Abstract
Type 2 endoleaks are the most common endoleak type following endovascular aneurysm repair. The natural history of these endoleaks can vary, with some demonstrating a self-limited or indolent course, while others can contribute to aneurysm sac enlargement and rupture. A variety of embolization techniques, including transarterial catheterization and direct sac puncture techniques, have been developed for the treatment of type 2 endoleaks. In this article, the authors review the indications, techniques, and outcomes of current treatment strategies for type 2 endoleaks.
Keywords: interventional radiology, endoleak, aortic aneurysm, embolization, stent graft
Type 2 endoleak after endovascular aneurysm repair (EVAR) of abdominal aortic aneurysms (AAA) remains the most prevalent endoleak type, occurring in 20 to 40% of cases 1 and comprising over half of all endoleaks. These endoleaks develop from retrograde collateral flow into the aneurysm sac, most commonly via the inferior mesenteric artery (IMA) and lumbar arteries, with other less common sources being the median sacral artery or accessory renal arteries. Risk factors for the development of type 2 endoleak include older age, patent IMA, larger number of patent lumbar arteries, and larger aneurysm sac diameter. 2
In contrast to type 1 and type 3 endoleaks, for which intervention is well agreed upon, the management of type 2 endoleaks remains controversial. The prevailing motivation for the treatment of type 2 endoleaks is based on concern that retrograde arterial flow into the aneurysm sac can increase sac pressure, lead to sac enlargement, and increase risk of aneurysm rupture. 3 Aneurysm rupture occurs in approximately 1% of post-EVAR aneurysms with type 2 endoleak. 4 However, studies have shown that more than half of type 2 endoleaks can spontaneously resolve, 5 the presence of a type 2 endoleak does not significantly increase aneurysm rupture rate, 6 and postembolization aneurysm rupture rate is similar to overall EVAR population. 7 Therefore, there continues to be disagreement on optimal management for type 2 endoleaks, with some providers advocating early intervention, while others support conservative observation.
There has been recognition that type 2 endoleaks have subtypes with varying pathophysiology that may explain differences in outcomes between historical studies. The timing of endoleak onset has been associated with significant differences in aneurysm sac behavior. Early type 2 endoleaks, defined as onset < 1 year post-EVAR, spontaneously self-resolve in 75% cases, whereas late type 2 endoleaks, defined as onset > 1 year post-EVAR, self-resolve in only 29% cases. 8 In a retrospective series by Pineda et al, 8% of early endoleaks required embolization, while 55% of late endoleaks required embolization, 8 suggesting more aggressive physiology, and need for more vigilant monitoring of the late endoleak group. Persistent type 2 endoleaks, defined as endoleaks lasting > 6 months, have been associated with older patient age, larger diameter of IMA and lumbar arteries, coil embolization of the internal iliac arteries, and distal graft extension. 1 The anatomy of the endoleak is another potential variable contributing to differences in natural history. Complex type 2 endoleaks with multiple communicating vessels, presence of a nidus, and larger diameter feeder/draining vessels have been associated with higher risk of aneurysm sac enlargement. 9 Endoleaks seen only on the venous phase of computed tomography angiography (CTA) imaging have also been associated with lower rates of technically successful embolization, and higher rate of repeat embolizations. 10 Patient factors including low platelets in the perioperative period have also been associated with greater risk of type 2 endoleak–associated aneurysm sac enlargement. 11
Considering these evolving concepts in type 2 endoleak physiology, and wide variations in provider practice patterns, it is unsurprising that there remains no definitive consensus on optimal management. The most recent Society of Vascular Surgery guidelines recommend treatment of type 2 endoleaks for aneurysm sac expansion ≥5 mm, 12 while European Society of Vascular Surgery guidelines recommend treatment for aneurysm sac expansion ≥1 cm, 1 both based on level C evidence. Other proposed criteria for intervention include endoleak persistence >6 months, 3 the presence of large nidus, 13 14 15 more than three feeding or draining arteries, 13 14 15 feeding artery diameter >4 mm, 13 14 and high-flow velocities in aneurysm sac. 16
Embolization Strategies
A variety of endovascular and percutaneous embolization strategies have been developed for the treatment of type 2 endoleaks. The most established approaches can be broadly classified into transarterial and direct sac puncture techniques. 17 18 19 Newer, alternative approaches including transgraft 20 and perigraft 21 endoleak catheterizations have also been described. Determination of optimal approach is based on analysis of endoleak and feeder branch anatomy as well as available imaging equipment and provider experience. For patients who need endoleak intervention based on post-EVAR imaging surveillance with Doppler ultrasound only, it is recommended to obtain CTA to delineate endoleak and arterial branch anatomy prior to any planned intervention.
