Abstract
The current global Severe Acute Respiratory Syndrome- Coronavirus-2 (SARS-CoV-2) epidemic has heightened calls for studies to evaluate respiratory exposure for wastewater treatment workers. In this global first study, we assess occupational health risks to wastewater treatment plant (WWTP) operators from inhalation of aerosolized SARS-CoV-2 using a Quantitative Microbiological Risk Assessment (QMRA) framework. The following considerations were used to develop the QMRA and assess the illness risks to workers: a) the proportion of the population who are infected and thus responsible for shedding SARS-CoV-2 into raw wastewater; b) the concentration of SARS-CoV-2 in raw and treated wastewater; c) the volume of aerosolized water inhaled by a WWTP operator during work; d) humidity and temperature-dependent viability of coronaviruses in aerosolized waste water; e) estimation of the amount, frequency, and duration of exposure; and f) exposure doses. The variables were then fed into an exponential dose response model to estimate the risks in three scenarios representing low-grade, moderate and aggressive outbreaks. These scenarios were designed on the assumption of 0.03%, 0.3% and 3% of the wastewater-generating population being infected with SARS-CoV-2. In terms of averaged-out illness risk profiles, the individual illness risks for low grade, moderate and aggressive outbreak scenarios respectively are 0.036, 0.32 and 3.21 illness cases per 1000 exposed WWTP operators. Our study suggests that the risk of accidental occupational exposure to SARS-CoV-2 in raw wastewater, via inhalation at the WWTP environment, is negligible, particularly when less than 0.3% of the population served by the plant are actively infected.
Keywords: SARS-CoV-2 in wastewater, QMRA, Health risks, Wastewater operators
Graphical abstract
1. Introduction
A novel coronavirus disease named COVID-19 emerged from China in 2019 and rapidly spread throughout the world (WHO, 2020a, WHO, 2020b). The International Committee on Taxonomy of Viruses named the virus responsible for this outbreak as Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) (Nghiem et al., 2020; Wu et al., 2020). Despite several public health strategies, the daily total number of COVID-19 cases globally has not slowed down (WHO, 2020a). Eleven months on, this virus has caused 35.5 million illness cases and 1.04 million deaths, as of October 2020 (Worldometer, 2020).
Published studies have reported faecal shedding of the SARS-CoV-2 virus from symptomatic and asymptomatic individuals before and during the onset of symptoms (Lescure et al., 2020; Nghiem et al., 2020; Wu et al., 2020). Several studies also detected SARS-CoV-2 RNA in stools and wastewater (Ahmed et al., 2020; Bogler et al., 2020; Medema et al., 2020; Randazzo et al., 2020; Singer and Wray, 2020). Given these considerations, there have been concerns about the possibility of indirect or direct transmission of SARS-CoV-2 via exposure to untreated or treated wastewater (Ashour et al., 2020; Bogler et al., 2020; Cheung et al., 2020; Kitajima et al., 2020).
A Water Environment Federation blue-ribbon panel of experts commissioned to evaluate the safety of wastewater workers during the coronavirus pandemic recommended that studies should be conducted to evaluate respiratory exposure during tasks performed by workers in wastewater collection and treatment (TPO, 2020). Other independent studies have also called for QMRAs to evaluate respiratory exposure associated with wastewaters (Amoah et al., 2020; Kitajima et al., 2020). This is particularly relevant to aerosolization of wastewater and thus accidental exposure of wastewater operators to SARS-CoV-2 during daily workplace activities such as manual cleaning, coarse screening, wastewater sampling for microbiological and chemical analysis, and process supervision.
Reference pathogens including norovirus, enterovirus and adenovirus were used for QMRAs in relation to WWTP influents (Dada, 2018a, Dada, 2018b, Dada, 2019a, Dada, 2019b, Dada, 2020a, Dada, 2020b; McBride, 2007, McBride, 2011, McBride, 2016a, McBride, 2016b; McBride et al., 2013). Recently, Zaneti et al. (2020) applied SARS-CoV-2 as the reference QMRA pathogen considering ingestion via the faecal oral route. There is a dearth of published information on risk assessments focused on coronavirus spread through exposure to SARS-CoV-2 via inhalation. In this study, SARS-CoV-2 was used as the reference QMRA pathogen to assess health risks during occupational exposure by inhalation in wastewater treatment environments
2. Materials and methods
2.1. Data collection
Information related to treatment type, population covered and location of each wastewater treatment plant (WWTP) was extracted from an online inventory collated during the National Performance Review for 2018. Information from over 300 publicly owned WWTPs in New Zealand was collected and analysed for QMRA purposes.
2.2. Hazard analysis
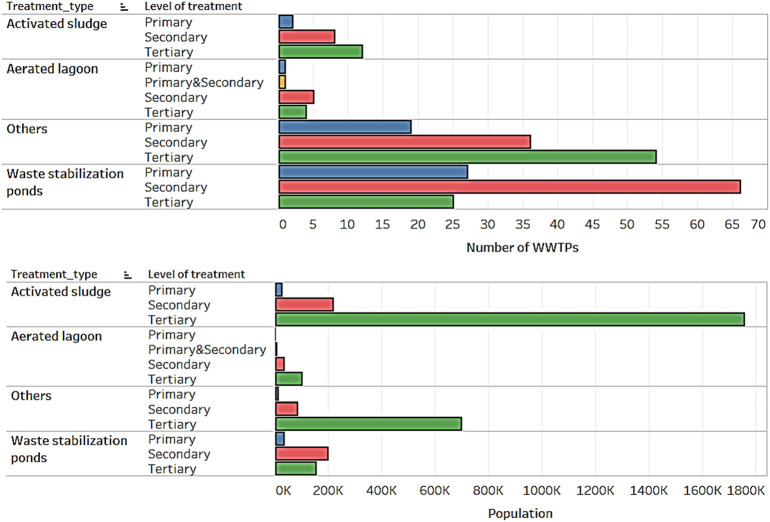
Wastewater stabilization ponds (WSPs) are the most prevalent form of wastewater treatment used for treating domestic sewage from small to medium-sized communities in New Zealand (Fig. 1 ). WSPs generally encompass three different pond systems: oxidation (mechanical), aerated, and anaerobic ponds (WaterNZ, 2005). WSPs are used either wholly or in combination with other forms of treatment (e.g. aeration lagoons, wetlands, sand filters, membrane filtration, and ultraviolet based disinfection). In terms of populations served by WWTPs, activated sludge (AS) systems are the most popular treatment systems (Fig. 1). Activated sludge (AS) systems are also the most widely used biological wastewater treatment process in the developed world (Scholz, 2015).
Fig. 1.
Types of treatments available at New Zealand WWTPs.
