Abstract
Background: Tongxinluo (TXL) capsule, a polypharmacy derived from traditional Chinese medicine (TCM), has been widely used in coronary heart disease (CHD), while the underlying mechanism of TXL capsule is still unclear. The present study aimed at investigating the underlying mechanism of TXL acting on CHD patients and providing substantial evidence in molecular evidence by means of a network pharmacological analysis.
Method: Active compounds and targeted genes of TXL were retrieved from TCM systems pharmacology (TCMSP) and TCM integrative database (TCMID). CHD and coronary artery disease were treated as search queries in GeneCards and Online Mendelian Inheritance in Man (OMIM) databases to obtain disease-related genes. Visualization of disease–targets network was performed under administration of Cytoscape software. Besides, Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses were administered. H9c2 cells were used to validate the predicted results in cardiomyocytes/reoxygenation model, and anti-inflammatory ability was examined.
Results: A network of a total of 212 nodes and 1016 edges was obtained. Peptide and ubiquitin-like protein ligase binding occupied a leading position of GO enrichment. For KEGG analysis, fluid shear stress and atherosclerosis, as well as inflammation-related pathways were enriched. Cellular validation revealed the anti-inflammatory effect of β-sitosterol, eriodictyol, odoricarpin, and tirucallol as active compounds of TXL.
Conclusion: Our study provided substantial molecular evidence that TXL capsule possessed the characteristics of multitargets with safe profile, and the main component is capable of regulating cytokine level in CHD patients.
Keywords: Coronary heart disease, Molecular mechanism, Network pharmacology, TXL
Introduction
Coronary heart disease (CHD), one of the most common cardiovascular diseases is caused by reduction in blood flow to cardiomyocyte owing to build-up of plaque in arteries of heart [1,2]. CHD has become a leading cause of death and the mortality increased from 5.2 million to over 7 million between 1990 and 2010 [3]. It affects individuals at any age while becomes approximately triple in progressively elder populations compared with other age groups, and the morbidity in males is larger than that in female population [4]. Statin, as the cornerstone in anti-atherosclerotic regimen, has demonstrated the substantial efficacy at reducing cardiovascular events. However, even with intensive statin therapy, many patients still suffered from high residual risks in cardiovascular events [5]. Thus, exploration of alternative anti-atherosclerotic medications with high efficacy as well as low side-effect is needed.
Traditional Chinese medicine (TCM) plays an important role in Asian population and has been popular in Western countries for its efficacy as well as less side-effects [6]. Tongxinluo (TXL) capsule, which consists of 12 principal components from plants and animal products, was approved by Food and Drug Administration (FDA) of China for treating angina pectoris and ischemic stroke [7]. Several clinical studies revealed that TXL has the ability to attenuate and stabilize atherosclerotic plaque by means of lowering serum lipid, anti-oxidation and anti-inflammation [8,9]. Furthermore, a recent multicenter randomized controlled trial, CAPITAL, demonstrated that TXL in addition to routine anti-atherosclerotic therapy could prevent the progression of intima-media thickness (IMT), plaque area and vascular remodeling [10], which provided clinic-based evidence of TXL on CHD patients. Nevertheless, the exact pharmacological effects of TXL are still unclear due to its complex formula.
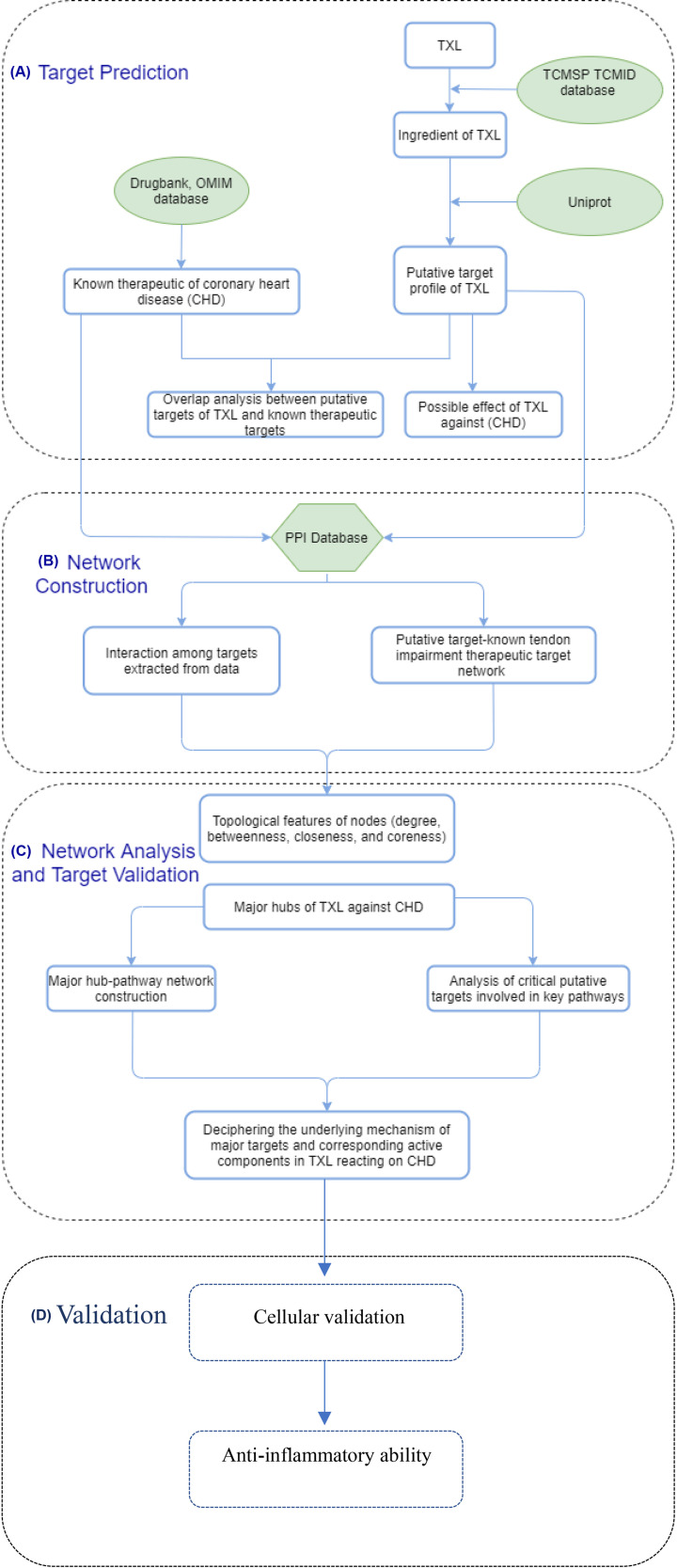
With the rapid development of bioinformatics, system biology and polypharmacology, network pharmacology-based analysis has been proved to be a potent method to investigate the mechanism of TCM with complex formula [11,12]. In the present study, we aimed at investigating the mechanism of TXL exerted on CHD patients in molecular level by means of constructing a comprehensive network pharmacology-based analysis. The complete flowchart of the present study is displayed in Figure 1.
Figure 1. Flowchart of this network pharmacology analysis.
(A) Target prediction. (B) Network construction. (C) Network analysis and target validation. (D) Validation model.
