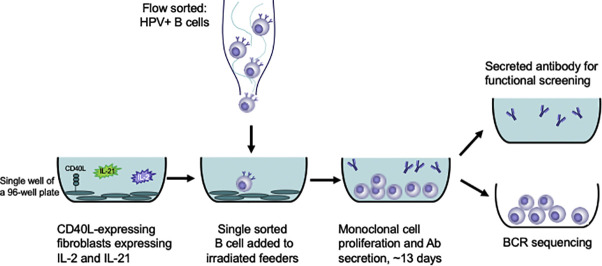
Abstract
The isolation of human monoclonal antibodies (mAbs) arising from natural infection with human pathogens has proven to be a powerful technology, facilitating the understanding of the host response to infection at a molecular level. mAbs can reveal sites of vulnerability on pathogens and illuminate the biological function of the antigenic targets. Moreover, mAbs have the potential to be used directly for therapeutic applications such as passive delivery to prevent infection in susceptible target populations, and as treatment of established infection. The isolation of antigen-specific B cells from vaccine trials can also assist in deciphering whether the desired B cells are being targeted by a given vaccine. Several different processes have been developed to isolate mAbs, but all are generally labor-intensive and result in varying degrees of efficiency. Here, we describe the development of a cost-effective feeder cell line that stably expresses CD40-ligand, interleukin-2 and interleukin-21. Sorting of single B cells onto a layer of irradiated feeder cells sustained antibody production that permits functional screening of secreted antibodies in a manner that enables subsequent recovery of B cells for recombinant antibody cloning. As a proof of concept, we show that this approach can be used to isolate B cells that secrete antibodies that neutralize human papilloma virus (HPV) from participants of an HPV vaccine study.
Keywords: B cells, Antibodies, High-throughput, Screening, Human papillomavirus, Cytokines
Graphical abstract
Highlights
-
•
Development of a cell line that provides signals for human B cell growth and antibody secretion.
-
•
50% cultures seeded from single B cells produce detectable IgG.
-
•
B cells secreting HPV-neutralizing antibodies from a vaccine study are identified.
1. Introduction
The isolation of pathogen-specific monoclonal antibodies (mAbs) generated during natural human infections with human immunodeficiency virus (HIV), influenza virus, respiratory syncytial virus, human cytomegalovirus (CMV), Epstein-Barr virus (EBV), Zika virus and dengue virus has helped define critical epitopes on these pathogens and advanced vaccine design (Rappuoli et al., 2016; Burton, 2017; Snijder et al., 2018; Robbiani et al., 2017). Moreover pathogen-specific mAbs can provide critical proof of concept to establish the protective efficacy of antibodies against pathogenic challenge (Mascola et al., 2000; Hessell et al., 2009; Hessell et al., 2007; Parren et al., 2001; Wang et al., 2018; Shingai et al., 2014; Magnani et al., 2017; Wang et al., 2017; Renegar and Small Jr., 1991; Pinto et al., 2020), with demonstrated prophylactic or therapeutic potential if delivered passively (Caskey et al., 2019). Importantly, the rapid isolation of mAbs is a powerful method that can be used to understand the human immune response to infection in the context of rapidly emerging infections, such as the current COVID-19 pandemic (Pinto et al., 2020; Traggiai et al., 2004; Seydoux et al., 2020; Ju et al., 2020; Shi et al., 2020).
Several approaches exist to identify and isolate antigen-specific B cells and monoclonal antibodies. Some of these are based on binding, such as the construction and panning of phage display libraries (Ying et al., 2014; Prabakaran et al., 2006; Chen et al., 2012; Sui et al., 2004; Burton et al., 1994; Roben et al., 1994; Bugli et al., 2001; McCafferty et al., 1990), or using fluorescence activated cell sorting (FACS) to identify and isolate individual antigen-specific B cells for single-cell sequencing of antibody heavy and light chain transcripts which are then reconstructed as mAbs (Scheid et al., 2008; Tiller et al., 2008). Another approach to identify vaccine-specific antibodies is to isolate antibody-secreting plasmablasts (without antigen-specific sorting) for single-cell BCR sequencing to reconstruct recombinant antibodies (Smith et al., 2009). These techniques select for antigen-specific B cells and require subsequent BCR sequencing and generation of recombinant mAbs to test for an antibody activity of interest, such as neutralization or the ability to recruit effector cells and mediate antibody-dependent cellular phagocytosis (ADCP) or antibody-dependent cellular cytotoxicity (ADCC).
Other approaches are based on immortalizing B cells and then culturing them in multi-well plates and screening culture supernatants for activity. These include B cell immortalization with Epstein-Barr virus (Steinitz et al., 1977; Traggiai et al., 2004), electrofusion with a human B cell/murine myeloma hybrid (Li et al., 2006), or genetic reprogramming through retroviral delivery of anti-apoptotic transcription factor genes (Kwakkenbos et al., 2010). An alternative to these immortalization strategies is culturing of B cells on feeder cells in the presence of cytokines to promote differentiation and short-term growth (Huang et al., 2013; Walker et al., 2009; Huang et al., 2012). The major advantage of these approaches is that they permit a forward screen for activity prior to recombinant mAb generation. This can be advantageous, if not critical, to identify antibodies that only recognize quaternary epitopes or native epitopes that are not present on recombinant proteins. Disadvantages of these immortalization and culture-based approaches are inefficiencies of immortalization (e.g., EBV) and the cost of recombinant cytokines to supplement B cell cultures in a high throughput format.
Here, we describe the development and characterization of a feeder cell line based on the one described by Huang and colleagues (Huang et al., 2013) that provides B cells the necessary stimuli to proliferate in culture for approximately 2 weeks without the requirement for recombinant cytokine supplementation. We constructed a lentiviral delivery vector capable of delivering the genes for IL-2 and IL-21, and transduced CD40L-expressing 3T3 cells. We demonstrate that this cell line maintains long-term expression of CD40L, IL-2, and IL-21, and when irradiated, supports growth and secretion of IgG from B cells after 13 days of co-culture. To further illustrate the utility of this feeder cell line, we used it to identify and isolate binding and neutralizing mAbs from a participant in a nonavalent human papilloma virus (HPV) vaccine trial.
2. Materials and methods
2.1. Production of lentiviral construct to deliver recombinant human IL-2 and IL-21
Human IL-2 cDNA containing a C153A mutation (Stauber et al., 2006) followed by human IL-21 cDNA separated by a T2A cleavage site was used to replace the eGFP ORF downstream of the MND promoter in pRRL.MND.eGFP-WPRE (Sather et al., 2011) to yield pRRL-MND-IL2-T2-IL21-WPRE.
Lentiviruses were produced by co-transfecting the pRRL-MND-IL2-T2-IL21-WPRE, psPAX2 (Addgene #12260) and pMD2.G (Addgene #12259) plasmids at a 4:2:1 ratio (6, 3, and 1.5 μg, respectively) into 293T cells in DMEM +10% FBS and glutamine using PEI MAX (Polysciences) at a 4:1 PEI to DNA ratio. After incubation for 18–24 h at 37 °C in the presence of 5% CO2, the media was aspirated, the cells were washed once with 1× phosphate buffered saline (PBS) and then overlaid with DMEM without phenol red, supplemented with 3% FBS and glutamine. Twenty-four hours later, the supernatant was passed through a 0.22 μm filter. Viral particles were concentrated by centrifugation at 8500 ×g overnight at 4 °C. The media was then aspirated off and the viruses were resuspended at 100× in HBSS and stored at −80 °C.
