Abstract
Background
Canine parvovirus type 2 (CPV-2) and feline parvovirus (FPV) are known as the main causes of several serious diseases and have a severe impact on puppies and kittens, respectively. FPV and new CPV-2 variants are all able to infect cats, causing diseases indistinguishable from feline panleukopenia. However, FPV only replicates efficiently in feline cells in vitro and replicates in dogs in the thymus and bone marrow without being shed in feces. In our previous study, the genotypes of six parvoviral isolates were unable to be identified using a SimpleProbe® real-time PCR assay.
Methods
In the present study, we characterized previously unidentified FPV-like viruses isolated from dogs in Vietnam. The six isolates were utilized to complete VP2 gene sequencing and to conduct phylogenetic analyses.
Results
Sequence analysis of the six parvoviral strains identified the species as being similar to FPV. Phylogenetic analysis demonstrated that the complete VP2 genes of the strains are similar to those of FPV. The FPV-like strains contain a Thr101 mutation in the VP2 protein, which is different from prototype FPV strains.
Discussion
Our data provide evidence for the existence of changes in the charge, protein contact potential and molecular surface of the core of the receptor-binding size with an Ile101 to Thr101 mutation. This is also the first study to provide reliable evidence that FPV may be a threat to the Vietnamese dog population.
Keywords: Feline parvovirus, Canine parvovirus type 2, Sequences analysis, 3D structure
Introduction
Canine parvoviral enteritis is characterized by acute gastroenteritis and/or bloody diarrhea and is one of the most common infectious diseases in puppies (Appel, Scott & Carmichael, 1979; Black et al., 1979). The causative agent canine parvovirus type 2 (CPV-2) is a single-stranded, nonenveloped, small DNA virus with a genome size of approximately 5 kb (Reed, Jones & Miller, 1988). It is antigenically and genetically unrelated to canine parvovirus type 1, which is currently called canine minute virus (Appel, Scott & Carmichael, 1979). CPV belongs to the genus Protoparvovirus in the family Parvoviridae and according to the International Committee on Taxonomy of Viruses, it is included with the species Carnivore protoparvovirus 1 together with feline parvovirus virus (FPV), mink enteritis virus (MEV) and raccoon parvovirus (RPV) (Cotmore et al., 2019).
FPV was first identified to have a viral cause in 1928 (Verge & Cristoforoni, 1928) and isolated in tissue culture in 1964 (Johnson, 1965). CPV-2 is believed to have originated from FPV or a closely related FPV-like parvovirus of wild carnivores (Allison et al., 2014); however, these viruses are very different in terms of host cell specificity. CPV-2 can replicate in both canine and feline cells in vitro and in vivo (Truyen & Parrish, 1992). Conversely, FPV can only replicate efficiently in the thymus but loses its efficiency in mesenteric lymph nodes or in the small intestine of FPV-inoculated dogs (Truyen & Parrish, 1992). CPV-2 shows several missense mutations within the VP2 protein compared to FPV, including Lys80Arg, Lys93Asn, Val103Ala, Val232Ile, Asp323Asn, Asp375Asn, Asn564Ser and Ala568Gly (Parrish, 1991; Parrish, Aquadro & Carmichael, 1988; Truyen et al., 1995). Shortly after CPV-2 was first identified in the late 1970s, CPV-2 was replaced in the dog population by strains carrying small antigenic variations of the VP2 protein (Decaro & Buonavoglia, 2012). Variants CPV-2a, 2b and 2c can be distinguished by monoclonal antibodies and molecular analysis based on residue 426 of the VP2 protein (Decaro et al., 2005; Parrish et al., 1991; Parrish et al., 1985). The functions of VP2 protein include facilitating transferrin receptor binding (Chang, Sgro & Parrish, 1992), controlling host range (Chang, Sgro & Parrish, 1992) and eliciting an immune response (Lopez de Turiso et al., 1991). Transferrin receptor type-1 (TfR) serves as a receptor for FPV and CPV-2 infection (Hueffer et al., 2003a). Physiologically, TfR mediates iron uptake into cells by binding and importing iron-loaded transferrin, which also binds to the hereditary hemochromatosis protein in the intestine to regulate iron uptake by blocking transferrin binding (Kawabata, 2019). Apical domain residues are reported to be critical for controlling parvovirus binding in FPV and CPV-2 interaction with canine and feline TfR, respectively (Goodman et al., 2010; Hueffer et al., 2003a; Kaelber et al., 2012; Palermo, Hueffer & Parrish, 2003).
According to research surveillance of CPV-2, a novel CPV-2c variant was identified in several Asian countries in the past few years, including China (Geng et al., 2015; Wang et al., 2016; Zhang et al., 2010; Zhao et al., 2015). , Taiwan (Chiang et al., 2016; Lin et al., 2017), Laos (Vannamahaxay et al., 2017), Thailand (Charoenkul et al., 2019) and Vietnam (Hoang et al., 2019a; Nakamura et al., 2004). An antigenic variant of this CPV-2c with a few residue substitutions (Ala5Gly, Phe267Tyr, Tyr324Ile and Gln370Arg) compared to the prototype CPV-2c was detected (Chiang et al., 2016; Geng et al., 2015; Lin et al., 2017; Wang et al., 2016; Zhao et al., 2015). This novel CPV-2c variant is prevalent on the Asian continent. Surprisingly, a few FPV strains have been detected from naturally infected dogs in Pakistan (Ahmed et al., 2018) or Thailand (Charoenkul et al., 2019). In a previous study, we developed a reliable and sensitive tool for differentiating between the CPV-2 genotypes (Hoang et al., 2019b). Interestingly, there were some parvoviral isolates with genotypes that could not be identified using this SimpleProbe® real-time PCR assay (Hoang et al., 2019a), raising the question of whether any FPV variants can occur and become emerging viruses in dog populations. In the present study, we characterized previously unidentified FPV-like viruses in dogs in Vietnam. Here, the complete VP2 gene sequences and 3D structures of FPV-like viruses are analyzed and discussed.
Materials & Methods
Specimen preparation
Six parvoviral isolates with genotypes that could not be identified using the SimpleProbe® real-time PCR assay (Hoang et al., 2019b). Details with respect to the clinical histories of individual animals are provided in Table 1.