Methods
Chemical ingredients searching
In order to obtain the chemical ingredients of components in TXL capsule, we performed a comprehensive search on TCM systems pharmacology database (TCMSP, https://tcmspw.com/tcmsp.php) and TCM integrative database (TCMID, https://www.megabionet.org/tcmid/) by using the following queries: Ginseng radix et rhizoma (Araliaceae; Chinese ginseng), Paeoniaeradixrubra (Paeoniaceae; Chinese peony), Ziziphispinosae semen (Rhamnaceae; jujube seed) (fried), Dalbergiaeodoriferae lignum (Dalbergia odorifera T.C.Chen; Huanghuali wood), Santalum album L. (Santalaceae; sandalwood), Olibanum (Burseraceae; Boswellia)(prepared), Borneolum (Blumea balsamifera DC.), Hirudo (Haemopidae, leech), Scorpio (Buthidae; Chinese scorpion), Scolopendra (Scolopendrasubspinipesmutilans L. Koch), Cicadae periostracum (Cicadidae; cicada), Eupolyphaga Steleophaga (Corydiidae; Woodlouse) which are principal components of TXL [13,14]. Ingredients, molecule name, molecular weight, water partition coefficient, number of hydrogen bond donors and receptors, human oral bioavailability (OB), half-life, blood–brain barrier (BBB) and drug-likeness (DL) of each principal component were obtained from abovementioned database. Active compounds were screened out on the basis of absorption, distribution, metabolism, and excretion (ADME) protocols, with criteria of OB ≥ 30% and DL ≥ 0.18.
Targets of active compounds
We comprehensively searched the direct targeted receptors of each active compound via DrugBank database, a specific bioinformatics and cheminformatics resource with detailed drug data, as well as targeted receptors (https://www.drugbank.ca). Full names of targeted protein receptors were obtained and converted into gene symbol on the basis of UniProt ID (https://www.uniprot.org/) for following analysis.
Disease-related genes retrieval
GeneCards (https://www.genecards.org/) and Online Mendelian Inheritance in Man (OMIM) databases (https://www.omim.org/) were retrieved for acquiring CHD-related genes using the keywords of CHD and coronary artery disease. Intersection of retrieved targets of active compounds and disease-related genes were obtained under the administration of R (version 3.6.2) for downstream analysis.
Visualization of ingredient-target genes-pathway and protein–protein interaction network
All intersected targets of active compounds and disease-related genes were put into Cytoscape software (Version 3.7.2) for visualization of ingredient-target genes-pathway network. To obtain interactions between intersected genes, overlapped genes were used for construction of protein–protein interaction (PPI) network in STRING database (https://string-db.org/) with the cut-off criteria of confidence > 0.4 and hiding disconnected nodes.
Gene Ontology and Kyoto Encyclopedia of Genes and Genomes enrichment analysis
Overlapped genes were retrieved for GO and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis with the criterion of P-value <0.05. Bar plots of GO and KEGG were exported and signal pathways involved in this network analysis were visualized in forms of diagram.
Reagents used in validation
β-sitosterol, ellagic acid, formononetin, eriodictyol, were purchased from MedChemExpress (MCE, Shanghai, China) with the purity > 98%. Odoricarpin was purchased from TASLY PHARM (Tianjing, China) with the purity > 98%. Tirucallol was purchased from Shanghai Institute of Biotechnology Co., Ltd. (Shanghai, China) with the purity > 98%.
Cells
H9c2 cells were purchased from Tongpai Technology Company (Shanghai, China) and cultured in Dulbecco’s modified Eagle’s medium (DMEM) bought from Thermo Fisher Scientific (Guangzhou, China), with the supplement of 10% v/v FBS and 1% v/v penicillin/streptomycin in CO2 incubator at 37°C and 95% relative humidity.
Cell models
Regarding the investigation of the protective effect of TXL, hypoxia/reoxygenation (H/R) model was administered. Cells were put into an incubator with Krebs–Ringer bicarbonate buffer medium saturated with 99.99% N2 for 140 min [15]. Cells were reoxygenated through changing the DMEM back and cultured under normal oxygen level (21%) for 1 h. The molecules were applied for 48 h before hypoxia until the end of oxygenation.
Cell viability test
Cell viability test was performed under the assistance of cell counting kit-8 (CCK-8) after the administration of abovementioned active components of TXL. Cells with different molecules were seeded in a 96-well plate at a density of 1 × 104 cells for 24 h. Then, 10% CCK-8 was added and OD value was read at 450 nm after 1 h. In addition, optimal concentration of each molecule was explored ranging from 5 to 100 μM [16–19]. Each cell viability test with different molecules was repeated five times and measurement of relative cell viability was recorded.
Investigation of anti-inflammatory effect
For anti-inflammatory effect, cells were seeded in a 96-well plate incubated for 24 h, and treated with 0.01 μg/ml LPS 30 min after incubation with optimal concentration of abovementioned molecules was obtained. Then, supernatant was collected by adding 150 μl dimethyl sulfoxide (DMSO) and stored at −80°C for downstream analysis. Concentration of cytokine was measured by enzyme-linked immunosorbent assay (ELISA) under corresponding protocol and IL-6 (K4144-100, Biovision) and IL-8 (K4169-100, Biovision) ELISA kits were administered in the presentstudy. Each test with different molecules was repeated five times and average concentration of corresponding results was recorded.
Results
Identification of putative ingredient targets
With the mentioned search queries of Panax Ginseng C. A. Mey., Radix Paeoniae Rubra, Ziziphi Spinosae Semen, Dalbergiae Odoriferae lignum, Santalum Album L., Olibanun, Cicadae Periostracum, Borneolum Syntheticum, hirudo, Scorpio, Scolopendra, Cicadae periostracum and criteria of OB ≥ 30% as well as DL ≥ 0.18, a total of 111 chemical ingredients were collected within TXL prescription from TCMSP and TCMID databases. Besides, the targeted genes of each retrieved chemical ingredients were explored and a total of 1205 targeted genes were obtained. The names of targeted genes were converted into gene ID on basis of UniProt database, and eventually 861 eligible targeted genes with molecular names and symbol ID were acquired. The active compounds involved in the present study with the amount as well as ratio of each component [20] were shown in Table 1 and detailed information of putative ingredients with targeted genes were documented in Supplementary Table S1.
Table 1. Detailed information of active ingredients of TXL.
