Abstract
Purpose
We hypothesized that radiation-induced lymphopenia could be predicted by the effective dose to the circulating immune cells (EDIC) in advanced esophageal squamous cell carcinoma treated with trimodality therapy according to the Dutch ChemoRadiotherapy for Oesophageal cancer followed by Surgery Study (CROSS) trial regimen. To test this hypothesis, we examined the effect of EDIC on the degree of lymphocyte drop (lymphocyte nadir).
Methods and Materials
Patients with advanced nonmetastatic esophageal squamous cell carcinoma treated in a single tertiary cancer center from 2012 to 2018 were eligible for this study. All patients had to have a radiation therapy plan available for EDIC computation and received neoadjuvant chemoradiation according to the Dutch CROSS trial regimen before radical esophagectomy. The EDIC was calculated as a function of integral doses to the lung, heart, and total body with a verified mathematical model. The association between EDIC and lymphocyte nadir was studied, and the relationships of overall survival (OS) with lymphocyte nadir and EDIC were assessed using multivariable Cox regression model.
Results
This analysis included 92 eligible consecutive patients (77 men and 15 women). The mean EDIC was 2.8 Gy (range, 0.6-4.4). EDIC was significantly correlated with lymphocyte nadir (Spearman coefficient = –0.505; P < .01), and lymphocyte nadir was a significant independent factor for shorter OS (hazard ratio = 0.63; P < .001). Lymphocyte nadir was also the most significant factor in determining OS among other clinical parameters. Exploratory analysis showed significant OS differences between EDIC groups (<2, 2-4, and >4 Gy). The 2–year OS rates were 66.7%, 42.7%, and 16.7% for EDIC <2, 2 to 4, and >4 Gy, respectively.
Conclusions
There was a significant correlation between radiation dose to circulating immune cells and lymphocyte nadir, which in turn affected OS in patients with advanced nonmetastatic esophageal squamous cell carcinoma treated by trimodality therapy.
Introduction
Radiation is well known to have a potent lymphocyte-killing effect. Radiation can destroy mature circulating lymphocytes at as low as 1 Gy.1 The importance of this has been overlooked for decades, until recently when immunotherapy emerged as a standard treatment for many cancers. Because lymphocytes play a major role in exerting antitumor immunity, many clinical studies have demonstrated a correlation between absolute lymphocyte counts and survival.2, 3, 4
Radiation-induced lymphopenia has been reported to be adversely associated with overall survival (OS) and recurrence-free survival in various cancers including glioblastoma, non-small cell lung cancer (NSCLC), pancreatic cancer, and head and neck cancer.5, 6, 7, 8, 9 One of the possible mechanisms of lymphopenia in radiation therapy (RT) is the large volume of low-dose bath that kills a vast number of circulating lymphocytes in both systemic and pulmonary circulation.7 Yovino et al10 first reported that a single fraction of typical RT plan (60 Gy in 30 fractions) for patients with glioblastoma resulted in 0.5 Gy exposure to 5% of circulating cells. Ninety-nine percent of circulating lymphocytes would be exposed to at least 0.5 Gy after the whole course of 30 fractions. Tang et al7 further reported a significant correlation between low-dose lung dosimetry V1-V5 with the degree of lymphopenia in patients with NSCLC. They hypothesized that as all cardiac output must go through pulmonary circulation, the large volume low-dose bath radiation to pulmonary vasculature would kill most circulating lymphocytes, contributing to worse outcomes in patients with higher V5 value. Jin et al11 further studied the contributing factors and calculated the dose to circulating lymphocyte in patients with lung cancer. They developed a model to estimate the effective dose to circulating immune cells (EDIC) and found that higher EDIC was correlated with poorer survival in patients with lung cancer in the Radiation Therapy Oncology Group (RTOG) 0617 trial.
The esophagus is an organ mainly confined in the thorax. The majority of entry and exit radiation beams have to pass through lung tissue during treatment of esophageal cancer with either the conformal three-dimensional or intensity modulated radiation therapy (IMRT) photon technique. Recently published studies12, 13, 14 in the United States suggested that higher lymphocyte nadir predicts better pathologic and survival outcomes for patients with esophageal cancer. However, these studies included a majority of patients with adenocarcinoma treated with radical chemoradiation, and there was heterogeneity regarding radiation therapy doses and chemotherapy regimens. Therefore, it is worth studying the dosimetry and clinical parameters that cause lymphopenia in esophageal squamous cell cancer (ESCC) during neoadjuvant chemoradiation with the ChemoRadiotherapy for Oesophageal cancer followed by Surgery Study (CROSS) regimen,15 a standard of care employed in Europe and Asia.
As immunotherapy is increasingly incorporated into the systemic treatment of various cancers, it is important to devise a better lymphocyte-sparing radiation technique. For example, stereotactic body radiation therapy is associated with significantly less radiation-induced lymphopenia than fractionated RT in locally advanced pancreatic cancer.16 A lymphocyte-sparing radiation technique may be crucial for subsequent immunotherapy if there were metastases later. For instance, there are reported phase II and III studies on the use of immunotherapy including checkpoint inhibitors in advanced ESCC. The landmark phase III Keynote 181 study17 demonstrated improved clinical outcomes with second-line pembrolizumab in advanced esophageal cancer with a programmed death-ligand 1 combined proportional score ≥10. Thus, studying factors in preserving lymphocytes during initial RT for esophageal cancer may have far-reaching importance in subsequent metastatic settings.
