Abstract
Global environmental pollution has led to human exposure to ultraviolet (UV) radiation due to the damaged ozone layer, thereby increasing the incidence and death rate of skin cancer including both melanoma and non-melanoma. Overexpression and activation of V-akt murine thymoma viral oncogene homolog (AKT, also known as protein kinase B) and related signaling pathways are major factors contributing to many cancers including lung cancer, esophageal squamous cell carcinoma and skin cancer. Although BRAF inhibitors are used to treat melanoma, further options are needed due to treatment resistance and poor efficacy. Depletion of AKT expression and activation, and related signaling cascades by its inhibitors, decreases the growth of skin cancer and metastasis. Here we have focused the effects of AKT and related signaling (PI3K/AKT/mTOR) pathways by regulators derived from plants and suggest the need for efficient treatment in skin cancer therapy.
Keywords: AKT, skin cancer, AKT inhibitor, signal transduction
1. Introduction
Skin cancer is one of the most frequent cancers worldwide [1,2], with increasing annual costs and morbidity [3,4]. Based on their cellular origin, skin cancers are mainly divided into melanoma (derived from melanocytes) and non-melanoma skin cancer (NMSC, from epithelial cells). NMSC includes basal cell carcinoma (~80%) and squamous cell carcinoma (~16%) and melanoma accounts for ~4% of all skin cancer [5]. Even though it accounts for a small portion of skin cancer, melanoma is the most malignant cause of invasive metastasis with a five year survival rate of ~20% [6]. An estimated 7,700,000 new cases of NMSC, including 5,900,000 basal cell carcinomas and 1,800,000 squamous cell carcinomas, have been reported globally including 195 countries accounting for 65,000 cancer deaths [2,7]. An estimated ~100,350 new cases of melanoma and 6850 related deaths have been reported in the USA during 2020 [1].
The major environmental risk factors for skin cancer (melanoma and NMSC) include UVA, UVB and UVC [6,8]. UVC has the shortest wavelengths spectrum (100–280 nm) and after absorption by the ozone layer and atmosphere, it barely reaches the earth. Generally, the human skin is affected by solar UV (100–400 nm) radiation composed of UVA (315–400 nm, ~95%) and UVB (280–315 nm, ~5%) rays triggering skin inflammation, carcinogenesis and cancer development [9,10].
Genetic mutation of p53, BRAF, RAS, CDKN2A and PTEN, and abnormal expression/activation of T-LAK cell-originated protein kinase (TOPK), mitogen-activated protein kinase kinase (MEK), 90 kDa ribosomal S6 kinase (RSK) and AKT in melanoma and NMSC induce cancer cell signal transduction thereby promoting skin carcinogenesis and cancer cell proliferation, migration and invasion [6,9,11,12,13,14]. Solar UV triggers PTEN mutations. Other environmental stimuli induce overexpression and/or activation of AKT and related signaling pathways in skin cancer proliferation and survival. Therefore, it is not surprising that AKT-mediated signaling is one of the main mechanisms in skin cancer therapy. The focus of the article is on AKT and related signaling pathways, and their implications in skin cancer therapy.
Strategies for the skin cancer management include surgery, radiation, chemotherapy and cutting-edge targeted therapies [13,15]. Dacarbazine, temozolomide, paclitaxel, cisplatin and carboplatin are generally used to treat melanoma, whereas vismodegib, sonidegib and cetuximab are used to target basal and squamous cell carcinoma. Vemurafenib is a competitive inhibitor of BRAF kinase activity, and especially inhibits melanoma with a V600 mutation. However, treatment with these therapeutic agents is associated with severe side effects and a low quality of life including nausea, hair loss and increased risk of infection. It also increases the risk of resistance immediately after treatment for several months. Therefore, natural compounds are the preferred choice due to their reduced risk of toxicity and cost-effectiveness.
2. AKT and Related Signaling Pathways Are Important in Skin Cancer Regulation
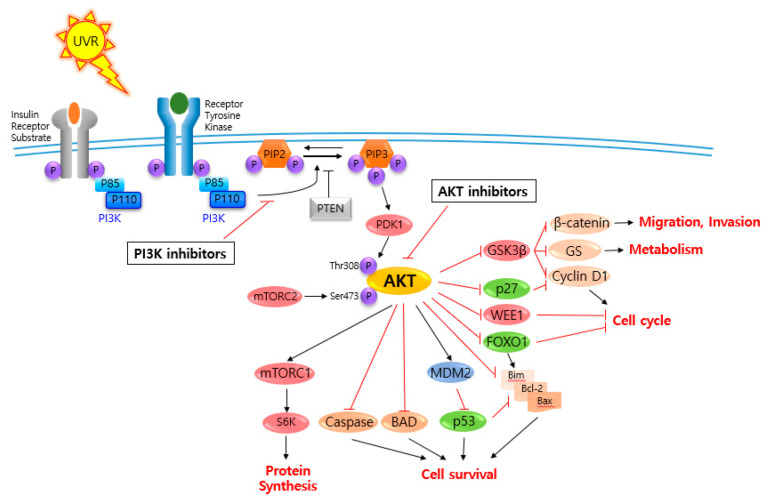
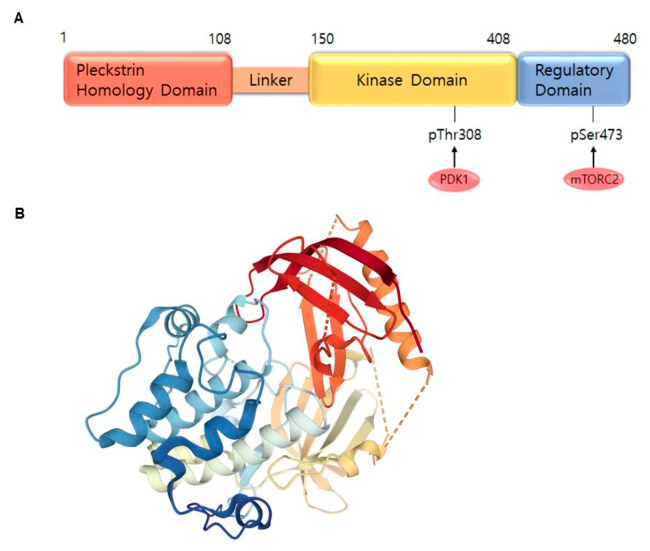
AKT is a serine/threonine-specific protein kinase and alternatively named in protein kinase B (PKB). It plays a key role in multiple cellular processes such as protein synthesis, cell proliferation, cell cycle progression, survival and migration as well as metabolism (Figure 1) [16,17]. The AKT subtypes include AKT1, AKT2 and AKT3 which contain conserved domains such as pleckstrin homology (PH) domain for protein–protein interaction or interaction with phosphatidylinositol phosphate (PIP)-containing lipid bilayers, linker, kinase domain for catalytic activity and regulatory (hydrophobic motif, HM) domain for compete activation with high homology (~80%) as shown in Figure 2. Most of the direct AKT inhibitors target the ATP binding pocket in the kinase and HM domain. In the AKT knock-out (KO) mouse model, each AKT isoform showed different phenotypes such as growth retardation and altered placental development phenotype with increasing lethality in AKT1 KO, insulin resistance and mild growth retardation in AKT2 KO mice, and abnormal development of skin and bone/muscle defect in AKT3 KO [18,19]. AKT is highly activated and expressed in cancers including lung, ovarian, pancreatic and esophageal squamous cell carcinoma [16,20]. In skin cancer, the active form of phospho-AKT (pAKT) was overexpressed in 22 (54%) of 41 benign nevi and 112 (71.3%) of 157 primary melanoma tumors compared to normal adjacent tissue, resulting in a reciprocal five-year survival rate in human metastatic melanoma [21,22]. Together with downstream target protein mTOR, the activated AKT1 induces highly metastatic melanomas involving lung (67%) and brain (17%) in BRAFV600E/Cdkn2aNull mice [23]. Thus, AKT is implicated in melanoma metastasis. AKT protein is activated by the phosphorylation of PI3K (p110/p85 heterodimer), which recruits AKT to the cell membrane through phosphatidylinositol (3,4,5)-trisphosphate (PIP3)-PH domain binding. It’s also stimulated by receptor tyrosine kinase (RTK), phosphatidylinositol-dependent kinase (PDK) 1 or solar UV-induced signaling. The active AKT (p-AKT) mediates the signal transduction to downstream molecules such as the mTOR complex (mTORC)/p70S6 kinase (S6K), caspases, BCL2-associated agonist of cell death (BAD), mouse double minute 2 homolog (MDM2, E3 ubiquitin-protein ligase)/p53, forkhead box protein O1 (FOXO1)/B-cell lymphoma 2 (Bcl-2)/Bcl-2-like protein 11 (Bim)/Bcl-2-associated X protein (Bax), WEE1(G2 Checkpoint Kinase), p27 and glycogen synthase kinase 3 beta (GSK3β/β-catenin/glutamine synthetase (GS)/cyclin D1 for protein synthesis, cell survival, cell cycle, metabolism, migration and invasion (Figure 1). The other hands, PTEN/PI3K/AKT-mediated pathways promote cancer cell migration and invasion by the upregulation of matrix metalloproteinase (MMP)-2, MMP-9 and vascular endothelial growth factor (VEGF) [24,25]. Therefore, it is desirable to regulate the AKT kinase expression and activation and related signaling pathways for skin cancer therapy.
