Abstract
Overexpression of podocalyxin (PODXL) is associated with progression, metastasis, and poor outcomes in several cancers. PODXL also plays an important role in the development of normal tissues. For antibody-based therapy to target PODXL-expressing cancers using monoclonal antibodies (mAbs), cancer-specificity is necessary to reduce the risk of adverse effects to normal tissues. In this study, we developed an anti-PODXL cancer-specific mAb (CasMab), named as PcMab-60 (IgM, kappa) by immunizing mice with soluble PODXL, which is overexpressed in LN229 glioblastoma cells. The PcMab-60 reacted with the PODXL-overexpressing LN229 (LN229/PODXL) cells and MIA PaCa-2 pancreatic cancer cells in flow cytometry but did not react with normal vascular endothelial cells (VECs), whereas one of non-CasMabs, PcMab-47 showed high reactivity for not only LN229/PODXL and MIA PaCa-2 cells but also VECs, indicating that PcMab-60 is a CasMab. Next, we engineered PcMab-60 into a mouse IgG2a-type mAb, named as 60-mG2a, to add antibody-dependent cellular cytotoxicity (ADCC). We further developed a core fucose-deficient type of 60-mG2a, named as 60-mG2a-f, to augment its ADCC activity. In vivo analysis revealed that 60-mG2a-f exerted antitumor activity in MIA PaCa-2 xenograft models at a dose of 100 μg/mouse/week administered three times. These results suggested that 60-mG2a-f could be useful for antibody-based therapy against PODXL-expressing pancreatic cancers.
Keywords: Podocalyxin, PODXL, Monoclonal antibody, Cancer-specific mAb, Pancreatic cancer
Abbreviations: ADCC, antibody-dependent cellular cytotoxicity; BSA, bovine serum albumin; CasMab, cancer-specific monoclonal antibody; CBIS, Cell-Based Immunization and Screening; CDC, complement-dependent cytotoxicity; PBS, phosphate-buffered saline; PODXL, podocalyxin
Highlights
-
•
PODXL is associated with poor outcomes in several cancers.
-
•
We developed an anti-PODXL cancer-specific mAb (PcMab-60).
-
•
A core fucose-deficient IgG2a type of PcMab-60 (60-mG2a-f) exerted antitumor activity in MIA PaCa-2 xenograft models.
-
•
60-mG2a-f could be useful for antibody-based therapy against PODXL-expressing pancreatic cancers.
1. Introduction
Podocalyxin (PODXL), also known as TRA-1-60 and TRA-1-81 antigens, is a highly N- or O-glycosylated type I transmembrane protein with a molecular weight of 150,000–200,000 [[1], [2], [3], [4]], and is used as pluripotent stem cell markers [3,[5], [6], [7], [8], [9]]. PODXL plays an important biological role in the kidney, heart, pancreatic, and breast tissues [10], and is also a prognostic indicator and a diagnostic marker for several malignant tumors such oral cancer [11], brain tumors [4], colorectal cancer [12], and renal cancer [13]. Because PODXL has been suggested to promote tumor growth, invasion, and metastasis [14,15], high PODXL expression could have adverse effects on overall survival (OS), disease-specific survival (DSS), and disease-free survival (DFS) in several cancers.
Despite the development of anti-PODXL monoclonal antibodies (mAbs), the efficacy of these treatments against cancers remains to be fully explained [5,16]. We previously immunized mice with recombinant soluble PODXL, which was purified from the culture supernatant of LN229/ectodomain-PODXL cells, and developed PcMab-47 of mouse IgG1 subclass [17]. We then produced a mouse-human chimeric mAb (chPcMab-47) and demonstrated its antitumor activity against colorectal cancers [18]. Furthermore, we established a chimeric anti-PODXL mAb (47-mG2a) by combining the variable region of PcMab-47 of mouse IgG1 and the constant region of mouse IgG2a [19]. Moreover, we produced 47-mG2a-f, a core fucose-deficient 47-mG2a to augment antibody-dependent cellular cytotoxicity (ADCC), and demonstrated high antitumor activity of 47-mG2a-f against oral cancers [19]. Although PcMab-47 and its modified mAbs are very useful for detecting PODXL in immunohistochemistry, they reacted with both cancer cells and normal cells, including vascular endothelial cells [[19], [20], [21], [22]]. Therefore, we could not transfer PcMab-47 into clinical use because its chimeric mAb or humanized mAb might show severe adverse effects.
For antibody-based therapy to target PODXL-expressing cancers, cancer-specificity is necessary to reduce the risk of adverse effects to normal tissues. In this study, we developed a cancer-specific anti-PODXL mAb, PcMab-60 (IgM, kappa) by immunizing mice with soluble PODXL. We engineered PcMab-60 into a mouse IgG2a-type mAb (60-mG2a) to add ADCC, and further produced a core fucose-deficient type of 60-mG2a (60-mG2a-f) to augment its ADCC activity. We then examined the antitumor activity of 60-mG2a-f against a mouse xenograft of pancreatic cancer.
2. Materials and methods
2.1. Cell lines
The MIA PaCa-2 was obtained from the Cell Resource Center for the Biomedical Research Institute of Development, Aging and Cancer Tohoku University (Miyagi, Japan). P3U1 (mouse myeloma cell line) and LN229 (glioblastoma cell line) were obtained from the American Type Culture Collection (Manassas, VA). In our previous study (17), the LN229/PODXL and LN229/ectodomain-PODXL were produced. LN229, LN229/PODXL, LN229/ectodomain-PODXL, and MIA PaCa-2 were cultured in DMEM medium (Nacalai Tesque, Inc., Kyoto, Japan) and P3U1 was cultured in RPMI1640 medium (Nacalai Tesque, Inc.). The media were supplemented with 10% heat-inactivated fetal bovine serum (FBS; Thermo Fisher Scientific Inc., Waltham, MA), 100 units/ml of penicillin, 100 μg/mL of streptomycin, and 0.25 μg/mL of amphotericin B (Nacalai Tesque, Inc.). The Vascular endothelial cells-1 and Vascular endothelial cells-2 were purchased from Cambrex Corp. (Walkersville, MD), and were cultured in endothelial cell medium EGM-2MV, supplemented with 5% FBS (Cambrex Corp.). All cells were cultured at 37 °C in a humidified atmosphere containing 5% CO2 and 95% air.
2.2. Animals
All animal experiments were performed in accordance with relevant guidelines and regulations to minimize animal suffering and distress in the laboratory. The Animal Care and Use Committee of Tohoku University approved all the animal experiments for hybridoma production. Animal experiments for antitumor activity were approved by the Institutional Committee for Experiments of the Institute of Microbial Chemistry. Mice were maintained in a pathogen-free environment (23 ± 2 °C, 55 ± 5% humidity) on 11 h light/13 h dark cycle with food and water supplied ad libitum across the experimental period. Mice were monitored for health and weight every 2 or 5 days during the 3-week period of each experiment. The loss of original body weight to a point >25% and/or a maximum tumor size >3000 mm3 were identified as humane endpoints for euthanasia. Mice were euthanized by cervical dislocation; death was verified by respiratory and cardiac arrest.