| Local name | Latin scientific names | Mol ID | Molecule name | Ratio* | MW | AlogP | OB (%) | DL |
|---|---|---|---|---|---|---|---|---|
| Ginseng radix et rhizoma | Panax Ginseng C. A. Mey | 0.024 (9.4) | ||||||
| MOL002879 | Diop | 390.62 | 7.44 | 43.59 | 0.39 | |||
| MOL000449 | Stigmasterol | 412.77 | 7.64 | 43.83 | 0.76 | |||
| MOL000358 | Beta-sitosterol | 414.79 | 8.08 | 36.91 | 0.75 | |||
| MOL003648 | Inermin | 284.28 | 2.44 | 65.83 | 0.54 | |||
| MOL000422 | Kaempferol | 286.25 | 1.77 | 41.88 | 0.24 | |||
| MOL004492 | Chrysanthemaxanthin | 584.96 | 8.24 | 38.72 | 0.58 | |||
| MOL005308 | Aposiopolamine | 271.34 | 1.39 | 66.65 | 0.22 | |||
| MOL005314 | Celabenzine | 379.55 | 2.29 | 101.88 | 0.49 | |||
| MOL005317 | Deoxyharringtonine | 515.66 | 3.13 | 39.27 | 0.81 | |||
| MOL005318 | Dianthramine | 289.26 | 2.05 | 40.45 | 0.2 | |||
| MOL005320 | Arachidonate | 304.52 | 6.41 | 45.57 | 0.2 | |||
| MOL005321 | Frutinone A | 264.24 | 2.7 | 65.9 | 0.34 | |||
| MOL005344 | Ginsenoside rh2 | 622.98 | 4.04 | 36.32 | 0.56 | |||
| MOL005348 | Ginsenoside-Rh4_qt | 458.8 | 5.59 | 31.11 | 0.78 | |||
| MOL005356 | Girinimbin | 263.36 | 4.6 | 61.22 | 0.31 | |||
| MOL005357 | Gomisin B | 514.62 | 2.73 | 31.99 | 0.83 | |||
| MOL005360 | Malkangunin | 432.56 | 1.84 | 57.71 | 0.63 | |||
| MOL005376 | Panaxadiol | 460.82 | 5.46 | 33.09 | 0.79 | |||
| MOL005384 | Suchilactone | 368.41 | 3.73 | 57.52 | 0.56 | |||
| MOL005399 | Alexandrin_qt | 414.79 | 8.08 | 36.91 | 0.75 | |||
| MOL005401 | Ginsenoside Rg5_qt | 442.8 | 6.8 | 39.56 | 0.79 | |||
| MOL000787 | Fumarine | 353.4 | 2.95 | 59.26 | 0.83 | |||
| Paeoniaeradixrubra | Paeoniaceae | 0.022 (8.6) | ||||||
| MOL001918 | Paeoniflorgenone | 318.35 | 0.79 | 87.59 | 0.37 | |||
| MOL001925 | Paeoniflorin_qt | 318.35 | 0.46 | 68.18 | 0.4 | |||
| MOL007016 | Paeoniflorigenone | 318.35 | 0.79 | 65.33 | 0.37 | |||
| MOL006996 | 1-o-beta-d-glucopyranosylpaeonisuffrone_qt | 332.38 | 0.51 | 65.08 | 0.35 | |||
| MOL007022 | EvofolinB | 318.35 | 2.07 | 64.74 | 0.22 | |||
| MOL007018 | 9-ethyl-neo-paeoniaflorin A_qt | 334.4 | 1.48 | 64.42 | 0.3 | |||
| MOL006992 | (2R,3R)-4-methoxyl-distylin | 318.3 | 1.89 | 59.98 | 0.3 | |||
| MOL007008 | 4-ethyl-paeoniflorin_qt | 332.38 | 1.02 | 56.87 | 0.44 | |||
| MOL007012 | 4-o-methyl-paeoniflorin_qt | 332.38 | 0.87 | 56.7 | 0.43 | |||
| MOL000492 | (+)-catechin | 290.29 | 1.92 | 54.83 | 0.24 | |||
| MOL001924 | Paeoniflorin | 480.51 | −1.28 | 53.87 | 0.79 | |||
| MOL001921 | Lactiflorin | 462.49 | −0.57 | 49.12 | 0.8 | |||
| MOL007005 | Albiflorin_qt | 318.35 | 0.42 | 48.7 | 0.33 | |||
| MOL000449 | Stigmasterol | 412.77 | 7.64 | 43.83 | 0.76 | |||
| MOL001002 | Ellagic acid | 302.2 | 1.48 | 43.06 | 0.43 | |||
| MOL004355 | Spinasterol | 412.77 | 7.64 | 42.98 | 0.76 | |||
| MOL002776 | Baicalin | 446.39 | 0.64 | 40.12 | 0.75 | |||
| MOL005043 | Campest-5-en-3beta-ol | 400.76 | 7.63 | 37.58 | 0.71 | |||
| MOL006999 | Stigmast-7-en-3-ol | 414.79 | 8.08 | 37.42 | 0.75 | |||
| MOL000358 | Beta-sitosterol | 414.79 | 8.08 | 36.91 | 0.75 | |||
| MOL000359 | Sitosterol | 414.79 | 8.08 | 36.91 | 0.75 | |||
| MOL006994 | 1-o-beta-d-glucopyranosyl-8-o -benzoylpaeonisuffrone_qt | 302.35 | 0.44 | 36.01 | 0.3 | |||
| MOL002714 | Baicalein | 270.25 | 2.33 | 33.52 | 0.21 | |||
| MOL002883 | Ethyl oleate (NF) | 310.58 | 7.44 | 32.4 | 0.19 | |||
| MOL007014 | 8-debenzoylpaeonidanin | 390.43 | -3.28 | 31.74 | 0.45 | |||
| MOL007003 | Bnzoyl paeoniflorin | 584.62 | 0.76 | 31.14 | 0.54 | |||
| MOL007025 | Isobenzoylpaeoniflorin | 584.62 | 0.76 | 31.14 | 0.54 | |||
| MOL006990 | (1S,2S,4R)-trans-2-hydroxy-1,8-cineole-B-d-glucopyranoside | 332.44 | −0.57 | 30.25 | 0.27 | |||
| MOL007004 | Albiflorin | 480.51 | −1.33 | 30.25 | 0.77 | |||
| Ziziphispinosae semen | Rhamnaceae | 0.024 (9.4) | ||||||
| MOL001522 | (S)-Coclaurine | 285.37 | 2.83 | 42.35 | 0.24 | |||
| MOL001546 | Zizyphusine | 342.45 | 3.12 | 41.53 | 0.55 | |||
| MOL001527 | Jujuboside A_qt | 472.78 | 4.39 | 34.96 | 0.62 | |||
| MOL001542 | Swertisin | 446.44 | 0.19 | 31.83 | 0.75 | |||
| MOL001525 | Daucosterol | 414.79 | 8.08 | 36.91 | 0.75 | |||
| MOL001532 | Phytosterol | 414.79 | 8.08 | 36.91 | 0.75 | |||
| MOL001521 | Ceanothic acid | 486.76 | 5.36 | 33.41 | 0.77 | |||
| MOL000211 | Mairin | 456.78 | 6.52 | 55.38 | 0.78 | |||
| MOL001539 | Sanjoinenine | 489.67 | 4.23 | 67.28 | 0.79 | |||
| Dalbergiaeodoriferae lignum | Dalbergia odorifera T.C.Chen | 0.020 (7.8) | ||||||
| MOL002958 | 3′-Hydroxymelanettin | 300.28 | 2.56 | 30.69 | 0.27 | |||
| MOL001792 | DFV | 256.27 | 2.57 | 32.76 | 0.18 | |||
| MOL002957 | 9-O-Methylcoumestrol | 282.26 | 3.26 | 33.73 | 0.38 | |||
| MOL002982 | (3R,4R)-3′,7-dihydroxy-2′,4′-dimethoxy-4-[(2S)-4′,5,7-trihydroxyflavanone-6-yl]isoflavan | 572.6 | 4.88 | 33.96 | 0.63 | |||
| MOL002967 | 7-hydroxy-4′-methoxy-2′,5′-dioxo-4-[(3R)-2′,7-dihydroxy-4′-methoxyisoflavan-5′-yl]isoflavane | 556.6 | 4.26 | 34.78 | 0.7 | |||
| MOL003000 | Stevein | 284.28 | 2.83 | 36.54 | 0.24 | |||
| MOL000359 | Sitosterol | 414.79 | 8.08 | 36.91 | 0.75 | |||