Pond-based systems are considered as self-sufficient treatment units, because the efficacy of treatment is contingent upon the maintenance of the overall microbial communities (Hosetti and Frost, 1998; Hosetti and Frost, 1995), and the proper balance of organics, light, dissolved oxygen, nutrients, algal presence (Amengual-Morro et al., 2012), and temperature. Because pond-based treatment systems are self-sufficient, there is a reduction in operator responsibilities to manage the treatment unit (Butler et al., 2017; Hosetti and Frost, 1998). However, WWTP operators may still be required to perform routine activities including manual cleaning, coarse screening, and wastewater sampling for microbiological and chemical analysis. WWTP operators working with activated sludge systems also perform similar activities. As a result, WWTP operators can be exposed to aerosolized form of SARS-CoV-2 via wind or mechanically generated aeration. The formation of wastewater aerosols and droplets was confirmed as a key mechanism for faecal–droplet–respiration transmission during previous coronavirus outbreaks and is suspected in the current COVID-19 outbreak caused by SARS-CoV-2 (Ding et al., 2020; Gormley et al., 2020; Yu et al., 2004). Aerosolized human coronaviruses remain viable for up to 16 h (Fears et al., 2020; Van Doremalen et al., 2020). Although dispersal of larger droplets is limited, as they deposit close to the source, larger droplets can cause local contamination of surfaces due to their enhanced capacity to carry pathogens, and are a major vector for pathogen transmission, including SARS-CoV-2 (Barker and Jones, 2005; Ding et al., 2020).
2.3. Exposure assessment
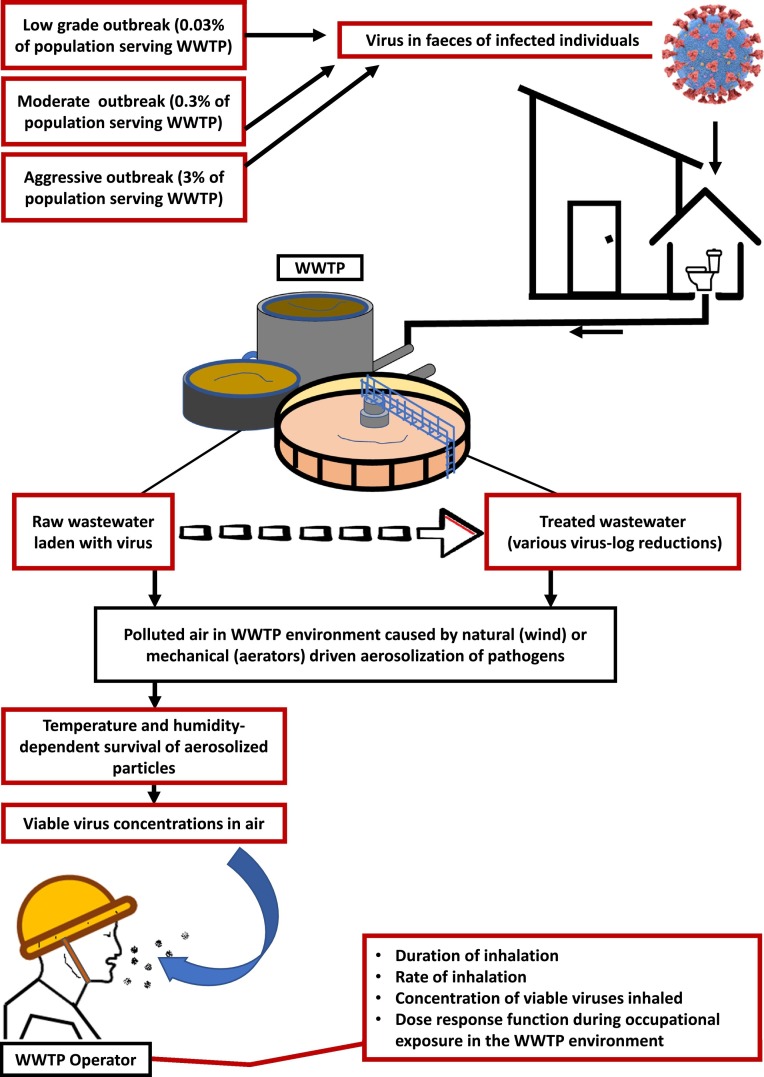
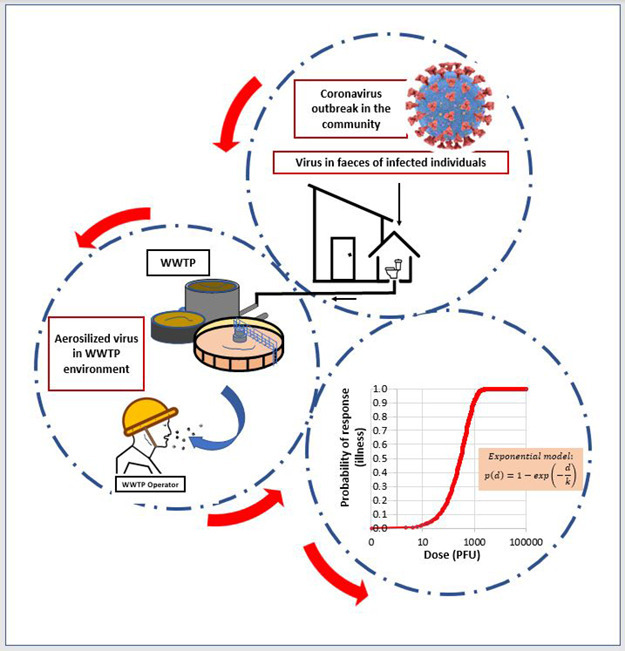
To assess the potential level of occupational exposure of WWTP operators to SARS-CoV-2, the following factors were considered (Fig. 2 ):
-
i.
Infected population responsible for faecal shedding of SARS-CoV-2 in raw wastewater;
-
ii.
SARS-CoV-2 concentrations in raw wastewater;
-
iii.
How much aerosolized water a WWTP operator will inhale during a working day;
-
iv.
Humidity and temperature-dependent viability of coronaviruses in aerosolized form; and,
-
v.
Estimation of the amount, frequency, length of time of exposure, and doses for exposure (see Table 1 ).
Fig. 2.
Schematic representation of the QMRA. Red boxes indicate QMRA inputs. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Table 1.