2.3. Hybridoma production
We immunized four-week-old female BALB/c mice (CLEA, Tokyo, Japan) with the purified ectodomain of human PODXL (100 μg) together with Imject Alum (Thermo Fisher Scientific Inc.) by intraperitoneal (i.p.) injection. After several additional immunizations, a booster i.p. injection of LN229/PODXL was given 2 days before the mice were euthanized by cervical dislocation, and spleen cells were harvested. The spleen cells were fused with P3U1 cells using PEG1500 (Roche Diagnostics, Indianapolis, IN). Hybridomas were grown in RPMI 1640 medium including L-glutamine with hypoxanthine, aminopterin, and thymidine selection medium supplement (Thermo Fisher Scientific Inc.). Culture supernatants were screened using enzyme-linked immunosorbent assay (ELISA) for binding to the purified ectodomain of PODXL.
2.4. ELISA
Proteins were immobilized on Nunc Maxisorp 96-well immuno plates (Thermo Fisher Scientific, Inc.) at 1 μg/mL for 30 min. After blocking with 1% bovine-serum albumin (BSA) in 0.05% Tween 20/phosphate buffered saline (PBS, Nacalai Tesque, Inc.), the plates were incubated with culture supernatant followed by 1:2000 diluted peroxidase-conjugated anti-mouse immunoglobulins (Agilent Technologies, Inc., Santa Clara, CA). The enzymatic reaction was produced with a 1-Step Ultra TMB-ELISA (Thermo Fisher Scientific, Inc.). The optical density was measured at 655 nm using an iMark microplate reader (Bio-Rad Laboratories, Inc., Berkeley, CA).
2.5. Antibodies
PcMab-47, a mouse anti-PODXL mAb (IgG1, kappa), was developed as previously described [17]. The mouse IgG was purchased from Sigma-Aldrich Corp. (St. Louis, MO). To generate 60-mG2a, appropriate VH cDNA of PcMab-60 and CH of mouse IgG2a were subcloned into pCAG-Ble vector (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan), and VL and CL cDNAs of PcMab-60 were subcloned into pCAG-Neo vector (FUJIFILM Wako Pure Chemical Corporation). To generate 60-mG2a, antibody expression vectors were transfected into ExpiCHO-S cells using the ExpiCHO Expression System (Thermo Fisher Scientific). To generate 60-mG2a-f, antibody expression vectors were also transfected into BINDS-09 (FUT8-knocked out ExpiCHO-S cells) using the ExpiCHO Expression System [23]. PcMab-60, 60-mG2a, and 60-mG2a-f were purified using Protein G-Sepharose (GE Healthcare Bio-Sciences, Pittsburgh, PA).
2.6. Flow cytometry
Cell lines were harvested via a brief exposure to 0.25% trypsin/1 mM ethylenediaminetetraacetic acid (EDTA; Nacalai Tesque, Inc.). After washing with 0.1% BSA in PBS, the cells were treated with 10 μg/mL of primary mAbs for 30 min at 4 °C, followed by treatment with Alexa Fluor 488-conjugated anti-mouse IgG (1:1000; Cell Signaling Technology, Danvers, MA). Fluorescence data were collected using an EC800 Cell Analyzer (Sony Corp., Tokyo, Japan).
2.7. Determination of binding affinity using flow cytometry
The MIA PaCa-2 cells (2 × 105) were resuspended in 100 μL of serially diluted PcMab-60 and 60-mG2a-f (6 ng/mL to 100 μg/mL), followed by the addition of Alexa Fluor 488-conjugated anti-mouse IgG (1:200; Cell Signaling Technology). Fluorescence data were collected using a cell analyzer (EC800). The dissociation constant KD was obtained by fitting the binding isotherms using the built-in one-site binding models in GraphPad PRISM 6 (GraphPad Software, La Jolla, CA).
2.8. Antitumor activity of anti-PODXL antibodies
Five-week-old female BALB/c nude mice were purchased from Charles River and used in experiments at 7 weeks of age. The cells (0.3 mL of 1.33 × 108/mL in DMEM) were mixed with 0.5 mL of BD Matrigel Matrix Growth Factor Reduced (BD Biosciences, San Jose, CA). A 100-μL suspension (containing 5 × 106 cells) was injected subcutaneously into the right flanks of nude mice. After 1 day, 100 μg of 60-mG2a-f or mouse IgG in 100 μL PBS were injected into the peritoneal cavity of each mouse. Additional antibodies were injected at days 8 and 15. The mice were euthanized 22 days after cell implantation. All data were expressed as the mean ± SEM. Statistical analysis was performed using the Tukey-Kramer test. P < 0.05 was considered statistically significant.