Methods and Materials
Hypothesis and objectives
We performed 2 analyses to test 2 related hypotheses. First, radiation-induced lymphopenia can be predicted by the EDIC that was computed by modeling the doses to the total body, lung, and heart. Second, EDIC had an effect on survival in patients with ESCC treated with trimodality therapy.
The primary objective of our study was to validate the EDIC model in ESCC and to study the correlation of EDIC with lymphocyte nadir (lowest absolute lymphocyte count). Secondary objectives were to explore the relationships of EDIC, lymphocyte nadir, and OS.
Study population
This was a retrospective study in patients with ESCC treated with neoadjuvant chemoradiation therapy according to the CROSS regimen. After approval by our institutional review board, clinical and dosimetric data of all patients with ESCC at our hospital from June 2012 to February 2018 were retrospectively reviewed. Patient confidentiality was maintained according to our institution regulations.
Included subjects had to have a histologic diagnosis of ESCC and they must have received neoadjuvant chemoradiation with the CROSS regime15 (5 weekly carboplatin area under the curve = 2 and paclitaxel 50 mg/m2, radiation dose = 41.4 Gy in 23 daily fractions) and subsequent surgery. All subjects had to have records of weekly blood tests available during chemoradiation and at 2 months after the completion of chemoradiation and complete dosimetry data available for EDIC computation. Subjects with distant metastasis or dropouts before the end of chemoradiation were excluded.
Data collection and dosimetric computation
For each eligible patient, results of blood tests including total white blood cell count, neutrophil count, lymphocyte count, and platelet count during neoadjuvant chemoradiation were retrospectively retrieved. Lymphocyte nadir was defined as the lowest absolute value of lymphocyte count among the set of lymphocyte values during chemoradiation and at 2 months after the completion of chemoradiation. Demographic and clinical data were retrieved from a prospectively maintained database. Dosimetry data were obtained from our planning system.
The EDIC was computed as a function of mean heart dose, mean lung dose, integral dose, and number of fractions according to the method reported by Jin et al.11 In this model, estimated dose to the immune system was computed by using the dose to the circulating immune cells as a surrogate. The model assumed that the radiation dose was uniformly delivered to all rapidly circulating immune cells in the heart, lung, and great vessels but also to the slowly circulating immune cells within the irradiated volumes. With regard to chemoradiation for ESCC, major organs to consider include the lung, heart, large vessels, and other organs. Integral dose of the total body was used to approximate the mean organ dose (MOD) for large vessels and other organs. The blood dose contributions of the heart (mean heart dose) and lungs (mean lung dose) were derived from the respective MODs and the estimated percentage of cardiac output and blood volume they received. Then the equivalent uniform dose (EUD) was calculated from the dose-volume histogram (DVH). EDIC is the sum of EUDs of all organs in the irradiated volume. EUD could be estimated by a simple function of MOD of these 4 organs.
In summary, EDIC was calculated using this equation11:
where n is the fraction number (23) and k = 45.
Statistical consideration
For the primary endpoint of lymphocyte nadir, study variables included patient demographics, tumor factors, and treatment factors such as radiation dosimetry to the major organs at risk. Spearman’s correlation coefficient between EDIC and lymphocyte nadir was analyzed.
Further univariable and multivariable linear regression analyses were used to correlate other variables with lymphocyte nadirs. Planning target volume (PTV) was converted into log PTV to obtain normal distribution.7 Parameters with P values <.1 in univariable model were considered for multivariable analysis. Normal distribution assumption was tested using the Shapiro-Wilk normality test. The significance of low-dose lung dosimetry with lymphocyte nadirs was similarly analyzed.
For secondary endpoint of survival, Cox regression was used to study the prognostic factors (including lymphocyte nadir) of OS and recurrence-free survival (RFS). Similar to linear regression, the multivariable analysis included parameters with P values <.1 in univariable models. We also performed survival analysis in different lymphocyte nadir and EDIC subgroups. Statistical analyses were conducted by Statistical Package for the Social Sciences version 25.0 (IBM) and R version 3.5.1. P less than .05 was considered significant.
Results
Patient characteristics
Ninety-two patients were eligible for final analysis. Baseline demographics and tumor and treatment characteristics of these patients are listed in Table 1. All patients were ethnically Chinese. Around half of the patients (54.3%) were age 65 or older, and 84% were male. All 92 patients had squamous cell carcinoma, which reflected the disease pattern in Asia as opposed to the Western population where adenocarcinoma predominates. Seventy-nine patients (86%) had normal baseline lymphocyte counts before chemoradiation. All patients but 1 (99%) had T3 disease and all of them had at least 1 regional lymph node involvement (N1, 50%; N2, 42.4%; and N3, 7.6%). Only 60.8% of patients finished the planned 5 cycles of weekly chemotherapy (4 cycles = 30.4%; 3 cycles = 7.6%; 2 cycles = 0%; 1 cycle = 1.1%), and the most common reason for noncompliance was suboptimal marrow tolerance. More patients were treated with three-dimensional conformal technique than with IMRT technique (56.5% vs 40%).
