Abstract
Thrombotic antiphospholipid syndrome (APS) is characterised by venous, arterial and/or small vessel thrombosis in the context of persistently positive antiphospholipid antibodies (aPL). The diagnosis and management of thrombotic APS continues to prove challenging for clinicians. We provide a practical guide to the diagnosis of APS including who to test for aPL and which tests to do. We also consider clinical practice points on the management of venous, arterial and small vessel thrombosis, in the context of first and recurrent thrombotic events. Non-criteria manifestations of APS are reviewed. An approach to recurrent thrombosis and anticoagulant-refractory APS is discussed, with options including increasing the anticoagulation intensity of vitamin K antagonists, switching to low-molecular-weight-heparin, the use of fondaparinux and/or the addition of antiplatelet treatment. Adjunctive options such as vitamin D, hydroxychloroquine and statins are also addressed.
Keywords: Thrombotic antiphospholipid syndrome, Vitamin K antagonists, Direct oral anticoagulants, Recurrent thrombosis, Anticoagulant-refractory, Clinical practice points
Highlights
-
•
Approaches to diagnosis and management of thrombotic APS considered in recent guidelines are summarised.
-
•
Clinical practice points are suggested.
-
•
Recurrent thrombotic events while on anticoagulation are reviewed.
1. Introduction
Antiphospholipid syndrome (APS) is characterised by thrombotic (venous, arterial and/or microvascular) and/or obstetric morbidity in the context of persistently positive antiphospholipid antibodies (aPL; lupus anticoagulant [LA], IgG and/or IgM anti-beta-2 glycoprotein-1 [aβ2GP1] and anticardiolipin antibodies [aCL]) [1,2]. The criteria for APS diagnosis are being updated [3]. APS may be associated with other autoimmune conditions and has been estimated to be present in approximately 7–15% of patients with systemic lupus erythematosus (SLE) [4,5]. In this context it is associated with a more severe course [5]. The estimated prevalence of APS is 1 in 2000 of the general population [6].
APL are detected in 1–5% of the general population [7,8]. A ‘two hit’ pathogenic model of APS has been proposed, whereby aPL which are initially present, combine with a second stimulus such as an infection or inflammatory condition to provoke the clinical consequences [9]. Genetic background may predispose to the development of aPL, and epigenetic variation may contribute to clinical heterogeneity [10]. Associations between aPL and multiple human leucocyte antigen (HLA)-DR or -DQ are reported as well as a valine (247)/leucine polymorphism on β2GP1 that could represent a genetic risk for production of aβ2GP1 antibodies and APS [11].
Evidence from clinical and experimental studies, in vitro and in vivo, confirm that certain aPL are pathogenic, not just diagnostic for APS [[12], [13], [14]]. APL are directed against antiphospholipid binding proteins. Anti-β2GP1 appears to play a particularly important role [15]. A key initiating pathogenic process is the exposure of negatively charged endothelial surface phospholipid. Exogenous and circulating β2GP1 can bind to this phospholipid surface and change its conformation, exposing a cryptic Arg39-Arg43 epitope in domain I that is recognised by pathologic aPL [16,17]. The aPL-β2GP1 complex can bind to and activate endothelial cells, platelets and monocytes [18] and aPL binding can upregulate monocyte expression of tissue factor, a potent initiator of coagulation [19]. Other mechanisms include aPL interfering with the activated protein C pathway leading to acquired resistance to APC [17,20] and aPL-induced decreased fibrinolysis [21]. Neutrophils play a role in arterial and venous thrombosis through the extracellular release of material, mainly DNA and histones, known as neutrophil extracellular traps (NETs) [22]. Mice treated with APS IgG develop thrombi rich in NETs [22]. APL can activate the complement cascade. Blocking C3 and C5 activation in mice appears to block aPL induced thrombosis [23].
In this review, we summarise current approaches to the diagnosis and management of thrombotic APS, with suggested clinical practice points based on available guidance, evidence and clinical experience.
2. Diagnosis of APS
2.1. Who to test for APS
Antiphospholipid antibody testing should be performed when there are clinical features suggestive of APS. Guidance on this topic is available from the British Society for Haematology (BSH) and ISTH [2,24,25]. Suggested indications for aPL testing include patients with recurrent thrombosis unexplained by subtherapeutic anticoagulation, patient non-adherence, malignancy, and thrombosis in unusual sites (i.e. other than lower limb DVT or PE). Following a provoked venous thromboembolism (VTE) where the provoking environmental factor is disproportionately mild or in younger patients (<50 years) with an unprovoked thrombotic event [25], aPL testing should be considered, particularly if it is a major thrombotic event. In this regard, the presence of aPL strengthens the decision to offer life-long anticoagulation following a first unprovoked VTE. A systematic review reported that a positive aPL test appears to predict an increased risk of recurrence in patients with a first VTE, however, the quality of evidence was very low [26]. A more recent prospective study suggested that aPL and raised D-dimer levels are independent risk factors for recurrence after a first unprovoked VTE [27]. The risk of recurrent thrombosis following a provoked first VTE associated with aPL and the duration of anticoagulation in this situation is undefined. APL testing should be considered in younger patients (<50 years) with ischaemic stroke, transient ischaemic attack or other evidence of brain ischaemia and may be useful in new stroke patients in whom APS is clinically suspected, to decide whether the patient would benefit from an anticoagulant rather than antiplatelet therapy, current standard care [25]. Conventional cardiovascular risk factors may be present in patients with APS-related stroke. The adjusted Global APS score (aGAPSS), that incorporates independent cardiovascular risk factors along with aPL status, could aid risk stratification based on the likelihood of recurrent arterial thrombosis [28].
2.2. Which aPL tests to do and when
Table 1 provides a summary of the aPL tests that should be performed. Confirmation of a diagnosis of APS requires demonstration of persistent aPL, on two occasions, at least 12 weeks apart [1,2,25]. It is important that all three aPL tests (LA, aCL and aβ2GP1) are performed as the aPL phenotype influences thrombotic risk. Triple aPL-positivity (i.e. the presence of LA, IgG and/or IgM aβ2GP1 and IgG and/or IgM aCL positivity) is correlated with the highest risk of thrombosis [29]. Medium to high titres are considered to be clinically significant for thrombosis [1,30], whereas low IgG or IgM titres are not, although they may be relevant for pregnancy morbidity [31]. Indeed, the role of IgM aCL and aβ2GP1 is uncertain. A critical review was unable to evaluate IgM antibodies as a single serologic marker, because of unavailability of separate IgG and IgM results [32]. A multicentre study reported that there was no added value in testing for IgM in thrombotic APS (although the data supported testing in obstetric APS), but, combined positivity for LA, IgG, and IgM was highly associated with thrombosis and pregnancy morbidity, therefore IgM could be useful for risk stratification. However, only 55 patients had arterial thrombosis [33].
Table 1.
Testing for antiphospholipid antibodies.
| General principles | ||||
| ||||
| aCL IgG/IgM: | ||||
| ELISA/Chemiluminescence | Present in medium to high titre:
Local verification of manufacturer's reference ranges |
|||
| aβ2GP1 IgG/IgM: | ||||
| ELISA/Chemiluminescence | Present in medium to high titre:
Local verification of manufacturer's reference ranges |
|||
| No anticoagulation | LMWH/UFH | Vitamin K antagonist/DOAC | ||
| LA: Two tests using two different principles | DRVVT aPTT (PL sensitive reagents) SCT |
DRVVT | TVT/ECT are less affected by VKAs and anti-FXa DOACs. Their general use is pending upon the provision of independent evidence from collaborative studies with standardised kits | |
| Extended aPL testing | ||||
| IgA aCL/β2GP1 | ||||
| Antiphosphatidylserine/prothrombin antibodies | ||||
| Domain-1 and 5 β2GP1 | ||||
Abbreviations: aβ2GP1, anti-beta-2 glycoprotein-1 antibodies; aCL, anticardiolipin; aPL, antiphospholipid antibodies; LA, lupus anticoagulant; DRVVT, dilute Russell's viper venom time; aPTT, activated partial thromboplastin time; PL: phospholipid; TVT/ECT, Taipan snake venom time/Ecarin clotting time; UFH: unfractionated heparin.
