Highlights
-
•
SARS-CoV-2 infection is associated with thrombotic complications.
-
•
Mesenteric thrombosis is rare in general population but not in COVID-19.
-
•
Thrombosis can occur in both hospitalized and non-hospitalized patients.
-
•
Early diagnosis is crucial to achieve a good.
Abbreviations: COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; RT-PCR, real-time polymerase chain reaction; ACE2, angiotensin-converting enzyme 2
Keywords: COVID-19, SARS-CoV-2, Mesenteric ischemia, Thrombosis
Abstract
Introduction
The coronavirus disease 2019 (COVID-19) affects mainly the respiratory system, other organs may be involved, usually due to coagulation disturbances that lead to a high rate of thrombotic complications.
Case presentation
The first patient is 45 years-old who has been exposed to SARS CoV-2. Upon admission due to acute abdomen evidence surgery is performed in which an intestinal resection with an adequate post-surgical evolution takes place so the patient is discharged after 4 days with a prescription for oral anticoagulants. The second one is 42 years-old and has comorbidities. The patient is admitted upon evidence of COVID-19, after showing signs of vein mesenteric ischemia in a CT scan surgery is performed, however the patient dies 24 h after the intervention.
Discussion
Within severe cases of patients with COVID-19 the incidence of a symptomatology or gastrointestinal complications is high (39–73.8%). Thromboprophylaxis is recommended to all patients admitted for COVID-19, starting with heparin of low molecular weight as prophylaxis, as well as continuing with oral anticoagulants after being discharged.
Conclusion
Despite the fact that knowledge of the disease is rapidly advancing, all available treatments are still nonspecific to SARS-CoV-2 and the optimal management of COVID-19 remains unclear. An unexplained clinical picture should raise the suspicion for rare conditions such as mesenteric thrombosis. Adequate prophylactic measures should be implemented both during hospitalization and after discharge.
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has spread over more than 200 countries worldwide. Although this disease affects mainly the respiratory system, other organs may be involved, usually due to coagulation disturbances that lead to a high rate of thrombotic complications. In this regard, deep vein thrombosis has been reported in up to 25–50% of COVID-19 patients requiring intensive care, with mortality rates being as high as 30–40%. Although less frequent, other thrombotic events such as intestinal, cerebral, and peripheral limb thrombosis have been reported, usually in critical patients [[1], [2], [3]].
The prothrombotic effects of the virus may be directly related to its structure. It is known that the membrane of SARS-CoV-2 contains a Spike protein (protein S) which binds to the angiotensin-converting enzyme 2 (ACE2) receptor located on the membrane of host cells. ACE2 is most abundant in the lungs, intestine, oral mucosa, liver, and endothelium [[4], [5], [6], [7]]. The binding of SARS-CoV-2 to ACE2 reduces the degradation of angiotensin II, which in turn stimulates the production of IL-6 and promotes a cytokine storm. Moreover, it has been shown that angiotensin II induces the expression of tissue factor and plasminogen activator inhibitor-1 by endothelial cells, leading to a hypercoagulable state [6]. The histology of the small intestine secondary to a mesenteric thrombosis revealed a prominent endothelitis of the submucosa with an evidence of direct viral infection of the endothelial cells as well as diffuse endothelial swelling with mononuclear cell infiltrate. It is believed that there is an activation of alternate and lectin complement pathways (C5b-9 [membrane attack complex], C4d, and mannose-binding protein-associated serine protease 2 [MASP2]), which injures the endothelial cells [8]. Finally, SARS-CoV-2 infection is associated with increases in levels of not only angiotensin but also other prothrombotic proteins such as tissue factor, factor VIII, von Willebrand factor, and plasminogen activation inhibitor-1 [2,[8], [9], [10]].
Concerning intestinal damage, the prothrombotic effects of the virus rise the incidence of mesenteric ischemia from a mere 0.09–0.2% in the general population to 1.9–3.8% in patients with COVID-19 [11,12]. Furthermore, complement activation through the alternative and lectin pathways results in endothelial injury and arteriolar thrombosis [[4], [5], [6],10,13,14]. Hence, it is believed than microvascular changes, rather than major embolic events, play a central role in intestinal damage. However, as we show in this case report, large-vessel events (both thrombotic and embolic) can occur; it is important to note that venous thromboembolic complications are generally more common than arterial thrombosis [3,4]. Finally, patients with severe COVID-19 and hemodynamic instability or shock are at risk of suffering non-occlusive mesenteric ischemia [6].
