Abstract
Purpose
Few studies have evaluated the methodology by which radiation therapy (RT) for thyroid eye disease and compressive optic neuropathy is performed. The objective of this study was to retrospectively review our experience from a radiation planning standpoint and to determine whether current treatment methods provide adequate dose to target and collateral structures.
Methods
A retrospective review of 52 patients (104 orbits) with bilateral thyroid eye disease and compressive optic neuropathy treated with RT (20 Gy in 10 fractions) at our institution. RT plans were analyzed for target volumes and doses. Visual fields, color plates, and visual acuity were assessed pretreatment and at last available follow-up post RT. A standardized, anatomic contour of the retro-orbital space was applied to these retrospective plans to determine dose to the entire space, rather than the self-selected target structure.
Results
Compared with the anatomic retro-orbital space, the original contour overlapped by only 68%. Maximum and mean dose was 2134 cGy and 1910 cGy to the anatomic retro-orbital space. Consequently, 39.8% of the orbits had a mean dose <19 Gy (<17 Gy 16.4%, <18 Gy 27.6% <19 Gy 37.8%, <20 Gy 59.2%, 20-21 Gy 35.8%, >21 Gy 5%). There was no significant association of improvement in color plates (P = .07), visual fields (P = .77), and visual acuity (P = .62), based on these dose differences. When beam placement was retrospectively adjusted to include a space of 0.5 cm between the lens and the anterior beam edge, there was a 39.4% and 20.3% decrease in max and mean dose to the lens.
Conclusions
Without a standardized protocol for contouring in thyroid eye disease, target delineation was found to be rather varied, even among the same practitioner. Differences in dose to the anatomic retro-orbital space did not affect outcomes in the follow-up period. Although precise contouring of the retro-orbital space may be of little clinical consequence overall, a >0.5 cm space from the lens may significantly reduce or delay cataractogenesis.
Introduction
The pathogenesis of thyroid eye disease (TED) is subject to a variety of postulated mechanisms including the activation of immune-related cells and fibroblasts. This auto-immune activation causes inflammation and adipogenesis, resulting in the well-known signs of TED, such as soft tissue inflammation and proptosis. Radiation therapy (RT), in conjunction with corticosteroids, has been used to curtail these immunomodulatory mechanisms, shortening the active disease phase and reducing the chance of poststeroid rebound inflammation.1, 2, 3
Although the efficacy of RT in TED continues to be debated,4, 5, 6, 7, 8 its utility in cases complicated by compressive optic neuropathy (CON) appears more definitive. CON is infrequent, occurring in 1% to 8% of patients with TED.1,9,10 When RT is combined with oral corticosteroids, patients have shown a robust >90% response rate, with improvements in visual acuity, visual field, and dyschromatopsia.3 The use of RT also appears to prevent the subsequent development of compressive disease and reduce the incidence of disease relapse.10,11
The majority of studies evaluating the efficacy of RT in TED use a prescription dose first established by Donaldson et al in 1973, of 20 Gy in 10 fractions, instituted over several weeks.12 With its perceived efficacy and low complication rate, this study has become the historical standard by which patients receive treatment.1 As a heterogeneous disease process, however, no clearly defined protocol exists for target delineation. In addition, several small studies have deviated from the historical standard, comparing the response of TED patients (without compression) to lower doses of RT, ranging from 2.4 Gy to 16 Gy, with no significant difference in outcomes.13, 14, 15
The primary objective of this study was to assess contour variability in RT for TED-CON by comparing the dose and volume metrics of practitioner demarcated targeted volumes of the retro-bulbar space, to a standardized anatomic definition of the retro-bulbar space.
Methods
This was an institutional review board–approved retrospective review of 104 orbits from 52 patients who received a diagnosis of bilateral TED-CON and who were treated with combined RT and oral corticosteroids. Patients with possible concomitant causes for CON other than TED, such as malignancy or sarcoidosis, were excluded. Patients with prior orbital surgery or subsequent orbital surgery, including orbital decompression, were also excluded.
Disease status was updated in the institutional database for all patients in the electronic medical record or physical chart using data from patient visits and imaging. Demographic data was not routinely collected for all patients and were thus not included in this analysis. CON was determined by the cardinal signs of optic nerve dysfunction, which include decreased visual acuity (VA), the presence of an afferent pupillary defect, visual field deficits by mean deviation on 24 to 2 Humphrey visual fields (MD), and dyschromatopsia on Hardy-Rand-Rittler pseudoisochromatic plates (CP). MD, CP, and VA were assessed at the pretreatment visit and last available follow-up, an average of 305 days post-RT (49-1008 days), after the last fraction.