Figure 1.
V-akt murine thymoma viral oncogene homolog (AKT) and related signaling pathways are associated with several features of skin cancer.
Figure 2.
AKT1 structure. (A) Domains of the AKT1. AKT consists of an N-terminal pleckstrin homology (PH) domain (residues 5–108), linker, a catalytic kinase domain (residues 150–408) and a hydrophobic C-terminal tail (HM) (residues 409–480). (B) Crystal structure of AKT1 (PDB ID:3O96). Red indicates N-terminal domains consisting of PH; blue represents HM (https://www.rcsb.org/) (accessed on 18 August 2020).
3. AKT and Related Signaling Pathway Inhibitors for Skin Cancer Regulation
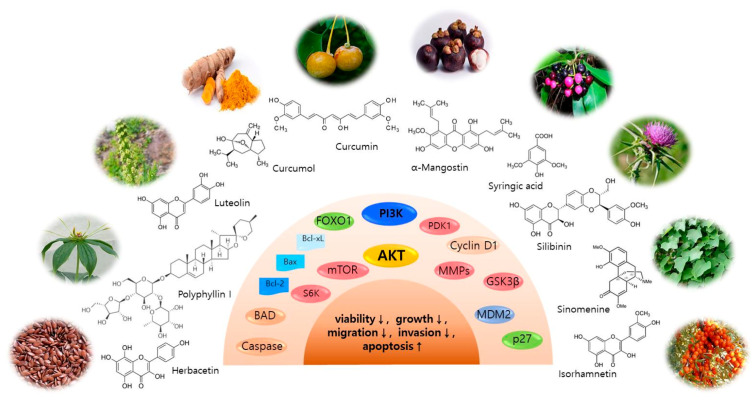
Numerous phytomedicines derived from natural plant or fruit extracts exhibit anticancer activities against cell proliferation, survival, migration, metastasis and angiogenesis in vitro and in vivo. It has been previously reported that acacetin, isoangustone A, sulforaphane and tryptanthrin inhibited melanoma cell proliferation and tumor growth, and induced cell cycle arrest and apoptosis by directly or indirectly targeting PI3K/AKT/mTOR signaling pathways [26,27,28,29]. Here we describe the mechanisms of the latest natural inhibitors targeting AKT kinase and related signaling pathways in skin cancer preclinical studies in vitro and in vivo (Tables 1 and 2, Figure 3).
Figure 3.
Natural compounds for effective treatment through various signaling factors in skin cancer therapy. Image Source: https://en.wikipedia.org/, https://www.naturalmedicinefacts.info/plant/sinomenium-acutum.html, https://www.wikiwand.com/it/Elaeagnus_rhamnoides, https://www.selleckchem.com, https://www.sigmaaldrich.com/. (accessed on 18 August 2020)
3.1. Isorhamnetin
The flavonoid 3′-methoxy-3,4′,5,7-tetrahydroxyflavone is derived from Persicaria thunbergii H. and Elaeagnus rhamnoides (L.) with reported anticancer effects on liver, colorectal, breast and lung cancers [30]. In melanoma, B16F10 cells, isorhamnetin treatment at 10 to 100 μM concentrations in different time points inhibited cell viability (72 h) and migration (12 and 24 h), and induced apoptosis (24 h) via downregulation of pAKT expression [31]. Mechanically, the 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 4 (PFKFB4) metabolic enzyme was inhibited by isorhamnetin, which decreased pAKT expression and was verified by silencing the PFKFB4 expression. Additionally, treatment of C57BL/6 mice bearing B16F10 tumors with 20 mg/kg isorhamnetin retarded the growth and inhibited the expression of cell proliferation marker Ki-67.
3.2. Curcumol
C15H24O2-(3s-(3a,3aa,5a,6a,8ab))-octahydro-3-methyl-8-methylene-5-(1-methylethyl)-6h-3a,6-epoxyazulen-6-ol, is a polyphenol compound derived from Curcuma Wenyujin (ethanol fraction), with pharmacological anticancer activities in liver, lung and gastric cancer [32]. Curcumol inhibited B16 cell viability, colony formation and tumor growth, in vitro (at 50–200 μM) and in vivo (20 mg/kg, intraperitoneal (i.p.)), as well as migration and invasion at 50 and 100 μM and lung metastasis [33]. Regulation of miR-152-3p by curcumol treatment suppressed mesenchymal epithelial transition factor (c-MET) expression following the downstream signal pAKT expression.
3.3. Polyphyllin I
Diosgenyl alpha-L-rhamnopyranosyl-(1-2)-(beta-L-ara-binofuranosyl-(1-4)-beta-D-glucopyranoside), is a major component of Paris polyphylla and inhibits the growth of gastric and ovarian cancers, and osteosarcoma [34,35]. Treatment with polyphyllin I at 1.5, 3.0 and 6.0 mg/L concentrations suppressed A375 melanoma cell progression by reducing cell proliferation, migration and invasion, and the induction of G1 cell cycle accumulation, apoptosis and autophagy at 48 h [36]. Polyphyllin I-induced apoptosis and autophagy was mediated via the downregulation of pPI3K, pAKT and pmTOR expression. It was validated by A375 cell xenograft mouse experiments demonstrating that polyphyllin I (5 mg/kg, i.p.) inhibited tumor growth in both volume and weight. The levels of proliferation marker, Ki-67 and apoptosis feature, TUNEL-positive cells, were inhibited and increased in polyphyllin I-treated tissues compared with vehicle-treated controls, respectively.
3.4. Herbacetin
The flavonoid 3,5,7,8-tetrahydroxy-2-(4-hydroxyphenyl) chromen-4-one is a compound derived from flaxseed and ramose scouring rush, which suppresses breast and colon cancers and hepatocellular carcinoma [37]. Herbacetin showed anticarcinogenic and anticancer activities in A431 cutaneous squamous cell carcinoma and SK-MEL-5 melanoma in vitro at 10 and 20 μM concentrations, and in a mouse model of two-stages skin carcinogenesis induced by 7,12-dimethylbenz[α]-anthracene (DMBA)/12-O-tetradecanoylphorbol-13-acetate (TPA) and solar UV (48 kJ/m2 UVA, 2.9 kJ/m2 UVB) as well as in a SK-MEL-5 cell xenograft mouse model in vivo following topical treatment (100 and 500 nmol) and i.p. injection (0.2 and 1 mg/kg) [38]. Based on the AKT kinase assay, ex vivo pull-down assay and in silico docking analysis, herbacetin directly binds to AKT and inhibits its activity as well as the related signaling pathways such as GSK3β and RSK2.
3.5. Luteolin
The flavonoid 3′,4′,5,7-tetrahydroxyflavone is a compound derived from Reseda luteola, celery, broccoli and onions, and inhibits lung, breast and colon cancers [39,40]. Luteolin suppressed UVB (0.05 J/cm2)-induced COX-2 expression, AP-1 and NF-κB activation by the downregulation of pERK, pp38, pJNK and pAKT in JB6 P+ cells via directly targeting PKCε and c-Src kinase activity at 10 and 20 μM, and attenuated UVB (0.18 J/cm2)-induced skin tumorigenesis in SKH-1 hairless mice topically treated with 10 and 40 nmol [41]. Yao X et al. reported that luteolin inhibited A375 melanoma cell proliferation and induced apoptosis as well as migration and invasion at 10, 15 and 20 μM by decreasing MMP-2 and MMP-9 and increasing TIMP-1 and TIMP-2 expression through reducing the pPI3K and pAKT1 levels [42]. In the A375 cell xenograft mouse model, luteolin (100 mg/kg, i.p.) retarded the tumor growth and weight, and inhibited the expression of MMP-2 and MMP-9, and pPI3K and pAKT1.