This article discusses two cases of COVID-19 who presented with acute mesenteric thrombosis and required urgent surgical intervention. This work has been reported in line with the SCARE criteria [15].
2. Presentation of cases
2.1. Case 1
A 45-year-old man with a history of untreated vitiligo presented to the emergency room with severe mesogastric pain that had started 48 h earlier without a clear trigger. The pain was colic in nature, worsened with movements, radiated to both iliac fossa, and had an intensity of 10/10. Nausea and diaphoresis were present and prompted admission. The patient had been exposed to SARS-CoV-2 14 days prior to admission, and subsequently had presented dyspnea, fever, and adynamia, with no hypoxia. COVID-19 was confirmed with a positive real-time polymerase chain reaction (RT-PCR test) for SARS-CoV-2.
Physical examination revealed normal oxygen saturation, diffuse abdominal pain that worsened with deep palpation of the right iliac fossa, positive appendicular cutoff points, positive superior and medium urethral points, and a negative Giordano sign.
Laboratory results upon admission showed leukocytosis with neutrophilia (16.4 × 103/μL white blood cells, neutrophils 86%), thrombocytosis (685,000/μL), hypercholesterolemia (214 mg/dL), hypertriglyceridemia (523 mg/dL), and elevated acute phase reactants (D-dimer 1450 mcg/L, ferritin 970 ng/mL, and C-reactive protein 367 mg/L). The liver function tests, serum electrolytes, and clotting time tests were within normal parameters except for an elevated fibrinogen level (579 mg/dL). The urine test was abnormal (cloudy appearance, pH 6.5, protein 100, and abundant bacteria).
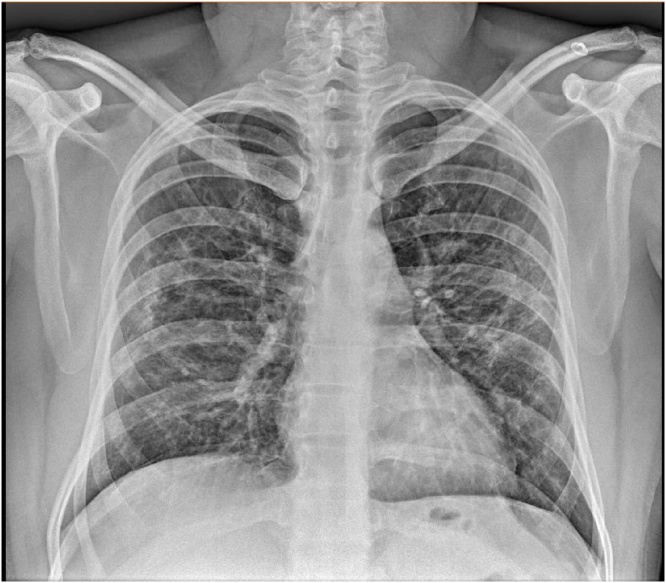
A chest radiograph (Image 1) showed bilateral parahiliar linear streaking of basal predominance, ground-glass opacities, and scarce consolidations with air bronchograms of left predominance.
Image 1.
Simple x-ray scan.
Considering acute appendicitis as a likely diagnosis (he scored high on scales used in suspected acute appendicitis such as Alvarado, Ripasa, and Appendicitis Inflammatory Response Scores), the patient was transferred to the operative room. Exploratory laparotomy revealed hemoperitoneum and a necrotic bowel loop located 30 cm proximal to the ileocecal valve. An intestinal resection with entero-enteral anastomosis was performed.