Practitioner demarcated target volume
The retro-bulbar target contoured by the treating radiation oncologist, referred to as the practitioner contour, was reviewed. The plans were analyzed for volumes and doses to target and collateral structures. All patients were planned and treated in a similar fashion. The patient was laid supine with the head midline. The isocenter was aligned with the patient’s lateral canthus, usually ensuring isocenter placement posterior to the lens. Two lateral (and sometimes oblique) beams (6 Mv) were placed oppositional to one another in a horizontal fashion, with the anterior beam edges matched posterior to the lens so that they appear parallel. Multicollimeter leaflet blocking was added posteriorly to reduce dose to the central nervous system. Plans were normalized to coverage of the planning target volume with a minimum of 95%, maximum less than 107%, and lenses under 4 Gy. There were no gross tumor volume or clinical target volume considerations. Maximum dose and mean dose were reported for the planning target volume, not as point dosing.
Identification of anatomic retro-bulbar space
Practitioner contours were then compared with the dose received when a standardized anatomic definition of the retro-bulbar space was retrospectively instituted.16,17 The anatomic contour started at the most superior opening of the orbit, excluding bone. Once the orbital aperture was reached, a straight line was drawn from the medial to lateral orbital wall, from the tip of the zygomatic bone to the point at which the frontal bone reflects back toward the orbit. As the demarcation moves inferiorly, this straight line eventually connects the posterior edge of the frontal process of the maxillary bone to the zygoma, and even more inferiorly, from the anterior lacrimal crest to the zygoma. This straight line marked the anterior most portion of the orbital contour, with exclusion of the globe. The posterior demarcation of the orbital contour is rounded at the posterior edge of the zygomatic bone, or once the diameter of the optic canal is <4 mm. The contour is continued inferiorly till closure of the inferior orbital space, excluding bone. Although minimal in volume, the nasolacrimal duct and superior orbital fissure were excluded. Within the anatomic contour, additional measurements were taken for total muscle volume and total fat volume.
Statistical analysis
Disease and patient characteristics were summarized using descriptive statistics. Percent overall improvement in CP, MD, and VA were reported by comparing changes in mean values from pretreatment to last available follow-up. The practitioner contour was compared with the volume of the anatomic contour. The percent volume overlap was described. Maximum and mean dose (in Gy) to the practitioner contour and the anatomic contour were reported for all orbits when keeping with the original beam placement and dosimetry. Based on the overall dose to the retro-bulbar space using the anatomic contour, subjects were divided into 5 subgroups; retro-bulbar spaces receiving less than 17 Gy, 17 to 18 Gy, 18 to 19.5 Gy, 19.5-20.5 Gy (~prescription dose), and >20.5 Gy. All analyses were performed on Philips Pinnacle Systems, Amsterdam, Netherlands (V.2-V.10). Outcomes with respect to mean differences in MD, CP, and VA among the 5 subgroups were assessed using one-way analysis of variance with an α of 0.05.
Results
The final analytical sample consisted of 52 patients and 104 orbits. The average age of the analytical sample was 62 (54% female). There was an overall 12.5% improvement in CP (mean, 4.05; range, 0-6), 21.4% improvement in MD (mean, –4.1; range, –25.94 to 1.48), and 38.3% improvement in VA (mean, LogMAR 0.202; range, 0-1) between pretreatment and last follow-up. Average pretreatment steroid dose was 55.3 mg (0-100 mg) and average posttreatment steroid dose was 3.46 mg (0-40 mg), resulting in a 93.7% reduction in corticosteroids.