Table 1. Clinical status of the 6 dogs and corresponding FPV analysis.
| Strain | Sampling time | Region of Vietnam | Agea | Sex | Vaccination | Clinical signs | Results of the rapid testc | Outcome | CPV-like or FPV-like | Accession number |
|---|---|---|---|---|---|---|---|---|---|---|
| HN39AA | 25.11.2017 | North | 5 M | M | No | Diarrhea, Vomiting | + | Alive | FPV-like | MK357738 |
| HN3X | 16.12.2017 | North | 3 M | N/Ab | No | Diarrhea, Vomiting | + | Alive | FPV-like | MK357739 |
| HN40AA | 23.12.2017 | North | 4 M | M | N/Ab | Diarrhea, Vomiting | + | Alive | FPV-like | MK357740 |
| HN10X | 13.1.2018 | North | 2 M | N/A | N/A | Diarrhea, Vomiting | + | Alive | FPV-like | MK357741 |
| HN41AA | 4.1.2018 | North | 9 M | M | N/A | Diarrhea, Vomiting | + | Alive | FPV-like | MK357742 |
| HN11X | 6.1.2018 | North | 3 M | N/A | N/A | Diarrhea, Vomiting | + | Alive | FPV-like | MK357743 |
Notes.
Age of the dog at presentation (in months).
N/A: not available.
Canine parvovirus Ag test, GREENAGE CO., LTD, VIETMAN.
Complete VP2 gene amplification and sequencing
The entire VP2 gene of parvovirus was PCR amplified by as described by Hoang et al. (Hoang et al., 2019a).
Sequence and phylogenetic analysis
These six VP2 DNA sequences were compared to reference FPVs (EU659114, EU659113, AB000066, M38246, KT240134, EU659112, X55115, M24002, JX475256, HQ184195, EU498716, EU498686, EU498692, DQ474237, AB054226), CPV-2 (M38245), CPV-2a (M24000), new CPV-2a (AY742953), CPV-2b (M74852), new CPV-2b (AY742955) and CPV-2c (FJ222821, MK357736, AB120727). The Clustal W and MegAlign programs (DNASTAR, Madison, WI, USA) were utilized for multiple alignments of nucleic acid and amino acid sequence. A total of 507 complete VP2 gene sequences (FPV-like, CPV-2-like, MEV-like and RPV strains) were obtained from the National Center for Biotechnology Information database for phylogenetic analysis. Phylogenetic analyses were processed properly by the maximum likelihood methods using Molecular Evolutionary Genetics Analysis X software based on the Tamura-Nei model under the assumption of a uniform rate of evolution (Tamura et al., 2013). The initial tree of the heuristic search were obtained automatically by applying Neighbor-Join and BioNJ algorithms to a pairwise distance matrix estimated using the maximum composite likelihood (MCL) method.
Sequence conservation analysis
Sequences conservation an amino acid levels was determined by calculating the information content of each position in a multiple alignment of 600 Carnivore protoparvoviruses, including 471 CPV-2 strains from dogs, 19 CPV-2 strains from cats, 90 FPV strains from cats and 21 FPV strains from dogs. The sequence logo of the critical VP2 amino acid residues was generated using SeqLogo in TBtools (https://github.com/CJ-Chen/TBtools).
Analysis of the transferrin receptor-binding site of FPV capsid VP2
An FPV (MK357738) VP2 structure comprising the sequence from amino acid position 37-583 was created using homology modeling (https://swissmodel.expasy.org/) based on feline panleukopenia virus strain 193 (X55115). The 3D structure was visualized using the open-source software PyMol (https://github.com/schrodinger/pymol-open-source). Intramolecular hydrogen bonds were displayed using the action popup (via Find -¿Polar Contacts). The electrostatic distribution on the surface was generated by automated PyMol representation (via the action popup -¿Generate -¿Vacuum Electrostatics -¿Protein Contact Potential) to visualize protein contact potential.
Results
PCR amplification and genotype analysis
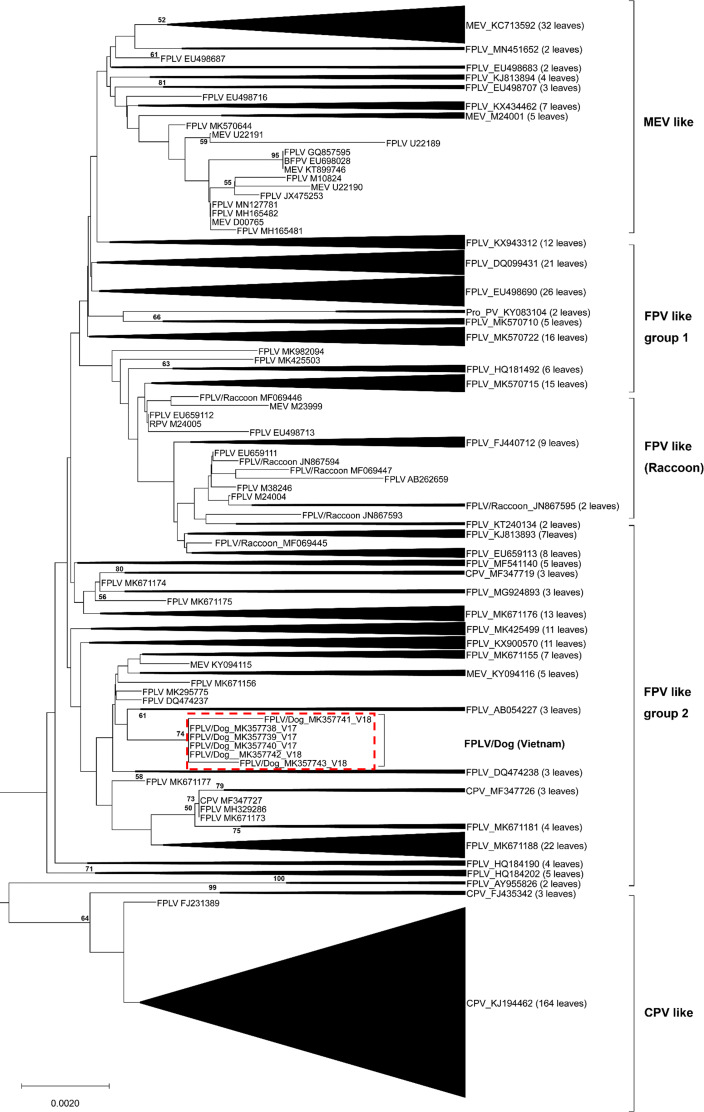
The complete VP2 genes of the 6 Vietnamese untypeable parvoviral DNAs from North Vietnam were collected (Table 1). A broader phylogenetic analysis based on the nucleotide sequences of the 507 full-length VP2 gene revealed that our six parvoviral DNA sequences cluster with FPV-like groups and not CPV-2-like, MEV-like or RPV groups (Fig. 1). Interestingly, several Chinese CPV-2-like strains isolated from cats were also clustered in this group (MF347726 and MF347727). These samples were ruled out the possibility of contamination by checking the feline housekeeping gene (data not shown). Notably, the results of this study revealed the appearance of FPV-like strains from dogs.
