Abstract
Purpose
Interest and application of stereotactic radiosurgery for multiple brain metastases continue to increase. Various planning systems are available for linear accelerator (linac)–based single-isocenter multiple metastasis radiosurgery. Two of the most advanced systems are BrainLAB Multiple Metastases Elements (MME), a dynamic conformal arc (DCA) approach, and Varian RapidArc (RA), a volumetric modulated arc therapy (VMAT) approach. In this work, we systematically compared plan quality between the 2 techniques.
Methods and Materials
Thirty patients with 4 to 10 metastases (217 total; median 7.5; Vmin = 0.014 cm3; Vmax = 17.73 cm3) were planned with both Varian RA and MME at 2 different institutions with extensive experience in each respective technique. All plans had a single isocenter and used Varian linac equipped with high-definition multileaf collimator. RA plans used 2 to 4 noncoplanar VMAT arcs with 10 MV flattening filter-free beam. MME plans used 4 to 9 noncoplanar DCAs and 6 MV flattening filter-free beam, (minimum planning target volume [PTVmin] = 0.49 cm3; PTVmax = 27.32 cm3; PTVmedian = 7.05 cm3). Prescriptions were 14 to 24 Gy in a single fraction. Target coverage goal was 99% of volume receiving prescription dose (D99% ≥ 100%). Plans were evaluated by Radiation Therapy Oncology Group/Paddick conformity index (CI) score, 12 Gy volume (V12Gy), V8Gy, V5Gy, mean brain dose (Dmean), and beam-on time.
Results
Conformity was favorable among RA plans (median: MME CIRTOG = 1.38; RA CIRTOG = 1.21; P < .0001). V12Gy and V8Gy were lower for RA plans (median: MME V12 = 23.7 cm3; RA V12 = 19.2 cm3; P = .0001; median: MME V8Gy = 53.6 cm3; RA V8Gy = 44.1 cm3; P = .024). V5Gy was lower for MME plans (median: MME V5Gy = 141.4 cm3; RA V5Gy = 142.8 cm3; P = .009). Mean brain was lower for MME plans (median: MME Dmean = 2.57 Gy; RA Dmean = 2.76 Gy; P < .0001).
Conclusions
For linac-based multiple metastasis stereotactic radiosurgery, RapidArc VMAT facilitates favorable conformity and V12Gy/V8Gy volume compared with the MME DCA plan. MME planning facilitates reduced dose spill at levels ≤V5Gy.
Introduction
Brain metastases are the most common form of brain tumor and remain a challenge for multidisciplinary cancer care. Surgery, whole brain radiation treatment, and stereotactic radiosurgery (SRS) are all used in the treatment of brain metastases. Radiosurgery has become a staple in the management of brain metastases.1, 2, 3, 4, 5 Gamma Knife, Cyberknife, and linear accelerator (linac)–based SRS are all widely used modalities.
Linac-based SRS methods for the treatment of a single lesion include circular arcs with stereotactic cones, dynamic conformal arcs (DCA) with multileaf collimator (MLC), intensity modulated radiosurgery, and volumetric modulated arc radiosurgery with a linac isocenter positioned at each target. For multiple brain metastases, the planning effort and delivery time of this technique are proportional to the number of lesions. The treatment time for a single target is approximately 15 to 20 minutes and increases with the treatment of additional metastases.
Many institutions now treat multiple metastases with a single isocenter using a volumetric modulated arc therapy (VMAT) technique.6, 7, 8, 9 Clark et al. were the first to publish the feasibility of this technique using the Varian Eclipse RapidArc (RA) in 2010,7 and provided a planning recipe in a subsequent paper in 2012.8 An initial group of works implied that VMAT should always produce poorer dosimetric quality in terms of conformity index (CI) and low dose spill.9, 10, 11 However, later work compared the dosimetric parameters using an updated version of the Clark 2012 technique on 28 patients with multiple metastases with the dosimetry of a Gamma Knife 4C unit and achieved clinically equivalent results between the modalities.12
Liu et al13 performed a similar dosimetric comparison to Perfexion, the newer generation of Gamma Knife, and achieved similar results. However, the quality of single isocenter VMAT is heavily dependent on planner experience and the particular treatment planning system. Planning time is typically an initial 15 to 30 minutes of optimization configuration and an additional 10 to 15 minutes of computation per iteration of optimization.