| MOL000358 | β-sitosterol | 414.79 | 8.08 | 36.91 | 0.75 | |||
| MOL002991 | (6aR,11aR)-3,9-dimethoxy-6a,11a-dihydro -6H-benzofurano[3,2-c]chromene-4,10-diol | 316.33 | 2.37 | 38.96 | 0.48 | |||
| MOL002963 | 4′,5′,7-trimethyl-3-methoxyflavone | 294.37 | 4.1 | 40.66 | 0.25 | |||
| MOL002914 | Eriodyctiol (flavanone) | 288.27 | 2.03 | 41.35 | 0.24 | |||
| MOL001040 | (2R)-5,7-dihydroxy-2-(4-hydroxyphenyl) chroman-4-one | 272.27 | 2.3 | 42.36 | 0.21 | |||
| MOL002962 | (3S)-7-hydroxy-3-(2,3,4-trimethoxyphenyl) chroman-4-one | 330.36 | 2.67 | 48.23 | 0.33 | |||
| MOL002989 | 4-Hydroxyhomopterocarpin | 300.33 | 2.64 | 48.41 | 0.43 | |||
| MOL002959 | 3′-Methoxydaidzein | 284.28 | 2.32 | 48.57 | 0.24 | |||
| MOL002565 | Medicarpin | 270.3 | 2.66 | 49.22 | 0.34 | |||
| MOL002940 | (3R)-3-(2,3-dihydroxy-4-methoxyphenyl) -7-hydroxychroman-4-one | 302.3 | 2.16 | 52.06 | 0.27 | |||
| MOL003001 | Vestitone | 286.3 | 2.43 | 52.83 | 0.24 | |||
| MOL002996 | Odoricarpin | 330.36 | 2.63 | 55.02 | 0.53 | |||
| MOL000228 | (2R)-7-hydroxy-5-methoxy-2- phenylchroman-4-one | 270.3 | 2.82 | 55.23 | 0.2 | |||
| MOL002973 | Bowdichione | 298.26 | 0.64 | 55.78 | 0.28 | |||
| MOL000380 | (6aR,11aR)-9,10-dimethoxy-6a,11a-dihydro- 6H-benzofurano[3,2-c]chromen-3-ol | 300.33 | 2.64 | 64.26 | 0.42 | |||
| MOL002990 | (6aR,11aR)-3,9,10-trimethoxy-6a,11a-dihydro-6H-benzofurano[3,2-c]chromen-4-ol | 330.36 | 2.63 | 66.86 | 0.53 | |||
| MOL002938 | (3R)-4′-Methoxy-2′,3, 7-trihydroxyisoflavanone | 302.3 | 1.83 | 68.86 | 0.27 | |||
| MOL002950 | (3R)-7,2′,3′-trihydroxy-4 -methoxyisoflavan | 288.32 | 2.21 | 69.65 | 0.24 | |||
| MOL000392 | Formononetin | 268.28 | 2.58 | 69.67 | 0.21 | |||
| MOL002975 | Butin | 272.27 | 2.3 | 69.94 | 0.21 | |||
| MOL002961 | (-)-Vestitol | 272.32 | 3.15 | 70.29 | 0.21 | |||
| MOL002981 | Duartin | 332.38 | 3.11 | 70.63 | 0.34 | |||
| MOL003003 | Xenognosin B | 284.28 | 2.32 | 72.71 | 0.24 | |||
| MOL002985 | Isoduartin | 332.38 | 3.11 | 74.11 | 0.34 | |||
| MOL002966 | Dalbergin | 268.28 | 3.1 | 78.18 | 0.2 | |||
| MOL003002 | Violanone | 316.33 | 2.42 | 80.24 | 0.3 | |||
| MOL002941 | (3R)-3-(2,3-dihydroxy-4-methoxyphenyl) chroman-7,8-diol | 304.32 | 2.61 | 82.35 | 0.27 | |||
| MOL002939 | (3R)-5′-Methoxyvestitol | 302.35 | 3.13 | 83.06 | 0.26 | |||
| MOL002999 | Sativanone | 300.33 | 2.68 | 85.63 | 0.27 | |||
| MOL002997 | 3-(2-hydroxy-3,4-dimethoxyphenyl)-2H-chromen-7-ol | 300.33 | 2.95 | 86.18 | 0.27 | |||
| Santalaceae | Santalum album L. | 0.008 (3.1) | ||||||
| MOL000354 | Isorhamnetin | 316.28 | 1.76 | 49.6 | 0.31 | |||
| MOL000006 | luteolin | 286.25 | 2.07 | 36.16 | 0.25 | |||
| MOL002322 | Isovitexin | 432.41 | −0.06 | 31.29 | 0.72 | |||
| Olibanum | Burseraceae | 0.008 (3.1) | ||||||
| MOL001215 | Tirucallol | 426.8 | 8.12 | 42.12 | 0.75 | |||
| MOL001241 | O-acetyl-α-boswellic acid | 498.82 | 6.8 | 42.73 | 0.7 | |||
| MOL001243 | 3alpha-Hydroxy-olean-12-en- 24-oic-acid | 456.78 | 6.42 | 39.32 | 0.75 | |||
| MOL001255 | Boswellic acid | 456.78 | 6.47 | 39.55 | 0.75 | |||
| MOL001263 | 3-oxo-tirucallic,acid | 454.76 | 6.99 | 42.86 | 0.81 | |||
| MOL001265 | Acetyl-alpha-boswellic,acid | 498.82 | 6.8 | 42.73 | 0.7 | |||
| MOL001272 | Incensole | 306.54 | 4.97 | 45.59 | 0.22 | |||
| MOL001295 | Phyllocladene | 272.52 | 5.63 | 33.4 | 0.27 | |||
| Borneolum | Blumea balsamifera DC. | 0.006 (2.3) | ||||||
| MOL006862 | Bronyl acetate | 447.55 | 4.02 | 59.3 | 0.51 | |||
| MOL006861 | Asiatic acid | 488.78 | 4.3 | 41.38 | 0.71 | |||
| MOL006865 | Dipterocarpol | 442.8 | 6.92 | 41.71 | 0.76 | |||
| Cicadidae | Cicadae Periostracum | 0.031 (11.7) | ||||||
| Hirudo | Haemopidae | 0.049 (18.8) | ||||||
| Scorpio | Buthidae | 0.031 (11.7) | ||||||
| Scolopendra | Scolopendrasubspinipesmutilans L. Koch | 0.006 (2.4) | ||||||
| EupolyphagaSteleophaga | Corydiidae | 0.031 (11.7) |
Ratio is displayed in form of g (%), and one tablet of TXL is 0.26 g.
Abbreviations: AlogP, partition coefficient of concentration of drug in octanol/concentration of drug in aqueous solution; MW, molecular weight.
Identification of disease-related genes
Since the application of TXL is to lower serum lipid level, anti-oxidation and anti-inflammation, which are standard management in CHD [21], the CHD and coronary artery disease were treated as keywords to acquire relevant genes. After the administration of search queries in GeneCards and OMIM databases, a total of 7389 CHD-relevant genes were obtained. Furthermore, intersection between ingredients-targeted and CHD-relevant genes were performed and 138 overlapped genes were obtained eventually. The Venn diagram of overlapped genes were displayed in Supplementary Figure S1.
Network visualization
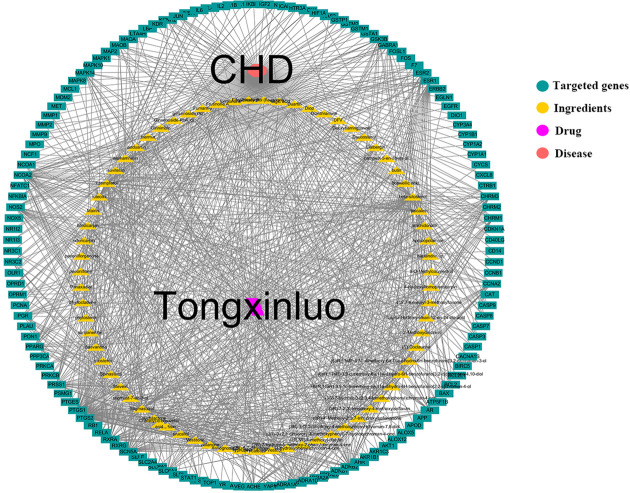
A complete ingredient–target network consisting of a total of 212 nodes and 1016 edges (138 target nodes, 72 putative ingredients nodes, 1 disease node and 1 TXL node) was obtained after administration of Cytoscape software as shown in Figure 2. For detailed information, each node included in this ingredient–target network was documented in Supplementary Table S2.