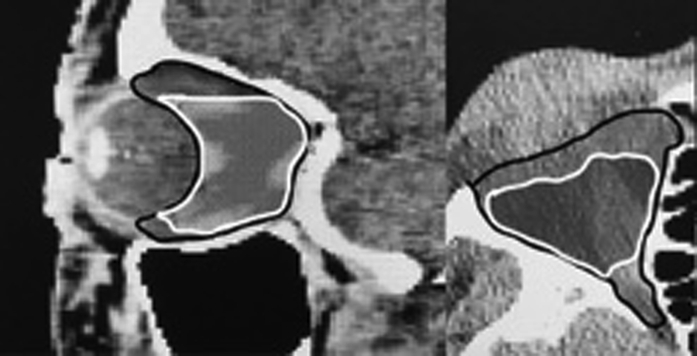
Dose to the practitioner demarcated target tissue was a max of 21.6 Gy (range, 20.5-28.7; standard deviation [SD] of 1.15) and mean of 20.7 Gy (range, 16.9-25.9; SD 1.09). Mean planning volume was 14.4 cm3 (4.6-24.0), with a standard deviation of 4.6. Mean muscle volume was 6.0 cm3 (2.9-13.3) with a standard deviation of 2.2. Mean fat volume was 15.0 cm3 (10.7-23.2) with a standard deviation of 2.3. The mean volume of the anatomic retro-bulbar space was 23.2 cm3 (16-34.1) with a standard deviation of 4.0. The practitioner contour overlapped with the anatomic contour by only 68% (25%-90%; Fig 1).
Figure 1.
Practitioner contour (white outline) versus anatomic contour (black outline).
Maximum and mean dose to the anatomic retro-bulbar space was 21.5 Gy (range, 20.4 Gy-26.7 Gy; SD of 2.3) and 19.1 Gy (range, 11.6 Gy-23.4 Gy; SD of 2.1), respectively. Of all or 76.8% received a maximum dose >20 Gy. Maximum and mean dose to the retina was 20.3 Gy and 8.0 Gy, with a maximum dose range of 11.5 to 28.7 Gy. Maximum and mean dose to the lens was 2.9 Gy and 1.4 Gy, with a maximum dose range of 0.36 Gy to 16.5 Gy (only 2 >8 Gy).
Using the anatomic contour of the retro-bulbar space, 38.9% of the orbits had a mean dose <19 Gy (16.6% <17, 12.5% 17-18 Gy, 15.3% 18-19.5 Gy, 27.8% 19.5-20.5 Gy, 26.4% >20.5 Gy). Six orbits received 16 Gy, 1 received 13 Gy, and 2 received 11 Gy. There was no significant association with outcome in CP (P = .07), VF (P = .77), VA (P = .62), or in steroid dose (P = .34) by subgroup. Incidentally, when anterior beam edges were retrospectively adjusted to 0.5 cm posterior to the lens there was a reduction in maximum dose by –39.4% and a reduction in mean dose by –20.3% to the lens, without compromising overall dose to the target tissue.
Discussion
Without a standardized protocol for contouring in TED, target delineation was found to be rather varied, even among the same practitioner, overlapping the anatomic retro-bulbar space by only 68% (25%-90%). Although the objective of this study was not to establish guidelines for the contouring and treatment of patients in TED-CON, the authors did note improved homogeneity in mean dose when practitioner contours overlapped more with the anatomic retro-bulbar space. The meticulous and specific nature of the anatomic contour is not feasible in a practical setting but does involve improved coverage of the superior, inferior, and apical regions, which are of particular importance in the development of CON and visual field deficits.18, 19, 20 There is repeated support in the literature regarding the benefit of established contouring guidelines. It significantly improves reproducibility and accuracy in treatment planning across a myriad of sites and allows for the treatment of patients in centers with limited experience.21,22 It also reduces the risk of dosage error, target delineation error, and human error, which in the context of RT for TED, has led to poor outcomes, such as radiation retinopathy.23,24
Contour variability in RT is not unique to TED. In the malignant setting, studies evaluating breast, prostate, esophageal, and lung cancer, for example, have found similarly wide degrees of overlap. These same studies have found that variability in the malignant setting can result in clinically meaningful underdosage, and a reduction in tumor control.25, 26, 27 The variability in TED-CON, on the other hand, appears to be less concerning, with orbits that received less than 17 Gy overall versus those at 21 Gy (a difference of 25% in prescription dose), demonstrating no significant difference in outcomes during the post-RT follow-up period. This is consistent with findings from previous smaller studies on noncompressive TED and RT, at doses as low as 2.4 Gy.13, 14, 15
Although limited by our small sample size and retrospective nature, this is the first study to analyze the plausibility of lowering radiation doses to the retro-bulbar space in cases of TED-CON. Although a cumulative dose <35 Gy is usually considered safe for the development of optic neuropathy, there are several published instances of optic neuropathy occurring at lower treatment doses, especially in smokers and cases with nerve compression (2 common characteristics in the TED-CON population). Studies have also identified optic nerve sensitivity to fractionation, finding a significant reduction in optic neuropathy at fractions <2.0 Gy.28, 29, 30, 31, 32 Cases of radiation retinopathy have also been reported in the 10 to 40 Gy range in TED.24,29 The use of low dose RT, or fractions <2 Gy, could allow for retreatment in cases of recurrent TED-CON (which occurs in 15.7% of TED patients), while reducing the risk associated with cumulative damage to collateral tissues.33 This possibility would also allow for retreatment as a salvage option in cases of refractory or recurrent disease.