Figure 1. Phylogenetic relationship based on broad nucleotide sequences (n = 507) of the complete VP2 genes of FPV, CPV-2, MEV and RPV.
Phylogenetic relationship based on broad nucleotide sequences (n = 507) of the complete VP2 genes of FPV, CPV-2, MEV and RPV. The phylogenetic tree was constructed using the maximum likelihood method with bootstrap analysis (n = 1, 000) to determine the best fitting tree. Bootstrap support values greater than 50 are shown. FPV-like isolates in the present study are indicated as a red dash line box.
DNA and amino acid sequence analyses
The entire VP2 nucleotide sequences were analyzed using DNASTAR software, revealing 96.8–98.5%, 99.8–100% and 99.3–100% homology with CPV-2 (2a, 2b and 2c) between our isolates and reference FPV strains, respectively. Amino acid sequence comparisons among the 6 isolates and the 23 reference strains revealed that the 6 isolates are similar to FPV based on the critical VP2 amino acid residues Lys80, Lys93, Val103, Asp232, Asn564 and Ala568. The critical residues for host range are also identical to those in FPV (Lys93, Val103, Ala300, Asp323) (Fig. S1). Amino acid variation in FPV VP2 sequences mainly occurs in the receptor-binding region (Fig. S1). Surprisingly, Thr101 was found in all of our FPV-like isolates and some of the recent reference strains, but the prototype FPVs carry Ile101 in their VP2 protein (Fig. S1). Sequences conservation was studied by applying a sliding window analysis for the entire VP2 sequences. Sequence logo analysis of the amino acid sequence of FPV in dogs were similar to FPV in cats (Fig. 2). These results confirm that the six samples isolated from dog feces in Vietnam in the present study are FPV-like viruses.
Figure 2. Sequence logos showing the amino acid usage in the VP2 of Carnivore protoparvoviruses.
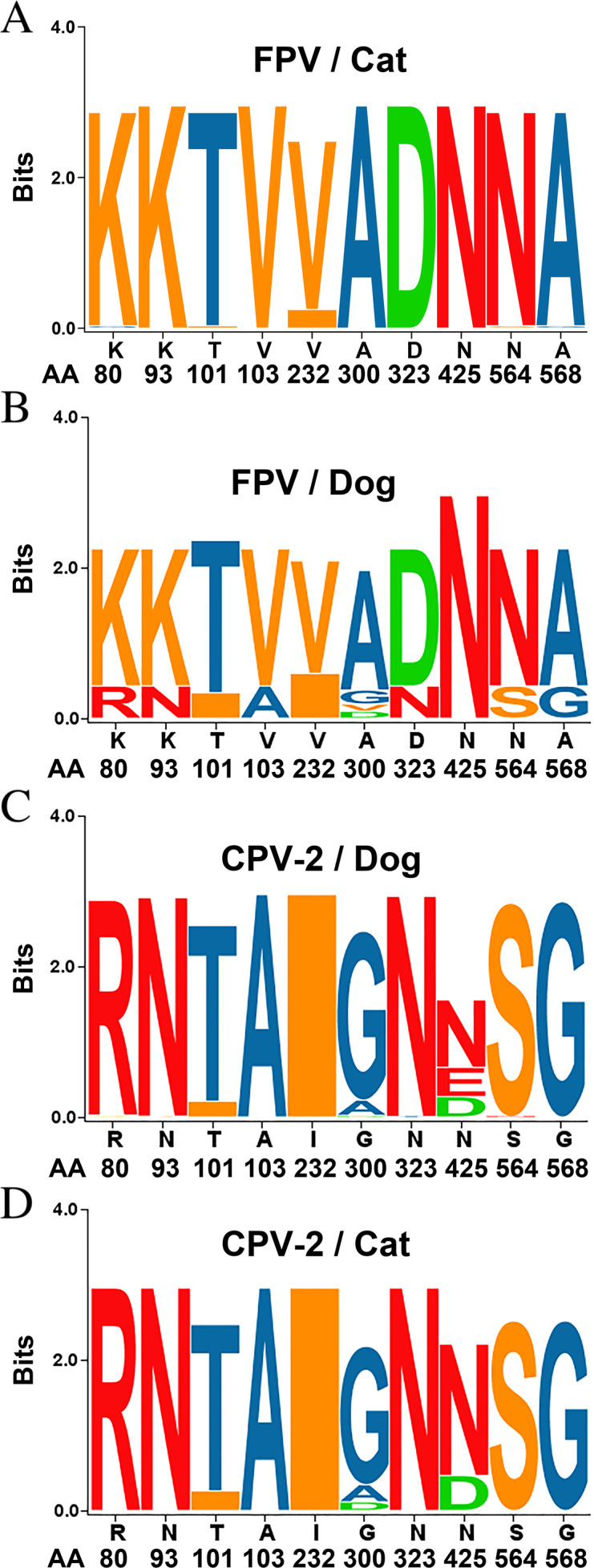
Sequence logos showing the amino acid usage in the VP2 of Carnivore protoparvoviruses (n = 600). (A) FPV in cat, (B) FPV in dog, (C) CPV-2 in dog and (D) CPV-2 in cat. The relative sizes of the letters in each stack represent their relative frequencies at each amino acid position. The height of the entire stack of residues is the information measured in bits (Y-axis): from minimum (0) to maximum conservation (3).
