Abstract
Cytokine storm induced by the coronavirus 19 (COVID-19) profoundly activates the coagulation cascade causing venous thromboembolism (VTE). Initial studies from Wuhan, China showed increased incidence of VTE in patients with no standard deep vein thrombosis (DVT) prophylaxis in COVID-19 pneumonia patients. Few have argued for high intensity or intermediate DVT prophylaxis in COVID-19 patients with the incidence of VTE ranging from 16 to 27% despite standard DVT prophylaxis. However, no guideline recommendations presently exist to prescribe augmented DVT prophylaxis in these patients due to lack of evidence although the risk of VTE was clearly demonstrated. While there are ongoing trials to demonstrate the efficacy of intermediate dosing against standard DVT prophylaxis in the prevention of VTE, we present a 36-year-old male admitted with COVID-19 pneumonia who developed acute high-risk pulmonary embolism (PE) requiring emergent thrombolytic therapy despite intermediate dosing DVT prophylaxis.
Keywords: Pulmonary embolism, COVID-19, Thrombolytic therapy, DVT prophylaxis, Venous thromboembolism
Abbreviations: VTE, Venous thromboembolism; COVID-19, Coronavirus disease 2019; PE, Pulmonary embolism
Highlights
-
•
Coronavirus 19 (COVID-19) patients are at risk of venous thromboembolism (VTE).
-
•
Incidence of VTE still high in with standard deep vein thrombosis prophylaxis in COVID-19 patients.
-
•
No societal guidelines exist for augmented thromboprophylaxis in COVID-19 patients.
-
•
Appropriate risk stratification tools and thromboprophylaxis regimes in these patients is paramount.
1. Introduction
Cytokine storm induced by the coronavirus 19 (COVID-19) profoundly activates the coagulation cascade causing venous thromboembolism (VTE) [1]. Tang et al. retrospectively analyzed that patients affected with COVID-19 showed increased incidence of VTE when compared to no standard deep vein thrombosis (DVT) prophylaxis [2]. Few studies argued for high intensity or intermediate DVT prophylaxis in COVID-19 patients with the incidence of VTE ranging from 16 to 27% despite standard DVT prophylaxis [3,4]. Subsequently many institutions created thromboprophylaxis regimes that were described as high intensity or Intermediate dosing to counteract the overwhelming effects of thrombosis in these patients while guideline recommendations were still awaited. However, here we present a 36-year-old male admitted with COVID-19 pneumonia who developed acute high-risk pulmonary embolism (PE) requiring emergent thrombolytic therapy despite intermediate dosing DVT prophylaxis.
A 36-year male airport baggage handler with no significant past medical history was admitted towards the end of April 2020 to our facility with symptoms of fever, cough, headache, and runny nose of 8 days duration. He has no significant past medical history and does not take any mediations. In the emergency department, he was noted to be in mild respiratory distress requiring 4 L/minute nasal cannula, respiratory rate of 23/min. He was febrile 39.1 °C degrees centigrade with a blood pressure (BP) of 125/83 mmHg, and heart rate of 103 beats/min. He weighed 84 kg and calculated BMI was 28. His mental status and other systemic examinations were unremarkable. Electrocardiogram showed sinus tachycardia. Chest x-ray showed opacities in the left mid lung zone. White blood cell count was 7.14 × 109, Hb 150 g/L, and Platelet count 201 × 109. He had normal renal and liver functions. He was admitted to the regular nursing floor for further management.