In this study, overall lens dose was well below the cataractogenic dose, although the fractionation model (dose tolerance of <2 Gy fractions) remains a risk factor.31,32 The authors noted that the use of a 0.5 cm space between the anterior beam edge and the lens appeared to reduce dose to the lens even further (−39.4% in maximum dose and −20.3% in mean dose), though the clinical benefit of this method is unknown (Fig 2). The average age of patients with TED-CON reported here coincides with the natural progression of senile cataract and therefore lowering RT dose even further may be of little consequence (cataract status pre and post RT was not uniformly collected).
Figure 2.
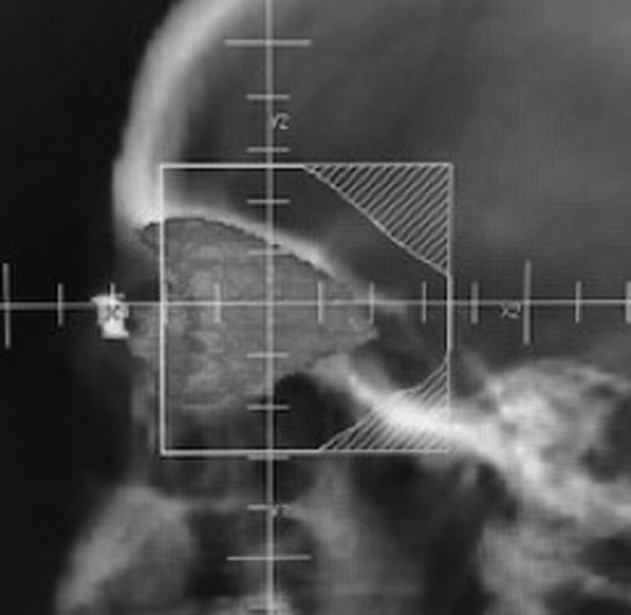
Lens-sparing method: approximately 0.5 cm from posterior edge of lens. Note the anatomic contour in 3-dimensions.
This study is limited by its retrospective nature. Considering the wide contour variability that allowed for the dose comparisons to be made, it is unclear whether previous studies evaluating RT efficacy (or lack thereof), were patients who received a full 20 Gy. A larger study instituting controlled contours and varied prescriptions in radiation dose, such as 10 Gy, 15 Gy, and 20 Gy, would help confirm the findings suggested here.
Footnotes
Sources of support: none.
Reseearch data are stored in an institutional repository and will be shared upon request to the corresponding author (unless otherwise destroyed as per institutional review board requirements upon study expiration).
Disclosures: Dr Wang reports personal fees from AstraZeneca, personal fees and nonfinancial support from Elekta, personal fees and nonfinancial support from Merck, personal fees from Doximity, personal fees from Wolters Kluwer, personal fees and nonfinancial support from Novocure, personal fees and nonfinancial support from Radiation Therapy Oncology Group Foundation, personal fees from Cancer Panels, personal fees and nonfinancial support from AbbVie, personal fees from Rutgers, and personal fees from University of Iowa, outside the submitted work. All other authors have no disclosures.