The results of LA testing in the acute thrombotic situation should be interpreted with caution as there may be false positives due to raised factor VIII [34], false negatives due to raised C-reactive protein [35] and false negatives or positives related to concomitant anticoagulation [36]. Postinfection aPL are usually transient and generally unassociated with thrombosis [37]. A frequent single LA positivity during (acute phase) has been observed in COVID-19 infection but is not clearly related to thrombotic complications. Triple aPL positivity and high aCL/aβ2GPI titres are rare. Repeat testing suggests aPL are mostly transient [38]. The timing of LA testing following a first VTE (where DOACs are standard treatment), is pertinent. The 16th International Congress on aPL treatment trends task force report advises that: it may be preferable to defer screening for aPL for most patients with a new VTE in the acute setting. For those patients in whom there is clinical concern for APS, however (e.g., patients with a new VTE and obstetric or non-criteria manifestations of APS), testing can be performed with appropriate interpretation of the laboratory results [39].
Several noncriteria aPL, such as antibodies against the domain 1 of aβ2GP1 and anti-phosphatidylserine/prothrombin, and IgA aCL and aβ2GP1 have been studied. However, extended aPL testing (not widely available) is not recommended in routine practice as these antibodies do not have diagnostic utility, although they might aid in risk stratification [40]. Automated chemiluminescent assays have replaced ELISA for aCL and aβ2GP1 in some centres. The advantages of chemiluminescence include faster turnaround times, random access rather than batched assays, and improved reproducibility of results and inter- and intra-laboratory variation with automation [41]. High-avidity anti-protein C antibodies are associated with resistance to activated protein C and may provide a marker for a severe thrombotic phenotype in APS [42].
3. Management of APS related thrombosis
Warfarin or other vitamin K antagonists (VKAs) at therapeutic intensity are the standard treatment for thrombotic APS. The European Medicines Agency (EMA) has recommended that direct oral anticoagulants (DOACs) should not be used in APS, especially those that are triple aPL-positive [43]. This recommendation, which followed a risk assessment triggered by the TRAPS (Rivaroxaban in Thrombotic APS) trial [44], has been widely adopted by regulatory authorities internationally. It should be noted that the EMA recommendation does not constitute a contraindication to the use of DOACs in APS [45].
3.1. First venous thromboembolic event
Direct oral anticoagulants are the standard treatment for a first VTE [24,39,45] episode in general population patients, based on large phase 3 trials [46]. The prevalence of aPL following a first unprovoked VTE is 9% [27,47], and thus it is likely that many of these patients will have undiagnosed APS, without any reported increased risk of thrombosis. Table 2 provides a summary of the guidance from professional bodies for antithrombotic treatment in thrombotic APS.
Table 2.
Antithrombotic treatment for first thrombotic event in APS.
| Guidance | Venous | Arterial | Small vessel thrombosisa |
|---|---|---|---|
| International Congress on Antiphospholipid Antibodies (2020) |
|
DOACs should be avoided First line therapy should be a VKA |
DOACs should be avoided Use VKA as first line if anticoagulation elected |
| International Society on Thrombosis and Haemostasis (2020) |
|
Use VKA instead of DOACs | Use VKA instead of DOACs |
| British Society for Haematology Addendum (2020) | VKA if known triple aPL-positive If on a DOAC and is triple aPL-positive:
|
Recommend VKA and do not recommend DOAC | N/A |
| British Society for Haematology (2012) | VKA range 2.0–3.0 | VKA range 2.0–3.0 or antiplatelet therapy | N/A |
| European League Against Rheumatism (2019) | Treatment with VKA with a target INR 2–3 is recommended Rivaroxaban should not be used in patients with triple aPL positivity due to the high risk of recurrent events. DOACs could be considered in patients not able to achieve a target INR despite good adherence to VKA or those with contraindications to VKA (e.g., allergy or intolerance to VKA) |
Treatment with VKA is recommended over treatment with LDA only Treatment with VKA with INR 2–3 or INR 3–4 is recommended, considering the individual's risk of bleeding and recurrent thrombosis. Treatment with VKA with INR 2–3 plus LDA may also be considered |
N/A |
| American College of Chest Physicians (2012) | VKA INR range (INR 2.0–3.0) rather than higher intensity (INR 3.0–4.5) | VKA INR range (INR 2.0–3.0) rather than higher intensity (INR 3.0–4.5) | N/A |
Abbreviations: aPL, antiphospholipid antibodies; APS, antiphospholipid syndrome; DOAC, direct oral anticoagulant; INR, international normalised ratio; LDA, low dose aspirin; LMWH, low-molecular-weight-heparin; N/A, not addressed; VKA, vitamin K antagonist; VTE, venous thromboembolism.
Very low quality data.
Two randomised controlled trials about 15 years ago, the first included 75/109 [48] with venous thrombosis and the second 87/114 [49], concluded that the optimal target INR for VTE in APS is 2.5 (range 2.0–3.0, standard-intensity). The RAPS (Rivaroxaban in Antiphospholipid Syndrome) trial which was not designed or powered for clinical outcomes randomised 116 patients with previous VTE, to rivaroxaban 20 mg once daily or standard intensity warfarin target INR 2.5. The RAPS trial reported no recurrent thrombotic or bleeding events in the rivaroxaban or warfarin arms in a 7 month follow up period. Overall, 28% were triple aPL-positive [50]. Another randomised controlled trial (RCT) trial suggested that DOACs are not associated with recurrent VTE [51]. A single arm pilot feasibility study of rivaroxaban in 82 APS patients with prior VTE which indicated that the rates of thrombosis and bleeding after at least a year of follow up, were comparable to previous RCTs with no new safety signals identified [52]. However, an individual patient data meta-analysis reported that 58% (18/31) of patient with recurrent arterial thrombosis while on a DOAC had previous VTE alone, with 18/31 of these patients triple aPL-positive [53]. Consideration of the use of DOACs following first APS-related VTE therefore requires a nuanced approach.
3.1.1. Clinical practice points (see Table 2 for further details)
-
•
Optimisation of risk factors for thrombosis and active management of bleeding risk factors
-
•
Initiate VKA in patients known to have aPL
-
•
For single or double aPL-positive patients on a DOAC for first VTE as standard of care, continuation of the DOAC may be considered, with patient involvement in the decision, based on discussion of perceived risks, benefits and uncertainties, for shared decision-making.
-
•
For triple aPL-positive patients on a DOAC for first VTE as standard of care, explain to the patient that it is recommended that the DOAC is switched to a VKA. For those who elect to remain on a DOAC, clinical surveillance, is important. This might include magnetic resonance imaging (MRI) brain imaging to identify ischaemic lesions, which if present merit consideration of a switch to an alternative anticoagulant, with the first option a VKA [39].
3.2. First arterial thrombotic event
The prevalence of stroke and transient ischaemic attack (TIA) at presentation with APS in a prospective cohort of 1000 patients was 13.1% and 7.0%, respectively [54]. Twenty percent of APS patients in this cohort developed a stroke [55]. Approximately 17% of strokes in patients under the age of 50 are associated with aPL [56] The spectrum of ischaemic brain lesions in APS encompasses white matter hyperintensities (WMH) of presumed vascular origin [57] which have face validity, being associated with clinically important outcomes of stroke, dementia and death [58,59]. APS patients are also at increased risk of myocardial infarction (MI) [60], with aPL reported in 11% of patients with MI [61]. Other arterial thrombotic events such as renal artery thrombosis [62] and peripheral arterial ischaemia [63] can occur. Table 2 provides a summary of current guidance for anticoagulation, including intensity, and antiplatelet treatment. VKAs remain the standard of care, although the optimal anticoagulation intensity is undefined. The variability in the guidance reflects the lack of appropriate, adequately powered studies to guide optimal antithrombotic and/or antiplatelet treatment in APS patients with arterial thrombotic manifestations.