His COVID-19 PCR nasopharyngeal swab test came back positive. C-reactive protein (CRP) on admission was 59 mg/L, ferritin 3122 μg/L, Interleukin-6499 ng/L and pro-calcitonin 0.14 μg/L. No anti-viral treatments were prescribed in view of his prolonged QTc (460 seconds). He received empiric piperacillin-tazobactam 4.5 g IV every 6 h for possible secondary bacterial pneumonia. His D-dimer was 0.57 μg/mL and fibrinogen 4.53 g/L on admission. He received enoxaparin 40 mg once daily subcutaneously. On Day 3 his oxygen requirements increased to 10–12 L/min on non-rebreathing mask although patient appeared the same clinically from admission. On day 3, his CRP was 116 mg/L, Ferritin 3104 μg/L, Interleukin-6698 ng/L and procalcitonin 0.15 μg/L. Also, his chest x-ray showed bilateral patchy infiltrates which was worse in comparison to the one on admission. He was moved to the intensive care unit (ICU) for closer monitoring. Infectious disease was consulted, and he was prescribed 400 mg IV Tocilizumab on Day 3. His D-dimer on day 2, 3 and 4 were 1.25 μg/mL, 1.86 μg/mL and greater than 4 μg/mL, respectively. He was switched from enoxaparin 40 mg daily subcutaneously to twice daily based on our institution policy of high intensity DVT prophylaxis (intermediate dosing) although no guideline recommendations from any society existed at the time. A Doppler of lower extremities was performed which was normal. Patient was able to prone position himself multiple times during his stay in the ICU. His oxygen requirements gradually came down to 3–4 L/min nasal cannula and he was appropriately moved out of the ICU on day 8. He was ambulatory on the regular floor showing good signs of recovery and improved symptoms. On day 11, patient suddenly felt lightheaded when he got up to go to the toilet. He was in visible respiratory distress, diaphoretic with a respiratory rate of 40/min, heart rate 120/min, oxygen requirements up to 15 L/min. His BP was 92/57 mmhg. Prior to the event his BP was 124/74 mmHg. His electrocardiogram showed sinus tachycardia with no ST deviation. Emergent computed tomography angiogram of the chest with Pulmonary embolism (CTPE) protocol (Fig. 1) showed a saddle embolus with extension to the right and left pulmonary arteries with significant clot burden. There was evidence of right ventricular (RV) strain with RV diameter/left ventricle diameter ratio ~3 (normal < 0.9). He was quickly moved back to the ICU. Cardiac point of care ultrasound showed lack of RV free wall movement with hyperdynamic apex as well septal flattening and bowing to suggest pressure overload in the RV. NT-BNP was 1187 ng/L (normal < 85 ng/L) and troponin 0.011 (normal < 0.010). His Lupus anti-coagulant was mildly positive. Both his Pulmonary Embolism Severity Index (PESI) was Class V (high risk mortality) and simplified PESI score was high. He was considered to have high-risk PE. With no contraindications to thrombolysis and after obtaining consent, he was thrombolysed with alteplase (100 mg over 2 h). No complications ensued. His hemodynamics stabilized as did his oxygen requirements. Repeat formal Echocardiogram showed no evidence of right heart strain or RV dysfunction. He was moved out of the ICU the next day and discharged on room air the following week with a 3-month prescription of apixaban. He was seen in our Tele-outpatient follow up clinic through our hospital network at about 8 weeks. He remained on room air at the time and was back working full time as an airport baggage handlder with excellent level of exercise tolerance. . Further tests revealed Factor V Leiden, Pro-thrombin gene mutation, anti-thrombin III, Protein C and S were negative. His Lupus anti-coagulant test was now normal as well as his anti-cardiolipin antibody test was negative.
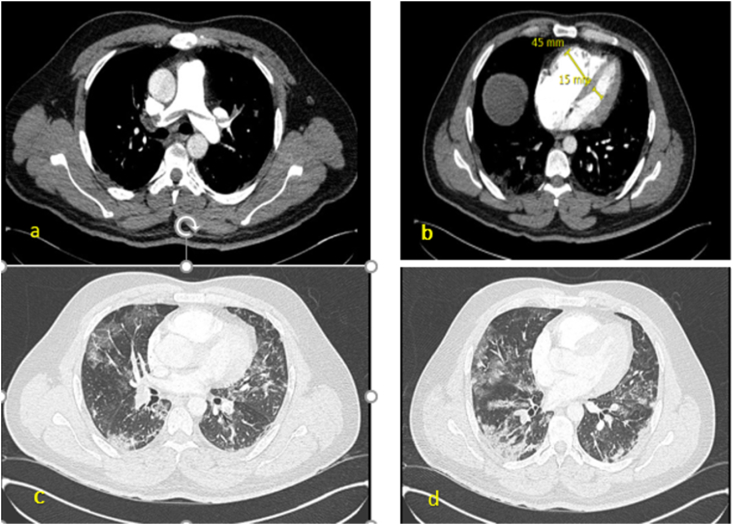
Fig. 1.