Transarterial Approach
Transarterial embolization is performed via retrograde catheterization of endoleak inflow vessels using coaxial microcatheter systems ( Fig. 1a–e ). The IMA can be catheterized from the superior mesenteric artery via the arc of Riolan or other middle colic and marginal artery branches ( Fig. 1c ). Lumbar arteries can be catheterized from the internal iliac artery through iliolumbar branches. Once the catheter reaches the endoleak, embolization of the sac and associated feeder vessels can be performed with coils 17 ( Fig. 1d ), and/or liquid embolic agents like n-butyl cyanoacrylate (nBCA) and ethylene vinyl alcohol copolymer (Onyx). 22 Regardless of embolic agent, it is important to embolize feeders close to their connection to the aneurysm sac to reduce risk of recanalization and avoid risk of nontarget ischemia to sensitive structures like the colon.
Fig. 1.
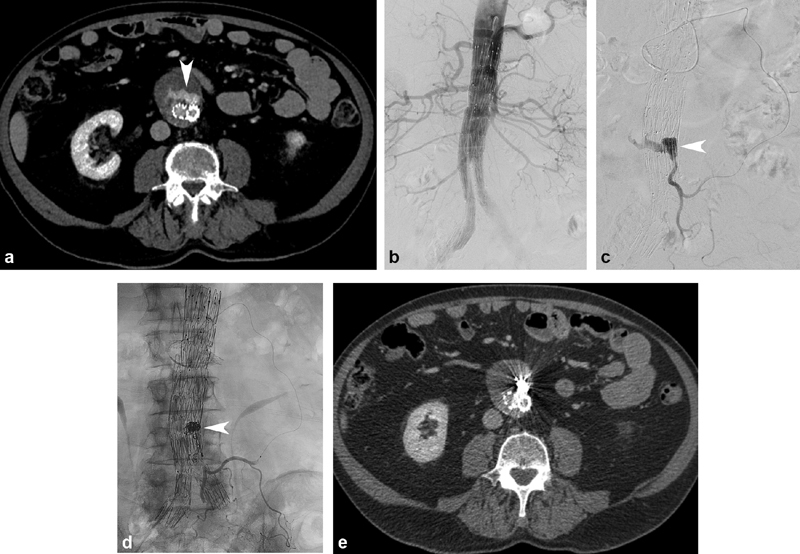
Type 2 endoleak embolization using transarterial approach. ( a ) CT angiography (CTA) demonstrates an anteriorly positioned endoleak (arrowhead). ( b ) Abdominal aortography demonstrated no concurrent type 1 or type 3 endoleak. ( c ) Retrograde catheterization of the inferior mesenteric artery (IMA) was performed via the superior mesenteric artery and arc of Riolan. Arteriography confirmed opacification of the endoleak cavity (arrowhead). ( d ) Following coil embolization (arrowhead), IMA arteriography demonstrated no further endoleak opacification, with preservation of flow to proximal IMA branches. ( e ) One month postembolization CTA demonstrated no further endoleak opacification, and stable aneurysm sac size.
Advantages of transarterial approach include familiar patient and operator positioning to other transarterial interventions, and the ability to perform simultaneous aortography to exclude the presence of combined type 1 or 3 endoleaks ( Fig. 1b ). However, catheterization of the feeder arterial branches can be extremely challenging or impossible in patients with internal iliac or IMA occlusion, tortuous or diminutive feeder vessel anatomy, or prior transarterial embolization. The high degree of technical difficulty of transarterial embolization resulting from these anatomic factors is evidenced in lower technical success rates compared with translumbar technique. 23 Alternate approaches should be pursued if CTA demonstrates any of these findings, or the patient has undergone failed transarterial embolization attempt.
