FXR proteins are part of ribonucleoprotein granules controlling stability, translation, and cellular localization of target mRNAs. In the current issue, Agote‐Aran et al extend the repertoire of FXR protein action and show that they are also involved in the transport of nuclear pore complex components to the nuclear envelope and thereby prevent cytoplasmic aggregation of these proteins.
Subject Categories: Membrane & Intracellular Transport
New findings show that FXR proteins not only regulate stability, translation and cellular localization of mRNAs, but also transport of nuclear pore complex (NPC) components to the nuclear envelope to regulate NPC formation.
The fragile X‐related proteins are a family of RNA‐binding proteins highly expressed in the brain. The founding member of this protein family, fragile X mental retardation protein (FMRP), has attracted much attention because it is involved in fragile X syndrome (FXS), the most common genetic neurodevelopmental disorder and the single largest genetic contribution to autism (Bagni & Zukin, 2019). FMRP and its two paralogs, fragile X‐related protein 1 and 2 (FXR1 and FXR2), combine with other RNA‐binding proteins as well as coding and non‐coding RNAs to form large ribonucleoprotein particles or granules (Li & Zhao, 2014). Different types of fragile X‐granules are expressed in neurons, where they regulate transport, localization, stability, and translation of target RNAs. In the current study, Agote‐Aran et al (2020) expand our view on FXR proteins and show that they also control the assembly of granules containing nucleoporins, the building blocks of nuclear pore complexes (NPCs).
In a search for FXR1 interacting proteins, the authors identify several nucleoporins and corroborate this by co‐immunoprecipitations. Upon knockdown of FXR1, nucleoporins accumulate in granular structures in the cytoplasm. These granules contain nucleoporins of different functionality such as Nup133 and Nup85, part of the NPC structural backbone, the transmembrane nucleoporins gp210/Nup210 and POM121, which link NPCs to the nuclear envelope membranes, and the FG‐nucleoporins Nup98, Nup214, and Nup358/RanBP2. The latter contain phenylalanine–glycine repeats, which form a cohesive network within NPCs that acts as a diffusion barrier between cytoplasm and nucleoplasm and is responsible for the transport properties of NPCs. In turn, in FXR1 knockdown cells nucleoporin levels at the nuclear envelope are slightly reduced. In addition, the nuclear envelope shape is distorted despite the fact that the levels of proteins involved in nuclear envelope stability like lamins and several proteins of the inner nuclear membrane are not or only mildly affected by FXR1 downregulation. Interestingly, cytoplasmic nucleoporin granules also occur in untreated cells albeit at lower frequency and of smaller size. Here, FXR1 partially co‐localizes with these granules.
What is the nature of these cytoplasmic nucleoporin granules? Nucleoporin‐positive cytoplasmic structures are often annulate lamellae that consist of stacks of ER membrane sheets with numerous embedded NPCs. However, electron microscopy does not show membranes near the nucleoporin granules induced by FXR1 knockdown even though they contain transmembrane nucleoporins. Granule formation is also not a result of higher nucleoporin expression as FXR1 knockdown does not affect mRNA and protein levels of nucleoporins nor their degradation. Nucleoporin granules are seemingly distinct from stress granules as they do not contain typical markers such as TIA‐1 or G3BP1. Given that FXR proteins are RNA‐binding proteins, the authors rule out the possibility that the cytoplasmic nucleoporin granules are RNA dependent as they lack mRNAs and do not dissolve upon RNase treatment. Rather, the granules disappear upon incubation with hexandiol, a compound that dissolves aggregates formed by phase separation including the FG‐nucleoporin meshwork within NPCs. Thus, these granules probably arise from nucleoporins accumulating as condensates resulting from liquid–liquid phase separation in the cytosol. Indeed, many nucleoporins have the property to form such condensates (Schmidt & Görlich, 2016). The authors propose that the aggregates form upon cytoplasmic accumulation of nucleoporins, which, in normal conditions, are directed to the nuclear envelope. Indeed, GFP‐tagged Nup107 appears in the cytoplasm of untreated cells as small dots, which move toward the nuclear envelope. Downregulation of FXR1 increases the number of dots and their average size. Interestingly, these dots are able to fuse and divide in line with the idea that nucleoporin granules are condensates with liquid‐like properties.
To understand the mechanism behind nucleoporin granule formation, the authors focus on another binding partner of FXR1 they identified: the dynein complex. Dynein is a motor protein transporting cargo along microtubules. Downregulation of dynein and its adaptor BicD2 phenocopies FXR1 downregulation: The nuclear envelopes are malformed, and cytoplasmic nucleoporin granules accumulate. Upon treatment with the microtubule‐depolymerizing agent nocodazole, nucleoporins reversibly aggregate, an effect which is further increased by FXR1§ or dynein knockdown. Thus, nucleoporins can travel along microtubules to the nuclear envelope requiring FXR1 as partner; in the absence of FXR1, dynein, BicD2, or microtubules, nucleoporins accumulate in the cytoplasm and form larger condensates.
