J. Neurosurg. Spine. 2019;30(3):299–307. doi: 10.3171/2018.11.SPINE18709. [DOI] [PubMed] [Google Scholar]
- 22.Rothrock R., Pennington Z., Ehresman J. Hybrid therapy for spinal metastases. Neurosurg. Clin. N. Am. 2020 doi: 10.1016/j.nec.2019.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Szendroi M., Antal I., Szendroi A., Lazáry Á., Varga P.P. Diagnostic algorithm, prognostic factors and surgical treatment of metastatic cancer diseases of the long bones and spine. EFORT Open Rev. 2017;2(9):372–381. doi: 10.1302/2058-5241.2.170006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bilsky M.H., Laufer I., Burch S. Shifting paradigms in the treatment of metastatic spine disease. Spine. 2009;34(22 Suppl):101–107. doi: 10.1097/brs.0b013e3181bac4b2. [DOI] [PubMed] [Google Scholar]
- 25.Delgado-López P.D., Roldán-Delgado H., Corrales-García E.M. Stereotactic body radiation therapy and minimally invasive surgery in the management of spinal metastases: A change in the paradigm. Neurocirugía (Engl. Ed.) 2019 doi: 10.1016/j.neucie.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 26.Gasbarrini A., Cappuccio M., Mirabile L. Spinal metastases: Treatment evaluation algorithm. Eur. Rev. Med. Pharmacol. Sci. 2004 [PubMed] [Google Scholar]
- 27.Paton G., Frangou E., Fourney D. Contemporary treatment strategy for spinal metastasis: The “lMNOP” system. Can. J. Neurol. Sci. 2011 doi: 10.1017/S031716710001177X. [DOI] [PubMed] [Google Scholar]
- 28.Cofano F., di Perna G., Zenga F. The Neurology-Stability-Epidural compression assessment: a new score to establish the need for surgery in spinal metastases. Clin. Neurol. Neurosurg. 2020 doi: 10.1016/j.clineuro.2020.105896. [DOI] [PubMed] [Google Scholar]
- 29.Husain Z.A., Sahgal A., Chang E.L. Modern approaches to the management of metastatic epidural spinal cord compression. CNS Oncol. 2017;6(3):231–241. doi: 10.2217/cns-2016-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liberati A., Altman D.G., Tetzlaff J. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ (Clin. Res. Ed.) 2009 doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wright J.G., Swiontkowski M.F., Heckman J.D. Introducing levels of evidence to the journal. J. Bone Joint Surg. Series A. 2003;85(1):1–3. doi: 10.2106/00004623-200301000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Karnofsky D.A., Abelmann W.H., Craver L.F., Burchenal J.H. The use of the nitrogen mustards in the palliative treatment of carcinoma. Cancer. 1948 doi: 10.1002/1097-0142(194811)1:4<634::AID-CNCR2820010410>3.0.CO;2-L. [DOI] [Google Scholar]
- 33.Oken M.M., Creech R.H., Davis T.E. Toxicology and response criteria of the Eastern Cooperative Oncology Group. Am. J. Oncol.: Cancer Clin. Trials. 1982 doi: 10.1097/00000421-198212000-00014. [DOI] [PubMed] [Google Scholar]
- 34.Tomita K., Kawahara N., Kobayashi T., Yoshida A., Murakami H., Akamaru T. Surgical strategy for spinal metastases. Spine. 2001 doi: 10.1097/00007632-200102010-00016. [DOI] [PubMed] [Google Scholar]
- 35.Tokuhashi Y., Matsuzaki H., Oda H., Oshima M., Ryu J. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine. 2005 doi: 10.1097/01.brs.0000180401.06919.a5. [DOI] [PubMed] [Google Scholar]
- 36.Conti A., Acker G., Kluge A. Decision making in patients with metastatic spine. The role of minimally invasive treatment modalities. Front. Oncol. 2019;9:1–15. doi: 10.3389/fonc.2019.00915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bilsky M.H., Laufer I., Fourney D.R. Reliability analysis of the epidural spinal cord compression scale. J. Neurosurg. Spine. 2010 doi: 10.3171/2010.3.SPINE09459. [DOI] [PubMed] [Google Scholar]
