Abstract
The aim of this study was to investigate 28-day mortality after COVID-19 diagnosis in the European kidney replacement therapy population. In addition, we determined the role of patient characteristics, treatment factors, and country on mortality risk with the use of ERA-EDTA Registry data on patients receiving kidney replacement therapy in Europe from February 1, 2020, to April 30, 2020. Additional data on all patients with a diagnosis of COVID-19 were collected from 7 European countries encompassing 4298 patients. COVID-19–attributable mortality was calculated using propensity score–matched historic control data and after 28 days of follow-up was 20.0% (95% confidence interval 18.7%–21.4%) in 3285 patients receiving dialysis and 19.9% (17.5%–22.5%) in 1013 recipients of a transplant. We identified differences in COVID-19 mortality across countries, and an increased mortality risk in older patients receiving kidney replacement therapy and male patients receiving dialysis. In recipients of kidney transplants ≥75 years of age, 44.3% (35.7%–53.9%) did not survive COVID-19. Mortality risk was 1.28 (1.02–1.60) times higher in transplant recipients compared with matched dialysis patients. Thus, the pandemic has had a substantial effect on mortality in patients receiving kidney replacement therapy, a highly vulnerable population due to underlying chronic kidney disease and a high prevalence of multimorbidity.
Keywords: attributable mortality, COVID-19, dialysis, kidney replacement therapy, registries, transplantation
Graphical abstract
Editor’s Note.
This is one of several articles we think you will find of interest that are part of our special issue of Kidney International addressing the challenges of dialysis and transplantation during the COVID-19 pandemic. Please also find additional material in our commentaries and letters to the editor sections. We hope these insights will help you in the daily care of your own patients.
Since the initial outbreak in Wuhan, China, in December 2019, coronavirus disease 2019 (COVID-19) has spread rapidly across the world, prompting a global pandemic. The disease—caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus—causes pneumonia, but also affects other organs. According to the European Centre for Disease Prevention and Control, the number of reported COVID-19 cases in the European Union (EU) is 2783 (range 281–6648) per million general population (pmp), representing 0.28% (range 0.03%–0.66%) of the EU population, with variation in numbers depending on governmental control measures, definition of cases, and testing capacity.1 Mortality due to the SARS-CoV-2 virus is high compared with most other viral infections. Although a case fatality rate of 2.3% was reported from China,2 the average rate is 11.7% (range 0.6%–18.9%) in the EU general population.1 Among hospitalized patients in United Kingdom suffering from severe COVID-19, the case fatality rate reaches 26%.3
Patients treated with kidney replacement therapy (KRT; either dialysis or kidney transplantation) represent a vulnerable population. Under normal circumstances, age-standardized cardiovascular and noncardiovascular mortality rates in dialysis patients are already 8.8 and 8.1 times higher than in the general population, respectively,4 and compared with their age- and sex-matched counterparts in the general population, kidney transplant recipients experience a 30%–50% reduced life expectancy.5 It may be expected that COVID-19 causes substantial mortality in both dialysis and kidney transplant populations due to their underlying chronic kidney disease and a high prevalence of comorbid conditions such as diabetes mellitus and cardiovascular disease. In transplant recipients, the potential effect of their long-term use of immunosuppression is a matter of debate. Some argue they may be at greater risk of severe infection because of their impaired immune system,6 whereas others speculate that immunosuppressive therapy may be protective as it might address the COVID-19–induced cytokine storm.7
Although no deaths were reported among 5 COVID-19 cases on hemodialysis in a single Chinese center,8 several case series from Italy (n = 41, n = 94),9 , 10 Spain (n = 36),11 and the United States (n = 59)12 with varying follow-up suggest a high mortality in the dialysis population with rates ranging from 29% to 41%. Preliminary reports in transplant recipients seem to suggest a somewhat lower mortality, with estimates ranging from 13% (n = 15) in the United States to 25% in Italy (n = 20).13, 14, 15 The largest study to date is from Spain, reporting on a group of 868 KRT patients (67% dialysis patients and 33% transplant patients) with a mortality rate of 23%.16
Risk estimates from studies with small sample sizes are known to suffer from inaccuracy due to random variation. In addition, because some of the above-mentioned samples were derived from in-hospital populations, the estimates reflect risk in a selected group of more severely ill patients and may not be generalizable to the broader KRT patient population. Moreover, most of these studies, including the largest one, used the case fatality rate as a measure of mortality, which is often calculated while the individual outcome (recovery or death) is known only for a proportion of infected patients.17
To date, large population-based studies on mortality in the KRT population with complete follow-up information are lacking. Therefore, the first aim of the present study was to investigate the COVID-19 attributable mortality 28 days after diagnosis in European dialysis and kidney transplant recipients with the use of historic cohorts of prevalent dialysis and transplant patients without COVID-19. The second aim was to compare mortality between dialysis and transplant patients with COVID-19. Finally, we aimed to determine the role of patient characteristics, KRT treatment-related factors, and country as risk factors for death in both groups.
Results
Patient population
From February 1, 2020, to April 30, 2020, a total of 4298 KRT patients were diagnosed with COVID-19, of which 3285 (76.4%) were on dialysis—3160 on hemodialysis and 125 on peritoneal dialysis—and 1013 (23.6%) were living with a functioning transplant (Table 1 ). The majority of dialysis patients diagnosed with COVID-19 originated from France (49.6%) and Spain (29.7%). In dialysis patients, the median age at COVID-19 diagnosis was 71.7 years (interquartile range [IQR] 60.6–80.5), ranging from 63.2 years in Romania to 74.0 years in Spain. Two-thirds were ≥65 years of age, almost two-thirds were male, almost half suffered from either diabetes mellitus (25.5%) or hypertension/renovascular disease (RVD) (21.2%) as primary renal disease (PRD), and 96.2% were on hemodialysis. Sufficient numbers of transplant patients with COVID-19 were available from France (50.5%) and Spain (49.5%). Transplant recipients were younger than those on dialysis (P < 0.001), with a median age of 60.9 years (IQR 51.1–69.4) and 37.3% being ≥65 years. Similarly to dialysis patients, 65.4% were male (P = 0.23), however, the share of patients with diabetes mellitus (12.8%) and hypertension/RVD (10.6%) as PRD was lower.