Distributions and inputs for the QMRA.
| Parameter | QMRA statistics applied | Comments |
|---|---|---|
| Virus concentration in faeces of infected individuals, SARS-CoV-2 (genomes per mL) | Minimum = 2000 Median = 1.7 × 106 Maximum = 4.1 × 107 |
Consistent with Han et al. (2020), Amoah et al. (2020). Hockey stick distribution, as previously described (McBride, 2007, McBride, 2011, McBride, 2016a, McBride, 2016b). 1000 genomes = 1 PFU |
| Duration (hours) of occupational exposure of WWTP operators to aerosolized SARS-CoV-2 | Minimum = 2 Median = 4 Maximum = 8 |
Time spent by WWTP operators on a typical workday at the treatment plant. This includes routine activities such as manual cleaning, coarse screening, wastewater sampling for microbiological and chemical analysis, and process supervision. |
| Wastewater inhalation rate, mL per hour | Minimum = 2 Median = 10 Maximum = 20 |
PERT distribution for an adult, consistent with previous QMRA studies on inhalation risks associated with aerosolized adenoviruses (McBride, 2007, McBride, 2011, McBride, 2016a, McBride, 2016b, Dada, 2018a, Dada, 2020b, Dada, 2019a, Dada, 2019b, Dada, 2020a) |
| Air temperature (degC) | Minimum = 1.4 Median = 12.4 Maximum =20.2 |
PERT distribution. Data from NIWA mean monthly values for the 1981–2010. |
| Relative humidity | Minimum = 65.0% Median = 81.5% Maximum = 94.2% |
PERT distribution. Data from NIWA mean monthly values for the 1981–2010. |
| Dose response parameters | SARS-CoV-2 exponential model (k = 4.1 × 102). | Watanabe et al. (2010). |
2.4. SARS-CoV-2 concentrations in wastewater that arrive at the WWTP
This study assumes a conservative stance in which all individuals who have been previously or are currently infected (symptomatic or asymptomatic) continue to shed the virus in their stools. One of the key factors influencing the occurrence of coronaviruses in wastewater is the concentration of virus shed by infected individuals (Cai et al., 2020; Zhang et al., 2020). Literature has shown concentrations of SARS-CoV-2 in stool of infected individual ranged between 1.7 × 106 to 4.1 × 107 genome copies (Han et al. 2020 in Amoah et al., 2020). In accordance with previous WWTP QMRA reports and international literature (e.g. McBride, 2016a, McBride, 2016b), median (1.7 × 106 genomes per mL) and maximum (4.1 × 107 genomes per mL) stool virus concentrations were bounded in the hockey-stick distribution in such a way that the resulting data are strongly right-skewed with a hinge at the 95th percentile. This therefore presents, in the same population, the generally predominant lower virus concentrations (i.e. having higher probabilities) alongside the extreme concentrations (which may be rare, but substantial).
Generally, some levels of dilution occur given that greywater and other forms of wastewater become mixed with stools once flushed down the toilet. Two scenarios were thus considered.
-
I.
No dilution scenario: Stools from individuals shedding SARS-CoV-2, flushed down the toilet, are not diluted at all and reach the WWTP as influent. It was assumed to contain SARS-CoV-2 concentrations of most likely between 1.7 × 106 genomes per mL (median) and 4.1 × 107 genomes per mL (maximum) (i.e. dilution factor = 1).
-
II.
Dilution scenario: The average person passes about 30 mL of stool a day for every five kilograms of body weight. This translates to approximately 192 to 510 mL of stool per individual. Typically, the amount of water used in the toilet for one full flush is 6 L. That is, for every single toilet use per individual shedding SARS-CoV-2, the concentration is diluted roughly 12 to 31 times. A dilution factor (stool_DF) distributed between 12 and 31 was therefore included in the modelling.
2.5. Prevalence-dependent SARS-CoV-2 loading
Three different scenarios (low, moderate and aggressive), representing different proportions of the population infected with the virus, were applied in this study. Low outbreak, moderate outbreak, and aggressive outbreak scenarios assume that 0.03%, 0.3% and 3%, respectively, of the population being served by a WWTP are infected and are shedding SARS-CoV-2 in their stools.
Given this consideration, the virus concentration expected at the WWTP is:
where Pop infected is the number of individuals infected in a population being served by a WWTP, stool virusconc is the SARS-CoV-2 concentration in the stools of infected individuals, Total_pop is the total population being served by a WWTP, stool_DF is the stool dilution factor.
2.6. Virus reductions achieved before and during treatment at the WWTP
The final virus concentrations in WWTP wastewater on a random day were then subjected to varying log removals (depending on the wastewater treatment scenario considered) before they were incorporated into the QMRA. It was assumed that before aerosolization, human coronaviruses in raw wastewater and the residual coronavirus concentrations following treatment all remain viable.
Given these considerations, the SARS-CoV-2 virus concentration in wastewater for each WWTP treatment scenario is therefore:
where logreductionfactor is 1, 10, 100, 1000- and 10,000-fold reduction respectively for 0,1,2,3 and 4 log-removals achieved during treatment at the WWTP.
2.7. Temperature and relative humidity dependent viability of aerosolized viruses
Among the meteorological factors, humidity (relative humidity; RH and absolute humidity; AH) and temperature play an important role in the viability status of coronaviruses in the air. Published literature has shown that 60% of coronaviruses in droplets or aerosols remain infectious for up to an hour at a RH of 79% at 25 °C but at a higher temperature and lower RH of 38 °C and 24%, less than 5% remained infectious. Recent studies have also suggested that AH rather than RH modulates virus survival (Deyle et al., 2016; McDevitt et al., 2010; Shaman and Kohn, 2009; Shaman et al., 2010). Marr et al. (2019) published an equation with an r-squared value of ~0.9 that relates RH and air temperature to AH. The equation was used in this study to estimate AH and thereafter to estimate the viability of aerosolized coronaviruses. Mathematically, AH was calculated as:
where AH is absolute humidity in gm-3, RH is relative humidity in percent and T is in 0C, applicable over a temperature range of −40 to 50 °C, and:
Given these considerations, the viable concentration of aerosolized SARS-CoV-2 is therefore given as:
2.8. Rate of inhalation and dose calculation
The dose of the pathogen that an individual inhales is an important component of the dose-response models used to predict the probability of infection or illness. To convert pathogen concentrations into doses, reference was made to the inhalation rates applied in a previous QMRA that assessed health risks associated with stormwater (McBride et al., 2013). McBride et al. (2013) applied a synthesis of previous studies (Dorevitch et al., 2011; Dufour et al., 2006; Haas et al., 1999) to arrive at minimum, mode and maximum associated volumetric intake rates of 10, 50 and 100 mL for ingestion associated with primary contact. In this study, it was assumed that WWTP operators would be physically present at the WWTP vicinity for a median and maximum period of 4 and 8 h on any random day, with associated volumetric intake rates of 2, 10 and 20 mL per hour (Table 1). Consistent with McBride et al.'s (2013) stormwater QMRA study on risks due to inhalation of aerosolized adenoviruses, inhalation volume per individual per exposure event was taken as the simple product of the inhalation rate and exposure duration. The inhalation volume was then multiplied by the viable concentration of aerosolized SARS-CoV-2 to determine the dose of aerosolized SARS-CoV-2 that an individual WWTP operator was exposed to.