Figure 2. Ingredient–target network.
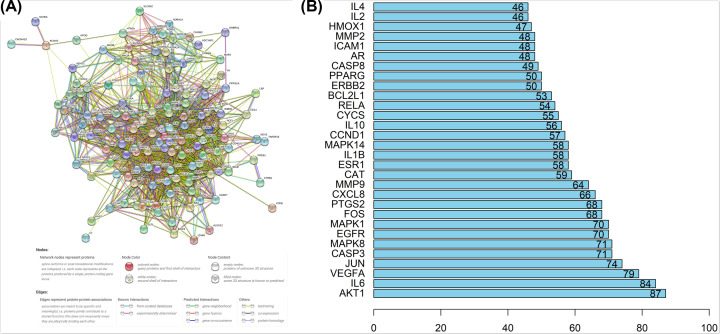
Overlapped genes were processed by STRING to produce a PPI network with confidence > 0.4 and shown in Figure 3A. PPIs were displayed by a total of 138 nodes and 1939 edges with average node degree of 28.1. Within the PPI network, AKT1 showed high degree in coreness of 87-times interaction, followed by IL6 (84 times), VEGFA (79-times), JUN (74-times), CASP3 (71-times), MAPK8 (71-times), respectively. Top 30 proteins with highest interaction time are shown in Figure 3B and the detailed information of PPI is documented in Suplementary Table S3.
Figure 3. Overlapped genes interaction.
(A) PPI network showing interactions between the involved genes. (B) Frequency of targets within PPI network.
GO and KEGG enrichment analyses
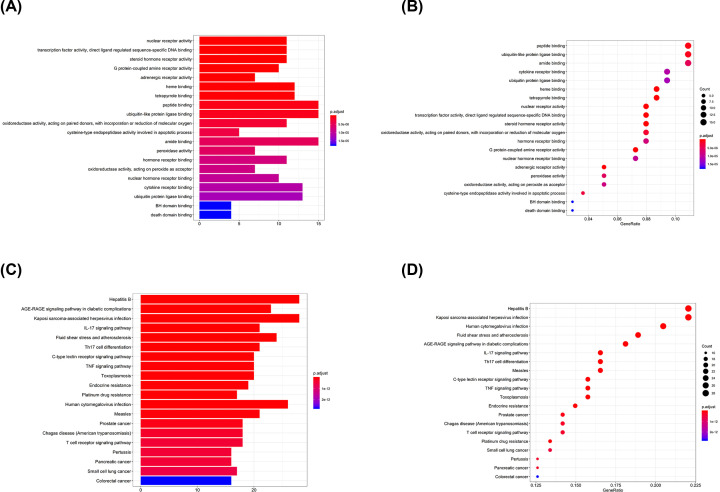
Overlapped genes’ names were converted into symbol ID via UniProt database for GO and KEGG enrichment analyses. Regarding GO enrichment analysis, function of peptide binding and ubiquitin-like protein ligase binding occupied the leading position among all relevant genes with adjusted P-value of 6.35e−7 and 1.00e−6, respectively. Heme binding and tetrapyrrole binding function were at second place of overlapped genes enrichment analysis with adjusted P-value of 3.49e−8 and 6.83e−8, respectively. Top 20 categories of GO enrichment analysis are shown in Figure 4A,B.
Figure 4. GO and KEGG enrichment analyses.
(A) Box plot of GO enrichment. (B) Dot plot of GO enrichment. (C) Box plot of KEGG enrichment. (D) Dot plot of KEGG enrichment.
When it comes to KEGG enrichment analysis, AGE-RAGE signaling pathway, and fluid shear stress and atherosclerosis pathway occupied the predominant position with adjusted P-value of 5.60e−19 and 3.88e−17, respectively. Moreover, inflammation-related pathways, such as IL-17, TNF and T-cell receptors signaling pathways, were principal pathways within the TXL-CHD overlapped genes enrichment, with the adjusted P-value of 3.19e−17, 1.13e−14, 3.73e-13, respectively. Top 20 categories of KEGG enrichment analysis are shown in Figure 4C,D. Furthermore, Pathviews of fluid shear stress and atherosclerosis, IL-6, TNF, toll-like receptor, and T-cell receptor signaling pathways are displayed in Supplementary Figure S2.
Active ingredients protect H9c2 cells from H/R injury
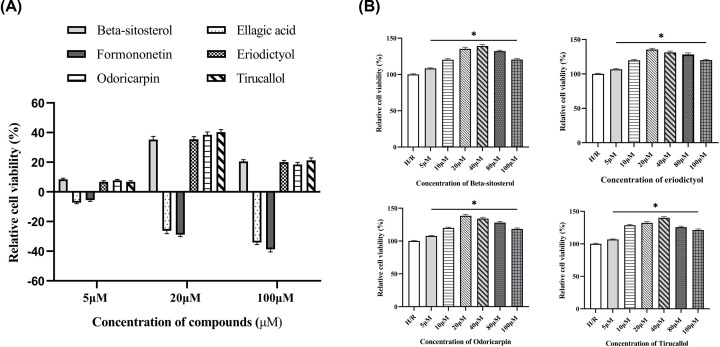
Six potential ingredients, β-sitosterol, ellagic acid, formononetin, eriodictyol, odoricarpin, tirucallol (detailed information shown in Table 2), were obtained and used for validation. Regarding to cell viability tests, β-sitosterol, eriodictyol, odoricarpin and tirucallol revealed positive improvement effect, while ellagic acid and formononetin were found to be cytotoxic to H9c2 cells in H/R model (Figure 5A). Improvement rate at different concentrations was investigated to obtain optimal dosage. From the results, the optimal dosage of β-sitosterol, eriodictyol, odoricarpin, tirucallol were 40, 20, 20 and 40 μM in this model, respectively, and decreased relative cell viability was observed in each test when concentration exceeded 50 μM (Figure 5B).