Table 1.
Characteristics of KRT patients diagnosed with COVID-19, by treatment modality and country
| Dialysis |
Transplant |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | HD | PD | Austria | French-speaking part of Belgiuma | France | Romania | Spain | Switzerland | The Netherlands | All | France | Spain | |
| No. of patients | 3285 | 3160 | 125 | 44 | 140 | 1631 | 270 | 976 | 87 | 137 | 1013 | 512 | 501 |
| Age at diagnosis, yr, median (IQR) | 71.7 (60.6–80.5) | 71.8 (60.8–80.6) | 70.2 (59.4–78.5) | 71.8 (62.8–81.3) | 71.2 (57.9–78.5) | 72 (60.8–81.2) | 63.2 (52.7–70.2) | 74 (63–81.2) | 73.7 (59.6–81.9) | 73.4 (61–81.5) | 60.9 (51.1–69.4) | 59.6 (49.9–67.9) | 62.5 (52.3–70.9) |
| 0–19 | 9 (0.3) | 8 (0.3) | 1 (0.8) | 0 (0) | NA | 3 (0.2) | 1 (0.4) | 5 (0.5) | 0 (0) | 0 (0) | 8 (0.8) | 4 (0.8) | 4 (0.8) |
| 20–44 | 225 (6.8) | 215 (6.8) | 10 (8.0) | 2 (4.5) | 11 (7.9) | 127 (7.8) | 26 (9.6) | 46 (4.7) | 7 (8.0) | 6 (4.4) | 128 (12.6) | 80 (15.6) | 48 (9.6) |
| 45–64 | 854 (26.0) | 817 (25.9) | 37 (29.6) | 10 (22.7) | 41 (29.3) | 401 (24.6) | 123 (45.6) | 222 (22.7) | 22 (25.3) | 35 (25.5) | 499 (49.3) | 263 (51.4) | 236 (47.1) |
| 65–74 | 857 (26.1) | 823 (26.0) | 34 (27.2) | 15 (34.1) | 34 (24.3) | 429 (26.3) | 82 (30.4) | 245 (25.1) | 16 (18.4) | 36 (26.3) | 260 (25.7) | 115 (22.5) | 145 (28.9) |
| ≥75 | 1340 (40.8) | 1297 (41.0) | 43 (34.4) | 17 (38.6) | 54 (38.6) | 671 (41.1) | 38 (14.1) | 458 (46.9) | 42 (48.3) | 60 (43.8) | 118 (11.6) | 50 (9.8) | 68 (13.6) |
| Sex | |||||||||||||
| Male | 2077 (63.2) | 1993 (63.1) | 84 (67.2) | 24 (54.5) | 97 (69.3) | 1042 (63.9) | 148 (54.8) | 629 (64.4) | 57 (65.5) | 80 (58.4) | 662 (65.4) | 338 (66) | 324 (64.7) |
| Female | 1208 (36.8) | 1167 (36.9) | 41 (32.8) | 20 (45.5) | 43 (30.7) | 589 (36.1) | 122 (45.2) | 347 (35.6) | 30 (34.5) | 57 (41.6) | 351 (34.6) | 174 (34.0) | 177 (35.3) |
| Primary renal disease | |||||||||||||
| Glomerulonephritis | 381 (11.6) | 363 (11.5) | 18 (14.4) | 7 (15.9) | 12 (8.6) | 196 (12.0) | 47 (17.4) | 103 (10.6) | 7 (8.0) | 9 (6.6) | 224 (22.1) | 128 (25.0) | 96 (19.2) |
| Diabetes | 839 (25.5) | 813 (25.7) | 26 (20.8) | 14 (31.8) | 44 (31.4) | 447 (27.4) | 53 (19.6) | 223 (22.8) | 23 (26.4) | 35 (25.5) | 130 (12.8) | 67 (13.1) | 63 (12.6) |
| Hypertension/RVD | 695 (21.2) | 671 (21.2) | 24 (19.2) | 8 (18.2) | 26 (18.6) | 427 (26.2) | 49 (18.1) | 116 (11.9) | 27 (31.0) | 42 (30.7) | 107 (10.6) | 73 (14.3) | 34 (6.8) |
| Other | 1370 (41.7) | 1313 (41.6) | 57 (45.6) | 15 (34.1) | 58 (41.4) | 561 (34.4) | 121 (44.8) | 534 (54.7) | 30 (34.5) | 51 (37.2) | 552 (54.5) | 244 (47.7) | 308 (61.5) |
| Treatment modality | |||||||||||||
| HD | 3160 (96.2) | NA | NA | 43 (97.7) | 133 (95.0) | 1586 (97.2) | 270 (100) | 927 (95.0) | 83 (95.4) | 118 (86.1) | NA | NA | NA |
| PD | 125 (3.8) | NA | NA | 1 (2.3) | 7 (5.0) | 45 (2.8) | 0 (0) | 49 (5.0) | 4 (4.6) | 19 (13.9) | NA | NA | NA |
| Year of KRT start, median (IQR) | 2017 (2014–2018) | 2017 (2013–2018) | 2018 (2017–2019) | 2017 (2013–2019) | 2017 (2014–2019) | 2016 (2013–2018) | 2016 (2012–2019) | 2017b (2015–2019) | 2016 (2013–2018) | 2016 (2014–2018) | 2011 (2005–2016) | 2009 (2003–2014) | 2014b (2007–2017) |
COVID-19, coronavirus disease 2019; HD, hemodialysis; IQR, interquartile range; KRT, kidney replacement therapy; NA, not applicable; PD, peritoneal dialysis; RVD, renovascular disease.