2.9. Dose-response models
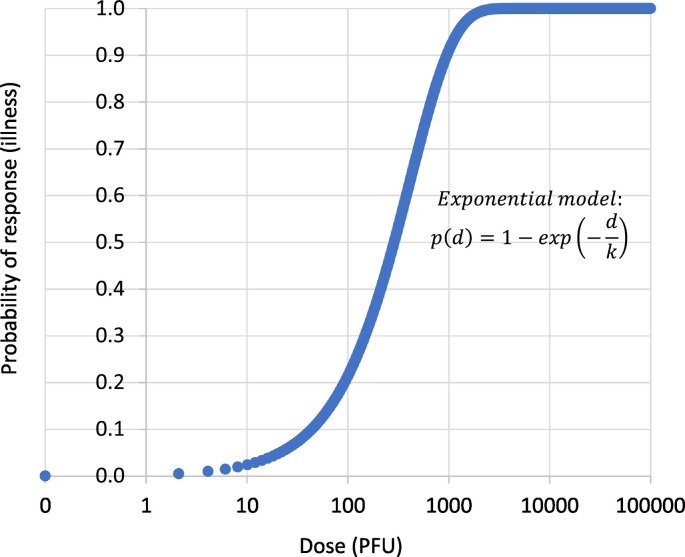
Dose-response models are mathematical functions that estimate the risk of a response (for example, infection or illness) given a known dose of a pathogen. Although there are no previously published dose-response models for SARS-CoV-2, Watanabe et al. (2010) reported an exponential model for SARS-CoV-1 based on datasets fitted for infections of transgenic mice that are susceptible to SARS-CoV-1. The exponential model was also applied to the analysis of the epidemiological data of SARS outbreak that occurred as a result of inhalation of SARS-CoV-1 associated with faulty plumbing at an apartment complex in Hong Kong in 2003. Considering the similarities of both coronaviruses, we applied the same dose response model to predict the risk of SARS-CoV-2 infection (Fig. 3 ).
where p(d) is the risk of illness at the dose of d. Parameter k in the exponential model equals 4.10 ∗ 105, the reciprocal of the probability that a single pathogen will initiate the response (Watanabe et al., 2010). It was assumed in this study that 1000 genomic copies correspond to one (1) plaque forming unit (PFU), consistent with previous studies (Aslan et al., 2011; Jensen et al., 2018).
Fig. 3.
Exponential dose-response model for SARS-CoV-2 infection.
2.10. Risk characterization
Information from the previous steps was incorporated into Monte Carlo simulations to determine the likelihood of illness from occupational exposure to SARS-CoV-2. The Monte Carlo simulation is a randomization method that applies multiple random sampling from distributions assigned to key input variables in a model, in a way that incorporates the uncertainty profiles of each key input variable into the uncertainty profile of the output. Typically, in a Monte Carlo model run, 100 individuals who do not have prior knowledge of existing contamination in the wastewater and who do not wear protective nose coverings are ‘exposed’ to potentially infectious aerosolised pathogens on a given day and this exposure is repeated 1000 times. Therefore, the total number of exposures is 100,000. The result of the analysis is a full range of possible risks, including average and worst-case scenarios, associated with exposure to pathogens during the identified recreational activities. Monte Carlo simulations were undertaken using @Risk software (Palisade, NY). QMRA results are reported in terms of illness, assuming a conservative stance in which every single infection leads to illness. The predicted risk is reported as the IIR (individual illness risk), calculated as the total number of illness cases divided by the total number of exposures, and is expressed as a percentage. The IIR is then compared with a threshold of 1 illness per 1000 exposed individuals as specified in the WHO interim guidance document. This threshold is considered the no observable adverse effects level (NOAEL).
3. Results
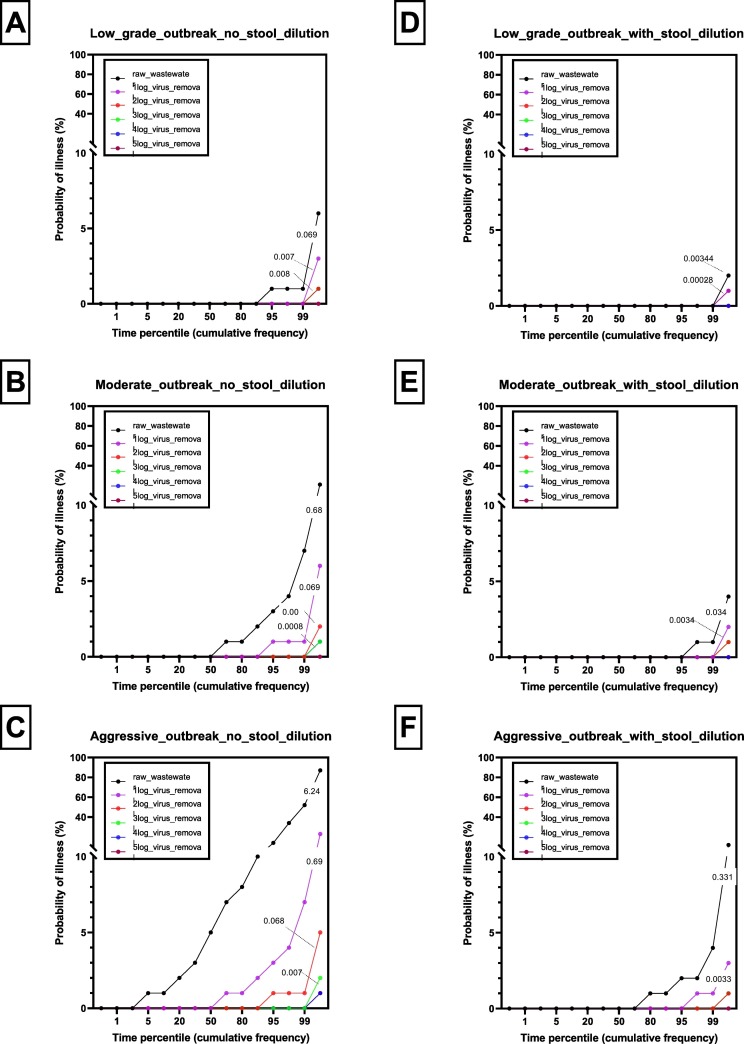
Presented in Fig. 4a-f are risk profiles for occupational exposure to SARS-CoV-2 via inhalation in the WWTP environment based on the virus concentrations in infected persons' stools (a) when diluted with toilet flush water; and (b) when not diluted with toilet flush water during conditions of low, moderate and aggressive outbreak. Aerosolized SARS-CoV-2 concentrations have also been corrected for RH and temperature-dependent viabilities. Depending on the nature of the treatment at the WWTP, the virus concentrations in the raw wastewater are reduced by between 1 and 5 logs. While the risk profiles are presented in terms of probabilities of illness plotted against time percentiles, prominent IIR values (%) are shown on the profiles.