References
- 1.Godfrey K.J., Kazim M. Radiotherapy for active thyroid eye disease. Ophthal Plastic Reconstr Surg. 2018;34(4S Suppl 1):S98–S104. doi: 10.1097/IOP.0000000000001074. [DOI] [PubMed] [Google Scholar]
- 2.Kazim M., Trokel S., Moore S. Treatment of acute graves orbitopathy. Ophthalmolo Gy. 1991;98:1443–1448. doi: 10.1016/s0161-6420(91)32114-6. [DOI] [PubMed] [Google Scholar]
- 3.Gold K.G., Scofield S., Isaacson S.R., Stewart M.W., Kazim M. Orbital radiotherapy combined with corticosteroid treatment for thyroid eye disease-compressive optic neuropathy. Ophthal Plastic Reconstr Surg. 2018;34:172–177. doi: 10.1097/IOP.0000000000001003. [DOI] [PubMed] [Google Scholar]
- 4.Prummel M.F., Berghout A., Wiersinga W.M., Mourits M.P., Koornneef L., Blank L. Randomised double-blind trial of prednisone versus radiotherapy in Graves' ophthalmopathy. The Lancet. 1993;342:949–954. doi: 10.1016/0140-6736(93)92001-a. [DOI] [PubMed] [Google Scholar]
- 5.Gorman C.A., Garrity J.A., Fatourechi V. A prospective, randomized, double-blind, placebo-controlled study of orbital radiotherapy for Graves’ ophthalmopathy. Ophthalmolo Gy. 2001;108:1523–1534. doi: 10.1016/s0161-6420(01)00632-7. [DOI] [PubMed] [Google Scholar]
- 6.Kahaly G., Böckmann H., Beyer J., Bischoff S. Long-term observation of endocrine ophthalmopathy and retrospective appraisal of therapeutic measures. J Endocrinol Invest. 1990;13:287–292. doi: 10.1007/BF03349564. [DOI] [PubMed] [Google Scholar]
- 7.Tsujino K., Hirota S., Hagiwara M. Clinical outcomes of orbital irradiation combined with or without systemic high-dose or pulsed corticosteroids for Graves’ ophthalmopathy. Int J Radiat Oncol Biol Phys. 2000;48:857–864. doi: 10.1016/s0360-3016(00)00668-4. [DOI] [PubMed] [Google Scholar]
- 8.Ng C.M., Yuen H.K., Choi K.L. Combined orbital irradiation and systemic steroids compared with systemic steroids alone in the management of moderate-to-severe Graves' ophthalmopathy: A preliminary study. Hong Kong Med J. 2005;11:322–330. [PubMed] [Google Scholar]
- 9.Bartley G.B. The epidemiologic characteristics and clinical course of ophthalmopathy associated with autoimmune thyroid disease in Olmsted County, Minnesota. Trans Am Ophthalmol Soc. 1994;92:477. [PMC free article] [PubMed] [Google Scholar]
- 10.Dolman P.J., Rath S. Orbital radiotherapy for thyroid eye disease. Curr Opin Ophthalmol. 2012;23:427–432. doi: 10.1097/ICU.0b013e3283560b2b. [DOI] [PubMed] [Google Scholar]
- 11.Shams P.N., Ma R., Pickles T., Rootman J., Dolman P.J. Reduced risk of compressive optic neuropathy using orbital radiotherapy in patients with active thyroid eye disease. Am J Ophthalmol. 2014;157:1299–1305. doi: 10.1016/j.ajo.2014.02.044. [DOI] [PubMed] [Google Scholar]
- 12.Donaldson S.S., Bagshaw M.A., Kriss J.P. Supervoltage orbital radiotherapy for Graves' ophthalmopathy. J Clin Endocrinol Metab. 1973;37:276–285. doi: 10.1210/jcem-37-2-276. [DOI] [PubMed] [Google Scholar]
- 13.Kahaly G.J., Ro¨sler H.P., Pitz S., Hommel G. Low-versus high-dose radiotherapy for Graves’ ophthalmopathy: A randomized, single blind trial. J Clin Endocrinol Metab. 2000;85:102–108. doi: 10.1210/jcem.85.1.6257. [DOI] [PubMed] [Google Scholar]
- 14.Gerling J., Kommerell G., Henne K., TAO Multicenter Study Group Retrobulbar irradiation for thyroid-associated orbitopathy: Double-blind comparison between 2.4 and 16 Gy. Int J Radiat Oncol Biol Phys. 2003;55:182–189. doi: 10.1016/s0360-3016(02)03795-1. [DOI] [PubMed] [Google Scholar]
- 15.Johnson K.T., Wittig A., Loesch C., Esser J., Sauerwein W., Eckstein A.K. A retrospective study on the efficacy of total absorbed orbital doses of 12, 16 and 20 Gy combined with systemic steroid treatment in patients with Graves’ orbitopathy. Graefes Arch Clin Exp Ophthalmol. 2010;248:103. doi: 10.1007/s00417-009-1214-3. [DOI] [PubMed] [Google Scholar]
