Abstract
Brucellosis is an important zoonosis worldwide. Equines are susceptible to the infection when in close contact with infected animals. The objective of our study was to update the existing knowledge and detect and differentiate the causative agent of brucellosis in breeding equines in Punjab, Pakistan. A cross-sectional study was designed to evaluate the occurrence and etiology of the infection in the equine population in three districts. A total of 448 equine sera were collected from three prefectures viz. Sahiwal, Khanewal, and Okara of the Punjab Province of Pakistan. Ninety-six (21.4%) samples were found positive by RBPT, 3.56% (16/448) by iELISA, and 4.24% (19/448) by CFT. Real-time PCR demonstrated the presence of Brucella abortus-DNA in sero-positive samples. Age and location were found as risk factors. The study concludes equine brucellosis seroprevalence in the country where Brucella abortus as the main etiology. Fistulous withers and poll evil cases should be treated with care as they could be hazardous and a source of zoonotic transmission. Routine screening at an early age, vaccination in ruminants, and consumption of pasteurized dairy milk in humans is recommended for prevention of the infection. Specific tests need to be standardized and validated.
Keywords: Brucella abortus, brucellosis, equines, zoonosis, Pakistan
1. Introduction
Brucellosis is an abortive bacterial zoonosis primarily seen in ruminants and is caused by Brucella (B.) abortus and B. melitensis [1,2,3]. Transmission occurs via direct contact with infected animals or indirectly via the contaminated environment. Humans contract brucellosis mainly through consumption of unpasteurized contaminated dairy milk [4]. “Nonpreferred” hosts (e.g., equids) are susceptible when in close contact with the infected “preferred” hosts [5,6,7,8]. In domestic ruminants, abortion storms during the last trimester, hyperthermia, and retention of fetal membranes and then intermittent fever, arthralgia, and myalgia in acute cases and neurologic disorders in chronic cases in humans are characteristic signs [9]. Equines are potential dead end-host of brucellosis as infection remains asymptomatic with secondary signs (e.g., supra-atlantal bursitis, fistulous withers, carpal hygroma, olecranon bursitis) and occasional epididymitis in stallions [10,11,12,13]. The bursal fluid from such equids may be a source of infection in humans. In Pakistan, brucellosis has been reported in ruminant herds caused by both B. abortus and B. melitensis [1,5]. In humans, occupational exposure to the infected animals has also been associated with transmission of the infection in the country [14,15,16]. Equines are a source of livelihood for poor families and are also status symbols for institutions and rich people in Pakistan [17]. The main purpose of equine rearing in Pakistan is to provide sports (horse riding, polo, tent pegging, etc.) and draft power. They are often raised in proximity with ruminants at the small animal holders’ level, whereas separate at the well managed institutional level. Scarce literature is available regarding brucellosis in equines [18,19]. The purpose of this study was to update the existing knowledge and detect and differentiate the causative agent for brucellosis in breeding equines with reproductive disorders in Pakistan.