CTPE study showing saddle embolism in the main pulmonary trunk extending in to both the right and left main pulmonary artery (a) with RV/LV diameter ratio close to 3 (b). Bilateral ground glass patchy opacities seen in the mid (c) and lower zones (d) of the lung suggestive of COVID-19 pneumonia.
2. Discussion
The pro-coagulant effects and its manifestations in severe COVID-19 patients have been well established [1,5]. Patho-physiologically there appears to be two distinct processes. Firstly, the virus causes direct infection of the endothelial cells causing diffuse inflammation which leads to widespread apoptosis from recruitment of immune -mediated cells. As a result, there is loss of vascular tone and homeostasis creating microvascular dysfunction primarily by a combination of inflammation, vasoconstriction, tissue edema and clot formation, all of which decrease the vessel lumen size and subsequent organ ischemia [5]. Secondly, due to the significantly increased fibrinogen levels there is a higher incidence of large vessel thrombosis such as DVT and PE [1]. Both these processes result in significantly elevated D-dimer levels.
Profound elevation in D-dimer levels in severe COVID-19 pneumonia patients suggest that the body's own fibrinolytic system is under enormous pressure to break down clot formation that are forming in many organs including the lungs. Therefore, prescribing anti-coagulation for thromboprophylaxis in these patients is paramount. Tang et al. retrospectively reported that prescribing heparin especially low molecular weight heparin (LMWH) provided mortality benefit against patients with no heparin treatment [2]. More specifically, this was observed in patients with D-dimer greater than 3.0 μg/mL when prescribing heparin showed a 20% reduction in mortality (P = 0.017) [2]. However, in a prospective multi-center French study of 150 ICU patients, 16.7% had Pulmonary embolism despite standard prophylactic anti-coagulation [3]. Similarly, another Dutch retrospective study of 184 ICU patients reported a cumulative incidence of VTE of 27% [4]. Objectively, Ranucci et al. demonstrated that clot firmness and clotting time were on the upper limit of normal on viscoelastic testing in a small study of 16 patients in very ill COVID-19 patients [6]. However, at the time and even presently, no guideline recommendations are available on increasing the intensity of DVT prophylaxis in these patient although the risks were clearly demonstrated. Based on the above, our hospital created a policy to provide high intensity or Intermediate dosing DVT prophylaxis for COVID-19 patients who had a D-dimer greater than 3 μg/mL. Our patient was placed on enoxaparin 40 mg daily on admission and then increased to 40 mg twice a day when his D-dimer levels went higher than 3 μg/mL. Despite intensifying his DVT prophylaxis regimen, he still developed a high-risk PE after 8 days. He was slightly overweight but not obese or fell into any category where subcutaneous doses of enoxaparin were seemingly not efficacious and required factor Xa monitoring. Our patient was young and ambulatory with no co-morbidities. He had no personal or family history of VTE. His thrombophilia screen was negative. The occurrence of VTE in this case suggests that our understanding of the process and degree of thrombosis is still extremely limited. Presently there are many registered trials on clinicalltrials.gov but only two active (recruiting) studies comparing the efficacy of intermediate and standard dosing of LMWH in COVID-19 patients on the incidence of VTE [7,8]. Another study is comparing the efficacy of therapeutic anticoagulation with augmented thromboprophylaxis for COVID-19 patients admitted to the ICU [9]. Lastly, one study is comparing the prevalence of bleeding in patients treated with higher than recommended thromboprophylaxis [10].These clinical studies will certainly shed more light on the appropriate choice of different anticoagulation regimes in COVID-19 patients and their results are eagerly awaited. Obviously, management of massive PE should not differ from any non-COVID-19 patient once diagnosed. Bedside echocardiography and CTPE study revealed RV strain and coupled with his unstable hemodynamics, elevated PESI score, he promptly received thrombolytic therapy. Our patient responded well to thrombolysis and was discharged the following week with a 3-month prescription of apixaban with follow up arranged with the hematologist.