Data are presented as n (%) or median (IQR), unless otherwise specified. Percentages are column percentages. A country was included only if it had >25 dialysis or transplant patients with COVID-19.
Data on patients younger than 20 years were not included.
Year of starting current treatment modality.
On May 1, 2020, COVID-19 cases represented ∼2.9% of all prevalent patients on dialysis (country range 1.0%–3.7%) and 1.4% of those living with a functioning graft (country range 1.3%–1.6%) (Supplementary Table S1). Compared with dialysis and transplant patients without COVID-19, those with COVID-19 were 2–3 years older. The proportion of male patients was slightly increased, with 2.0% more men among dialysis patients, and 3.0% more men among transplant recipients (Supplementary Table S2). In both dialysis and transplant patients with COVID-19 there were more patients with diabetes mellitus as PRD (4.7% and 3.9%, respectively).
Crude mortality
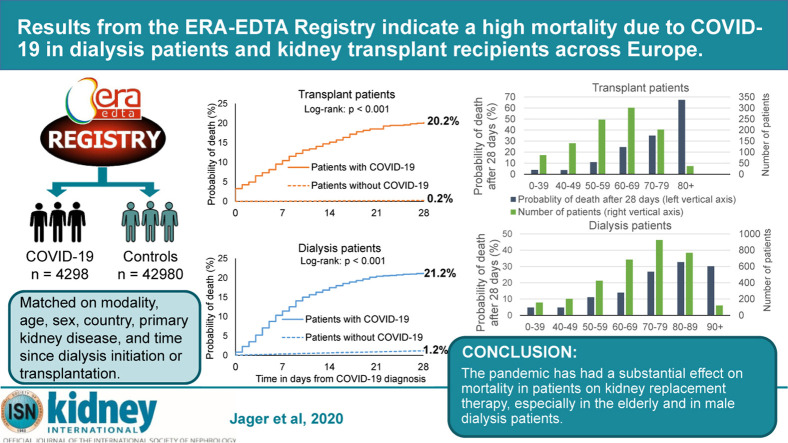
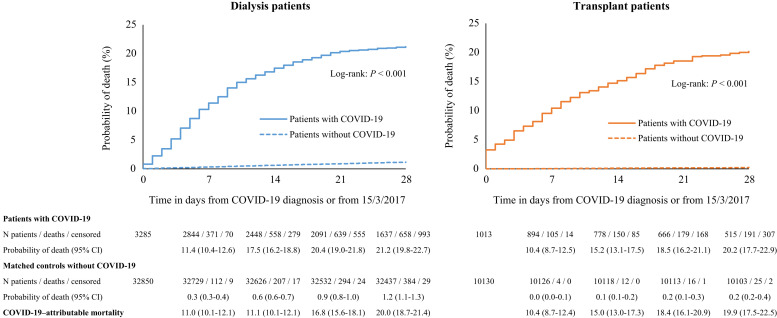
Twenty-eight days after COVID-19 diagnosis, 628 out of 3160 hemodialysis patients and 30 of 125 patients on peritoneal dialysis had died. Figure 1 shows that in the dialysis group as a whole, 21.2% had died 28 days after diagnosis, with 0.3% of deaths occurring on the day of diagnosis. In transplant recipients, 191 out of 1013 had died after 28 days. Their crude 28-day probability of death of 20.2% was similar to that in the dialysis cohort (Figure 1), with 3.3% of deaths taking place on the day of diagnosis. At 28 days, the survival curves start to level out, reflecting that most of the deaths due to COVID-19 had occurred within this period.
Figure 1.
Probability of death among dialysis patients (left panel) and transplant patients (right panel) with coronavirus disease 2019 (COVID-19) and a propensity score–matched control group without COVID-19 (alive at, and followed from, March 15, 2017). CI, confidence interval.
COVID-19 attributable mortality
Compared with the expected 1.2% mortality in the matched control group of dialysis patients without COVID-19, the COVID-19 attributable mortality was 20.0% and mortality risk was 21.1 (95% confidence interval [CI] 18.6–23.9) times higher in dialysis patients diagnosed with COVID-19 (Figure 1; Supplementary Table S3). In transplant recipients diagnosed with COVID-19, the attributable mortality was 19.9% over the expected 0.2% mortality in the matched control group. Because mortality is generally far lower in transplant patients compared with dialysis patients, their mortality risk was 92.7 (95% CI 61.0–140.7) times higher compared with their non–COVID-19 matched control patients (Figure 1).
Mortality risk in transplant recipients versus dialysis patients
Supplementary Figure S1 shows that the mortality risk in transplant recipients with COVID-19 was 28% higher (hazard ratio 1.28, 95% CI 1.02–1.60) compared with the selected group of dialysis patients that could be matched (Supplementary Table S4).