2. Materials and Methods
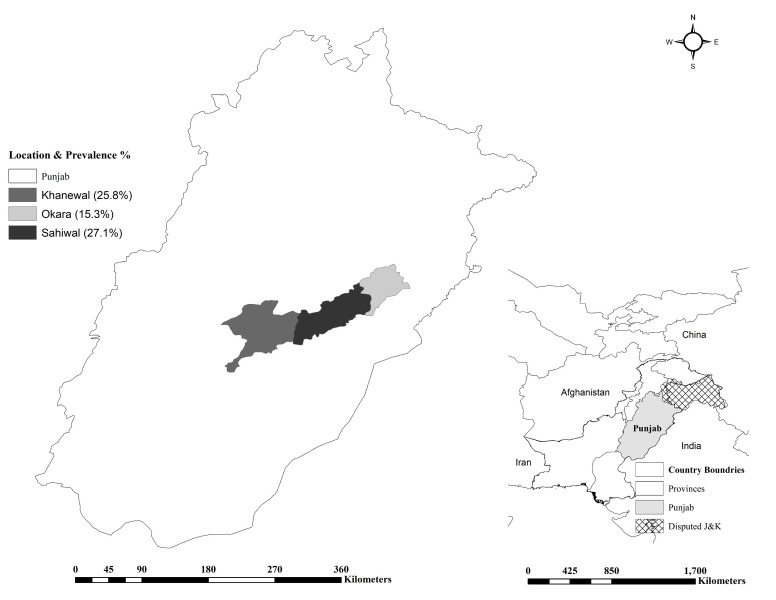
A total of 448 (427 mares, 13 stallions, and 8 Jackasses) of the 4564 “Ghori-Pal” equine population in the three prefectures of Punjab (Pakistan), including Okara (n = 977), Khanewal (n = 1204), and Sahiwal (N = 2383), were recruited. The sampling period spanned over 4 months from December 2017–March 2018 (Figure 1). These animals had a previous history of reproductive disorders, i.e., abortion (≤17% in pregnant mares) and infertility, but no history of articular bursitis (supra-atlantal bursitis, fistulous withers, hygromas, etc.). Owing to unavailability of prevalence estimates of equine brucellosis in the study locales, an expected prevalence of 50% with a 95% confidence interval (CI) and 5% desired absolute precision was taken for sample size estimation [20]. Thus, a minimum of 384 samples had to be taken from the equid population. Finally, 448 animals displaying clinical signs were sampled to be on the safe side. Blood samples (3 mL) were withdrawn directly into gel-clot serum vials. These equids had minimum to no contact with prevailing ruminant herds in the neighborhood. Animal related factors, e.g., age, sex, and breed and environment related factors, e.g., feeding, housing, and breeding practice were noted on a proforma invoice.
Figure 1.
Map for equine brucellosis in Punjab, Punjab.
The sera were tested with Pourquier® Rose Bengale Ag (IDEXX, Montpellier, France) for Rose Bengal Plate Test (RBPT), followed by parallel testing with ID Screen® Brucellosis Serum Indirect Multi-species (IDVet, Grabels, France) for indirect-Enzyme Linked Immunosorbent Assay (iELISA) and Complement Fixation Test (CFT) in the presence of positive and negative controls (provided by Friedrich-Loeffler-Institut (FLI), Jena, Germany) as per standard/manufacturer’s recommendations [7,21,22]. A titer >20 ICFTU/mL was considered as positive for CFT. These tests were applied to detect anti-smooth-lipopolysaccharide (LPS) antibodies produced by exposure to B. abortus and/or B. melitensis.
DNA was extracted from serum samples by QIAamp DNA Mini Qiacube kit (Qiagen, Hilden, Germany) in the presence of E. coli controls. The DNA was detected/differentiated (B. abortus and B. melitensis) by using genus and species-specific primers in a real-time PCR system along with B. abortus (ATCC 23448) and B. melitensis (ATCC 23456) as positive controls [23]. Nuclease free water was used as a no template control (NTC). Briefly, decontamination for 2 min at 50 °C followed by initial denaturation for 10 min at 95 °C, 1 cycle each, denaturation for 25 secs at 95 °C, and 1 min at 57 °C for primers annealing and elongation, 50 cycles for each. A ≤38 cycle threshold (Ct) value was considered positive based on previous in-house validation experience [24].
Univariate and multivariate analysis was done to determine the association of the factors with the infection by using SPSS version 10.0 (IBM, Armonk, NY, USA) and a map was built using ArcGIS (Esri, Redlands, CA, USA).
Ethical statement: The animals were treated and handled for blood collection as per bio-ethical and standard procedures of the “Institutional Bio-Ethics Committee, University of Baluchistan, Quetta, Pakistan” (vide letter no. UOB/ORIC/19/258).