3. Results
3.1. Development of anti-PODXL mAbs
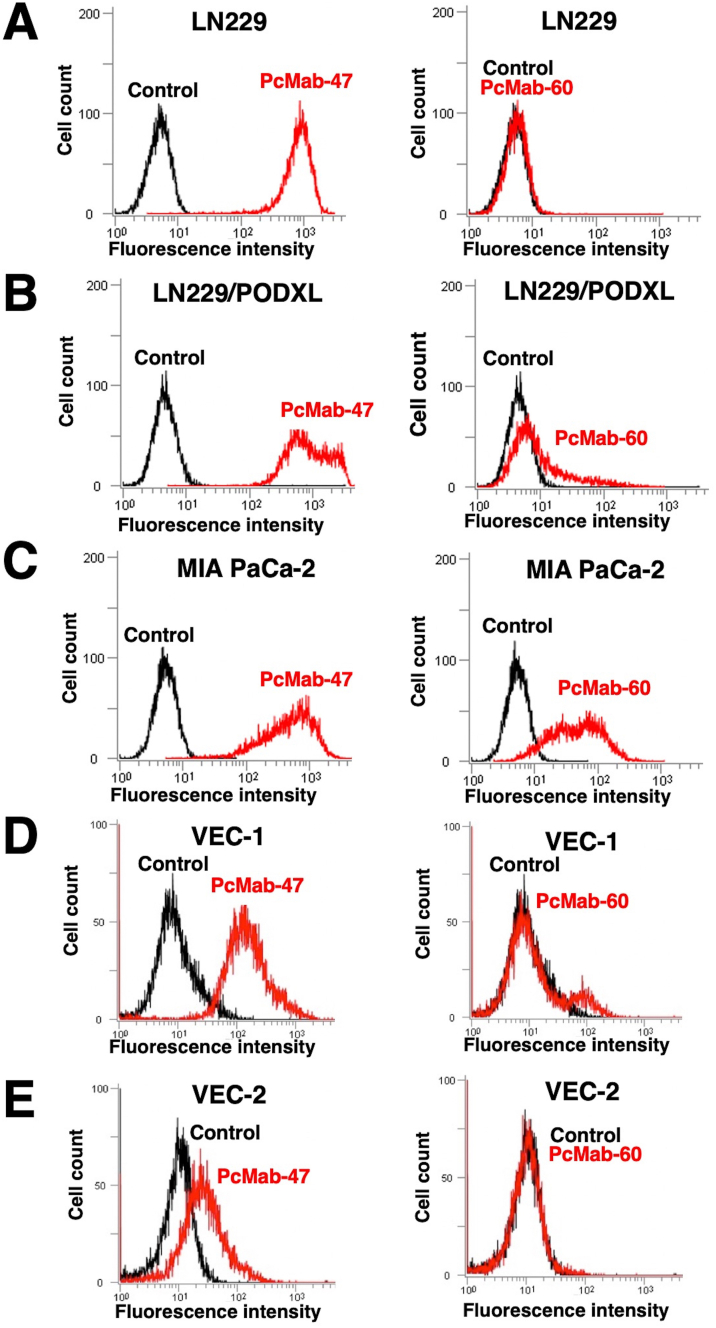
First, we immunized mice with recombinant PODXL, which was purified from the culture supernatant of LN229/ectodomain-PODXL. A booster i.p. injection of LN229/PODXL was administered. The culture supernatants were screened using ELISA for binding to purified PODXL. As a second screening, we performed flow cytometry for reaction with LN229 and LN229/PODXL, and a stronger reaction against LN229/PODXL than against LN229 was necessary. As a positive control, we used PcMab-47 [17], which strongly reacted with endogenous PODXL of LN229 glioblastoma cell line (Fig. 1A, left). The reaction of PcMab-47 to LN229/PODXL was slightly higher than that to LN229 (Fig. 1B, left). In this study, we developed a novel anti-PODXL mAb, PcMab-60 (IgM, kappa), which did not react with LN229 (Fig. 1A, right), but weakly reacted with LN229/PODXL (Fig. 1B, right). In contrast, PcMab-60 showed a higher reaction with MIA PaCa-2 pancreatic cancer cell line (Fig. 1C, right), although PcMab-47 reaction to MIA PaCa-2 is weaker than that of LN229 (Fig. 1C, left), indicating that PcMab-60 might be specific to pancreatic cancers. Because PODXL is highly expressed in vascular endothelial cells, PcMab-47 stained PODXL of two vascular endothelial cells (VEC-1 and VEC-2) as shown in Fig. 1D (left) and Fig. 1E (left). In contrast, PcMab-60 did not react with VEC-1 (Fig. 1D, right) and VEC-2 (Fig. 1E, right), indicating that PcMab-60 is cancer-specific.
Fig. 1.
Flow cytometry against cancer cells and normal cells using anti-PODXL mAbs (PcMab-47 and PcMab-60). LN229 (A), LN229/PODXL (B), MIA PaCa-2 (C), VEC-1 (D), and VEC-2 (E) cells were treated with PcMab-47 (10 μg/mL), PcMab-60 (10 μg/mL), or control, followed by secondary antibodies. Vascular endothelial cells, VEC.
3.2. Production of the mouse IgG2a-type antibody PcMab-60
Because the mouse IgG2a possesses high ADCC and complement-dependent cytotoxicity (CDC) activities [24], we next developed IgG2a-type of PcMab-60, which was named as 60-mG2a. To generate 60-mG2a, appropriate VH cDNA of PcMab-60 and CH of mouse IgG2a were subcloned into pCAG-Ble vector, and VL and CL cDNAs of PcMab-60 were subcloned into pCAG-Neo vector. Both vectors were transfected into ExpiCHO-S cells, and 60-mG2a was purified from the supernatant. We further produced a core-fucose-deficient type of 60-mG2a, which was named as 60-mG2a-f using the BINDS-09 cell line (FUT8-knockout Expi–CHO–S cell line). Next, the reactivity of 60-mG2a and 60-mG2a-f was confirmed by flow cytometry using MIA PaCa-2 cells. As shown in Fig. 2, PcMab-60, 60-mG2a, and 60-mG2a-f reacted strongly with MIA PaCa-2 cells.
Fig. 2.
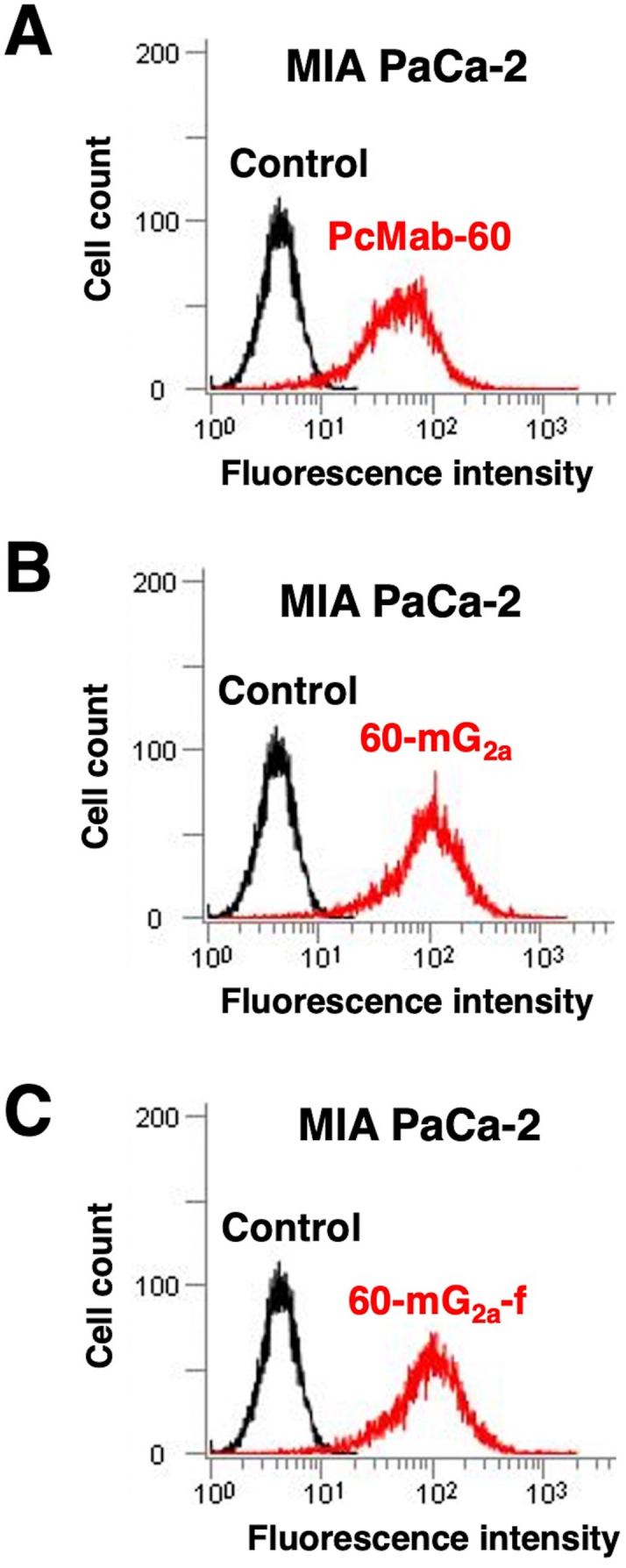
Flow cytometry against MIA PaCa-2 cells using anti-PODXL mAbs (PcMab-60, 60-mG2a, and 60-mG2a-f). MIA PaCa-2 cells were treated with PcMab-60 (10 μg/mL), 60-mG2a (10 μg/mL), 60-mG2a-f (10 μg/mL), or control, followed by secondary antibodies.