Direct Sac Puncture Approaches
Percutaneous Left Translumbar
Of the direct sac puncture techniques, percutaneous translumbar approach ( Fig. 2a–e ) is the most established. Patients are positioned prone and a percutaneous left translumbar access is planned using anatomic landmarks based on CTA ( Fig. 2b ). An access needle is advanced under fluoroscopic guidance through the aneurysm sac and importantly into the endoleak cavity. Desired needle tip position within the endoleak is confirmed with blood return and arteriography. Newer imaging technologies including cone beam CT 24 ( Fig. 2c ) and image fusion software 25 can be effective adjuncts to fluoroscopy for needle trajectory planning and confirmation of needle tip position. Access to the endoleak cavity can be obtained with a GrebSet (Vascular Solutions, Minnesota) or other similar access set, a 21G needle is positioned within the endoleak sac, and exchange is made for a coaxial access catheter to perform arteriography and embolization ( Fig. 2d ). An alternate access set, the Introducer Sheath/Needle (Argon Medical Devices, Texas), has a needle stylet with metal stiffener inside a 5-Fr catheter, which allows arteriography and embolization directly through the access catheter, obviating the need for an exchange.
Fig. 2.
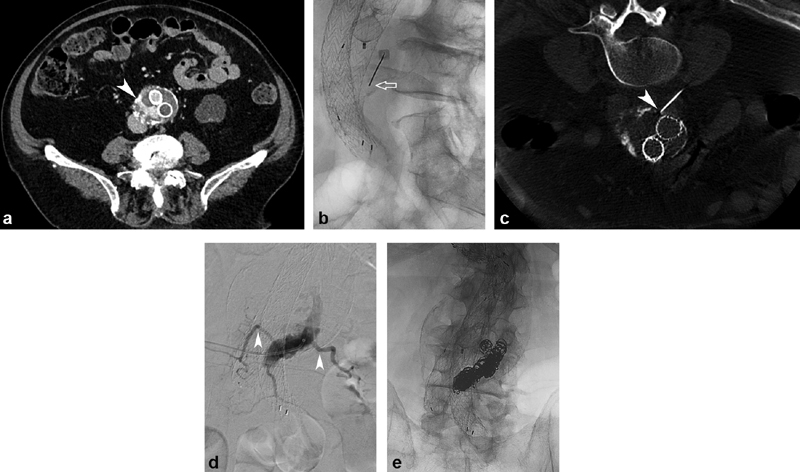
Type 2 endoleak embolized using translumbar approach. ( a ) CTA demonstrates endoleak (arrowhead) along the right posterior aspect of the aneurysm sac. ( b ) Using fluoroscopic landmarks, a 21G 20-cm needle (arrow) was advanced via a left translumbar approach. ( c ) Intraprocedural cone beam CT was used to confirm needle trajectory and tip position (arrowhead). ( d ) A GrebSet was placed, and arteriography confirmed good position within the endoleak cavity, with opacification of feeding lumbar arteries (arrowheads). ( e ) Fluoroscopic image demonstrates coil pack position following embolization.
Percutaneous Right Transcaval
If the endoleak is positioned along the right side of the aneurysm sac, a right sided transcaval approach can be performed. Traversal of the inferior vena cava (IVC) with the access needle/sheath is a safe approach and has not been associated with increased risk of hemorrhage, but coil prolapse into the IVC has been reported. 26
Percutaneous Transabdominal
In patients with suitable habitus, an anterior percutaneous transabdominal approach can be a feasible alternative to translumbar approach. This technique can facilitate catheterization of lumbar artery feeders, which originate from the posterior wall of the aorta, and are more in line with a transabdominal access. Some patients also have respiratory difficulty when prone and can better tolerate supine position.