2.2. Viral transduction of 3T3-CD40L cell line
The murine 3T3-CD40L cell line (Huang et al., 2013) was acquired from the NIH AIDS Reagent Program (Cat.# 12535). Cryopreserved 3T3-CD40L cells were quickly warmed at 37 °C and added to warm DMEM complete growth media [DMEM supplemented with 10% FBS (Gemini), 1% l-glutamine (Gibco), 1% penicillin-streptomycin (Gibco), and 350 μg/mL geneticin (G418) (Gibco)]. Cells were washed by spinning at 300 ×g for 10 min and resuspended in growth media. Cells were then counted and seeded at 40,000 cells/mL in T25 flasks. Once the cells reached 90–95% confluency, media was removed, the cells were rinsed with PBS and detached from the flask by incubating with 5 mL 0.25% trypsin (Gibco) for 3 min at 37 °C. Cells were then combined in a 50 mL conical and washed twice with growth media. The 3T3-CD40L cells were counted, resuspended at 40,000 cells per mL in growth media and 50,000 cells were seeded in 1.25 mL per well in 12-well culture plates. Plates were incubated for 2 days at 37 °C with 5% CO2. The IL-2/IL-21 lentiviral stock was thawed and a range of dilutions (0.1–10 μL) of the lentivirus were made in DMEM complete with 8 μg/mL Polybrene (Sigma-Aldrich). The lentiviral dilutions were transferred to the 3T3-CD40L cells and incubated for 5 days at 37 °C. The IL-2 and IL-21 transduced 3T3-CD40L cells (3T3 IL-2/IL-21/CD40L cells) were expanded as follows: media was removed from the culture plates, cells were washed twice with 1× PBS and incubated in 3 mL trypsin for 3 min at 37 °C. Trypsin was quenched with 500 μL growth media and the cells were resuspended. Cells were seeded in T25 flasks with additional growth media and expanded. Once a sufficient number of cells were expanded, the cell line was cryopreserved at 5 × 106 cells/vial and stored in liquid nitrogen.
2.3. Characterization of the 3T3 IL-2/IL-21/CD40L cell line
2.3.1. IL-2 and IL-21 production by ELISA
IL-2 and IL-21 secretion was measured by enzyme-linked immunosorbent assay (ELISA) (Invitrogen). ELISAs were performed according to manufacturer instructions. In brief, 96-well high bind microplates (Corning) were coated with 100 μL coating antibody (anti-human IL-2 or IL-21) diluted 1:250 in 1× PBS and incubated at 4 °C overnight. Cell culture supernatants were collected and cryopreserved at −80 °C until the day of ELISA assays. Supernatant aliquots were thawed at room temperature (RT) and were diluted in the ELISA assay diluent at various dilutions (1:50–1:10,000) depending on the concentration of cytokines. The coated plates were washed 3 times with 250 μL PBS, 0.05% Tween 20 buffer and blotted to remove excess liquid. Plates were blocked for 1 h at RT with 200 μL 1× ELISA diluent. The plates were washed 2 times and diluted supernatants were transferred to the ELISA plates along with the serially diluted standards. Samples were incubated at RT for 2 h. Plates were washed and 100 μL detection antibody (biotin-conjugated anti-human IL-2 or IL-21) diluted to 1:250 was added to the plates and incubated for 1 h at RT. Plates were again washed followed by the addition of 100 μL avidin-HRP diluted to 1:250 and incubated for 30 min at RT. The plates were washed 6 times before the addition of 100 μL 1× TMB solution. The reaction was arrested with 1 N sulfuric acid after 15 min at RT and the plates were read at 450 nm on a SpectraMax i3X plate reader (Molecular Devices). Concentrations of IL-2 and IL-21 were interpolated from the appropriate standard curves.
2.3.2. CD40L expression assessed by flow cytometry
Initial characterization of the 3T3 IL-2/IL-21/CD40L cell line was performed to confirm continued CD40L expression. CD40L expression on non-irradiated 3T3 IL-2/IL-21/CD40L cells was confirmed by flow cytometry after 1 week (3 passages) and 8 weeks (24 passages). Cells were detached from the flask using cold PBS supplemented with 5 mM EDTA (Gibco) and a portion of the sample was transferred to FACS tubes. Cells were incubated with anti-CD154 PE (BD Biosciences, TRAP-1) in stain media (1× PBS, 10% FBS) for 20 min at 4 °C. The sample was washed with PBS and run on an LSR II (BD Biosciences) alongside an unstained cell sample. Data were analyzed using FlowJo v9.4 (BD Biosciences).
2.4. Preparation of irradiated 3T3 IL-2/IL-21/CD40L cells
2.4.1. Irradiation of 3T3 IL-2/IL-21/CD40L cells
A single-cell suspension of expanded 3T3 IL-2/IL-21/CD40L cells was transferred to 50 mL conical tubes. In order to prevent the feeder cells from overwhelming the B cell monocultures when co-cultured, the 3T3 IL-2/IL-21/CD40L cells were irradiated at 5000 rads for 5 min to prevent them from undergoing additional expansion when in co-culture. Aliquots of 5 × 106 irradiated 3T3 IL-2/IL-21/CD40L cells were cryopreserved in order to generate a stock of irradiated feeders readily available to prepare B cell culture plates.
2.4.2. Feeder cell culture plate preparation
When preparing feeder cell culture plates for B cell sorting, a vial of cryopreserved irradiated 3T3 IL-2/IL-21/CD40L cells was rapidly thawed to 37 °C until a small pellet of frozen cells remained, and the vial contents were transferred to warm culture media (IMDM, 10% FBS, 1% l-glutamine, 1% penicillin-streptomycin). The feeder cells were washed to remove any remaining DMSO, counted on an automated cell counter (Muse, Millipore) and plated on flat-bottom tissue culture-treated plates at a density of 87,500 live cells per cm2 of well area in culture media. The outer wells from each plate were filled with 1× PBS to prevent evaporation of media in culture wells. Before use, plates were incubated for 12–24 h at 37 °C to allow the cells to recover and begin producing cytokines.
2.5. Expansion of monoclonal memory B cells using 3T3 IL-2/IL-21/CD40L cells
2.5.1. Control participant cohort
Peripheral blood mononuclear cells (PBMCs) were obtained from HIV-1 seronegative donors who were recruited at the Seattle HIV Vaccine Trials Unit (Seattle, Washington, USA) as part of the study “Establishing Immunologic Assays for Determining HIV-1 Prevention and Control”. All participants signed informed consent, and the Fred Hutchinson Cancer Research Center IRB (Seattle, WA, USA) institutional human subjects review committee approved the protocol prior to study initiation.
2.5.2. PBMC preparation and single-cell sorting for memory B cells
Cryopreserved PBMCs were rapidly warmed at 37 °C until a small frozen pellet remained. Vial contents were then transferred to a conical tube containing warm R10 media (RPMI containing 10% FBS, 1% l-glutamine, and 1% Penicillin-Streptomycin) with benzonase at 50 U/mL (Millipore). PBMCs were centrifuged at 300 ×g for 10 min and washed twice with 20 mL R10 media. Thawed PBMCs were aliquoted to a FACS tube for staining. Cells were centrifuged at 650 ×g for 5 min and stained with anti-CD14 PE-Cy5 (eBioscience, 61D3), anti-CD56 PE-Cy5 (BD Biosciences, HCD56), anti-CD3 BV510 (BD Biosciences, OKT3), anti-CD19 ECD (Beckman Coulter, J3-119), anti-IgD BV786 (BD Biosciences, IA6-2), anti-IgG BV421 (BD Biosciences, G18-145) in stain buffer (1× PBS supplemented with 10% FBS). Cells were incubated with the antibodies for 20 min at 4 °C. The sample was washed twice with stain buffer and resuspended in R10 media containing viability dye (7AAD, Invitrogen). IgG+ memory B cells were sorted (one per well) into 96-well half-area culture plates containing the irradiated 3T3-CD40L feeder cells described above. Cells were sorted on a FACS Aria II (BD) using a 70 μm nozzle and the following gating strategy: CD3-, CD56-, CD14-, CD19+, IgD-, and IgG+ live singlet cells. Ten plates of sorted cells were placed in the incubator at 37 °C. Culture supernatants were harvested 13 days later by quickly pelleting the cells at 750 ×g for about 30 s and transferring the supernatant to a new 96-well culture plate, which was stored at −80 °C until use.