3.3. The binding affinity of anti-PODXL mAbs
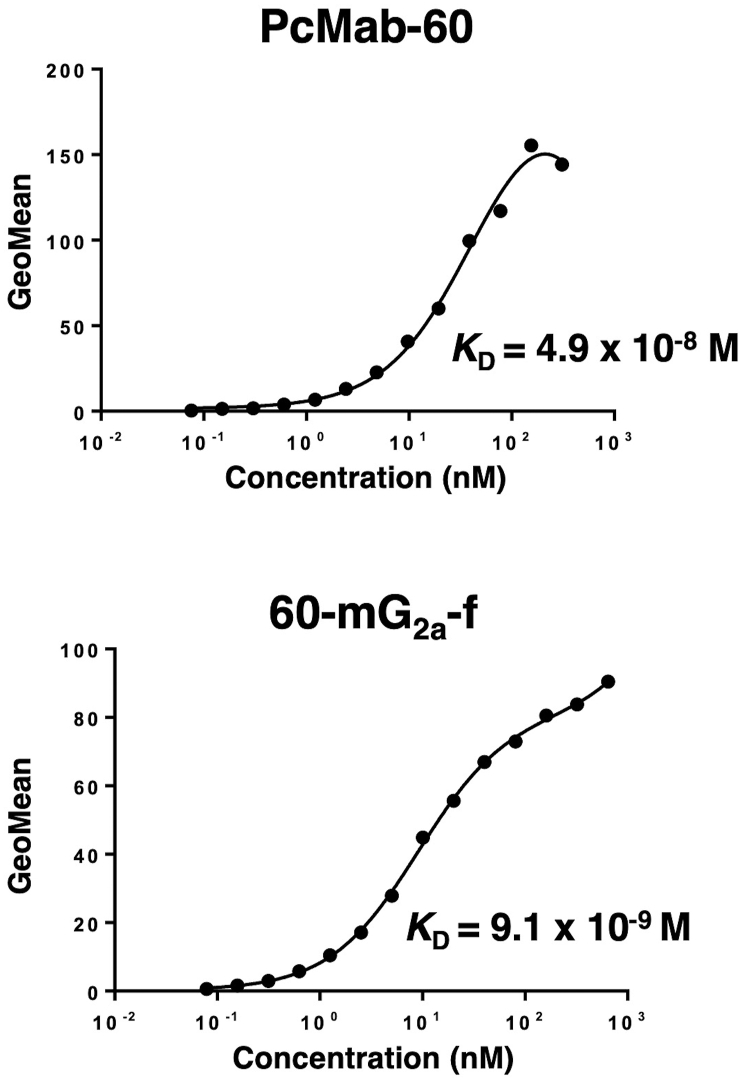
We performed a kinetic analysis of the interactions of PcMab-60 and 60-mG2a-f with MIA PaCa-2 cells using flow cytometry. As shown in Fig. 3, the dissociation constant (KD) for PcMab-60 against MIA PaCa-2 was 4.9 × 10-8 M. In contrast, the KD for 60-mG2a-f was 9.1 × 10−9 M. The binding affinity of 60-mG2a-f against MIA PaCa-2 was 5.4-fold higher than that of PcMab-60. These results are compatible with our previous observations that all the chimeric antibodies, including chPcMab-47, 47-mG2a, and 47-mG2a-f, also possess a higher affinity for PODXL than the original PcMab-47 [19].
Fig. 3.
Determination of binding affinity of anti-PODXL antibodies (PcMab-60 and 60-mG2a-f) using flow cytometry. MIA PaCa-2 cells were suspended in 100 μL of serially diluted antibodies (6 ng/mL to 100 μg/mL), and secondary antibodies were then added. Fluorescence data were collected using a cell analyzer. GeoMean, the geometric mean of fluorescence intensity.