Traversal of bowel loops during transabdominal access has potential to cause significant morbidity; so, careful patient selection and technique is crucial. Zener et al described the use of ultrasound-guided needle access for transabdominal endoleak access using an 18G needle, applying firm probe pressure to displace adjacent bowel loops along the access tract. 18 In their series of 30 patients, technical success was achieved in 97% cases, and freedom from aneurysm growth was achieved in 85%. 18 The complication rate was 9% including a case of nontarget nBCA embolization resulting in self-limited neuropraxia. 18
Embolization Technique
Once endoleak sac access is established using the aforementioned direct sac puncture techniques, embolization can be performed using a variety of approaches. The majority of operators exchange the access needle for a sheath or stiffened access set to allow use of a coaxial catheter and subsequent embolization with 0.035-inch coils and/or glue ( Fig. 2e ). If access is made with the earlier-mentioned Argon Introducer 5-Fr sheath/needle, embolization can be performed with 0.035-inch coils or glue through the 5-Fr catheter. To avoid the need for an exchange step, some groups have also directly advanced microcatheters through an 18G needle with valve adapter. 18
Selective catheterization and embolization of branch vessels is typically feasible in only a minority of cases, and studies have shown that the addition of branch vessel embolization does not produce superior clinical outcomes. In a series of 29 patients, Yu et al found that branch and nidus embolization resulted in significantly longer procedure time and greater radiation dose, but yielded no significant improvement in rates of aneurysm sac or residual endoleak as compared with nidus embolization alone. 27 This concurred with previous findings from Stavropoulos et al showing no significant difference in clinical outcomes between transarterial (nidus and branch vessel) and translumbar (nidus alone) embolizations. 17
Various nidus embolization techniques have been described, using combination of coils and liquid embolics or liquid embolics alone. Our group first deploys coils into the endoleak to reduce flow, and then follows with nBCA to completely occlude the nidus. 17 It is not necessary to reflux nBCA into the feeder branches, which can increase the risk of nontarget embolization. Alternative embolization agents to nBCA include thrombin 19 and Onyx, 18 28 all of which have varied administration characteristics. Relative to nBCA, Onyx allows increased control of the Onyx cast, but technical and cost considerations limit its broader use. Direct thrombin injection is not used by our group, as the lack of control of the thrombin after injection into the sac places the patient at theoretically higher risk of nontarget embolization compared with nBCA or Onyx.
Some groups have developed embolization approaches using only liquid embolic, which can obviate the need for an access sheath or catheter. Lagios et al described this approach in a series of 25 patients, using direct nBCA infusion through a 22G Chiba needle. 29 Cone beam CT was used to confirm needle position, and varying nBCA dilution was used, based on endoleak flow characteristics and estimated endoleak volume. Technical success was achieved in 22 of 25 (88%) patients, noting 3 of 25 (12%) patients had incomplete embolization on postoperative day 1 ultrasound, requiring repeat embolization. Painful nontarget glue embolization into the psoas occurred in 2 of 25 (8%) cases.
Other Embolization Approaches
Transvenous Transcaval
Transvenous transcaval embolization is a technique that can be especially useful in cases where percutaneous approach is not feasible and the endoleak is predominantly right sided. This approach requires detailed assessment of the preprocedural CTA, to determine the optimal endoleak access site from the IVC. In a series of 10 patients, Burley et al performed transcaval access with Rosch-Uchida sets, and embolized using coils, and, if needed, gelatin granules and thrombin slurry. 19 Nine of 10 (90%) patients had decreased aneurysm sac diameters on follow-up imaging at 6 months. Hyatt et al described the use of intravascular ultrasound (IVUS)-guided approach for Rosch-Uchida needle endoleak access from femoral approach. 28 The authors performed Onyx embolization through a coaxial microcatheter system and monitored embolization on IVUS, using cessation of endoleak Doppler flow as the embolization endpoint.
Transarterial Perigraft
The endoleak sac can also be accessed using a transarterial perigraft approach. In this technique, a catheter is wedged between the arterial wall and distal edge of the iliac stent graft, and retrograde catheterization of the potential space between the vessel lumen and stent graft is performed with a hydrophilic wire. Once the catheter and wire reach the endoleak sac, arteriography and embolization can be performed in similar fashion to other embolization approaches. Chivot et al described a modification on perigraft technique, using a balloon occlusion catheter at the entrance of the aneurysm sac to prevent reflux during Onyx embolization. 21 Perigraft technique has similar benefits to conventional transarterial approach including ability to perform aortography to evaluate for combined type 1 or 3 endoleak. A major disadvantage of perigraft approach is the risk of causing a type 1B endoleak from the access approach, requiring stent graft extension for treatment.
Transarterial Transgraft
Another new approach to endoleak access is transarterial transgraft endoleak embolization, in which the endograft is purposely punctured to attain endoleak access. 20 Nakai et al described this approach in a patient with continued aneurysm sac enlargement after translumbar embolization of a type 2 endoleak—nBCA embolization was followed by graft reinforcement/relining, and resulted in resolution of endoleak, with aneurysm sac stability at 6-month follow-up. 30
Imaging Follow-up
CTA and Doppler ultrasound are the most commonly used imaging modalities for follow-up after embolization of type 2 endoleaks. Even in the presence of streak artifact from metallic embolic material, CT affords visualization of residual endoleaks ( Fig. 1e ) and accurate measurement of AAA sac size. Doppler ultrasound can be limited by patient's body habitus and operator experience. Follow-up intervals at our institution are at 1 month, 6 months, and yearly after embolization.