Different patients exhibit various levels of cytokine storm activity and it is impossible for physicians to predict the degree of host response to the virus and subsequent activation of the coagulation cascade. It is unclear if D-dimer themselves will allow us to risk stratify patients appropriately. Nevertheless, it is however safe to say that profoundly elevated d-dimer and fibrinogen levels are a marker of severity and poor prognosis [11,12]. As we learn more from the pandemic, a detailed review of the appropriate anti-coagulation regime is warranted in severe COVID-19 patients especially when it can contribute significantly to the mortality. We urge physicians to be cognizant of the incidence of DVT and PE despite higher doses of DVT thromboprophylaxis and evaluate any COVID-19 patient for VTE who develop worsening respiratory symptoms and deteriorating hemodynamics in the absence of sepsis.
Author contributions
SS drafted the manuscript. JM and HE revised the manuscript for important intellectual content and gave final approval of the version to be published. All authors read and approved the final manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
Shameen Salam, Jihad Mallat and Hussam Elkambergy have no conflicts of interest to declare.
Acknowledgements
None.
References
- 1.Dolhnikoff M. Pathological evidence of pulmonary thrombotic phenomena in severe COVID-19. J. Thromb. Haemostasis. 2020;18(6):1517–1519. doi: 10.1111/jth.14844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang N. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemostasis. 2020;18(5):1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Helms J. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klok F.A. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Varga Z. Endothelial cell infection and endotheliitis in COVID-19. Lancet (London, England) 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ranucci M. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J. Thromb. Haemostasis. 2020;18:1747–1751. doi: 10.1111/jth.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.ClinicalTrials.gov. National Library of Medicine (US); Bethesda (MD): 2000 Feb 29. https://clinicaltrials.gov/ct2/show/NCT04366960?id=NCT04380779+OR+NCT04366960+OR+NCT04360824+OR+NCT04345848&draw=2&rank=2&load=cart [Internet] Identifier NCT 04366960, Comparison of Two Doses of Enoxaparin for Thromboprophylaxis in Hospitalized COVID-19 Patients; 2020 April 29; Available from. [Google Scholar]
- 8.ClinicalTrials.gov. National Library of Medicine (US); Bethesda (MD): 2000 Feb 29. https://clinicaltrials.gov/ct2/show/NCT04360824?id=NCT04380779+OR+NCT0ad4366960+OR+NCT04360824+OR+NCT04345848&draw=2&rank=3&load=cart [Internet] Identifier NCT04360824, Covid-19 associated Coagulopathy; 2020 April 24; Available from. [Google Scholar]
- 9.ClinicalTrials.gov. National Library of Medicine (US); Bethesda (MD): 2000 Feb 29. https://clinicaltrials.gov/ct2/show/NCT04345848?id=NCT04380779+OR+NCT04366960+OR+NCT04360824+OR+NCT04345848&draw=2&rank=4&load=cart [Internet] Identifier NCT04345848Preventing COVID-19 Complications with Low- and High-dose Anticoagulation (COVID-HEP); 2020 April 15; Available from. [Google Scholar]
- 10.ClinicalTrials.gov. National Library of Medicine (US); Bethesda (MD): 2000 Feb 29. https://clinicaltrials.gov/ct2/show/NCT04380779?id=NCT04380779+OR+NCT04366960+OR+NCT04360824+OR+NCT04345848&draw=2&rank=1&load=cart [Internet] Identifier NCT04380779 Prevalence of Severe Bleeding in COVID-19 Patients Treated With Higher Than Recommended Thromboprophylaxis Doses ; 2020 May 8; Available from. [Google Scholar]
- 11.Guan W.j. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang N. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemostasis. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]