3. Results
Overall, 21.4% (96/448) samples were found positive by RBPT, 3.56% (16/448) by iELISA, and 4.24% (19/448) reacted in CFT (Table 1 and Table 2). The reproductive disorders’ history was highest in equines of Sahiwal followed by Khanewal and Okara (data unpublished). The equids were raised and maintained in a 1000 m2 stable area that comprised of a grazing and an open area paddock. The mares were first mated at four years of age by natural breeding. No artificial insemination was practiced. These equids were fed on gram (Cicer arietinum) and barley (Hordeum vulgare) grains, wheat bran and oats (Avena sativa), and lucerne (Medicago sativa) as green fodder. Based on previous published literature, we considered RBPT as the final criteria for statistical analysis. Univariable analysis indicated a higher occurrence in horses than in donkeys with a nonsignificant association (Table 1). Age was found to be significantly associated (p < 0.05), with the highest occurrence 9/22 (40.9%; CI 20.7–63.6) at early age (≤5 years), followed by 51/207 (24.6%; CI 18.9–31.1) at younger age (5.1–10 years) and 36/219 (16.4%; CI 11.8–22) at older age (>10 years). Animal sex showed a nonsignificant association (p > 0.05), however prevalence was numerically higher in females (22.3%; CI 18.4–26.5) than the males (4.8%; CI 0.1–23.8). Geographical location was found to be significantly associated (p < 0.05) with the occurrence of the infection, with the highest numbers being found in Sahiwal 48/177 (27.1%; CI 20.7–34.3), followed by 16/62 (25.8%; CI 15.5–38.5) in Khanewal and 32/209 (15.3%; CI 10.7–20.9) in Okara (Figure 1). Breeds showed a nonsignificant (p > 0.05) association. Local breeds showed the highest 5/10 (50%; CI 18.7–81.3) seroprevalence followed by exotic breeds 71/341 (20.8%; CI 16.6–25.5) and the Arabian breed, with 20/97 (20.6%; CI 13.1–30). Abortion was insignificantly associated (p > 0.05) with seroprevalence 1/3 (33.3%; CI 0.8–90.6) and 95/445 (21.4%; CI 17.6–25.5) in animals with a prior history. The PCR analysis of samples revealed the presence of B. abortus in all seropositive animals.
Table 1.
Univariable analysis based on the RBPT results.
| Variable | Category | Pos./Tested | Prev. % (95%CI) | p-Value | OR | 95% CI |
|---|---|---|---|---|---|---|
| Species | Donkey | 0/8 | 0 (0–36.9) | 0.091 * | - | - |
| Horse | 96/440 | 21.8 (18–26) | - | - | ||
| Age | ≤5 Y | 9/22 | 40.9 (20.7–63.6) | χ2 = 9.464 p = 0.009 |
3.52 | 1.40–8.85 |
| 5.1–10 Y | 51/207 | 24.6 (18.9–31.1) | 1.67 | 1.03–2.68 | ||
| >10 Y | 36/219 | 16.4 (11.8–22) | Ref | - | ||
| Sex | Female | 95/427 | 22.3 (18.4–26.5) | χ2 = 3.635 p = 0.057 * |
5.72 | 0.76–43.19 |
| Male | 1/21 | 4.8 (0.1–23.8) | Ref | |||
| Location | Okara | 32/209 | 15.3 (10.7–20.9) | χ2 = 8.755 p = 0.013 |
Ref | - |
| Khanewal | 16/62 | 25.8 (15.5–38.5) | 1.92 | 0.97–3.81 | ||
| Sahiwal | 48/177 | 27.1 (20.7–34.3) | 2.06 | 1.25–3.39 | ||
| Breed | ArabX | 20/97 | 20.6 (13.1–30) | χ2 = 4.961 p = 0.084 |
Ref | - |
| ExoticX | 71/341 | 20.8 (16.6–25.5) | 1.01 | 0.58–1.77 | ||
| Desi | 5/10 | 50 (18.7–81.3) | 3.85 | 1.02–14.61 | ||
| Abortion | No | 1/3 | 33.3 (0.8–90.6) | χ2 = 0.257 p = 0.614 |
1.84 | 0.17–20.53 |
| Yes | 95/445 | 21.4 (17.6–25.5) | Ref | - | ||
| Total | 96/448 | 21.4 (17.7–25.5) | ||||
* calculated by Fisher’s exact test; Pos. = Positive; Prev. = prevalence; CI = Confidence Interval; OR = Odds ratio.