Embolization Outcomes
Outcomes with modern embolization techniques have generally yielded good technical and clinical outcomes. A recent meta-analysis of 1,073 patients with type 2 endoleak treated predominantly with transarterial or translumbar embolization revealed an overall technical success rate of 88%, clinical success rate of 68%, and perioperative complications in 3.8% cases. 7
It is important to be aware that there are wide variations in patient selection, procedure technique, and follow-up among available studies, which confound direct comparison between embolization approaches. The largest bodies of outcomes data are for transarterial and translumbar embolization, the two most established techniques. A recent meta-analysis of 354 patients reported a significantly higher technical success rate with translumbar embolization (odds ratio: 13.3, p = 0.0002), but saw no significant difference in clinical success between the two groups. 23 The lack of difference in clinical success rate was concordant with some prior series, 17 while discordant with a prior meta-analysis. 4 Notably, comparison of these two techniques is inherently confounded by the fact that translumbar embolization is often pursued only after transarterial embolization has failed.
Outcomes of more recently developed techniques such as transvenous transcaval and perigraft embolization are more difficult to compare, given limited evidence in the form of small series and case reports.
Surgical Treatment
Given the variety of percutaneous and endovascular techniques for embolization of type 2 endoleaks, surgical treatment is generally limited to the minority of cases that have failed embolization. Laparoscopic clipping of the IMA 31 and combined endovascular/laparoscopic procedures 32 33 have been described as less invasive approaches than open side branch ligation.
Conclusion
The management of type 2 endoleaks remains controversial, owing to complexities in natural history and unclear association with aneurysm sac growth and rupture. Current guidelines suggest embolization of type 2 endoleaks for aneurysm sac enlargement ≥5 to 10 mm. A variety of embolization techniques have been developed, the most established being transarterial and translumbar approaches. Newer strategies such as transvenous transcaval, perigraft, and transgraft embolization have demonstrated efficacy in small series and can be considered in select cases. The optimal role of type 2 endoleak intervention continues to evolve with our growing understanding of type 2 endoleak pathophysiology.
Footnotes
Conflict of Interest None declared.
References
- 1.ESVS Guidelines Committee . Wanhainen A, Verzini F, Van Herzeele I. Editor's choice - European Society for Vascular Surgery (ESVS) 2019 Clinical Practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur J Vasc Endovasc Surg. 2019;57(01):8–93. doi: 10.1016/j.ejvs.2018.09.020. [DOI] [PubMed] [Google Scholar]
- 2.Guo Q, Du X, Zhao J. Prevalence and risk factors of type II endoleaks after endovascular aneurysm repair: A meta-analysis. PLoS One. 2017;12(02):e0170600. doi: 10.1371/journal.pone.0170600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones J E, Atkins M D, Brewster D C. Persistent type 2 endoleak after endovascular repair of abdominal aortic aneurysm is associated with adverse late outcomes. J Vasc Surg. 2007;46(01):1–8. doi: 10.1016/j.jvs.2007.02.073. [DOI] [PubMed] [Google Scholar]
- 4.Sidloff D A, Stather P W, Choke E, Bown M J, Sayers R D. Type II endoleak after endovascular aneurysm repair. Br J Surg. 2013;100(10):1262–1270. doi: 10.1002/bjs.9181. [DOI] [PubMed] [Google Scholar]
- 5.Gelfand D V, White G H, Wilson S E. Clinical significance of type II endoleak after endovascular repair of abdominal aortic aneurysm. Ann Vasc Surg. 2006;20(01):69–74. doi: 10.1007/s10016-005-9382-z. [DOI] [PubMed] [Google Scholar]