2.5.3. Detection of secreted IgG by ELISA
Maxi-Sorp ELISA plates (Invitrogen) were coated with 100 μL coating antibody (anti-human IgG Fc Antibody, ICL) diluted to 1:200 in coating buffer (0.05 M Bicarbonate Buffer, pH 9.6). Plates were incubated for 1 h at RT and washed five times with 200 μL wash buffer (PBS, 0.05% Tween 20). Plates were then blocked with 300 μL blocking buffer (PBS, 1% BSA, 0.03% Tween 20) and incubated for 30 min at RT. Blocking buffer was decanted and 50 μL of supernatant diluted in sample diluent (ICL) to a 1:10 dilution was added to each well. Samples were incubated for 1 h at RT. Plates were washed 5 times with 200 μL wash buffer and 100 μL antibody conjugate (anti-human IgG h + l Antibody, ICL) diluted to 1:30,000 was added to each well. The plates were incubated at RT for 40 mins in the dark. Plates were again decanted and washed 5 times with wash buffer and 100 μL TMB substrate (Surmodics) was added to each well. ELISA plates were incubated for 8 min at RT in the dark after which the development was arrested with 100 μL 0.1 M sulfuric acid. Plates were read at 450 nm on a SpectraMax i3X plate reader (Molecular Devices).
2.6. Expansion of monoclonal HPV vaccine-specific memory B cells using 3T3 IL-2/IL-21/CD40L cells
2.6.1. Participants
The Fred Hutch institutional human subjects review committee approved all protocols prior to study initiation, and participants completed a thorough written informed consent process before study enrollment. Eligible participants were non-pregnant healthy adults, 18 through 26 years of age. As part of the enrollment criteria, participants were screened for seropositivity for Human Papilloma Virus (HPV) strains 16 and 18. HPV seronegative consented participants were vaccinated with the licensed HPV vaccine, Gardasil-9® (nHPV), at weeks 0, 8, and 24. nHPV contains self-assembling virus-like particles (VLPs) of the L1 major capsid proteins from HPV strains: 6, 11, 16, 18, 31, 33, 45, 52, and 58. Blood was collected for isolation and cryopreservation of PBMCs two weeks after the second vaccination (week 10).
2.6.2. Preparation of HPV pseudoviruses
HPV16 and HPV18 pseudoviruses (PsV) were prepared as previously described (Scherer et al., 2014). In brief, 293TT cells were co-transfected with pYSEAP (plasmid expressing secreted alkaline phosphatase) and either p16L1L2 or p18L1L2 (plasmid expressing HPV16 or HPV18 L1 and L2) using Lipofectamine2000 transfection reagent (Invitrogen). After 72 h, cell lysates were prepared by incubating the cells at 37 °C for 24 h in PBS-Mg solution (PBS supplemented with 1× antibiotic-antimycotic (Life Technologies) and 9.5 mM MgCl2), 0.5% Triton X-100, 40 mM sodium phosphate buffer pH 7.5 and 20 μg/mL RNAse A, then storing at −80 °C until use. In contrast to nHPV vaccine VLPs, the PsVs contain both the L1 and L2 major and minor capsid proteins and are infection competent. Thawed lysates were clarified twice prior to labeling. Each HPV PsV strain was conjugated to a unique fluorochrome (AF488 or AF647) by rotating diluted cell lysates with Alexa Fluor 488 or Alexa Fluor 647 dye (Life Technologies Cat #A10235, A20173) for 1 h at RT in the presence of 90 mM sodium bicarbonate buffer, pH 8.5. Labeled PsV were isolated from excess dye and cell lysate precipitate using Optiprep (Sigma-Aldrich) density gradient ultracentrifugation. Fractions containing the most L1 protein, as determined by SDS-PAGE gels, were used for staining (Scherer et al., 2014).
2.6.3. Sorting HPV vaccine-specific memory B cells
Cryopreserved PBMCs collected two weeks after the second nHPV vaccination were thawed and washed as described above. Samples were first incubated with AVID Live/Dead stain (Invitrogen) and a cocktail of unlabeled antibodies to biomolecules that may bind to HPV PsV in a non-antigen-specific manner [syndecan-1 (abcam Cat#ab24511), heparan sulfate (US Biological Life Sciences Cat#H1890), and integrin alpha 6 (eBioscience, eBioGoH3 Cat#14–0495-82)]. Cells were incubated in the cocktail in 1× PBS for 20 min at 4 °C to reduce background binding of HPV pseudoviruses (PsV). The blocking cocktail was washed off with stain buffer (1× PBS with 10% FBS) and the samples were centrifuged at 650 ×g for 5 min. HPV-specific cells were identified using the fluorochrome-conjugated PsVs and a panel of antibodies (Table 1 ) to identify B cell populations of interest. The PsV and antibody cocktail was added to the cells and incubated at 4 °C for 30 min. Cells were washed with stain buffer and resuspended for collection. Immediately before sorting, the sample was filtered into a FACS tube. The sample was collected and sorted on a FACS Aria II (BD Biosciences) using a 70 μm nozzle. PsV-specific memory B cells were single cell sorted into 96-well culture plates containing the transduced irradiated 3T3 IL-2/IL-21/CD40L cells. The gating strategy used for sorting was as follows: live, singlet, lymphocytes, CD3-, CD56-, CD14-,CD19+, IgD-, IgG+ or IgM+, HPV16 PsV+ or HPV18 PsV+. Because there are no documented cases of cross-reactive HPV16 and HPV18 mAbs (Bissett et al., 2014a; Bissett et al., 2014b), any cells that bound both PsV strains were gated out as non-specific binding. Culture plates containing single sorted B cells plus feeder cells were placed in a 37 °C incubator at 5% CO2 for 13 days. Supernatant was harvested as previously described. In the culture plates, supernatant was replaced with 10 μL lysis buffer [DNA Suspension Buffer (Teknova), 0.25% IGEPAL (Sigma-Aldrich), BSA at 1 mg/mL] for future sequencing. All plates were frozen immediately on dry ice and stored at −80 °C until binding and neutralization assays were performed.
Table 1.
Flow cytometry antibody panel.
| Antibody | Manufacturer | Clone |
|---|---|---|
| CD38 PerCP-Cy5.5 | BD Biosciences | HIT2 |
| IgM PE-Dazzle594 | BioLegend | MHM-88 |
| CD19 PE-Cy7 | BD Biosciences | SJ25C1 |
| CD73 BV421 | BD Biosciences | AD2 |
| CD3 BV510 | BD Biosciences | UCHT1 |
| CD56 BV510 | BD Biosciences | NCAM16.2 |
| CD14 BV510 | BD Biosciences | MϕP9 |
| CD27 BV605 | BD Biosciences | L128 |
| IgD BV650 | BD Biosciences | IA6-2 |
| CD20 BV711 | BD Biosciences | 2H7 |
| IgG BV786 | BD Biosciences | G18-145 |
2.6.4. HPV16 and HPV18 binding by Luminex
HPV16 and HPV18 binding was determined by a Luminex-based assay utilizing glutathione-S-transferase (GST)-HPV L1 fusion proteins that were coupled with magnetic beads, as previously described (Katzenellenbogen et al., 2015). In brief, GST, GST- HPV L1 (16, 18) and GST-Protein A (subcloned from a plasmid kindly provided by Dr. Alice Prince, Columbia University) fusion proteins were expressed from a modified pGex4T vector (Katzenellenbogen et al., 2015), and crude bacterial lysates prepared. Magnetic microspheres (beads) containing a unique combination of fluorescent dyes (Bio-Rad Laboratories, Hercules, CA) were covalently coupled with glutathione-linked casein. Modified beads were used to purify fusion proteins from the crude lysates with each bead set incubated with a different fusion protein. Beads were washed, mixed together and incubated with culture supernatants in 96-well plates (final dilution 1:10). Antibodies that bound to the fusion proteins were detected by incubation with biotinylated anti-human IgG or IgM (KPL, Gaithersburg, MD) followed by streptavidin-phycoerythrin (SAPE) (Invitrogen). Fluorescence was measured on a BioPlex 200 instrument (Bio-Rad Laboratories). The median fluorescent intensity (MFI) for GST was subtracted from the MFI of the other antigens. GST-protein A was included to detect the presence of any IgG in the culture supernatants.