Huang et al14 proposed single-isocenter DCA to treat multiple brain metastases and showed that this has similar plan quality as multi-isocenter DCA plans, with lower peripheral dose spread but worse conformity than VMAT plans. A commercially available planning software using a similar technique developed by BrainLAB AG (Feldkirchen, Germany), Multiple Metastases Element (MME), has been adopted by some institutions. MME uses a preconfigured set of DCAs with a single isocenter to treat up to 15 metastases in 1 session. MME then optimizes the weight for each arc to achieve the best overall conformity among all targets. Planning time for optimization and dose calculation is 2 to 4 minutes, which dramatically improves the planning efficiency. MME also reduces the variability in plan quality among different skill level planners owing to minimal adjustable parameters.
In this interinstitutional study involving Thomas Jefferson University (TJU) and the University of Alabama at Birmingham (UAB) radiation oncology departments, we compared the planning quality of these 2 single-isocenter multiple metastasis linac-based SRS planning approaches. A total of 30 patients with 217 cumulative brain metastases were included. Each institution contributed 15 patients with 4 to 10 brain metastases. This study is different from previous works9, 10, 11, 12, 13 because of the increased number of patients and metastases, and plans were generated by users who already had extensive experience using the planning techniques.
Methods and Materials
Case selection and characteristics
Thirty patients (15 from each institution) who had 4 to 10 brain metastases and were treated with single-isocenter linac-based SRS were selected for this study. Fifteen previously treated patients from TJU were identified with cases that had been planned with BrainLAB MME and treated on a Varian TrueBeam STx Linac system equipped with high-definition HD120 MLC using 6 MV photon flattening filter-free (FFF) mode.
Digital Imaging and Communications in Medicine (DICOM) files of the computed tomography images and structure sets were de-identified and sent to UAB for replanning with RA. Fifteen patients who were planned with RA and treated on either Varian TrueBeam STx or Edge with HD120-MLC using 10 MV FFF mode (these linear accelerators are identical platforms; differences in accessories that accompany the Edge are not germane to planning dosimetry) were identified from UAB. Digital Imaging and Communications in Medicine files of the images and structure sets were de-identified and sent to TJU for replanning with MME. Prescriptions for each target were not altered between the plans.
For most metastases, TJU prescription selections for each metastasis are largely based on the Radiation Therapy Oncology Group (RTOG) 9508 study.15 UAB practices are similar except that a maximum 20 Gy instead of 24 Gy at the highest dose level is favored. The prescription dose for each target in this series ranged from 14 to 24 Gy in a single fraction and was selected based on individual target volume and clinician preference. Plans were normalized according to a planning goal 99% of the target volume coverage with its prescription dose. Case characteristics were target numbers (min = 4, max = 10; median = 7.5), individual target volume (TVmin = 0.014 cm3; TVmax = 17.73 cm3; TVmedian = 0.35 cm3), and planning target volume (PTVmin = 0.49 cm3; PTVmax = 27.32 cm3; PTVmedian = 7.05 cm3).
Thomas Jefferson University planning technique for single-isocenter multiple metastases using BrainLAB Elements
TJU has been using MME to plan and treat patients with multiple brain metastases since 2015. More than 150 patients and 600 metastases have been treated. MME is a dedicated automatic planning software for multiple brain metastases designed to treat multiple targets (up to 15 in the current version) simultaneously with a single setup isocenter using multiple noncoplanar DCAs. The isocenter location is placed at the center of mass of all PTVs and is not adjustable by planner.
The couch angles of the noncoplanar DCA are preconfigured in planning templates. In this study, 6 different templates were used for each case, and the plan that achieved the best conformity was selected as the final plan. The first template consists of 5 couch angles with 40° separations at 0°, 40°, 80°, 340°, and 300°, respectively (IEC 61217 convention). The second one consists of 5 couch angles at 10°, 50°, 90°, 350°, and 310°. The third one consists of 6 couch angles at 0°, 35°, 70°, 355°, 320°, and 285° (35° separation). The fourth one consists of 6 couch angles at 10°, 42°, 74°, 350°, 318°, and 286° (32° separation). The fifth one consists of 6 couch angles at 0°, 30°, 60°, 90°, 330°, 300° (30° separation). The last one consists of 6 couch angles at 20°, 48°, 76°, 340°, 312°, 284° (28° separation). The MME optimizer selects couch angles from the available set and the final plan usually consists of 4 to 6 different couch angles.