Given the relationship of FXR1 to FMRP, which function is lost in the FXS, Agote‐Aran et al investigate whether the two other proteins of the FXR family would have similar function as FXR1. Interestingly, downregulation of FXR2 and FMRP also induces cytoplasmic nucleoporin granules and abnormal nuclear shape. Moreover, cellular models of the FXS lacking FMRP also exhibit cytoplasmic nucleoporin granules and a malformed nucleus.
Together, these data suggest that FXR proteins direct nucleoporins, probably as small condensates, toward the nuclear envelope (Fig 1). It is conceivable that they function here in NPC formation. Likewise, in drosophila oocytes nucleoporin condensates serve as reservoir for NPC assembly into annulate lamellae involving microtubules (Hampoelz et al, 2019). However, whether NPC number indeed decreases upon FXR protein downregulation or loss remains open. As this mechanism might be an auxiliary but not essential way to support NPC assembly, its contribution to total NPC number might be small. Indeed, FXR1 downregulation only mildly affects nuclear transport capacity. In animal cells, NPC assembly occurs at the end of mitosis, initiated on the decondensing chromatin, and during interphase, from the inner nuclear membrane (Weberruss & Antonin, 2016). FXR1 might be involved in either assembly (Fig 1). However, alternative functions of these nucleoporin condensates can be envisioned, e.g. exchange of nucleoporins from already existing NPCs. Especially, FG‐nucleoporins have a very low half time within NPCs (Rabut et al, 2004) and thus might be exchanged to maintain NPC functionality. Similarly, it is unclear how FXR proteins affect the shape of the nuclear envelope. One might speculate that FXR proteins might also deliver other nuclear envelope components apart from nucleoporins, and its loss of function thus compromises nuclear envelope stability.
Figure 1. FXR1 participates to NPC homeostasis.
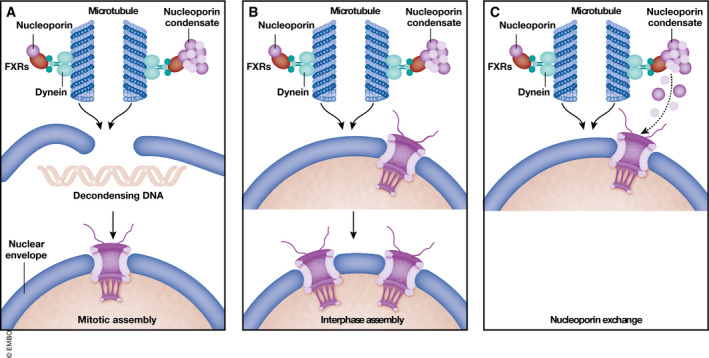
Agote‐Aran et al propose a novel role for FXR proteins (red) which links nucleoporins (violet) to dynein (green) for microtubule‐dependent transport toward the nuclear envelope. Whether this mechanism is relevant for mitotic (A) or interphase (B) NPC assembly, or has other functions like NPC repair (C) remains an interesting open question.
The EMBO Journal (2020) 39: e106510
See also A Agote‐Aran et al (October 2020)
Footnotes
Correction added on 15 October 2020, after first online publication: the term FRX has been corrected to FXR in the article.
References
- Agote‐Aran A, Schmucker S, Jerabkova K, Jmel Boyer I, Berto A, Pacini L, Ronchi P, Kleiss C, Guerard L, Schwab Y et al (2020) Spatial control of nucleoporin condensation by fragile X‐related proteins. EMBO J 39: e104467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagni C, Zukin RS (2019) A synaptic perspective of fragile X syndrome and autism spectrum disorders. Neuron 101: 1070–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampoelz B, Schwarz A, Ronchi P, Bragulat‐Teixidor H, Tischer C, Gaspar I, Ephrussi A, Schwab Y, Beck M (2019) Nuclear pores assemble from nucleoporin condensates during oogenesis. Cell 179: 671–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhao X (2014) Concise review: fragile X proteins in stem cell maintenance and differentiation. Stem Cells 32: 1724–1733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabut G, Doye V, Ellenberg J (2004) Mapping the dynamic organization of the nuclear pore complex inside single living cells. Nat Cell Biol 111: 4–21 [DOI] [PubMed] [Google Scholar]
- Schmidt HB, Görlich D (2016) Transport selectivity of nuclear pores, phase separation, and membraneless organelles. Trends Biochem Sci 41: 46–61 [DOI] [PubMed] [Google Scholar]
- Weberruss M, Antonin W (2016) Perforating the nuclear boundary ‐ how nuclear pore complexes assemble. J Cell Sci 129: 4439–4447 [DOI] [PubMed] [Google Scholar]