Table 2.
Comparison of the results of RBPT, iELISA, and CFT used to detect anti-Brucella antibodies in equines (n = 448).
| RBPT | iELISA | CFT | |||
|---|---|---|---|---|---|
| Negative | Positive | Negative | Positive | ||
| Negative | Observed | 345 | 7 | 334 | 18 |
| Expected | 339.4 | 12.6 | 337.1 | 14.9 | |
| Positive | Observed | 87 | 9 | 95 | 1 |
| Expected | 92.6 | 3.4 | 91.9 | 4.1 | |
| Total (Observed) | 432 | 16 | 429 | 19 | |
RBPT = Rose Bengal Plate Test; iELISA = indirect-Enzyme linked Immune sorbent Assay; CFT = Complement Fixation Test.
In multivariate analysis, age and location were connected with higher risks, i.e., ≤5 years (OR 3.37; CI 1.32–8.60) followed by 5.1–10 years (OR 1.68; CI 1.04–2.73) and Sahiwal (OR 2.05; CI 1.03–4.09) followed by Khanewal (OR 1.99; CI 1.20–3.31) (Table 3).
Table 3.
Multivariable analysis based upon the RBPT results.
| Variable | Exposure Variable | Comparison | OR | 95%CI | p-Value |
|---|---|---|---|---|---|
| Age | ≤5 Y | >10 Y | 3.37 | 1.32–8.60 | 0.01 |
| 5.1–10 Y | >10 Y | 1.68 | 1.04–2.73 | ||
| Location | Sahiwal | Okara | 2.05 | 1.03–4.09 | 0.02 |
| Khanewal | Okara | 1.99 | 1.20–3.31 |
Hosmer and Lemeshow Test Value = χ2 3.216, 4 df, p-Value 0.522; Nagelkerke R2 = 0.059; OR = Odds ratio; CI = Confidence Interval.
Of the 448 samples analysed by three different serologic assays, 96, 16, and 19 samples tested positive, respectively, in RBPT, iELISA, and CFT (Table 2). Out of the 96 RBPT positive samples, nine were iELISA and one was CFT positive also. An inter-rater reliability analysis using the Kappa statistics was performed to determine agreement among three tests (Table 4). There was poor agreement between RBPT and iELISA results, k = 0.106 (95% CI 0.022, 0.19), p = 0.001. Whereas, there was no agreement between RBPT and CFT results, k = −0.057 (95% CI −0.114, −0.0005), p = 0.079. Poor agreement was also observed between iELISA and CFT results, k = 0.197 (95% CI 0.10, 0.29), p < 0.01.
Table 4.
Agreement between RBPT, iELISA, and CFT used for serodiagnosis of brucellosis in equines (n = 448).
| Comparison | Observed Agreement | SE | Kappa Value | 95% CI of Kappa | p-Value | Strength |
|---|---|---|---|---|---|---|
| RBT vs. iELISA | 79.02% | 0.043 | 0.106 | 0.022, 0.19 | 0.001 | Poor |
| RBT vs. CFT | 74.78% | 0.021 | −0.057 | −0.11449, −0.00049 | 0.079 | No Agreement |
| iELISA vs. CFT | 93.97% | 0.097 | 0.197 | 0.100, 0.294 | <0.01 | Poor |
RBPT = Rose Bengal Plate Test; iELISA = indirect-Enzyme linked Immune sorbent Assay; CFT = Complement Fixation Test; SE = Standard error.
4. Discussion
Since long ago, ruminant brucellosis has been endemic in Pakistan [25]. Although equines are not primary hosts of the disease, they are considered dead-end hosts [26]. Previously, B. abortus has been reported in equines elsewhere and as a cause of bovine brucellosis in the country [1]. Hence, we conducted this study to investigate the burden and etiology of equine brucellosis in Pakistani Punjab.