2.6.5. HPV16 and HPV18 neutralization assays
Neutralization assays for HPV16 and HPV18 utilized unconjugated PsV, described above, following previously established procedures (Scherer et al., 2014; Scherer et al., 2016; Buck et al., 2005). In brief, 293TT cells were plated in a 96-well plate and incubated for 4 to 6 h at 37 °C. The HPV PsV were incubated with diluted supernatant (1:3.2) in a separate 96-well plate for 1 h at RT. The PsV supernatant mixture was transferred to the plates containing 293TT cells and incubated at 37 °C for 68 h. Once 293TT cells are infected by the PsV, the reporter plasmid (pYSEAP) is expressed and alkaline phosphatase is secreted into the media. The alkaline phosphatase levels in the media were quantified by transferring cell supernatant to Immulon 2 HB plates containing AP substrate and read at 405 nm after a 30 min incubation.
3. Results
3.1. Successful lentiviral vector transduction for the generation of a stable 3T3 IL-2/IL-21/CD40L cell line
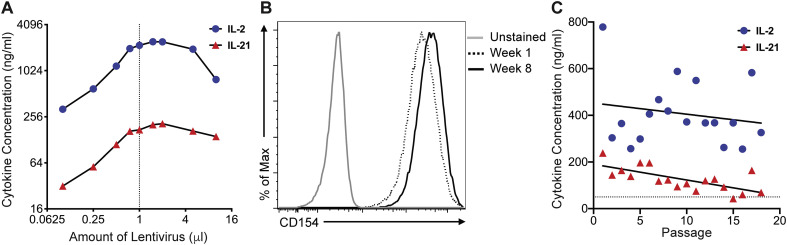
We generated a lentiviral vector capable of delivering a transcript encoding human IL-2 and IL-21, separated by T2A from a single transcript. This was packaged into lentiviral particles and used to transduce 3T3 cells stably expressing CD40L (Huang et al., 2013). Transgene expression results in a single transcript, while the self-cleaving T2A peptide liberates IL-21 from IL-2 co-translationally. 3T3-CD40L cells were transduced with various titers of IL-2/IL-21 lentivirus and cultured for 5 days, after which the cell line was expanded and frozen down for future use. Cell culture supernatant from the transduced 3T3-CD40L cells was tested for IL-2 and IL-21 cytokine secretion by ELISA. IL-2 concentrations were consistently higher than IL-21 and there was a clear dose-response, with cytokine levels increasing with the amount of lentivirus used for transduction. The concentration of secreted IL-2 and IL-21 plateaued with the addition of 1 μL of IL-2/IL-21 lentivirus and were not improved upon by the addition of more virus (Fig. 1A). Therefore, we proceeded to further characterize the 3T3-CD40L cell line transduced with 1 μL of IL-2/IL-21 lentivirus stock (3T3 IL-2/IL-21/CD40L cells).
Fig. 1.
Characterization of IL-2/IL-21-transduced 3T3-CD40L cell line cytokine secretion and CD40L receptor expression. A) Analysis of IL-2 and IL-21 cytokine secretion by 3T3-CD40L cells transduced with varying amounts of IL-2/IL-21 lentivirus. Samples were measured by ELISA 5 days post-transduction. Dashed line indicates 1 μL, which yielded optimal cytokine secretion with the lowest amount of virus. B) CD40L expression was assessed by flow cytometry on non-irradiated feeder cells after 1 and 8 weeks using anti-CD154 PE. Unstained cells were used as a control. C) Concentrations of IL-2 (blue circles) and IL-21 (red triangles) over 18 passages (~2 months) were measured in non-irradiated cells. Lines indicate the average concentration over time determined. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Successful long-term use of the 3T3 IL-2/IL-21/CD40L cell line for B cell culture requires the stable secretion of IL-21 and IL-2, and expression of CD40L over many passages. We verified that the 3T3 IL-2/IL-21/CD40L cells displayed high levels of CD40L expression after 3 and 24 passages (approximately 2 months) post-transduction by flow cytometry (Fig. 1B). Non-irradiated 3T3 IL-2/IL-21/CD40L cells stained with anti-CD40L had close to 100% expression of the receptor. We similarly assessed the stability of cytokine secretion by IL-2 and IL-21 ELISA over the course of 8 weeks (18 passages). Both cytokines were present in the culture media with concentrations ranging between 250 and 780 ng/mL for IL-2 and between 40 and 240 ng/mL for IL-21. IL-2 secretion was relatively stable over 2 months of culture, whereas IL-21 expression gradually declined in concentration over the 18 passages (Fig. 1C).
3.2. IL-2/IL-21/CD40L 3T3 cells produce sufficient IL-2 and IL-21 for B cell culture
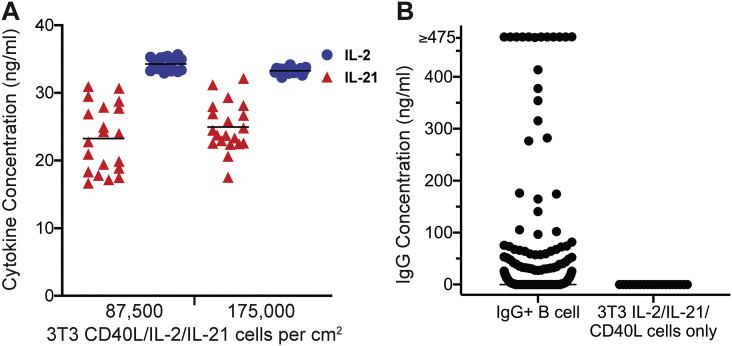
The stable secretion of cytokines is essential to support B cell survival, replication, and immunoglobulin production after the cells are irradiated. Previously irradiated and cryopreserved 3T3 IL-2/IL-21/CD40L cells were thawed, resuspended at 215,000 or 430,000 cells/mL and plated in 65uL per well in half-area 96-well plates. Supernatant was collected from 40 wells to assess IL-2 and IL-21 cytokine concentrations after 1 day in culture at the two plating densities, 87,500 cells per cm2 and 175,000 cells per cm2. The mean concentration of IL-2 measured after 24 h of incubation when cells were plated at 87,500 cells per cm2 was 34.27 ng/mL and did not change when the seeding density was doubled, at 33.27 ng/mL. The mean IL-21 concentration measured after 1 day was 23.24 ng/mL and increased slightly when the seeding density was doubled to 24.96 ng/mL (Fig. 2A). We confirmed that seeding culture plates at 87,500 cells per cm2 provided close to maximum culturing support through IL-2 and IL-21 cytokine secretion and therefore used that seeding density for all future culturing. Using our novel 3T3 IL-2/IL-21/CD40L cell line, we were able to demonstrate simultaneous production of IL-2 and IL-21 in the 20–40 ng/mL range following irradiation and cryopreservation. We note that these levels are slightly lower than those used in established protocols where the exogenous addition of 50 ng/mL IL-2 and IL-21 to complete IMDM media was found to be sufficient to support B cell growth (Huang et al., 2013).
Fig. 2.