Fig. 4.
Risk profiles for occupational exposure to SARS-CoV-2 via inhalation in the WWTP environment based on the virus concentrations in infected persons' stools (a) when diluted with toilet flush water and (b) when not diluted with toilet flush water during conditions of low, moderate and aggressive outbreak. Depending on the nature of the treatment at the WWTP, the virus concentrations in the raw wastewater are reduced by between 1 and 5 logs. Prominent IIR values (%) are shown on the profiles.
The risk profile was highest for raw wastewater but generally reduced with increasing orders of log reduction (Fig. 4a-f). In the worst case scenario, where dilution of the stool with toilet flush water was excluded from the risk assessment (Fig. 4a–c), 95% of the time, there would be no more than one illness case among 100 WWTP operators accidentally exposed to SARS-CoV-2 in raw wastewater, via inhalation at the WWTP environment, on any random day during low grade outbreak conditions (i.e. 0.03% of the population served by the WWTP is infected). In comparison, 95% of the time, there would be no more than three illness cases recorded during moderate outbreak conditions (i.e. 0.3% of the population served by the WWTP is infected). During aggressive outbreak conditions (i.e. when 3% of the population served by the WWTP is infected), the risk profiles are markedly higher with up 14 illness cases predicted among 100 WWTP operators accidentally exposed to SARS-CoV-2 in raw wastewater, via inhalation at the WWTP environment, 95% of the time on any random day (Fig. 4c). These elevated risks would also remain if, during conditions of aggressive outbreak, WWTP operators were exposed to partially treated wastewater in processing units where wastewater virus reductions of less than 1log had been achieved (Fig. 4c). Cumulatively, in terms of averaged-out illness risk profiles, the individual illness risks for low grade, moderate and aggressive outbreak scenarios, respectively, are 0.691, 6.8 and 62.4 illness cases per 1000 individuals (Fig. 4a–c).
In the more realistic scenario where dilution of the stool with toilet flush water was included in the risk assessment (Fig. 4d), 99% of the time there would be no illness case among 100 WWTP operators accidentally exposed to SARS-CoV-2 in raw wastewater, via inhalation at the WWTP environment, on any random day during low-grade outbreak conditions (i.e. 0.03% of the population served by the WWTP is infected). However, 99% of the time, there would be no more than 1 and 4 illness cases, respectively, per 100 exposed WWTP operators during moderate and aggressive outbreak conditions (Fig. 4e–f). Cumulatively, in terms of averaged-out illness risk profiles, the individual illness risks for low-grade, moderate and aggressive outbreak scenarios respectively are 0.036, 0.32 and 3.21 illness cases per 1000 exposed WWTP operators (Fig. 4d-f).
4. Discussion
This study was conducted in response to concerns that populations at greatest risk in the ongoing SARS-CoV-2 pandemic may be WWTP operators who may be exposed to raw wastewater. Our study is the first ever QMRA study that has assessed the potential level of occupational exposure of WWTP operators to SARS-CoV-2 via inhalation. This study is also the second QMRA study published that has assessed the potential level of occupational exposure of WWTP operators to SARS-CoV-2. A previously published QMRA report (Zaneti et al., 2020) used viral loads dependent on the proportions of individuals infected with the virus but applied an exposure scenario that assumes WWTP operators accidentally ingest 1 mL of raw wastewater containing SARS-CoV-2 through their mouth while performing routine activities. While focusing on ingestion as the route of transmission, the Zaneti et al. (2020) study also did not include consideration of contaminated surfaces in the modelling, despite these sources being potential vital links in the transfer of SARS-CoV-2 through the oral route. Our study adds to existing knowledge in that it focuses on human health risks during accidental inhalation of aerosolized forms of the wastewater.
In selecting QMRA input parameters, this QMRA included considerations for most of the factors that have been suggested as crucial to the fate of SARS-CoV-2 in the WWTP environment (Amoah et al., 2020). In addition to inhalation rate and exposure time, this study also included considerations of humidity and temperature-dependent survival of SARS-CoV-2 in aerosolized form based on a viability model for influenza viruses (Kong et al., 2020). The influenza viability model applied to estimate humidity and temperature-dependent survival of aerosolized SARS-CoV-2 is justified because it has been detected in patients with influenza-like illness (Kong et al., 2020).
This study also adds to the body of scholarly literature in that it considers the assessment of occupational health risks associated with accidental exposure to SARS-CoV-2 following log-removals that are typically achieved in WWTPs. The observation that risk profiles were highest for raw wastewater but generally reduced with increasing orders of log reduction is expected.
Overall, in the more realistic scenario where dilution of the stool with toilet flush water was included in the risk assessment, our results show cumulative illness risk (mean) was less than 1 per 1000 exposures for low-grade and moderate outbreak scenarios. This indicates that the risks of accidental occupational exposure to SARS-CoV-2 in raw wastewater, via inhalation at the WWTP environment are negligible. It is, however, important to mention that in terms of the upper limit (i.e. 99th percentile), a low level of risk was observed. These predicted health risks, however, only occur during extreme values of the QMRA input components were used. For instance, a WWTP operator exposed to raw or partially treated wastewater for 8 h continuously that is very unlikely unless WWTP staff are manually collecting and transporting sludge for off-site treatment or being involved in manual sludge dewatering. Previous literature has already reported the occurrence of human coronaviruses in both treated and untreated sludge (Annalaura et al., 2020; Bibby and Peccia, 2013; Bibby et al., 2011; Singer and Wray, 2020). This study thus suggests that there may be risks to WWTP operators involved in extensive periods of manual sludge handling, in agreement with Amoah et al. (2020), but only during conditions of aggressive outbreak. This may be particularly relevant in countries where the proportion of active cases in the population already exceeds 0.3% (for instance, the proportions of currently infected individuals compared to the population in the US, Peru and Bolivia are 1.03%, 0.5% and 0.5%, respectively, as at 25 August 2020 (Worldometer, 2020). Meanwhile, regardless of the proportion actively infected in the population served by a WWTP, we suggest that precautionary measures should still be applied to protect WWTP operators. The CDC already provides recommendations (CDC, 2020) including engineering and administrative controls, safe work practices, and use of PPE when handling untreated wastewater.