Serology is the main stay in diagnosing brucellosis, which relies on a variety of tests, including RBPT, serum agglutination test (SAT), milk ring test (MRT), ELISAs, fluorescence polarization assay (FPA), and CFT. Each of the tests has certain limitation in terms of diagnostic accuracy. RBPT and iELISA are highly sensitive compared to CFT, which is widely considered as a specific test. Similarly, the SAT shows great promise in terms of sensitivity, with a quantitation ability, especially in humans. MRT is a sensitive and practical tool for both individual and bulk milk tank screening in bovines. Having poor sensitivity, PCR is a highly specific test, which is used as a complementary identification and typing tool. Real-time format is superior to conventional PCR, which provides better sensitivity while maintaining the specificity. Most of these tests require standardization and validation depending upon the disease situation in the area, laboratory conditions, and local resource power [27]. The bacterial culture is a gold standard for diagnosis of brucellosis; nonetheless, it is less sensitive, requires advanced biosafety conditions, e.g., biosafety level-III, has a long test turnover time, and possesses serious human health hazards. Therefore, we chose serology and PCR for the present investigation.
A higher seroprevalence by RBPT may be ascribed to nonspecific reactions of the RBPT antigen to certain bacteria, e.g., Salmonella, Yersninia, and E. coli [28]. Since RBPT has been found to be a satisfactory diagnostic test in our previous reports from Pakistan, we considered RBPT results as final criteria and for statistical analysis [27,29].
This study did not identify statistically significant differences (p > 0.05) between horses and donkeys. However, a higher seroprevalence in horses (21.8%; CI 18–26) might be owing to the fact that the horses were overrepresented in the sample size. Moreover, comparatively more male horses were tested than male donkeys. This might have influenced the results and should be confirmed in further studies to evaluate the difference and influence of the species on results [18,30].
Age was found to be statistically significantly associated (p > 0.05) with a positive serological test (Table 1). Animals younger than <10 years were found to be more at risk (≤5 years, OR 3.37; CI 1.32–8.60 and 5.1–10 years, OR 1.68; CI 1.04–2.73) than older ones, i.e., >10 years (Table 3). These findings are supported by previous literature, suggesting the presence of antibodies without obvious signs at an earlier age and a decline in detectable antibody titer with growing age associated with latency [18,30].
Sex of the animals was not found to be significantly associated (p > 0.05) with positive serology. Females showed 22.3% (18.4–26.5) seroprevalence and males 4.8% (0.1–23.8). These findings may again be biased by the high number of female animals tested in this study because of the lower numbers of breeding horses in the population. A better comparison is possible when comparable numbers of both sexes can be recruited in studies. These findings are supported by previous studies, which found that abortive discharges and transmission of brucellosis were more often associated [11,12,18,30]. However, opposing arguments do exist.
Location was significantly associated (p < 0.05) with seroprevalence in this study (Table 1). The highest seroprevalence was noted in Sahiwal (27.1%; CI 20.7–34.3), followed by Khanewal (25.8%; CI 15.5–38.5) and Okara (15.3%; CI 10.7–20.9). The risk of occurrence was higher in Sahiwal (OR 2.05; CI 1.03–4.09) than the other two districts (Table 3). These results are in parallel with Wadood et al., who suggested management related factors to influence the results [18]. However, a significant association has also been found [31].
The breed was insignificantly associated (p > 0.05) with seropositivity, however a higher percentage of positives was recorded in local breeds (50%; CI 18.7–81.3) followed by exotic (20.8%; CI 16.6–25.5) and Arabian breeds (20.6%; CI 13.1–30). A similar pattern to that of Wadood et al. was found, with little variation [18]. However, a scientific explanation could not be found.
Surprisingly, history of abortion was not significantly (p > 0.05) associated with the outcome of the infection (Table 1). Even a higher seroprevalence (33.3%; CI 0.8–90.6) was found in animals without prior history of abortion (21.4%; CI 17.6–25.5). Rarity of abortion is well known in equine brucellosis [11].