Characterization of irradiated 3 T3 IL-2/IL-21/CD40L cell line. A) Concentrations of IL-2 (blue circles) and IL-21 (red triangles) secreted by irradiated feeder cells were measured in 96-well half-volume plates at 87,500 cells per cm2 (n = 20) and 175,000 cell per cm2 (n = 20). Bars indicate the mean. B) Concentration of IgG in culture supernatant from single-cell seeded IgG+ memory B cells (n = 580) after 13 days incubation and compared to wells containing only 3 T3 IL-2/IL-21/CD40L cells measured by ELISA. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.3. 3T3 IL-2/IL-21/CD40L cells to support memory B cell expansion and differentiation into antibody-secreting cells
To demonstrate the B cell supportive capacity of the 3T3 IL-2/IL-21/CD40L cell line, we single-cell sorted human IgG+ memory B cells onto plated 3T3 IL-2/IL-21/CD40L cells and cultured them for 13 days. We then performed IgG ELISAs on the culture supernatants to assess the proportion of B cells that survived, expanded and differentiated into antibody secreting cells and found that 52.41% of wells were positive for IgG (Fig. 2B), which is comparable to the efficiency of culturing when IL-2 and IL-21 are added exogenously to 50 ng/mL (Huang et al., 2013). The median concentration of IgG across the 304 wells with detectable IgG was 4.36 ng/mL. These results indicate that the irradiated 3T3 IL-2/IL-21/CD40L cell line secrets IL-2 and IL-21 to sufficient levels to support IgG secretion in cultures seeded by a single B cell.
3.4. Primary vaccine-specific memory B cells can be expanded using the 3T3 IL-2/IL-21/CD40L feeder cells and screened for IgG and neutralizing activity
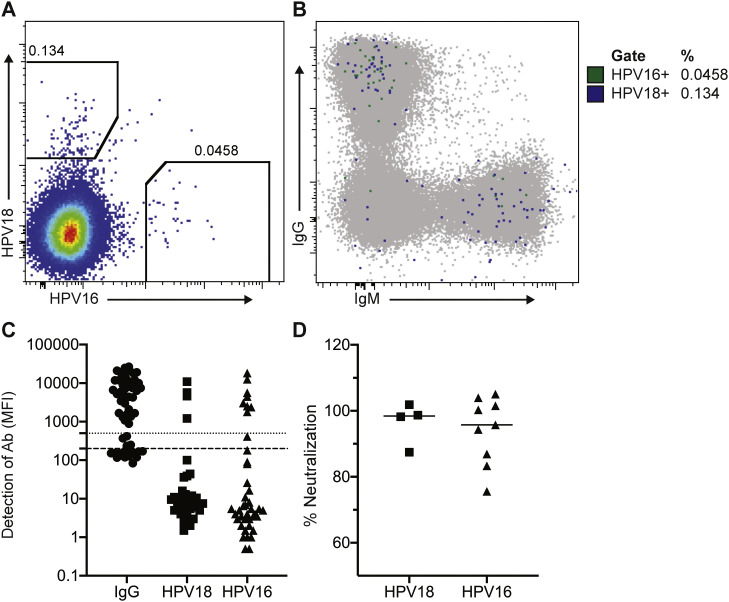
To evaluate whether the feeder cells support sufficient B cell proliferation and antibody secretion to perform functional screens, we evaluated whether antigen-specific HPV vaccine-induced memory B cells could be expanded and differentiated into antibody secreting cells. Furthermore, we evaluated whether we could identify monoclonal HPV-specific B cells that secrete antibodies capable of neutralizing HPV pseudoviruses. We evaluated HPV-specific memory B cells from a PBMC sample collected two weeks after the second HPV vaccination. By flow cytometry, HPV-specific B cells were 0.18% of IgD- B cells in this participant at only two weeks post-second immunization (Fig. 3A). Seventy-four percent of those B cells were specific for the HPV18 strain whereas 26% were specific for HPV16, and 48% of the HPV-specific memory B cells were IgG+. The next dominant isotype (39%) was IgM, although they were CD27+ and IgD-, indicating an antigen-experienced phenotype (Fig. 3B). We single-cell sorted HPV-specific IgG+ memory B cells onto 96-well plates containing the irradiated 3T3 IL-2/IL-21/CD40L cells. After 13 days incubation, we evaluated the supernatants for concentration of IgG, HPV-binding and neutralization of HPV pseudoviruses. Supernatant from each well was tested for binding to protein A as a measure of Ig secretion and to HPV16 and HPV18 L1 proteins using a multiplexed Luminex assay. Sixty-four percent (36/56) of wells with individual sorted IgG+ B cells had detectable IgG in the supernatant (Fig. 3C), and 36% (13/36) of monoclonal B cells with detectable IgG had detectable binding to HPV16 (n = 9) or HPV18 (n = 4) L1 proteins by Luminex (Fig. 3C), respectively. All of the HPV18-specific supernatants were able to neutralize HPV18, while 8 out of 9 of the HPV16-specific B cell supernatants were able to neutralize HPV16 (Fig. 3D). Thus, from the 56 HPV-specific IgG+ memory B cells sorted and screened, we identified 12 monoclonal HPV-specific B cells with neutralizing activity.
Fig. 3.
Analysis of HPV-specific memory B cell sorts and culture supernatant screening. HPV18 and HPV16-specific memory B cells were single cell sorted into culture plates prepared with irradiated transduced 3T3 IL-2/IL-21/CD40L cells. Cells were cultured for 13 days and supernatants were harvested and screened for binding and neutralization to HPV pseudoviruses. A) PBMCs from 2 weeks following a second nHPV vaccination stained with a panel of antibodies and gated on HPV16 or HPV18-specific IgD− memory B cells. B) HPV18 and HPV16-specific memory B cells overlaid on total IgD− B cells showing the isotype distribution. Supernatants harvested from a plate of 56 cultured B cells were tested for binding and neutralization. C) Supernatants harvested from a plate of 56 cultured B cells were tested for binding to HPV16 and HPV18 L1 using a Luminex-based assay. Median Fluorescence Intensity (MFI) is representative of the amount of antibody bound to the beads. Dotted line indicates the positivity threshold for IgG (500) and dashed line indicates positivity threshold for HPV18/16 (200). D) Culture supernatants that showed binding to HPV18 or HPV16 L1 proteins were tested for neutralization of HPV16 and HPV18 pseudoviruses. Lines indicate the median percent neutralization.
4. Discussion
High-throughput screening of human mAbs has facilitated the rapid discovery of numerous potent or broadly neutralizing antibodies against various viruses, including HIV, HPV, influenza, zika virus, EBV, SARS-CoV, SARS-CoV-2 and MERS, thus revolutionizing the infectious diseases field (Rappuoli et al., 2016; Snijder et al., 2018; Robbiani et al., 2017; Scherer et al., 2018; Scherer et al., 2014; Traggiai et al., 2004; Tang et al., 2014; Ju et al., 2020; Seydoux et al., 2020; Brouwer et al., 2020; Robbiani et al., 2020; Zost et al., 2020). Many screening methods have been developed, but each possess some key disadvantages that leave room for improvement.
The development of high-throughput culturing methods for monoclonal human primary B cells, enabled efficient functional screening via monoclonal culture supernatant before single-cell BCR sequencing and generation of recombinant antibodies, was a significant advancement to the HIV-1 field that yielded some of the most potent broadly neutralizing antibodies (Huang et al., 2013; Walker et al., 2009; Walker et al., 2011; Huang et al., 2012). Yet, this is an expensive and labor-intensive approach that can be cost-prohibitive. The generation of the 3T3 IL-2/IL-21/CD40L cell line described here allows marked improvement in affordable discovery of novel mAbs by reducing requirements for costly reagents and allowing for the selective sequencing of cells with desired phenotypic profiles.
In this report, we have detailed the production and characterization of the 3T3 IL-2/IL-21/CD40L cell line. We further demonstrate its utility in allowing the efficient detection and characterization of secreted antibodies. The 3T3 IL-2/IL-21/CD40L cells maintained stable surface expression of CD40L and secretion of IL-2 and IL-21 over extended passages in culture. In addition, when irradiated, cryopreserved and then thawed for use in co-culturing primary B cells, 3T3 IL-2/IL-21/CD40L cells quickly recovered and secreted IL-2 and IL-21. Importantly, when these irradiated 3T3 IL-2/IL-21/CD40L cells were co-cultured with primary memory B cells the overall efficiency of successful B cell expansion and detection of secreted antibodies (52–64%) was in line with previous reports (Huang et al., 2013), demonstrating that cytokines secreted by the cell line were functionally equivalent to the recombinant cytokines added exogenously in previous studies.