Mortality risk factors in dialysis patients diagnosed with COVID-19
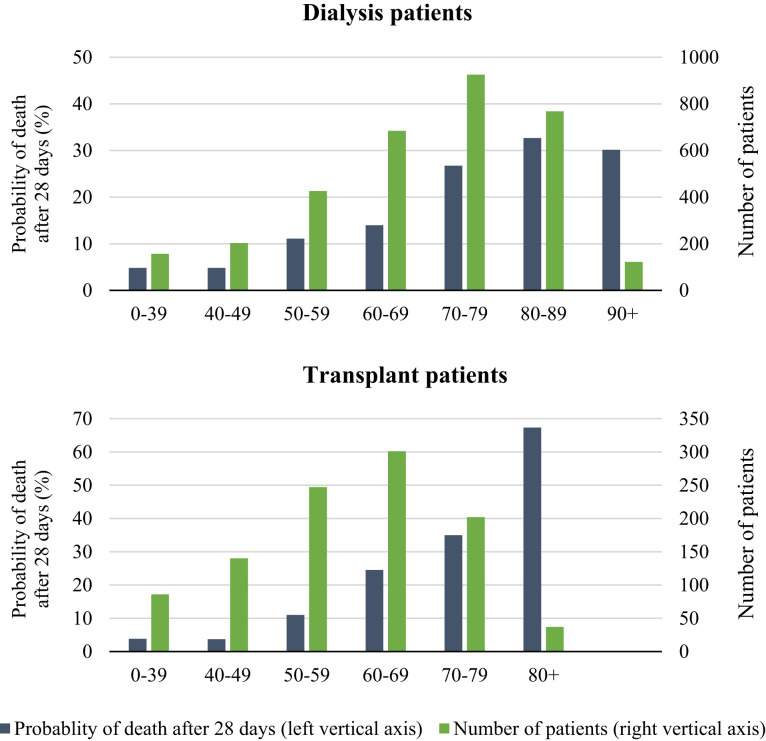
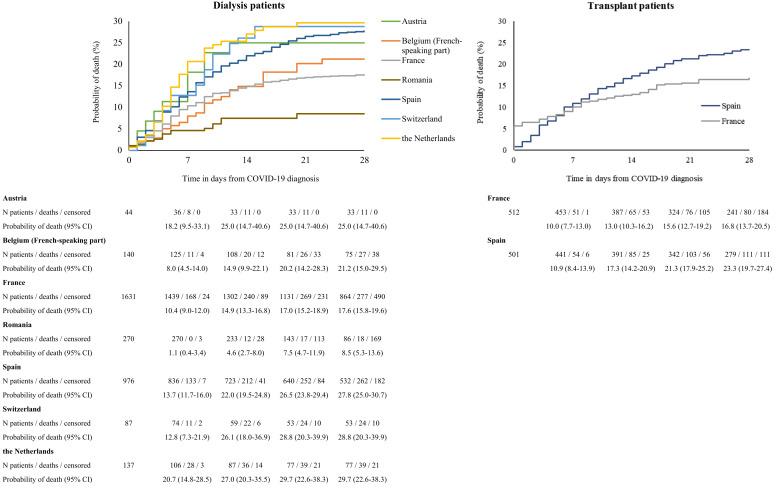
In dialysis patients, the analysis of crude mortality by age category revealed substantial differences across age groups, with 28-day mortality in patients ≥75 years of age as high as 31.4% (Table 2 ; Figure 2 ). The risk of death in men was 22.5% versus 19.0% in women. Dialysis patients with hypertension/RVD as PRD had the highest probability of death (24.3%), followed by diabetes mellitus (20.6%) and glomerulonephritis (16.7%). The 28-day probability of death was 25.0% in those treated with peritoneal dialysis and 23.8% in hemodialysis patients. There were substantial differences in mortality across the 7 participating countries; it was highest in the Netherlands (29.7%) and lowest in Romania (8.5%) (Figure 3 ). Multivariable analyses identified higher age and male sex as risk factors for 28-day mortality in COVID-19 dialysis patients (Table 2). After adjustment for all available confounders, dialysis patients in Romania and France had a lower mortality risk than those in Switzerland. The probability of death by age group, sex, and PRD is provided in Supplementary Figures S2–S4 and the COVID-19–attributable mortality is provided in Supplementary Table S5.
Table 2.
Probability of death in subgroups and risk factors in dialysis patients with COVID-19
| n | Probability of death within 28 days (95% CI) |
Hazard ratio (95% CI) |
|||
|---|---|---|---|---|---|
| Crude | Adjusteda | Crude | Adjusteda | ||
| Age at COVID-19 diagnosis, yr | |||||
| 0–64 | 1088 | 8.9 (7.2–10.8) | 8.7 (6.9–10.4) | 1 | 1 |
| 65–74 | 857 | 20.8 (18.1–23.8) | 20.0 (17.2–22.7) | 2.59 (2.00–3.35) | 2.54 (1.96–3.29) |
| ≥75 | 1340 | 31.4 (28.9–34.0) | 29.1 (26.5–31.7) | 4.10 (3.26–5.16) | 3.85 (3.06–4.86) |
| Sex | |||||
| Female | 1208 | 19.0 (16.8–21.5) | 16.0 (13.9–18.1) | 1 | 1 |
| Male | 2077 | 22.5 (20.7–24.4) | 19.2 (17.3–21.0) | 1.21 (1.03–1.43) | 1.23 (1.04–1.45) |
| Primary renal disease | |||||
| Glomerulonephritis | 381 | 16.7 (13.2–21.0) | 16.4 (12.4–20.2) | 1 | 1 |
| Diabetes | 839 | 20.6 (17.9–23.6) | 17.8 (15.1–20.4) | 1.28 (0.95–1.72) | 1.11 (0.82–1.49) |
| Hypertension/RVD | 695 | 24.3 (21.2–27.8) | 19.4 (16.4–22.3) | 1.55 (1.15–2.08) | 1.22 (0.90–1.65) |
| Other | 1370 | 21.2 (19.1–23.6) | 17.7 (15.5–19.8) | 1.33 (1.01–1.77) | 1.11 (0.84–1.48) |
| Year of KRT startb | |||||
| <2014 | 803 | 20.8 (18.0–23.9) | 19.0 (16.1–21.9) | 1.05 (0.86–1.28) | 1.13 (0.92–1.39) |
| 2014–2017 | 1128 | 22.8 (20.4–25.4) | 17.9 (15.5–20.2) | 1.08 (0.99–1.17) | 1.02 (0.94–1.12) |
| 2018–2020 | 1354 | 20.1 (18.0–22.4) | 17.3 (15.1–19.5) | 1 | 1 |
| Treatment modality | |||||
| HD | 3160 | 21.0 (19.6–22.6) | 18.0 (16.3–19.7) | 1 | 1 |
| PD | 125 | 25.0 (18.2–33.9) | 21.6 (14.1–28.5) | 1.23 (0.85–1.77) | 1.24 (0.85–1.80) |
| Country | |||||
| Austria | 44 | 25.0 (14.7–40.6) | 21.7 (9.4–32.4) | 0.87 (0.43–1.78) | 0.89 (0.43–1.81) |
| Belgium (French-speaking part) | 140 | 21.2 (15.0–29.5) | 20.3 (12.9–27.0) | 0.66 (0.38–1.15) | 0.71 (0.41–1.23) |
| France | 1631 | 17.6 (15.8–19.6) | 15.6 (13.6–17.5) | 0.57 (0.38–0.87) | 0.58 (0.38–0.88) |
| Romania | 270 | 8.5 (5.3–13.6) | 10.4 (5.4–15.2) | 0.27 (0.14–0.49) | 0.38 (0.21–0.71) |
| Spain | 976 | 27.8 (25.0–30.7) | 24.2 (21.0.2–27.1) | 0.93 (0.61–1.42) | 0.91 (0.60–1.39) |
| Switzerland | 87 | 28.8 (20.3–39.9) | 25.0 (15.5–33.4) | 1 | 1 |
| The Netherlands | 137 | 29.7 (22.6–38.3) | 25.7 (18.1–32.6) | 1.06 (0.64–1.76) | 1.06 (0.64–1.76) |
CI, confidence interval; COVID-19, coronavirus disease 2019; HD, hemodialysis; KRT, kidney replacement therapy; PD, peritoneal dialysis; RVD, renovascular disease.