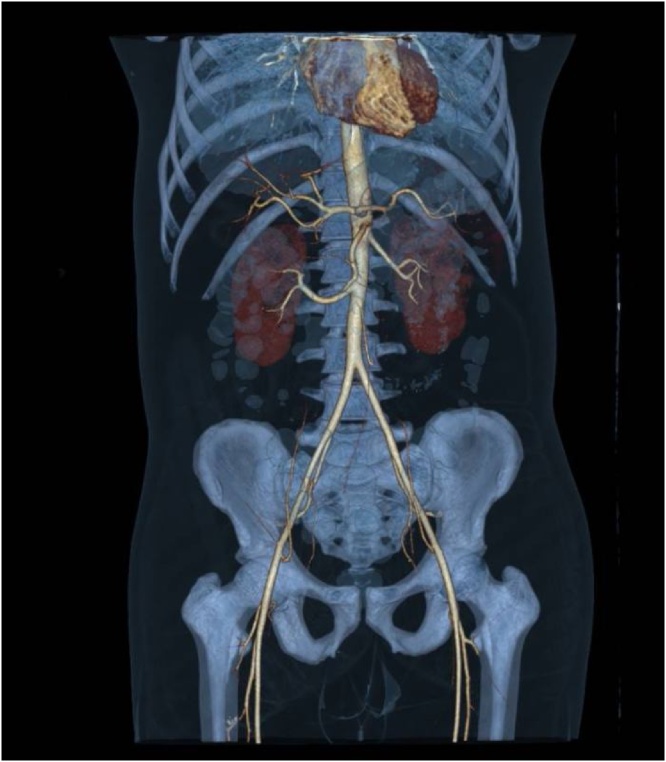
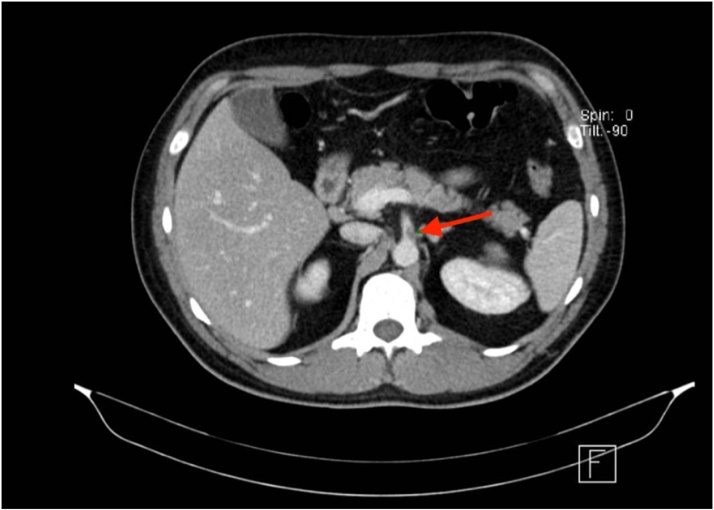
The patient was transferred to postoperative care where he received therapeutic doses of enoxaparin (1 mg/kg BID), started a liquid diet on the next day, and resumed normal diet and intestinal transit within 48 h after surgery. Repeat acute phase reactant measurements revealed a ferritin level of 1480 ng/mL, C-reactive protein of 90.4 mg/L, and leukocytes count within normal values. High-resolution chest and abdominal CT scans showed secondary lung involvement attributed to SARS-CoV-2, evidence of superior mesenteric ischemia of a likely thrombotic etiology with partial rechanneling through the middle colic artery, and hypoxic-ischemic changes in the distal ileum and the cecum (Image 2, Image 3).
Image 2.
Abdominopelvic angiography with 3D reconstruction.
Image 3.
Computed CT scan with a transversal plane where the likely thrombus in the superior mesenteric artery is visible.
Four days after surgery, both abdominal and lung involvements had improved considerably and the patient was discharged with rivaroxaban 10 mg every 24 h for 6 months.
2.2. Case 2
A 42-year-old woman with a history of extreme obesity (BMI 62.4 kg/m2) and a ventriculoperitoneal shunt due to a partially resected craniopharyngioma in 2012 started 48 h prior to admission with spontaneous colic abdominal pain. The pain was located in the epigastrium, had a maximal intensity, did not radiate, and was associated with a difficulty with passing gases and a weeklong constipation. On the next two days, she developed dyspnea that prompted a visit to the emergency service; she was then admitted to the pulmonology department as a suspected case of COVID-19.
On physical examination, the oxygen saturation ranged from 80% to 91% with oxygen delivery through a nasal cannula at 3 L/min. The abdomen was distended, depressible, and painful to deep palpation in the epigastrium and mesogastrium, with no evidence of peritoneal irritation. Upon rectal examination, rectorrhagia was evident; the tone was adequate and the ampulla empty. Grade 3 hemorrhoidal disease was identified.
Blood analysis showed leukocytosis with neutrophilia (18.8 × 103/uL white blood cells, 83.5% neutrophils), asymptomatic hypernatremia (150.5 mEq/L), an elevated C-reactive protein (239 mg/L), and normal kidney and liver function tests and procalcitonin levels. A prolonged prothrombin time (15.8 s) and elevated INR (1.4) were observed; fibrinogen levels were within normal values (338 mg/dl), while D-dimer levels were significantly elevated (14,407 mcg/L).