- 38.Fisher C.G., DiPaola C.P., Ryken T.C. A novel classification system for spinal instability in neoplastic disease. Spine. 2010 doi: 10.1097/brs.0b013e3181e16ae2. [DOI] [PubMed] [Google Scholar]
- 39.Barzilai O., Amato M.K., McLaughlin L. Hybrid surgery-radiosurgery therapy for metastatic epidural spinal cord compression: A prospective evaluation using patient-reported outcomes. Neuro-Oncol. Pract. 2018;5(2):104–113. doi: 10.1093/nop/npx017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pessina F., Navarria P., Riva M. Long-term follow-up of patients with metastatic epidural spinal cord compression from breast cancer treated with surgery followed by radiotherapy. World Neurosurg. 2018;110:e281–e286. doi: 10.1016/j.wneu.2017.10.156. [DOI] [PubMed] [Google Scholar]
- 41.Gerszten P.C., Burton S.A., Ozhasoglu C., Welch W.C. Radiosurgery for spinal metastases: Clinical experience in 500 cases from a single institution. Spine. 2007 doi: 10.1097/01.brs.0000251863.76595.a2. [DOI] [PubMed] [Google Scholar]
- 42.Maranzano E., Latini P. Effectiveness of radiation therapy without surgery in metastatic spinal cord compression: Final results from a prospective trial. Int. J. Radiat. Oncol. Biol. Phys. 1995 doi: 10.1016/0360-3016(95)00572-G. [DOI] [PubMed] [Google Scholar]
- 43.Gilbert R.W., Kim J.-H., Posner J.B. Epidural spinal cord compression from metastatic tumor: Diagnosis and treatment. Ann. Neurol. 1978 doi: 10.1002/ana.410030107. [DOI] [PubMed] [Google Scholar]
- 44.Gerszten P.C., Mendel E., Yamada Y. Radiotherapy and radiosurgery for metastatic spine disease: what are the options, indications, and outcomes? Spine. 2009 doi: 10.1201/9781315154053-7. [DOI] [PubMed] [Google Scholar]
- 45.Rades D., Fehlauer F., Schulte R. Prognostic factors for local control and survival after radiotherapy of metastatic spinal cord compression. J. Clin. Oncol. 2006 doi: 10.1200/JCO.2005.05.0542. [DOI] [PubMed] [Google Scholar]
- 46.Rades D., Huttenlocher S., Bajrovic A. Surgery followed by radiotherapy versus radiotherapy alone for metastatic spinal cord compression from unfavorable tumors. Int. J. Radiat. Oncol. Biol. Phys. 2011 doi: 10.1016/j.ijrobp.2010.11.056. [DOI] [PubMed] [Google Scholar]
- 47.Gasbarrini A., Boriani S., Capanna R. Management of patients with metastasis to the vertebrae: Recommendations from the Italian Orthopaedic Society (SIOT) Bone Metastasis Study Group. Expert Rev. Anticancer Ther. 2014 doi: 10.1586/14737140.2014.856532. [DOI] [PubMed] [Google Scholar]
- 48.Fridley J.S., Hepel J.T., Oyelese A.A. Current treatment of metastatic spine tumors - surgery and stereotactic radiosurgery. Rhode Island Med. J. 2013:2017. [PubMed] [Google Scholar]
- 49.Fanous A.A., Fabiano A.J. Surgical management of spinal metastatic disease. J. Neurosurg. Sci. 2016 doi: 10.23736/S0390-5616.16.03914-X. [DOI] [PubMed] [Google Scholar]
- 50.Alghamdi M., Tseng C.L., Myrehaug S. Postoperative stereotactic body radiotherapy for spinal metastases. Chin. Clin. Oncol. 2017 doi: 10.21037/cco.2017.06.27. [DOI] [PubMed] [Google Scholar]
- 51.Chow E., Zeng L., Salvo N., Dennis K., Tsao M., Lutz S. Update on the systematic review of palliative radiotherapy trials for bone metastases. Clin. Oncol. 2012 doi: 10.1016/j.clon.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 52.Rades D., Šegedin B., Conde-Moreno A.J. Radiotherapy with 4 Gy × 5 versus 3 Gy × 10 for metastatic epidural spinal cord compression: Final results of the SCORE-2 Trial (ARO 2009/01) J. Clin. Oncol. 2016 doi: 10.1200/JCO.2015.64.0862. [DOI] [PubMed] [Google Scholar]