The start and stop gantry angles for each arc are first set to default values (10° to 170° when couch angles are between 0° and 90°; 190° to 350° when couch angles are between 270° and 360° [IEC 61217 convention]). The arcs are automatically modified during optimization. Two arcs are delivered for each couch angle (one forward swept, one reverse swept). Collimator rotation is used to smear out the inter-MLC leaf radiation leakage. The MLC leaves are shaped to conform to each individual PTV, with an additional margin of up to 1 mm selected automatically during optimization. The target volumes irradiated by each arc are chosen to maximize the number of targets while minimizing leakage radiation to nontarget tissue.
Each leaf pair is only allowed to treat 1 PTV at any time. For each arc, MME will try to include as many metastases as possible to treat. However, if >1 target is in line with a single leaf pair, MME will select one to treat in one arc and then generate another arc in the reverse direction on the same table angle for each remaining lesion to prevent the phenomenon, referred to as “island-blocking,”16,17 from occurring, whereby a leaf pair is open across 2 in-line targets with nontarget tissue exposed in between.
After the automatic assignment of PTVs to each arc, the arc weights are optimized to achieve the best overall conformity. Because the dose prescriptions to the PTVs are enforced (>99% target volume receives prescription dose) during the optimization, the CI is given by the ratio between the volume surrounding the PTV receiving greater than the prescribed dose and the volume of the PTV. If perfect conformity is achieved, the CI score is 1.
Planning time for optimization and dose calculation was approximately 2 to 4 minutes per planning template, depending on the complexity of the case. The total planning time using all 6 templates was typically 20 to 30 minutes. Plans were developed using MME version 1.5 for the 6 MV FFF beam of a Varian TrueBeam STx equipped with an HD-120 MLC. The dose was calculated using a pencil-beam algorithm with a calculation grid of 1 mm.
University of Alabama at Birmingham planning technique for single-isocenter multiple metastases SRS using Eclipse RapidArc
The UAB technique was initially developed in 20107,8 and has since been refined. More than 1000 cases have been treated with this technique since 2010, targeting at least 3000 lesions with the current average annual of 150 patients with brain tumors in 2017. A large number of cases with >10 targets have been treated with this technique up to a maximum of 27.
At its core, this VMAT technique uses a single isocenter placed at the multiple target centroid, and 3 concentric rings of tuning structures encompassing each target to enforce dose falloff in a stepwise fashion. The inner ring drives conformity, the middle drives the 50% isodose volume, and the outer drives the 40% isodose volume. These ring structures were used to help achieve highly conformal prescription doses to targets while reducing moderate and low-dose spills. A separate constraint on the brain volume with targets excluded penalizes the low-dose spill. If a target is in close proximity to a critical structure, such as the brain stem or optic apparatus, additional optimization criteria are added to prioritize organ avoidance.
Plans consisted of 2 to 4 VMAT arcs: one 360° axial arc and up to three 180° noncoplanar half arcs at couch angles of 45°, 90°, and 315° (IEC convention). The collimator angles were set to 30° or 45° and modified as needed to minimize island blocking between lesions,16,17 and jaw tracking was enabled. The plans were normalized such that the prescription dose covered 99% of the composite target volume.
The detailed configuration and dose optimization criteria have been previously published as part of an earlier study,8,12 circulated widely and are also made available here in Appendix E1 (available online at https://doi.org/10.1016/j.adro.2019.10.007).
Setup of the tuning structures requires approximately 5 to 10 minutes for each plan, and optimization and calculation of the plan for a 1-mm dose grid require approximately 10 to 15 minutes. Optimization and calculation time are heavily dependent on calculation grid size and hardware specifications. Plans were developed using Eclipse version 13.6 (Varian Medical Systems, Palo Alto, CA) for the 10 MV FFF beam of a Varian Edge equipped with an HD-120 MLC. The HD-120 has a 2.5-mm leaf width in the central 8 cm and 5-mm leaf width in the remaining peripheral region. Dose was calculated using the analytical anisotropic algorithm, version 13.6.