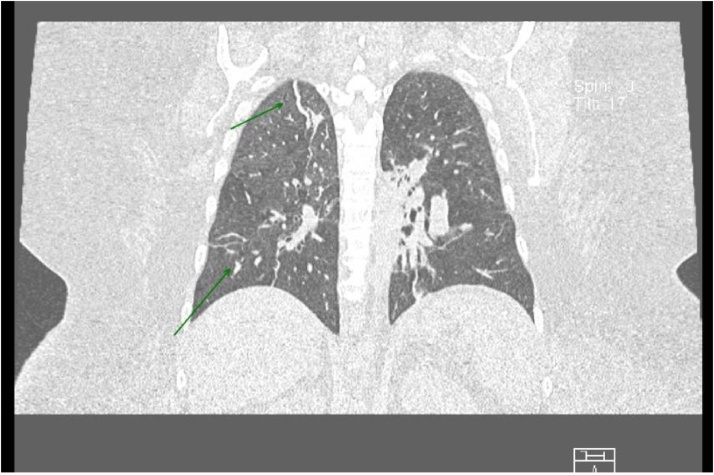
Conservative management for intestinal obstruction was initiated and 1000 ml per day of intestinal material were obtained through the nasogastric tube. Results of a SARS-CoV-2 RT-PCR test performed on the third day of admission were negative. A CT scan showed a mild atypical pneumonia highly suggestive of COVID-19 (Image 4). Abdominal findings included ileum, wall edema and perfusion alterations due to stress, absence of a defined transition zone, and peritoneal fat stripes. Considering the high sensitivity and specificity of the pulmonary findings, the patient was assumed as having COVID-19 and a new SARS-CoV-2 RT-PCR test was scheduled in 7 days.
Image 4.
Chest CT scan from a coronal plane with lung window.
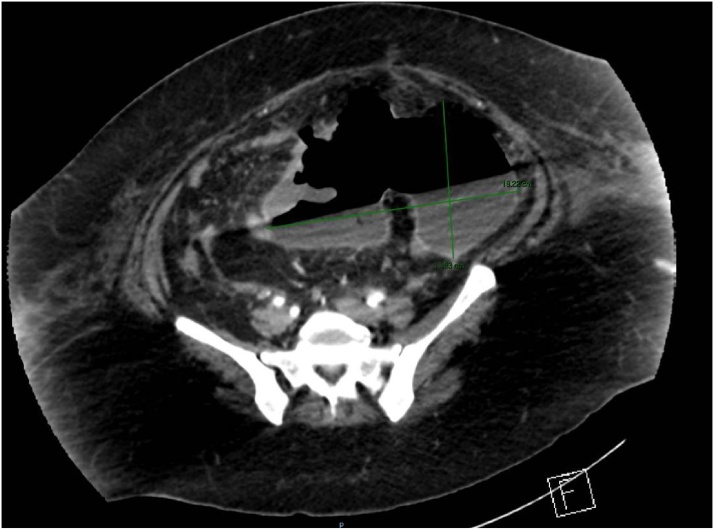
On day 7, partial intestinal obstruction persisted and fever developed. Blood and urine samples were taken for cultures and empiric antibiotic therapy initiated. Three days later, an extended-spectrum beta-lactamase-producing Escherichia coli was isolated in urine cultures and therapy was switched to ertapenem. A repeat abdominal CT scan revealed thrombosis of the portal and mesenteric veins and an abdominopelvic collection in the mesentery, 831 ml in volume, containing gas (Image 5).
Image 5.
Abdominal CT scan from a transversal plane.
An urgent exploratory laparotomy was performed which found fecal peritonitis, necrosis of the epiploon, and a jejunal perforation involving 40% of the circumference, located 20–30 cm distal to the angle of Treitz. Loop resection, entero-enteral manual anastomosis, partial omentectomy, and cavity wash were performed.
The patient was transferred to the intensive care unit due to septic shock with renal, cardiovascular, and respiratory failure, and died 24 h later.
3. Discussion
Since the onset of the SARS-CoV-2 pandemic, many extrapulmonary complications have been described and surgical ones are not an exception. Thrombotic events seem to play a central role in systemic involvement. Risk factors associated with thrombotic complications in SARS-CoV-2 infection include male gender, hypertension, cardiovascular disease, obesity, and prolonged immobilization [2,4,9,10].
