Abstract
Delirium, an acute alteration in mental status characterized by confusion, inattention and a fluctuating level of arousal, is a common problem in critically ill patients. Delirium prolongs hospital stay and is associated with higher mortality. The pathophysiology of delirium has not been fully elucidated. Neuroinflammation and neurotransmitter imbalance seem to be the most important factors for delirium development. In this review, we present the most important pathomechanisms of delirium in critically ill patients, such as neuroinflammation, neurotransmitter imbalance, hypoxia and hyperoxia, tryptophan pathway disorders, and gut microbiota imbalance. A thorough understanding of delirium pathomechanisms is essential for effective prevention and treatment of this underestimated pathology in critically ill patients.
Keywords: delirium, critical illness, hypoxia, hyperoxia, neuroinflammation, neurotransmitter agents, kynurenine pathway, tryptophan, gastrointestinal microbiome
1. Introduction
Acute non-traumatic brain injury, manifested as a type of neuropsychological dysfunction, is frequently noted in critically ill patients undergoing elective or emergency surgery and treated in the intensive care unit (ICU). It includes various types of behavioral disorders, commonly known as delirium, defined as an acute disturbance in attention and awareness with additional disturbances in cognition not resulting from pre-existing neuropsychological disorders, and caused by another medical condition [1,2]. Delirium has been observed in patients undergoing elective or emergency surgery, and in critically ill patients treated in the ICU. The rates of delirium vary between 68% and 80% in mechanically ventilated critically ill patients and between 9% and 70% in patients without artificial breathing support [3,4,5]. It has been documented that delirium contributes independently to poor outcome and prolongs the length of stay in the hospital [6,7]. The fact that delirium is a strong determinant of hospital stay and is an extremely common complication of ICU treatment implicates it as a contributing factor to the increased cost of hospitalization.
Generally, delirium is classified into three main subtypes: hyperactive and its extreme subtype, excitation, hypoactive and its extreme subtype, a form of catatonia, and mixed [8]. Based on clinical manifestations, delirium has five core domains: psychomotor disturbance, emotional dysregulation, cognitive deficits, attention deficits and disorders in circadian rhythm [9]. Importantly, hypoactive delirium is associated with worse long-term cognition than the hyperactive subtype [7]. Many patients remain undiagnosed when validated delirium screening tools are not used, both in the ICU and outside the ICU. Therefore, delirium monitoring policies should be implemented in every hospital ward [10].
More than 100 different risk factors have been described for delirium, and are categorized into predisposing factors (present before the admission to the hospital) and potentially modifiable precipitating factors (generated during the treatment period) [11]. Despite a large number of risk factors, only a few of them significantly increase the incidence of delirium in hospital settings. Hypoxia, prolonged mechanical ventilation, hyperbilirubinemia, raised creatinine and the use of benzodiazepines, as well as elderly age, sleep deprivation, alcohol or drug addiction, physical immobility, severe comorbidities and severe infection predispose to the development of delirium [8,9,12].
Pathomechanisms of acute non-traumatic brain injury have been studied for many recent years. Currently, there are a few leading theories that seem to explain the non-traumatic brain damage. Some of them seem to be very clear, whereas others are very controversial (Table 1). The aim of this review was to analyze the most popularly known pathomechanisms of delirium in critically ill patients treated in the ICU.
Table 1.
Studies regarding pathomechanisms of delirium—design and main characteristics.
| Pathomechanisms | Authors and Reference Number | Study Design | Number of Patients | Results |
|---|---|---|---|---|
| Hypoxia | Funk et al. [16] | Prospective controlled clinical study | 15 septic shock patients | Decrease in cerebral saturation corresponds to the incidence of delirium |
| Mikkelsen et al. [17] | Prospective, multicentre cohort clinical study | 406 adult patients treated for ARDS | Low PaO2 was associated with cognitive impairment | |
| Hopkins et al. [18] | Prospective controlled clinical study | 120 adult patients treated for ARDS | Hypoxia assessed as SaO2 < 90% is associated with long-term neurocognitive disorders | |
| Hyperoxia | Kupiec et al. [19] | Retrospective clinical study | 93 cardiac surgery patients | Hyperoxia defined as PaO2 > 120 mmHg is associated with the occurrence of postoperative delirium |
| Mutch et al. [20] | Prospective clinical study | 12 healthy volunteers | Disturbance in cerebral blood flow following hyperoxia corresponds with postoperative neuropsychological disorders | |
| Lopez et al. [24] | Prospective controlled clinical study | 310 cardiac surgery patients | Hyperoxia defined as any intraoperative cerebral oxygenation greater than baseline | |
| Neuroinflammation | Velagapudi et al. [36] | Experimental, behavioural and histological study | 61 animals undergoing orthopaedic surgery | Orthopaedic surgery leads to microglial activation, astrogliosis and brain blood-barrier disruption |
| Disorders in neurotransmitters | Adam et al. [47] | Prospective observational study | 114 cardiac surgery patients | Decrease in acetylcholine hydrolysing enzyme activity increases risk for delirium |
| John et al. [48] | Prospective observational study | 251 cardiac surgery patients | There are no correlations between acetylcholine hydrolysing enzyme activity and risk of delirium | |
| Yilmaz et al. [54] | Prospective observational study | 137 cardiac surgery patients | Dopamine infusion is an independent risk factor for delirium | |
| Yoshitaka et al. [57] | Prospective observational study | 40 critically ill patients | Plasma GABA activity is associated with delirium | |
| Wyrobek et al. [61] | Prospective observational study | 77 elderly patients undergoing spinal surgery | Decrease in the brain-derived neurotrophic factor is associated with delirium | |
| Madsen et al. [67] | Prospective observational study | 30 healthy volunteers | Disorders in 5-HT4 receptor correlate with impaired memory and risk for neuropsychiatric disorders | |
| Tryptophan metabolism and kynurenine pathway dysregulation | Kozak et al. [75] | Experimental, behavioural and histological study | Animal study | Elevated brain kynurenic acid impairs cognitive function |
| Valle et al. [77] | Prospective observational study | 62 HIV-infected patients | Elevated quinolinic acid is a risk factor for neurocognitive disorders | |
| Gulaj et al. [78] | Prospective observational study | 34 patients with Alzheimer dementia | Plasma kynurenic acid and quinolinic acid correlate with impaired cognitive function | |
| Solvang et al. [79] | Prospective observational study | 155 patients with dementia | Kynurenine had a nonlinear quadratic relationship with cognitive disorders | |
| Gut microbiota dysregulation | Zhang et al. [93] | Experimental, behavioural study | 11 pigs | Gut microbiota disorders induce delirium |
| Liufu et al. [94] | Experimental, behavioural study | 10 mice | Gut microbiota disorders induce delirium | |
| Liskiewicz et al. [96] | Prospective observational study | 16 patients with major depression | Disorders in gut microbiota are associated with the severity of depression | |
| Huang et al. [97] | Prospective observational study | 54 patients with major depression | Defects of the Firmicutes (gut bacteria) increase a risk for depression |
Legend: ARDS—adult respiratory distress syndrome; GABA—gamma-amino butyric acid; HIV—human immunodeficiency virus; 5-HT4—5-hydroxytryptamine; PaO2—partial pressure of oxygen; SaO2—oxygen saturation.
2. Hypoxia or Hyperoxia-Related Brain Injury
The brain is considered the most vulnerable organ at the highest risk of oxygen disorders. When oxygen delivery to the brain is decreased below a critical value, a biochemical cascade is induced that leads to neuronal damage. Cerebral hypoxia results in changes in the intra- and extracellular electrolyte concentrations. An anoxia-related increase in cell membrane permeability occurs between 60 and 180 s after the onset, leading to a decrease in extracellular sodium, chloride and calcium with an increase in potassium leaks from the neuronal cells. At the same time, calcium rapidly influxes into the neurons, leading to subsequent mitochondrial dysfunction and overproduction of reactive oxygen radicals [13]. The mitochondrial dysfunction causes further ATP depletion, which impairs osmotic pump activity. These disorders may induce neuronal apoptosis and necrosis within a few hours; necrotic neuronal damage is commonly observed early after severe ischemic events, whereas apoptotic cell death may occur with longer survival periods [14]. Additionally, prolonged or intermittent hypoxia activates microglia, which is a trigger for neuroinflammation manifested as a so-called delayed post-anoxic encephalopathy [15]. A decrease in cerebral oxygen saturation is associated with delirium in septic shock patients [16]. Mikkelsen and colleagues also found relationships between lower PaO2 on the day of admission to the ICU and cognitive impairment in general, and executive dysfunction specifically [17]. The duration of hypoxemia during admission correlated with attention, verbal memory and executive function in patients treated for severe acute respiratory distress syndrome with PaO2/FiO2 < 150 mmHg, but it did not correlate with any neurocognitive functions at two-year follow-up [18].