Plan comparison and dosimetric parameter extraction
Dose-volume results often vary between treatment planning systems for the same 3-dimensional dose distribution owing to algorithmic and beam modeling differences, particularly for very small targets.18, 19, 20 Therefore, we chose to use a third-party software platform for dosimetric analysis. Additionally, neither institution had made modifications to the default beam model other than the standard measurement values prescribed by the TG-51 normal commissioning process for the treatment plans generated here.21 Computed tomography images, structure sets, and 3-dimensional dose matrices (1-mm dose grid) of all 60 plans were sent to MIM after calculation (MIM Software Inc, Cleveland, OH, version 6.5.7) for dose-volume histogram (DVH) analysis. In this manner, inter-treatment planning system dose-volume dependency uncertainty was eliminated. Each institution has performed robust verification of its respective beam model and performs patient-specific dosimetric quality assurance for multiple targets in each of its radiosurgery plans.
At the level of the entire plan, the following parameters were extracted: V12Gy (cm3), V8Gy (cm3), V5Gy (cm3), and mean brain dose (Dmean). The nomenclature described by Mayo et al22 was followed for reporting dose-volume metrics. For each individual target, the following parameters were extracted: Paddick CI, RTOG CI, and percentage volume covered by prescription dose (coverage).
| (1) |
| (2) |
Both RTOG and Paddick CIs are included for reader preference and ease of reference. All parameters were compared using a paired 2-tailed Wilcoxon signed-rank test at a prespecified level of significance of .05. The Wilcoxon signed-rank test was chosen to avoid the population normality assumption required of a parametric paired test. Mean and median statistics are reported for each parameter, but statistics are reported upon the median because the Wilcoxon signed-rank test was used.
Results
Both MME and RA were able to achieve clinically satisfactory plans in all cases. Among individual targets (217 total), conformity was favorable for RA plans (median ± standard deviation: RTOG_CI (MME) = 1.38 ± 0.25; RTOG_CI (RA) = 1.23 ± 0.32; P < .0001; PCI (MME) = 0.70 ± 0.1; PCI (RA) = 0.77 ± 0.12; P < .0001). Mean target coverage by prescription dose was 97.5 ± 2.3% for MME and 96.9% ± 2.5% for RA plans (P = .007). Maximum target doses were not a studied parameter in this investigation, but no target dose exceed 175% of the prescription.
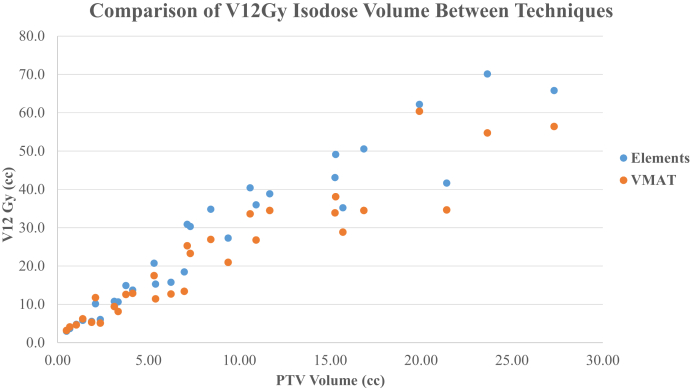
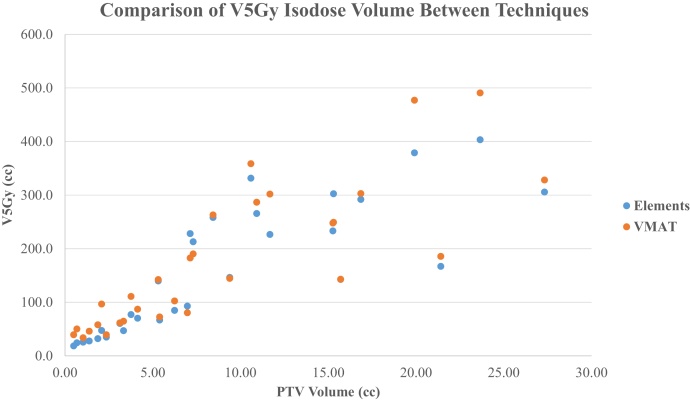
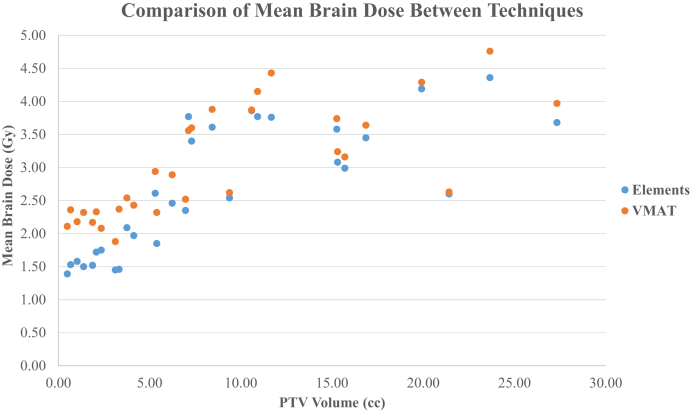