Gastrointestinal manifestations in patients with COVID-19 are usually nonspecific and include nausea, emesis, and diarrhea. Considering that chest CT scans are frequently performed in COVID-19 patients, it might be reasonable to extend them to the abdomen, specifically in patients who present with abdominal pain or have an unfavorable disease course [16]. As was evidenced in case 2, changes in chest and abdomen CT scans may precede RT-PCR positivity. Given that the sensitivity of RT-PCR is between 42% and 71%, a negative test can become positive within 4 days in a patient with COVID-19. On the other hand, the sensitivity and specificity of CT for the diagnosis of COVID-19 are 60%–98% and 25–53%, respectively [17].
Among seriously ill patients with COVID-19, the incidence of gastrointestinal symptoms or complications is high (39–73.8%) [11,12]. Approximately 57% of patients show abnormal abdominopelvic tomographic findings, with 18% being attributable to a hypercoagulability state [18].
Thromboprophylaxis with low-molecular-weight heparin is recommended for all hospitalized patients with COVID-19. The recommended dosages are 40 mg daily in patients with a creatinine clearance >30 mL/min, and 30 mg daily in patients with 15–30 mL/min; unfractionated heparin is recommended when the creatinine clearance is <15 mL/min [1,2,4,9,10,16,19]. Patients with COVID-19 who require intensive care are considered to be at highest risk of thrombotic complications and death, and for this reason they require higher doses of anticoagulants. Similarly, in patients with BMIs of >40 kg/m2, it is recommended to increase the dose by 30% 10]. Patients with preexisting or persistent risk factors should receive anticoagulants for at least three months after hospital discharge and undergo periodic monitoring of clotting time, platelet counts, and d-dimer levels. An alternative for ambulatory treatment of patients at high risk is rivaroxaban at 10 mg daily for 31–39 days or betrixaban 160 mg as the initial dose and 80 mg daily thereafter for 35–42 days [7,10].
Finally, as many patients with abdominal complications will likely undergo surgical interventions, it is worth mentioning that patients with SARS-CoV-2 infection have a higher risk of postoperative death than patients who are not infected. A study has shown that the postoperative mortality rates in this group of patients are 23.8% within 30 days, 18.9% in elective surgeries, 25.6% in emergency surgeries, 16.3% in minor surgeries, and 26.9% in major surgeries. The most affected patients were those who presented pulmonary involvement, who represented half of all cases [20].
4. Conclusion
In this article, we described the cases of two patients with COVID-19 who presented with intestinal thrombotic complications early in the course of the disease. The first case, having no significant comorbidities and a mild COVID-19, had a favorable postoperative course. Contrarily, the second patient suffered from important comorbidities that were determinant to a poor outcome within 24 h after surgical intervention.
Despite the fact that knowledge of the disease is rapidly advancing, all available treatments are still nonspecific to SARS-CoV-2 and the optimal management of COVID-19 remains unclear. Considering that thrombotic complications seem to account for a significant number of complications and deaths, an unexplained clinical picture should raise the suspicion for rare conditions such as mesenteric thrombosis. Moreover, performing clotting tests and imaging studies in a timely manner should result in an early diagnosis and improved chances of survival. Finally, adequate prophylactic measures should be implemented both during hospitalization and after discharge.
Declaration of Competing Interest
The authors report no declarations of interest.
Funding
No funding received.
Ethical approval
We have reported a case report with no requirement for ethical approval.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author’s contribution
Reiko M. Rodriguez-Nakamura: writing – original draft.
Mariel Gonzalez-Calatayud: Reviewing and Editing.
Antonio Ramiro Martinez Martinez: participate in the management of the patient.
Registration of research studies
This case report does not require registration as a research study.
Guarantor
Reiko Rodríguez-Nakamura.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Acknowledgement
We would like to thank Editage (www.editage.com) for English language editing.