Similar to hypoxia, hyperoxia may also be harmful and increases the risk for acute non-traumatic brain injury [19,20]. The most dramatic disorders in brain function and cerebral blood flow were observed in healthy volunteers with combined hyperoxia and hypocapnia during anesthesia [20]. Notably, hyperoxia induces hypocapnia following hyperventilation, which is explained by the Haldane effect (oxygenated hemoglobin binds less CO2). Unbounded CO2 must be transported as the dissolved ion, which increases pH and stimulates the brainstem nuclei to increase ventilation. Exposure to arterial hyperoxia correlates with worse outcome and higher mortality in stroke and traumatic brain injury patients [21]. Hyperoxia decreases phosphorylation of protein 3-kinase and increases activation of c-Jun N-terminal kinase, favoring apoptotic cell death [22]. Acute 6-hour hyper-oxygenation markedly down-regulates the brain-derived neurotrophic factors, neutrophins 3 and 4, and induces oxidative stress, leading to apoptotic neurodegeneration [23]. High blood oxygen tension increases F2-isoprostane and isofuran plasma concentrations—molecules reflecting the free radical-induced arachidonic acid peroxidation, which can induce brain arteriole vasoconstriction [24,25]. Clinical observations seem to confirm an unprofitable effect of high blood oxygen tension, suggesting hyperoxia may be an independent risk factor for postoperative delirium in cardiac surgery patients [19,24]. Hence, it can be speculated that both hypoxia and hyperoxia may be associated with increased risk of delirium.
3. Neuroinflammatory Hypothesis
Every systemic inflammatory event triggers the release of several pro- and anti-inflammatory mediators, which may affect neuronal activity. Experimental studies have documented that peripheral cytokines released following the systemic inflammatory response can penetrate the blood–brain barrier (BBB) directly via active transport or indirectly via vagal nerve stimulation, and this effect can be intensified by hypoxia [26,27,28,29]. Elevated plasma pro-inflammatory cytokine concentrations, such as IL-1β and tumor necrosis factor α (TNFα), activate receptors in the endothelial cells, which causes cyclo-oxygenase activation, resulting in increased BBB permeability [30,31]. Additionally, elevated plasma interferon-γ concentration following a general inflammatory response damages occludin (a tight junction protein), which enables macrophage transition to the intracellular space in the brain, and stimulates astrogliosis and microglial activation [31,32]. Of note, peripheral administration of lipopolysaccharide induces a rapid elevation of TNFα in the brain per se [33]. An increase in BBB permeability is associated with cerebral edema and activation of microglia, which play a crucial role in synaptic plasticity and produces behavioral adaptation to environmental signals. Microglial cells are the main macrophage cells representing the brain immune system. Activated microglia secrete proinflammatory cytokines, eicosanoids and excitatory amino acids and stimulate production of reactive oxygen radicals and nitric oxide. Microglial activation is also responsible for regenerative processes and releasing neuroprotective factors. Disorders in microglial signaling impair memory [34]. It has been suggested that sepsis-related cognitive decline results from neuroinflammatory cascade following microglial activation [35]. Similarly, microglial activation with BBB dysfunction following the systemic inflammatory response was observed in mice that underwent elective orthopedic surgery [36]. Activated microglia and inflammatory mediators released by them modulate cholinergic, β-adrenergic and GABA-ergic neurotransmission, as well as secretion of vasopressin, corticotropin-releasing factor and adrenocorticotropic hormone, leading in turn to non-traumatic neuronal injury in the brain [37]. Significant reduction in the risk of postoperative delirium in patients treated with anti-inflammatory medications seems to confirm this hypothesis [38,39]. Interestingly, statins also reduce the neuroinflammatory response following systemic inflammation and/or ischemia-related brain dysfunction. Statins have been suggested in the treatment of delirium [40]. Several clinical studies have confirmed a relationship between systemic inflammation with increased cytokines, particularly IL-6, which are associated with delirium in patients treated for hip fracture [41,42]. Therefore, the important role of neuroinflammation in the development of postoperative neurocognitive dysfunction has become apparent.
4. Neurotransmitter Disorders
The occurrence of delirium can also result from dysfunction of multiple neurotransmitter systems. Disorders of the cholinergic system have been suggested as a crucial pathomechanism for delirium. Activation of acetylcholine receptors is associated with better learning and memory, and an inhibition of postsynaptic acetylcholine muscarinic-1 receptor corresponds to cognitive dysfunction and hallucinations [43,44]. Indeed, post-synaptic muscarinic-1 receptors are responsible for perception, attention and cognitive function [44]. Cholinergic hypofunction in the basal forebrain results in vulnerability to the cognitive deficits and memory dysfunction following systemic inflammation [45]. Additionally, centrally administrated interleukin 1β impairs memory in a cholinesterase-sensitive manner with reduction of acetylcholine outflow [46]. On the other hand, the release of acetylcholine can decrease the neuroinflammatory response to systemic inflammation via inhibition of IL-6, IL-8 and TNF release [42,43]. Clinical study has shown a correlation between low blood acetylcholinesterase concentration and delirium in cardiac surgery patients, whereas others have negated such a relationship [47,48].
Risk for delirium is also related to an age-dependent loss of dopamine receptors, and the imbalance between dopamine synthesis and dopamine receptors leading to neuropsychological disorders. The dopamine (DA) receptors influence the activity of ion pumps affecting neuronal excitability in the brain. The DA-1 and the DA-2 receptors modulate intracellular calcium levels and their activation increases intracellular calcium in a different manner. The DA receptors affect behavioral and locomotion functions [48]. The activation of D-1 receptors produces maximal locomotor stimulation, whereas activation of D-2 receptors decreases dopamine release reducing activity [49]. The DA-3 receptors, which are mainly localized postsynaptically in the nucleus accumbens, inhibit locomotor function [50]. Thus, elevated cerebral dopamine may cause neurobehavioral changes with raised cognitive impairment, anxiety and working memory dysfunction in elderly patients [51,52]. Dopamine receptors play a crucial role in cortical acetylcholine release and systemic administration of dopamine 2 antagonists significantly attenuated acetylcholine efflux [53]. A clinical study has shown that dopamine infusion increased the risk for delirium in cardiac surgery patients in a dose-dependent manner [54].
The DA-2 receptors inhibit neuronal signals via regulation of gamma-amino butyric acid (GABA) release. Indeed, downregulation in GABA receptor sensitivity is also suggested as an important pathomechanism of delirium, particularly in patients with alcohol dependency [55]. GABA is the most important inhibitory neurotransmitter in the cortex, hippocampus, amygdala, basal ganglia, cerebellum, medulla and spinal cord [56]. GABA concentrations in the cerebrospinal fluid and plasma have been considered a useful marker of brain activity and delirium [57]. Moreover, GABA plays a crucial role in sleep regulation, and disorders in GABA activity following neuroinflammation may result in sleep deprivation, which is one of the most important risk factors for delirium [58].
Disorders in the glutamatergic system in the limbic area, which strongly contribute to depression and mood disorders, are another reason for delirium. The proinflammatory cytokines, which are released by activated microglia, reduce glutamate uptake via inhibition of glutamate transporters on glial cells leading in turn to an increase in extrasynaptic glutamate concentration [59]. This glutamate binds to N-methyl-D-aspartate (NMDA) receptors reducing synaptic neuroplasticity and neuronal activity via suppression of synthesis and release of brain-derived neutrophic factor [60]. This hypothesis seems to be confirmed by Wyrobek and colleagues’ clinical observation, who noted a relationship between the decline in plasma brain-derived neutrophic factor (BDNF) concentration and episodes of delirium in elderly patients >70 years old who underwent lumbar spine surgery [61]. The elevated glutamate concentration bound to extrasynaptic NMDA receptors also suppresses the mammalian target of rapamycin signaling pathway, which reduces the synaptic plasticity and consequently impairs memory and learning [62].
The hypothesis that serotonin (5-hydroxytryptamine (5-HT)) plays an important role in the development of delirium has been examined in several studies [63,64,65]. 5-HT is produced from tryptophan by hydroxylation followed by acetylation and methylation to melatonin in the pineal gland. This last step is vitamin B6 dependent. Currently, 5-HT is widely distributed in the brain, and seven types of serotonin receptors have been characterized [64,65]. The serotonin 1 and 3 receptors (5-HT1 and 5-HT3) are responsible for learning and memory, the 5-HT2 receptors are responsible for cognitive function, the 5-HT4 receptors are responsible for disorders in the mood and depression development, and 5HT7 receptors are responsible for circadian rhythm [65]. The inhibition of serotonergic neurotransmission intensifies impulsivity and reduces patience [63,65]. The decrease in 5-HT synthesis specifically impairs short-term and long-term memory [63,64]. Reducing serotonin availability in the brain leads to delirium-like syndromes [66]. Clinical study with positron-emission tomography showed an age-dependent decrease of 1% per decade in striatal 5-HT4 receptors, and 13% lower 5-HT4 receptor activity in the limbic system with the largest difference of 19% in the amygdala in women compared with men [67]. Given the prevalent influence of 5-HT on emotion, memory, learning and circadian rhythm, the hypothesis about their crucial role in the pathogenesis of delirium seems to be correct. Interestingly, stress and inflammatory states are triggers for serotonin deficiency, and disorders in the above described receptors are initiated by neuroinflammation following trauma or general inflammatory responses [68,69].