Our study has demonstrated the occurrence of B. abortus as the prevailing cause of brucellosis in the tested equines. Previously, B. abortus and to a lower extent, B. suis have been reported in equines [11]. Although equines were raised away from the ruminant herds, the infection might have transmitted via a route that could not be ascertained in this study. In Pakistan, endemicity of B. abortus is known in ruminants and the animals raised in close contact with ruminants have been reported to contract the infection from the existing herd [32]. Isolation of the etiologic agent(s) both from equines and ruminants could be helpful in determining if the same strain is causing the infection in this area.
5. Conclusions
Livestock brucellosis remains an endemic problem in the country. Fistulous withers and poll evil cases should be treated with care as they could be hazardous and a source of transmission to humans. Lateral transmission of infection to other domestic animals is unknown. Eradication of brucellosis in ruminants will positively affect prevalence in dead-end hosts, such as horses. Age and location were found to be risk factors. Routine screening starting at an age of 12 months should be considered in the brucellosis control policy in equines. Males should be the focus of future studies for a better assessment of the epidemiological situation. B. abortus was found to be the cause of brucellosis in equines in Pakistan, but this finding needs further proof, probably by isolation of the bacteria.
Acknowledgments
All veterinarians, technicians, and “Ghori-Pal” farmers are thanked for their cooperation in this study. The University of Baluchistan, Quetta, University of Agriculture, Faisalabad and University of Veterinary and Animal Sciences (sub-campus), Jhang in Pakistan and Friedrich-Loeffler-Institut, Jena in Germany are highly thanked for their technical support in this study.
Author Contributions
Conceptualization, A.H. and A.M.T.; methodology, A.H.; formal analysis, M.H.H.; investigation, A.H. and T.J.; resources, M.S., I.K., and F.M.; data curation, A.H. and T.J.; writing—original draft preparation, T.J.; writing—review and editing, A.Z., R.H., W.A., M.S., M.I., and H.N. All authors have read and agreed to the published version of the manuscript.
Funding
Authors highly acknowledge the funding from “German Federal Foreign Office” under the project “Development of lab networking under biosafety and biosecurity aspects in Pakistan”. Funding agencies played no role in publishing, conducting, and designing the study and results.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Ali S., Ali Q., Melzer F., Khan I., Akhter S., Neubauer H., Jamal S.M. Isolation and identification of bovine Brucella isolates from Pakistan by biochemical tests and PCR. Trop Anim. Health Prod. 2014;46:73–78. doi: 10.1007/s11250-013-0448-6. [DOI] [PubMed] [Google Scholar]
- 2.Khan M.Z., Usman T., Sadique U., Qureshi M.S., Farooque M., Hassan M.S., Khan A. Molecular characterization of Brucella abortus and Brucella melitensis in cattle and humans at the North West of Pakistan. Pak. Vet. J. 2017;37:360–363. [Google Scholar]
- 3.Mahmood R., Waheed U., Ali T., Gopaul K.K., Dainty A.C., Muchowski J.K., Koylass M.S., Brew S.D., Perrett L.L., Whatmore A.M. Serological and nucleic acid based detection of brucellosis in livestock species and molecular characterization of Brucella melitensis strains isolated from Pakistan. Int. J. Agric. Biol. 2016;18:311–318. doi: 10.17957/IJAB/15.0088. [DOI] [Google Scholar]