3.4. Antitumor activity of 60-mG2a-f against MIA PaCa-2 xenografts
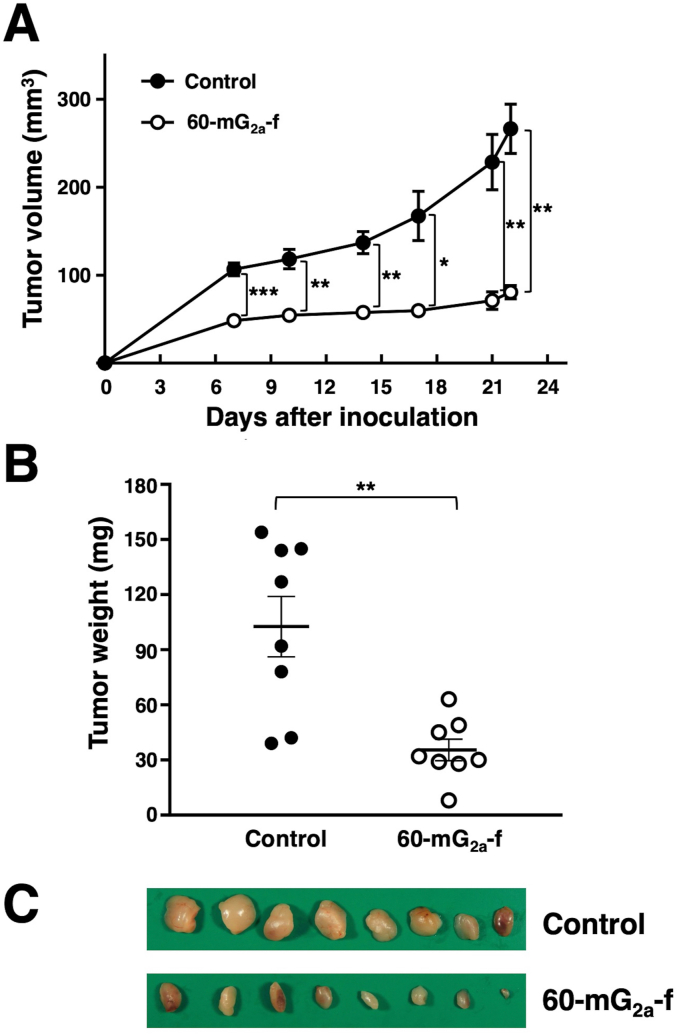
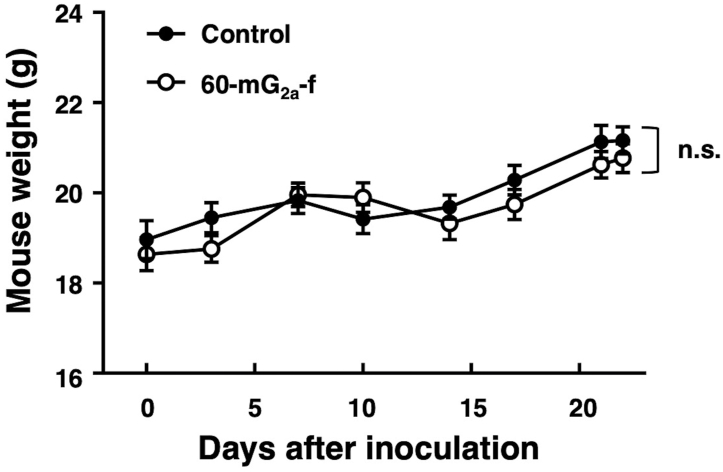
To investigate the antitumor effects of 60-mG2a-f on primary tumor growth in vivo, the MIA PaCa-2 cells were subcutaneously implanted into the flanks of nude mice. The 60-mG2a-f and the mouse IgG (control) were injected three times (100 μg of the antibodies on days 1, 8, and 15 after cell injections) into the peritoneal cavity of mice. Tumor formation was observed in all groups. The 60-mG2a-f significantly reduced tumor development compared with mouse IgG on days 7, 10, 14, 17, 21, and 22 (Fig. 4A). The tumor weights of mice in the 60-mG2a-f group were significantly lower than that in the IgG group on day 22 (Fig. 4B) The resected tumors of MIA PaCa-2 xenografts are depicted in Fig. 4C. Body weight was not significantly different between mouse IgG and 60-mG2a-f groups (Fig. 5).
Fig. 4.
Antitumor activity of 60-mG2a-f against MIA PaCa-2 xenograft. (A) Tumor volume of MIA PaCa-2 xenografts. MIA PaCa-2 cells were injected subcutaneously into female nude mice into the 60-mG2a-f group (n = 8) and the control IgG group (n = 8). The indicated antibodies (100 μg/day; 5 mg/kg) were administered intraperitoneally 1, 8, and 15 days after cancer cell inoculation. The tumor volume was measured at the indicated time points. The values are means ± SEM. (B) Tumor weight of MIA PaCa-2 xenografts (day 22). The values are means ± SEM. An asterisk indicates statistical significance (*P < 0.05, **P < 0.01, ***P < 0.005, Tukey-Kramer's test). (C) Resected tumors of MIA PaCa-2 xenografts (day 22).
Fig. 5.
Body weights of mice with MIA PaCa-2 xenografts. MIA PaCa-2 cells were injected subcutaneously into female nude mice into the 60-mG2a-f group (n = 8) and the control IgG group (n = 8). The indicated antibodies (100 μg/day; 5 mg/kg) were administered intraperitoneally 1, 8, and 15 days after cancer cell inoculation. Body weights of mice with MIA PaCa-2 xenografts were measured at the indicated time points. The values are means ± SEM. n.s., not significant by Tukey-Kramer's test.
4. Discussion
In our previous study, we developed the original technology for the production of cancer-specific monoclonal antibodies (CasMabs) against membrane proteins [[25], [26], [27]]. We have successfully produced anti-podoplanin CasMabs, such as LpMab-2 [25] and LpMab-23 [28] that specifically recognize cancer-type podoplanin in tumor tissues and not in normal-type podoplanin, which is expressed in lymphatic vessels. Moreover, the CasMab technology is useful for generating anti-glycopeptide mAbs (GpMabs). We have produced the following anti-podoplanin GpMabs: LpMab-3, LpMab-9, LpMab-12, LpMab-19, and LpMab-21 [[29], [30], [31]]. The other original technology for the production of sensitive and specific mAbs is the Cell-Based Immunization and Screening (CBIS) method [32]. We have developed several anti-podoplanin mAbs against pig [33], Tasmanian devil [34], alpaca [35], tiger [36], whale [37], goat [38], horse [39], and bear [40] PDPNs using the CBIS method.
In this study, we employed the CasMab technology, and successfully produced an anti-PODXL CasMab, PcMab-60, which highly reacts with MIA PaCa-2 pancreatic cell line, but did not react with PODXL-expressing vascular endothelial cells (Fig. 1). Although PcMab-60 was determined to be IgM, which usually shows low binding affinity, it showed moderate affinity (KD: 4.9 × 10-8 M) against MIA PaCa-2 cells (Fig. 3). Furthermore, 60-mG2a-f showed a higher binding affinity (KD: 9.1 × 10-9 M) compared with its original PcMab-60 (Fig. 3), indicating derivatives of PcMab-60, such as humanized mAbs of PcMab-60, could be useful for antibody-based therapy for pancreatic cancers. 60-mG2a-f and 60-mG2a showed no difference in the binding affinity (data not shown). We have sometimes experienced that chimeric antibodies possess much higher affinity or lower affinity when compared with original mAbs [18,[41], [42], [43], [44], [45], [46]]. The stability of antibodies might be different among constant regions. We produced 60-mG2a-f, a non-fucosylated version of 60-mG2a to augment its ADCC activities because non-fucosylated antibodies are known to show higher ADCC activities [47,48]. As expected, 60-mG2a-f exhibited high antitumor activities in a MIA PaCa-2 xenograft model (Fig. 4). In the future study, we need to combine PcMab-60 with anti-cancer drugs or include them in novel antitumor regimens, including T cells and viruses, to exert antitumor activity against cancer cells.
High PODXL expression was significantly associated with worse OS and was predictive of shorter OS in multiple cancers, especially pancreatic cancers [49]. It was also revealed that high PODXL expression predicted worse DSS and DFS. These results suggest that PODXL could be a prognostic factor, and diagnostic tools targeting this protein are expected. Unfortunately, we herein could not analyze PODXL expression in pancreatic cancer tissues because PcMab-60 was not useful for immunohistochemical analysis. In contrast, our previously established PcMab-47 was advantageous for immunohistochemical analysis [20]. The PODXL expression in pancreatic cancer tissues could be analyzed using PcMab-47, and then PODXL-positive patients should be treated using cancer-specific humanized PcMab-60.