5. Tryptophan Metabolism and Kynurenine Pathway Dysregulation
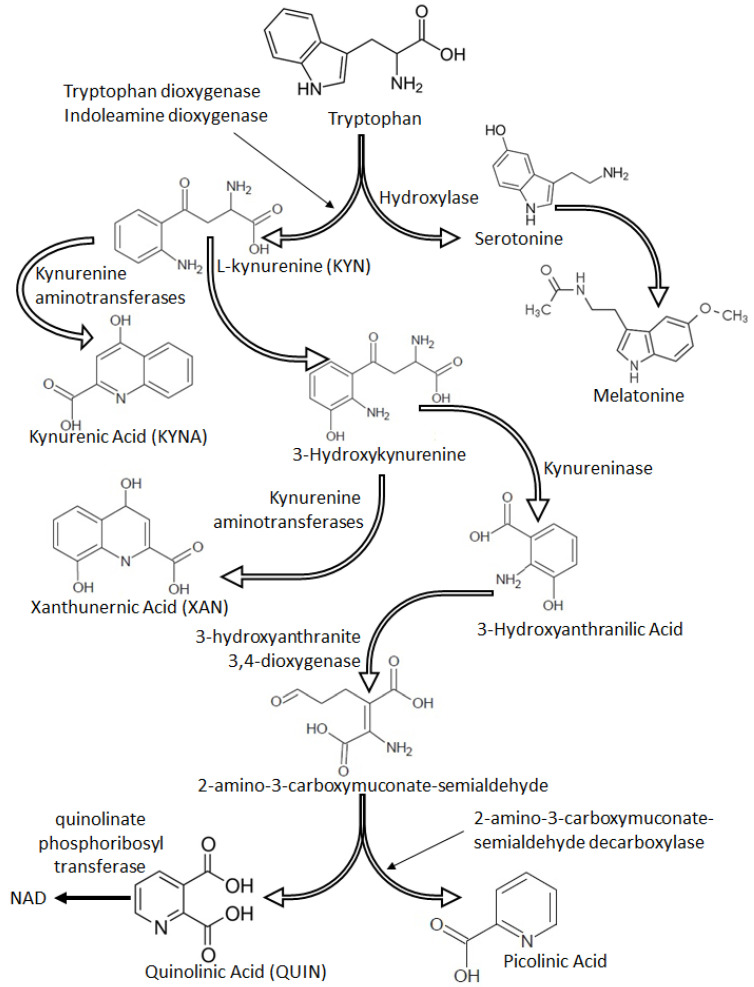
Tryptophan is an essential amino acid, which is metabolized within two main pathways: the serotonin pathway and the kynurenine pathway, leading to the synthesis of several neuroactive metabolites such as kynurenic acid (KYNA), 3-hydroxyanthranilic acid oxygenated to quinolinic acid (QUIN), picolinic acid, 5-hydroxyanthranilic acid, xanthurenic acid (XAN), kynurenine (KYN) and others (Figure 1). Some of the final metabolites in the kynurenine pathway present anti-excitatory activity, whereas others present pro-excitatory and pro-convulsive properties [68,70]. KYNA, a broad-spectrum antagonist of endogenous excitatory amino acids with generally accepted neuroprotective activity, blocks the strychnine-insensitive glycine recognition site in the NMDA receptor and the choline-induced increase in GABAergic function at the nanomolar and micromolar, physiological concentrations, respectively [71,72]. Elevated KYNA concentration was associated with myelin damage leading to neuronal dysfunction [73]. Indeed, accumulation of brain KYNA concentration induces learning and memory function, and a reduction of its level significantly improves cognitive function [74,75]. Therefore, it may be speculated that the physiological KYNA concentration in the brain has pronounced neuroprotective properties, whereas its elevated level induces cognitive disorders.
Figure 1.
Tryptophan pathways.
Another kynurenine pathway’s metabolite, QUIN, has neurotoxic activity via an increase in glutamate activity in the synaptic space by reducing the reuptake of glutamate in the presynaptic NMDA receptors. QUIN is co-localized with hyperphosphorylated tau protein and induces its phosphorylation in the cortical neurons [76]. A clinical study has documented a strong correlation between cerebrospinal QUIN concentration and the presence of dementia in AIDS patients [77]. Low plasma KYNA concentration and a marked increase in QUIN concentration is associated with a high risk for severe dementia in Alzheimer’s disease [78]. Several studies have documented that disorders of the tryptophan pathway are associated with memory dysfunction, dementia and delirium [79,80]. Additionally, QUIN is produced by activated macrophages, whereas elevated levels of KYNA may result from an inflammatory response [77,81]. Indeed, under stressful and inflammatory conditions tryptophan is quickly metabolised by indoleamine 2,3-dioxygenase (IDO), which plays a crucial role in the kynurenine pathway, and which is localized in the lung, the brain, the kidney and immune cells [82].
IDO activation has been shown to down-regulate neuroinflammation [83]. QUIN, picolinic acid, 3-hydroxykynurenine and 3-hydroxyanthranilic acids are neurotoxic and can cross the BBB during systemic inflammation [83,84]. Concededly, KYNA has no ability to cross the healthy BBB, however, general inflammation and inflammatory-related endothelial activation lead to BBB injury, opening the way for penetration of high amounts of KYNA to the brain [85]. KYNA is also produced and released by inflammation-activated astrocytes and microglia [86]. Therefore, we suggest that intra-cerebral activation of the kynurenine pathway and cerebral influx of neurotoxic kynurenine metabolites may lead to neuronal damage and in turn delirium. However, these pathomechanisms should be confirmed in further studies.
6. Gut Microbiota Dysregulation
Recently, the role of gut microbiota as a physiological regulator of essential processes including brain function has been the subject of many investigations. It has been documented that the brain–gut axis, a complex bi-directional signaling system, regulates brain function [87]. Abnormal composition of intestinal microbiota may contribute to the development of neurodegeneration and neuroinflammation, which are associated with depression or autism [88,89]. An experimental study has presented a modification of gut microbiota following gastrointestinal surgery, which required long-time transformation [90,91,92]. Changes in gut microbiota composition mainly included Enterobacteriaceae, Bacteroidaceae and Rhodospirillaceae [91]. This microbial dysbiosis is closely linked to disturbances of gene expression of inflammatory cytokines. It has been documented that abnormal intestinal microbiota composition may be an important risk factor of postoperative delirium [93]. An experimental study has shown an elevated amount of Escherichia and Shigella in animals with delirium [94]. A clinical observation seems to confirm this relationship, because mechanically ventilated patients with delirium had increased amounts of Firmicutes bacteria with concurrent reduction of Proteobacteria in the gut, and these changes did not correspond to early nutrition, microbiome composition, and the type of delirium [95]. Interestingly, treatment of postoperative dysbiosis with Lactobacillus or other probiotics mitigated delirium [95]. It has been documented, that disorders in gut microbiota are associated with the severity of depressive syndromes [96,97]. Hence, it can be suggested that the microbiome affects neurocognitive dysfunction, however this hypothesis needs further study.
7. Treatment of Delirium
Independent of the type of delirium, early identification of the risk factors of non-traumatic brain injury and their modification or elimination are the most important elements of management for reduction of delirium severity. It has been documented that approximately 30% of delirium cases are preventable [98]. Prevention and treatment of delirium should be based on the implementation of the routine daily practice care bundle presented by the Society of Critical Care Medicine (SCCM) called the ABCDEF bundle (A—Assess, Prevent, and Manage Pain, B—Both Spontaneous Awakening Trials (SAT) and Spontaneous Breathing Trials (SBT), C—Choice of analgesia and sedation, D—Delirium: Assess, Prevent, and Manage, E—Early mobility and Exercise, F—Family engagement and empowerment) [99]. The implementation of early mobility activities combined with an appropriate level of sedation and adequate pain management is a challenge in critically ill patients, but the combined efforts seem to be effective methods of delirium prevention and treatment [100,101]. It must also be emphasized that the mainstay of delirium treatment is early and focused management of disrupted homeostasis that can lead to delirium. This approach should focus on treatable or reversible conditions, including treatment of hypoxia, correction of underlying electrolyte disorders (i.e., hypo- or hypernatremia), early detection and treatment of infections, maintaining adequate volemia and preventing gastrointestinal disorders.
Adequate management of hypoxia in a patient with delirium should be the primary goal of the ICU team. It should not only be based on providing supplemental oxygen or mechanical ventilation, but also on ensuring adequate cerebral blood flow, avoidance of anemia or avoidance of a range of factors potentially leading to cerebral vasoconstriction (i.e., hypocarbia). Continuous monitoring of cerebral oxygenation seems to reduce the risk of delirium effectively. Different clinical studies and meta-analyses have shown close relationships between disorders in cerebral oximetry and the severity of delirium, suggesting that cerebral oximetry is an easy and feasible method to measure risk of postoperative neuropsychological disorders in cardiac surgery patients [24,102,103,104]. Likewise, continuous measurement of cerebral oximetry can help to identify an episode of cerebral hypoxia allowing quick intervention, which may reduce the risk of delirium in critically ill ICU patients [16,105]. Hence, monitoring of cerebral oximetry should be widely used in clinical practice, especially in patients at increased risk of delirium.
Monitoring of hyperoxemia is difficult and commonly requires regular blood gas analysis because pulsoximetry and arterial saturation (SpO2 and SaO2, respectively) are not credible when arterial partial oxygen pressure (PaO2) increases above 100 mmHg. Recently, the oxygen reserve index (ORI) has been implemented into clinical practice to avoid hyperoxemia [106,107]. The ORI is a new multiple-wavelength pulse oximetry reflecting the oxygenation status in the moderate range of hyperoxia with a PaO2 of approximately 100–200 mmHg [106]. Although this technology is new and not commonly applied to routine clinical practice, it seems to make oxygen therapy significantly safer and easier. Nevertheless, the usefulness of the ORI in prevention of hyperoxia-related delirium requires further studies.