The model for age was adjusted for sex and country; the model for sex was adjusted for age and country; the model for primary renal disease (PRD) was adjusted for age, sex, and country; the model for year of KRT start was adjusted for age, sex, PRD, and country; the model for treatment modality was adjusted for age, sex, PRD, year of KRT start, and country; and the model for country was adjusted for age, sex, PRD, and year of KRT start.
For Spain this was year of dialysis start.
Figure 2.
Probability of death 28 days after diagnosis of coronavirus disease 2019 and number at risk for dialysis patients (top) and transplant patients (bottom), by age at diagnosis. Axis scales differ for dialysis and transplant graphs. Two transplant patients >90 years of age at diagnosis were included in the 80+ age group.
Figure 3.
Probability of death among dialysis patients (left) and transplant patients (right) with coronavirus disease 2019 (COVID-19), by country. CI, confidence interval.
Mortality risk factors in transplant recipients diagnosed with COVID-19
In kidney transplant recipients, the analysis of crude mortality by age group showed a high 44.3% probability of death in those ≥75 years of age, which accounted for almost half of the patients (Table 3 ). The probability of death was 19.1% in men and 22.2% in women, and highest in patients suffering from diabetes mellitus as PRD (30.6%), followed by hypertension/RVD (27.9%), and lowest in those with glomerulonephritis (16.4%). The probability of death was higher in Spain (23.3%) than in France (16.8%) (Figure 3). In multivariable analyses, only higher age was identified as a risk factor for 28-day mortality (Table 3). The probability of death by age group, sex, and PRD is provided in Supplementary Figures S2–S4, and the COVID-19–attributable mortality is provided in Supplementary Table S5.
Table 3.
Probability of death in subgroups and risk factors in transplant patients with COVID-19
| n | Probability of death within 28 days (95% CI) |
Hazard ratio (95% CI) |
|||
|---|---|---|---|---|---|
| Crude | Adjusteda | Crude | Adjusteda | ||
| Age at COVID-19 diagnosis, yr | |||||
| 0–64 | 635 | 11.9 (9.5–14.9) | 11.9 (9.2–14.5) | 1 | 1 |
| 65–74 | 260 | 29.3 (23.9–35.6) | 28.6 (22.6–34.1) | 2.78 (2.00–3.88) | 2.72 (1.95–3.80) |
| ≥75 | 118 | 44.3 (35.7–53.9) | 43.2 (33.3–51.6) | 5.16 (3.59–7.42) | 5.10 (3.55–7.34) |
| Sex | |||||
| Female | 351 | 22.2 (18.0–27.1) | 18.4 (14.2–22.5) | 1 | 1 |
| Male | 662 | 19.1 (16.2–22.5) | 15.8 (12.8–18.7) | 0.83 (0.62–1.11) | 0.82 (0.61–1.1) |
| Primary renal disease | |||||
| Glomerulonephritis | 224 | 16.4 (12.0–22.3) | 14.9 (10.0–19.5) | 1 | 1 |
| Diabetes | 130 | 30.6 (23.0–40.1) | 21.7 (14.4–28.3) | 2.03 (1.27–3.24) | 1.52 (0.95–2.44) |
| Hypertension/RVD | 107 | 27.9 (20.1–38.0) | 20.1 (12.4–27.2) | 1.88 (1.14–3.10) | 1.44 (0.86–2.39) |
| Other | 552 | 17.8 (14.8–21.4) | 15.8 (12.5–18.9) | 1.13 (0.76–1.67) | 1.10 (0.74–1.64) |
| Year of KRT start (France only) | |||||
| <2014 | 370 | 18.7 (14.9–23.2) | 18.3 (13.0–23.3) | 2.39 (0.59–9.76) | 2.89 (0.7–11.92) |
| 2014–2017 | 115 | 12.7 (7.5–21.0) | 14.2 (8.3–19.6) | 1.24 (0.59–2.61) | 1.26 (0.60–2.67) |
| 2018–2020 | 27 | 7.6 (1.9–27.0) | 21.2 (13.4–28.2) | 1 | 1 |
| Year of transplantation (Spain only) | |||||
| <2014 | 241 | 22.6 (17.7–28.6) | 18.3 (13.0–23.3) | 0.70 (0.45–1.08) | 0.83 (0.53–1.30) |
| 2014–2017 | 143 | 18.3 (12.8–26.0) | 14.2 (8.3–19.6) | 0.76 (0.58–0.98) | 0.81 (0.63–1.06) |
| 2018–2020 | 117 | 30.8 (23.0–40.4) | 21.2 (13.4–28.2) | 1 | 1 |
| Country | |||||
| France | 512 | 16.8 (13.7–20.5) | 14.2 (10.2–17.9) | 0.73 (0.55–0.97) | 0.78 (0.58–1.05) |
| Spain | 501 | 23.3 (19.7–27.4) | 19.6 (15.5–23.5) | 1 | 1 |
CI, confidence interval; COVID-19, coronavirus disease 2019; HD, hemodialysis; KRT, kidney replacement therapy; PD, peritoneal dialysis; RVD, renovascular disease.