Declaration of competing interest
The authors declare no conflicts of interest involving this article.
Acknowledgments
This research was supported in part by AMED under Grant Numbers: JP20am0401013 (Y.K.), JP20am0101078 (Y.K.), JP20ae0101028 (Y.K.), and JP20bm1004001 (Y.K.), and by JSPS KAKENHI Grant Numbers: 17K07299 (M.K.K.) and 19K07705 (Y.K.).
References
- 1.McNagny K.M., Pettersson I., Rossi F., Flamme I., Shevchenko A., Mann M., Graf T. Thrombomucin, a novel cell surface protein that defines thrombocytes and multipotent hematopoietic progenitors. J. Cell Biol. 1997;138:1395–1407. doi: 10.1083/jcb.138.6.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schopperle W.M., Kershaw D.B., DeWolf W.C. Human embryonal carcinoma tumor antigen, Gp200/GCTM-2, is podocalyxin. Biochem. Biophys. Res. Commun. 2003;300:285–290. doi: 10.1016/s0006-291x(02)02844-9. [DOI] [PubMed] [Google Scholar]
- 3.Schopperle W.M., DeWolf W.C. The TRA-1-60 and TRA-1-81 human pluripotent stem cell markers are expressed on podocalyxin in embryonal carcinoma. Stem Cell. 2007;25:723–730. doi: 10.1634/stemcells.2005-0597. [DOI] [PubMed] [Google Scholar]
- 4.Hayatsu N., Kaneko M.K., Mishima K., Nishikawa R., Matsutani M., Price J.E., Kato Y. Podocalyxin expression in malignant astrocytic tumors. Biochem. Biophys. Res. Commun. 2008;374:394–398. doi: 10.1016/j.bbrc.2008.07.049. [DOI] [PubMed] [Google Scholar]
- 5.Toyoda H., Nagai Y., Kojima A., Kinoshita-Toyoda A. Podocalyxin as a major pluripotent marker and novel keratan sulfate proteoglycan in human embryonic and induced pluripotent stem cells. Glycoconj. J. 2017;34:139–145. doi: 10.1007/s10719-016-9757-0. [DOI] [PubMed] [Google Scholar]
- 6.Richards M., Tan S.P., Tan J.H., Chan W.K., Bongso A. The transcriptome profile of human embryonic stem cells as defined by SAGE. Stem Cell. 2004;22:51–64. doi: 10.1634/stemcells.22-1-51. [DOI] [PubMed] [Google Scholar]
- 7.Bhattacharya B., Miura T., Brandenberger R., Mejido J., Luo Y., Yang A.X., Joshi B.H., Ginis I., Thies R.S., Amit M., Lyons I., Condie B.G., Itskovitz-Eldor J., Rao M.S., Puri R.K. Gene expression in human embryonic stem cell lines: unique molecular signature. Blood. 2004;103:2956–2964. doi: 10.1182/blood-2003-09-3314. [DOI] [PubMed] [Google Scholar]
- 8.Zeng X., Miura T., Luo Y., Bhattacharya B., Condie B., Chen J., Ginis I., Lyons I., Mejido J., Puri R.K., Rao M.S., Freed W.J. Properties of pluripotent human embryonic stem cells BG01 and BG02. Stem Cell. 2004;22:292–312. doi: 10.1634/stemcells.22-3-292. [DOI] [PubMed] [Google Scholar]
- 9.Hayman M.W., Przyborski S.A. Proteomic identification of biomarkers expressed by human pluripotent stem cells. Biochem. Biophys. Res. Commun. 2004;316:918–923. doi: 10.1016/j.bbrc.2004.02.141. [DOI] [PubMed] [Google Scholar]
- 10.Doyonnas R., Kershaw D.B., Duhme C., Merkens H., Chelliah S., Graf T., McNagny K.M. Anuria, omphalocele, and perinatal lethality in mice lacking the CD34-related protein podocalyxin. J. Exp. Med. 2001;194:13–27. doi: 10.1084/jem.194.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin C.W., Sun M.S., Wu H.C. Podocalyxin-like 1 is associated with tumor aggressiveness and metastatic gene expression in human oral squamous cell carcinoma. Int. J. Oncol. 2014;45:710–718. doi: 10.3892/ijo.2014.2427. [DOI] [PubMed] [Google Scholar]
- 12.Larsson A., Johansson M.E., Wangefjord S., Gaber A., Nodin B., Kucharzewska P., Welinder C., Belting M., Eberhard J., Johnsson A., Uhlen M., Jirstrom K. Overexpression of podocalyxin-like protein is an independent factor of poor prognosis in colorectal cancer. Br. J. Canc. 2011;105:666–672. doi: 10.1038/bjc.2011.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsu Y.H., Lin W.L., Hou Y.T., Pu Y.S., Shun C.T., Chen C.L., Wu Y.Y., Chen J.Y., Chen T.H., Jou T.S. Podocalyxin EBP50 ezrin molecular complex enhances the metastatic potential of renal cell carcinoma through recruiting Rac 1 guanine nucleotide exchange factor ARHGEF7. Am. J. Pathol. 2010;176:3050–3061. doi: 10.2353/ajpath.2010.090539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flores-Tellez T.N., Lopez T.V., Vasquez Garzon V.R., Villa-Trevino S. Co-expression of ezrin-CLIC5-podocalyxin is associated with migration and invasiveness in hepatocellular carcinoma. PloS One. 2015;10 doi: 10.1371/journal.pone.0131605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Snyder K.A., Hughes M.R., Hedberg B., Brandon J., Hernaez D.C., Bergqvist P., Cruz F., Po K., Graves M.L., Turvey M.E., Nielsen J.S., Wilkins J.A., McColl S.R., Babcook J.S., Roskelley C.D., McNagny K.M. Podocalyxin enhances breast tumor growth and metastasis and is a target for monoclonal antibody therapy. Breast Cancer Res. 2015;17:46. doi: 10.1186/s13058-015-0562-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang L., Yao C., Khodadadi-Jamayran A., Xu W., Zhang R., Banerjee N.S., Chang C.W., Chow L.T., Townes T., Hu K. The universal 3D3 antibody of human PODXL is pluripotent cytotoxic, and identifies a residual population after extended differentiation of pluripotent stem cells. Stem Cell. Dev. 2016;25:556–568. doi: 10.1089/scd.2015.0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ogasawara S., Kaneko M.K., Yamada S., Honma R., Nakamura T., Saidoh N., Yanaka M., Yoshida K., Fujii Y., Kato Y. PcMab-47: novel antihuman podocalyxin monoclonal antibody for immunohistochemistry. Monoclon. Antibodies Immunodiagn. Immunother. 2017;36:50–56. doi: 10.1089/mab.2017.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaneko M.K., Kunita A., Yamada S., Nakamura T., Yanaka M., Saidoh N., Chang Y.W., Handa S., Ogasawara S., Ohishi T., Abe S., Itai S., Harada H., Kawada M., Nishioka Y., Fukayama M., Kato Y. Antipodocalyxin antibody chPcMab-47 exerts antitumor activity in mouse xenograft models of colorectal adenocarcinomas. Monoclon. Antibodies Immunodiagn. Immunother. 2017;36:157–162. doi: 10.1089/mab.2017.0020. [DOI] [PubMed] [Google Scholar]