Maintaining the circadian rhythm of wakefulness and sleep in the ICU is difficult; therefore, attention to normalize the circadian rhythm is of uttermost importance. It has been shown by Skrobik et al. that introducing a low nocturnal dose of dexmedetomidine reduces the incidence of delirium but does not improve sleep quality [108]. Treatment with melatonin to correct the circadian rhythm is commonly used in patients with elevated risk of delirium [109,110,111]. Physiologically, melatonin secretion is low during daytime and increases early in the evening and at night with the peak in the middle of the night [112]. The circadian rhythm of melatonin secretion inversely corresponds to cortisol secretion [113]. The desynchronization of the melatonin secretion rhythm has been reported in sedated critically ill patients [110]. This desynchronization may result from disturbances in cortisol secretion in critically ill patients or may be associated with a disturbed production of tryptophan, because an elevated level of plasma interferon-γ following an inflammatory response induces IDO activity leading to intensive tryptophan degradation in the kynurenine pathway [114,115]. Notably, increased IDO activity has been described in patients with major depression [115,116]. Hence, treatment with melatonin should be implemented in depressive critically ill patients, in whom hypoactive or mixed delirium has been diagnosed.
Immuno-inflammatory activation also plays a crucial role in the pathophysiology of major depression, and elevated levels of inflammatory markers have been noted in patients with delirium [117,118,119]. Therefore, some advocate the use of anti-inflammatory medications for consideration in patients with non-traumatic brain injury. Importantly, treatment with dexamethasone did not reduce the incidence of delirium, and intra-articular administration of corticosteroids induced hyperactive delirium in elderly patients with moderate dementia [120,121]. Experimental and clinical studies documented the anti-inflammatory and immunomodulatory effect of statins, which was associated with a reduction of delirium in critically ill patients [40,122,123,124,125]. Administration of atorvastatin/simvastatin decreased systemic and brain tissue levels of proinflammatory cytokines and reduced lipid peroxidation, preventing the development of long-term cognitive dysfunction [122]. Another study also documented that simvastatin reduced the severity of depression via reduction of microglia and astrocyte activation in the hippocampus after experimental traumatic brain injury [124]. Based on these observations, some authors postulate a protective effect of statins connected with their anti-inflammatory activity in the brain. The possibility of a therapeutic anti-neuroinflammatory effect of statins in patients with delirium should be confirmed in future clinical trials.
Treating agitation in the ICU has always been challenging and difficult, therefore antipsychotics and anti-convulsive medications are commonly used in patients with hyperactive and mixed delirium [126,127,128,129]. Antipsychotics are thought to work by nonspecific blockade and restoration of the imbalanced neurotransmission in the brain. Haloperidol, the most popular neuroleptic agent, is not recommended for routine use in delirium [130] but may have a role in the hyperactive subtype. If used it should only be continued until agitation is controlled and no longer thereafter, because it may cause several extrapyramidal symptoms and may be associated with increased mortality in elderly hospitalized patients [126]. A clinical study including 68 mechanically ventilated patients with subsyndromal delirium documented that a low dose of haloperidol administrated early during the ICU stay did not prevent delirium and had little therapeutic advantage [127]. A retrospective analysis of the effectiveness in the treatment of delirium showed no significant differences in delirium duration and secondary outcome in geriatric patients treated with different antipsychotic agents [128]. A comparison of haloperidol, ziprasidone and placebo in a randomized, double-blind trial of 566 patients with ICU delirium found no effect of either antipsychotic medication on the number of coma-free and delirium-free days [131]. Of note, antipsychotics also present extracerebral adverse effects such as QTc prolongation, which is an independent risk factor for life threatening cardiac arrhythmia and sudden cardiac death [132]. Antipsychotics cannot be suggested as the first line in treatment of delirium. Valproic acid presents similar effectiveness to antipsychotics in the treatment of agitation associated with hyperactive delirium, and the adverse effects of its administration are lower than antipsychotics; overall this agent is well tolerated [129,133]. Hence, the use of valproic acid as an adjuvant for treatment of hyperactive delirium can be a promising alternative to antipsychotics.
It must be stressed that effective delirium management should be based on its prevention and the use of non-pharmacological measures, rather than pharmacological treatment. Management strategies should include noise reduction, exposure to natural light during the day, limiting exposure to light at night, avoiding extremes of temperature, ensuring undisturbed night rest. It is extremely important to ensure efficient communication with the environment, which includes the patient’s daily orientation in time, place and the condition and support of their senses (e.g., provision of glasses and hearing aids). Moreover, the presence of family and friends to support the patient at the bedside and provide a link with the reality outside of the ICU is of uttermost importance when dealing with delirium in critically ill patients.
8. Conclusions
This review briefly presents the most important pathomechanisms for delirium in critically ill patients. Many of them are the basis for the application of targeted treatment in delirium. However, the effect of pharmacological treatment may depend on brain reserve, cognitive reserve, intellectual and non-intellectual activity and severity of pre-existing drug or alcohol addiction (if present) [134]. Therefore, the detailed recognition of the pathomechanisms of the non-traumatic brain injury requires further study and shall lead to effective therapeutic options.
Author Contributions
Conceptualization, W.D. and K.K.; Methodology, W.D., D.S.-G., M.J. and M.G.-B.; Validation, W.D., K.K., S.W.R., and E.W.E.; Resources, W.D., M.G.-B., C.P., S.Z. and K.K.; Writing—Original Draft Preparation, W.D., D.S.-G. and K.K.; Writing—Review & Editing, K.K., S.Z., S.W.R. and E.W.E.; Visualization, M.J., C.P. and S.Z.; Supervision, W.D., E.W.E. and K.K.; Project Administration, K.K.. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Pub; Arlington, VA, USA: 2013. [Google Scholar]
- 2.European Delirium Association. American Delirium Society The DSM-5 criteria, level of arousal and delirium diagnosis: Inclusiveness is safer. BMC Med. 2014;12:141–148. doi: 10.1186/s12916-014-0141-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shehabi Y., Riker R.R., Bokesch P.M., Wisemandle W., Shintani A., Ely E.W., SEDCOM Study Group Delirium duration and mortality in lightly sedated, mechanically ventilated intensive care patients. Crit. Care Med. 2010;38:2311–2318. doi: 10.1097/CCM.0b013e3181f85759. [DOI] [PubMed] [Google Scholar]
- 4.Ely E.W., Shintani A., Truman B., Speroff T., Gordon S.M., Harrell J.F.E., Inouye S.K., Bernard G.R., Dittus R.S. Delirium as a Predictor of Mortality in Mechanically Ventilated Patients in the Intensive Care Unit. JAMA. 2004;291:1753–1762. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 5.Kotfis K., Marra A., Ely E.W. ICU delirium—A diagnostic and therapeutic challenge in the intensive care unit. Anaesthesiol. Intensive Ther. 2018;50:160–167. doi: 10.5603/AIT.a2018.0011. [DOI] [PubMed] [Google Scholar]
- 6.Lindroth H., Khan B.A., Carpenter J.S., Gao S., Perkins A.J., Khan S.H., Wang S., Jones R.N., Boustani M.A. Delirium Severity Trajectories and Outcomes in ICU Patients: Defining a Dynamic Symptom Phenotype. Ann. Am. Thorac. Soc. 2020;17:1094–1103. doi: 10.1513/annalsats.201910-764oc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayhurst C.J., Marra A., Han J.H., Patel M.B., Brummel N.E., Thompson J.L., Jackson J.C., Chandrasekhar R., Ely E.W., Pandharipande P.P., et al. Association of Hypoactive and Hyperactive Delirium with Cognitive Function After Critical Illness. Crit. Care Med. 2020;48:e480–e488. doi: 10.1097/CCM.0000000000004313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maldonado J.R. Delirium pathophysiology: An updated hypothesis of the etiology of acute brain failure. Int. J. Geriatr. Psychiatry. 2018;33:1428–1457. doi: 10.1002/gps.4823. [DOI] [PubMed] [Google Scholar]