The model for age was adjusted for sex and country; the model for sex was adjusted for age and country; the model for primary renal disease (PRD) was adjusted for age, sex, and country; the model for year of KRT start and year of transplant was adjusted for age, sex, PRD, and country; and the model for country was adjusted for age, sex, PRD, and year of KRT start (or transplantation in Spain).
Discussion
In this article, we present complete population-based data on more than 4000 KRT patients affected by COVID-19 collected through national and regional renal registries in Europe. We report the probability of death at 28 days after diagnosis and related risk factors in dialysis patients from 7 European countries and in transplant recipients from 2 European countries. In both the dialysis and the transplant recipient groups, one-fifth of patients had died by 28 days after diagnosis. A head-to-head matched comparison showed that transplant recipients had a 28% higher risk of death compared with their dialysis counterparts. Further analysis in dialysis patients revealed higher age, male sex, and country as risk factors, whereas in transplant recipients only higher age was associated with an increased risk of death.
The data suggest that the incidence of diagnosed COVID-19 in the KRT population was low. Nevertheless, as 2.9% of the prevalent dialysis population and 1.4% of those living on a functioning graft were affected by COVID-19, this disease seems to have had a greater impact on the KRT population compared to the general population,1 which may be due to their older age, or perhaps the consequence of more frequent testing.
Even though our COVID-19 patients were sourced from population-based registries, they may not represent all KRT patients with COVID-19. The majority of infections are asymptomatic or mild and do not require hospitalization, perhaps not even consultation of a general practitioner or a nephrologist. Those patients may not have been tested and may therefore have remained undiagnosed. As testing in dialysis centers became more common, even standard, during the unfolding of the pandemic, this is less likely the case for hemodialysis patients who visit their dialysis center a few times a week. The proportions of patients with COVID-19 were considerably lower in patients on peritoneal dialysis and in transplant recipients. We speculate that in these groups, testing may have been restricted to the symptomatic and more severe cases, and therefore our data for these populations likely represent a sicker group of patients. This is supported by the relatively high number of transplant recipients who died on the day of diagnosis (3.3%). This sampling bias may explain our finding that transplant patients are at higher risk of death than dialysis patients of similar propensity score. On the other hand, being immunocompromised may still have been more of a disadvantage while countering the infection than an advantage through reducing the cytokine storm.6 , 7
Although the absolute risk of contracting COVID-19 was low in KRT patients, 28-day mortality in COVID-19 patients far exceeded the mortality that may be expected for KRT patients of similar propensity score based on the historic control data. Information on the 28-day probability of death due to COVID-19 by age category in the general population is lacking. However, data on the case-fatality rate in the Italian general population amounted to 3.5% in 60–69-year-olds and to 12.8% for those 70–79 years of age.18 Similar data from Spain indicate slightly higher percentages: 5.2% and 14.6%, respectively.19 This may suggest that mortality from COVID-19 in the dialysis population (median age 71.7 years) is ∼2 times higher and in transplant patients (median age 60.9 years) ≥6 times higher, compared with non-KRT patients with COVID-19 of similar age. Undoubtedly, multimorbidity in dialysis and transplant patients will have played an important role in explaining this substantial mortality, but unfortunately our data did not permit further investigation on this topic. We should, however, keep in mind that in both the dialysis and the transplant groups almost 80% of patients survived COVID-19 at least up to 28 days after diagnosis, despite the fact that a substantial number of them may not have been admitted to the intensive care unit owing to their supposedly high risk of death.
In both dialysis and transplant patients with COVID-19, higher age remained the most important risk factor for mortality in our multivariable analysis. The finding that male sex was a risk factor in dialysis patients with COVID-19 is of interest. It confirms previous findings in the general population and also the slightly increased cardiovascular mortality found in elderly men compared with women on dialysis without COVID-19.20 Previous studies found preexisting heart disease to be a risk factor in dialysis patients.10 We did not have access to comorbidity data, but PRD can be considered as a proxy for comorbidity. In our study, the point estimates of the additional risk induced by diabetes mellitus and hypertension/RVD did suggest a rise in mortality. However, an additional independent effect of PRD on top of age could not be detected, possibly as a consequence of insufficient statistical power. In dialysis patients with COVID-19, we found that the probability of death varied across countries. Although it is interesting, we do not wish to draw any conclusions from that finding, because much of the variation may be attributed to dissimilarities in the identification of COVID-19 cases (as a result of varying testing strategies), differences in the severity of infections, and the inability to adjust for unmeasured country- and patient-level confounders.
Strengths and limitations
This study reports data from renal registries that aim to include complete data with full national coverage, eliminating the sampling bias found in smaller and non–population-based studies. Notwithstanding this unique strength, when it comes to reporting on patient populations with COVID-19, renal registries, too, cannot avoid the effects of impaired testing strategies resulting from test kit shortages. Underreporting of cases, regardless of the reason (no symptoms, lack of care seeking behavior, lack of testing, or not reporting to the KRT treatment center), will have led to an overestimation of mortality. This overestimation is likely to be small for hemodialysis patients, but it may be more important for peritoneal dialysis patients and transplant recipients, where possibly more severe cases were included. The extent of this sampling bias, induced by varying testing strategies, may have differed across countries and centers. Furthermore, using registry data as a source, we had no access to additional information on patient and treatment characteristics that could potentially be important to the outcome of COVID-19 patients on KRT. Finally, even if this study includes the highest number of COVID-19 patients on KRT to date, it may still suffer from problems of statistical power, resulting in an inability to identify truly existing associations.