Ile101Thr mutation changes the binding surface of the VP2 protein
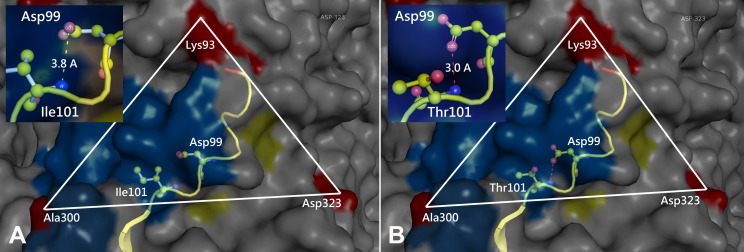
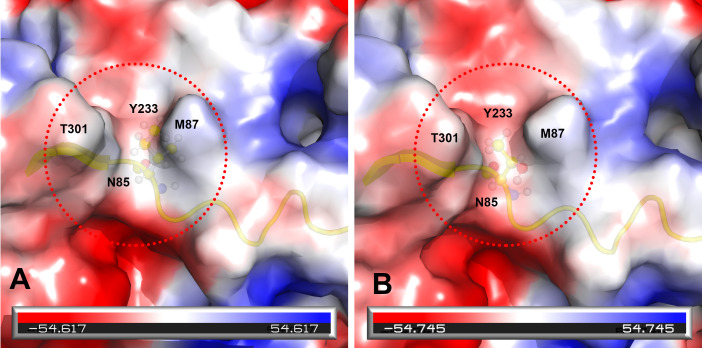
Prototype FPV and our isolate were compared by protein structure modeling with SWISS-MODEL to analyze the transferrin receptor-binding surface. The 3D structure of the VP2 protein was constructed to identify residue 101 within the receptor-binding region (Fig. 3). Our results showed that residue 101 is located not only at the core of the receptor-binding region but also at the antibody-binding site (Fig. 3). Interestingly, the Ile101 to Thr101 mutation leads to the formation of a polar contact between Asp99 and Thr101, resulting in a change to the molecular surface of the core of the receptor-binding region (Fig. 3). Our results demonstrated that Thr101 contacts more negative charges than does Ile101 within this region (Fig. 4). The molecular surface near residue 101 forms a canyon structure surrounded by amino acids Asn85, Met87, Tyr233 and Thr301. The deep-buried mutated residue (101Ile → Thr) affects the charge distribution in this canyon area (dotted circular scale), which is located at the edge of the receptor binding site (Fig. 4). As this mutation may affect binding ability to the virus host cell receptor, Ile101Thr appears to alter the local protein contact potential of the VP2 protein.
Figure 3. Comparison of the 3D structure of the transferrin receptor-binding site of the prototype FPV (A) and our isolate (B) capsid protein VP2.
Comparison of the 3D structure of the transferrin receptor-binding site of the prototype FPV (A) and our isolate (B) capsid protein VP2. Red residues represent the transferrin receptor-binding site. Triangle areas indicate the VP2 footprint for the transferrin receptor. Blue residues represent capsid protein binding to Fab fragments of neutralizing antibodies. Schematic diagram of amino acids interactions are shown in the upper left corner.
Figure 4. Analysis of electrostatic interactions on the molecular surface near residue Ile101 (A) compared to Thr101 (B) of the VP2 protein.
Analysis of electrostatic interactions on the molecular surface near residue Ile101 (A) compared to Thr101 (B) of the VP2 protein. Red and blue represent negative and positive charge areas, respectively. Residues 93 to 104 of the VP2 protein are highlighted in yellow. Residue 101 is shown in ball & stick presentation.
Discussion
FPV has been known as a contagious disease of cats since 1920s (Verge & Cristoforoni, 1928). CPV-2, considered a prominent host variant of the feline virus (Truyen, Platzer & Parrish, 1996), started to appear in dogs in the late 1970s. Although FPV has been revealed to be genetically stable (Decaro et al., 2008), CPV-2 demonstrates higher rates of nucleotide changes (Decaro et al., 2009; Pereira, Leal & Durigon, 2007; Shackelton et al., 2005). A few years after its discovery, the original CPV-2 was replaced by two antigenic variants, CPV-2a and CPV-2b, which can be differentiated by 5-6 amino acids of the main capsid protein VP2 (Parrish, 1991; Parrish et al., 1985). CPV-2c, the latest variant, was detected in 2000 in Italy (Buonavoglia et al., 2001), and its rapid spread to other continents has attracted increasing attention (Calderon et al., 2011; Chiang et al., 2016; Hong et al., 2007; Martella et al., 2004; Nakamura et al., 2004; Vannamahaxay et al., 2017; Wang et al., 2016; Yi et al., 2014).
To date, the transmission of CPV-2 to cats and FPV to dogs has been an interesting topic for researchers, and many in vivo and in vitro experiments have been conducted to provide more detail regarding the host ranges of these viruses. Based on abundant in vitro results, FPV efficiently replicates only in feline cells, whereas CPV-2 can replicate in both canine and feline cells (Hueffer et al., 2003b; Truyen & Parrish, 1992). Under natural conditions, CPV-2a, 2b and 2c have all been isolated from cats, with feline panleukopenia reported in many countries (Battilani et al., 2006; Decaro et al., 2010; Decaro et al., 2011; Ikeda et al., 2000; Mochizuki, Harasawa & Nakatani, 1993; Nakamura et al., 2001; Truyen, Platzer & Parrish, 1996). In contrast, information about FPV in dogs in the field is lacking. The host ranges of FPV and CPV-2 are quite sophisticated in vivo. Specifically, FPV can replicate in feline tissues, including the thymus, spleen, lymph nodes, and intestinal epithelial cells, and high viral loads are shed in feces. In dogs, the virus is found only in the thymus and bone marrow but is not detected in the mesenteric lymph nodes or gut, with no virus shedding in feces (Truyen & Parrish, 1992). Regarding viral evolution, the CPV-2 ancestor can only infect the gut, meaning it can be shed and spread widely among dogs (Truyen & Parrish, 1992).