Table 2. Molecules used in validation.
| Mole ID | Molecule name | MW | OB (%) | DL | Structure |
|---|---|---|---|---|---|
| MOL000358 | β-sitosterol | 414.79 | 36.91 | 0.75 | 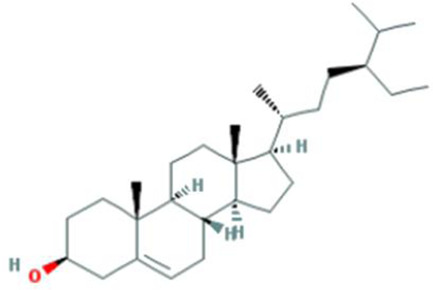 |
| MOL001002 | Ellagic acid | 302.2 | 43.06 | 0.43 | 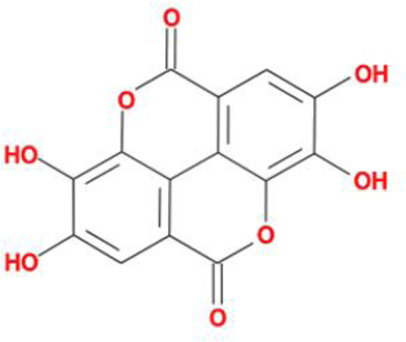 |
| MOL000392 | Formononetin | 268.28 | 69.67 | 0.21 | 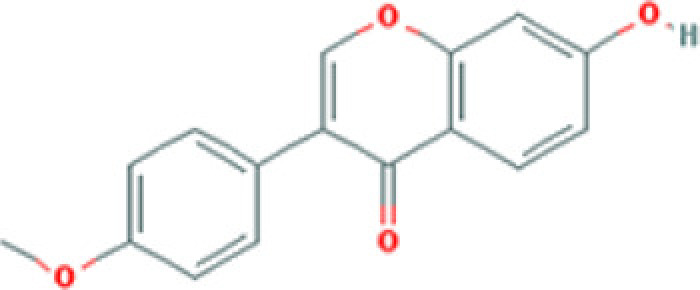 |
| MOL002914 | Eriodictyol | 288.27 | 41.35 | 0.24 | 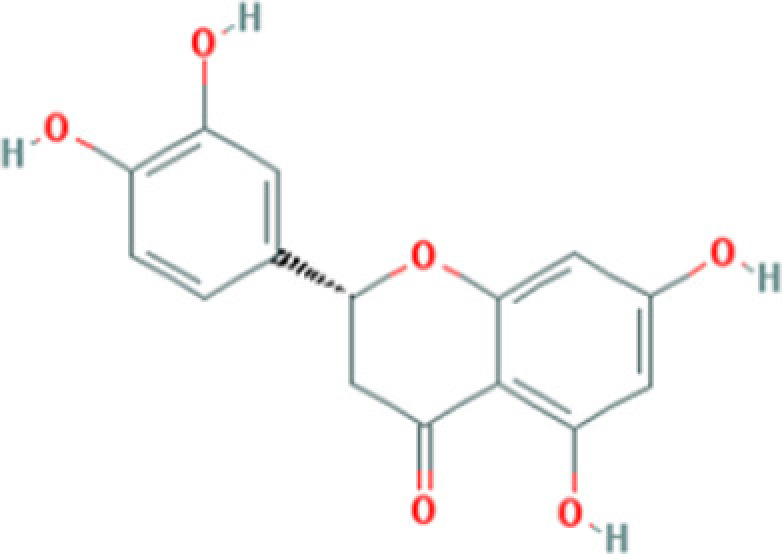 |
| MOL002996 | Odoricarpin | 330.36 | 55.02 | 0.53 | 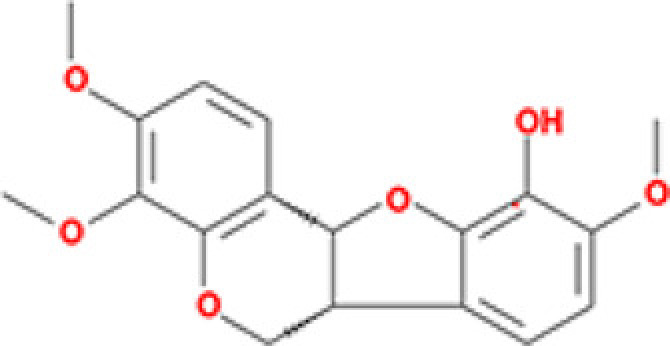 |
| MOL001215 | Tirucallol | 426.8 | 42.12 | 0.75 | 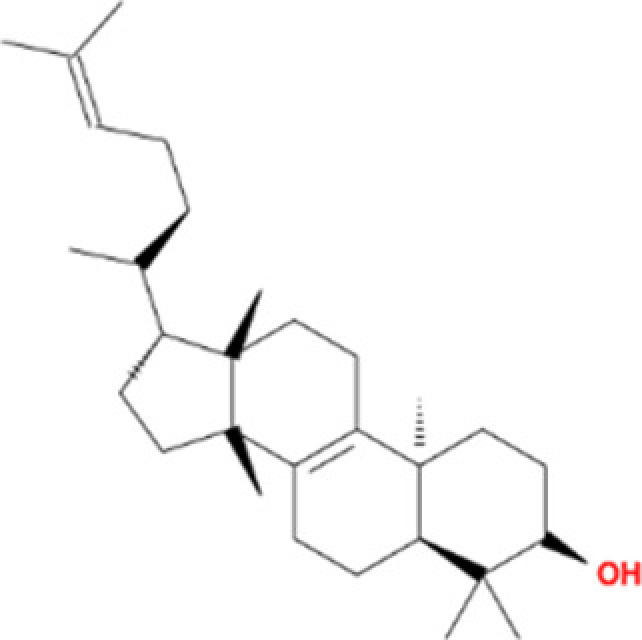 |
Abbreviation: MW, molecular weight.
Figure 5. Cell viability test of active compounds of TXL.
(A) Different concentrations of active compounds on improvement rate (n=5). (B) Exploration of optimal dosage of active compounds on improvement rate (n=5). * indicates existence of significance (P<0.05).
Anti-inflammatory effect of TXL
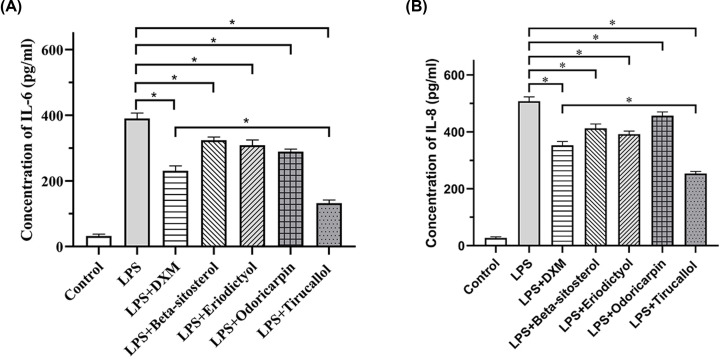
Due to the significance of anti-inflammatory regulation in CHD management, the anti-inflammatory effect of TXL was investigated. Since the enriched pathways in anti-inflammatory regulation (Supplementary Figure S2), concentrations of IL-6 (Figure 6A) and IL-8 (Figure 6B) were investigated with the abovementioned optimal concentration of four compounds. β-sitosterol, eridictyol, odoricarpin and tirucallol indicated significant inhibition on concentration of IL-6 as well as IL-8 (P<0.05). Moreover, tirucallol revealed to have a significant anti-inflammation effect compared with DXM group (P<0.05). Collectively, active compounds of TXL is capable of regulating anti-inflammation.
Figure 6. Anti-inflammatory ability of active compounds.
(A) Effects of active compounds on IL-6 concentration (n=5). (B) Effects of active compounds on IL-8 concentration (n=5). * indicates existence of significance (P<0.05).
Discussion
In previous study, resistance to statin regimen led to rapid progression of atheroma, indicating warranted alternative to lipid-lowering medication [22]. As indicators of plaque progression, IMT and maximal plaque area are favored indicators for CHD assessment. In CAPITAL trial, as the additional anti-atherosclerotic regimen to routine CHD therapy, TXL revealed superiority compared with control group in slowing down the progression of CHD significantly [10]. However, the underlying anti-atherosclerotic effects of TXL were unclear. After this research, substantial evidences might be provided at the molecular level.
Network pharmacology was designed for investigating single-medication targeting on multiple targets so as to enhance efficacy as well as reducing toxicity to patients [23]. Besides, TXL capsule was a mixture of 12 plant and animal products with multiple ingredients and targets, which conformed to the abovementioned perspective and was proved to be effective in cellular level in the present study.