Conclusion
The COVID-19 pandemic has had a substantial effect on mortality in all subgroups of KRT patients affected by the disease, culminating in elderly KRT patients and in transplant recipients. It is conceivable that early in the pandemic, hemodialysis centers may have served as important foci of infection. It is of vital importance that in future pandemics the nephrology community have crisis management and control protocols in place and be able to act swiftly to increase the safety of their patients and mitigate the damage to their health as much as possible. Recommendations in this direction have been published, and many studies on prevention of COVID-19 in KRT patients are still underway.
Patients and Methods
Data collection and participants
The European Renal Association–European Dialysis and Transplant Association (ERA-EDTA) Registry collects data annually on patients starting KRT from national and regional renal registries in Europe. When the COVID-19 pandemic reached our continent, the registries providing individual-level patient data on KRT patients were asked to provide additional data on all KRT patients with a diagnosis of COVID-19, either a clinical diagnosis or one proven by testing. Data items included patient and KRT treatment characteristics (month and year of birth, sex, PRD, year of start of KRT, current treatment modality), supplemented with the date of COVID-19 diagnosis, and the date of death. Data from 7 renal registries with at least 25 patients on dialysis or 25 patients living with a functioning kidney transplant with COVID-19 diagnoses were included in this study (Austria, French-speaking Belgium, France, Romania, Spain, Switzerland, and the Netherlands). The year of KRT start was not available for Spain; instead the year of start of the current treatment modality was provided. PRD was categorized into 4 groups: glomerulonephritis, diabetes mellitus, hypertension/RVD, and other PRDs. Patients with missing year of death (n = 2) and treatment modality (n = 8) were deleted from the analysis. Missing PRD (n = 5) was included in the “other” PRD category. Sex and date of detection were complete for all patients. All national and regional renal registries contributing data for this study followed national legislation and European and national regulations for data protection.
Study outcomes and statistical analysis
The end point studied was all-cause mortality within 28 days after diagnosis of COVID-19. Continuous variables were given as mean (SD) or median (IQR) and were compared between groups with the use of the (paired) t test or the Wilcoxon rank sums test. Categoric variables were presented as frequencies and percentages and were compared by means of Fisher exact test. The percentage of dialysis and transplant patients diagnosed with COVID-19 was estimated by dividing the number of cases by the number of prevalent patients at December 31, 2017, multiplied by 100.
To compare the probability of death between patients with COVID-19 and those without COVID-19 among dialysis and transplant patients, we selected historic control patients from the ERA-EDTA Registry database who were alive and on dialysis or living with a functioning transplant on March 15, 2017. COVID-19 patients were matched to 10 non–COVID-19 patients based on their estimated propensity scores. This propensity score was calculated using logistic regression including COVID-19 as dependent variable and the following independent variables: modality (either dialysis or transplantation, 100% match required), age, sex, PRD (4 groups), time since start of KRT (or since last treatment modality change for Spain), and country.
Survival analyses were used to calculate the probability of death. For patients with COVID-19, the date of diagnosis was taken as the starting point and all-cause mortality was the event studied. Follow-up time was censored after 28 days of follow-up or on May 1, 2020, whichever occurred first. For dialysis and transplant patients without COVID-19 the follow-up started on March 15, 2017 and ended at death or at 28 days after March 15, 2017. The Kaplan-Meier method was used to calculate unadjusted probabilities of death for dialysis patients and transplant patients with COVID-19 and their matched historic control patients without COVID-19, allowing for for censored observations. The log-rank test was used to compare the distribution of time to death between groups. The COVID-19–attributable mortality was defined as the probability of death in the COVID-19 patient population minus the probability of death in the non–COVID-19 patient population (i.e., historic control patients). Attributable mortality measures the proportion of the probability of death in the COVID-19 population that can be attributed to COVID-19.
The comparison of mortality in dialysis and transplant patients with COVID-19 also made use of propensity score matching as a method to control for confounding. Patients from these groups were matched 1 to 1, and propensity scores were based on age, sex, PRD (4 groups), time since start of KRT (or since last treatment modality change for Spain) and country. Patients who could not be matched were deleted from the analyses. Again, the Kaplan-Meier method was used to compare the probabilities of death for patients with COVID-19 on dialysis and their matched control patients living on a functioning transplant. We performed a sensitivity analysis of the main results to ascertain the accuracy of the propensity score with the use of multivariable Cox regression (adjusting for age, sex, year of start of KRT, PRD, and country). Results from this analysis did not differ meaningfully from the main results.
In dialysis and transplant patients with COVID-19, crude and adjusted probabilities of death were studied for age categories (<65 years, 65–74 years, and ≥75 years), for men and women, by PRD category, by treatment modality (dialysis vs. transplantation), and by country. We used Cox regression analysis to investigate the association of COVID-19 with the probability of death. In COVID-19 patients, we applied Cox regression to adjust for age, sex, PRD, year of start of KRT or year of transplant, treatment modality, and country, where appropriate.
All analyses were performed with SAS software version 9.4.
Disclosure
All the authors declared no competing interests.
Acknowledgements
The ERA-EDTA Registry is funded by the European Renal Association–European Dialysis and Transplant Association (ERA-EDTA). The ERA-EDTA had no involvement in the conduct of this study. This article was written by KJJ, AK, et al. on behalf of the ERA-EDTA Registry, which is an official body of the ERA-EDTA.
The authors thank the patients and the staff of the dialysis and transplant units for contributing the data via their national and regional renal registries. Furthermore, they gratefully acknowledge the following registries and persons for their contribution of the data: the Austrian Dialysis and Transplant Registry (R. Kramar); the French-speaking Belgian Society of Nephrology (J.M. des Grottes); the French Epidemiology and Information Network in Nephrology (M. Lassalle); the Dutch Renal Registry (L. Heuveling, S. Vogelaar); all of the regional registries of Spain; the Spanish Society of Nephrology; and the Spanish Renal Replace Therapy National Registry at the National Transplantation Organization; as well as the ERA-EDTA Registry committee members not mentioned above for their advice in the analysis and the drafting of this article: M. Arici, J. de Meester, M. Evans, P. Finne, J. Harambat, L. Mercadal, M. Nordio, S.S. Sørensen, and E. Vidal.