- 4.Aparicio E.D. Epidemiology of brucellosis in domestic animals caused by Brucella melitensis, Brucella suis and Brucella abortus. Rev. Sci. Tech. 2013;32:53–60. doi: 10.20506/rst.32.1.2187. [DOI] [PubMed] [Google Scholar]
- 5.Saeed U., Ali S., Khan T.M., El-Adawy H., Melzer F., Khan A.U., Iftikhar A., Neubauer H. Seroepidemiology and the molecular detection of animal brucellosis in Punjab, Pakistan. Microorganisms. 2019;7:449. doi: 10.3390/microorganisms7100449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fatima S., Khan I., Nasir A., Younus M., Saqib M., Melzer F., Neubauer H., El-Adawy H. Serological, molecular detection and potential risk factors associated with camel brucellosis in Pakistan. Trop. Anim. Health Prod. 2016;48:1711–1718. doi: 10.1007/s11250-016-1148-9. [DOI] [PubMed] [Google Scholar]
- 7.Jamil T., Melzer F., Khan I., Iqbal M., Saqib M., Hammad Hussain M., Schwarz S., Neubauer H. Serological and molecular investigation of Brucella species in dogs in Pakistan. Pathogens. 2019;8:294. doi: 10.3390/pathogens8040294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saleem M.Z., Akhtar R., Aslam A., Rashid M.I., Chaudhry Z.I., Manzoor M.A., Shah B.A., Ahmed R., Yasin M. Evidence of Brucella abortus in non-preferred caprine and ovine hosts by real-time PCR Assay. Pak. J. Zool. 2019;51:1187–1189. doi: 10.17582/journal.pjz/2019.51.3.sc3. [DOI] [Google Scholar]
- 9.Galinska E.M., Zagórski J. Brucellosis in humans-etiology, diagnostics, clinical forms. Ann. Agric. Environ. Med. 2013;20:233–238. [PubMed] [Google Scholar]
- 10.Sellon D.C., Nicoletti P.L. Chapter 37—Brucellosis. In: Sellon D.C., Long M.T., editors. Equine Infectious Diseases. 2nd ed. W.B. Saunders; St. Louis, MO, USA: 2014. pp. 337–339.e331. [DOI] [Google Scholar]
- 11.Mair T., Divers T. Brucellosis in the Horse. [(accessed on 12 May 2020)];2009 Available online: https://astableconnection.com/wp-content/uploads/2019/02/Brucellosis-in-the-horse.pdf.
- 12.Karthik K., Prabakar G., Bharathi R., Kumar S. Equine Brucellosis: Review on epidemiology, pathogenesis, clinical signs, prevention and control. J. Exp. Biol. Agric. Sci. 2016;4:151–160. [Google Scholar]
- 13.Giles R., Donahue J., Hong C., Tuttle P., Petrites-Murphy M., Poonacha K., Roberts A., Tramontin R., Smith B., Swerczek T. Causes of abortion, stillbirth, and perinatal death in horses: 3527 cases (1986–1991) J. Am. Vet. Med. Assoc. 1993;203:1170–1175. [PubMed] [Google Scholar]
- 14.Mukhtar F., Kokab F. Brucella serology in abattoir workers. J. Ayub. Med. Coll Abbottabad. 2008;20:57–61. [PubMed] [Google Scholar]
- 15.Asif M., Waheed U., Farooq M., Ali T., Khan Q.M. Frequency of brucellosis in high risk human groups in Pakistan detected through polymerase chain reaction and its comparison with conventional slide agglutination test. Int. J. Agric. Biol. 2014;16:986–990. [Google Scholar]
- 16.Ali S., Ali Q., Neubauer H., Melzer F., Elschner M., Khan I., Abatih E.N., Ullah N., Irfan M., Akhter S. Seroprevalence and risk factors associated with brucellosis as a professional hazard in Pakistan. Foodborne Pathog. Dis. 2013;10:500–505. doi: 10.1089/fpd.2012.1360. [DOI] [PubMed] [Google Scholar]
- 17.Shah S.Z.A., Nawaz Z., Nawaz S., Carder G., Ali M., Soomro N., Compston P.C. The Role and welfare of cart donkeys used in waste management in Karachi, Pakistan. Animals. 2019;9:159. doi: 10.3390/ani9040159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wadood F., Ahmad M., Khan A., Gul S., Rehman N. Seroprevalence of brucellosis in horses in and around Faisalabad. Pak. Vet. J. 2009;29:196–198. [Google Scholar]