As mentioned above, there is limited information to date on FPV infection in dogs in the field. In 1993, an isolate was recovered from a typical clinical parvoviral infection in a dog, but the virus possessed properties more similar to FPV than to CPV-2 or CPV-2a. The results suggest the possibility that transmission of FPV to dogs may have occurred (Mochizuki, Harasawa & Nakatani, 1993). Recently, transmission of FPV from cats to dogs in clinics was detected in Pakistan in 2018 (Ahmed et al., 2018) and in Thailand in 2019 (Charoenkul et al., 2019). Just a few FPV cases found in dogs during the past two decades. This means FPV has only occasionally infected dogs. In addition, mutation of apical domain residues in host TfR was observed to be critical for controlling parvovirus binding (Goodman et al., 2010; Hueffer et al., 2003a; Kaelber et al., 2012; Palermo, Hueffer & Parrish, 2003). The sequence of TfR from FPV-infected dogs needs to be further elucidated. Interestingly, according to phylogenetic analysis, the six specimens in our study cluster with FPV (Fig. 1), and all six isolates were collected from feces of dogs suspected of having canine parvoviral infection (Table 1). Comparison of the sequences of the six samples with the prototype FPV (M38246) and previously identified FPVs from Italy (EU498686) and China (DQ474237) showed high similarity in FPV VP2 among Vietnamese samples compared to other samples from around the world, with only some minor changes in the nucleic acid sequences. The important amino acid residues (80, 93, 103, 232, 323, 564, 568) and their corresponding nucleotide positions (nts 238-240, 277-279, 307-309, 694-696, 1690-1692, 1702-1704) can distinguish between FPV from CPV-2, with results similar to the careful examination by Decaro (Decaro & Buonavoglia, 2012). Taken together, our results are consistent with previous studies, indicating that FPV-like viruses can infect dogs in the field.
Due to the antigenic and genetic similarity between CPV-2 and FPV (Appel, Scott & Carmichael, 1979), both viruses can be classified as host-range variants of FPV (Ikeda et al., 2000). Moreover, other reports emphasize that the canine and feline host ranges can be determined by VP2 protein residues 93, 103, 300 and 323 (Truyen et al., 1995). Amino acid variations of FPV VP2 mainly occur in the receptor-binding region (Fig. S1), indicating that those variations may be involved with the interaction between the virus and its receptor. Residues Met87Leu, Ile101Thr, Ala300Gly, Asp305Tyr and Val555Ile of the VP2 protein have been identified as important for host range (Miranda & Thompson 2016). Surprisingly, our results showed that a Thr101 mutation in all of our FPV-like isolates but not in prototype FPV strains, as prototype FPV and CPV-2 encode Ile101 (Miranda & Thompson, 2016). According to the concept of consecutive genetic evolution of CPV-2, the Ile101 to Thr101 mutation was found in all CPV-2 variants (CPV-2a, 2b and 2c). Interestingly, the Thr101 mutation was also observed in recent FPV isolates in nature. Therefore, this particular mutation, Thr101, has been reported in circulating FPV and CPV-2 populations (Hoelzer et al., 2008). Whether the Thr101 variant is able to replicate efficiently in the gastrointestinal tract of dogs needs to be further investigated. Overall, there is limited information about the 3D structure or binding surface for Thr101 in the VP2 protein. Our 3D structure results indicated that the Thr101 mutation leads to the formation of a polar contact between Asp99 and Thr101. This may result in a change to the molecular surface of the core of the receptor-binding region, subsequently influencing receptor-binding affinity between the transferrin receptor and the virus. In addition, our data agree with previous studies indicating that residue 101 is located not only at the core of the receptor-binding region (Lee et al., 2019) but also at the antibody-binding site (Organtini et al., 2016). Taken together, the impact of the Thr101 mutation will require further investigation.
Conclusions
This study indicates the appearance of FPV-like strains in Vietnamese dogs. These FPV-like isolates carry an Ile101Thr mutation that is different from prototype FPV strains. Our data provide evidence for the existence of changes in the charge, protein contact potential and molecular surface of the core of the receptor-binding size with an Ile101 to Thr101 mutation. This is also the first study to provide reliable evidence that the FPV may be a threat to the Vietnamese dog population, which emphasizes the necessity of raising awareness for surveillance of this virus.
Supplemental Information
Stars represent residues that can be used to distinguish between FPVs and CPV-2 variants. The red color represents residues that can determine the canine or feline host range. Bold font represents residue Ile101 or Thr101. FPV-like isolates in the present study are indicated as a solid line box. The numbering at the top of the alignment is based on the FPV VP2 sequence.
Acknowledgments
The authors would like to thanks the dog owners without whose help this study could not have been completed.
Funding Statement
The authors received no funding for this work.
Contributor Information
Ming-Tang Chiou, Email: mtchiou@mail.npust.edu.tw.
Chao-Nan Lin, Email: cnlin6@mail.npust.edu.tw.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Minh Hoang and Cheng-Nan Wu performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Chuen-Fu Lin performed the experiments, analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Huong Thanh Thi Nguyen performed the experiments, analyzed the data, prepared figures and/or tables, and approved the final draft.
Van Phan Le conceived and designed the experiments, prepared figures and/or tables, and approved the final draft.
Ming-Tang Chiou conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Chao-Nan Lin conceived and designed the experiments, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Animal Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
The study did not involve any animal experiment. The Institutional Animal Care and Use Committee (IACUC) of National Pingtung University of Science and Technology did not deem it necessary for this research group to obtain formal approval to conduct this study.