Table 1.
Baseline characteristics
| Characteristics | N = 92 (%) |
|---|---|
| Age at diagnosis | |
| Under 65 | 42 (45.7%) |
| 65 and above | 50 (54.3%) |
| Sex | |
| Male | 77 (83.7%) |
| Female | 15 (16.3%) |
| Baseline lymphocyte level | |
| Normal | 79 (85.9%) |
| Low | 13 (14.1%) |
| RT technique | |
| 3DCRT | 52 (56.5%) |
| IMRT | 40 (43.5%) |
| T-staging | |
| T1 | 0 (0%) |
| T2 | 1(1.1%) |
| T3 | 91 (98.9%) |
| T4 | 0 (0%) |
| N-staging | |
| N0 | 0 (0) |
| N1 | 46 (50%) |
| N2 | 39 (42.4%) |
| N3 | 7 (7.6%) |
| Number of chemotherapy courses | |
| 5 | 56 (60.8%) |
| 4 | 28 (30.4%) |
| 3 | 7 (12.5%) |
| 2 | 0 (0%) |
| 1 | 1 (1.1%) |
| EDIC group | |
| <2 Gy | 18 (19.6%) |
| 2 to <4 Gy | 68 (73.9%) |
| >4 Gy | 6 (6.5%) |
Abbreviations: EDIC = effective dose to the circulating immune cells; IMRT = intensity modulated radiation therapy; RT = radiation therapy; 3DCRT = 3-dimensional conformal radiation therapy.
EDIC and lymphocyte nadir
Univariable and multivariable linear regressions demonstrated that larger PTV volume was significantly associated with lower lymphocyte nadirs (Table 2), in which normality was not rejected for the Studentized residuals (https://www.jmp.com/en_us/statistics-knowledge-portal/what-is-multiple-regression/mlr-residual-analysis-and-outliers.html) of the multiple linear regression with P = .44 using the Shapiro-Wilk normality test. In addition, baseline lymphocyte count was also associated with lower lymphocyte nadir. No other demographic or clinical parameter correlated significantly with lymphocyte nadir. Neither RT technique nor the number of courses of chemotherapy affected lymphocyte nadirs.
Table 2.
Univariable and multivariable linear regression associating baseline variables with lymphocyte nadirs (109 cells/L) during radiation treatment
|
Characteristic |
Univariable analysis |
Multivariable analysis |
||||
|---|---|---|---|---|---|---|
| Regression coefficient | 95% CI | P | Regression coefficient | 95% CI | P | |
| Male (vs female) | 0.048 | –0.039 to 0.136 | .278 | NI | ||
| Age (1 y) | –0.001 | –0.005 to 0.002 | .537 | NI | ||
| Stage | ||||||
| N3 (vs N2 and N1) | –0.085 | –0.206 to 0.037 | .168 | NI | ||
| Baseline lymphocyte (109 cells/L) | 0.112 | 0.068-0.156 | <.001 | 0.098 | 0.060-0.135 | <.001 |
| Chemotherapy | ||||||
| 5 courses (vs fewer than 5 courses) | 0.060 | –0.005 to –0.126 | .071 | 0.044 | –0.005 to 0.093 | .081 |
| Radiation therapy | ||||||
| Log PTV | –0.393 | –0.629 to –0.158 | .001 | –0.205 | –0.399 to –0.011 | .038 |
| EDIC | –0.085 | –0.115 to –0.055 | <.001 | –0.061 | –0.089 to –0.034 | <.001 |
| IMRT (vs 3DCRT) | 0.019 | –0.047 to 0.084 | .567 | NI | ||
Abbreviations: 3DCRT = 3-dimensional conformal radiation therapy; CI = confidence interval; EDIC = effective dose to the circulating immune cells; IMRT = intensity modulated radiation therapy; NI = not included; PTV = planning target volume.
The result of the primary outcome is shown in Table 3. The estimated EDIC, which is a function of mean heart dose, mean lung dose, and mean doses to large vessels and other thoracic organs (approximated by mean total body dose), had a significantly negative correlation with the lymphocyte nadir (Spearman coefficient = -0.505; P < .01). Individual mean doses to the lung, heart, and total body were also highly significant in predicting lymphocyte nadirs (Spearman coefficients = –0.34, –0.502, and –0.36 respectively; P < .01).
Table 3.
OAR dosimetry and EDIC versus lymphocyte nadir
| Spearman coefficient | P | |
|---|---|---|
| Mean total body dose | –0.36 | <.001 |
| Mean lung dose | –0.34 | .01 |
| Mean heart dose | –0.502 | <.001 |
| EDIC | –0.505 | <.01 |
Abbreviations: EDIC = effective dose to the circulating immune cells; OAR = organs at risk.