3.6. Sinomenine
The alkaloid component 7,8-didehydro-4-hydroxy-3,7-dimethoxy-17-methylmorphinane-6-one is derived from Sinomenium acutum which is used for rheumatoid arthritis [43]. The anticancer activities of sinomenine have been reported in ovarian, gastric and lung cancers [31,44]. Sinomenine significantly inhibited caspase-3 activity and the viability of B16-F10 mouse melanoma cells and induced apoptosis and related proteins expression (Bax/Bcl-2 ratio) at 25, 50 and 100 μM in a CCK-8 assay and Annexin V stained cell counting as well as in Western blot analysis [45]. In detail, sinomenine increased autophagy by the induction of beclin1 expression and LC3II/LC3I level ratio and reduction in p-p62/SQSTM1 expression by the inhibition of pAKT and pmTOR expression, and thereby suppressed the cell growth and increased apoptosis/autophagy. The results were confirmed by treatment of cells with chloroquine, an autophagy inhibitor and a LY294002, PI3K/AKT inhibitor. Moreover, sinomenine (100 mg/kg, s.c.) retarded the melanoma tumor growth in vivo.
3.7. Syringic Acid
The active compound 4-hydroxy-3,5-dimethoxybenzoic acid is derived from Euterpe oleracea and Rhus javanica extract, and inhibits colorectal cancer and oral squamous cell carcinoma [46,47]. Syringic acid (0.2 and 1 mM) prevented UVB (0.2 J/cm2)-induced skin papilloma and tumors in SKH-1 hairless mice [48]. Mechanically syringic acid (40 μM) inhibited UVB (0.04 J/cm2)-induced reactive oxygen species (ROS) production by regulation of NADPH oxidase (NoX) activity and enhanced the interaction between phosphatase (PTP)-κ and epidermal growth factor receptor (EGFR), which was then decreased following signaling cascade pBraf and pAKT expression in human skin epithelial HaCaT cells.
3.8. Ginkgo Biloba Exocarp Extract
Derived from Ginkgo biloba L. (Ginkgo nuts) via the water solution–alcohol precipitation method, suppressed Lewis lung cancer cell growth or angiogenesis by regulation of mitogen-activated protein kinase (MAPK) signaling pathways and Wnt/β-catenin-vascular endothelial growth factor (VEGF) signaling pathways [49,50]. Ginkgo biloba exocarp extract inhibited B16-F10 cell proliferation, migration and adhesion to HUVEC cells in vitro at 10, 20 and 40 μg/mL by decreasing the expression of pPI3K and pAKT, and reduced B16F10 cells grafted tumor growth and lung metastasis in C57BL/6J mice, in vivo at 50, 100 and 200 mg/kg [51].
3.9. α-Mangostin
An active component of Garcinia mangostana Linn, 1,3,6-Trihydroxy-7-methoxy-2,8-bis(3-methylbut-2-en-1-yl)-9H-xanthen-9-one inhibits cervical cancer and hepatocellular carcinoma by the suppression of cancer stemness and signal transducer and activator of transcription (STAT3) signaling via Src homology region 2 domain-containing phosphatase (SHP)-1 stabilization [52,53]. α-Mangostin (5 and 10 mg/kg, i.p.) reduced the tumor incidence and the average numbers in a DMBA/TPA-induced skin carcinogenesis mouse model compared to a control group treated with olive oil [54]. The expression of proinflmmatory cytokines, IL-4, IL-18 and IL-1β was decreased and that of IL-10 was increased in both skin tumor and blood samples, and the level of apoptosis markers, cleaved PARP, cleaved caspase-3, Bad and Bax were induced and Bcl-xL and Bcl-2 were reduced by α-mangostin treatment. Moreover, pPI3K, pAKT and pmTOR expression were decreased by α-mangostin administration in mouse tissues of DMBA/TPA-induced skin carcinogenesis.
3.10. Silibinin (Silybin)
The bioactive compound (2R,3R)-3,5,7-trihydroxy-2-[(2R,3R)-3-(4-hydroxy-3-methoxyphenyl)-2-(hydroxymethyl)-2,3-dihydro-1,4-benzodioxin-6-yl]-2,3-dihydrochromen-4-one is derived from Silybum marianum (L.) and Gaertn. (Asteraceae) and inhibits melanoma and nasopharyngeal carcinoma by the downregulation of MEK/MAPK signaling pathways and PD-L1 expression [14,55]. Silibinin (25, 50 and 100 μM) and its 2,3-dehydro-derivative (DHS, 20 and 30 μM) suppressed ASZ001 (ASZ) and BSZ basal cell carcinoma cells proliferation and colony formation, and induced apoptosis [56]. Silibinin (50 and 100 μM) and DHS (50 and 100 μM) also inhibited signaling pathways including pEGFR, pERK1/2, pAKT and pSTAT3 expression in ASZ cells at 72 h, thereby abrogating NF-κB and AP-1 activities. Based on the results of ASZ cells in a mouse allograft model, the oral administration of silibinin (200 mg/kg) and DHS (200 mg/kg) significantly reduced the tumor weight. Dheeraj A et al., also reported that silibinin inhibited hedgehog inhibitor SANT-1 or GDC-0449- resistance ASZ001 basal cell carcinoma cell growth and colony formation and induced apoptosis by the regulation of pEGFR, pAKT and pERK expression [57].
3.11. Curcumin
The turmeric flavonoid compound 1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione is a from rhizome of Curcuma longa, regulating breast and bladder cancer development [58,59]. The proliferation and invasion of A375 and C8161 melanoma cells was inhibited by treatment with curcumin (25 or 15 μM), which induced G2/M phase cell cycle accumulation and autophagy by a reduction in pAKT, pmTOR and pP70S6K expression [60]. Additionally, curcumin (25 mg/kg, i.p.) suppressed the tumor growth of A375 cell-xenografted mice.
3.12. Other Bioactive Components
The viability of human uveal melanoma UM-1 cells was inhibited by treatment with pristimerin, a quinine methide triterpenoid compound derived from Celastraceae and Hippocrateaceae and the apoptosis induction was mediated by disrupting the mitochondrial membrane potential and increasing the ROS production. Furthermore, pristimerin reduced migration and invasion by the regulation of pAKT and pFoxO3a expression with confirming the knock-down of AKT in UM-1 cells [61]. In human A375 and mouse B16F10 melanoma cells, bioactive compounds such as gambogic acid [62], melittin [63], kaempferol [64], euplotin C [65], lycorine [66], oxyfadichalcone C [67], isoliquiritigenin [68], muniziqi granule/harmine [69], apigenin [70] and casticin [71] inhibited cell proliferation, colony formation, migration and invasion by downregulating the expression of pPI3K, pAKT, pmTOR and pGSK3β together with the expression of related biomarkers including p27, cyclin D1, LC3, 4EBP1, Bax, Bcl-2 and MMPs. A375.S2 cells, which are investigated in studies involving metastasis, chrysin [72] and berberine [73], significantly reduced cell mobility, migration and invasion by decreasing the level of MMPs, N-cadherin and uPA expression through the inhibition of PKC and pAKT. In A431 non-melanoma skin cancer cells, treatment with caffeic acid n-butyl ester induced G2/M phase of cell cycle arrest and apoptosis and inhibited migration by decreasing the expression of pPI3K, pAKT and pmTOR [74].