Data Availability
References
- Ahmed et al. (2018).Ahmed N, Riaz A, Zubair Z, Saqib M, Ijaz S, Nawaz-Ul-Rehman MS, Al-Qahtani A, Mubin M. Molecular analysis of partial VP-2 gene amplified from rectal swab samples of diarrheic dogs in Pakistan confirms the circulation of canine parvovirus genetic variant CPV-2a and detects sequences of feline panleukopenia virus (FPV) Virology Journal. 2018;15:45. doi: 10.1186/s12985-018-0958-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison et al. (2014).Allison AB, Kohler DJ, Ortega A, Hoover EA, Grove DM, Holmes EC, Parrish CR. Host-specific parvovirus evolution in nature is recapitulated by in vitro adaptation to different carnivore species. PLOS Pathogens. 2014;10:e1004475. doi: 10.1371/journal.ppat.1004475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appel, Scott & Carmichael (1979).Appel MJ, Scott FW, Carmichael LE. Isolation and immunisation studies of a canine parco-like virus from dogs with haemorrhagic enteritis. Veterinary Record. 1979;105:156–159. doi: 10.1136/vr.105.8.156. [DOI] [PubMed] [Google Scholar]
- Battilani et al. (2006).Battilani M, Scagliarini A, Ciulli S, Morganti L, Prosperi S. High genetic diversity of the VP2 gene of a canine parvovirus strain detected in a domestic cat. Virology. 2006;352:22–26. doi: 10.1016/j.virol.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Black et al. (1979).Black JW, Holscher MA, Powell HS, Byerly CS. Parvoviral enteritis and panleukopenia in dogs. Veterinary medicine, small animal clinician. 1979;74:47–50. [PubMed] [Google Scholar]
- Buonavoglia et al. (2001).Buonavoglia C, Martella V, Pratelli A, Tempesta M, Cavalli A, Buonavoglia D, Bozzo G, Elia G, Decaro N, Carmichael L. Evidence for evolution of canine parvovirus type 2 in Italy. Journal of General Virology. 2001;82:3021–3025. doi: 10.1099/0022-1317-82-12-3021. [DOI] [PubMed] [Google Scholar]
- Calderon et al. (2011).Calderon MG, Romanutti C, D’Antuono A, Keller L, Mattion N, La Torre J. Evolution of canine parvovirus in Argentina between years 2003 and 2010: CPV2c has become the predominant variant affecting the domestic dog population. Virus Research. 2011;157:106–110. doi: 10.1016/j.virusres.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, Sgro & Parrish (1992).Chang SF, Sgro JY, Parrish CR. Multiple amino acids in the capsid structure of canine parvovirus coordinately determine the canine host range and specific antigenic and hemagglutination properties. Journal of Virology. 1992;66:6858–6867. doi: 10.1128/JVI.66.12.6858-6867.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charoenkul et al. (2019).Charoenkul K, Tangwangvivat R, Janetanakit T, Boonyapisitsopa S, Bunpapong N, Chaiyawong S, Amonsin A. Emergence of canine parvovirus type 2c in domestic dogs and cats from Thailand. Transboundary and Emerging Diseases. 2019;66:1518–1528. doi: 10.1111/tbed.13177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang et al. (2016).Chiang SY, Wu HY, Chiou MT, Chang MC, Lin CN. Identification of a novel canine parvovirus type 2c in Taiwan. Virology Journal. 2016;13(160) doi: 10.1186/s12985-016-0620-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotmore et al. (2019).Cotmore SF, Agbandje-McKenna M, Canuti M, Chiorini JA, Eis-Hubinger AM, Hughes J, Mietzsch M, Modha S, Ogliastro M, Penzes JJ, Pintel DJ, Qiu J, Soderlund-Venermo M, Tattersall P, Tijssen P, Ictv Report C. ICTV Virus Taxonomy Profile: Parvoviridae. Journal of General Virology. 2019;100:367–368. doi: 10.1099/jgv.0.001212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro & Buonavoglia (2012).Decaro N, Buonavoglia C. Canine parvovirus: a review of epidemiological and diagnostic aspects, with emphasis on type 2c. Veterinary Microbiology. 2012;155:1–12. doi: 10.1016/j.vetmic.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro et al. (2010).Decaro N, Buonavoglia D, Desario C, Amorisco F, Colaianni ML, Parisi A, Terio V, Elia G, Lucente MS, Cavalli A, Martella V, Buonavoglia C. Characterisation of canine parvovirus strains isolated from cats with feline panleukopenia. Research in Veterinary Science. 2010;89:275–278. doi: 10.1016/j.rvsc.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro et al. (2011).Decaro N, Desario C, Amorisco F, Losurdo M, Colaianni ML, Greco MF, Buonavoglia C. Canine parvovirus type 2c infection in a kitten associated with intracranial abscess and convulsions. Journal of Feline Medicine and Surgery. 2011;13:231–236. doi: 10.1016/j.jfms.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro et al. (2008).Decaro N, Desario C, Miccolupo A, Campolo M, Parisi A, Martella V, Amorisco F, Lucente MS, Lavazza A, Buonavoglia C. Genetic analysis of feline panleukopenia viruses from cats with gastroenteritis. Journal of General Virology. 2008;89:2290–2298. doi: 10.1099/vir.0.2008/001503-0. [DOI] [PubMed] [Google Scholar]
- Decaro et al. (2009).Decaro N, Desario C, Parisi A, Martella V, Lorusso A, Miccolupo A, Mari V, Colaianni ML, Cavalli A, DiTrani L, Buonavoglia C. Genetic analysis of canine parvovirus type 2c. Virology. 2009;385:5–10. doi: 10.1016/j.virol.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Decaro et al. (2005).Decaro N, Elia G, Campolo M, Desario C, Lucente MS, Bellacicco AL, Buonavoglia C. New approaches for the molecular characterization of canine parvovirus type 2 strains. Journal of veterinary medicine. B. Infectious diseases and veterinary public health. 2005;52:316–319. doi: 10.1111/j.1439-0450.2005.00869.x. [DOI] [PubMed] [Google Scholar]