Table 1 summarizes in detail the number of metastases, prescription dose, total PTV volume, V12Gy, V5Gy, and mean brain dose of the MME and RA plans. When comparing each case, V12Gy was favorable among RA plans (median: V12Gy [cm3]; MME = 23.7 cm3; V12Gy [cm3]; RA = 19.2 cm3; P < .0001). As shown in Figure 1, RA plans achieved less V12Gy for 26 of 30 cases. The few cases in which MME plans achieved comparable or less V12Gy were all among those with small total PTV (VPTV < 2.1 cm3). V8Gy was favorable among RA plans (median: V8Gy [cm3]; MME = 53.6 cm3; V8Gy [cm3]; RA = 44.1 cm3; P = .024). V5Gy was favorable among the MME plans (median: V5Gy [cm3]; MME = 141.4 cm3; V5Gy [cm3]; RA = 142.8 cm3; P = .009). As shown in Figure 2, MME plans achieved less V5Gy (cm3) for 25 of 30 cases. Figure 3 shows the distributions of mean brain doses between techniques plotted against total target volume. We observed a slightly lower mean brain dose in MME plans compared with RA (median: MME Dmean = 2.57 Gy; RA Dmean 2.76 Gy; P < .0001) is evident.
Table 1.
Number of metastases, prescription dose, total planning target volume, V12Gy volume, V8Gy, V5Gy, and mean brain dose of the Element and VMAT plans of the 30 cases in this study
| No. Metastases/ case | Rx |
VPTV |
V12Gy (cm3) |
V8Gy (cm3) |
V5Gy (cm3) |
Mean brain dose (Gy) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (Gy) | (cm3) | Elements | VMAT | Elements | VMAT | Elements | VMAT | Elements | VMAT | ||
| mean | 7.4 | 17.8 | 8.96 | 27.2 | 22.4 | 57.8 | 52.4 | 158.3 | 174.8 | 2.66 | 3.03 |
| median | 7.5 | 18 | 7.05 | 24.0 | 19.2 | 53.6 | 44.1 | 141.4 | 142.8 | 2.57 | 2.76 |
| P-value | < .0001 | .024 | .009 | < .0001 | |||||||
Abbreviations: Rx = prescription; VMAT = volumetric modulated arc therapy; VPTV = total PTV volume.
Figure 1.
Comparison of 12 Gy isodose volume for 30 cases between 2 different techniques.
Figure 2.
Comparison of 5 Gy isodose volume for 30 cases between 2 different techniques.
Figure 3.
Comparison of mean brain dose for 30 cases between 2 techniques.
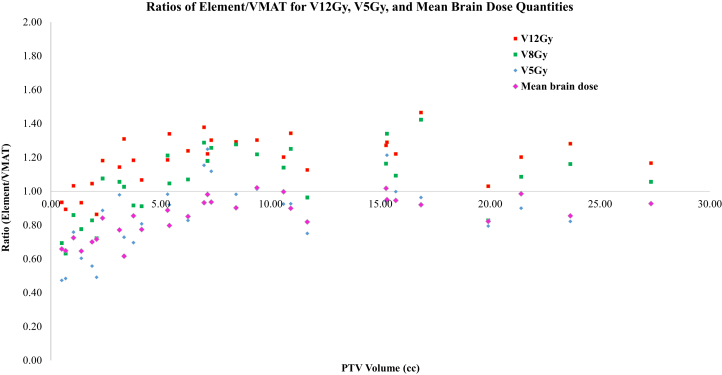
Figure 4 shows the ratio of 4 parameters (V12Gy, V8Gy, V5Gy, and Dmean) between MME and RA plans. For the 6 cases in our data set in which total target volume was <2.1 cm3, absolute differences were small, MME was favorable for each of the V8, V5, and mean brain dose parameters. For the V12 parameter, MME was favorable in 4 of 6 cases. For the remaining 24 cases with PTV >2.1 cm3, V12 favored RA plans (ratio mean 1.24). In these cases, while MME plans still predominantly had favorable V5 and mean brain dose, the respective relative differences were much smaller.