- 6.van Marrewijk C, Buth J, Harris P L, Norgren L, Nevelsteen A, Wyatt M G. Significance of endoleaks after endovascular repair of abdominal aortic aneurysms: the EUROSTAR experience. J Vasc Surg. 2002;35(03):461–473. doi: 10.1067/mva.2002.118823. [DOI] [PubMed] [Google Scholar]
- 7.Ultee K HJ, Büttner S, Huurman R. Editor's choice - systematic review and meta-analysis of the outcome of treatment for Type II endoleak following endovascular aneurysm repair. Eur J Vasc Endovasc Surg. 2018;56(06):794–807. doi: 10.1016/j.ejvs.2018.06.009. [DOI] [PubMed] [Google Scholar]
- 8.Pineda D M, Calligaro K D, Tyagi S, Troutman D A, Dougherty M J. Late type II endoleaks after endovascular aneurysm repair require intervention more frequently than early type II endoleaks. J Vasc Surg. 2018;67(02):449–452. doi: 10.1016/j.jvs.2017.05.124. [DOI] [PubMed] [Google Scholar]
- 9.Müller-Wille R, Borgmann T, Wohlgemuth W A. Dual-energy computed tomography after endovascular aortic aneurysm repair: the role of hard plaque imaging for endoleak detection. Eur Radiol. 2014;24(10):2449–2457. doi: 10.1007/s00330-014-3266-y. [DOI] [PubMed] [Google Scholar]
- 10.McDonald M D, Paik H H, Fairman R. Outcomes of type 2 endoleaks detected on venous phase CT arteriography. Diagn Interv Imaging. 2018;99(04):225–229. doi: 10.1016/j.diii.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Inoue K, Furuyama T, Kurose S. Platelets reflect the fate of type II endoleak after endovascular aneurysm repair. J Vasc Surg. 2020;72(02):541–5480. doi: 10.1016/j.jvs.2019.09.062. [DOI] [PubMed] [Google Scholar]
- 12.Chaikof E L, Dalman R L, Eskandari M K. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67(01):2–7700. doi: 10.1016/j.jvs.2017.10.044. [DOI] [PubMed] [Google Scholar]
- 13.Keedy A W, Yeh B M, Kohr J R, Hiramoto J S, Schneider D B, Breiman R S. Evaluation of potential outcome predictors in type II Endoleak: a retrospective study with CT angiography feature analysis. AJR Am J Roentgenol. 2011;197(01):234–240. doi: 10.2214/AJR.10.4566. [DOI] [PubMed] [Google Scholar]
- 14.Parent F N, Meier G H, Godziachvili V. The incidence and natural history of type I and II endoleak: a 5-year follow-up assessment with color duplex ultrasound scan. J Vasc Surg. 2002;35(03):474–481. doi: 10.1067/mva.2002.121848. [DOI] [PubMed] [Google Scholar]
- 15.Bargellini I, Napoli V, Petruzzi P. Type II lumbar endoleaks: hemodynamic differentiation by contrast-enhanced ultrasound scanning and influence on aneurysm enlargement after endovascular aneurysm repair. J Vasc Surg. 2005;41(01):10–18. doi: 10.1016/j.jvs.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 16.Arko F R, Filis K A, Siedel S A. Intrasac flow velocities predict sealing of type II endoleaks after endovascular abdominal aortic aneurysm repair. J Vasc Surg. 2003;37(01):8–15. doi: 10.1067/mva.2003.55. [DOI] [PubMed] [Google Scholar]
- 17.Stavropoulos S W, Park J, Fairman R, Carpenter J. Type 2 endoleak embolization comparison: translumbar embolization versus modified transarterial embolization. J Vasc Interv Radiol. 2009;20(10):1299–1302. doi: 10.1016/j.jvir.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Zener R, Oreopoulos G, Beecroft R, Rajan D K, Jaskolka J, Tan K T. Transabdominal direct sac puncture embolization of Type II endoleaks after endovascular abdominal aortic aneurysm repair. J Vasc Interv Radiol. 2018;29(08):1167–1173. doi: 10.1016/j.jvir.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 19.Burley C G, Kumar M H, Bhatti W A, Boyd C, Sales C M. Transcaval embolization as the preferred approach. J Vasc Surg. 2019;69(04):1309–1313. doi: 10.1016/j.jvs.2018.08.177. [DOI] [PubMed] [Google Scholar]