- Geng et al. (2015).Geng Y, Guo D, Li C, Wang E, Wei S, Wang Z, Yao S, Zhao X, Su M, Wang X, Wang J, Wu R, Feng L, Sun D. Co-Circulation of the rare CPV-2c with unique Gln370Arg substitution, new CPV-2b with unique Thr440Ala substitution, and new CPV-2a with high prevalence and variation in Heilongjiang Province, Northeast China. PLOS ONE. 2015;10:e0137288. doi: 10.1371/journal.pone.0137288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman et al. (2010).Goodman LB, Lyi SM, Johnson NC, Cifuente JO, Hafenstein SL, Parrish CR. Binding site on the transferrin receptor for the parvovirus capsid and effects of altered affinity on cell uptake and infection. Journal of Virology. 2010;84:4969–4978. doi: 10.1128/jvi.02623-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang et al. (2019a).Hoang M, Lin WH, Le VP, Nga BTT, Chiou MT, Lin CN. Molecular epidemiology of canine parvovirus type 2 in Vietnam from November 2016 to February 2018. Virology Journal. 2019a;16:52. doi: 10.1186/s12985-019-1159-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang et al. (2019b).Hoang M, Wu HY, Lien YX, Chiou MT, Lin CN. A SimpleProbe® real-time PCR assay for differentiating the canine parvovirus type 2 genotype. Journal of Clinical Laboratory Analysis. 2019b;33(1):e22654. doi: 10.1002/jcla.22654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoelzer et al. (2008).Hoelzer K, Shackelton LA, Holmes EC, Parrish CR. Within-host genetic diversity of endemic and emerging parvoviruses of dogs and cats. Journal of Virology. 2008;82:11096–11105. doi: 10.1128/jvi.01003-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong et al. (2007).Hong C, Decaro N, Desario C, Tanner P, Pardo MC, Sanchez S, Buonavoglia C, Saliki JT. Occurrence of canine parvovirus type 2c in the United States. Journal of Veterinary Diagnostic Investigation. 2007;19:535–539. doi: 10.1177/104063870701900512. [DOI] [PubMed] [Google Scholar]
- Hueffer et al. (2003a).Hueffer K, Govindasamy L, Agbandje-McKenna M, Parrish CR. Combinations of two capsid regions controlling canine host range determine canine transferrin receptor binding by canine and feline parvoviruses. Journal of Virology. 2003a;77:10099–10105. doi: 10.1128/jvi.77.18.10099-10105.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hueffer et al. (2003b).Hueffer K, Parker JS, Weichert WS, Geisel RE, Sgro JY, Parrish CR. The natural host range shift and subsequent evolution of canine parvovirus resulted from virus-specific binding to the canine transferrin receptor. Journal of Virology. 2003b;77:1718–1726. doi: 10.1128/JVI.77.3.1718-1726.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda et al. (2000).Ikeda Y, Mochizuki M, Naito R, Nakamura K, Miyazawa T, Mikami T, Takahashi E. Predominance of canine parvovirus (CPV) in unvaccinated cat populations and emergence of new antigenic types of CPVs in cats. Virology. 2000;278:13–19. doi: 10.1006/viro.2000.0653. [DOI] [PubMed] [Google Scholar]
- Johnson (1965).Johnson RH. Feline Panleucopaenia: I.—identification of a virus associated with the syndrome. Research in Veterinary Science. 1965;6:466–471. doi: 10.1016/S0034-5288(18)34726-X. [DOI] [PubMed] [Google Scholar]
- Kaelber et al. (2012).Kaelber JT, Demogines A, Harbison CE, Allison AB, Goodman LB, Ortega AN, Sawyer SL, Parrish CR. Evolutionary reconstructions of the transferrin receptor of Caniforms supports canine parvovirus being a re-emerged and not a novel pathogen in dogs. PLOS Pathogens. 2012;8:e1002666. doi: 10.1371/journal.ppat.1002666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabata (2019).Kawabata H. Transferrin and transferrin receptors update. Free Radical Biology and Medicine. 2019;133:46–54. doi: 10.1016/j.freeradbiomed.2018.06.037. [DOI] [PubMed] [Google Scholar]
- Lee et al. (2019).Lee H, Callaway HM, Cifuente JO, Bator CM, Parrish CR, Hafenstein SL. Transferrin receptor binds virus capsid with dynamic motion. Proceedings of the National Academy of Sciences. 2019;116:20462–20471. doi: 10.1073/pnas.1904918116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin et al. (2017).Lin YC, Chiang SY, Wu HY, Lin JH, Chiou MT, Liu HF, Lin CN. Phylodynamic and genetic diversity of canine parvovirus type 2c in Taiwan. International Journal of Molecular Sciences. 2017;18 doi: 10.3390/ijms18122703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martella et al. (2004).Martella V, Cavalli A, Pratelli A, Bozzo G, Camero M, Buonavoglia D, Narcisi D, Tempesta M, Buonavoglia C. A canine parvovirus mutant is spreading in Italy. Journal of Clinical Microbiology. 2004;42:1333–1336. doi: 10.1128/JCM.42.3.1333-1336.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda & Thompson (2016).Miranda C, Thompson G. Canine parvovirus: the worldwide occurrence of antigenic variants. Journal of General Virology. 2016;97:2043–2057. doi: 10.1099/jgv.0.000540. [DOI] [PubMed] [Google Scholar]
- Mochizuki, Harasawa & Nakatani (1993).Mochizuki M, Harasawa R, Nakatani H. Antigenic and genomic variabilities among recently prevalent parvoviruses of canine and feline origin in Japan. Veterinary Microbiology. 1993;38:1–10. doi: 10.1016/0378-1135(93)90070-N. [DOI] [PubMed] [Google Scholar]
- Nakamura et al. (2001).Nakamura K, Sakamoto M, Ikeda Y, Sato E, Kawakami K, Miyazawa T, Tohya Y, Takahashi E, Mikami T, Mochizuki M. Pathogenic potential of canine parvovirus types 2a and 2c in domestic cats. Clinical and Diagnostic Laboratory Immunology. 2001;8:663–668. doi: 10.1128/cdli.8.3.663-668.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura et al. (2004).Nakamura M, Tohya Y, Miyazawa T, Mochizuki M, Phung HT, Nguyen NH, Huynh LM, Nguyen LT, Nguyen PN, Nguyen PV, Nguyen NP, Akashi H. A novel antigenic variant of Canine parvovirus from a Vietnamese dog. Archives of Virology. 2004;149:2261–2269. doi: 10.1007/s00705-004-0367-y. [DOI] [PubMed] [Google Scholar]