References
- 1.Görlinger K., Dirkmann D., Gandhi A., Simioni P. COVID-19 associated coagulopathy and inflammatory response: what do we know already and what are the knowledge gaps? Anesth. Analg. 2020 doi: 10.1213/ANE.0000000000005147. Published online 2020 Jul 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miesbach W., Makris M. COVID-19: coagulopathy, risk of thrombosis, and the rationale for anticoagulation. Clin. Appl. Thromb. Hemost. 2020;(January–December) doi: 10.1177/1076029620938149. Published online 2020 Jul 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Incidence of venous and arterial thromboembolic complications in COVID-19: a systematic review and meta-analysis. Thromb. Res. 2020;196:27–30. doi: 10.1016/j.thromres.2020.08.022. Letter to the editor in chief, Published online 2020 August 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singhania N., Bansal S., Nimmatoori D.P., Ejaz A.A., McCullough P.A., Singhania G. Current overview on hypercoagulability in COVID-19. Am. J. Cardiovasc. Drugs. 2020 doi: 10.1007/s40256-020-00431-z. Published online 2020 august 04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jara G.F.E., Jimenez A.G.F., Sananay A.E.L., Murillo S.J.C., Molina V.P.A. Hipercoagulabilidad, trombosis intravascular y trombocitosis asociada al COVID-19. Reporte de un caso. 2020;5(2) http://www.revistabionatura.com [Google Scholar]
- 6.Hussain P.A., Haseeb W.A., Yaseen M. ELSEVIER; 2020. Acute Mesenteric Ischemia in Severe Coronavirus-19 (COVID-19): Possible Mechanism and Diagnostic Pathway. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hatami F., Valizadeh Niloufar, Mostafa A.R.M. Emerging mechanism for the new coronavirus-related myocardial injury and ischemia: a review of the literature. Anatol. J. Cardiol. 2020;24:7–12. doi: 10.14744/AnatolJCardiol.2020.68166. Published online 2020 July 02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pons S., Fodil S., Azoulay E., Zafrani L. The vascular endothelium: the cornerstone of organ dysfuntion in severe SARS-CoV-2 infection. Crit. Care. 2020;24:353. doi: 10.1186/s13054-020-03062-7. Published online 2020 June 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.COVID-19 coagulopathy: an evolving story. Lancet Haematol. 2020;7(June (6)):e425. doi: 10.1016/S2352-3026(20)30151-4. Published online 2020 May 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galanis N., Stavraka C., Agathangelidis F., Petsatodis Ev, Giankoulof C., Givissis P. Coagulopathy in COVID-19 infection: a case of acute upper limb ischemia. J. Surg. Case Rep. 2020;6:1–4. doi: 10.1093/jscr/rjaa204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seeliger B., Philouze G., Cherkaoui Z., Felli E., Mutter D. Acute abdomen in patients with SARS-CoV-2 infection or co-infection. Langenbecks Arch. Surg. 2020 doi: 10.1007/s00423-020-01948-2. Published online 2020 July 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cano-Matías A., Marenco-de L.C.B., Sánchez-Ramírez N., Retamar-Gentil M., Pérez-Margallo E. Isquemia mesentérica aguda: un desafío aún no resuelto. Cirugía Andaluza. 2019;30(1):57–65. [Google Scholar]
- 13.Azouz E., Monnier-Cholley L., Arrivé L. Systemic arterial thrombosis and acute mesenteric ischemia in a patient with COVID-19. Intensive Care Med. 2020;46:1464–1465. doi: 10.1007/s00134-020-06079-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Helms J., Tacquard C., Severac F., Leonard-Lorant I., Ohana M. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a prospective cohort study. Intensive Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agha R.A., Borrelli M.R., Farwana R., Koshy K., Fowler A., Orgill D.P., For the SCARE Group The SCARE 2018 statement: updating consensus Surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2018;60:132–136. doi: 10.1016/j.ijsu.2018.10.028. [DOI] [PubMed] [Google Scholar]
- 16.Abou-Ismail M.Y., Diamond A., Kapoor S., Arafah Y., Nayak L. The hypercoagulable state in COVID-19: incidence, pathophysiology, and management. Thromb. Res. 2020;194:101–115. doi: 10.1016/j.thromres.2020.06.029. Available online 20 June 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simpson D.O.S., Kay F.U., Abbara S., Bhalla S., Chung J.H. Radiological Society of North America expert consensus statement on reporting chest CT findings related to COVID-19. Endorsed by the Society of Thoracic Radiology, the American College of Radiology, and RSNA. Radiol. Cardiothoracic Imaging. 2020 doi: 10.1148/ryct.2020200152. Published Online 2020 Mar 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldberg-Stein S., Fink A., Paroder V., Kobi M., Yee J. Abdominopelvic CT findings in patients with novel coronavirus disease 2019 (COVID-19) Abdom. Radiol. 2020;45:2613–2623. doi: 10.1007/s00261-020-02669-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheung S., Quiwa J.C., Pillai A., Onwu C., Tharayil Z.J., Gupta R. Superior mesenteric artery thrombosis and acute intestinal ischemia as a consequence of COVID-19 infection. Am. J. Case Rep. 2020;21 doi: 10.12659/AJCR.925753. Published online 2020 July 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.COVIDSurg Collaborative Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet. 2020;XXX doi: 10.1016/S0140-6736(20)31182-X. [DOI] [PMC free article] [PubMed] [Google Scholar]