4. Perspectives
Multisteps of skin carcinogenesis are processed by initiation, promotion and progression [75]. UV is itself both initiator and promoter, and chemicals such as DMBA and TPA are initiators or initiator/promoters. Initiators trigger the DNA damage or ROS production. It can be removed by repair system in healthy cells, however, when the cells fail to recover from DNA damage or oxidative stress then the cells are transformed into neoplastic cells. This initiation and promotion steps indicated by inflammation markers including COX-2, NF-kB and AP-1, and PI3K/AKT/mTOR signaling pathways. Transformed cells continuously progress to cancer by proliferation and spread to other organs by migration and invasion. In these stages, PI3K/AKT/mTOR signaling pathways mediate to induce the survival and migration/invasion biomarkers including cyclins, Bcl-2 family and MMPs. Phytochemicals derived from plants can control each step of carcinogenesis, cancer proliferation and metastasis. In Table 1 and Table 2, most of natural compounds showed antiproliferation, antisurvival, antimigration and anti-invasion of skin cancers by the regulation of AKT-mediated signaling while syringic acid, herbacetin and α-mangostin inhibited DMBA/TPA or UV-induced skin carcinogenesis. Herbacetin directly targeted the ATP-binding pocket of the AKT catalytic domain and others are indirectly affected by AKT up- or downstream signaling pathways. Most effective phytochemicals contain the structure of flavonoid and polyphenols; however, it is difficult to dissolve in water and therefore limited to effect on target organs in vivo. Therefore, the formulation of natural compounds for improving the permeability could be modified such as ethosome. Binary ethosome of evodiamine and fisetin derived from Evodia rutaecarpa and onion enhanced the inhibitory activities of B16 melanoma cell proliferation and UVB-induced inflammation in mice [76,77]. Additionally, the combination therapy of clinical agents and natural compounds is expected to yield positive results. Application of natural compounds in skin cancer therapy requires the standardization of the plant-derived components and elucidation of their action mechanisms. On the other hand, we should consider other options with natural compounds mediated not only via canonical AKT-mediated signaling pathways but also new AKT-mediated signaling mechanisms such as miR-152-3p/c-MET/AKT and AKT/PFKFB4 pathways [31,33].
Table 1.
List of natural compounds targeting PI3K/AKT/mTOR signaling pathway in various skin cancers (in vitro).
| Compounds | Plants | Cancer Types | Cell Lines | Mechanisms | Ref. |
|---|---|---|---|---|---|
| Isorhamnetin | Persicaria thunbergii H., Elaeagnus rhamnoides (L.) |
melanoma | B16F10 | proliferation↓, migration↓ pAKT↓, PFKFB4↓ |
[31] |
| Curcumol | Curcuma wenyujin | melanoma | B16F10 | viability↓, colony formation↓ migration↓, pAKT↓, c-MET↓, miR-152-3p↓ |
[33] |
| Polyphyllin I | Paris polyphylla | melanoma | A375 | cells growth↓, migration↓, invasion↓, cell cycle progression↓, apoptosis↑, Bax↑, cleaved caspases-3↑, Bcl-2↓ autophagy↑, Beclin 1↑, LC3II ↑, p62 ↓ pPI3K↓, pAKT↓, pmTOR↓ |
[36] |
| Herbacetin | Flaxseed, ramose scouring rush herb | SCC, melanoma | JB6, A431, SK-MEL-5, SK-MEL-28 |
AKT1/2 activity↓, ODC activity↓, growth↓, neoplastic transformation↓, pGSK3β↓, ODC activity↓, AP1 activity↓, NF-κB activity↓, pERK1/2↓, p65↓ |
[38] |
| melanoma | A375, Hs294T | tumor growth↓, angiogenesis↓, pEGFR↓, pAKT↓, pERK ↓ pGSK3β↓, ODC activity↓, AP1 activity↓, NF-κB activity↓ |
|||
| Luteolin | Reseda luteola | melanoma | A375.S2 | proliferation↓, migration↓, invasion↓, apoptosis↑, MMP-2↓, MMP-9↓, TIMP-1↑, TIMP-2↑, pAKT1↓, pPI3K↓ |
[41] |
| Sinomenine | Sinomenium acutum | melanoma | B16F10 | cell viability↓, apoptosis↑, Bax↑, Bcl-2↓, caspase-3 activity↑, autophagy↑, Beclin-1↑, LC3II/LC3I ratio↑, pp62/SQSTM1↓, pAKT↓, pmTOR↓ |
[45] |
| Syringic acid | Euterpe oleracea, Rhus javanica | non-melanoma | HaCaT | UVB-induced COX-2↓, UVB-induced MMP-1↓, UVB-induced PGE2 generation↓, UVB-induced AP-1 activity↓, pERK1/2↓, pJNK1/2↓, pp38↓, pMEK1/2↓, p-MKK4/7↓, pMKK3/6↓, pB-Raf↓, pAKT↓, pSrc↓, EGFR↓, UVB-induced cyclooxygenase-2↓, matrix metalloproteinase-1↓, prostaglandin E2↓ | [48] |
| Ginkgo biloba Exocarp Extract | Ginkgo biloba L. | melanoma | B16F10 | proliferation↓, migration↓, heterogeneous adhesion↓, pPI3K↓, pAKT↓, NF-κB↓, MMP-9↓ | [51] |
| Silibinin | Milk thistle plant (Silybum marianum) | BCC | ASZ001, Sant-1, GDC-0449 resistance ASZ001 | growth↓, colony formation ↓, pEGFR↓, pAKT↓, cyclin D1↓, Gli-1↓, SMO↓, SUFU↓, apoptosis↑, caspase-3↑, Bcl-2↓ | [56] |
| Silybum marianum (L.) Gaertn., Asteraceae | BCC | ASZ, BSZ | cell growth↓, clonogenicity↓, apoptosis↑, pEGFR↓, pERK1/2↓, pAKT↓, pSTAT3↓ | [57] | |
| Curcumin | rhizome of Curcuma longa | melanoma | A375 and C8161 | proliferation↓, invasion↓, G2/M phase cell-cycle arrest↑, autophagy↑, pAKT↓, pmTORC1↓, pp70S6K↓ | [60] |
| Pristimerin | Celastraceae, Hippocrateacea |
uveal melanoma | UM-1 | apoptosis↑, viability↓, colony formation↓, mitochondrial membrane potential↓, ROS level↑, G0/G1 phase arrest↑ migration↓, invasion↓ pAKT↓, pFoxO3a ↓, Bim↑, p27Kip1↑, cleaved caspase-3↑, PARP↑, Bax↑, Cyclin D1↓, Bcl-2↓ |
[61] |
| Gambogic acid | resin of Garciania hanburyi | melanoma | A375, B16F10, |
proliferation↓, migration↓, invasion↓, adhesion↓, EMT↓, angiogenesis processes↓ MMP-2 and MMP-9 activities↓ PI3K–AKT–mTOR signaling pathway↓ |
[62] |
| Melittin /Bee Venom | honey bees (Apis mellifera) | melanoma | B16F10, A375SM, SK-MEL-28 | growth↓, colony-forming ability↓, migration↓, invasion↓, apoptosis↑, cleaved caspase-3 and -9↑, pPI3K↓, pAKT↓, mTOR↓, ERK↓, p38↓ |
[63] |
| Kaempferol | piper | melanoma | A375 | proliferation↓, migration↓, colony formation↓, apoptosis↑, G2/M cell cycle arrest↑, pmTOR↓, pPI3K↓, pAKT↓ | [64] |
| Euplotin C | Euplotes crassus | melanoma | A375, 501Mel, MeWo, HDFa | viability↓, apoptosis↑, migration↓, B-Raf↓, pERK 1/2↓, pAKT↓ | [65] |
| Lycorine |
Lycoris radiate spider lilies (Lycoris), daffodils (Narcissus) and snowdrops (Galanthus) |
malignant melanoma | HEMa, A375 | proliferation↓, cell migration↓, invasion↓, apoptosis↑, caspase-3↑, Bax↑, Bcl-2↓, pAKT↓, pmTOR↓, 4EBP1↓ | [66] |
| Oxyfadichalcone C | Oxytropis falcate | melanoma | A375 | proliferation↓, G1 phase arrest↑, apoptosis↑, migration↓, invasion↓, p27↑, cyclin D1↓, ppRb↓, pIntegrin β1↓, MMP-2/9↓, metastasis↓, pPDK1↓, pAKT↓, pGSK-3β↓, pmTOR↓, pp70s6k↓, pERK↓ | [67] |
| Isoliquiritigenin | Glycyrrhizae Radix | melanoma | A375 | proliferation↓, G2/M cell cycle arrest↑, mTOR↓, RICTOR↓, pAKT↓, pGSK-3β↓ | [68] |
| Muniziqi granule/harmine | Peganum harmala, Cichorium intybus, Dracocephalum moldavica, Ocimum basilicum, Althaea rosea, and Nigella glandulifera | melanoma | B16F10 | proliferation↓, autophagy, autophagosome formation↑, LC3-II↑, P62↓, apoptosis↑, G1 cell cycle arrest↑, pAKT↓, pmTOR↓, pERK1/2↓ | [69] |
| Apigenin | Various fruits and vegetables | A375, C8161 | proliferation↓, migration↓, invasion↓, apoptosis↑, G2/M cell cycle arrest↑, cleaved caspase-3↑, cleaved PARP↑, pERK1/2↓, pAKT↓, pmTOR↓ | [70] | |
| Casticin | Fructus viticis | melanoma | B16F10 | migration↓, invasion↓, MMP-9↓, MMP-2↓, MMP-1↓, FAK↓, 14-3-3↓, GRB2↓, AKT↓, NF-κB↓, p65↓, SOS-1↓, p-EGFR↓, p-JNK 1/2↓, uPA↓, Rho A↓ | [71] |
| Chrysin | passionflower, silver linden, honey, propolis | melanoma | A375.S2 | mobility↓, migration↓, invasion↓, MMP-2 activity↓, GRB2↓, SOS-1↓, PKC↓, pAKT (Thr308)↓, NF-κBp65↓, NF-κBp50↓ uPA↓, N-cadherin↓, MMP-1↓, MMP-2↓, VEGF↓, E-cadherin↑, NF-κBp65↓ |
[72] |
| Berberine | the roots and bark of Berberis genus | melanoma | A375.S2 | morphological changes↑, viability↓, mobility↓, migration↓, invasion↓, MMP-9 activity↓, MMP-1↓, MMP-13↓, E-cadherin↑, N-cadherin↓, RhoA↓, ROCK1↓, SOS-1↓, GRB2↓, Ras↓, pERK1/2↓, pc-Jun↓, p-FAK↓, pAKT↓, NF-κB↓, uPA↓, PKC↓, PI3K↓ | [73] |
| caffeic acid n -butyl ester | skin carcinoma | A431 | Apoptosis↑, Bax↑, Bcl-2↓, ROS↑, MMP↓, G2 phase arrest↑, migration↓, pmTOR↓, pPI3K, pAKT↓ | [74] |
↓; decrease ↑; increase.