Two randomised controlled trials [48,49] concluded that the optimal target INR for thrombotic APS is 2.5 (range 2.0–3.0). In both studies, patients with arterial thrombosis were under-represented with 44/109 previous arterial (34 had arterial only) [48] and 27/114 in the other [49]. A third study, the Antiphospholipid Antibodies and Stroke Study (APASS), was a prospective cohort study which reported no benefit of warfarin anticoagulation (INR target range: 1.4–2.8) over aspirin (325 mg/day) in stroke prevention [64]. Laboratory criteria for aPL did not fulfil the international consensus criteria for a diagnosis of APS [1]. The TRAPS trial compared rivaroxaban 20 mg once daily with warfarin, target INR 2.5, in 120 triple aPL-positive thrombotic APS patients [44]. Seven patients in the rivaroxaban arm (approximate annualised recurrent thrombosis rate 7.5%) had new arterial thrombotic events (four ischaemic stroke and three myocardial infarctions) compared with 0% in the warfarin arm. Four of the seven patients had previous arterial thrombosis [44].
A systematic review of 16 studies on secondary thromboprophylaxis in patients with aPL found that most recurrent thromboses (venous and arterial) occur in patients not receiving anticoagulation, or on antiplatelet treatment alone. Recurrent events were least likely to occur in those on warfarin, INR >3 [65]. A recent meta-analysis reported that patients with APS who had an initial arterial thrombotic event had a recurrence rate over 2 years of 16% and 18% when taking anticoagulation or aspirin alone, respectively. The majority of patients taking anticoagulation were taking either a DOAC at a standard therapeutic dose, or warfarin with an INR range of 2.0–3.0 [66]. The numbers of patients on DOAC versus VKA were not specified. The doses of DOACs reported in the literature in APS patients have been shown to be efficacious vs. standard-intensity warfarin in the phase three trials in general population patients with a first VTE [46]. These doses may not however, be effective to prevent arterial thrombosis [67]. The RISAPS (Rivaroxaban in Stroke Patients with APS) phase 2/3 RCT aims to assess the efficacy of high-intensity rivaroxaban 15 mg twice daily versus high-intensity warfarin, target INR 3.5 (range 3.0–4.0) in patients with APS with previous ischaemic stroke or other ischaemic brain manifestations (ClinicalTrials.gov Identifier: NCT03684564).
3.2.1. Clinical practice points (see Table 2 for further details)
-
•
Conventional arterial risk factors such as hypertension, hyperlipidaemia, being overweight, poor glycaemic control and smoking, should be optimised to minimise the risk of recurrent arterial thrombosis
-
•
Patients known to have aPL should be initiated on anticoagulation – the current standard treatment for APS patients with arterial thrombosis is VKA
-
•
Antithrombotic options comprise VKA at target INR range 2.0–3.0, with or without low dose aspirin, or target INR 3.0–4.0
-
•
The INR target should be determined on an individual basis, balancing the risk of permanent disability including cognitive impairment and/or death due to recurrent stroke/ischaemic brain lesions versus the risk of bleeding.
3.3. Small vessel thrombosis
Small vessel thrombosis is best described in the context of catastrophic antiphospholipid syndrome (CAPS), where it is a defining criteria [68,69], but is outwith the scope of this review. Small vessel thrombosis also occurs in the subacute/chronic setting, although the literature in this area is scant. Histology is desirable for diagnostic accuracy, but is not always feasible depending on the risk of biopsy which varies with site, severity of illness and bleeding risk [68].
Thrombosis of the major renal arteries or veins can occur, but small vessel disease affecting the kidneys is a distinct phenomenon known as aPL-related nephropathy [70,71]. Hypertension, proteinuria, hematuria, and renal insufficiency are the most common manifestations of aPL-related nephropathy [71]. Small vessel thrombosis can have cutaneous manifestations, for example cutaneous digital gangrene, or necrotic skin ulceration which on biopsy is secondary to diffuse non-inflammatory thrombosis [72,73]. Cognitive dysfunction in APS is associated with the presence of WMH on MRI, which are presumed to be secondary to small vessel thrombosis [58]. Other small vessel thrombosis has been described including the pulmonary vasculature [74] and osteonecrosis [75].
Patients with small vessel thrombosis in the presence of persistent aPL antibodies fulfil the criteria for APS. Anticoagulation is widely used although without a strong evidence base.
3.3.1. Clinical practice points (see Table 2 for further details)
-
•
Anticoagulation is reasonable to use on an empirical basis
-
•
Anticoagulant options include VKA and, particularly if thrombocytopenia is present, LWMH
-
•
DOACs should be avoided, unless in the context of a clinical trial
-
•
Co-existent lupus nephritis, if present, should be actively managed
-
•
Empirical options that may be considered in severe cases include rituximab, intravenous immunoglobulins, plasma exchange, eculizumab, vasodilators, surgical interventions such as sympathectomy and hyperbaric oxygen therapy [76].
3.4. Non-criteria APS manifestations
Non-criteria APS manifestations include thrombocytopenia, aPL-related cardiac valve disease (vegetation, valve thickening and dysfunction), aPL-related nephropathy, skin ulcers (pyoderma gangrenosum-like or livedoid vasculitis) or cognitive dysfunction [1,77]. These are generally refractory to anticoagulation. Thrombocytopenia, platelet count <150 × 109/L, has a prevalence of between 16 and 53% [78] in APS patients and appears to be associated with increased risk of thrombosis [79]. A meta-analysis of 23 primary echocardiographic studies demonstrated that compared with SLE patients without aPL (n = 988), the overall pooled odds ratios for cardiac valve disease and Libman-Sacks endocarditis in aPL-positive patients (n = 668) were 3.13 (95% CI, 2.31–4.24) and 3.51 (95% CI, 1.93–6.38), respectively. LA and IgG aCL aPL subtypes conferred the greatest risk of cardiac valve disease at 5.88 (95% CI, 2.92–11.84) and 5.63 (95% CI, 3.53–8.97) respectively [80].
The RITAPS (Rituximab in APS) phase 2 study of rituximab, an anti-CD20 monoclonal antibody, in 19 APS patients with non-criteria APS manifestations, including thrombocytopenia, cardiac valve disease, skin ulceration, aPL nephropathy, and/or cognitive dysfunction reported that rituximab showed benefit for some non-criteria manifestations, notably skin ulceration and cognitive dysfunction, with the safety in aPL positive patients consistent with the safety profile of rituximab. The authors concluded that despite no substantial change in aPL profiles, rituximab may be effective in controlling some but not all non-criteria manifestations of APS [81].
3.4.1. Clinical practice points
-
•
Management is empirical and rituximab merits consideration
-
•
Anticoagulation to be considered if small vessel thrombosis might be implicated
4. Recurrent and anticoagulant-refractory thrombotic APS
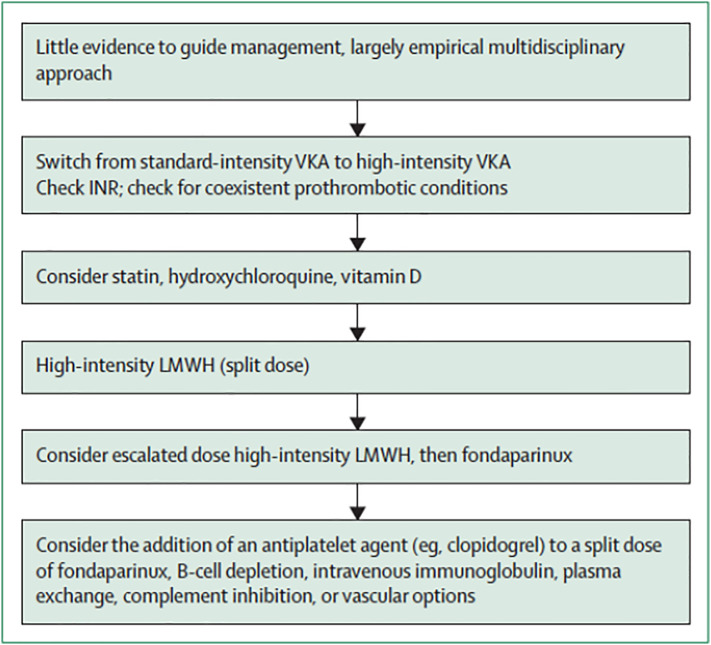
Anticoagulant-refractory APS is defined as thrombotic APS breakthrough thrombosis on therapeutic anticoagulation. This is a different entity to recurrent thromboses that occur due to a subtherapeutic INR, which could be related to non-adherence or a spurious therapeutic INR due to LA effect on thromboplastin producing a false elevation of the INR [82]. If re-thrombosis occurs while on therapeutic intensity VKA, further management is empirical and extrapolated from management of similar situations in patients with other disorders, such as cancer related thrombosis [76]. Antithrombotic treatment options include high intensity VKA, LMWH and fondaparinux, with or without the addition of an antiplatelet agent (see Fig. 1 ).