- 19.Gul S.T., Khan A., Ahmad M., Hussain I. Seroprevalence of brucellosis and associated hematobiochemical changes in Pakistani horses. Pak. J. Agric. Sci. 2013;50:745–750. [Google Scholar]
- 20.Thrusfield M., Christley R. Veterinary Epidemiology. Wiley; Hoboken, NJ, USA: 2018. [Google Scholar]
- 21.Jamil T., Melzer F., Saqib M., Shahzad A., Khan Kasi K., Hammad Hussain M., Rashid I., Tahir U., Khan I., Haleem Tayyab M., et al. Serological and molecular detection of bovine brucellosis at institutional livestock farms in Punjab, Pakistan. Int. J. Environ. Res. Public Health. 2020;17:1412. doi: 10.3390/ijerph17041412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.OIE . Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. World Organization for Animal Health OIE; Paris, France: 2016. Brucellosis (Brucella abortus, B. melitensis and B. suis) (Infection with B. abortus, B. melitensis and B. suis) pp. 355–398. [Google Scholar]
- 23.Probert W.S., Schrader K.N., Khuong N.Y., Bystrom S.L., Graves M.H. Real-time multiplex PCR assay for detection of Brucella spp., B. abortus, and B. melitensis. J. Clin. Microbial. 2004;42:1290–1293. doi: 10.1128/JCM.42.3.1290-1293.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gwida M.M., El-Gohary A.H., Melzer F., Tomaso H., Rösler U., Wernery U., Wernery R., Elschner M.C., Khan I., Eickhoff M., et al. Comparison of diagnostic tests for the detection of Brucella spp. in camel sera. BMC Res. Notes. 2011;4:525. doi: 10.1186/1756-0500-4-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farooq U., Fatima Z., Afzal M., Anwar Z., Jahangir M. Sero-prevalence of brucellosis in bovines at farms under different management conditions. Br. J. Dairy Sci. 2011;2:35–39. [Google Scholar]
- 26.Moreno E. Retrospective and prospective perspectives on zoonotic brucellosis. Front. Microbiol. 2014;5 doi: 10.3389/fmicb.2014.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ducrotoy M.J., Muñoz P.M., Conde-Álvarez R., Blasco J.M., Moriyón I. A systematic review of current immunological tests for the diagnosis of cattle brucellosis. Prev. Vet. Med. 2018;151:57–72. doi: 10.1016/j.prevetmed.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 28.Abubakar M., Mansoor M., Arshed M.J. Bovine brucellosis: Old and new concepts with Pakistan perspective. Pak. Vet. J. 2012;32:147–155. [Google Scholar]
- 29.Gusi A.M., Bertu W.J., Jesús de Miguel M., Dieste-Pérez L., Smits H.L., Ocholi R.A., Blasco J.M., Moriyón I., Muñoz P.M. Comparative performance of lateral flow immunochromatography, iELISA and Rose Bengal tests for the diagnosis of cattle, sheep, goat and swine brucellosis. PLoS Negl. Trop. Dis. 2019;13:e0007509. doi: 10.1371/journal.pntd.0007509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tijjani A., Junaidu A., Salihu M., Farouq A., Faleke O., Adamu S., Musa H., Hambali I. Serological survey for Brucella antibodies in donkeys of north-eastern Nigeria. Trop. Anim. Health Prod. 2017;49:1211–1216. doi: 10.1007/s11250-017-1318-4. [DOI] [PubMed] [Google Scholar]
- 31.Bandara A., Mahipala M. Incidence of brucellosis in Sri Lanka: An overview. Vet. Microbial. 2002;90:197–207. doi: 10.1016/S0378-1135(02)00254-7. [DOI] [PubMed] [Google Scholar]
- 32.Cohen N.D., Carter G.K., McMullan W.C. Fistulous withers in horses: 24 cases (1984–1990) J. Am. Vet. Med. Assoc. 1992;201:121–124. [PubMed] [Google Scholar]