Lymphocyte nadir and low-dose bath in lung
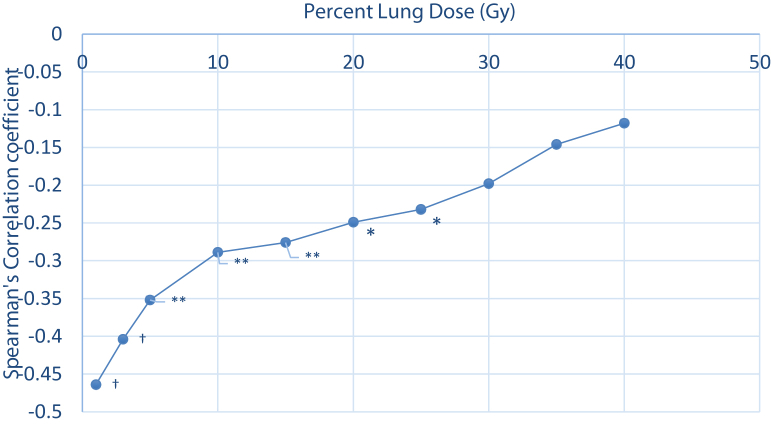
A previous study established a correlation between low-dose bath to lung and lymphocyte nadir in NSCLC.11 We performed a similar analysis to see whether this relationship also exists in our patients with ESCC. Spearman’s correlation coefficients (R) between lymphocyte nadir and different lung DVH parameters, namely V1 to V40, are shown in Figure 1. The coefficients were highly significant at low-dose lung DVH zones from V1 to V25 (coefficient from -0.47 to -0.27; P < .05). The coefficients lost statistical significance at V30 level onward (P > .05). The absolute values of correlation coefficients decreased together with incremental increase in P value from low-dose zone level (V1) to high-dose zone level (V40).
Figure 1.
Lung dose-volume histogram and correlation with lymphocyte nadir. Lymphocyte nadir (primary outcome) was correlated negatively with low-dose lung dosimetry only, reflecting dose to circulating lymphocytes. Significance was lost above V25. † P < .001, ∗∗ P < .01, ∗P < .05
Lymphocyte nadir, EDIC, and survivals
Our secondary outcome was the survival effect of lymphocyte nadir and EDIC. Median OS for the whole population was 17.5 months (95% confidence interval, 9.30-25.77 months) after a median follow-up of 16.9 months (range, 1.3-79.3). Univariable and multivariable Cox regression results of clinical variables on OS and RFS are shown in Table 4. Multicollinearity was not found with variance inflation factor ≤1.18 in either OS or RFS. Multivariable models suggested that lymphocyte nadir was the only variable with statistical significance (P < .05) in both OS and RFS. Higher lymphocyte nadir was found to have lower risk on both OS and RFS (hazard ratio [HR] = 0.75 per 108 cells/L, P = .003; and 0.78 per 108 cells/L, P = .022, respectively) after adjustment in the multivariable Cox regression models. Log PTV, in contrast to common belief that larger PTV adversely affects OS, did not reach statistically significant effect on either OS or RFS in univariable or multivariable models. Nadirs of total white cell count, age, sex, RT techniques, number of courses of chemotherapy given, and N stage did not have an effect on survival.
Table 4.
Cox regression association baseline variables with outcomes during radiation treatment
| Overall survival |
HR | Univariable |
P | HR | Multivariable |
P |
|---|---|---|---|---|---|---|
| Variables | 95% CI | 95% CI | ||||
| Lymphocyte nadir (108 cells/L) | 0.72 | 0.58-0.89 | .003 | 0.75 | 0.58-0.96 | .022 |
| Age (1 y) | 0.99 | 0.97-1.02 | .68 | NI | ||
| WCC nadir (109 cells/L) | 1.02 | 0.83-1.27 | .84 | NI | ||
| Male (vs female) | 1.002 | 0.51-1.98 | .995 | NI | ||
| Stage | ||||||
| N3 (vs N2 and N1) | 0.66 | 0.24-1.83 | .43 | NI | ||
| Baseline lymphocyte | 0.56 | 0.36-0.87 | .010 | 0.67 | 0.41-1.11 | .12 |
| Chemotherapy | ||||||
| ≥5 courses (vs <5 courses) | 0.66 | 0.40-1.10 | .11 | NI | ||
| Radiation therapy | ||||||
| Log10 PTV | 6.41 | 0.80-51.16 | .080 | 5.32 | 0.56-50.51 | .15 |
| EDIC | 1.18 | 0.89-1.57 | .25 | NI | ||
| IMRT (vs 3DCRT) | 0.74 | 0.44-1.24 | .25 | NI |
| Recurrence-free survival |
HR | Univariable |
P | HR | Multivariable |
P |
|---|---|---|---|---|---|---|
| Variables | 95% CI | 95% CI | ||||
| Lymphocyte nadir (108 cells/L) | 0.74 | 0.60-0.91 | .004 | 0.78 | 0.62-0.99 | .043 |
| Age (1 y) | 0.99 | 0.96-1.01 | .30 | NI | ||
| WCC nadir (109 cells/L) | 1.01 | 0.82-1.23 | .96 | NI | ||
| Male (vs female) | 1.14 | 0.58-2.24 | .71 | NI | ||
| Stage | ||||||
| N3 (vs N2 and N1) | 0.60 | 0.22-1.64 | .32 | NI | ||
| Baseline lymphocyte | 0.52 | 0.33-0.82 | .005 | 0.63 | 0.37-1.06 | .079 |
| Chemotherapy | ||||||
| ≥5 courses (vs <5 courses) | 0.72 | 0.44-1.19 | .20 | NI | ||
| Radiation therapy | ||||||
| Log10 PTV | 6.07 | 0.76-47.45 | .086 | 4.79 | 0.52-44.47 | .17 |
| EDIC | 1.19 | 0.91-1.56 | .21 | NI | ||
| IMRT (vs 3DCRT) | 0.70 | 0.43-1.18 | .19 | NI |
Abbreviations: 3DCRT = 3-dimensional conformal radiation therapy; CI = confidence interval; EDIC = effective dose to the circulating immune cells; HR = hazard ratio; IMRT = intensity modulated radiation therapy; NI = not included; PTV = planning target volume; WCC = white cell count.