Regarding enrichment analysis, several pathways revealed the potential mechanism of TXL capsule acting on anti-atherosclerotic events. Peptide and ubiquitin-like protein ligase binding occupied the predominant position among GO enrichment analysis, in which rising ubiquitin was reported as positively correlated indicators with the severity of pathologies such as trauma, burn, and especially in CHD and acute myocardial infarction (AMI) patients [24–26]. Also, extracellular ubiquitin was shown to be elevated in CHD patients, especially in patients with acute coronary syndrome (ACS) attack, and it was positively related to Gensini score reflecting the degree of atherosclerosis in CHD [27]. Moreover, ubiquitin was suggested to be positively related to inflammatory markers CRP, CK-MB and cTnl, which were associated with progression of atherosclerosis as well as AMI [28]. To sum up, ubiquitin is an alternative biomarker to predict the severity of CHD. Predominant function of targeted genes on ubiquitin-like protein ligase binding might hint that TXL capsule had the capacity on regulating extracellular ubiquitin level to prevent the progression of atherosclerosis.
Fluid shear stress and atherosclerosis pathway was enriched in KEGG analysis, and it was found to be associated with microvascular and epicardial endothelial dysfunction in CHD patients. Coronary arteries exposed to abnormal microvascular endothelial function exhibited significantly lower shear stress compared with normal coronary arteries [29]. Apart from systemic risk factors, local factors as low shear stress might contribute to promotion of early focal epicardial endothelial dysfunction and potential plaque progression [30,31]. A fall in shear stress might be triggered by microvascular endothelial dysfunction which induced by established systemic risk factors like inflammation and oxidative stress at early stage of disease, further provoking as well as exacerbating inflammatory processes of coronary endothelium. Moreover, inflammation plays an indispensable role in the progression of atherosclerosis [32,33], and inflammation-related pathways such as IL-17, TNF, toll-like receptor, T-cell receptor signaling pathways, were enriched among KEGG analysis. Targeted anti-inflammatory regimen and reduction in CRP have been shown to reduce major adverse cardiovascular events in established CHD patients [34,35]. As discussed above, TXL was also capable of regulating ubiquitin to adjust CRP level, and the active compounds of TXL were validated to be effective in regulating inflammation-related pathway, which further confirmed the theory of anti-inflammatory effects of TXL capsule on CHD patients.
However, several limitations should be considered in the present study. First, retrieved active ingredients might be inconsistent with the exact compounds absorbed by patients. Second, only targeted genes of active ingredients could be found but the exploration of predominantly targeted genes by active compounds is difficult. Third, errors might occur in GO and KEGG enrichment analyses due to the complex formula of TXL capsule and enriched pathway might be confused. Last but not the least, validation is performed at cellular level and the verification in animal model to investigate more indicators is still necessary in future research.
Conclusion
Our study provided substantial molecular evidence that TXL capsule possessed the characteristics of multitargets with safe profile, and its main component is effective in regulating cytokine level as well as improving hypoxia to protect myocardial cells on CHD patients.
Supplementary Material
Abbreviations
- AMI
acute myocardial infarction
- CCK-8
cell counting kit-8
- CHD
coronary heart disease
- CK-MB
creatine kinase-MB
- CRP
C-reactive protein
- cTn1
cardiac troponin 1
- DL
drug-likeness
- DMEM
Dulbecco’s modified Eagle’s medium
- ELISA
enzyme-linked immunosorbent assay
- GO
gene ontology
- H/R
hypoxia/reoxygenation
- IMT
intima-media thickness
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- LPS
lipopolysaccharide
- OB
oral bioavailability
- OMIM
Online Mendelian Inheritance in Man
- PPI
protein–protein interaction
- TCM
Traditional Chinese medicine
- TCMID
TCM integrative database
- TCMSP
TCM systems pharmacology
- TXL
tongxinluo
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
The authors declare that there are no sources of funding to be acknowledged.
Author Contribution
The detailed contributions of each author is listed as follows:
Guode Li: conceptualization, methodology, data analysis, manuscript writing. Qingbo Xu: methodology, data analysis. Kedong Han: investigation. Wenhe Yan: investigation. Chaopei Huang: investigation.
Consent for Publication
All authors gave their consent to publish the present study.
References
- 1.GBD 2013 Mortality and Causes of Death Collaborators (2015) Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 385, 117–171 10.1016/S0140-6736(14)61682-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (2019) Coronary artery disease (CAD). National Center for Chronic Disease Prevention and Health Promotion, Division for Heart Disease and Stroke Prevention, 9 December 2019 https://www.cdc.gov/heartdisease/coronary_ad.htm [Google Scholar]
- 3.Lozano R., Naghavi M., Foreman K., Lim S., Shibuya K., Aboyans V. et al. (2012) Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380, 2095–2128 10.1016/S0140-6736(12)61728-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finegold J.A., Asaria P. and Francis D.P. (2013) Mortality from ischaemic heart disease by country, region, and age: statistics from World Health Organisation and United Nations. Int. J. Cardiol. 168, 934–945 10.1016/j.ijcard.2012.10.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.HPS2-THRIVE Collaborative Group (2013) HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. Eur. Heart J. 34, 1279–1291 10.1093/eurheartj/eht055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li B., Xu X., Wang X., Yu H., Li X., Tao W. et al. (2012) A systems biology approach to understanding the mechanisms of action of chinese herbs for treatment of cardiovascular disease. Int. J. Mol. Sci. 13, 13501–13520 10.3390/ijms131013501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karalliedde L.D. and Kappagoda C.T. (2009) The challenge of traditional Chinese medicines for allopathic practitioners. Am. J. Physiol. Heart Circ. Physiol. 297, H1967–H1969 10.1152/ajpheart.00944.2009 [DOI] [PubMed] [Google Scholar]
- 8.Zhang L., Liu Y., Lu X.T., Wu Y.L., Zhang C., Ji X.P. et al. (2009) Traditional Chinese medication Tongxinluo dose-dependently enhances stability of vulnerable plaques: a comparison with a high-dose simvastatin therapy. Am. J. Physiol. Heart Circ. Physiol. 297, H2004–H2014 10.1152/ajpheart.00208.2009 [DOI] [PubMed] [Google Scholar]