- 53.Howell D.D., James J.L., Hartsell W.F. Single-fraction radiotherapy versus multifraction radiotherapy for palliation of painful vertebral bone metastases - Equivalent efficacy, less toxicity, more convenient: A subset analysis of Radiation Therapy Oncology Group trial 97–14. Cancer. 2013 doi: 10.1002/cncr.27616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ghogawala Z., Mansfield F.L., Borges L.F. Spinal radiation before surgical decompression adversely affects outcomes of surgery for symptomatic metastatic spinal cord compression. Spine. 2001 doi: 10.1097/00007632-200104010-00025. [DOI] [PubMed] [Google Scholar]
- 55.Ho J.C., Tang C., Deegan B.J. The use of spine stereotactic radiosurgery for oligometastatic disease. J. Neurosurg. Spine. 2016 doi: 10.3171/2016.1.SPINE151166. [DOI] [PubMed] [Google Scholar]
- 56.J.H. Chang, J.H. Shin, Y.J. Yamada, et al. SBRT for Spinal Mets: What are the risks and how do we minimize them? 2017;41(Suppl 20):1–15. doi:10.1097/BRS.0000000000001823.Stereotactic. [DOI] [PMC free article] [PubMed]
- 57.Kim Y.J., Kim J.H., Kim K. The feasibility of spinal stereotactic radiosurgery for spinal metastasis with epidural cord compression. Cancer Res. Treat. 2019;51(4):1324–1335. doi: 10.4143/crt.2018.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Al-Omair A., Masucci L., Masson-Cote L. Surgical resection of epidural disease improves local control following postoperative spine stereotactic body radiotherapy. Neuro-Oncol. 2013 doi: 10.1093/neuonc/not101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Redmond K.J., Sciubba D., Khan M. A phase 2 study of post-operative stereotactic body radiation therapy (SBRT) for solid tumor spine metastases. Int. J. Radiat. Oncol. Biol. Phys. 2020;106(2):261–268. doi: 10.1016/j.ijrobp.2019.10.011. [DOI] [PubMed] [Google Scholar]
- 60.Ito K., Nihei K., Shimizuguchi T. Postoperative re-irradiation using stereotactic body radiotherapy for metastatic epidural spinal cord compression. J. Neurosurg. Spine. 2018 doi: 10.3171/2018.1.SPINE171155. [DOI] [PubMed] [Google Scholar]
- 61.Radiation Therapy Oncology Group. Image-guided radiosurgery or stereotactic body radiation therapy in treating patients with localized spine metastasis (ClinicalTrials.gov NLM Identifier: NCT00922974). Bethesda, MD: National Library of Medicine; 2000. https://clinicaltrials.gov/ct2/show/NCT00922974. Accessed February 1, 2017.
- 62.Kelly P.D., Zuckerman S.L., Than K.D., Attia A., Jaboin J.J. Metastatic spine disease in lung cancer patients: national patterns of radiation and surgical care. J. Spine Surg. 2019;5(3):320–328. doi: 10.21037/jss.2019.08.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Meleis A., Jhawar S.R., Weiner J.P. Stereotactic body radiation therapy in nonsurgical patients with metastatic spinal disease and epidural compression: A retrospective review. World Neurosurg. 2019;122:e198–e205. doi: 10.1016/j.wneu.2018.09.210. [DOI] [PubMed] [Google Scholar]
- 64.Redmond K.J., Lo S.S., Soltys S.G. Consensus guidelines for postoperative stereotactic body radiation therapy for spinal metastases: Results of an international survey. J. Neurosurg. Spine. 2017 doi: 10.3171/2016.8.SPINE16121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Anand A.K., Venkadamanickam G., Punnakal A.U. Hypofractionated stereotactic body radiotherapy in spinal metastasis - with or without epidural extension. Clin. Oncol. 2015 doi: 10.1016/j.clon.2015.01.035. [DOI] [PubMed] [Google Scholar]
- 66.Bate B.G., Khan N.R., Kimball B.Y., Gabrick K., Weaver J. Stereotactic radiosurgery for spinal metastases with or without separation surgery. J. Neurosurg. Spine. 2015 doi: 10.3171/2014.10.SPINE14252. [DOI] [PubMed] [Google Scholar]