- 9.Maldonado J.R. Acute brain failure: Pathophysiology, diagnosis, management and sequelae of delirium. Crit. Care Clin. 2017;33:461–519. doi: 10.1016/j.ccc.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 10.Marra A., Kotfis K., Hosie A., MacLullich A.M.J., Pandharipande P., Ely E.W., Pun B.T. Delirium Monitoring: Yes or No? That Is The Question. Am. J. Crit. Care. 2019;28:127–135. doi: 10.4037/ajcc2019874. [DOI] [PubMed] [Google Scholar]
- 11.Vasilevskis E.E., Han J.H., Hughes C.G., Ely E.W. Epidemiology and risk factors for delirium across hospital settings. Best Pr. Res. Clin. Anaesthesiol. 2012;26:277–287. doi: 10.1016/j.bpa.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sampath H., Jayaswal A.K., Soohinda G., Dutta S. Delirium in medical intensive care units: Incidence, subtypes, risk factors, and outcome. Indian J. Psychiatry. 2019;61:352–358. doi: 10.4103/psychiatry.IndianJPsychiatry_583_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie Y., Zacharias E., Hoff P., Tegtmeier F. Ion channel involvement in anoxic depolarization induced by cardiac arrest un rat brain. J. Cereb. Blood Flow Metab. 1995;15:587–594. doi: 10.1038/jcbfm.1995.72. [DOI] [PubMed] [Google Scholar]
- 14.Chen J., Nagayama T., Jin K., Stetler R.A., Zhu R.L., Graham S.H., Simon R.P. Induction of Caspase-3-Like Protease May Mediate Delayed Neuronal Death in the Hippocampus after Transient Cerebral Ischemia. J. Neurosci. 1998;18:4914–4928. doi: 10.1523/JNEUROSCI.18-13-04914.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weinstein J.R., Koerner I.P., Möller T. Microglia in ischemic brain injury. Future Neurol. 2010;5:227–246. doi: 10.2217/fnl.10.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Funk D.J., Kumar A., Klar G. Decreases in cerebral saturation in patients with septic shock are associated with increased risk of death: A prospective observational single center study. J. Intensiv. Care. 2016;4:42. doi: 10.1186/s40560-016-0167-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mikkelsen M.E., Christie J.D., Lanken P.N., Biester R.C., Thompson B.T., Bellamy S.L., Localio A.R., Demissie E., Hopkins R.O., Angus D.C., et al. Faculty Opinions recommendation of The adult respiratory distress syndrome cognitive outcomes study: Long-term neuropsychological function in survivors of acute lung injury. Am. J. Respir. Crit. Care Med. 2012;185:1307–1315. doi: 10.1164/rccm.201111-2025OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hopkins R.O., Weaver L.K., Collingridge D., Parkinson R.B., Chan K.J., Orme J.F. Two-Year Cognitive, Emotional, and Quality-of-Life Outcomes in Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2005;171:340–347. doi: 10.1164/rccm.200406-763OC. [DOI] [PubMed] [Google Scholar]
- 19.Kupiec A., Adamik B., Forkasiewicz-Gardynik K., Gozdzik W. Intra-operative hyperoxia and the risk of delirium in eldery patients after cardiac surgery. Aging. 2020;12:7006–7014. doi: 10.18632/aging.103058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mutch W.A.C., El-Gabalawy R., Ryner L., Puig J., Essig M., Kilborn K., Fidler K., Graham M.R. Brain BOLD MRI O2 and CO2 stress testing: Implications for perioperative neurocognitive disorder following surgery. Crit. Care. 2020;24:1–13. doi: 10.1186/s13054-020-2800-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Damiani E., Adrario E., Girardis M., Romano R., Pelaia P., Singer M., Donati A. Arterial hyperoxia and mortality in critically ill patients: A systematic review and meta-analysis. Crit. Care. 2014;18:711. doi: 10.1186/s13054-014-0711-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Terraneo L., Samaja M. Comparative Response of Brain to Chronic Hypoxia and Hyperoxia. Int. J. Mol. Sci. 2017;18:1914. doi: 10.3390/ijms18091914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Felderhoff-Mueser U., Bittigau P., Sifringer M., Jarosz B., Korobowicz E., Mahler L., Piening T., Moysich A., Grune T., Thor F., et al. Oxygen causes cell death in the developing brain. Neurobiol. Dis. 2004;17:273–282. doi: 10.1016/j.nbd.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 24.Lopez M.G., Pandharipande P., Morse J., Shotwell M.S., Milne G., Pretorius M., Shaw A.D.S., Roberts L.J., Billings F.T. Intraoperative cerebral oxygenation, oxidative injury, and delirium following cardiac surgery. Free Radic. Biol. Med. 2017;103:192–198. doi: 10.1016/j.freeradbiomed.2016.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hou X., Roberts II L.J., Gobeil F., Taber D., Kanai K., Abran D., Brault S., Checchin D., Sennlaub F., Lachapelle P., et al. Isomer-specific contractile effects of a series of synthetic F2-isoprostanes on retinal and cerebral microvasculature. Free Radic. Biol. Med. 2004;36:163–172. doi: 10.1016/j.freeradbiomed.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 26.Kotfis K., Biernawska J., Zegan-Barańska M., Żukowski M. Peripheral Blood Lymphocyte Subsets (CD4+, CD8+ T Cells, NK Cells) in Patients with Cardiovascular and Neurological Complications after Carotid Endarterectomy. Int. J. Mol. Sci. 2015;16:10077–10094. doi: 10.3390/ijms160510077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song T.-T., Bi Y.-H., Gao Y.-Q., Huang R., Hao K., Xu G., Tang J.-W., Ma Z.-Q., Kong F.-P., Coote J.H., et al. Systemic pro-inflammatory response facilitates the development of cerebral edema during short hypoxia. J. Neuroinflamm. 2016;13:63. doi: 10.1186/s12974-016-0528-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matt S.M., Johnson R.W. Neuro-immune dysfunction during brain aging: New insights in microglial cell regulation. Curr. Opin. Pharmacol. 2016;26:96–101. doi: 10.1016/j.coph.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang S., Dong H., Zhang X., Li N., Sun J., Qian Y.-N. Cerebral mast cells contribute to postoperative cognitive dysfunction by promoting blood brain barrier disruption. Behav. Brain Res. 2016;298:158–166. doi: 10.1016/j.bbr.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 30.Skelly D.T., Hennessy E., Dansereau M.-A., Cunningham C. A Systematic Analysis of the Peripheral and CNS Effects of Systemic LPS, IL-1Β, TNF-α and IL-6 Challenges in C57BL/6 Mice. PLoS ONE. 2013;8:e69123. doi: 10.1371/journal.pone.0069123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Minagar A., Long A., Ma T., Jackson T.H., Kelley R.E., Ostanin D.V., Sasaki M., Warren A.C., Jawahar A., Cappell B., et al. Interferon (IFN)-beta 1a and IFN-beta 1b block IFN-gamma disinterration of endothelial junction integrity and barrier. Endothelium. 2003;10:299–307. doi: 10.1080/10623320390272299. [DOI] [PubMed] [Google Scholar]
- 32.Rahman M.T., Ghosh C., Hossain M., Linfield D., Rezaee F., Janigro D., Marchi N., Van Boxel-Dezaire A.H. IFN-γ, IL-17A, or zonulin rapidly increase the permeability of the blood-brain and small intestinal epithelial barriers: Relevance for neuro-inflammatory diseases. Biochem. Biophys. Res. Commun. 2018;507:274–279. doi: 10.1016/j.bbrc.2018.11.021. [DOI] [PubMed] [Google Scholar]
- 33.Qin L., Wu X., Block M.L., Liu Y., Breese G.R., Hong J.-S., Knapp D.J., Crews F.T. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Savage J.C., Carrier M., Tremblay M.-È. Morphology of Microglia across Contexts of Health and Disease. Methods Mol. Biol. 2019;2034:13–26. doi: 10.1007/978-1-4939-9658-2_2. [DOI] [PubMed] [Google Scholar]
- 35.Perry V.H., Holmes C. Microglial priming in neurodegenerative disease. Nat. Rev. Neurol. 2014;10:217–224. doi: 10.1038/nrneurol.2014.38. [DOI] [PubMed] [Google Scholar]
- 36.Velagapudi R., Subramaniyan S., Xiong C., Porkka F., Rodriguiz R.M., Wetsel W.C., Terrando N. Orthopedic Surgery Triggers Attention Deficits in a Delirium-Like Mouse Model. Front. Immunol. 2019;10:2675. doi: 10.3389/fimmu.2019.02675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pavlov V.A., Ochani M., Gallowitsch-Puerta M., Ochani K., Huston J.M., Czura C.J., Al-Abed Y., Tracey K.J. Central muscarinic cholinergic regulation of the systemic inflammatory response during endotoxemia. Proc. Natl. Acad. Sci. USA. 2006;103:5219–5223. doi: 10.1073/pnas.0600506103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mariscalco G., Mariani S., Biancari F., Banach M. Effects of statins on delirium following cardiac surgery—Vidence from literature. Psychiatr. Pol. 2015;49:1359–1370. doi: 10.12740/PP/60139. [DOI] [PubMed] [Google Scholar]