We note that the levels of IL-21 secreted by our 3T3 IL-2/IL-21/CD40L cell line have been previously demonstrated to induce class switching in naïve B cell cells that are co-stimulated by CD40L (Pène et al., 2004; Avery et al., 2008; Cohen et al., 2014). Although we focused our efforts on sorting IgG+ B cells in this study, it is possible that the culture conditions could induce class-switching. If this were the case, the flow cytometry panel could be designed to interrogate the initial antibody isotype and/or subclass of the sorted B cells.
We have demonstrated our application of this method for the discovery of vaccine-induced monoclonal antibodies that bind and neutralize HPV viruses 16 and 18. Approximately 12% of the sorted HPV-specific IgG+ memory B cells gave rise to cultures that displayed neutralizing activity following 2 weeks of culture. Among the cultures that showed specific binding to HPV16 or HPV18 L1 proteins, 92% were neutralizing. This is within the expected range of neutralizing antibodies identified using B cell sorting approaches followed by direct B cell sequencing and recombinant mAb reconstruction, which range from ~25–100%, depending on the donor and time of B cell isolation following vaccination (Scherer et al., 2014; Scherer et al., 2018). In brief, we believe that this technology marks an advancement for the field for isolation of mAbs and interrogation of human B cell responses to infectious diseases and related vaccines.
Acknowledgements
This work was supported by the National Institutes of Health R01AI147846, 5UM1AI068618 and UM1AI14462. We also acknowledge the use of New Development Funds from the Fred Hutchinson Cancer Research Center. We thank Laura Richert-Spuhler and Stephen P. Voght for assistance with reading and preparing this manuscript.
References
- Avery D.T., Bryant V.L., Ma C.S., de Waal Malefyt R., Tangye S.G. IL-21-induced isotype switching to IgG and IgA by human naive B cells is differentially regulated by IL-4. J. Immunol. 2008;181:1767–1779. doi: 10.4049/jimmunol.181.3.1767. [DOI] [PubMed] [Google Scholar]
- Bissett S.L., Draper E., Myers R.E., Godi A., Beddows S. Cross-neutralizing antibodies elicited by the Cervarix(R) human papillomavirus vaccine display a range of Alpha-9 inter-type specificities. Vaccine. 2014;32:1139–1146. doi: 10.1016/j.vaccine.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissett S.L., Mattiuzzo G., Draper E., Godi A., Wilkinson D.E., Minor P., Page M., Beddows S. Pre-clinical immunogenicity of human papillomavirus alpha-7 and alpha-9 major capsid proteins. Vaccine. 2014;32:6548–6555. doi: 10.1016/j.vaccine.2014.07.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer P.J.M., Caniels T.G., van der Straten K., Snitselaar J.L., Aldon Y., Bangaru S., Torres J.L., Okba N.M.A., Claireaux M., Kerster G., Bentlage A.E.H., van Haaren M.M., Guerra D., Burger J.A., Schermer E.E., Verheul K.D., van der Velde N., van der Kooi A., van Schooten J., van Breemen M.J., Bijl T.P.L., Sliepen K., Aartse A., Derking R., Bontjer I., Kootstra N.A., Wiersinga W.J., Vidarsson G., Haagmans B.L., Ward A.B., de Bree G.J., Sanders R.W., van Gils M.J. Potent neutralizing antibodies from COVID-19 patients define multiple targets of vulnerability. Science. 2020;369:643–650. doi: 10.1126/science.abc5902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck C.B., Pastrana D.V., Lowy D.R., Schiller J.T. Generation of HPV pseudovirions using transfection and their use in neutralization assays. Methods Mol. Med. 2005;119:445–462. doi: 10.1385/1-59259-982-6:445. [DOI] [PubMed] [Google Scholar]
- Bugli F., Bastidas R., Burton D.R., Williamson R.A., Clementi M., Burioni R. Molecular profile of a human monoclonal antibody fab fragment specific for Epstein-Barr virus gp350/220 antigen. Hum. Immunol. 2001;62:362–367. doi: 10.1016/s0198-8859(01)00216-6. [DOI] [PubMed] [Google Scholar]
- Burton D.R. What are the Most powerful immunogen design vaccine strategies? Reverse vaccinology 2.0 shows great promise. Cold Spring Harb. Perspect. Biol. 2017;9 doi: 10.1101/cshperspect.a030262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton D.R., Pyati J., Koduri R., Sharp S.J., Thornton G.B., Parren P.W., Sawyer L.S., Hendry R.M., Dunlop N., Nara P.L. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science. 1994;266:1024–1027. doi: 10.1126/science.7973652. [DOI] [PubMed] [Google Scholar]
- Caskey M., Klein F., Nussenzweig M.C. Broadly neutralizing anti-HIV-1 monoclonal antibodies in the clinic. Nat. Med. 2019;25:547–553. doi: 10.1038/s41591-019-0412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Streaker E.D., Russ D.E., Feng Y., Prabakaran P., Dimitrov D.S. Characterization of germline antibody libraries from human umbilical cord blood and selection of monoclonal antibodies to viral envelope glycoproteins: implications for mechanisms of immune evasion and design of vaccine immunogens. Biochem. Biophys. Res. Commun. 2012;417:1164–1169. doi: 10.1016/j.bbrc.2011.12.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen K., Altfeld M., Alter G., Stamatatos L. Early preservation of CXCR5+ PD-1+ helper T cells and B cell activation predict the breadth of neutralizing antibody responses in chronic HIV-1 infection. J. Virol. 2014;88:13310–13321. doi: 10.1128/JVI.02186-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessell A.J., Hangartner L., Hunter M., Havenith C.E., Beurskens F.J., Bakker J.M., Lanigan C.M., Landucci G., Forthal D.N., Parren P.W., Marx P.A., Burton D.R. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449:101–104. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- Hessell A.J., Poignard P., Hunter M., Hangartner L., Tehrani D.M., Bleeker W.K., Parren P.W., Marx P.A., Burton D.R. Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat. Med. 2009;15:951–954. doi: 10.1038/nm.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Ofek G., Laub L., Louder M.K., Doria-Rose N.A., Longo N.S., Imamichi H., Bailer R.T., Chakrabarti B., Sharma S.K., Alam S.M., Wang T., Yang Y., Zhang B., Migueles S.A., Wyatt R., Haynes B.F., Kwong P.D., Mascola J.R., Connors M. Broad and potent neutralization of HIV-1 by a gp41-specific human antibody. Nature. 2012;491:406–412. doi: 10.1038/nature11544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Doria-Rose N.A., Longo N.S., Laub L., Lin C.L., Turk E., Kang B.H., Migueles S.A., Bailer R.T., Mascola J.R., Connors M. Isolation of human monoclonal antibodies from peripheral blood B cells. Nat. Protoc. 2013;8:1907–1915. doi: 10.1038/nprot.2013.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju B., Zhang Q., Ge J., Wang R., Sun J., Ge X., Yu J., Shan S., Zhou B., Song S., Tang X., Yu J., Lan J., Yuan J., Wang H., Zhao J., Zhang S., Wang Y., Shi X., Liu L., Zhao J., Wang X., Zhang Z., Zhang L. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature. 2020;584:115–119. doi: 10.1038/s41586-020-2380-z. [DOI] [PubMed] [Google Scholar]