In order to optimize public health protection, a precautionary approach to this QMRA has been applied through the entire process. For instance, this QMRA assumed that all infected individuals in the population that feeds the WWTP shed SARS-CoV-2. This study also assumed that all the SARS-CoV-2 particles within the stools of infected individuals are infective. A meta-analysis conducted by Parasa et al. (2020) showed that only 40.5% of patients tested positive for SARS-CoV-2 had viral RNA in their faeces (95% confidence interval, 27.4%–55.1%). The duration of viral shedding in faeces lasted up to 18 days after hospitalization. However, WHO-China Joint Mission on Coronavirus Disease reported detecting SARS-CoV-2 viral RNA in the faeces of 30% patients for up to 5 weeks (WHO, 2020b). There is still uncertainty as to whether these viral RNA detection rates actually correlate with the presence of infectious virus. In a recent study (Wölfel et al., 2020), high concentrations (up to 108) of viral fragments similar to those in sputum were reported based on 13 stool samples taken within 12 days from 4 patients. Unlike the case for sputum, an attempt to grow SARS-CoV-2 from stool was not successful. However, contrasting results were published in another study where SARS-CoV-2 was successfully cultured from faeces of a critically ill patient in China (Xiao et al., 2020). While the number of samples analysed in this study is very small (n = 3), it points to the possibility of the RNA in faeces being infective. Wölfel et al. (2020) submitted that more studies would be needed to address whether SARS-CoV-2 shed in stool is rendered non-infectious though contact with the gut environment. Given this possibility of infectivity of RNA viruses in stools, albeit at levels not currently known, it was considered safe to assume in this QMRA that all viral RNA in stool was infectious.
This QMRA also excluded opportunities for dilution of stools from infected individuals; hence it involved higher SARS-CoV-2 concentrations in the raw wastewater that reached the WWTP. Generally, in addition to the levels of dilution that occur at the toilet level (i.e. with toilet flush water), more dilution can occur within the sewerage system. For instance, greywater and other forms of wastewater (e.g. from baths, sinks, washing machines, and other kitchen appliances) from houses connected to reticulated sewerage systems become mixed with the stools once flushed down the toilet, en route to the WWTP. As the available literature suggests that dish, shower, sink, and laundry water comprise 50–80% of residential wastewater (Al-Jayyousi, 2003), higher dilution possibilities indicate that the reported risks in this QMRA may be overstated.
It should be noted that the coronavirus dose response models used in this study was based on an infection endpoint, as reported by Watanabe et al. (2010). In our study, we have assumed a worst-case scenario where every infected individual becomes ill. In reality, infection may not necessarily progress to illness. For instance, while healthy individuals may not necessarily become ill, the elderly and immune-compromised citizens may present with a different response effect for the same dose of SARS-CoV-2. Even among the healthy, differential immunity status may exist (McBride et al., 2013).
Although this study also considered risks following treatment at the WWTP, this study did not focus on a specific wastewater treatment but used a more generalizable range of treatments. That could be potentially achieved at various WWTPs in New Zealand and elsewhere. These included scenarios for virus removal (i.e. raw wastewater) as well as 1, 2, 3, and 4 log10 virus removals equivalent to 10, 100, 1000 and 10,000-fold reductions in viral loads, respectively. These represent the range of virus log reductions that have been associated with various forms of wastewater treatment, e.g. 1 log10 in wastewater pond treatment systems (Verbyla and Mihelcic, 2015) and between 2 and 4 log10 removals in activated sludge and membrane bioreactor systems (De Luca et al., 2013; Wen et al., 2009; Zhang and Farahbakhsh, 2007).
More studies are still needed to characterize SARS-CoV-2 in the faeces of active and recovered individuals, as well as in WWTP influents and effluents. WWTP virus concentrations and infectivity analysis of a larger sampling scale would be useful to improve the quality of future QMRAs. Care should be exercised to ensure a harmonization of laboratory-generated genomic concentrations of SARS-CoV-2 and doses applied in future QMRAs. For instance, while the coronavirus dose response model in Watanabe et al. (2010) was based on plaque-forming units, there may be multiple virus particles for each PFU as viral particles that are defective or which fail to infect their target cell will not produce a plaque and thus will be inadvertently excluded from the plaque count.
5. Conclusion
Our study suggests that the risk of accidental occupational exposure of SARS-CoV-2 in raw wastewater via inhalation at the WWTP environment is low. Despite the highly conservative stance that this QMRA adopts, generally low individual illness risks were predicted during low-grade and moderate outbreak conditions (when less than 0.03% and 0.3% respectively, of the population served by a WWTP are actively infected).
CRediT authorship contribution statement
Christopher Ayokunle Dada: Conceptualization, Formal analysis, Writing - original draft, Writing - review & editing. Pradip Gyawali: Writing - original draft, Writing - review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Editor: Damia Barcelo
References
- Ahmed, W., Angel, N., Edson, J., Bibby, K., Bivins, A., O'Brien, J. W., … Li, J. 2020. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 138764. [DOI] [PMC free article] [PubMed]
- Al-Jayyousi O.R. Greywater reuse: towards sustainable water management. Desalination. 2003;156(1–3):181–192. [Google Scholar]
- Amengual-Morro C., Niell G.M., Martínez-Taberner A. Phytoplankton as bioindicator for waste stabilization ponds. J. Environ. Manag. 2012;95:S71–S76. doi: 10.1016/j.jenvman.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Amoah I.D., Kumari S., Bux F. Coronaviruses in wastewater processes: source, fate and potential risks. Environ. Int. 2020;105962 doi: 10.1016/j.envint.2020.105962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annalaura C., Ileana F., Dasheng L., Marco V. Making waves: coronavirus detection, presence and persistence in the water environment: state of the art and knowledge needs for public health. Water Res. 2020;115907 doi: 10.1016/j.watres.2020.115907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashour H.M., Elkhatib W.F., Rahman M., Elshabrawy H.A. Insights into the recent 2019 novel coronavirus (SARS-CoV-2) in light of past human coronavirus outbreaks. Pathogens. 2020;9(3):186. doi: 10.3390/pathogens9030186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslan A., Xagoraraki I., Simmons F.J., Rose J.B., Dorevitch S. Occurrence of adenovirus and other enteric viruses in limited-contact freshwater recreational areas and bathing waters. Journal of applied microbiology. J. Appl. Microbiol. 2011;111(5):1250–1261. doi: 10.1111/j.1365-2672.2011.05130.x. [DOI] [PubMed] [Google Scholar]
- Barker J., Jones M.V. The potential spread of infection caused by aerosol contamination of surfaces after flushing a domestic toilet. J. Appl. Microbiol. 2005;99(2):339–347. doi: 10.1111/j.1365-2672.2005.02610.x. [DOI] [PubMed] [Google Scholar]
- Bibby K., Peccia J. Identification of viral pathogen diversity in sewage sludge by metagenome analysis. Envir. Sci. Tech. 2013;47(4):1945–1951. doi: 10.1021/es305181x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibby K., Viau E., Peccia J. Viral metagenome analysis to guide human pathogen monitoring in environmental samples. Lett. Appl. Microbiol. 2011;52(4):386–392. doi: 10.1111/j.1472-765X.2011.03014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogler, A., Packman, A., Furman, A., Gross, A., Kushmaro, A., Ronen, A., … Morgenroth, E. 2020. Rethinking wastewater risks and monitoring in light of the COVID-19 pandemic. Nature Sustainability, 1–10.