Table 2.
List of natural compounds targeting PI3K/Akt/mTOR signaling pathway in various skin cancers (in vivo).
| Compounds | Plants | Cancer Types | Model | Treatment | Mechanisms | Ref. |
|---|---|---|---|---|---|---|
| Isorhamnetin | Hippophae rhamnoides L. | melanoma | C57BL/6 mice injected with B16F10 cells, 1 × 105 | 20 mg/kg per day; for 7 days | Proliferation↓, Ki67↓ | [31] |
| Curcumol | Curcuma wenyujin | melanoma | C57BL/6 mice injected (s.c. into the right lower paw and i.v. into the tail vein) with B16 cells, 2 × 106 | 20 mg/kg, i.p.; 3 times per week; for 30 days | proliferation↓, growth↓ invasion↓, metastasis↓ |
[33] |
| Polyphyllin I | Paris polyphylla | melanoma | male BALB/c -nude mice with A375 cells, 2 × 106 | Polyphyllin I 5 mg/kg; i.p.; once a day for 35 days | tumor weight↓, tumor size↓ apoptosis↑, TUNEL positive cells↑, Ki67↓ |
[36] |
| Herbacetin | Flaxseed, ramose scouring rush herb | SCC | -DMBA/TPA model; Hairless SKH:HR-1-hrBr (SKH-1) (8–9 weeks old), initiation with DMBA (200 nmol), and promotion with 17 nmol of TPA in acetone, topically applied twice weekly for 20 weeks -solar–UV induced-skin carcinogenesis model; exposed to solar–UV (48 kJ/UVA/2.9 kJ/UVB) three times weekly for 12 weeks -xenograft model; Athymic mice (Cr:NIH(S), NIH Swiss nude, 6–9-wk-old) with SK-MEL-5 cells, 3 × 106 |
-DMBA/TPA model; 100 or 500 nmol of herbacetin applied to dorsal mouse skin at 30 min before TPA treatment. -solar–UV induced-skin tumor mouse model; after 20 weeks later, herbacetin 100 or 500 nmol for an additional 7 weeks -xenograft model; herbacetin 0.2 and 1 mg/kg; i.p. injected three times per week for 15 days |
skin papillomas↓, tumor volume↓, Ki67↓, pAKT↓, pGSK3β↓, pRSK↓, ODC↓ | [38] |
| Luteolin | Reseda luteola | Melanoma | Female BALB/c -nude mice with A375 cells, 1 × 107 | 100 mg/kg/day, i.p. for 22 days | tumor growth↓ PI3K/AKT↓, MMP-2↓, MMP-9↓ |
[41] |
| Sinomenine | Sinomenium acutum | Melanoma | xenograft model; BALB/c nude mice (6-week-old) by subcutaneously injection with B16-F10 cells | 100 mg/kg/day; s.c., daily for 35 days. | tumor weight↓, tumor volume↓, Ki67↓, PCNA↓ | [45] |
| Syringic acid | Euterpe oleracea, Rhus javanica | non-melanoma | SKH-1 hairless mouse, UVB (0.2 J/cm2) exposure (three times per week for 22 weeks) | 0.2 or 1 mM per mouse in 200 μL acetone on the dorsal surface 1 h before UVB irradiation | UVB-induced skin tumor↓, COX-2↓, MMP-13↓, | [48] |
| Ginkgo biloba Exocarp Extract | C57BL/6J female mice (6-week-old) by subcutaneously injection with B16-F10, 2.0 × 106 cells | 50, 100, 200 mg/kg by intragestic gavage, once a day for 17 days | tumor growth↓, lung metastasis↓, MMP-9↓ |
[51] | ||
| α-Mangostin | pericarp of mangosteen | Skin cancer | DMBA (60 μg)/TPA (4 μg) induced skin carcinogenesis model in ICR female mice, once a week for 20 weeks | 5 and 20 mg/kg, (dissolved in 0.2 mL olive oil) once a day, starting from the day after TPA was topically applied, i.p. for 20 weeks | Skin papilloma↓, growth↓, LC3↑, LC3-II↑, Beclin1↑, LC3-I↓, p62↓, Bax↑, cleaved caspase-3↑, cleaved PARP↑, Bad↑, Bcl-2↓, Bcl-xl↓, apoptosis↑, p-PI3K↓, p-AKT↓, p-mTOR↓ | [54] |
| Silibinin and its 2,3-dehydro-derivative | Silybum marianum (L.) Gaertn., Asteraceae | BCC | ectopic allograft model; five weeks old nude mice (Foxn1nu/nu) by subcutaneously injection with 1 × 106 ASZ cells | silibinin (200 mg/kg in 0.5% CMC) or DHS (200 mg/kg); oral administration, 6 days per week for a total of 7 weeks | tumor growth↓, PCNA↓, cyclin D1↓, proliferation↓, NF-κB↓, AP-1↓, c-Fos↓ | [56] |
| Curcumin | rhizome of Curcuma longa | Melanoma | BALB/c nude female mice (6-week-old) by subcutaneously injection with A375 cells (1 × 107/mL) | 25 mg/kg by i.p. injections, every day for 3 weeks | growth↓ | [60] |
↓; decrease ↑; increase.
Acknowledgments
We greatly appreciated it using the Convergence Research Laboratory (established by the MNU Innovation Support Project in 2019) to conduct this review.
Author Contributions
S.-Y.H. and J.-I.C. contributed to the literature search and collection of articles, assisted with designing the figures and writing; A.-W.K. assisted designing figures; and J.-H.S. and M.-H.L. designed the structure of manuscript and edited manuscript, and supervised the studies and allocated the funding. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Basic Science Research program of National Research Foundation Korea, grant number 2019R1A2C1005899 (J.H.S); 2020R1I1A3070556 (M.H.L).
Conflicts of Interest
The authors declare no conflict of interest.