Fig. 1.
Proposed management for anticoagulant-refractory thrombotic antiphospholipid syndrome (Ref: [76] Reproduced from Cohen et al.).
Abbreviations: LMWH, low-molecular-weight heparin; VKA, vitamin K antagonist.
4.1. Recurrent thrombotic events while on anticoagulation
4.1.1. Oral anticoagulants
Several studies have reported on recurrent thrombotic events while on oral anticoagulation. In patients on VKA, a prospective observational study of 1000 APS patients reported that 25% of patients on antithrombotic treatment (80% on anticoagulation with or without antiplatelet treatment and 20% on antiplatelet treatment alone) developed thrombosis after 5 to 10 years of follow up [55]. A retrospective study of triple-aPL APS patients, demonstrated that 29% (36/123) of patients treated with VKA, target INR 2.5 (range 2.0–3.0) experienced recurrent thrombotic events, 44% venous and 56% arterial [29]. Recurrent thrombosis rates in two RCTs evaluating standard-intensity versus high-intensity warfarin were 3.4% (2/58) and 10.7% (6/56) [49]; and 5.5% (3/55) and 11.1% (6/54) [48] in the standard and high intensity warfarin arms, respectively. Notably, in the first study, the majority (6/8) of recurrent thrombotic events occurred while the INR was <3.0 [49]. In the second study recurrent thrombosis was observed in 6/54 (11.1%) assigned to high-intensity warfarin and 3/55 (5.5%) to standard-intensity, but the INR at the time of re-thrombosis was not reported [48]. A systematic review of APS patients treated with a DOAC (290 rivaroxaban, 144 dabigatran and 13 apixaban) showed that 16% (73/447) developed one or more recurrent thromboses with the mean time before thrombotic event 12.5 months. Recurrent VTE occurred in 28 patients, arterial thromboses in 31 patients, small vessel thrombosis in 13 patients and site of recurrence missing in 8 patients. Of those who were triple aPL positive, the rate of recurrence was 56% [53].
4.1.2. Parenteral anticoagulants
Considering recurrent thrombosis on parenteral anticoagulation, one study evaluated the use of LMWH in venous or arterial disease 24 APS patients, 16 of these had failed warfarin therapy. It highlighted that LMWH was safe and an effective alternative [83]. A further retrospective review examined the use of LMWH in 23 APS patients who had intolerance or lack of response to warfarin. The mean duration of LMWH therapy was 36 months. Only three patients treated with LMWH had no clinical improvement/recurrent thrombosis suggesting the LMWH may be an alternative to warfarin [84]. Fondaparinux, a synthetic analogue of heparin pentasaccharide required for antithrombin binding, is considered when warfarin and LMWH have failed. We reported the use of fondaparinux in three patients with anticoagulant-refractory thrombotic APS who remained event free over 40 months of follow-up [85].
4.1.3. Clinical practice points (see Table 3 for further details)
Table 3.
Antithrombotic treatment for recurrent thrombotic event in APS.
| Guidance | Recurrent venous thrombosis | Recurrent arterial thrombosis |
|---|---|---|
| International Congress on Antiphospholipid Antibodies (2020) | DOACs should not be used for recurrent thrombosis while on standard-intensity VKA. Other treatment options include increased INR target range, standard treatment dose LMWH, fondaparinux if VKA/LMWH not suitable, or the addition of antiplatelet therapy | |
| International Society on Thrombosis and Haemostasis (2020) | DOACs should not be used for recurrent thrombosis while on therapeutic intensity VKA In this circumstance, other therapeutic options may include an increased target INR range, treatment dose LMWH, or the addition of antiplatelet therapy |
|
| British Society for Haematology Addendum (2020) | N/A | N/A |
| British Society for Haematology (2012) | N/A | N/A |
| European League Against Rheumatism (2019) | Investigation of, and education on, adherence to VKA treatment, along with frequent INR testing, should be considered If the target INR of 2–3 had been achieved, addition of LDA, increase of INR target to 3–4 or change to LMWH may be considered |
In patients with recurrent arterial thrombosis despite adequate treatment with VKA, after evaluating for other potential causes, an increase of INR target to 3–4, addition of LDA or switch to LMWH can be considered |
| American College of Chest Physicians (2016) (not specific for APS) | If not on LMWH consider switching to LMWH If recurrent VTE on LMWH, suggests increasing the dose of LMWH (by a quarter to a third) |
N/A |
-
•
Suspected recurrent thrombosis requires appropriate objective imaging and documentation with comparison made with previous available imaging where possible
-
•
If the patient is being treated with a VKA, the INR at the time of recurrence should be checked to assess whether or not the thrombosis occured on therapeutic anticoagulation. Chromogenic factor X levels provide an LA-independent measure of anticoagulation intensity but are not widely available and a therapeutic range is not established [82]
-
•
Additional provoking factors for thrombosis need to be considered, such as malignancy
-
•
Prior to making any adjustment to anticoagulation treatment, reassess bleeding risk factors and evaluate full blood count, renal function and weight, to inform appropriate anticoagulation dosing
-
•
Following recurrent thrombosis while on therapeutic VKA, options include high-intensity VKA, LMWH, fondaparinux and/or addition of antiplatelet treatment
-
•
If recurrent thrombosis occurs on standard-treatment dose LMWH, this may be increased by one-quarter to one-third [86,87], using split dose (i.e. divided total dose given twice-daily) and consideration of monitoring with anti-Xa levels
4.2. Adjunctive treatment for APS-related thrombosis
Table 4 outlines the adjunctive treatment options for the management of APS-related thrombosis.
Table 4.
Adjunctive treatment.
| Pathophysiology | Evidence for clinical use |
|---|---|
| Vitamin D | |
Protect against thrombosis through:
|
|
| Hydroxychloroquinea | |
HCQ has immunomodulatory and antithrombotic effects mediated through:
|
|
| Statins | |
| Fluvastatin and simvastatin can prevent aβ2GP1-antibodies inducing endothelial cell adhesive properties via NF-κB binding to DNA which plays a central role in inflammation | Elevated levels of VEGF, soluble TF and TNF-α were identified in APS patients and that fluvastatin was able to significantly reduce those markers in the majority of treated patients |
Abbreviations: APS, antiphospholipid syndrome; β2GP1, βeta-2 glycoprotein-1; GPIIbIIIa, glycoprotein IIaIIIb; HCQ, hydroxychloroquine; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; TF, tissue factor; TLR, toll-like receptor; MyDD88, differentiation primary response gene 88; TNF-α, tumour necrosis factor-α; VEGF, vascular endothelial growth factor; VKA, vitamin K antagonist(s).
Hydroxychloroquine is standard treatment in patients with SLE if no contraindications.
4.2.1. Obstetric APS
The clinical manifestations of obstetric APS include three or more consecutive pregnancy losses at less than ten-week gestation, one or more fetal death at ≥10 weeks gestation or ≥one preterm delivery for severe preeclampsia and/or placental insufficiency. The pathogenesis of this condition is not yet fully understood. The management consists of LMWH combined with low dose aspirin. A more detailed review of the management of obstetric APS has been published elsewhere [88].