- 9.Mao C., Chung V.C., Yuan J.Q., Yu Y.Y., Yang Z.Y., Wu X.Y. et al. (2013) Evaluation of the add-on effect of chinese patent medicine for patients with stable or unstable angina: a systematic review and meta-analysis. Evid. Based Complement. Alternat. Med. 2013, 673193 10.1155/2013/673193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang M., Liu Y., Xu M., Zhang L., Liu Y., Liu X. et al. (2019) Carotid artery plaque intervention with Tongxinluo capsule (CAPITAL): A multicenter randomized double-blind parallel-group placebo-controlled study. Sci. Rep. 9, 4545 10.1038/s41598-019-41118-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao F., Guochun L., Yang Y., Shi L., Xu L. and Yin L. (2015) A network pharmacology approach to determine active ingredients and rationality of herb combinations of Modified-Simiaowan for treatment of gout. J. Ethnopharmacol. 168, 1–16 10.1016/j.jep.2015.03.035 [DOI] [PubMed] [Google Scholar]
- 12.Fang H.Y., Zeng H.W., Lin L.M., Chen X., Shen X.N., Fu P. et al. (2017) A network-based method for mechanistic investigation of Shexiang Baoxin Pill’s treatment of cardiovascular diseases. Sci. Rep. 7, 43632 10.1038/srep43632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ru J., Li P., Wang J., Zhou W., Li B., Huang C. et al. (2014) TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J. Cheminformatics 6, 13 10.1186/1758-2946-6-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang L., Xie D., Yu Y., Liu H., Shi Y., Shi T. et al. (2017) TCMID 2.0: a comprehensive resource for TCM. Nucleic Acids Res. 46, 10.1093/nar/gkx1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiu P.Y., Luk K.F., Leung H.Y., Ng K.M. and Ko K.M. (2008) Schisandrin B stereoisomers protect against hypoxia/reoxygenation-induced apoptosis and inhibit associated changes in Ca2+-induced mitochondrial permeability transition and mitochondrial membrane potential in H9c2 cardiomyocytes. Life Sci. 82, 1092–1101 10.1016/j.lfs.2008.03.006 [DOI] [PubMed] [Google Scholar]
- 16.Cui S., Jiang H., Chen L., Xu J., Sun W., Sun H. et al. (2020) Design, synthesis and evaluation of wound healing activity for β-sitosterols derivatives as potent Na(+)/K(+)-ATPase inhibitors. Bioorg. Chem. 98, 103150 10.1016/j.bioorg.2019.103150 [DOI] [PubMed] [Google Scholar]
- 17.Yousuf M., Shamsi A., Khan P., Shahbaaz M., AlAjmi M.F., Hussain A. et al. (2020) Ellagic acid controls cell proliferation and induces apoptosis in breast cancer cells via inhibition of cyclin-dependent kinase 6. Int. J. Mol. Sci. 21, 10.3390/ijms21103526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li T., Zhong Y., Tang T., Luo J., Cui H., Fan R. et al. (2018) Formononetin induces vasorelaxation in rat thoracic aorta via regulation of the PI3K/PTEN/Akt signaling pathway. Drug Des. Dev. Ther. 12, 3675–3684 10.2147/DDDT.S180837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seo C.S., Lim H.S., Ha H., Jin S.E. and Shin H.K. (2015) Quantitative analysis and anti-inflammatory effects of Gleditsia sinensis thorns in RAW 264.7 macrophages and HaCaT keratinocytes. Mol. Med. Rep. 12, 4773–4781 10.3892/mmr.2015.3936 [DOI] [PubMed] [Google Scholar]
- 20.Yiling W. (1997) Tongxinluo medicinal capsule for curing coronary heart disease and angina pectoris and preparation method thereof, CN1198332A https://patents.google.com/patent/CN1198332A/en [Google Scholar]
- 21.Miyauchi K. and Daida H. (2010) Clinical significance of intensive lipid-lowering therapy using statins in patients with coronary artery disease: LDL-cholesterol: the lower, the better; is it true for Asians? (Pro) Circ. J. 74, 1718–1730 10.1253/circj.CJ-10-0565 [DOI] [PubMed] [Google Scholar]
- 22.Kataoka Y., St John J., Wolski K., Uno K., Puri R., Tuzcu E.M. et al. (2015) Atheroma progression in hyporesponders to statin therapy. Arterioscler. Thromb. Vasc. Biol. 35, 990–995 10.1161/ATVBAHA.114.304477 [DOI] [PubMed] [Google Scholar]
- 23.Hopkins A.L. (2008) Network pharmacology: the next paradigm in drug discovery. Nat. Chem. Biol. 4, 682–690 10.1038/nchembio.118 [DOI] [PubMed] [Google Scholar]
- 24.Baker T.A., Davis C.S., Bach H.H.T., Romero J., Burnham E.L., Kovacs E.J. et al. (2012) Ubiquitin and stromal cell-derived factor-1alpha in bronchoalveolar lavage fluid after burn and inhalation injury. J. Burn Care Res. 33, 57–64 10.1097/BCR.0b013e31823dc559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poillucci G., Podda M., Pisanu A., Mortola L., Dalla Caneva P., Massa G. et al. (2019) Risk factors for postoperative morbidity following appendectomy in the elderly: a nationwide prospective cohort study. Eur. J. Trauma Emerg. Surg. 10.1007/s00068-019-01186-2 [DOI] [PubMed] [Google Scholar]
- 26.Chen S.M., Zhang H.X., Li Y.G., Wang D.M., Zhang G.H. and Tan C.J. (2008) Expression of ubiquitin in peripheral inflammatory cells from patients with coronary artery disease. J. Int. Med. Res. 36, 1227–1234 10.1177/147323000803600609 [DOI] [PubMed] [Google Scholar]
- 27.Ji Y., Yao J., Zhao Y., Zhai J., Weng Z. and He Y. (2020) Extracellular ubiquitin levels are increased in coronary heart disease and associated with the severity of the disease. Scand. J. Clin. Lab. Invest. 1–9 10.1080/00365513.2020.1728783 [DOI] [PubMed] [Google Scholar]
- 28.Esser N., Paquot N. and Scheen A.J. (2015) Inflammatory markers and cardiometabolic diseases. Acta Clin. Belg. 70, 193–199 10.1179/2295333715Y.0000000004 [DOI] [PubMed] [Google Scholar]
- 29.Siasos G., Sara J.D., Zaromytidou M., Park K.H., Coskun A.U., Lerman L.O. et al. (2018) Local low shear stress and endothelial dysfunction in patients with nonobstructive coronary atherosclerosis. J. Am. Coll. Cardiol. 71, 2092–2102 10.1016/j.jacc.2018.02.073 [DOI] [PubMed] [Google Scholar]
- 30.Koskinas K.C., Feldman C.L., Chatzizisis Y.S., Coskun A.U., Jonas M., Maynard C. et al. (2010) Natural history of experimental coronary atherosclerosis and vascular remodeling in relation to endothelial shear stress: a serial, in vivo intravascular ultrasound study. Circulation 121, 2092–2101 10.1161/CIRCULATIONAHA.109.901678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koskinas K.C., Sukhova G.K., Baker A.B., Papafaklis M.I., Chatzizisis Y.S., Coskun A.U. et al. (2013) Thin-capped atheromata with reduced collagen content in pigs develop in coronary arterial regions exposed to persistently low endothelial shear stress. Arterioscler. Thromb. Vasc. Biol. 33, 1494–1504 10.1161/ATVBAHA.112.300827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang R., Brennan M.L., Fu X., Aviles R.J., Pearce G.L., Penn M.S. et al. (2001) Association between myeloperoxidase levels and risk of coronary artery disease. JAMA 286, 2136–2142 10.1001/jama.286.17.2136 [DOI] [PubMed] [Google Scholar]
- 33.Moriwaki K., Takeuchi T., Fujimoto N., Sawai T., Sato Y., Kumagai N. et al. (2018) Effect of Sitagliptin on coronary flow reserve assessed by magnetic resonance imaging in type 2 diabetic patients with coronary artery disease. Circ. J. 82, 2119–2127 10.1253/circj.CJ-18-0083 [DOI] [PubMed] [Google Scholar]
- 34.Ridker P.M., Danielson E., Fonseca F.A., Genest J., Gotto A.M. Jr, Kastelein J.J. et al. (2008) Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N. Engl. J. Med. 359, 2195–2207 10.1056/NEJMoa0807646 [DOI] [PubMed] [Google Scholar]
- 35.Ridker P.M., Everett B.M., Thuren T., MacFadyen J.G., Chang W.H., Ballantyne C. et al. (2017) Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 377, 1119–1131 10.1056/NEJMoa1707914 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.