Author Contributions
KJJ, AK, NCC, and VSS contributed to the study design, data collection, data analysis, interpretation, and writing of the article. All of the other authors contributed to study design, data collection, interpretation, and writing of the article.
Footnotes
see commentaries on pages 1402 and 1404
Table S1. Kidney replacement therapy (KRT) patients diagnosed with COVID-19 up to May 1, 2020, as percentage of prevalent KRT patients on December 31, 2017, by treatment modality and country.
Table S2. Prevalent Kidney replacement therapy (KRT) patients on December 31, 2017, by treatment modality and country.
Table S3. Comparison of COVID-19 patients and propensity score–matched control patients without COVID-19.
Table S4. Comparison of transplant and propensity score–matched dialysis COVID-19 patients.
Table S5. COVID-19–attributable mortality for dialysis and transplant patients with COVID-19.
Figure S1. Probability of death among age- and sex-matched dialysis and transplant patients with COVID-19.
Figure S2. Probability of death among dialysis patients (left) and transplant patients (right) with COVID-19, by age group.
Figure S3. Probability of death among dialysis patients (left) and transplant patients (right) with COVID-19, by sex.
Figure S4. Probability of death among dialysis patients (left) and transplant patients (right) with COVID-19, by primary renal disease.
Supplementary Material
References
- 1.European Centre for Disease Prevention and Control COVID-19 situation update for the EU/EEA and the UK. https://www.ecdc.europa.eu/en/cases-2019-ncov-eueea Available at:
- 2.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 3.Docherty A.B., Harrison E.M., Green C.A. Features of 16,749 hospitalised UK patients with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol. medRxiv. 2020;04(23):20076042. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Jager D.J., Grootendorst D.C., Jager K.J. Cardiovascular and noncardiovascular mortality among patients starting dialysis. JAMA. 2009;302:1782–1789. doi: 10.1001/jama.2009.1488. [DOI] [PubMed] [Google Scholar]
- 5.ERA-EDTA Registry ERA-EDTA Registry Annual Report 2017. 2019. https://www.era-edta.org/en/registry/publications/annual-reports/#2017 Available at:
- 6.Coates P.T., Wong G., Drueke T. Early experience with COVID-19 in kidney transplantation. Kidney Int. 2020;97:1074–1075. doi: 10.1016/j.kint.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tay M.Z., Poh C.M., Rénia L. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20:363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang R., Liao C., He H. COVID-19 in hemodialysis patients: a report of 5 cases. Am J Kidney Dis. 2020;76:141–143. doi: 10.1053/j.ajkd.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scarpioni R., Manini A., Valsania T. Covid-19 and its impact on nephropathic patients: the experience at Ospedale “Guglielmo da Saliceto” in Piacenza. G Ital Nefrol. 2020;37(2):4. [PubMed] [Google Scholar]
- 10.Alberici F., Delbarba E., Manenti C. A report from the Brescia Renal COVID Task Force on the clinical characteristics and short-term outcome of hemodialysis patients with SARS-CoV-2 infection. Kidney Int. 2020;98:20–26. doi: 10.1016/j.kint.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goicoechea M., Sánchez Cámara L.A., Macías N. COVID-19: clinical course and outcomes of 36 maintenance hemodialysis patients from a single center in Spain. Kidney Int. 2020;98:27–34. doi: 10.1016/j.kint.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valeri A.M., Robbins-Juarez S.Y., Stevens J.S. Presentation and outcomes of patients with ESKD and COVID-19. J Am Soc Nephrol. 2020;31:1409–1415. doi: 10.1681/ASN.2020040470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohan S. Early description of coronavirus 2019 disease in kidney transplant recipients in New York. J Am Soc Nephrol. 2020;31:1150–1156. doi: 10.1681/ASN.2020030375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banerjee D., Popoola J., Shah S. COVID-19 infection in kidney transplant recipients. Kidney Int. 2020;97:1076–1082. doi: 10.1016/j.kint.2020.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alberici F., Delbarba E., Manenti C. A single center observational study of the clinical characteristics and short-term outcome of 20 kidney transplant patients admitted for SARS-CoV2 pneumonia. Kidney Int. 2020;97:1083–1088. doi: 10.1016/j.kint.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sánchez-Álvarez J.E., Fontán M.P., Martín C.J. Status of SARS-CoV-2 infection in patients on renal replacement therapy. Report of the COVID-19 Registry of the Spanish Society of Nephrology (SEN) Nefrologia. 2020;40:272–278. doi: 10.1016/j.nefro.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Natale F., Ghio D., Tarchi D., Goujon A., Conte A. COVID-19 cases and case fatality rate by age. https://ec.europa.eu/knowledge4policy/publication/covid-19-cases-case-fatality-rate-age_en Available at:
- 18.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323:1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 19.Equipo COVID-19, Red Nacional de Vigiancia Epidemiológica; Centro Nacional de Epidemiología; Centro Nacional de Microbiología, Instituto de Salud Carlos III Informe no 32. Situación de COVID-19 en España a 21 de mayo de 2020. https://www.isciii.es/QueHacemos/Servicios/VigilanciaSaludPublicaRENAVE/EnfermedadesTransmisibles/Documents/INFORMES/Informes%20COVID-19/Informe%20n%C2%BA%2032.%20Situaci%C3%B3n%20de%20COVID-19%20en%20Espa%C3%B1a%20a%2021%20de%20mayo%20de%202020.pdf
- 20.Carrero J.J., de Jager D.J., Verduijn M. Cardiovascular and noncardiovascular mortality among men and women starting dialysis. Clin J Am Soc Nephrol. 2011;6:1722–1730. doi: 10.2215/CJN.11331210. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.