- 39.Marra A., McGrane T.J., Henson C.P., Pandharipande P. Melatonin in Critical Care. Crit. Care Clin. 2019;35:329–340. doi: 10.1016/j.ccc.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 40.Fracassi A., Marangoni M., Rosso P., Pallottini V., Fioramonti M., Siteni S., Segatto M. Statins and the Brain: More than Lipid Lowering Agents? Curr. Neuropharmacol. 2019;17:59–83. doi: 10.2174/1570159X15666170703101816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Munster B.C., Korevaar J.C., Zwinderman A.H., Levi M., Wiersinga W.J., De Rooij S.E. Time-Course of Cytokines during Delirium in Elderly Patients with Hip Fractures. J. Am. Geriatr. Soc. 2008;56:1704–1709. doi: 10.1111/j.1532-5415.2008.01851.x. [DOI] [PubMed] [Google Scholar]
- 42.Cerejeira J., Firmino H., Vaz-Serra A., Mukaetova-Ladinska E.B. The neuroinflammatory hypothesis of delirium. Acta Neuropathol. 2010;119:737–754. doi: 10.1007/s00401-010-0674-1. [DOI] [PubMed] [Google Scholar]
- 43.Umholtz M., Nader N.D. Anesthetic Immunomodulation of the Neuroinflammation in Postoperative Cognitive Dysfunction. Immunol. Investig. 2017;46:805–815. doi: 10.1080/08820139.2017.1373898. [DOI] [PubMed] [Google Scholar]
- 44.Jardine K.H., Wideman C.E., MacGregor C., Sgarbossa C., Orr D., Mitchnick K.A., Winters B.D. Activation of cortical M1 muscarinic receptors and related intracellular signaling is necessary for reactivation-induced object memory updating. Sci. Rep. 2020;10:9209. doi: 10.1038/s41598-020-65836-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Field R.H., Gossen A., Cunningham C. Prior pathology in the basal forebrain cholinergic system predisposes to inflammation-induced working memory deficits: Reconciling inflammatory and cholinergic hypotheses of delirium. J. Neurosci. 2012;32:6288–6294. doi: 10.1523/JNEUROSCI.4673-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taepavarapruk P., Song C. Reductions of acetylcholine release and nerve growth factor expression are correlated with memory impairment induced by interleukin-1β administrations: Effects of omega-3 fatty acid EPA treatment. J. Neurochem. 2010;112:1054–1064. doi: 10.1111/j.1471-4159.2009.06524.x. [DOI] [PubMed] [Google Scholar]
- 47.Adam E.H., Haas V., Lindau S., Zacharowski K., Scheller B. Cholinesterase alterations in delirium after cardiosurgery: A German monocentric prospective study. BMJ Open. 2020;10:e031212. doi: 10.1136/bmjopen-2019-031212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.John M., Ely E.W., Halfkann D., Schoen J., Sedemund-Adib B., Klotz S., Radtke F., Stehr S., Hueppe M. Acetylcholinesterase and butyrylcholinesterase in cardiosurgical patients with postoperative delirium. J. Intensiv. Care. 2017;5:29. doi: 10.1186/s40560-017-0224-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jackson D.M., Westlind-Danielsson A. Dopamine receptors: Molecular biology, biochemistry and behalioural aspects. Pharmacol. Ther. 1994;64:291–369. doi: 10.1016/0163-7258(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 50.Sokoloff P., Schwartz J.-C. Novel dopamine receptors half a decade later. Trends Pharmacol. Sci. 1995;16:270–275. doi: 10.1016/S0165-6147(00)89044-6. [DOI] [PubMed] [Google Scholar]
- 51.Pedrosa R., Soares-da-Silva P. Oxidative and non-oxidative mechanisms of neuronal cel death and apoptosis by 1-3,4-dihydroxyphenylalanine (L-dopa) and dopamine. Br. J. Pharmacol. 2002;137:1305–1313. doi: 10.1038/sj.bjp.0704982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ebrahimi-Ghiri M., Nasehi M., Zarrindast M.-R. The modulatory role of accumbens and hippocampus D2 receptors in anxiety and memory. Naunyn Schmiedebergs Arch. Pharmacol. 2018;391:1107–1118. doi: 10.1007/s00210-018-1534-0. [DOI] [PubMed] [Google Scholar]
- 53.Moore H., Fadel M., Sarter M., Bruno J.P. Role of accumbens and cortical dopamine receptors in the regulation of cortical acetycholine release. Neuroscience. 1999;88:811–822. doi: 10.1016/S0306-4522(98)00261-9. [DOI] [PubMed] [Google Scholar]
- 54.Yilmaz S., Aksoy E., Diken A.I., Yalçınkaya A., Erol M.E., Cagli K. Dopamine Administration is a Risk Factor for Delirium in Patients Undergoing Coronary Artery Bypass Surgery. Heart Lung Circ. 2016;25:493–498. doi: 10.1016/j.hlc.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 55.Masood B., Lepping P., Romanov D., Poole R. Treatment of alcohol-induced psychotic disorder (alcoholic hallucinosis)—A systematic review. Alcohol Alcohol. 2018;53:259–267. doi: 10.1093/alcalc/agx090. [DOI] [PubMed] [Google Scholar]
- 56.Jembrek M., Vlainić J. GABA Receptors: Pharmacological Potential and Pitfalls. Curr. Pharm. Des. 2015;21:4943–4959. doi: 10.2174/1381612821666150914121624. [DOI] [PubMed] [Google Scholar]
- 57.Yoshitaka S., Egi M., Kanazawa T., Toda Y., Kiyoshi M. The association of plasma gamma-aminobutyric acid concentration with postoperative delirium in critically ill patients. Crit. Care Resusc. 2014;16:269–273. [PubMed] [Google Scholar]
- 58.Wisden W., Yu X., Franks N.P. GABA Receptors and the Pharmacology of Sleep. Handb. Exp. Pharmacol. 2019;253:279–304. doi: 10.1007/164_2017_56. [DOI] [PubMed] [Google Scholar]
- 59.Tilleux S., Hermans E. Neuroinflammation and regulation of glial glutamate uptake in neurological disorders. J. Neurosci. Res. 2007;85:2059–2070. doi: 10.1002/jnr.21325. [DOI] [PubMed] [Google Scholar]
- 60.Carvalho A.L., Caldeira M.V., Santos S.D., Duarte C.B. Role of the brain-derived neutrophic factor at glutamateric synapses. Br. J. Pharmacol. 2008;153:S310–S324. doi: 10.1038/sj.bjp.0707509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wyrobek J., LaFlam A., Max L., Tian J., Neufeld K., Kebaish K., Walston J., Hogue C., Riley L., Everett A., et al. Association of intraoperative changes in brain-derived neurotrophic factor and postoperative delirium in older adults. Br. J. Anaesth. 2017;119:324–332. doi: 10.1093/bja/aex103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lipton J.O., Sahin M. The neurology of mTOR. Neuron. 2014;84:275–291. doi: 10.1016/j.neuron.2014.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Meneses A. Neural activity, memory, and dementias: Serotonergic markers. Behav. Pharmacol. 2017;28:132–141. doi: 10.1097/FBP.0000000000000279. [DOI] [PubMed] [Google Scholar]
- 64.Rebholz H., Friedman E., Castello J. Alterations of Expression of the Serotonin 5-HT4 Receptor in Brain Disorders. Int. J. Mol. Sci. 2018;19:3581. doi: 10.3390/ijms19113581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Terry A.V., Buccafusco J.J., Wilson C. Cognitive dysfunction in neuropsychiatric disorders: Selected serotonin receptor subtypes as therapeutic targets. Behav. Brain Res. 2008;195:30–38. doi: 10.1016/j.bbr.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 66.Sternbach H. The serotonin syndrome. Am. J. Psychiatry. 1991;148:705–713. doi: 10.1176/ajp.148.6.705. [DOI] [PubMed] [Google Scholar]
- 67.Madsen K., Haahr M.T., Marner L., Keller S.H., Baaré W.F., Svarer C., Hasselbalch S.G., Knudsen G.M. Age and sex effects on 5-HT4receptors in the human brain: A [11C]SB207145 PET study. J. Cereb. Blood Flow Metab. 2011;31:1475–1481. doi: 10.1038/jcbfm.2011.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim Y.-K., Jeon S.W. Neuroinflammation and the Immune-Kynurenine Pathway in Anxiety Disorders. Curr. Neuropharmacol. 2018;16:574–582. doi: 10.2174/1570159X15666170913110426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Felger J.C. Imaging the Role of Inflammation in Mood and Anxiety-related Disorders. Curr. Neuropharmacol. 2018;16:533–558. doi: 10.2174/1570159X15666171123201142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stone T.W., Forrest C.M., Mackay G.M., Stoy N., Darlington L.G. Tryptophan, adenosine, neurodegeneration and neuroprotection. Metab. Brain Dis. 2007;22:337–352. doi: 10.1007/s11011-007-9064-3. [DOI] [PubMed] [Google Scholar]
- 71.Schurr A. Neuroprotection against ischemic/hypoxic brain damage: Blockers of ionotropic glutamate receptor and voltage sensitive calcium channels. Curr. Drug Targets. 2004;5:603–618. doi: 10.2174/1389450043345209. [DOI] [PubMed] [Google Scholar]