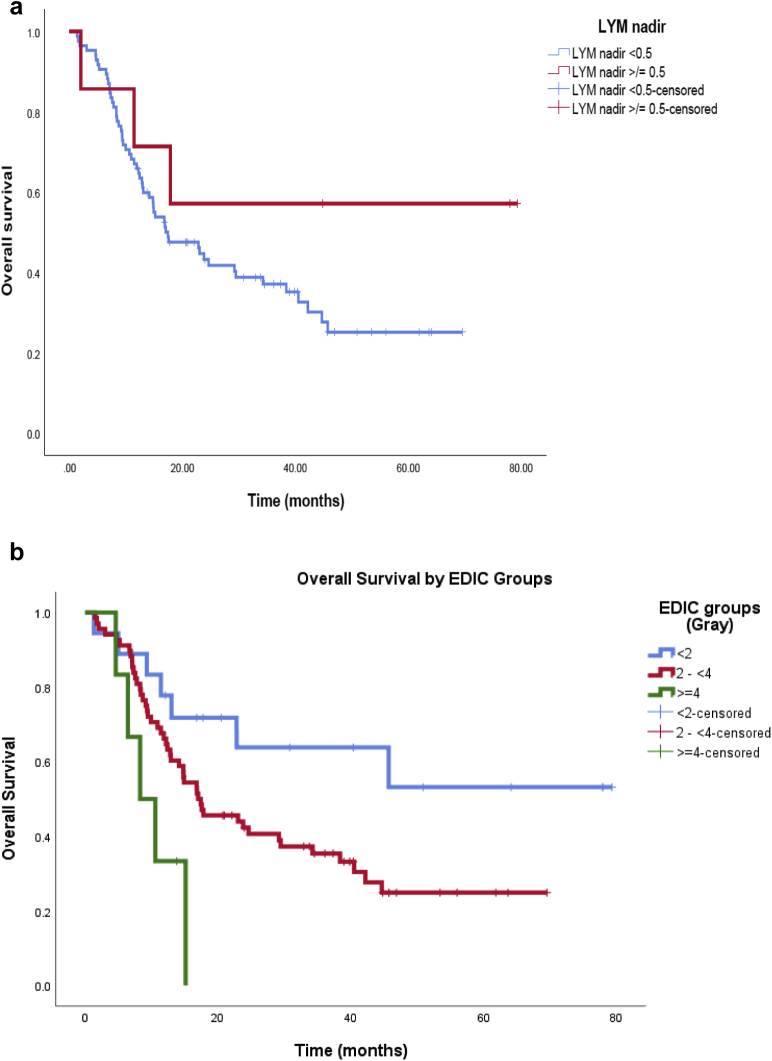
We then performed further analysis to explore relationships between OS with different groups of lymphocyte nadir and EDIC. Following previously reported studies,5 we used lymphocyte count 0.5 (109 cells/L) as the cutoff. The Kaplan-Meier curve is shown in Figure 2a. There was a trend of better survival for the group with lymphocyte nadir ≥0.5 than the group <0.5 (P = .192).
Figure 2.
Overall survival relationship with (a) lymphocyte nadir and (b) effective dose to the circulating immune cells (EDIC) groups.
EDIC of our 92 patients ranged from 0.64 to 4.39 Gy. We divided the patients into 3 groups according to EDIC value, namely <2 Gy, 2 to <4 Gy, and ≥4 Gy. There was significant difference in OS with different EDIC groups, with higher EDIC predicting poorer survival (P = .01; Fig 2b). In addition, higher EDIC also predicted lower probability of 2-year OS.
Discussion
Our study demonstrated the importance of lymphocyte count in determining the outcomes of locally advanced ESCC, and we advise additional dose constraints in RT planning. Lower lymphocyte nadir was associated with poorer OS and RFS in this group of patients. Similar results have been shown in other solid tumors including lung, pancreatic, head and neck, and glioblastoma.5 In our study, the HR of lymphocyte nadir on OS was 0.63 per 105 lymphocytes/mL (or 108 cells/L). In other words, there was 37% relative reduction in death per 105 lymphocytes/mL (or 108 cells/L) increment in lymphocyte nadir. The size of the effect was comparable to a similar study on NSCLC reported by Tang et al,7 in which the corresponding HR was 0.51 per 103 lymphocytes/mL (P = .01) on univariate analysis. Contrary to previous reports, PTV was not a predictor of survival, with statistical significance after adjusting of lymphocyte nadirs in the multivariable (Table 4). Log PTV had a large HR (>6), but it was not statistically significant (P = 0080/0.10 in uni-/multivariable for OS). Similar to RFS, log PTV was close to being statistically significant, with P values = 0.086 and 0.15, respectively. The findings suggested that log PTV might trend toward clinical significance with high HR, but it cannot reach statistical significance. We believe that PTV effect on survival may likely be indirect, and PTV might exert its effect on survival through its contribution to lymphocyte nadirs.