- 19.Itai S., Ohishi T., Kaneko M.K., Yamada S., Abe S., Nishioka Y., Kawada M., Harada H., Kato Y. Anti-podocalyxin antibody exerts antitumor effects via antibody-dependent cellular cytotoxicity in mouse xenograft models of oral squamous cell carcinoma. Oncotarget. 2018;9:22480–22497. doi: 10.18632/oncotarget.25132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Itai S., Yamada S., Kaneko M.K., Harada H., Kato Y. Immunohistochemical analysis using antipodocalyxin monoclonal antibody PcMab-47 demonstrates podocalyxin expression in oral squamous cell carcinomas. Monoclon. Antibodies Immunodiagn. Immunother. 2017;36:220–223. doi: 10.1089/mab.2017.0030. [DOI] [PubMed] [Google Scholar]
- 21.Yamada S., Itai S., Kaneko M.K., Kato Y. Anti-podocalyxin monoclonal antibody 47-mG2a detects lung cancers by immunohistochemistry. Monoclon. Antibodies Immunodiagn. Immunother. 2018;37:91–94. doi: 10.1089/mab.2018.0002. [DOI] [PubMed] [Google Scholar]
- 22.Kaneko M.K., Itai S., Yamada S., Kato Y. 47-mG2a: a mouse IgG2a-type of PcMab-47 useful for detecting podocalyxin in esophageal cancers by immunohistochemistry. Monoclon. Antibodies Immunodiagn. Immunother. 2018;37:158–161. doi: 10.1089/mab.2018.0003. [DOI] [PubMed] [Google Scholar]
- 23.Kato Y., Mizuno T., Yamada S., Nakamura T., Itai S., Yanaka M., Sano M., Kaneko M.K. Establishment of P38Bf, a core-fucose-deficient mouse-canine chimeric antibody against dog podoplanin. Monoclon. Antibodies Immunodiagn. Immunother. 2018;37:218–223. doi: 10.1089/mab.2018.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kato Y., Kunita A., Fukayama M., Abe S., Nishioka Y., Uchida H., Tahara H., Yamada S., Yanaka M., Nakamura T., Saidoh N., Yoshida K., Fujii Y., Honma R., Takagi M., Ogasawara S., Murata T., Kaneko M.K. Antiglycopeptide mouse monoclonal antibody LpMab-21 exerts antitumor activity against human podoplanin through antibody-dependent cellular cytotoxicity and complement-dependent cytotoxicity. Monoclon. Antibodies Immunodiagn. Immunother. 2017;36:20–24. doi: 10.1089/mab.2016.0045. [DOI] [PubMed] [Google Scholar]
- 25.Kato Y., Kaneko M.K. A cancer-specific monoclonal antibody recognizes the aberrantly glycosylated podoplanin. Sci. Rep. 2014;4:5924. doi: 10.1038/srep05924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Itai S., Fujii Y., Kaneko M.K., Yamada S., Nakamura T., Yanaka M., Saidoh N., Chang Y.W., Handa S., Takahashi M., Suzuki H., Harada H., Kato Y. H2Mab-77 is a sensitive and specific anti-HER2 monoclonal antibody against breast cancer. Monoclon. Antibodies Immunodiagn. Immunother. 2017;36:143–148. doi: 10.1089/mab.2017.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Itai S., Kaneko M.K., Fujii Y., Yamada S., Nakamura T., Yanaka M., Saidoh N., Handa S., Chang Y.W., Suzuki H., Harada H., Kato Y. Development of EMab-51, a sensitive and specific anti-EGFR monoclonal antibody in Western blot and immunohistochemistry. Monoclon. Antibodies Immunodiagn. Immunother. 2017;36:214–219. doi: 10.1089/mab.2017.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamada S., Ogasawara S., Kaneko M.K., Kato Y. LpMab-23: a cancer-specific monoclonal antibody against human podoplanin. Monoclon. Antibodies Immunodiagn. Immunother. 2017;36:72–76. doi: 10.1089/mab.2017.0001. [DOI] [PubMed] [Google Scholar]
- 29.Oki H., Ogasawara S., Kaneko M.K., Takagi M., Yamauchi M., Kato Y. Characterization of monoclonal antibody LpMab-3 recognizing sialylated glycopeptide of podoplanin. Monoclon. Antibodies Immunodiagn. Immunother. 2015;34:44–50. doi: 10.1089/mab.2014.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaneko M.K., Oki H., Hozumi Y., Liu X., Ogasawara S., Takagi M., Goto K., Kato Y. Monoclonal antibody LpMab-9 recognizes O-glycosylated N-terminus of human podoplanin. Monoclon. Antibodies Immunodiagn. Immunother. 2015;34:310–317. doi: 10.1089/mab.2015.0022. [DOI] [PubMed] [Google Scholar]
- 31.Kato Y., Ogasawara S., Oki H., Goichberg P., Honma R., Fujii Y., Kaneko M.K. LpMab-12 established by CasMab technology specifically detects sialylated O-glycan on Thr52 of platelet aggregation-stimulating domain of human podoplanin. PloS One. 2016;11 doi: 10.1371/journal.pone.0152912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Itai S., Fujii Y., Nakamura T., Chang Y.W., Yanaka M., Saidoh N., Handa S., Suzuki H., Harada H., Yamada S., Kaneko M.K., Kato Y. Establishment of CMab-43, a sensitive and specific anti-CD133 monoclonal antibody, for immunohistochemistry. Monoclon. Antibodies Immunodiagn. Immunother. 2017;36:231–235. doi: 10.1089/mab.2017.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kato Y., Yamada S., Furusawa Y., Itai S., Nakamura T., Yanaka M., Sano M., Harada H., Fukui M., Kaneko M.K. PMab-213: a monoclonal antibody for immunohistochemical analysis against pig podoplanin. Monoclon. Antibodies Immunodiagn. Immunother. 2019;38:18–24. doi: 10.1089/mab.2018.0048. [DOI] [PubMed] [Google Scholar]