- 72.Dobelis P., Staley K.J., Cooper D.C. Lack of modulation of nicotinic acetylcholine alpha-7 receptor currents by kynurenic acid in adult hippocampal interneurons. PLoS ONE. 2012;7:e41108. doi: 10.1371/journal.pone.0041108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dabrowski W., Kwiecień J.M., Rola R., Klapec M., Stanisz G.J., Kotlińska-Hasiec E., Oakden W., Janik R., Coote M., Frey B.N., et al. Prolonged Subdural Infusion of Kynurenic Acid Is Associated with Dose-Dependent Myelin Damage in the Rat Spinal Cord. PLoS ONE. 2015;10:e0142598. doi: 10.1371/journal.pone.0142598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vohra M., Lemieux G.A., Lin L., Ashrafi K. Kynurenic acid accumulation underlies learning and memory impairment associated with aging. Genes Dev. 2018;32:14–19. doi: 10.1101/gad.307918.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kozak R., Campbell B.M., Strick C.A., Horner W., Hoffmann W.E., Kiss T., Chapin D.S., McGinnis D., Abbott A.L., Roberts B.M., et al. Reduction of Brain Kynurenic Acid Improves Cognitive Function. J. Neurosci. 2014;34:10592–10602. doi: 10.1523/JNEUROSCI.1107-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Anderson A.M., Croteau D., Ellis R.J., Rosario D., Potter M., Guillemin G.J., Brew B.J., Woods S.P., Letendre S.L. HIV, prospective memory, and cerebrospinal fluid concentrations of quinolinic acid and phosphorylated Tau. J. Neuroimmunol. 2018;319:13–18. doi: 10.1016/j.jneuroim.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Valle M., Price R.W., Nilsson A., Heyes M., Verotta D. CSF quinolinic acid levels are determined by local HIV infection: Cross-sectional analysis and modelling of dynamics following antiretroviral therapy. Brain. 2004;127:1047–1060. doi: 10.1093/brain/awh130. [DOI] [PubMed] [Google Scholar]
- 78.Gulaj E., Pawlak K., Bien B., Pawlak D. Kynurenine and its metabolites in Alzheimer’s sidease patients. Adv. Med. Sci. 2010;55:204–211. doi: 10.2478/v10039-010-0023-6. [DOI] [PubMed] [Google Scholar]
- 79.Solvang S.-E.H., Nordrehaug J.E., Aarsland D., Lange J., Ueland P.M., McCann A., Midttun Ø., Tell G.S., Giil L.M. Kynurenines, Neuropsychiatric Symptoms, and Cognitive Prognosis in Patients with Mild Dementia. Int. J. Tryptophan Res. 2019;12:1178646919877883. doi: 10.1177/1178646919877883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Voils S.A., Shoulders B.R., Singh S., Solberg L.M., Garrett T.L., Frye R.F. Intensive care unit delirium in surgical patients is associated with upreguloation in tryptophan metabolism. Pharmacotherapy. 2020;40:500–506. doi: 10.1002/phar.2392. [DOI] [PubMed] [Google Scholar]
- 81.Leonard B.E. Inflammation and depression: A causal or coincidental link to the pathophysiology? Acta Neuropsychiatr. 2018;30:1–16. doi: 10.1017/neu.2016.69. [DOI] [PubMed] [Google Scholar]
- 82.Taylor M.W., Feng G.S. Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. FASEB J. 1991;5:2516–2522. doi: 10.1096/fasebj.5.11.1907934. [DOI] [PubMed] [Google Scholar]
- 83.Smith A., Stone T.W., Smith R. Neurotoxicity of tryptophan metabolites. Biochem. Soc. Trans. 2007;35:1287–1289. doi: 10.1042/BST0351287. [DOI] [PubMed] [Google Scholar]
- 84.Kwidzinski E., Bechmann I. IDO expression in the brain: A double-edged sword. J. Mol. Med. 2007;85:1351–1359. doi: 10.1007/s00109-007-0229-7. [DOI] [PubMed] [Google Scholar]
- 85.Ouma B.J., Ssenkusu J.M., Shabani E., Datta D., Opoka R.O., Idro R., Bangirana P., Park G., Joloba M.L., Kain K.C., et al. Endothelial Activation, Acute Kidney Injury, and Cognitive Impairment in Pediatric Severe Malaria. Crit. Care Med. 2020;48:e734–e743. doi: 10.1097/ccm.0000000000004469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tao X., Yan M., Wang L., Zhou Y., Wang Z., Xia T., Liu X.-M., Pan R.-L., Chang Q. Homeostasis Imbalance of Microglia and Astrocytes Leads to Alteration in the Metabolites of the Kynurenine Pathway in LPS-Induced Depressive-Like Mice. Int. J. Mol. Sci. 2020;21:1460. doi: 10.3390/ijms21041460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ridaura V., Belkaid Y. Gut microbiota: The link to your second brain. Cell. 2015;161:193–194. doi: 10.1016/j.cell.2015.03.033. [DOI] [PubMed] [Google Scholar]
- 88.Mayer E.A., Tillisch K., Gupta A. Gut/brain axis and the microbiota. J. Clin. Investig. 2015;125:926–938. doi: 10.1172/JCI76304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schmidt C. Mental Health: Thinking from the Gut. Nature. 2015;518:S12–S15. doi: 10.1038/518S13a. [DOI] [PubMed] [Google Scholar]
- 90.Aron-Wisnewsky J., Clément K. The Effects of Gastrointestinal Surgery on Gut Microbiota: Potential Contribution to Improved Insulin Sensitivity. Curr. Atheroscler. Rep. 2014;16:454. doi: 10.1007/s11883-014-0454-9. [DOI] [PubMed] [Google Scholar]
- 91.Lapthorne S., Bines J.E., Fouhy F., Dellios N.L., Wilson G., Thomas S.L., Scurr M., Stanton C., Cotter P.D., Pereira-Fantini P. Changes in the colon microbiota and intestinal cytokine gene expression following minimal intestinal surgery. World J. Gastroenterol. 2015;21:4150–4158. doi: 10.3748/wjg.v21.i14.4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lederer A.-K., Pisarski P., Kousoulas L., Fichtner-Feigl S., Hess C., Huber R. Postoperative changes of the microbiome: Are surgical complications related to the gut flora? A systematic review. BMC Surg. 2017;17:125. doi: 10.1186/s12893-017-0325-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang J., Bi J.-J., Guo G.-J., Yang L., Zhu B., Zhan G.-F., Li S., Huang N.-N., Hashimoto K., Yang C., et al. Abnormal composition of gut microbiota contributes to delirium-like behaviors after abdominal surgery in mice. CNS Neurosci. Ther. 2019;25:685–696. doi: 10.1111/cns.13103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liufu N., Liu L., Shen S., Jiang Z., Dong Y., Wang Y., Culley D., Crosby G., Cao M., Shen Y., et al. Anesthesia and surgery induce age-dependent changes in behaviors and microbiota. Aging. 2020;12:1965–1986. doi: 10.18632/aging.102736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kitsios G., Fair K., Xie M., Shah F., Fitch A., Rapport S., Huwe J., Alexander S., Morris A., Girard T.D., et al. Critical Care: Microbiome, Genetics, and Other Biomarkers in Acute Critical Illness. American Thoracic Society; New York, NY, USA: 2018. Gut microbiome dysbiosis and delirium in mechanically ventilated adults patients: A prospective cohort study; p. A2777. [Google Scholar]
- 96.Liskiewicz P., Kaczmarczyk M., Misiak B., Wronski M., Baba-Kubis A., Skonieczna-Zydecka K., Marlicz W., Bienkowski P., Misera A., Pelka-Wysiecka J., et al. Analysis of gut microbiota and intestinal integrity markers of inpatients with major depressive disorder. Prog. Neuropharmacol. Biol. Psychiatry. 2020;19:110076. doi: 10.1016/j.pnpbp.2020.110076. [DOI] [PubMed] [Google Scholar]
- 97.Huang Y., Shi X., Li Z., Shen Y., Shi X., Wang L., Li G., Yuan Y., Wang J., Zhang Y., et al. Possible association of Firmicutes in the gut microbiota of patients with major depressive disorder. Neuropsychiatr. Dis. Treat. 2018;14:3329–3337. doi: 10.2147/NDT.S188340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Inouye S.K., Bogardus S.T., Jr., Charpentier P.A., Leo-Summers L., Acampora D., Holford T.R., Cooney L.M., Jr. A multicomponent intervention to prevent delirium in hospitalized ilder patients. N. Engl. J. Med. 1999;340:669–676. doi: 10.1056/NEJM199903043400901. [DOI] [PubMed] [Google Scholar]
- 99.Barr J., Fraser G.L., Puntillo K., Ely E.W., Gélinas C., Dasta J.F., Davidson J.E., Devlin J.W., Kress J.P., Joffe A.M., et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit. Care Med. 2013;41:263–306. doi: 10.1097/CCM.0b013e3182783b72. [DOI] [PubMed] [Google Scholar]
- 100.Morandi A., Piva S., Ely E.W., Myatra S.N., Salluh J.I.F., Amare D., Azoulay E., Bellelli G., Csomos A., Fan E., et al. Worldwide survey of the “Assessing pain, both spontaneous awakening and breathing trialsm choice of drugs, delirium monitoring/management, early exercise/mobility, and family empowerment” (ABCDEF) bundle reply. Crit. Care Med. 2017;45:e1111–e1122. doi: 10.1097/CCM.0000000000002640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Banerjee A., Girard T.D., Pandharipande P. The complex interplay between delirium, sedation, and early mobility during critical illness: Applications in the trauma unit. Curr. Opin. Anaesthesiol. 2011;24:195–201. doi: 10.1097/ACO.0b013e3283445382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mailhot T., Cossette S., Lambert J., Cournoyer A., Denault A. Cerebral oximetry as a biomarker of postoperative delirium in cardiac surgery patients. J. Crit. Care. 2016;34:17–23. doi: 10.1016/j.jcrc.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 103.Lei L., Katznelson R., Fedorko L., Carroll J., Poonawala H., Machina M., Styra R., Rao V., Djaiani G. Cerebral oximetry and postoperative delirium after cardiac surgery: A randomised, controlled trial. Anaesthesia. 2017;72:1456–1466. doi: 10.1111/anae.14056. [DOI] [PubMed] [Google Scholar]
- 104.Zorrilla-Vaca A., Healy R., Grant M.C., Joshi B., Rivera-Lara L., Brown C., Mirski M.A. Intraoperative cerebral oximetry-based management for optimizing perioperative outcomes: A meta-analysis of randomized controlled trials. Can. J. Anesth. 2018;65:529–542. doi: 10.1007/s12630-018-1065-7. [DOI] [PubMed] [Google Scholar]