Search Strategy and Selection Criteria
This review was prepared by searching in PubMed with querying key words: AKT, skin cancer, AKT inhibitor and AKT signaling pathway. The search was conducted for research articles including clinical reports up to 30 July 2020 within recent five years and we especially summarized research articles in each cancer type.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA Cancer J. Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Global Burden of Disease Cancer Collaboration. Fitzmaurice C., Abate D., Abbasi N., Abbastabar H., Abd-Allah F., Abdel-Rahman O., Abdelalim A., Abdoli A., Abdollahpour I., et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2019 doi: 10.1001/jamaoncol.2019.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tran D.A., Coronado A.C., Sarker S., Alvi R. Estimating the health care costs of non-melanoma skin cancer in Saskatchewan using physician billing data. Curr. Oncol. 2019;26:114–118. doi: 10.3747/co.26.4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guy G.P., Jr., Machlin S.R., Ekwueme D.U., Yabroff K.R. Prevalence and costs of skin cancer treatment in the U.S., 2002–2006 and 2007–2011. Am. J. Prev. Med. 2015;48:183–187. doi: 10.1016/j.amepre.2014.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Apalla Z., Nashan D., Weller R.B., Castellsague X. Skin Cancer: Epidemiology, Disease Burden, Pathophysiology, Diagnosis, and Therapeutic Approaches. Dermatol. Ther. 2017;7:5–19. doi: 10.1007/s13555-016-0165-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wrobel S., Przybylo M., Stepien E. The Clinical Trial Landscape for Melanoma Therapies. J. Clin. Med. 2019;8:368. doi: 10.3390/jcm8030368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 8.Khavari P.A. Modelling cancer in human skin tissue. Nat. Rev. Cancer. 2006;6:270–280. doi: 10.1038/nrc1838. [DOI] [PubMed] [Google Scholar]
- 9.Roh E., Lee M.H., Zykova T.A., Zhu F., Nadas J., Kim H.G., Bae K.B., Li Y., Cho Y.Y., Curiel-Lewandrowski C., et al. Targeting PRPK and TOPK for skin cancer prevention and therapy. Oncogene. 2018;37:5633–5647. doi: 10.1038/s41388-018-0350-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee M.H., Lim D.Y., Kim M.O., Lee S.Y., Shin S.H., Kim J.Y., Kim S.H., Kim D.J., Jung S.K., Yao K., et al. Genetic ablation of caspase-7 promotes solar-simulated light-induced mouse skin carcinogenesis: The involvement of keratin-17. Carcinogenesis. 2015;36:1372–1380. doi: 10.1093/carcin/bgv110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim J.E., Lee K.W. Molecular Targets of Phytochemicals for Skin Inflammation. Curr. Pharm. Des. 2018;24:1533–1550. doi: 10.2174/1381612824666180426113247. [DOI] [PubMed] [Google Scholar]
- 12.Zhao R., Choi B.Y., Lee M.H., Bode A.M., Dong Z. Implications of Genetic and Epigenetic Alterations of CDKN2A (p16(INK4a)) in Cancer. EBioMedicine. 2016;8:30–39. doi: 10.1016/j.ebiom.2016.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pal H.C., Hunt K.M., Diamond A., Elmets C.A., Afaq F. Phytochemicals for the Management of Melanoma. Mini Rev. Med. Chem. 2016;16:953–979. doi: 10.2174/1389557516666160211120157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee M.H., Huang Z., Kim D.J., Kim S.H., Kim M.O., Lee S.Y., Xie H., Park S.J., Kim J.Y., Kundu J.K., et al. Direct targeting of MEK1/2 and RSK2 by silybin induces cell-cycle arrest and inhibits melanoma cell growth. Cancer Prev. Res. 2013;6:455–465. doi: 10.1158/1940-6207.CAPR-12-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nikas I.P., Paschou S.A., Ryu H.S. The Role of Nicotinamide in Cancer Chemoprevention and Therapy. Biomolecules. 2020;10:477. doi: 10.3390/biom10030477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song M., Bode A.M., Dong Z., Lee M.H. AKT as a Therapeutic Target for Cancer. Cancer Res. 2019;79:1019–1031. doi: 10.1158/0008-5472.CAN-18-2738. [DOI] [PubMed] [Google Scholar]
- 17.Shariati M., Meric-Bernstam F. Targeting AKT for cancer therapy. Expert Opin. Investig. Drugs. 2019;28:977–988. doi: 10.1080/13543784.2019.1676726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar C.C., Madison V. AKT crystal structure and AKT-specific inhibitors. Oncogene. 2005;24:7493–7501. doi: 10.1038/sj.onc.1209087. [DOI] [PubMed] [Google Scholar]
- 19.Fayard E., Tintignac L.A., Baudry A., Hemmings B.A. Protein kinase B/Akt at a glance. J. Cell Sci. 2005;118:5675–5678. doi: 10.1242/jcs.02724. [DOI] [PubMed] [Google Scholar]
- 20.Song M., Liu X., Liu K., Zhao R., Huang H., Shi Y., Zhang M., Zhou S., Xie H., Chen H., et al. Targeting AKT with Oridonin Inhibits Growth of Esophageal Squamous Cell Carcinoma In Vitro and Patient-Derived Xenografts In Vivo. Mol. Cancer Ther. 2018;17:1540–1553. doi: 10.1158/1535-7163.MCT-17-0823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soares C.D., Borges C.F., Sena-Filho M., Almeida O.P., Stelini R.F., Cintra M.L., Graner E., Zecchin K.G., Jorge J. Prognostic significance of cyclooxygenase 2 and phosphorylated Akt1 overexpression in primary nonmetastatic and metastatic cutaneous melanomas. Melanoma Res. 2017;27:448–456. doi: 10.1097/CMR.0000000000000368. [DOI] [PubMed] [Google Scholar]
- 22.Slipicevic A., Holm R., Nguyen M.T., Bohler P.J., Davidson B., Florenes V.A. Expression of activated Akt and PTEN in malignant melanomas: Relationship with clinical outcome. Am. J. Clin. Pathol. 2005;124:528–536. doi: 10.1309/YT58WWMTA6YR1PRV. [DOI] [PubMed] [Google Scholar]
- 23.Cho J.H., Robinson J.P., Arave R.A., Burnett W.J., Kircher D.A., Chen G., Davies M.A., Grossmann A.H., VanBrocklin M.W., McMahon M., et al. AKT1 Activation Promotes Development of Melanoma Metastases. Cell Rep. 2015;13:898–905. doi: 10.1016/j.celrep.2015.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X., Yang Z., Song W., Zhou L., Li Q., Tao K., Zhou J., Wang X., Zheng Z., You N., et al. Overexpression of Bmi-1 contributes to the invasion and metastasis of hepatocellular carcinoma by increasing the expression of matrix metalloproteinase (MMP)2, MMP-9 and vascular endothelial growth factor via the PTEN/PI3K/Akt pathway. Int. J. Oncol. 2013;43:793–802. doi: 10.3892/ijo.2013.1992. [DOI] [PubMed] [Google Scholar]
- 25.Egeblad M., Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 26.Song N.R., Lee E., Byun S., Kim J.E., Mottamal M., Park J.H., Lim S.S., Bode A.M., Lee H.J., Lee K.W., et al. Isoangustone A, a novel licorice compound, inhibits cell proliferation by targeting PI3K, MKK4, and MKK7 in human melanoma. Cancer Prev. Res. 2013;6:1293–1303. doi: 10.1158/1940-6207.CAPR-13-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jung S.K., Kim J.E., Lee S.Y., Lee M.H., Byun S., Kim Y.A., Lim T.G., Reddy K., Huang Z., Bode A.M., et al. The P110 subunit of PI3-K is a therapeutic target of acacetin in skin cancer. Carcinogenesis. 2014;35:123–130. doi: 10.1093/carcin/bgt266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Antony J., Saikia M., Vinod V., Nath L.R., Katiki M.R., Murty M.S., Paul A., Shabna A., Chandran H., Joseph S.M., et al. DW-F5: A novel formulation against malignant melanoma from Wrightia tinctoria. Sci. Rep. 2015;5:11107. doi: 10.1038/srep11107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arcidiacono P., Stabile A.M., Ragonese F., Pistilli A., Calvieri S., Bottoni U., Crisanti A., Spaccapelo R., Rende M. Anticarcinogenic activities of sulforaphane are influenced by Nerve Growth Factor in human melanoma A375 cells. Food Chem. Toxicol. 2018;113:154–161. doi: 10.1016/j.fct.2018.01.051. [DOI] [PubMed] [Google Scholar]