- Organtini et al. (2016).Organtini LJ, Lee H, Iketani S, Huang K, Ashley RE, Makhov AM, Conway JF, Parrish CR, Hafenstein S. Near-atomic resolution structure of a highly neutralizing fab bound to canine parvovirus. Journal of Virology. 2016;90:9733–9742. doi: 10.1128/jvi.01112-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palermo, Hueffer & Parrish (2003).Palermo LM, Hueffer K, Parrish CR. Residues in the apical domain of the feline and canine transferrin receptors control host-specific binding and cell infection of canine and feline parvoviruses. Journal of Virology. 2003;77:8915–8923. doi: 10.1128/jvi.77.16.8915-8923.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish (1991).Parrish CR. Mapping specific functions in the capsid structure of canine parvovirus and feline panleukopenia virus using infectious plasmid clones. Virology. 1991;183:195–205. doi: 10.1016/0042-6822(91)90132-U. [DOI] [PubMed] [Google Scholar]
- Parrish, Aquadro & Carmichael (1988).Parrish CR, Aquadro CF, Carmichael LE. Canine host range and a specific epitope map along with variant sequences in the capsid protein gene of canine parvovirus and related feline, mink, and raccoon parvoviruses. Virology. 1988;166:293–307. doi: 10.1016/0042-6822(88)90500-4. [DOI] [PubMed] [Google Scholar]
- Parrish et al. (1991).Parrish CR, Aquadro CF, Strassheim ML, Evermann JF, Sgro JY, Mohammed HO. Rapid antigenic-type replacement and DNA sequence evolution of canine parvovirus. Journal of Virology. 1991;65:6544–6552. doi: 10.1128/JVI.65.12.6544-6552.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish et al. (1985).Parrish CR, O’Connell PH, Evermann JF, Carmichael LE. Natural variation of canine parvovirus. Science. 1985;230:1046–1048. doi: 10.1126/science.4059921. [DOI] [PubMed] [Google Scholar]
- Pereira, Leal & Durigon (2007).Pereira CA, Leal ES, Durigon EL. Selective regimen shift and demographic growth increase associated with the emergence of high-fitness variants of canine parvovirus. Infection, Genetics and Evolution. 2007;7:399–409. doi: 10.1016/j.meegid.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Reed, Jones & Miller (1988).Reed AP, Jones EV, Miller TJ. Nucleotide sequence and genome organization of canine parvovirus. Journal of Virology. 1988;62:266–276. doi: 10.1128/JVI.62.1.266-276.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackelton et al. (2005).Shackelton LA, Parrish CR, Truyen U, Holmes EC. High rate of viral evolution associated with the emergence of carnivore parvovirus. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:379–384. doi: 10.1073/pnas.0406765102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura et al. (2013).Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truyen et al. (1995).Truyen U, Gruenberg A, Chang SF, Obermaier B, Veijalainen P, Parrish CR. Evolution of the feline-subgroup parvoviruses and the control of canine host range in vivo. Journal of Virology. 1995;69:4702–4710. doi: 10.1128/JVI.69.8.4702-4710.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truyen & Parrish (1992).Truyen U, Parrish CR. Canine and feline host ranges of canine parvovirus and feline panleukopenia virus: distinct host cell tropisms of each virus in vitro and in vivo. Journal of Virology. 1992;66:5399–5408. doi: 10.1128/JVI.66.9.5399-5408.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truyen, Platzer & Parrish (1996).Truyen U, Platzer G, Parrish CR. Antigenic type distribution among canine parvoviruses in dogs and cats in Germany. Veterinary Record. 1996;138:365–366. doi: 10.1136/vr.138.15.365. [DOI] [PubMed] [Google Scholar]
- Lopez de Turiso et al. (1991).Lopez de Turiso JA, Cortes E, Ranz A, Garcia J, Sanz A, Vela C, Casal JI. Fine mapping of canine parvovirus B cell epitopes. Journal of General Virology. 1991;72(Pt 10):2445–2456. doi: 10.1099/0022-1317-72-10-2445. [DOI] [PubMed] [Google Scholar]
- Vannamahaxay et al. (2017).Vannamahaxay S, Vongkhamchanh S, Intanon M, Tangtrongsup S, Tiwananthagorn S, Pringproa K, Chuammitri P. Molecular characterization of canine parvovirus in Vientiane. Laos. Arch Virol. 2017;162:1355–1361. doi: 10.1007/s00705-016-3212-1. [DOI] [PubMed] [Google Scholar]
- Verge & Cristoforoni (1928).Verge J, Cristoforoni N. La gastroenterite infectieuse des chats estelle due a un virus filtrable? Comptes Rendus Seances Biologies. 1928;99:312–314. [Google Scholar]
- Wang et al. (2016).Wang J, Lin P, Zhao H, Cheng Y, Jiang Z, Zhu H, Wu H, Cheng S. Continuing evolution of canine parvovirus in China: isolation of novel variants with an Ala5Gly mutation in the VP2 protein. Infection, Genetics and Evolution. 2016;38:73–78. doi: 10.1016/j.meegid.2015.12.009. [DOI] [PubMed] [Google Scholar]
- Yi et al. (2014).Yi L, Tong M, Cheng Y, Song W, Cheng S. Phylogenetic analysis of canine parvovirus VP2 gene in China. Transboundary and Emerging Diseases. 2014;63:262–269. doi: 10.1111/tbed.12268. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2010).Zhang R, Yang S, Feng H, Gui C, Xia X. The first detection of canine parvovirus type 2c in China. Zhongguo Bingyuan Shengwuxue Zazhi / Journal of Pathogen Biology. 2010;5:246–249. [Google Scholar]
- Zhao et al. (2015).Zhao HWJ, Jiang Y, Cheng Y, Lin P, Zhu H, Han G, Yi L, Zhang S, Guo L, Cheng S. Typing of Canine Parvovirus strains circulating in North-East China. Transboundary and Emerging Diseases. 2015;64:495–503. doi: 10.1111/tbed.12390. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Stars represent residues that can be used to distinguish between FPVs and CPV-2 variants. The red color represents residues that can determine the canine or feline host range. Bold font represents residue Ile101 or Thr101. FPV-like isolates in the present study are indicated as a solid line box. The numbering at the top of the alignment is based on the FPV VP2 sequence.
Data Availability Statement
The following information was supplied regarding data availability:
Our raw data are the viral sequences, which submitted to GenBank accession number MK357738 to MK357743.