- Katzenellenbogen R.A., Carter J.J., Stern J.E., Butsch Kovacic M.S., Mehta P.A., Sauter S.L., Galloway D.A., Winer R.L. Skin and mucosal human papillomavirus seroprevalence in persons with Fanconi anemia. Clin. Vaccine Immunol. 2015;22:413–420. doi: 10.1128/CVI.00665-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwakkenbos M.J., Diehl S.A., Yasuda E., Bakker A.Q., van Geelen C.M.M., Lukens M.V., van Bleek G.M., Widjojoatmodjo M.N., Bogers W.M.J.M., Mei H., Radbruch A., Scheeren F.A., Spits H., Beaumont T. Generation of stable monoclonal antibody-producing B cell receptor-positive human memory B cells by genetic programming. Nat. Med. 2010;16:123–128. doi: 10.1038/nm.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Sai T., Berger M., Chao Q., Davidson D., Deshmukh G., Drozdowski B., Ebel W., Harley S., Henry M., Jacob S., Kline B., Lazo E., Rotella F., Routhier E., Rudolph K., Sage J., Simon P., Yao J., Zhou Y., Kavuru M., Bonfield T., Thomassen M.J., Sass P.M., Nicolaides N.C., Grasso L. Human antibodies for immunotherapy development generated via a human B cell hybridoma technology. Proc. Natl. Acad. Sci. U. S. A. 2006;103:3557–3562. doi: 10.1073/pnas.0511285103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnani D.M., Rogers T.F., Beutler N., Ricciardi M.J., Bailey V.K., Gonzalez-Nieto L., Briney B., Sok D., Le K., Strubel A., Gutman M.J., Pedreno-Lopez N., Grubaugh N.D., Silveira C.G.T., Maxwell H.S., Domingues A., Martins M.A., Lee D.E., Okwuazi E.E., Jean S., Strobert E.A., Chahroudi A., Silvestri G., Vanderford T.H., Kallas E.G., Desrosiers R.C., Bonaldo M.C., Whitehead S.S., Burton D.R., Watkins D.I. Neutralizing human monoclonal antibodies prevent Zika virus infection in macaques. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aan8184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascola J.R., Stiegler G., VanCott T.C., Katinger H., Carpenter C.B., Hanson C.E., Beary H., Hayes D., Frankel S.S., Birx D.L., Lewis M.G. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat. Med. 2000;6:207–210. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- McCafferty J., Griffiths A.D., Winter G., Chiswell D.J. Phage antibodies: filamentous phage displaying antibody variable domains. Nature. 1990;348:552–554. doi: 10.1038/348552a0. [DOI] [PubMed] [Google Scholar]
- Parren P.W., Marx P.A., Hessell A.J., Luckay A., Harouse J., Cheng-Mayer C., Moore J.P., Burton D.R. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J. Virol. 2001;75:8340–8347. doi: 10.1128/JVI.75.17.8340-8347.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pène J., Gauchat J.F., Lécart S., Drouet E., Guglielmi P., Boulay V., Delwail A., Foster D., Lecron J.C., Yssel H. Cutting edge: IL-21 is a switch factor for the production of IgG1 and IgG3 by human B cells. J. Immunol. 2004;172:5154–5157. doi: 10.4049/jimmunol.172.9.5154. [DOI] [PubMed] [Google Scholar]
- Pinto D., Park Y.-J., Beltramello M., Walls A.C., Tortorici M.A., Bianchi S., Jaconi S., Culap K., Zatta F., De Marco A., Peter A., Guarino B., Spreafico R., Cameroni E., Case J.B., Chen R.E., Havenar-Daughton C., Snell G., Telenti A., Virgin H.W., Lanzavecchia A., Diamond M.S., Fink K., Veesler D., Corti D. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature. 2020;583:290–295. doi: 10.1038/s41586-020-2349-y. [DOI] [PubMed] [Google Scholar]
- Prabakaran P., Gan J., Feng Y., Zhu Z., Choudhry V., Xiao X., Ji X., Dimitrov D.S. Structure of severe acute respiratory syndrome coronavirus receptor-binding domain complexed with neutralizing antibody. J. Biol. Chem. 2006;281:15829–15836. doi: 10.1074/jbc.M600697200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappuoli R., Bottomley M.J., D’Oro U., Finco O., De Gregorio E. Reverse vaccinology 2.0: human immunology instructs vaccine antigen design. J. Exp. Med. 2016;213:469–481. doi: 10.1084/jem.20151960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renegar K.B., Small P.A., Jr. Passive transfer of local immunity to influenza virus infection by IgA antibody. J. Immunol. 1991;146:1972–1978. [PubMed] [Google Scholar]
- Robbiani D.F., Bozzacco L., Keeffe J.R., Khouri R., Olsen P.C., Gazumyan A., Schaefer-Babajew D., Avila-Rios S., Nogueira L., Patel R., Azzopardi S.A., Uhl L.F.K., Saeed M., Sevilla-Reyes E.E., Agudelo M., Yao K.H., Golijanin J., Gristick H.B., Lee Y.E., Hurley A., Caskey M., Pai J., Oliveira T., Wunder E.A., Jr., Sacramento G., Nery N., Jr., Orge C., Costa F., Reis M.G., Thomas N.M., Eisenreich T., Weinberger D.M., de Almeida A.R.P., West A.P., Jr., Rice C.M., Bjorkman P.J., Reyes-Teran G., Ko A.I., MacDonald M.R., Nussenzweig M.C. Recurrent potent human neutralizing antibodies to Zika virus in Brazil and Mexico. Cell. 2017;169:597–609. doi: 10.1016/j.cell.2017.04.024. e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbiani D.F., Gaebler C., Muecksch F., Lorenzi J.C.C., Wang Z., Cho A., Agudelo M., Barnes C.O., Gazumyan A., Finkin S., Hägglöf T., Oliveira T.Y., Viant C., Hurley A., Hoffmann H.H., Millard K.G., Kost R.G., Cipolla M., Gordon K., Bianchini F., Chen S.T., Ramos V., Patel R., Dizon J., Shimeliovich I., Mendoza P., Hartweger H., Nogueira L., Pack M., Horowitz J., Schmidt F., Weisblum Y., Michailidis E., Ashbrook A.W., Waltari E., Pak J.E., Huey-Tubman K.E., Koranda N., Hoffman P.R., West A.P., Jr., Rice C.M., Hatziioannou T., Bjorkman P.J., Bieniasz P.D., Caskey M., Nussenzweig M.C. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature. 2020;584:437–442. doi: 10.1038/s41586-020-2456-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roben P., Moore J.P., Thali M., Sodroski J., Barbas C.F., 3rd, Burton D.R. Recognition properties of a panel of human recombinant fab fragments to the CD4 binding site of gp120 that show differing abilities to neutralize human immunodeficiency virus type 1. J. Virol. 1994;68:4821–4828. doi: 10.1128/jvi.68.8.4821-4828.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sather B.D., Ryu B.Y., Stirling B.V., Garibov M., Kerns H.M., Humblet-Baron S., Astrakhan A., Rawlings D.J. Development of B-lineage predominant lentiviral vectors for use in genetic therapies for B cell disorders. Mol. Ther. 2011;19:515–525. doi: 10.1038/mt.2010.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid J.F., Mouquet H., Feldhahn N., Walker B.D., Pereyra F., Cutrell E., Seaman M.S., Mascola J.R., Wyatt R.T., Wardemann H., Nussenzweig M.C. A method for identification of HIV gp140 binding memory B cells in human blood. J. Immunol. Methods. 2008;343(2):65–67. doi: 10.1016/j.jim.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer E.M., Smith R.A., Simonich C.A., Niyonzima N., Carter J.J., Galloway D.A. Characteristics of memory B cells elicited by a highly efficacious HPV vaccine in subjects with no pre-existing immunity. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1004461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer E.M., Smith R.A., Gallego D.F., Carter J.J., Wipf G.C., Hoyos M., Stern M., Thurston T., Trinklein N.D., Wald A., Galloway D.A. A single human papillomavirus vaccine dose improves B cell memory in previously infected subjects. EBioMedicine. 2016;10:55–64. doi: 10.1016/j.ebiom.2016.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer E.M., Smith R.A., Carter J.J., Wipf G.C., Gallego D.F., Stern M., Wald A., Galloway D.A. Analysis of memory B-cell responses reveals suboptimal dosing schedule of a licensed vaccine. J. Infect. Dis. 2018;217:572–580. doi: 10.1093/infdis/jix566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seydoux E., Homad L.J., MacCamy A.J., Parks K.R., Hurlburt N.K., Jennewein M.F., Akins N.R., Stuart A.B., Wan Y.H., Feng J., Whaley R.E., Singh S., Boeckh M., Cohen K.W., McElrath M.J., Englund J.A., Chu H.Y., Pancera M., McGuire A.T., Stamatatos L. Analysis of a SARS-CoV-2-infected individual reveals development of potent neutralizing antibodies with limited somatic mutation. Immunity. 2020;53:98–105. doi: 10.1016/j.immuni.2020.06.001. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi R., Shan C., Duan X., Chen Z., Liu P., Song J., Song T., Bi X., Han C., Wu L., Gao G., Hu X., Zhang Y., Tong Z., Huang W., Liu W.J., Wu G., Zhang B., Wang L., Qi J., Feng H., Wang F.-s., Wang Q., Gao G.F., Yuan Z., Yan J. A human neutralizing antibody targets the receptor binding site of SARS-CoV-2. Nature. 2020;584:120–124. doi: 10.1038/s41586-020-2381-y. [DOI] [PubMed] [Google Scholar]