5. Conclusion
We provide clinical guidance to facilitate the diagnosis and optimal management of thrombotic APS. Testing for aPL antibodies is appropriate in certain situations, notably where the provoking factor is disproportionately mild compared to the thrombotic event. APL testing while on anticoagulation is particularly challenging. Arterial presentations of APS are commonly TIA and stroke. Patients with a first VTE are usually treated with a DOAC. It is agreed, however, that DOACs should be avoided in APS-related arterial thrombosis. There is a lack of evidence on how best to manage small vessel thrombosis and the non-criteria manifestations of APS. Recurrent and anticoagulant-refractory thrombotic APS must first be confirmed with imaging but the options for management are limited, though they include increasing the intensity of VKA, switching to LMWH, fondaparinux and/or the addition of an antiplatelet agent. Adjunctive agents such as hydroxychloroquine, statins and vitamin D may also help to improve outcomes for thrombotic APS patients.
References
- 1.Miyakis S., Lockshin M.D., Atsumi T. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) J. Thromb. Haemost. 2006;4(2):295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 2.Devresse K.M.J, Ortel T., Pengo V., De Laat B. Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibodies. Laboratory criteria for antiphospholipid syndrome: communication from the SSC of the ISTH. J. Thromb. Haemost. 2018;16(4):809–813. doi: 10.1111/jth.13976. [DOI] [PubMed] [Google Scholar]
- 3.Barbhaiya M., Zuily S., Ahmadzadeh Y., Naden R., Costenbader K., Erkan D., On behalf of the New APS Classification Criteria Collaborators Development on new international classification criteria for antiphospholipid syndrome: phase II results. Am. Coll. Rheumatol. 2019 Abstract Number 145. [Google Scholar]
- 4.Gracia-Tello B., Jones A., Raine C., Isenberg D. Systemic Lupus Erythematosus: Detailed Anatomy of a Cohort (Follow-up for More Than 35 Years) Arthritis Rheum. 2016;68(suppl 10) [Google Scholar]
- 5.Ruiz-Irastorza G., Egurbide M., Ugalde J., Aguirre C. High impact of antiphospholipid syndrome on irreversible organ damage and survival of patients with systemic lupus erythematosus. Arch. Intern. Med. 2004;164(1):77–82. doi: 10.1001/archinte.164.1.77. [DOI] [PubMed] [Google Scholar]
- 6.Duarte-Garcia A., Pham M., Crowson C. 189 epidemiology of antiphospholipid syndrome: a population-based study. 189 Epidemiology of Antiphospholipid Syndrome: A Population-based Study. 2019 doi: 10.1002/art.40901. A143-A143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi W., Krilis S., Chong B., Gordon S., Chesterman C. Prevalence of lupus anticoagulant and anticardiolipin antibodies in a healthy population. Aust. NZ J. Med. 1990;20(3):231–236. doi: 10.1111/j.1445-5994.1990.tb01025.x. [DOI] [PubMed] [Google Scholar]
- 8.Levine J.S., Branch D.W., Rauch J. The antiphospholipid syndrome. N. Engl. J. Med. 2002;346(10):752–763. doi: 10.1056/NEJMra002974. [DOI] [PubMed] [Google Scholar]
- 9.Bordin G., Boldorini R., Meroni P. The two hit hypothesis in the antiphospholipid syndrome: acute ischaemic heart involvement after valvular replacement despite anticoagulation in a patient with secondary APS. Lupus. 2003;12(11):851–853. doi: 10.1191/0961203303lu445cr. [DOI] [PubMed] [Google Scholar]
- 10.Ortiz-Fernández L., Sawalha A.H. Genetics of antiphospholipid syndrome. Curr. Rheumatol. Rep. 2019;21(12):65. doi: 10.1007/s11926-019-0869-y. [DOI] [PubMed] [Google Scholar]
- 11.Castro-Marrero J., Balada E., Vilardell-Tarrés M., Ordi-Ros J. Genetic risk factors of thrombosis in the antiphospholipid syndrome. Br. J. Haematol. 2009;147(3):289–296. doi: 10.1111/j.1365-2141.2009.07831.x. [DOI] [PubMed] [Google Scholar]
- 12.Giannakopoulos B., Krilis S.A. The pathogenesis of the antiphospholipid syndrome. N. Engl. J. Med. 2013;368(11):1033–1044. doi: 10.1056/NEJMra1112830. [DOI] [PubMed] [Google Scholar]
- 13.Pierangeli S.S., Chen P.P. Raschi E, et al. Antiphospholipid antibodies and the antiphospholipid syndrome: pathogenic mechanisms. 2008;34(03):236–250. doi: 10.1055/s-0028-1082267. [DOI] [PubMed] [Google Scholar]
- 14.Meroni P.L., Borghi M.O., Raschi E., Tedesco F. Pathogenesis of antiphospholipid syndrome: understanding the antibodies. Nat. Rev. Rheumatol. 2011;7(6):330. doi: 10.1038/nrrheum.2011.52. [DOI] [PubMed] [Google Scholar]
- 15.McDonnell T., Wincup C., Buchholz I. The role of beta-2-glycoprotein I in health and disease associating structure with function: more than just APS. Blood Reviews. 2020;39:100610. doi: 10.1016/j.blre.2019.100610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agar C., van Os G.M., Morgelin M. Beta2-glycoprotein I can exist in 2 conformations: implications for our understanding of the antiphospholipid syndrome. Blood. 2010;116(8):1336–1343. doi: 10.1182/blood-2009-12-260976. [DOI] [PubMed] [Google Scholar]
- 17.Schreiber K., Sciascia S., de Groot P.G. Antiphospholipid syndrome. Nat. Rev. Dis. Primers. 2018;4:17103. doi: 10.1038/nrdp.2017.103. [DOI] [PubMed] [Google Scholar]
- 18.De Groot P., Urbanus R., Derksen R. Pathophysiology of thrombotic APS: where do we stand? Lupus. 2012;21(7):704–707. doi: 10.1177/0961203312438631. [DOI] [PubMed] [Google Scholar]
- 19.Zhou H., Wolberg A.S., Roubey R.A. Characterization of monocyte tissue factor activity induced by IgG antiphospholipid antibodies and inhibition by dilazep. Blood. 2004;104(8):2353–2358. doi: 10.1182/blood-2004-01-0145. [DOI] [PubMed] [Google Scholar]
- 20.Ramirez G.A, Efthymiou M., Isenberg D.A. Under crossfire: thromboembolic risk in systemic lupus erythematosus. Rheumatology (Oxford) 2019;58:940–952. doi: 10.1093/rheumatology/key307. [DOI] [PubMed] [Google Scholar]
- 21.Vikerfors A., Svenungsson E., Ågren A. Studies of fibrin formation and fibrinolytic function in patients with the antiphospholipid syndrome. Thromb. Res. 2014;133(5):936–944. doi: 10.1016/j.thromres.2014.02.023. [DOI] [PubMed] [Google Scholar]