- 16.Osaki T.H. Comparison of methodologies in volumetric orbitometry. Ophthalmic Plast Reconstr Surg. 2013;29:431–436. doi: 10.1097/IOP.0b013e31829d028a. [DOI] [PubMed] [Google Scholar]
- 17.Petris C., Kazim M.K. Standardization of orbital volume measurement. Abstract presented at: American Society of Ophthalmic Plastic and Reconstructive Surgery. 2015 [Google Scholar]
- 18.Neigel J.M., Rootman J., Belkin R.I. Dysthyroid optic neuropathy: The crowded orbital apex syndrome. Ophthalmolo Gy. 1988;95:1515–1521. doi: 10.1016/s0161-6420(88)32978-7. [DOI] [PubMed] [Google Scholar]
- 19.Choi C.J., Oropesa S., Callahan A.B. Patterns of visual field changes in thyroid eye disease. Orbit. 2017;36:201–207. doi: 10.1080/01676830.2017.1314510. [DOI] [PubMed] [Google Scholar]
- 20.Giaconi J.A., Kazim M., Rho T., Pfaff C. CT scan evidence of dysthyroid optic neuropathy. Ophthalmic Plast Reconstr Surg. 2002;18:177–182. doi: 10.1097/00002341-200205000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Li X.A., Tai A., Arthur D.W., Buchholz T.A. Variability of target and normal structure delineation for breast cancer radiotherapy: An RTOG Multi-Institutional and Multiobserver Study. Int J Radiat Oncol Biol Phys. 2009;73:944–951. doi: 10.1016/j.ijrobp.2008.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Njeh C.F. Tumor delineation: The weakest link in the search for accuracy in radiotherapy. J Med Phys. 2008;33:136. doi: 10.4103/0971-6203.44472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nikoskelainen E., Joensuu H. Retinopathy after irradiation for Graves' ophthalmopathy. The Lancet. 1989;334:690–691. doi: 10.1016/s0140-6736(89)90944-6. [DOI] [PubMed] [Google Scholar]
- 24.Miller M.L., Goldberg S.H., Bullock J.D. Radiation retinopathy after standard radiotherapy for thyroid-related ophthalmopathy. Am J Ophthalmol. 1991;112:600–601. doi: 10.1016/s0002-9394(14)76869-2. [DOI] [PubMed] [Google Scholar]
- 25.Rasch C.R., Steenbakkers R.J., Fitton I. Decreased 3D observer variation with matched CT-MRI, for target delineation in Nasopharynx cancer. Radiation Oncolo Gy. 2010;5:21. doi: 10.1186/1748-717X-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rasch C., Steenbakkers R., van Herk M. Target definition in prostate, head, and neck. Semin Radiat Oncol. 2005;15:136–145. doi: 10.1016/j.semradonc.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 27.Wong E.K., Truong P.T., Kader H.A. Consistency in seroma contouring for partial breast radiotherapy: Impact of guidelines. Int J Radiat Oncol Biol Phys. 2006;66:372–376. doi: 10.1016/j.ijrobp.2006.05.066. [DOI] [PubMed] [Google Scholar]
- 28.Merriam G.R., Jr., Szechter A., Focht E.F. Columbia-Presbyterian Medical Center, New York. Columbia University, New York; 1 January, 1972. Effects of Ionizing Radiations on the Eye. [Google Scholar]
- 29.Peeler C.E., Cestari D.M. Radiation optic neuropathy and retinopathy with low dose (20 Gy) radiation treatment. Am J Ophthalmol Case Rep. 2016;3:50–53. doi: 10.1016/j.ajoc.2016.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferguson I., Huecker J., Huang J., McClelland C., Van Stavern G. Risk factors for radiation-induced optic neuropathy: A case–control study. Clin Exp Ophthalmol. 2017;45:592–597. doi: 10.1111/ceo.12927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Finger P.T. Radiation therapy for orbital tumors: Concepts, current use, and ophthalmic radiation side effects. Surv Ophthalmol. 2009;54:545–568. doi: 10.1016/j.survophthal.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 32.Jeganathan V.S., Wirth A., MacManus M.P. Ocular risks from orbital and periorbital radiation therapy: A critical review. Int J Radiat Oncol Biol Phys. 2011;79:650–659. doi: 10.1016/j.ijrobp.2010.09.056. [DOI] [PubMed] [Google Scholar]
- 33.Patel P., Khandji J., Kazim M. Recurrent thyroid eye disease. Ophthalmic Plast Reconstr Surg. 2015;31:445–448. doi: 10.1097/IOP.0000000000000371. [DOI] [PubMed] [Google Scholar]