- 67.Xiaozhou L., Xing Z., Xin S. Efficacy analysis of separation surgery combined with SBRT for spinal metastases—A long-term follow-up study based on patients with spinal metastatic tumor in a single-center. Orthop. Surg. 2019;2020:404–420. doi: 10.1111/os.12594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Siegal T., Siegal T. Surgical decompression of anterior and posterior malignant epidural tumors compressing the spinal cord: A prospective study. Neurosurgery. 1985 doi: 10.1227/00006123-198509000-00005. [DOI] [PubMed] [Google Scholar]
- 69.di Martino A., Caldaria A., de Vivo V., Denaro V. Metastatic epidural spinal cord compression. Expert Rev. Anticancer Ther. 2016;16(11):1189–1198. doi: 10.1080/14737140.2016.1240038. [DOI] [PubMed] [Google Scholar]
- 70.Patchell R.A., Tibbs P.A., Regine W.F. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: A randomised trial. Lancet. 2005 doi: 10.1016/S0140-6736(05)66954-1. [DOI] [PubMed] [Google Scholar]
- 71.Tateiwa D., Oshima K., Nakai T. Clinical outcomes and significant factors in the survival rate after decompression surgery for patients who were non-ambulatory due to spinal metastases. J. Orthop. Sci. 2019 doi: 10.1016/j.jos.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 72.Fehlings M.G., Nater A., Tetreault L. Survival and clinical outcomes in surgically treated patients with metastatic epidural spinal cord compression: Results of the prospective multicenter AOSpine study. J. Clin. Oncol. 2016 doi: 10.1200/JCO.2015.61.9338. [DOI] [PubMed] [Google Scholar]
- 73.Bilsky M.H., Laufer I., Matros E., Yamada J., Rusch V.W. Advanced lung cancer. Aggressive surgical therapy vertebral body involvement. Thorac. Surg. Clin. 2014;24(4):423–431. doi: 10.1016/j.thorsurg.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 74.Komagata M., Nishiyama M., Imakiire A., Kato H. Total spondylectomy for en bloc resection of lung cancer invading the chest wall and thoracic spine: Case report. J. Neurosurg. 2004;100(4 Suppl.):353–357. doi: 10.3171/spi.2004.100.4.0353. [DOI] [PubMed] [Google Scholar]
- 75.Vega R.A., Traylor J.I., Habib A., Rhines L.D., Tatsui C.E., Rao G. minimally invasive separation surgery for metastases in the vertebral column: A technical report. Oper. Neurosurg. 2019:1–8. doi: 10.1093/ons/opz233. [DOI] [PubMed] [Google Scholar]
- 76.Zuckerman S.L., Laufer I., Sahgal A. When less is more: The indications for mis techniques and separation surgery in metastatic spine disease. Spine. 2016 doi: 10.1097/BRS.0000000000001824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cofano F., di Perna G., Monticelli M. Carbon fiber reinforced vs titanium implants for fixation in spinal metastases: A comparative clinical study about safety and effectiveness of the new “carbon-strategy”. J. Clin. Neurosci. 2020 doi: 10.1016/j.jocn.2020.03.013. [DOI] [PubMed] [Google Scholar]
- 78.Boriani S., Tedesco G., Ming L. Carbon-fiber-reinforced PEEK fixation system in the treatment of spine tumors: a preliminary report. Eur. Spine J. 2018 doi: 10.1007/s00586-017-5258-5. [DOI] [PubMed] [Google Scholar]
- 79.Tedesco G., Gasbarrini A., Bandiera S., Ghermandi R., Boriani S. Composite PEEK/Carbon fiber implants can increase the effectiveness of radiotherapy in the management of spine tumors. J. Spine Surg. 2017 doi: 10.21037/jss.2017.06.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jackson J.B., Crimaldi A.J., Peindl R., Norton H.J., Anderson W.E., Patt J.C. Effect of polyether ether ketone on therapeutic radiation to the spine. Spine. 2017 doi: 10.1097/BRS.0000000000001695. [DOI] [PubMed] [Google Scholar]