- Shingai M., Donau O.K., Plishka R.J., Buckler-White A., Mascola J.R., Nabel G.J., Nason M.C., Montefiori D., Moldt B., Poignard P., Diskin R., Bjorkman P.J., Eckhaus M.A., Klein F., Mouquet H., Cetrulo Lorenzi J.C., Gazumyan A., Burton D.R., Nussenzweig M.C., Martin M.A., Nishimura Y. Passive transfer of modest titers of potent and broadly neutralizing anti-HIV monoclonal antibodies block SHIV infection in macaques. J. Exp. Med. 2014;211:2061–2074. doi: 10.1084/jem.20132494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K., Garman L., Wrammert J., Zheng N.Y., Capra J.D., Ahmed R., Wilson P.C. Rapid generation of fully human monoclonal antibodies specific to a vaccinating antigen. Nat. Protoc. 2009;4:372–384. doi: 10.1038/nprot.2009.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijder J., Ortego M.S., Weidle C., Stuart A.B., Gray M.D., McElrath M.J., Pancera M., Veesler D., McGuire A.T. An antibody targeting the fusion machinery neutralizes dual-tropic infection and defines a site of vulnerability on Epstein-Barr virus. Immunity. 2018;48:799–811. doi: 10.1016/j.immuni.2018.03.026. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauber D.J., Debler E.W., Horton P.A., Smith K.A., Wilson I.A. Crystal structure of the IL-2 signaling complex: paradigm for a heterotrimeric cytokine receptor. Proc. Natl. Acad. Sci. U. S. A. 2006;103:2788–2793. doi: 10.1073/pnas.0511161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinitz M., Klein G., Koskimies S., Makel O. EB virus-induced B lymphocyte cell lines producing specific antibody. Nature. 1977;269:420–422. doi: 10.1038/269420a0. [DOI] [PubMed] [Google Scholar]
- Sui J., Li W., Murakami A., Tamin A., Matthews L.J., Wong S.K., Moore M.J., Tallarico A.S., Olurinde M., Choe H., Anderson L.J., Bellini W.J., Farzan M., Marasco W.A. Potent neutralization of severe acute respiratory syndrome (SARS) coronavirus by a human mAb to S1 protein that blocks receptor association. Proc. Natl. Acad. Sci. U. S. A. 2004;101:2536–2541. doi: 10.1073/pnas.0307140101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X.C., Agnihothram S.S., Jiao Y., Stanhope J., Graham R.L., Peterson E.C., Avnir Y., Tallarico A.S., Sheehan J., Zhu Q., Baric R.S., Marasco W.A. Identification of human neutralizing antibodies against MERS-CoV and their role in virus adaptive evolution. Proc. Natl. Acad. Sci. U. S. A. 2014;111:E2018–E2026. doi: 10.1073/pnas.1402074111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiller T., Meffre E., Yurasov S., Tsuiji M., Nussenzweig M.C., Wardemann H. Efficient generation of monoclonal antibodies from single human B cells by single cell RT-PCR and expression vector cloning. J. Immunol. Methods. 2008;329:112–124. doi: 10.1016/j.jim.2007.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traggiai E., Becker S., Subbarao K., Kolesnikova L., Uematsu Y., Gismondo M.R., Murphy B.R., Rappuoli R., Lanzavecchia A. An efficient method to make human monoclonal antibodies from memory B cells: potent neutralization of SARS coronavirus. Nat. Med. 2004;10:871–875. doi: 10.1038/nm1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker L.M., Phogat S.K., Chan-Hui P.Y., Wagner D., Phung P., Goss J.L., Wrin T., Simek M.D., Fling S., Mitcham J.L., Lehrman J.K., Priddy F.H., Olsen O.A., Frey S.M., Hammond P.W., Protocol G.P.I., Kaminsky S., Zamb T., Moyle M., Koff W.C., Poignard P., Burton D.R. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science. 2009;326:285–289. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker L.M., Huber M., Doores K.J., Falkowska E., Pejchal R., Julien J.P., Wang S.K., Ramos A., Chan-Hui P.Y., Moyle M., Mitcham J.L., Hammond P.W., Olsen O.A., Phung P., Fling S., Wong C.H., Phogat S., Wrin T., Simek M.D., Protocol G.P.I., Koff W.C., Wilson I.A., Burton D.R., Poignard P. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature. 2011;477:466–470. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Tomaras G.D., Jegaskanda S., Moody M.A., Liao H.X., Goodman K.N., Berman P.W., Rerks-Ngarm S., Pitisuttithum P., Nitayapan S., Kaewkungwal J., Haynes B.F., Cohen J.I. Monoclonal antibodies, derived from humans vaccinated with the RV144 HIV vaccine containing the hvem binding domain of herpes simplex virus (HSV) Glycoprotein D, Neutralize HSV infection, mediate antibody-dependent cellular cytotoxicity, and protect mice from ocular challenge with HSV-1. J. Virol. 2017;91 doi: 10.1128/JVI.00411-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Shi W., Chappell J.D., Joyce M.G., Zhang Y., Kanekiyo M., Becker M.M., van Doremalen N., Fischer R., Wang N., Corbett K.S., Choe M., Mason R.D., Van Galen J.G., Zhou T., Saunders K.O., Tatti K.M., Haynes L.M., Kwong P.D., Modjarrad K., Kong W.P., McLellan J.S., Denison M.R., Munster V.J., Mascola J.R., Graham B.S. Importance of neutralizing monoclonal antibodies targeting multiple antigenic sites on MERS-CoV spike to avoid neutralization escape. J. Virol. 2018;92(10):e02002–e02017. doi: 10.1128/JVI.02002-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying T., Du L., Ju T.W., Prabakaran P., Lau C.C., Lu L., Liu Q., Wang L., Feng Y., Wang Y., Zheng B.J., Yuen K.Y., Jiang S., Dimitrov D.S. Exceptionally potent neutralization of Middle East respiratory syndrome coronavirus by human monoclonal antibodies. J. Virol. 2014;88:7796–7805. doi: 10.1128/JVI.00912-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zost S.J., Gilchuk P., Case J.B., Binshtein E., Chen R.E., Nkolola J.P., Schäfer A., Reidy J.X., Trivette A., Nargi R.S., Sutton R.E., Suryadevara N., Martinez D.R., Williamson L.E., Chen E.C., Jones T., Day S., Myers L., Hassan A.O., Kafai N.M., Winkler E.S., Fox J.M., Shrihari S., Mueller B.K., Meiler J., Chandrashekar A., Mercado N.B., Steinhardt J.J., Ren K., Loo Y.M., Kallewaard N.L., McCune B.T., Keeler S.P., Holtzman M.J., Barouch D.H., Gralinski L.E., Baric R.S., Thackray L.B., Diamond M.S., Carnahan R.H., Crowe J.E., Jr. Potently neutralizing and protective human antibodies against SARS-CoV-2. Nature. 2020;584:443–449. doi: 10.1038/s41586-020-2548-6. [DOI] [PMC free article] [PubMed] [Google Scholar]