- 30.Olas B., Skalski B., Ulanowska K. The Anticancer Activity of Sea Buckthorn [Elaeagnus rhamnoides (L.) A. Nelson] Front. Pharmacol. 2018;9:232. doi: 10.3389/fphar.2018.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duan R., Liang X., Chai B., Zhou Y., Du H., Suo Y., Chen Z., Li Q., Huang X. Isorhamnetin Induces Melanoma Cell Apoptosis via the PI3K/Akt and NF-kappaB Pathways. Biomed. Res. Int. 2020;2020:1057943. doi: 10.1155/2020/1057943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo P., Wang Y.W., Weng B.X., Li X.K., Yang S.L., Ye F.Q. Synthesis, anti-tumor activity, and structure-activity relationships of curcumol derivatives. J. Asian Nat. Prod. Res. 2014;16:53–58. doi: 10.1080/10286020.2013.857660. [DOI] [PubMed] [Google Scholar]
- 33.Ning N., Liu S., Liu X., Tian Z., Jiang Y., Yu N., Tan B., Feng H., Feng X., Zou L. Curcumol inhibits the proliferation and metastasis of melanoma via the miR-152-3p/PI3K/AKT and ERK/NF-kappaB signaling pathways. J. Cancer. 2020;11:1679–1692. doi: 10.7150/jca.38624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dong R., Guo J., Zhang Z., Zhou Y., Hua Y. Polyphyllin I inhibits gastric cancer cell proliferation by downregulating the expression of fibroblast activation protein alpha (FAP) and hepatocyte growth factor (HGF) in cancer-associated fibroblasts. Biochem. Biophys. Res. Commun. 2018;497:1129–1134. doi: 10.1016/j.bbrc.2018.02.193. [DOI] [PubMed] [Google Scholar]
- 35.Chang J., Li Y., Wang X., Hu S., Wang H., Shi Q., Wang Y., Yang Y. Polyphyllin I suppresses human osteosarcoma growth by inactivation of Wnt/beta-catenin pathway in vitro and in vivo. Sci. Rep. 2017;7:7605. doi: 10.1038/s41598-017-07194-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Long J., Pi X. Polyphyllin I Promoted Melanoma Cells Autophagy and Apoptosis via PI3K/Akt/mTOR Signaling Pathway. Biomed. Res. Int. 2020;2020:5149417. doi: 10.1155/2020/5149417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim D.J., Roh E., Lee M.H., Oi N., Lim D.Y., Kim M.O., Cho Y.Y., Pugliese A., Shim J.H., Chen H., et al. Herbacetin Is a Novel Allosteric Inhibitor of Ornithine Decarboxylase with Antitumor Activity. Cancer Res. 2016;76:1146–1157. doi: 10.1158/0008-5472.CAN-15-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim D.J., Lee M.H., Liu K., Lim D.Y., Roh E., Chen H., Kim S.H., Shim J.H., Kim M.O., Li W., et al. Herbacetin suppresses cutaneous squamous cell carcinoma and melanoma cell growth by targeting AKT and ODC. Carcinogenesis. 2017;38:1136–1146. doi: 10.1093/carcin/bgx082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Potocnjak I., Simic L., Gobin I., Vukelic I., Domitrovic R. Antitumor activity of luteolin in human colon cancer SW620 cells is mediated by the ERK/FOXO3a signaling pathway. Toxicol. In Vitro. 2020;66:104852. doi: 10.1016/j.tiv.2020.104852. [DOI] [PubMed] [Google Scholar]
- 40.Masraksa W., Tanasawet S., Hutamekalin P., Wongtawatchai T., Sukketsiri W. Luteolin attenuates migration and invasion of lung cancer cells via suppressing focal adhesion kinase and non-receptor tyrosine kinase signaling pathway. Nutr. Res. Pract. 2020;14:127–133. doi: 10.4162/nrp.2020.14.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Byun S., Lee K.W., Jung S.K., Lee E.J., Hwang M.K., Lim S.H., Bode A.M., Lee H.J., Dong Z. Luteolin inhibits protein kinase C(epsilon) and c-Src activities and UVB-induced skin cancer. Cancer Res. 2010;70:2415–2423. doi: 10.1158/0008-5472.CAN-09-4093. [DOI] [PubMed] [Google Scholar]
- 42.Yao X., Jiang W., Yu D., Yan Z. Luteolin inhibits proliferation and induces apoptosis of human melanoma cells in vivo and in vitro by suppressing MMP-2 and MMP-9 through the PI3K/AKT pathway. Food Funct. 2019;10:703–712. doi: 10.1039/C8FO02013B. [DOI] [PubMed] [Google Scholar]
- 43.Xia X., May B.H., Zhang A.L., Guo X., Lu C., Xue C.C., Huang Q. Chinese Herbal Medicines for Rheumatoid Arthritis: Text-Mining the Classical Literature for Potentially Effective Natural Products. Evid. Based Complement Alternat Med. 2020;2020:7531967. doi: 10.1155/2020/7531967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Y., Liu C., Tan T., Li S., Tang S., Chen X. Sinomenine sensitizes human gastric cancer cells to cisplatin through negative regulation of PI3K/AKT/Wnt signaling pathway. Anticancer Drugs. 2019;30:983–990. doi: 10.1097/CAD.0000000000000834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun Z., Zheng L., Liu X., Xing W., Liu X. Sinomenine inhibits the growth of melanoma by enhancement of autophagy via PI3K/AKT/mTOR inhibition. Drug Des. Devel. Ther. 2018;12:2413–2421. doi: 10.2147/DDDT.S155798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Periyannan V., Annamalai V., Veerasamy V. Syringic acid modulates molecular marker-involved cell proliferation, survival, apoptosis, inflammation, and angiogenesis in DMBA-induced oral squamous cell carcinoma in Syrian hamsters. J. Biochem. Mol. Toxicol. 2020:e22574. doi: 10.1002/jbt.22574. [DOI] [PubMed] [Google Scholar]
- 47.Cho H.D., Kim J.H., Won Y.S., Moon K.D., Seo K.I. Inhibitory Effects of Pectinase-Treated Prunus Mume Fruit Concentrate on Colorectal Cancer Proliferation and Angiogenesis of Endothelial Cells. J. Food Sci. 2019;84:3284–3295. doi: 10.1111/1750-3841.14824. [DOI] [PubMed] [Google Scholar]
- 48.Ha S.J., Lee J., Park J., Kim Y.H., Lee N.H., Kim Y.E., Song K.M., Chang P.S., Jeong C.H., Jung S.K. Syringic acid prevents skin carcinogenesis via regulation of NoX and EGFR signaling. Biochem. Pharmacol. 2018;154:435–445. doi: 10.1016/j.bcp.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 49.Cao C., Han D., Su Y., Ge Y., Chen H., Xu A. Ginkgo biloba exocarp extracts induces autophagy in Lewis lung cancer cells involving AMPK/mTOR/p70S6k signaling pathway. Biomed. Pharmacother. 2017;93:1128–1135. doi: 10.1016/j.biopha.2017.07.036. [DOI] [PubMed] [Google Scholar]
- 50.Han D., Cao C., Su Y., Wang J., Sun J., Chen H., Xu A. Ginkgo biloba exocarp extracts inhibits angiogenesis and its effects on Wnt/beta-catenin-VEGF signaling pathway in Lewis lung cancer. J. Ethnopharmacol. 2016;192:406–412. doi: 10.1016/j.jep.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 51.Cao C., Su Y., Gao Y., Luo C., Yin L., Zhao Y., Chen H., Xu A. Ginkgo biloba Exocarp Extract Inhibits the Metastasis of B16-F10 Melanoma Involving PI3K/Akt/NF-kappaB/MMP-9 Signaling Pathway. Evid. Based Complement Alternat Med. 2018;2018:4969028. doi: 10.1155/2018/4969028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang H., Tan Y.P., Zhao L., Wang L., Fu N.J., Zheng S.P., Shen X.F. Anticancer activity of dietary xanthone alpha-mangostin against hepatocellular carcinoma by inhibition of STAT3 signaling via stabilization of SHP1. Cell Death Dis. 2020;11:63. doi: 10.1038/s41419-020-2227-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chien H.J., Ying T.H., Hsieh S.C., Lin C.L., Yu Y.L., Kao S.H., Hsieh Y.H. alpha-Mangostin attenuates stemness and enhances cisplatin-induced cell death in cervical cancer stem-like cells through induction of mitochondrial-mediated apoptosis. J. Cell. Physiol. 2020;235:5590–5601. doi: 10.1002/jcp.29489. [DOI] [PubMed] [Google Scholar]