- 34.Furusawa Y., Yamada S., Itai S., Nakamura T., Takei J., Sano M., Harada H., Fukui M., Kaneko M.K., Kato Y. Establishment of a monoclonal antibody PMab-233 for immunohistochemical analysis against Tasmanian devil podoplanin. Biochem Biophys Rep. 2019;18:100631. doi: 10.1016/j.bbrep.2019.100631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kato Y., Furusawa Y., Yamada S., Itai S., Takei J., Sano M., Kaneko M.K. Establishment of a monoclonal antibody PMab-225 against alpaca podoplanin for immunohistochemical analyses. Biochem Biophys Rep. 2019;18:100633. doi: 10.1016/j.bbrep.2019.100633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Furusawa Y., Kaneko M.K., Nakamura T., Itai S., Fukui M., Harada H., Yamada S., Kato Y. Establishment of a monoclonal antibody PMab-231 for tiger podoplanin. Monoclon. Antibodies Immunodiagn. Immunother. 2019;38:89–95. doi: 10.1089/mab.2019.0003. [DOI] [PubMed] [Google Scholar]
- 37.Kato Y., Furusawa Y., Itai S., Takei J., Nakamura T., Sano M., Harada H., Yamada S., Kaneko M.K. Establishment of an anticetacean podoplanin monoclonal antibody PMab-237 for immunohistochemical analysis. Monoclon. Antibodies Immunodiagn. Immunother. 2019;38:108–113. doi: 10.1089/mab.2019.0013. [DOI] [PubMed] [Google Scholar]
- 38.Furusawa Y., Yamada S., Nakamura T., Sano M., Sayama Y., Itai S., Takei J., Harada H., Fukui M., Kaneko M.K., Kato Y. PMab-235: a monoclonal antibody for immunohistochemical analysis against goat podoplanin. Heliyon. 2019;5 doi: 10.1016/j.heliyon.2019.e02063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Furusawa Y., Yamada S., Itai S., Nakamura T., Yanaka M., Sano M., Harada H., Fukui M., Kaneko M.K., Kato Y. PMab-219: a monoclonal antibody for the immunohistochemical analysis of horse podoplanin. Biochem Biophys Rep. 2019;18:100616. doi: 10.1016/j.bbrep.2019.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Furusawa Y., Takei J., Sayama Y., Yamada S., Kaneko M.K., Kato Y. Development of an anti-bear podoplanin monoclonal antibody PMab-247 for immunohistochemical analysis. Biochem Biophys Rep. 2019;18:100644. doi: 10.1016/j.bbrep.2019.100644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abe S., Morita Y., Kaneko M.K., Hanibuchi M., Tsujimoto Y., Goto H., Kakiuchi S., Aono Y., Huang J., Sato S., Kishuku M., Taniguchi Y., Azuma M., Kawazoe K., Sekido Y., Yano S., Akiyama S., Sone S., Minakuchi K., Kato Y., Nishioka Y. A novel targeting therapy of malignant mesothelioma using anti-podoplanin antibody. J. Immunol. 2013;190:6239–6249. doi: 10.4049/jimmunol.1300448. [DOI] [PubMed] [Google Scholar]
- 42.Abe S., Kaneko M.K., Tsuchihashi Y., Izumi T., Ogasawara S., Okada N., Sato C., Tobiume M., Otsuka K., Miyamoto L., Tsuchiya K., Kawazoe K., Kato Y., Nishioka Y. Antitumor effect of novel anti-podoplanin antibody NZ-12 against malignant pleural mesothelioma in an orthotopic xenograft model. Canc. Sci. 2016;107:1198–1205. doi: 10.1111/cas.12985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaneko M.K., Abe S., Ogasawara S., Fujii Y., Yamada S., Murata T., Uchida H., Tahara H., Nishioka Y., Kato Y. Chimeric anti-human podoplanin antibody NZ-12 of lambda light chain exerts higher antibody-dependent cellular cytotoxicity and complement-dependent cytotoxicity compared with NZ-8 of kappa light chain. Monoclon. Antibodies Immunodiagn. Immunother. 2017;36:25–29. doi: 10.1089/mab.2016.0047. [DOI] [PubMed] [Google Scholar]
- 44.Kaneko M.K., Kunita A., Abe S., Tsujimoto Y., Fukayama M., Goto K., Sawa Y., Nishioka Y., Kato Y. Chimeric anti-podoplanin antibody suppresses tumor metastasis through neutralization and antibody-dependent cellular cytotoxicity. Canc. Sci. 2012;103:1913–1919. doi: 10.1111/j.1349-7006.2012.02385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaneko M.K., Yamada S., Nakamura T., Abe S., Nishioka Y., Kunita A., Fukayama M., Fujii Y., Ogasawara S., Kato Y. Antitumor activity of chLpMab-2, a human-mouse chimeric cancer-specific antihuman podoplanin antibody, via antibody-dependent cellular cytotoxicity. Canc. Med. 2017;6:768–777. doi: 10.1002/cam4.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaneko M.K., Nakamura T., Kunita A., Fukayama M., Abe S., Nishioka Y., Yamada S., Yanaka M., Saidoh N., Yoshida K., Fujii Y., Ogasawara S., Kato Y. ChLpMab-23: cancer-specific human-mouse chimeric anti-podoplanin antibody exhibits antitumor activity via antibody-dependent cellular cytotoxicity. Monoclon. Antibodies Immunodiagn. Immunother. 2017;36:104–112. doi: 10.1089/mab.2017.0014. [DOI] [PubMed] [Google Scholar]
- 47.Niwa R., Shoji-Hosaka E., Sakurada M., Shinkawa T., Uchida K., Nakamura K., Matsushima K., Ueda R., Hanai N., Shitara K. Defucosylated chimeric anti-CC chemokine receptor 4 IgG1 with enhanced antibody-dependent cellular cytotoxicity shows potent therapeutic activity to T-cell leukemia and lymphoma. Canc. Res. 2004;64:2127–2133. doi: 10.1158/0008-5472.can-03-2068. [DOI] [PubMed] [Google Scholar]
- 48.Shinkawa T., Nakamura K., Yamane N., Shoji-Hosaka E., Kanda Y., Sakurada M., Uchida K., Anazawa H., Satoh M., Yamasaki M., Hanai N., Shitara K. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J. Biol. Chem. 2003;278:3466–3473. doi: 10.1074/jbc.M210665200. [DOI] [PubMed] [Google Scholar]
- 49.Wang J., Zhao Y., Qi R., Zhu X., Huang C., Cheng S., Wang S., Qi X. Prognostic role of podocalyxin-like protein expression in various cancers: a systematic review and meta-analysis. Oncotarget. 2017;8:52457–52464. doi: 10.18632/oncotarget.14199. [DOI] [PMC free article] [PubMed] [Google Scholar]