- 81.Cofano F., di Perna G., Marengo N. Transpedicular 3D endoscope-assisted thoracic corpectomy for separation surgery in spinal metastases: feasibility of the technique and preliminary results of a promising experience. Neurosurg. Rev. 2020 doi: 10.1007/s10143-019-01204-2. [DOI] [PubMed] [Google Scholar]
- 82.Cofano F., Zenga F., Mammi M. Intraoperative neurophysiological monitoring during spinal surgery: technical review in open and minimally invasive approaches. Neurosurg. Rev. 2018 doi: 10.1007/s10143-017-0939-4. [DOI] [PubMed] [Google Scholar]
- 83.Miller D.J., Lang F.F., Walsh G.L., Abi-Said D., Wildrick D.M., Gokaslan Z.L. Coaxial double-lumen methylmethacrylate reconstruction in the anterior cervical and upper thoracic spine after tumor resection. J. Neurosurg. 2000 doi: 10.3171/spi.2000.92.2.0181. [DOI] [PubMed] [Google Scholar]
- 84.Davarski A.N., Kitov B.D., Zhelyazkov C.B. Surgical management of metastatic tumors of the cervical spine. Folia Med. 2013 doi: 10.2478/folmed-2013-0026. [DOI] [PubMed] [Google Scholar]
- 85.Jandial R., Kelly B., Chen M.Y. Posterior-only approach for lumbar vertebral column resection and expandable cage reconstruction for spinal metastases clinical article. J. Neurosurg. Spine. 2013 doi: 10.3171/2013.4.SPINE12344. [DOI] [PubMed] [Google Scholar]
- 86.Barzilai O., McLaughlin L., Amato M.K. Predictors of quality of life improvement after surgery for metastatic tumors of the spine: prospective cohort study. Spine J. 2018 doi: 10.1016/j.spinee.2017.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Drakhshandeh D., Miller J.A., Fabiano A.J. Instrumented spinal stabilization without fusion for spinal metastatic disease. World Neurosurg. 2018;111:e403–e409. doi: 10.1016/j.wneu.2017.12.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Amankulor N.M., Xu R., Iorgulescu J.B. The incidence and patterns of hardware failure after separation surgery in patients with spinal metastatic tumors. Spine J. 2014 doi: 10.1016/j.spinee.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 89.Thind H., Fabiano A.J. The C7 pedicle as a superior fixation point in spinal stabilization for spinal metastatic disease. J. Spine Surg. 2018;4(1):156–161. doi: 10.21037/jss.2018.03.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang J.C., Boland P., Mitra N. Single-stage posterolateral transpedicular approach for resection of epidural metastatic spine tumors involving the vertebral body with circumferential reconstruction: results in 140 patients. J. Neurosurg. Spine. 2009 doi: 10.3171/spi.2004.1.3.0287. [DOI] [PubMed] [Google Scholar]
- 91.Miscusi M., Polli F.M., Forcato S. Comparison of minimally invasive surgery with standard open surgery for vertebral thoracic metastases causing acute myelopathy in patients with short- or mid-term life expectancy: Surgical technique and early clinical results. J. Neurosurg. Spine. 2015 doi: 10.3171/2014.10.SPINE131201. [DOI] [PubMed] [Google Scholar]
- 92.Zhou X., Cui H., He Y., Qiu G., Zhou D., Liu Y. Treatment of spinal metastases with epidural cord compression through corpectomy and reconstruction via the traditional open approach versus the mini-open approach: A multicenter retrospective study. J. Oncol. 2019;2019 doi: 10.1155/2019/7904740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Greenwood T.J., Wallace A., Friedman M.V., Hillen T.J., Robinson C.G., Jennings J.W. Combined ablation and radiation therapy of spinal metastases: A novel multimodality treatment approach. Pain Phys. 2015 [PubMed] [Google Scholar]
- 94.Vega R.A., Ghia A.J., Tatsui C.E. Percutaneous hybrid therapy for spinal metastatic disease: laser interstitial thermal therapy and spinal stereotactic radiosurgery. Neurosurg. Clin. N. Am. 2020 doi: 10.1016/j.nec.2019.11.006. [DOI] [PubMed] [Google Scholar]