Interestingly, the large-scale study INT 0123 published in 2002 proved there was no survival benefit or locoregional control of esophageal cancer by escalating the radiation dose above 50.4 Gy.18 There were controversies over the trial design and RT techniques adopted in that study, and our results may uncover the missing link between RT dose and survival. Higher radiation dose might have detrimental effect on OS as a result of lymphocyte killing. A recent analysis of radiation dose escalation in esophageal cancer19 concluded that although local control might benefit from dose escalation, there was no benefit in OS. Similar results were also shown in the dose escalation study RTOG 0617 for NSCLC. The study20 showed an unexpected detrimental effect on OS for higher dose of 74 Gy compared with the control arm of 60 Gy. This was postulated to be due to lower lymphocyte nadir in the higher dose arm in the study by Jin et al.11 As both gross tumor volumes and PTV of esophageal cancer and NSCLC are mainly confined to the thorax, and lymphopenia was shown to affect the outcomes in both cases, it is reasonable to hypothesize that the lack of survival benefit with dose escalation in ESCC might be due to higher dose to immune cells.
Low-dose parameters of lung DVH (namely V1-V25) are relevant to the survival of circulating lymphocytes as almost all circulating blood goes through the pulmonary circulation. Lung V5 has been shown to be correlated with survival in NSCLC.7 The low-dose bath could have effectively killed off a large number of circulating lymphocytes. Similarly, PTV was shown to correlate with lymphocyte nadir. This was likely caused by a larger radiation field, resulting in more circulating immune cells being exposed to radiation. We postulate that this might impair antitumor immunity and decrease the ability to mount immunity response to infection. Thus, this might explain a poorer survival in subjects with lower lymphocyte nadir.
Besides the lung DVH, dose to other previously undefined organs and tissues might also contribute to the lymphocyte drop. For instance, the heart, large vessels, and the thoracic duct, which house the return of lymphocytes into systemic circulation, are all in the thorax. In addition, the atrophic thymus in adult could have retained some lymphatic function as well. A validated mathematical model is needed to account for dose received by these organs in the thorax.
Therefore, we adopted the formula of predicting EDIC from our coauthors (J.J. and S.K.) in their study on lymphopenia and survival in NSCLC in RTOG 0617.11 We found that EDIC strongly correlated with the lymphocyte nadir with a Spearman coefficient of –0.505 (P < .01). In our study, EDIC ranged from 0.64 to 4.39 Gy, which was significantly lower than that reported in the RTOG 0617 cohort (2.05-12.20 Gy). This can be explained by a much lower dose of 41.4 Gy prescribed in our study according to the Dutch CROSS trial. Despite the difference in the EDIC range, both studies demonstrated the importance of EDIC in determining the lymphocyte nadir.
Ladbury et al21 published their results in adopting the EDIC formula for patients with stage III NSCLC. They retrospectively reviewed 117 patients and calculated their EDIC. They found that EDIC was independently associated with OS (HR, 1.17; P = .03), local progression-free survival (HR, 1.17; P = .02), and disease-free survival (HR, 1.15; P = .04). As both lung cancers and esophageal cancers are mainly located within the thoracic region, we believe that the EDIC formula would be a valid model in esophageal cancer too. On the other hand, Saito et al22 found that splenic dose volumes but not bone marrow dose volumes were predictive in treatment-related lymphopenia during chemoradiotherapy for esophageal cancer. We believe future studies can incorporate dose to the spleen as a better estimate of dose to EDIC.
Although lymphocyte nadir has been proven to affect OS in multivariable Cox regression, the KM survival curve only showed a trend of better survival in lymphocyte nadir ≥0.5 compared with that of <0.5. We think that because the 0.5 cutoff as reported by other studies is arbitrary, a significant Kaplan-Meier curve may be shown with other lymphocyte cutoffs or with larger sample size. Furthermore, we were able to show significant difference in 2-year survival probability in different EDIC subgroups. This was in line with the RTOG 0617 cohort.
Similar studies were reported by Fang et al13 from the MD Anderson Cancer Center. In their cohort of 313 patients with esophageal cancer who received neoadjuvant chemoradiotherapy, a higher lymphocyte nadir correlated with a higher pathologic complete response rate (odds ratio [OR] = 1.82; P < .003). They also found that mean body dose was inversely related to high absolute lymphocyte counts nadir (OR = 0.77 per Gy; P < .001). Although this is consistent with our findings, there are substantial differences between the 2 studies. Ninety-five percent of patients in their study had adenocarcinoma, and there was significant heterogeneity in terms of RT dose, use of neoadjuvant chemotherapy, and chemotherapy regimens in their concurrent chemoradiotherapy phase. In our study, all patients had squamous cell carcinoma, and all received the same chemotherapy and RT dose.