- 22.Meng H., Yalavarthi S., Kanthi Y. In vivo role of neutrophil extracellular traps in antiphospholipid antibody–mediated venous thrombosis. Arthritis & rheumatology. 2017;69(3):655–667. doi: 10.1002/art.39938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pierangeli S.S., Girardi G., Vega-Ostertag M., Liu X., Espinola R.G., Salmon J. Requirement of activation of complement C3 and C5 for antiphospholipid antibody–mediated thrombophilia. Arthritis & Rheumatism. 2005;52(7):2120–2124. doi: 10.1002/art.21157. [DOI] [PubMed] [Google Scholar]
- 24.Arachchillage D.R., Gomez K., Alikhan R. Addendum to British Society for Haematology guidelines on investigation and management of antiphospholipid syndrome, 2012 (Br. J. Haematol. 2012; 157: 47–58): use of direct acting oral anticoagulants. Br. J. Haematol. 2020;189(2):212–215. doi: 10.1111/bjh.16308. [DOI] [PubMed] [Google Scholar]
- 25.Devreese K.M.J., de Groot P.G., de Laat B. Guidance from the Scientific and Standardization Committee for lupus anticoagulant/antiphospholipid antibodies of the International Society on Thrombosis and Haemostasis: Update of the guidelines for lupus anticoagulant detection and interpretation. J. Thromb. Haemost. 2020;18:2828–2839. doi: 10.1111/jth.15047. [DOI] [PubMed] [Google Scholar]
- 26.Garcia D., Akl E.A., Carr R., Kearon C. Antiphospholipid antibodies and the risk of recurrence after a first episode of venous thromboembolism: a systematic review. Blood, The Journal of the American Society of Hematology. 2013;122(5):817–824. doi: 10.1182/blood-2013-04-496257. [DOI] [PubMed] [Google Scholar]
- 27.Kearon C., Parpia S., Spencer F.A. Antiphospholipid antibodies and recurrent thrombosis after a first unprovoked venous thromboembolism. Blood. 2018;131(19):2151–2160. doi: 10.1182/blood-2017-09-805689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Radin M., Sciascia S., Erkan D. 2019. The Adjusted Global Antiphospholipid Syndrome Score (aGAPSS) and the Risk of Recurrent Thrombosis: Results From the APS ACTION Cohort. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pengo V., Ruffatti A., Legnani C. Clinical course of high-risk patients diagnosed with antiphospholipid syndrome. J. Thromb. Haemost. 2010;8(2):237–242. doi: 10.1111/j.1538-7836.2009.03674.x. [DOI] [PubMed] [Google Scholar]
- 30.Devresse K.M.J, Pierangeli S., De Laat B. Testing for antiphospholipid antibodies with solid phase assays: guidance from the SSC of the ISTH. J. Thromb. Haemost. 2014;12(5):792–795. doi: 10.1111/jth.12537. [DOI] [PubMed] [Google Scholar]
- 31.Arachchillage D.R.J., Machin S.J., Mackie I.J., Cohen H. Diagnosis and management of non-criteria obstetric antiphospholipid syndrome. Thromb. Haemost. 2015;113(01):13–19. doi: 10.1160/TH14-05-0416. [DOI] [PubMed] [Google Scholar]
- 32.Kelchtermans H., Pelkmans L., De Laat B., Devreese K. IgG/IgM antiphospholipid antibodies present in the classification criteria for the antiphospholipid syndrome: a critical review of their association with thrombosis. J. Thromb. Haemost. 2016;14(8):1530–1548. doi: 10.1111/jth.13379. [DOI] [PubMed] [Google Scholar]
- 33.Chayoua W., Kelchtermans H., Gris J. The (non-) sense of detecting anti-cardiolipin and anti-β2glycoprotein I IgM antibodies in the antiphospholipid syndrome. J. Thromb. Haemost. 2020;18(1):169–179. doi: 10.1111/jth.14633. [DOI] [PubMed] [Google Scholar]
- 34.Ten Boekel E., Böck M., Vrielink G., Liem R., Hendriks H., de Kieviet W. Detection of shortened activated partial thromboplastin times: an evaluation of different commercial reagents. Thromb. Res. 2007;121(3):361–367. doi: 10.1016/j.thromres.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 35.Devreese K.M., Verfaillie C.J., De Bisschop F., Delanghe J.R. Interference of C-reactive protein with clotting times. Clinical Chemistry and Laboratory Medicine (CCLM). 2015;53(5):e141–e145. doi: 10.1515/cclm-2014-0906. [DOI] [PubMed] [Google Scholar]
- 36.Tripodi A., Cohen H., Devreese K.M. Lupus anticoagulant detection in anticoagulated patients. Guidance from the Scientific and Standardization Committee for Lupus Anticoagulant/Antiphospholipid Antibodies of the International Society on Thrombosis and Haemostasis. Journal of Thrombosis and Haemostasis. 2020;18:1569–1575. doi: 10.1111/jth.14846. [DOI] [PubMed] [Google Scholar]
- 37.Martirosyan A., Aminov R., Manukyan G. Environmental triggers of autoreactive responses: induction of antiphospholipid antibody formation. Front. Immunol. 2019;10:1609. doi: 10.3389/fimmu.2019.01609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Devreese K.M., Linskens E.A., Benoit D., Peperstraete H. Antiphospholipid antibodies in patients with COVID-19: a relevant observation? J. Thromb. Haemost. 2020;18(9):2191–2201. doi: 10.1111/jth.14994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cohen H., Cuadrado M.J., Erkan D. 16th International Congress on Antiphospholipid Antibodies Task Force report on antiphospholipid syndrome treatment trends. Lupus. 2020;29:1571–1593. doi: 10.1177/0961203320950461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Devreese K.M. Testing for antiphospholipid antibodies: advances and best practices. Int. J. Lab. Hematol. 2020;42:49–58. doi: 10.1111/ijlh.13195. [DOI] [PubMed] [Google Scholar]
- 41.Zhou J., Hou X., Zhang H., Wang T., Cui L. The clinical performance of a new chemiluminescent immunoassay in measuring anti-beta2 glycoprotein 1 and anti-cardiolipin antibodies. Med. Sci. Monit. 2018;24:6816–6822. doi: 10.12659/MSM.910369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arachchillage D., Efthymiou M., Mackie I., Lawrie A., Machin S., Cohen H. Anti-protein C antibodies are associated with resistance to endogenous protein C activation and a severe thrombotic phenotype in antiphospholipid syndrome. J. Thromb. Haemost. 2014;12(11):1801–1809. doi: 10.1111/jth.12722. [DOI] [PubMed] [Google Scholar]
- 43.European Medicines Agency. EMA/PRAC/219985/2019. Pharmacovigilance Risk Assessment Committee (PRAC). https://www.ema.europa.eu/en/documents/prac-recommendation/prac-recommendations-signals-adopted-8-11-april-2019-prac-meeting_en.pdf. Updated 2019.