- 20.Mewissen M W, Jan M F, Kuten D, Krajcer Z. Laser-assisted transgraft embolization: a technique for the treatment of Type II endoleaks. J Vasc Interv Radiol. 2017;28(11):1600–1603. doi: 10.1016/j.jvir.2017.07.029. [DOI] [PubMed] [Google Scholar]
- 21.Chivot C, Bouzerar R, Yzet T, Reix T. Transarterial perigraft balloon-assisted Onyx embolization of Type II endoleak. J Vasc Interv Radiol. 2019;30(04):617–619. doi: 10.1016/j.jvir.2018.11.030. [DOI] [PubMed] [Google Scholar]
- 22.Khaja M S, Park A W, Swee W. Treatment of type II endoleak using Onyx with long-term imaging follow-up. Cardiovasc Intervent Radiol. 2014;37(03):613–622. doi: 10.1007/s00270-013-0706-z. [DOI] [PubMed] [Google Scholar]
- 23.Guo Q, Zhao J, Ma Y. A meta-analysis of translumbar embolization versus transarterial embolization for type II endoleak after endovascular repair of abdominal aortic aneurysm. J Vasc Surg. 2020;71(03):1029–10340. doi: 10.1016/j.jvs.2019.05.074. [DOI] [PubMed] [Google Scholar]
- 24.van Bindsbergen L, Braak S J, van Strijen M J, de Vries J P. Type II endoleak embolization after endovascular abdominal aortic aneurysm repair with use of real-time three-dimensional fluoroscopic needle guidance. J Vasc Interv Radiol. 2010;21(09):1443–1447. doi: 10.1016/j.jvir.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 25.Rhee R, Oderich G, Hertault A.Multicenter experience in translumbar type II endoleak treatment in the hybrid room with needle trajectory planning and fusion guidanceJ Vasc Surg 2019; Doi: S0741-5214(19)32626-6 (epub ahead of print) [DOI] [PubMed]
- 26.Stavropoulos S W, Carpenter J P, Fairman R M, Golden M A, Baum R A.Inferior vena cava traversal for translumbar endoleak embolization after endovascular abdominal aortic aneurysm repair J Vasc Interv Radiol 20031409, Pt 11191–1194. [DOI] [PubMed] [Google Scholar]
- 27.Yu H, Desai H, Isaacson A J, Dixon R G, Farber M A, Burke C T. Comparison of Type II endoleak embolizations: embolization of endoleak nidus only versus embolization of endoleak nidus and branch vessels. J Vasc Interv Radiol. 2017;28(02):176–184. doi: 10.1016/j.jvir.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 28.Hyatt E, McLaughlin J N, Shah H, Kalva S P. Transcaval approach for embolization of type II Endoleak following endovascular aortic aneurysm repair. CVIR Endovasc. 2019;2(01):3. doi: 10.1186/s42155-018-0047-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lagios K, Karaolanis G, Bazinas T, Perdikides T, Bountouris I. Translumbar infusion of N-butyl cyanoacrylate for the treatment of Type II endoleaks. J Vasc Interv Radiol. 2018;29(06):826–832. doi: 10.1016/j.jvir.2018.01.788. [DOI] [PubMed] [Google Scholar]
- 30.Nakai M, Ikoma A, Loffroy R. Transgraft sac embolization combined with graft reinforcement for refractory mixed-type endoleak. Cardiovasc Intervent Radiol. 2019;42(04):620–624. doi: 10.1007/s00270-018-2144-4. [DOI] [PubMed] [Google Scholar]
- 31.Porta M, Cova M, Segreti S. Laparoscopic clipping of the inferior mesenteric artery and intraoperative indocyanine green angiography for Type II endoleak following endovascular aneurysm repair. J Laparoendosc Adv Surg Tech A. 2020;30(04):413–415. doi: 10.1089/lap.2019.0766. [DOI] [PubMed] [Google Scholar]
- 32.Zhou W, Lumsden A B, Li J. IMA clipping for a Type II endoleak: combined laparoscopic and endovascular approach. Surg Laparosc Endosc Percutan Tech. 2006;16(04):272–275. doi: 10.1097/00129689-200608000-00018. [DOI] [PubMed] [Google Scholar]
- 33.Ling A J, Pathak R, Garbowski M, Nadkarni S. Treatment of a large type II endoleak via extraperitoneal dissection and embolization of a collateral vessel using ethylene vinyl alcohol copolymer (Onyx) J Vasc Interv Radiol. 2007;18(05):659–662. doi: 10.1016/j.jvir.2007.02.006. [DOI] [PubMed] [Google Scholar]