Although lower lymphocyte count predicts worse survival outcomes in other solid tumors, there have been contradicting reports in the literature for esophageal cancer. One study2 in the United States showed that in patients with stage I to III esophageal cancer (85% adenocarcinoma), the lymphopenia group (defined as lymphocyte <0.5 × 109/L) had a higher risk of death (HR for death, 1.6; P = .027) compared with the nonlymphopenia group. This is supported by another study23 comparing proton beam therapy with IMRT in 448 patients with stage I-IVA esophageal cancer, which showed improved survival with higher lymphocyte count (HR for survival, 1.551; P = .01 per 1 unit of lymphocyte). However, another study24 showed that in 395 patients with stage I to III esophageal cancer, 5-year OS was not significantly different between patients with grade 4 and nongrade 4 lymphopenia (34% vs 41%; P = .47). As in our study, the Cox regression model results show that lymphocyte nadir strongly predicts survival. We believe the inconsistencies were mostly due to the arbitrary cutoff value of lymphocyte count and the statistical power of the respective sample sizes.24
Some other groups have proposed special radiation techniques to preserve lymphocytes in other solid tumors. These techniques involve the use of stereotactic body radiation therapy16 to minimize the number of fractions and the PTV, to reduce the low-dose bath effect to circulating lymphocytes. The use of proton therapy instead of photons has also been suggested.23 Because there is minimal exit dose beyond the Bragg peak, the total integral dose is reduced in proton therapy. This has been shown in the MD Anderson patient cohort, in which proton therapy was a predictor of higher lymphocyte nadir (compared with IMRT, OR = 4.18; P < .001). However, neither of these was standard for RT in esophageal cancer. Drug therapy in preserving circulating lymphocytes is also an attractive option. However, there is no well-established drug therapy at the time of this writing.
Our study was unique in that the tumor histology of ESCC and treatment regimens of the Dutch CROSS trial were homogenous, in contrast to many other studies in the United States in which adenocarcinoma predominated. Our hospital is the major tertiary referral center for patients with esophageal cancer in Hong Kong, which has a population of over 7 million, and we have included all locally advanced ESCC treated from 2012 to 2018. We believe the results could be generalized to Asian patients in whom ESCC predominates.
Our study shared the intrinsic weakness of all retrospective studies. We minimized the selection bias by including only patients with ESCC treated by the Dutch CROSS regimen. Another limitation was that the lymphocyte count obtained was total lymphocyte count. Lymphocyte subsets such as CD4+ ve and CD8+ ve cell counts were not available. Future prospective trials shall address this issue by collecting weekly cell counts of lymphocyte subsets.
Conclusions
Higher EDIC is associated with lower lymphocyte nadir, and lymphocyte nadir predicts OS. These findings shall be confirmed with larger scale prospective data in future studies. Perhaps the retrospective analysis of dosimetry data and lymphocyte nadirs in the INT–012318 and CROSS trials15 might be able to validate our findings.
Footnotes
Sources of support: No external funding received.
Disclosures: none.
References
- 1.Sellins K.S., Cohen J.J. Gene induction by gamma-irradiation leads to DNA fragmentation in lymphocytes. J Immunol. 1987;139:3199–3206. [PubMed] [Google Scholar]
- 2.Davuluri R., Jiang W., Fang P. Absolute lymphocyte count nadir during chemoradiation as a prognostic indicator of esophageal cancer survival outcomes. Int J Radiat Oncol Biol Phys. 2016;96:E177. doi: 10.1016/j.ijrobp.2017.05.037. [DOI] [PubMed] [Google Scholar]
- 3.Karantanos T., Karanika S., Seth B., Gignac G. The absolute lymphocyte count can predict the overall survival of patients with non-small cell lung cancer on nivolumab: A clinical study. Clin Transl Oncol. 2019;21:206–212. doi: 10.1007/s12094-018-1908-2. [DOI] [PubMed] [Google Scholar]
- 4.Pike L.R.G., Bang A., Mahal B.A. The impact of radiation therapy on lymphocyte count and survival in metastatic cancer patients receiving PD-1 immune checkpoint inhibitors. Int J Radiat Oncol Biol Phys. 2019;103:142–151. doi: 10.1016/j.ijrobp.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 5.Grossman S.A., Ellsworth S., Campian J. Survival in patients with severe lymphopenia following treatment with radiation and chemotherapy for newly diagnosed solid tumors. J Natl Compr Canc Netw. 2015;13:1225–1231. doi: 10.6004/jnccn.2015.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mendez J.S., Govindan A., Leong J., Gao F., Huang J., Campian J.L. Association between treatment-related lymphopenia and overall survival in elderly patients with newly diagnosed glioblastoma. J Neurooncol. 2016;127:329–335. doi: 10.1007/s11060-015-2037-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang C., Liao Z., Gomez D. Lymphopenia association with gross tumor volume and lung V5 and its effects on non-small cell lung cancer patient outcomes. Int J Radiat Oncol Biol Phys. 2014;89:1084–1091. doi: 10.1016/j.ijrobp.2014.04.025. [DOI] [PubMed] [Google Scholar]