- 44.Pengo V., Denas G., Zoppellaro G. Rivaroxaban vs warfarin in high-risk patients with antiphospholipid syndrome. Blood. 2018;132(13):1365–1371. doi: 10.1182/blood-2018-04-848333. [DOI] [PubMed] [Google Scholar]
- 45.Zuily S., Cohen H., Isenberg D. Use of direct oral anticoagulants in patients with thrombotic antiphospholipid syndrome: guidance from the scientific and standardization committee of the international society on thrombosis and haemostasis. J. Thromb. Haemost. 2020;18:2126–2137. doi: 10.1111/jth.14935. [DOI] [PubMed] [Google Scholar]
- 46.van Es N., Coppens M., Schulman S., Middeldorp S., Buller H.R. Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: evidence from phase 3 trials. Blood. 2014;124(12):1968–1975. doi: 10.1182/blood-2014-04-571232. [DOI] [PubMed] [Google Scholar]
- 47.Miranda S., Park J., Le Gal G. Prevalence of confirmed antiphospholipid syndrome in 18–50 years unselected patients with first unprovoked venous thromboembolism. J. Thromb. Haemost. 2019;18:926–930. doi: 10.1111/jth.14720. [DOI] [PubMed] [Google Scholar]
- 48.Finazzi G., Marchioli R., Brancaccio V. A randomized clinical trial of high-intensity warfarin vs. conventional antithrombotic therapy for the prevention of recurrent thrombosis in patients with the antiphospholipid syndrome (WAPS) 1. J. Thromb. Haemost. 2005;3(5):848–853. doi: 10.1111/j.1538-7836.2005.01340.x. [DOI] [PubMed] [Google Scholar]
- 49.Crowther M.A., Ginsberg J.S., Julian J. A comparison of two intensities of warfarin for the prevention of recurrent thrombosis in patients with the antiphospholipid antibody syndrome. N. Engl. J. Med. 2003;349(12):1133–1138. doi: 10.1056/NEJMoa035241. [DOI] [PubMed] [Google Scholar]
- 50.Cohen H., Hunt B.J., Efthymiou M. Rivaroxaban versus warfarin to treat patients with thrombotic antiphospholipid syndrome, with or without systemic lupus erythematosus (RAPS): a randomised, controlled, open-label, phase 2/3, non-inferiority trial. Lancet Haematol. 2016;3:e426–36. doi: 10.1016/S2352-3026(16)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ordi-Ros J., Sáez-Comet L., Pérez-Conesa M. Rivaroxaban versus vitamin K antagonist in antiphospholipid syndrome: a randomized noninferiority trial. Ann. Intern. Med. 2019;171:685–694. doi: 10.7326/M19-0291. [DOI] [PubMed] [Google Scholar]
- 52.Legault K., Blostein M., Carrier M. A single-arm feasibility cohort study of rivaroxaban in antiphospholipid syndrome. Pilot and Feasibility Studies. 2020;6(1):1–7. doi: 10.1186/s40814-020-00594-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dufrost V., Risse J., Reshetnyak T. Increased risk of thrombosis in antiphospholipid syndrome patients treated with direct oral anticoagulants. results from an international patient-level data meta-analysis. Autoimmun. Rev. 2018 doi: 10.1016/j.autrev.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 54.Cervera R., Piette J., Font J. Antiphospholipid syndrome: clinical and immunologic manifestations and patterns of disease expression in a cohort of 1,000 patients. Arthritis & Rheumatism: Official Journal of the American College of Rheumatology. 2002;46(4):1019–1027. doi: 10.1002/art.10187. [DOI] [PubMed] [Google Scholar]
- 55.Cervera R., Serrano R., Pons-Estel G.J. Morbidity and mortality in the antiphospholipid syndrome during a 10-year period: a multicentre prospective study of 1000 patients. Ann. Rheum. Dis. 2015;74(6):1011–1018. doi: 10.1136/annrheumdis-2013-204838. [DOI] [PubMed] [Google Scholar]
- 56.Sciascia S., Sanna G., Khamashta M.A. The estimated frequency of antiphospholipid antibodies in young adults with cerebrovascular events: a systematic review. Ann. Rheum. Dis. 2015;74(11):2028–2033. doi: 10.1136/annrheumdis-2014-205663. [DOI] [PubMed] [Google Scholar]
- 57.Kaichi Y., Kakeda S., Moriya J. Brain MR findings in patients with systemic lupus erythematosus with and without antiphospholipid antibody syndrome. AJNR Am. J. Neuroradiol. 2014;35(1):100–105. doi: 10.3174/ajnr.A3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tektonidou M.G., Varsou N., Kotoulas G., Antoniou A., Moutsopoulos H.M. Cognitive deficits in patients with antiphospholipid syndrome: association with clinical, laboratory, and brain magnetic resonance imaging findings. Arch. Intern. Med. 2006;166(20):2278–2284. doi: 10.1001/archinte.166.20.2278. [DOI] [PubMed] [Google Scholar]
- 59.Debette S., Markus H.S. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2010;341:c3666. doi: 10.1136/bmj.c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nazir S., Tachamo N., Lohani S., Hingorani R., Poudel D.R., Donato A. Acute myocardial infarction and antiphospholipid antibody syndrome: a systematic review. Coron. Artery Dis. 2017;28(4):332–335. doi: 10.1097/MCA.0000000000000476. [DOI] [PubMed] [Google Scholar]
- 61.Andreoli L., Chighizola C.B., Banzato A. Estimated frequency of antiphospholipid antibodies in patients with pregnancy morbidity, stroke, myocardial infarction, and deep vein thrombosis: a critical review of the literature. Arthritis care & research. 2013;65(11):1869–1873. doi: 10.1002/acr.22066. [DOI] [PubMed] [Google Scholar]
- 62.Tektonidou M.G. Antiphospholipid syndrome nephropathy: from pathogenesis to treatment. Front. Immunol. 2018;9:1181. doi: 10.3389/fimmu.2018.01181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gavier B., Vazquez F., Gandara E. Antiphospholipid antibodies and lower extremity peripheral arterial disease–a systematic review and meta-analysis. Vasa. 2016;45(4):325–330. doi: 10.1024/0301-1526/a000545. [DOI] [PubMed] [Google Scholar]
- 64.Levine S.R., Brey R.L., Tilley B.C. Antiphospholipid antibodies and subsequent thrombo-occlusive events in patients with ischemic stroke. JAMA. 2004;291(5):576–584. doi: 10.1001/jama.291.5.576. [DOI] [PubMed] [Google Scholar]
- 65.Ruiz-Irastorza G., Hunt B.J., Khamashta M.A. A systematic review of secondary thromboprophylaxis in patients with antiphospholipid antibodies. Arthritis Care & Research. 2007;57(8):1487–1495. doi: 10.1002/art.23109. [DOI] [PubMed] [Google Scholar]
- 66.Ortel T.L., Meleth S., Catellier D. Recurrent thrombosis in patients with antiphospholipid antibodies and an initial venous or arterial thromboembolic event: a systematic review and meta-analysis. J. Thromb. Haemost. 2020 doi: 10.1111/jth.14936. [DOI] [PubMed] [Google Scholar]
- 67.Cohen H., Efthymiou M., Isenberg D.A. Use of direct oral anticoagulants in antiphospholipid syndrome: reply. J. Thromb. Haemost. 2020;18(1):259–261. doi: 10.1111/jth.14674. [DOI] [PubMed] [Google Scholar]
- 68.Legault K., Schunemann H., Hillis C. McMaster RARE-Bestpractices clinical practice guideline on diagnosis and management of the catastrophic antiphospholipid syndrome. J. Thromb. Haemost. 2018;16(8):1656–1664. doi: 10.1111/jth.14192. [DOI] [PubMed] [Google Scholar]
- 69.Cervera R., Bucciarelli S., Plasín M.A. Catastrophic antiphospholipid syndrome (CAPS): descriptive analysis of a series of 280 patients from the “CAPS registry”. J. Autoimmun. 2009;32(3–4):240–245. doi: 10.1016/j.jaut.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 70.Taghavi M., Barbhaiya M., Tektonidou M., Fortin P., Andrade D., Knight J., Artim-Esen B., Atsumi T., Cohen H., Ji L., Sciascia S., Seshan S., Erkan D., on behalf of APS ACTION 14 Descriptive analysis of biopsy-proven antiphospholipid antibody-associated nephropathy patients included in the AntiPhospholipid syndrome alliance for clinical trials and InternatiOnal networking (APS ACTION) clinical database and repository (“Registry”) Am. Coll. Rheumatol. 2019 (Abstract Number: 1793) [Google Scholar]
- 71.Tektonidou M.G., Sotsiou F., Moutsopoulos H.M. Antiphospholipid syndrome (APS) nephropathy in catastrophic, primary, and systemic lupus erythematosus-related APS. J. Rheumatol. 2008;35(10):1983–1988. [PubMed] [Google Scholar]