- 105.Lee K.F., Wood M.D., Maslove D.M., Muscedere J., Boyd J.G. Dysfunctional cerebral autoregulation is associated with delirium in critically ill adults. J. Cereb. Blood Flow Metab. 2019;39:2512–2520. doi: 10.1177/0271678X18803081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Scheeren T.W.L., Belda F.J., Perel A. Correction to: The oxygen reserve index (ORI): A new tool to monitor oxygen therapy. J. Clin. Monit. 2018;32:379–389. doi: 10.1007/s10877-017-0049-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen S.-T., Min S. Oxygen reserve index, a new method of monitoring oxygenation status: What do we need to know? Chin. Med. J. 2020;133:229–234. doi: 10.1097/CM9.0000000000000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Skrobik Y., Duprey M.S., Hill N.S., Devlin J.W. Low-dose nocturnal dexmedetomidyne prevents ICU delirium. A randomized, placebo-controlles trial. Am. J. Respir. Crit. Care Med. 2018;197:1147–1156. doi: 10.1164/rccm.201710-1995OC. [DOI] [PubMed] [Google Scholar]
- 109.Dessap A.M., Roche-Campo F., Launay J.-M., Charles-Nelson A., Katsahian S., Brun-Buisson C., Brochard L. Delirium and Circadian Rhythm of Melatonin During Weaning From Mechanical Ventilation: An ancillary study of a weaning trial. Chest. 2015;148:1231–1241. doi: 10.1378/chest.15-0525. [DOI] [PubMed] [Google Scholar]
- 110.Olofsson K., Alling C., Lundberg D., Malmros C. Abolished circadian rhythm of melatonin secretion in sedated and artificially ventilated intensive care patients. Acta Anaesthesiol. Scand. 2004;48:679–684. doi: 10.1111/j.0001-5172.2004.00401.x. [DOI] [PubMed] [Google Scholar]
- 111.Baumgartner L., Lam K., Lai J., Barnett M., Thompson A., Gross K., Morris A. Effectiveness of Melatonin for the Prevention of Intensive Care Unit Delirium. J. Hum. Pharmacol. Drug Ther. 2019;39:280–287. doi: 10.1002/phar.2222. [DOI] [PubMed] [Google Scholar]
- 112.Weitzman E.D., Weinberg U., D’Eletto R., Lynch H., Wurtman R.J., Czeisler C., Erlich S. Studies of the 24 h rhythm of melatonin in man. J. Neural Transm. Suppl. 1978;13:325–337. [PubMed] [Google Scholar]
- 113.Rajaratnam S.M., Dijk D.J., Middleton B., Stone B.M., Arendt J. Melatonin phase-shifts humen circadiad rhythms with no evidence of changes in the duration of endogenous melatonin secretion or the 24-h production of reproductive hormones. J. Clin. Endocrinol. Metab. 2003;88:4303–4309. doi: 10.1210/jc.2003-030460. [DOI] [PubMed] [Google Scholar]
- 114.Venkatesh B., Cohen J., Hickman I.J., Nisbet J., Thomas P., Ward G., Hall J., Prins J., Prins J.B. Evidence of altered cortisol metabolism in critically ill patients: A prospective study. Intensiv. Care Med. 2007;33:1746–1753. doi: 10.1007/s00134-007-0727-7. [DOI] [PubMed] [Google Scholar]
- 115.Xu Y., Sheng H., Tang Z., Lu J., Ni X. Inflammation and increased IDO in hippocampus contribute to depression-like behaviour induced by estrogen deficiency. Behav. Brain Res. 2015;288:71–78. doi: 10.1016/j.bbr.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 116.Catena-Dell’Osso M., Rotella F., Dell’Osso A., Fagiolini A., Marazziti D. Inflammation, serotonin and major depression. Curr. Drug Targets. 2013;14:571–577. doi: 10.2174/13894501113149990154. [DOI] [PubMed] [Google Scholar]
- 117.Zhao X., Cao F., Liu Q., Li X., Xu G., Liu G., Zhang Y., Yang X., Yi S., Xu F., et al. Behavioral, inflammatory and neurochemical disturbances in LPS and UCMS-induced mouse models of depression. Behav. Brain Res. 2019;364:494–502. doi: 10.1016/j.bbr.2017.05.064. [DOI] [PubMed] [Google Scholar]
- 118.Rioli G., Tassi S., Mattei G., Ferrari S., Galeazzi G.M., Mancini S., Alboni S., Roncucci L. The Association Between Symptoms of Anxiety, Depression, and Cardiovascular Risk Factors: Rresults from an Italian cross-sectional study. J. Nerv. Ment. Dis. 2019;207:340–347. doi: 10.1097/NMD.0000000000000969. [DOI] [PubMed] [Google Scholar]
- 119.Kotfis K., Bott-Olejnik M., Szylińska A., Listewnik M., Rotter I. Characteristic, risk factor and outcome of early-onset delirium in elderly patients with first ever acute ischemic stroke—A prospective observational cohort study. Clin. Interv. Aging. 2019;14:1771–1782. doi: 10.2147/CIA.S227755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li L.-Q., Wang C., Fang M.-D., Xu H.-Y., Lu H.-L., Zhang H.-Z. Effects of Dexamethasone on Post-Operative Cognitive Dysfunction and Delirium in Adults Following General Anaesthesia: A Meta-Analysis of Randomised Controlled Trials. BMC Anesthesiol. 2019;19:113. doi: 10.1186/s12871-019-0783-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lally L., McCarthy G.M., Meehan K. Hyperactive delirium following administration of intra-articular corticosteroid. BMJ Case Rep. 2017;2017:2014217483. doi: 10.1136/bcr-2016-217483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Reis P.A., Alexandre P.C., D’Avila J.C., Siqueira L.D., Antunes B., Estato V., Tibiriça E.V., Verdonk F., Sharshar T., Chrétien F., et al. Statins prevent cognitive impairment after sepsis by reverting neuroinflammation, and microcirculatory/endothelial dysfunction. Brain Behav. Immun. 2017;60:293–303. doi: 10.1016/j.bbi.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 123.Alam A., Hana Z., Jin Z., Suen K.C., Ma D. Surgery, neuroinflammation and cognitive impairment. EBioMedicine. 2018;37:547–556. doi: 10.1016/j.ebiom.2018.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lim S.W., Shiue Y.L., Liao J.C., Wee H.Y., Wang C.C., Chio C.C., Chang C.H., Hu C.Y., Kuo J.R. Simvastatin therapy in the acute stage of traumatic brain injury attenuates brain trauma-induced depression-like behaviour in rats by reducing neuroinflammation in the hippocampus. Neurocrit. Care. 2017;26:122–132. doi: 10.1007/s12028-016-0290-6. [DOI] [PubMed] [Google Scholar]
- 125.Morandi A., Hughes C.G., Thompson J.L., Pandharipande P., Shintani A.K., Vasilevskis E.E., Han J.H., Jackson J.C., Laskowitz D.T., Bernard G.R., et al. Statins and Delirium During Critical Illness. Crit. Care Med. 2014;42:1899–1909. doi: 10.1097/CCM.0000000000000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Salluh J.I.F., Latronico N. Does this critically ill patient with delirium require any drug treatment? Intensiv. Care Med. 2019;45:501–504. doi: 10.1007/s00134-018-5310-x. [DOI] [PubMed] [Google Scholar]
- 127.Al-Qadheeb N.S., Skrobik Y., Schumaker G., Pacheco M.N., Roberts R.J., Ruthazer R.R., Devlin J.W. Preventing ICU Subsyndromal Delirium Conversion to Delirium With Low-Dose IV Haloperidol: A double-blind, placebo-controlles pilot study. Crit. Care Med. 2016;44:583–591. doi: 10.1097/CCM.0000000000001411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Felton M.A., Jarrett J.B., Hoffmaster R., D’Amico F.J., Sakely H., Proskowski J. Comparison of haloperidol, non-haloperidol antipsychotics, and no pharnmacotherapy for the management of delirium in an inpatient geriatric palliative care population. J. Pain Palliat. Care Pharmacother. 2018;32:141–148. doi: 10.1080/15360288.2018.1513434. [DOI] [PubMed] [Google Scholar]
- 129.Sher Y., Cramer A.C.M., Ament A., Lolak S., Maldonado J.R. Valproic Acid for Treatment of Hyperactive or Mixed Delirium: Rationale and Literature Review. Psychosomatics. 2015;56:615–625. doi: 10.1016/j.psym.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 130.Devlin J.W., Skrobik Y., Gélinas C., Needham D.M., Slooter A.J.C., Pandharipande P.P., Watson P.L., Weinhouse G.L., Nunnally M.E., Rochwerg B., et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit. Care Med. 2018;46:e825–e873. doi: 10.1097/CCM.0000000000003299. [DOI] [PubMed] [Google Scholar]
- 131.Girard T.D., Exline M.C., Carson S.S., Hough C.L., Rock P., Gong M.N., Douglas I.S., Malhotra A., Owens R.L., Feinstein D.J., et al. Haloperidol and Ziprasidone for Treatment of Delirium in Critical Illness. N. Engl. J. Med. 2018;379:2506–2516. doi: 10.1056/NEJMoa1808217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shah A.A., Aftab A., Coverdale J. QTc Prolongation with Antipsychotics: Is routine ECG monitoring recommended? J. Psychiatr. Pract. 2014;20:196–206. doi: 10.1097/01.pra.0000450319.21859.6d. [DOI] [PubMed] [Google Scholar]
- 133.Sher Y., Miller A.C., Lolak S., Ament A., Maldonado J.R. Adjunctive Valproic Acid in Management-Refractory Hyperactive Delirium: A Case Series and Rationale. J. Neuropsychiatry Clin. Neurosci. 2015;27:365–370. doi: 10.1176/appi.neuropsych.14080190. [DOI] [PubMed] [Google Scholar]
- 134.Cutuli D., De Guevara-Miranda D.L., Castilla-Ortega E., Santín L., Sampedro-Piquero P. Highlighting the Role of Cognitive and Brain Reserve in the Substance use Disorder Field. Curr. Neuropharmacol. 2019;17:1056–1070. doi: 10.2174/1570159X17666190617100707. [DOI] [PMC free article] [PubMed] [Google Scholar]