- 8.Chadha A.S., Liu G., Chen H.C. Does unintentional splenic radiation predict outcomes after pancreatic cancer radiation therapy? Int J Radiat Oncol Biol Phys. 2017;97:323–332. doi: 10.1016/j.ijrobp.2016.10.046. [DOI] [PubMed] [Google Scholar]
- 9.Campian J.L., Sarai G., Ye X., Marur S., Grossman S.A. Association between severe treatment-related lymphopenia and progression-free survival in patients with newly diagnosed squamous cell head and neck cancer. Head Neck. 2014;36:1747–1753. doi: 10.1002/hed.23535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yovino S., Kleinberg L., Grossman S.A., Narayanan M., Ford E. The etiology of treatment-related lymphopenia in patients with malignant gliomas: Modeling radiation dose to circulating lymphocytes explains clinical observations and suggests methods of modifying the impact of radiation on immune cells. Cancer Invest. 2013;31:140–144. doi: 10.3109/07357907.2012.762780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jin J.Y., Hu C., Xiao Y. Higher radiation dose to immune system is correlated with poorer survival in patients with stage III non-small cell lung cancer: a secondary study of a phase 3 cooperative group trial (NRG Oncology RTOG 0617) Int J Radiat Oncol Biol Phys. 2017;99:S151–S152. [Google Scholar]
- 12.Davuluri R., Jiang W., Fang P. Lymphocyte nadir and esophageal cancer survival outcomes after chemoradiation therapy. Int J Radiat Oncol Biol Phys. 2017;99:128–135. doi: 10.1016/j.ijrobp.2017.05.037. [DOI] [PubMed] [Google Scholar]
- 13.Fang P., Jiang W., Davuluri R. High lymphocyte count during neoadjuvant chemoradiotherapy is associated with improved pathologic complete response in esophageal cancer. Radiother Oncol. 2018;128:584–590. doi: 10.1016/j.radonc.2018.02.025. [DOI] [PubMed] [Google Scholar]
- 14.Shiraishi Y., Fang P., Xu C. Severe lymphopenia during neoadjuvant chemoradiation for esophageal cancer: A propensity matched analysis of the relative risk of proton versus photon-based radiation therapy. Radiother Oncol. 2018;128:154–160. doi: 10.1016/j.radonc.2017.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shapiro J., van Lanschot J.J.B., Hulshof M. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): Long-term results of a randomised controlled trial. Lancet Oncol. 2015;16:1090–1098. doi: 10.1016/S1470-2045(15)00040-6. [DOI] [PubMed] [Google Scholar]
- 16.Crocenzi T., Cottam B., Newell P. A hypofractionated radiation regimen avoids the lymphopenia associated with neoadjuvant chemoradiation therapy of borderline resectable and locally advanced pancreatic adenocarcinoma. J Immunother Cancer. 2016;4:45. doi: 10.1186/s40425-016-0149-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doi T., Bennouna J., Shen L. KEYNOTE-181: Phase 3, open-label study of second-line pembrolizumab vs single-agent chemotherapy in patients with advanced/metastatic esophageal adenocarcinoma. J Clin Oncol. 2016;34 TPS4140-TPS. [Google Scholar]
- 18.Minsky B.D., Pajak T.F., Ginsberg R.J. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: High-dose versus standard-dose radiation therapy. J Clin Oncol. 2002;20:1167–1174. doi: 10.1200/JCO.2002.20.5.1167. [DOI] [PubMed] [Google Scholar]
- 19.Brower J.V., Chen S., Bassetti M.F. Radiation dose escalation in esophageal cancer revisited: A contemporary analysis of the national cancer data base, 2004 to 2012. Int J Radiat Oncol Biol Phys. 2016;96:985–993. doi: 10.1016/j.ijrobp.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 20.Bradley J.D., Paulus R., Komaki R. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): A randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16:187–199. doi: 10.1016/S1470-2045(14)71207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ladbury C.J., Rusthoven C.G., Camidge D.R., Kavanagh B.D., Nath S.K. Impact of radiation dose to the host immune system on tumor control and survival for stage III non-small cell lung cancer treated with definitive radiation therapy. Int J Radiat Oncol Biol Phys. 2019;105:346–355. doi: 10.1016/j.ijrobp.2019.05.064. [DOI] [PubMed] [Google Scholar]
- 22.Saito T., Toya R., Yoshida N. Spleen dose-volume parameters as a predictor of treatment-related lymphopenia during definitive chemoradiotherapy for esophageal cancer. In vivo (Athens, Greece) 2018;32:1519–1525. doi: 10.21873/invivo.11409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fang P., Shiraishi Y., Verma V. Lymphocyte-sparing effect of proton therapy in patients with esophageal cancer treated with definitive chemoradiation. Int J Part Ther. 2018;4:23–32. doi: 10.14338/IJPT-17-00033.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang M., Oh P., Brady P. Lack of validation of lymphopenia as a prognostic factor in esophageal cancer chemoradiation. Int J Radiat Oncol Biol Phys. 2018;102:e46. [Google Scholar]