- 95.Pennington Z., Ahmed A.K., Molina C.A., Ehresman J., Laufer I., Sciubba D.M. Minimally invasive versus conventional spine surgery for vertebral metastases: a systematic review of the evidence. Ann. Transl. Med. 2018 doi: 10.21037/atm.2018.01.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lau D., Chou D. Posterior thoracic corpectomy with cage reconstruction for metastatic spinal tumors: Comparing the mini-open approach to the open approach. J. Neurosurg. Spine. 2015 doi: 10.3171/2014.12.SPINE14543. [DOI] [PubMed] [Google Scholar]
- 97.Jung J.M., Chung C.K., Kim C.H., Yang S.H. Minimally invasive surgery without decompression for hepatocellular carcinoma spinal metastasis with epidural spinal cord compression grade 2. J. Kor. Neurosurg. Soc. 2019;62(4):467–475. doi: 10.3340/jkns.2018.0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Almeida Bastos D.C., Everson R.G., de Oliveira Santos B.F. A comparison of spinal laser interstitial thermotherapy with open surgery for metastatic thoracic epidural spinal cord compression. J. Neurosurg. Spine. 2020 doi: 10.3171/2019.10.spine19998. [DOI] [PubMed] [Google Scholar]
- 99.Tatsui C.E., Stafford R.J., Li J. Utilization of laser interstitial thermotherapy guided by real-time thermal MRI as an alternative to separation surgery in the management of spinal metastasis. J. Neurosurg. Spine. 2015 doi: 10.3171/2015.2.SPINE141185. [DOI] [PubMed] [Google Scholar]
- 100.Tatsui C.E., Lee S.H., Amini B. Spinal laser interstitial thermal therapy: A novel alternative to surgery for metastatic epidural spinal cord compression. Clin. Neurosurg. 2016;79(6):S73–S82. doi: 10.1227/NEU.0000000000001444. [DOI] [PubMed] [Google Scholar]
- 101.Hadzipasic M., Giantini-Larsen A.M., Tatsui C.E., Shin J.H. Emerging percutaneous ablative and radiosurgical techniques for treatment of spinal metastases. Neurosurg. Clin. N. Am. 2020;31(1):141–150. doi: 10.1016/j.nec.2019.08.017. [DOI] [PubMed] [Google Scholar]
- 102.Hikata T., Isogai N., Shiono Y. A retrospective cohort study comparing the safety and efficacy of minimally invasive versus open surgical techniques in the treatment of spinal metastases. Clin. Spine Surg. 2017 doi: 10.1097/BSD.0000000000000460. [DOI] [PubMed] [Google Scholar]
- 103.Chou D., Lu D.C. Mini-open transpedicular corpectomies with expandable cage reconstruction: Technical note. J. Neurosurg. Spine. 2011 doi: 10.3171/2010.10.SPINE091009. [DOI] [PubMed] [Google Scholar]
- 104.Turel M.K., Kerolus M.G., O’Toole J.E. Minimally invasive “separation surgery” plus adjuvsant stereotactic radiotherapy in the management of spinal epidural metastases. J. Craniovertebr. Junct. Spine. 2017 doi: 10.4103/jcvjs.JCVJS_13_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Moussazadeh N., Rubin D.G., McLaughlin L., Lis E., Bilsky M.H., Laufer I. Short-segment percutaneous pedicle screw fixation with cement augmentation for tumor-induced spinal instability. Spine J. 2015 doi: 10.1016/j.spinee.2015.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhou Z., Wang Y., Sun Z., Qian Z. Safety of cement distribution patterns in metastatic vertebral tumors: A retrospective study. Med. Sci. Monit. 2019;25:7228–7234. doi: 10.12659/MSM.918212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kelly P.D., Zuckerman S.L., Yamada Y. Image guidance in spine tumor surgery. Neurosurg. Rev. 2019 doi: 10.1007/s10143-019-01123-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vasudeva V.S., Abd-El-Barr M., Pompeu Y.A., Karhade A., Groff M.W., Lu Y. Use of intraoperative ultrasound during spinal surgery. Glob. Spine J. 2017 doi: 10.1177/2192568217700100. [DOI] [PMC free article] [PubMed] [Google Scholar]