- 72.Weinstein S., Piette W. Cutaneous manifestations of antiphospholipid antibody syndrome. Hematol. Oncol. Clin. North Am. 2008;22(1):67–77. doi: 10.1016/j.hoc.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 73.Shiba M., Ieko M., Kawarada O. Symmetric peripheral gangrene in antiphospholipid syndrome. Heart Asia. 2016;8(2):8. doi: 10.1136/heartasia-2016-010768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Asherson R.A., Cervera R. Pulmonary hypertension, antiphospholipid antibodies, and syndromes. Clin. Rev. Allergy Immunol. 2007;32(2):153–158. doi: 10.1007/s12016-007-0012-0. [DOI] [PubMed] [Google Scholar]
- 75.Yoo W.H. Multiple rib infarcts: a rare form of osteonecrosis in antiphospholipid syndrome. Ann. Rheum. Dis. 2004;63(4):457–458. doi: 10.1136/ard.2003.006197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cohen H., Sayar Z., Efthymiou M. Management of anticoagulant-refractory antiphospholipid syndrome. Lancet Haematology. 2020;7(8):e613–e623. doi: 10.1016/S2352-3026(20)30116-2. [DOI] [PubMed] [Google Scholar]
- 77.Erkan D., Lockshin M. Non-criteria manifestations of antiphospholipid syndrome. Lupus. 2010;19(4):424–427. doi: 10.1177/0961203309360545. [DOI] [PubMed] [Google Scholar]
- 78.Cervera R., Tektonidou M., Espinosa G. Task force on catastrophic antiphospholipid syndrome (APS) and non-criteria APS manifestations (II): thrombocytopenia and skin manifestations. Lupus. 2011;20(2):174–181. doi: 10.1177/0961203310395052. [DOI] [PubMed] [Google Scholar]
- 79.Hisada R., Kato M., Sugawara E. Thrombotic risk stratification by platelet count in patients with antiphospholipid antibodies: a longitudinal study. J. Thromb. Haemost. 2017;15(9):1782–1787. doi: 10.1111/jth.13763. [DOI] [PubMed] [Google Scholar]
- 80.Zuily S., Huttin O., Mohamed S., Marie P., Selton-Suty C., Wahl D. Valvular heart disease in antiphospholipid syndrome. Curr. Rheumatol. Rep. 2013;15(4):320. doi: 10.1007/s11926-013-0320-8. [DOI] [PubMed] [Google Scholar]
- 81.Erkan D., Vega J., Ramón G., Kozora E., Lockshin M.D. A pilot open-label phase II trial of rituximab for non-criteria manifestations of antiphospholipid syndrome. Arthritis & Rheumatism. 2013;65(2):464–471. doi: 10.1002/art.37759. [DOI] [PubMed] [Google Scholar]
- 82.Tripodi A., de Laat B., Wahl D. Monitoring patients with the lupus anticoagulant while treated with vitamin K antagonists: communication from the SSC of the ISTH. J. Thromb. Haemost. 2016;14(11):2304–2307. doi: 10.1111/jth.13481. [DOI] [PubMed] [Google Scholar]
- 83.Bick R.L., Rice J. Long-term outpatient dalteparin (fragmin) therapy for arterial and venous thrombosis: efficacy and safety—a preliminary report. Clin. Appl. Thromb. Hemost. 1999;5(1_suppl):S67–S71. doi: 10.1177/10760296990050s112. [DOI] [PubMed] [Google Scholar]
- 84.Vargas-Hitos J.A., Ateka-Barrutia O., Sangle S., Khamashta M.A. Efficacy and safety of long-term low molecular weight heparin in patients with antiphospholipid syndrome. Ann. Rheum. Dis. 2011;70(9):1652–1654. doi: 10.1136/ard.2011.150268. [DOI] [PubMed] [Google Scholar]
- 85.Sayar Z., Burke S., Cohen H. The use of fondaparinux for recurrent thrombotic events. International Society on Thrombosis and Haemostasis. 2020 PB2410. [Google Scholar]
- 86.Carrier M., Le Gal G., Cho R., Tierney S., Rodger M., Lee A.Y. Dose escalation of low molecular weight heparin to manage recurrent venous thromboembolic events despite systemic anticoagulation in cancer patients. J. Thromb. Haemost. 2009;7(5):760–765. doi: 10.1111/j.1538-7836.2009.03326.x. [DOI] [PubMed] [Google Scholar]
- 87.Kearon C., Akl E.A., Ornelas J. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315–352. doi: 10.1016/j.chest.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 88.Arslan E., Branch D.W. Antiphospholipid syndrome: diagnosis and management in the obstetric patient. Best Practice & Research Clinical Obstetrics & Gynaecology. 2020;64:31–40. doi: 10.1016/j.bpobgyn.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 89.Chayoua W., Yin D., Kelchtermans H. 2020. Is there an additional value in detecting anticardiolipin and anti-β2 glycoprotein I IgA antibodies in the antiphospholipid syndrome? Thromb Haemost. [DOI] [PubMed] [Google Scholar]
- 90.Bertolaccini M.L. Sanna G. The clinical relevance of noncriteria antiphospholipid antibodies. 2018;44(05):453–457. doi: 10.1055/s-0037-1601328. [DOI] [PubMed] [Google Scholar]
- 91.Keeling D., Mackie I., Moore G.W., Greer I.A., Greaves M. British Committee for Standards in Haematology. Guidelines on the investigation and management of antiphospholipid syndrome. Br. J. Haematol. 2012;157(1):47–58. doi: 10.1111/j.1365-2141.2012.09037.x. [DOI] [PubMed] [Google Scholar]
- 92.Tektonidou M.G., Andreoli L., Limper M. EULAR recommendations for the management of antiphospholipid syndrome in adults. Ann Rheum Dis. 2019;78:1296–1304. doi: 10.1136/annrheumdis-2019-215213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Holbrook A., Schulman S., Witt D.M. Evidence-based management of anticoagulant therapy: antithrombotic therapy and prevention of thrombosis: American college of chest physicians evidence-based clinical practice guidelines. Chest. 2012;141(2):e152S–e184S. doi: 10.1378/chest.11-2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Garcia-Carrasco M., Jimenez-Herrera E., Galvez-Romero J. The anti-thrombotic effects of vitamin D and their possible relationship with antiphospholipid syndrome. Lupus. 2018;27(14):2181–2189. doi: 10.1177/0961203318801520. [DOI] [PubMed] [Google Scholar]
- 95.Andreoli L., Piantoni S., Dall’Ara F., Allegri F., Meroni P., Tincani A. Vitamin D and antiphospholipid syndrome. Lupus. 2012;21(7):736–740. doi: 10.1177/0961203312446386. [DOI] [PubMed] [Google Scholar]
- 96.Agmon-Levin N., Blank M., Zandman-Goddard G. Vitamin D: an instrumental factor in the anti-phospholipid syndrome by inhibition of tissue factor expression. Ann. Rheum. Dis. 2011;70(1):145–150. doi: 10.1136/ard.2010.134817. [DOI] [PubMed] [Google Scholar]
- 97.Belizna C. Hydroxychloroquine as an anti-thrombotic in antiphospholipid syndrome. Autoimmun. Rev. 2015;14(4):358–362. doi: 10.1016/j.autrev.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 98.Mehdi A.A., Uthman I., Khamashta M. Antiphospholipid syndrome: pathogenesis and a window of treatment opportunities in the future. Eur. J. Clin. Investig. 2010;40(5):451–464. doi: 10.1111/j.1365-2362.2010.02281.x. [DOI] [PubMed] [Google Scholar]
- 99.Ruiz-Irastorza G., Ramos-Casals M., Brito-Zeron P., Khamashta M.A. Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: a systematic review. Ann. Rheum. Dis. 2010;69(1):20–28. doi: 10.1136/ard.2008.101766. [DOI] [PubMed] [Google Scholar]
- 100.Schmidt-Tanguy A., Voswinkel J., Henrion D. Antithrombotic effects of hydroxychloroquine in primary antiphospholipid syndrome patients. J. Thromb. Haemost. 2013;11(10):1927–1929. doi: 10.1111/jth.12363. [DOI] [PubMed] [Google Scholar]
- 101.Meroni P.L., Raschi E., Testoni C. Statins prevent endothelial cell activation induced by antiphospholipid (anti–β2-glycoprotein I) antibodies: effect on the proadhesive and proinflammatory phenotype. Arthritis & Rheumatism: Official Journal of the American College of Rheumatology. 2001;44(12):2870–2878. doi: 10.1002/1529-0131(200112)44:12<2870::aid-art475>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 102.Jajoria P., Murthy V., Papalardo E., Romay-Penabad Z., Gleason C., Pierangeli S.S. Statins for the treatment of antiphospholipid syndrome? Ann. N. Y. Acad. Sci. 2009;1173(1):736–745. doi: 10.1111/j.1749-6632.2009.04815.x. [DOI] [PubMed] [Google Scholar]
- 103.Fanouriakis A., Kostopoulou M., Alunno A. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann. Rheum. Dis. 2019;78(6):736–745. doi: 10.1136/annrheumdis-2019-215089. [DOI] [PubMed] [Google Scholar]