- Butler E., Hung Y.-T., Al Ahmad M.S., Yeh R.Y.-L., Liu R.L.-H., Fu Y.-P. Oxidation pond for municipal wastewater treatment. Appl. Wat. Sci. 2017;7(1):31–51. [Google Scholar]
- Cai, J., Xu, J., Lin, D., Xu, L., Qu, Z., Zhang, Y., … Ge, Y. 2020. A case series of children with 2019 novel coronavirus infection: clinical and epidemiological features. Clin. Infect. Dis.. 71(6):1547–1551. [DOI] [PMC free article] [PubMed]
- CDC 2020. https://www.epa.gov/coronavirus/should-wastewater-workers-take-extra-precautions-protect-themselves-covid-19-virus (accessed 1 June 2020)
- Cheung K.S., Hung I.F., Chan P.P., Lung K., Tso E., Liu R.…Tam A.R. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from the Hong Kong cohort and systematic review and meta-analysis. Gastroenterology. 2020;159(1):81–95. doi: 10.1053/j.gastro.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dada A.C. Streamlined Environmental; Hamilton: 2018. Quantitative Microbial Risk Assessment for the Discharge of Treated Wastewater at Army Bay. Report WSL1701. (73 pp) [Google Scholar]
- Dada A.C. Streamlined Environmental; Hamilton: 2018. Quantitative Microbial Risk Assessment for the Discharge of Treated Wastewater Into Whitford Embayment through Turanga Creek, LCL1702. (41 pp) [Google Scholar]
- Dada A.C. Prepared/Presented on Behalf of Beef and Lamb New Zealand. 2019. Microbial evidence for Waikato region plan change (WRPC1) hearing, 2019. (33 pp) [Google Scholar]
- Dada A.C. Streamlined Environmental; Hamilton: 2019. Quantitative Microbial Health Risk Assessment for Wet Weather Wastewater Discharges Into City Rivers and Poverty Bay, Gisborne. Report GDC 1801. (49 pp) [Google Scholar]
- Dada A.C. Streamlined Environmental; Hamilton: 2020. Quantitative Microbial Risk Assessment for the Discharge of Treated Wastewater From the Opononi WWTP into the Hokianga Harbour, FNDC1802. (48 pp) [Google Scholar]
- Dada A.C. Streamlined Environmental; Hamilton: 2020. Quantitative Modelling of Public Health Risk Associated With Stormwater Network Improvements, Gisborne. Report GDC 1802. (49 pp) [Google Scholar]
- De Luca G., Sacchetti R., Leoni E., Zanetti F. Removal of indicator bacteriophages from municipal wastewater by a full-scale membrane bioreactor and a conventional activated sludge process: implications to water reuse. Bioresour. Technol. 2013;129:526–531. doi: 10.1016/j.biortech.2012.11.113. [DOI] [PubMed] [Google Scholar]
- Deyle E., Maher M., Hernandez R., Basu S., Sugihara G. Global environmental drivers of influenza. Proc. Natl. Acad. Sci. U. S. A. 2016;113 doi: 10.1073/pnas.1607747113. (13 081-013 086) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, Z., Qian, H., Xu, B., Huang, Y., Miao, T., Yen, H.-L., …. Shao, W. 2020. Toilets dominate environmental detection of SARS-CoV-2 virus in a hospital. Sci. Total Env. 141710. [DOI] [PMC free article] [PubMed]
- Dorevitch S., Panthi S., Huang Y., Li H., Michalek A.M., Pratap P.…Li A. Water ingestion during water recreation. Water Res. 2011;45(5):2020–2028. doi: 10.1016/j.watres.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Dufour A.P., Evans O., Behymer T.D., Cantu R. Water ingestion during swimming activities in a pool: a pilot study. J. Water Health. 2006;4(4):425–430. [PubMed] [Google Scholar]
- Fears, A. C., Klimstra, W. B., Duprex, P., Hartman, A., Weaver, S. C., Plante, K., … Fernandez, D. 2020. Comparative Dynamic Aerosol Efficiencies of Three Emergent Coronaviruses and the Unusual Persistence of SARS-CoV-2 in Aerosol Suspensions. medRxiv. 20063784.
- Gormley M., Aspray T.J., Kelly D.A. COVID-19: mitigating transmission via wastewater plumbing systems. Lancet Glob. Health. 2020;8(5):e643. doi: 10.1016/S2214-109X(20)30112-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas C.N., Rose J.B., Gerba C.P. John Wiley & Sons; 1999. Quantitative microbial risk assessment. [Google Scholar]
- Han M.S., Seong M.W., Heo E.Y., Park J.H., Kim N., Shin S., Cho S.I., Park S.S., Choi E.H. Sequential analysis of viral load in a neonate and her mother infected with SARS-CoV-2. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosetti B.B., Frost S. A review of the sustainable value of effluents and sludges from wastewater stabilization ponds. Ecol. Eng. 1995;5(4):421–431. [Google Scholar]
- Hosetti B., Frost S. A review of the control of biological waste treatment in stabilization ponds. Crit. Rev. Env. Sci. Tec. 1998;28(2):193–218. [Google Scholar]
- Jensen K.S., Adams R., Bennett R.S., Bernbaum J., Jahrling P.B., Holbrook M.R. Development of a novel real-time polymerase chain reaction assay for the quantitative detection of Nipah virus replicative viral RNA. PLoS One. 2018;13(6) doi: 10.1371/journal.pone.0199534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima, M., Ahmed, W., Bibby, K., Carducci, A., Gerba, C. P., Hamilton, K. A., … Rose, J. B. 2020. SARS-CoV-2 in wastewater: state of the knowledge and research needs. Sci. Total Env. 139076. [DOI] [PMC free article] [PubMed]
- Kong W.-H., Li Y., Peng M.-W., Kong D.-G., Yang X.-B., Wang L., Liu M.-Q. SARS-CoV-2 detection in patients with influenza-like illness. Nat. Microbiol. 2020;5(5):675–678. doi: 10.1038/s41564-020-0713-1. [DOI] [PubMed] [Google Scholar]
- Lescure F.-X., Bouadma L., Nguyen D., Parisey M., Wicky P.-H., Behillil S.…Le Hingrat Q. Clinical and virological data of the first cases of COVID-19 in Europe: a case series. Lancet Inf. Dis. 2020;20(6):697–706. doi: 10.1016/S1473-3099(20)30200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr L.C., Tang J.W., Van Mullekom J., Lakdawala S.S. Mechanistic insights into the effect of humidity on airborne influenza virus survival, transmission and incidence. J. R. Soc. Interface. 2019;16(150) doi: 10.1098/rsif.2018.0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride G. Statistical Framework for Recreational Water Quality Criteria and Monitoring. 2007. Microbial risk assessment modeling; pp. 135–151. [Google Scholar]
- McBride G. 2011. A Quantitative Microbial Risk Assessment for Napier City’s Ocean Outfall Wastewater Discharge. Report Prepared by NIWA for Napier City Council. HAM2011–016. [Google Scholar]