- 54.Wang F., Ma H., Liu Z., Huang W., Xu X., Zhang X. alpha-Mangostin inhibits DMBA/TPA-induced skin cancer through inhibiting inflammation and promoting autophagy and apoptosis by regulating PI3K/Akt/mTOR signaling pathway in mice. Biomed. Pharmacother. 2017;92:672–680. doi: 10.1016/j.biopha.2017.05.129. [DOI] [PubMed] [Google Scholar]
- 55.Sellam L.S., Zappasodi R., Chettibi F., Djennaoui D., Yahi-Ait Mesbah N., Amir-Tidadini Z.C., Touil-Boukoffa C., Ouahioune W., Merghoub T., Bourouba M. Silibinin down-regulates PD-L1 expression in nasopharyngeal carcinoma by interfering with tumor cell glycolytic metabolism. Arch. Biochem. Biophys. 2020;690:108479. doi: 10.1016/j.abb.2020.108479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tilley C., Deep G., Agarwal C., Wempe M.F., Biedermann D., Valentova K., Kren V., Agarwal R. Silibinin and its 2,3-dehydro-derivative inhibit basal cell carcinoma growth via suppression of mitogenic signaling and transcription factors activation. Mol. Carcinog. 2016;55:3–14. doi: 10.1002/mc.22253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dheeraj A., Rigby C.M., O’Bryant C.L., Agarwal C., Singh R.P., Deep G., Agarwal R. Silibinin Treatment Inhibits the Growth of Hedgehog Inhibitor-Resistant Basal Cell Carcinoma Cells via Targeting EGFR-MAPK-Akt and Hedgehog Signaling. Photochem. Photobiol. 2017;93:999–1007. doi: 10.1111/php.12727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pourhanifeh M.H., Mottaghi R., Razavi Z.S., Shafiee A., Hajighadimi S., Mirzaei H. Therapeutic Applications of Curcumin and its Novel Formulations in the Treatment of Bladder Cancer: A Review of Current Evidence. Anticancer Agents Med. Chem. 2020 doi: 10.2174/1871520620666200807223832. [DOI] [PubMed] [Google Scholar]
- 59.Avila-Galvez M.A., Gimenez-Bastida J.A., Espin J.C., Gonzalez-Sarrias A. Dietary Phenolics against Breast Cancer. A Critical Evidence-Based Review and Future Perspectives. Int. J. Mol. Sci. 2020;21:5718. doi: 10.3390/ijms21165718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhao G., Han X., Zheng S., Li Z., Sha Y., Ni J., Sun Z., Qiao S., Song Z. Curcumin induces autophagy, inhibits proliferation and invasion by downregulating AKT/mTOR signaling pathway in human melanoma cells. Oncol. Rep. 2016;35:1065–1074. doi: 10.3892/or.2015.4413. [DOI] [PubMed] [Google Scholar]
- 61.Yan F., Liao R., Silva M., Li S., Jiang Y., Peng T., Lazarovici P., Zheng W. Pristimerin-induced uveal melanoma cell death via inhibiting PI3K/Akt/FoxO3a signalling pathway. J. Cell. Mol. Med. 2020;24:6208–6219. doi: 10.1111/jcmm.15249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li C.Y., Wang Q., Wang X.M., Li G.X., Shen S., Wei X.L. Gambogic acid exhibits anti-metastatic activity on malignant melanoma mainly through inhibition of PI3K/Akt and ERK signaling pathways. Eur. J. Pharmacol. 2019;864:172719. doi: 10.1016/j.ejphar.2019.172719. [DOI] [PubMed] [Google Scholar]
- 63.Lim H.N., Baek S.B., Jung H.J. Bee Venom and Its Peptide Component Melittin Suppress Growth and Migration of Melanoma Cells via Inhibition of PI3K/AKT/mTOR and MAPK Pathways. Molecules. 2019;24:929. doi: 10.3390/molecules24050929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang J., Xiao P., Sun J., Guo L. Anticancer effects of kaempferol in A375 human malignant melanoma cells are mediated via induction of apoptosis, cell cycle arrest, inhibition of cell migration and downregulation of m-TOR/PI3K/AKT pathway. J. BUON. 2018;23:218–223. [PubMed] [Google Scholar]
- 65.Carpi S., Polini B., Poli G., Alcantara Barata G., Fogli S., Romanini A., Tuccinardi T., Guella G., Frontini F.P., Nieri P., et al. Anticancer Activity of Euplotin C, Isolated from the Marine Ciliate Euplotes crassus, Against Human Melanoma Cells. Mar. Drugs. 2018;16:166. doi: 10.3390/md16050166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jiang Q.Q., Liu W.B. Lycorine inhibits melanoma A375 cell growth and metastasis through the inactivation of the PI3K/AKT signaling pathway. Med. Sci. 2018;34:33–38. doi: 10.1051/medsci/201834f106. [DOI] [PubMed] [Google Scholar]
- 67.Peng X., Wang Z., Liu Y., Peng X., Liu Y., Zhu S., Zhang Z., Qiu Y., Jin M., Wang R., et al. Oxyfadichalcone C inhibits melanoma A375 cell proliferation and metastasis via suppressing PI3K/Akt and MAPK/ERK pathways. Life Sci. 2018;206:35–44. doi: 10.1016/j.lfs.2018.05.032. [DOI] [PubMed] [Google Scholar]
- 68.Chen X.Y., Li D.F., Han J.C., Wang B., Dong Z.P., Yu L.N., Pan Z.H., Qu C.J., Chen Y., Sun S.G., et al. Reprogramming induced by isoliquiritigenin diminishes melanoma cachexia through mTORC2-AKT-GSK3beta signaling. Oncotarget. 2017;8:34565–34575. doi: 10.18632/oncotarget.16655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zou N., Wei Y., Li F., Yang Y., Cheng X., Wang C. The inhibitory effects of compound Muniziqi granule against B16 cells and harmine induced autophagy and apoptosis by inhibiting Akt/mTOR pathway. BMC Complement Altern Med. 2017;17:517. doi: 10.1186/s12906-017-2017-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhao G., Han X., Cheng W., Ni J., Zhang Y., Lin J., Song Z. Apigenin inhibits proliferation and invasion, and induces apoptosis and cell cycle arrest in human melanoma cells. Oncol. Rep. 2017;37:2277–2285. doi: 10.3892/or.2017.5450. [DOI] [PubMed] [Google Scholar]
- 71.Shih Y.L., Chou H.M., Chou H.C., Lu H.F., Chu Y.L., Shang H.S., Chung J.G. Casticin impairs cell migration and invasion of mouse melanoma B16F10 cells via PI3K/AKT and NF-kappaB signaling pathways. Environ. Toxicol. 2017;32:2097–2112. doi: 10.1002/tox.22417. [DOI] [PubMed] [Google Scholar]
- 72.Chen H.Y., Jiang Y.W., Kuo C.L., Way T.D., Chou Y.C., Chang Y.S., Chung J.G. Chrysin inhibit human melanoma A375.S2 cell migration and invasion via affecting MAPK signaling and NF-kappaB signaling pathway in vitro. Environ. Toxicol. 2019;34:434–442. doi: 10.1002/tox.22697. [DOI] [PubMed] [Google Scholar]
- 73.Liu J.F., Lai K.C., Peng S.F., Maraming P., Huang Y.P., Huang A.C., Chueh F.S., Huang W.W., Chung J.G. Berberine Inhibits Human Melanoma A375.S2 Cell Migration and Invasion via Affecting the FAK, uPA, and NF-kappaB Signaling Pathways and Inhibits PLX4032 Resistant A375.S2 Cell Migration In Vitro. Molecules. 2018;23:2019. doi: 10.3390/molecules23082019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zeng N., Hongbo T., Xu Y., Wu M., Wu Y. Anticancer activity of caffeic acid nbutyl ester against A431 skin carcinoma cell line occurs via induction of apoptosis and inhibition of the mTOR/PI3K/AKT signaling pathway. Mol. Med. Rep. 2018;17:5652–5657. doi: 10.3892/mmr.2018.8599. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 75.Basu A.K. DNA Damage, Mutagenesis and Cancer. Int. J. Mol. Sci. 2018;19:970. doi: 10.3390/ijms19040970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lin H., Lin L., Choi Y., Michniak-Kohn B. Development and in-vitro evaluation of co-loaded berberine chloride and evodiamine ethosomes for treatment of melanoma. Int. J. Pharm. 2020;581:119278. doi: 10.1016/j.ijpharm.2020.119278. [DOI] [PubMed] [Google Scholar]
- 77.Moolakkadath T., Aqil M., Ahad A., Imam S.S., Praveen A., Sultana Y., Mujeeb M., Iqbal Z. Fisetin loaded binary ethosomes for management of skin cancer by dermal application on UV exposed mice. Int. J. Pharm. 2019;560:78–91. doi: 10.1016/j.ijpharm.2019.01.067. [DOI] [PubMed] [Google Scholar]