- McBride G. 2016. Quantitative Microbial Risk Assessment for the Discharge of Treated Wastewater: Snells Beach Wastewater Treatment Plan. Report Prepared by NIWA for Watercare Services Limited. HAM2016-038. [Google Scholar]
- McBride G. 2016. Quantitative Microbial Risk Assessment for the Discharge of Treated Wastewater: Warkworth Wastewater Treatment Plan. Report Prepared by NIWA for Watercare Services Limited. HAM2016-037. [Google Scholar]
- McBride G.B., Stott R., Miller W., Bambic D., Wuertz S. Discharge-based QMRA for estimation of public health risks from exposure to stormwater-borne pathogens in recreational waters in the United States. Water Res. 2013;47(14):5282–5297. doi: 10.1016/j.watres.2013.06.001. [DOI] [PubMed] [Google Scholar]
- McDevitt J., Rudnick S., First M., Spengler J. Role of absolute humidity in the inactivation of influenza viruses on stainless steel surfaces at elevated temperatures. Appl. Environ. Microbiol. 2010;76:3943–3947. doi: 10.1128/AEM.02674-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. 2020. Presence of SARS-Coronavirus-2 in Sewage. medRxiv. 20045880. [DOI] [PubMed] [Google Scholar]
- Nghiem L.D., Morgan B., Donner E., Short M.D. The COVID-19 pandemic: considerations for the waste and wastewater services sector. Case Studies in Chemical Environmental Engineering. 2020:100006. doi: 10.1016/j.cscee.2020.100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parasa, S., Desai, M., Chandrasekar, V. T., Patel, H. K., Kennedy, K. F., Roesch, T., . . . Artifon, E. L. 2020. Prevalence of gastrointestinal symptoms and fecal viral shedding in patients with coronavirus disease 2019: a systematic review and meta-analysis. JAMA Netw. Open, 3(6), e2011335-e2011335. [DOI] [PMC free article] [PubMed]
- Randazzo W., Truchado P., Cuevas-Ferrando E., Simón P., Allende A., Sánchez G. SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Wat. Res. 2020;115942 doi: 10.1016/j.watres.2020.115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz M. Elsevier; 2015. Wetland Systems to Control Urban Runoff. (ISBN: 9780444636126. 556 pp) [Google Scholar]
- Shaman J., Kohn M. Absolute humidity modulates influenza survival, transmission, and seasonality. Proc. Natl. Acad. Sci. U. S. A. 2009;106:3243–3248. doi: 10.1073/pnas.0806852106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaman J., Pitzer V.E., Viboud C., Grenfell B.T., Lipsitch M. Absolute humidity and the seasonal onset of influenza in the continental United States. PLoS Biol. 2010;8:e1000316. doi: 10.1371/journal.pbio.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer A., Wray R. 2020. Detection and Survival of SARS-Coronavirus in Human Stool, Urine, Wastewater and Sludge. doi: 10.20944. [Google Scholar]
- TPO . 2020. Expert Panel Finds Low Risk of Coronavirus for Wastewater Workers. Treatment Plant Operator. [Google Scholar]
- Van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N.…Gerber S.I. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. New Engl. J. Med. 2020;382(16):1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbyla M.E., Mihelcic J.R. A review of virus removal in wastewater treatment pond systems. Wat. Res. 2015;71:107–124. doi: 10.1016/j.watres.2014.12.031. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Bartrand T.A., Weir M.H., Omura T., Haas C.N. Development of a dose-response model for SARS coronavirus. Risk Anal. 2010;30(7):1129–1138. doi: 10.1111/j.1539-6924.2010.01427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WaterNZ Oxidation pond guildeines 2005. 2005. https://www.waternz.org.nz/Attachment?Action=Download&Attachment_id=120 Retrieved from.
- Wen Q., Tutuka C., Keegan A., Jin B. Fate of pathogenic microorganisms and indicators in secondary activated sludge wastewater treatment plants. J. Environ. Manag. 2009;90(3):1442–1447. doi: 10.1016/j.jenvman.2008.09.002. [DOI] [PubMed] [Google Scholar]
- WHO Corona virus situation updates. 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200211-sitrep-22-ncov.pdf?sfvrsn=6f80d1b9_4 Retrieved from.
- WHO Report of the WHO-China joint mission on coronavirus disease 2019 (COVID-19) 2020. https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf Retrieved from.
- Wölfel R., Corman V., Guggemos W., Seilmaier M., Zange S., Müller M.…Vollmar P., Rothe C, Hoelscher M, Bleicker T, Brünink S, Schneider J, Ehmann R, Zwirglmaier K, Drosten C, Wendtner 337 C. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581(7809):465–469. doi: 10.1038/s41586-020-2196-x. 336. [DOI] [PubMed] [Google Scholar]
- Worldometer. 2020. Coronavirus update (live). Reported cases and deaths by country, territory, or conveyance. Retrieved from https://www.worldometers.info/coronavirus/.
- Wu, Y., Guo, C., Tang, L., Hong, Z., Zhou, J., Dong, X., … Qu, X. 2020. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroent. Hepatol. 5(5), 434–435. [DOI] [PMC free article] [PubMed]
- Xiao F., Sun J., Xu Y., Li F., Huang X., Li H.…Zhao J. Infectious SARS-CoV-2 in feces of patient with severe COVID-19. Emerg. Infect. Dis. 2020;26(8):1920. doi: 10.3201/eid2608.200681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu I.T., Li Y., Wong T.W., Tam W., Chan A.T., Lee J.H.…Ho T. Evidence of airborne transmission of the severe acute respiratory syndrome virus. New Engl. J. Med. 2004;350(17):1731–1739. doi: 10.1056/NEJMoa032867. [DOI] [PubMed] [Google Scholar]
- Zaneti, R. N., Girardi, V., Spilki, F. R., Mena, K., Westphalen, A. P. C., da Costa Colares, E. R., … Etchepare, R. G. 2020. QMRA of SARS-CoV-2 for Workers in Wastewater Treatment Plants. medRxiv. 20116277. [DOI] [PMC free article] [PubMed]
- Zhang K., Farahbakhsh K. Removal of native coliphages and coliform bacteria from municipal wastewater by various wastewater treatment processes: implications to water reuse. Wat. Res. 2007;41(12):2816–2824. doi: 10.1016/j.watres.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Zhang J., Wang S., Xue Y. Fecal specimen diagnosis 2019 novel coronavirus–infected pneumonia. J. Med. Virol. 2020;92(6):680–682. doi: 10.1002/jmv.25742. [DOI] [PMC free article] [PubMed] [Google Scholar]