Figure 4.
Ratio of V12Gy (cm3), V5Gy (cm3), and Dmean_brain between the BrainLAB Multiple Metastases Elements and Varian RapidArc plans.
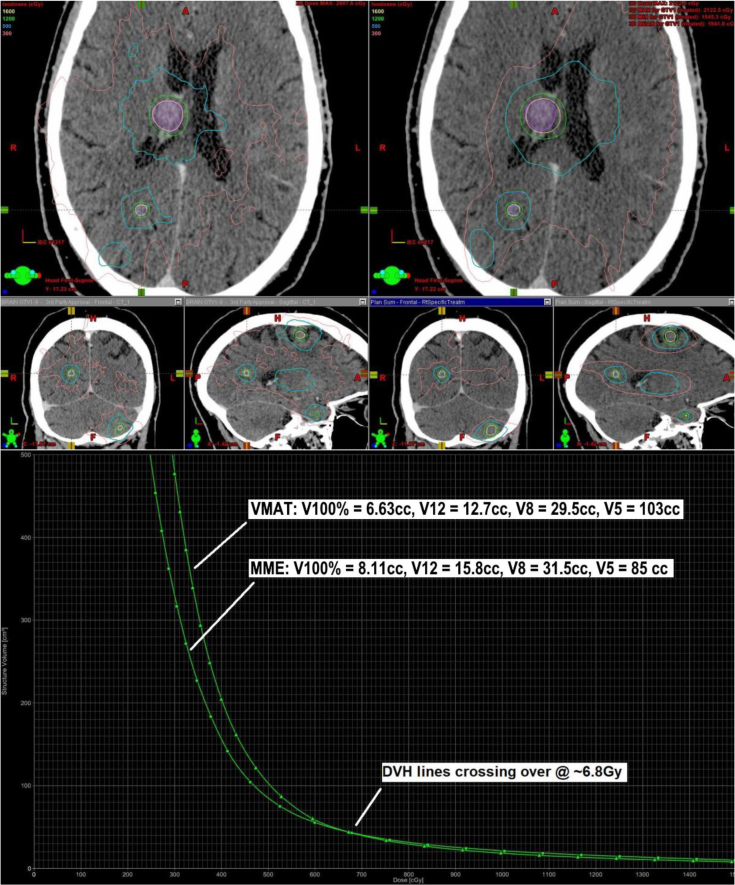
Figure 5 depicts the relationship between the 2 modalities for a representative case (n = 9 targets, total PTV = 6.24 cm3; Rx = 16 Gy to all targets). Figure 5 (top) shows a side-by-side comparison of dose distribution on the axial, sagittal, and coronal planes. Figure 5 (bottom) shows the DVH of normal brain tissue of this case. Note that the normal brain DVHs cross at 6.8 Gy, which represents the dose level below which MME has reduced low-dose spill. This case was representative of the majority of cases in which small but persistent and statistically significant differences persisted between the 2 modalities For dose levels ranging from approximately 6 Gy to the prescription dose, RA produced favorable isodose spill and for dose levels below approximately 6 Gy, MME produced favorable isodose spill.
Figure 5.
(Top) Side-by-side comparison of (left) volumetric modulated arc therapy Varian RapidArc and (right) BrainLAB Multiple Metastases Elements dose distributions on axial, sagittal, and coronal computed tomography slice for a representative case having 9 brain metastases, each prescribed 16 Gy with a total planning target volume of 6.24 cm3, (bottom) dose-volume histogram of normal brain tissue for each plan in this case.
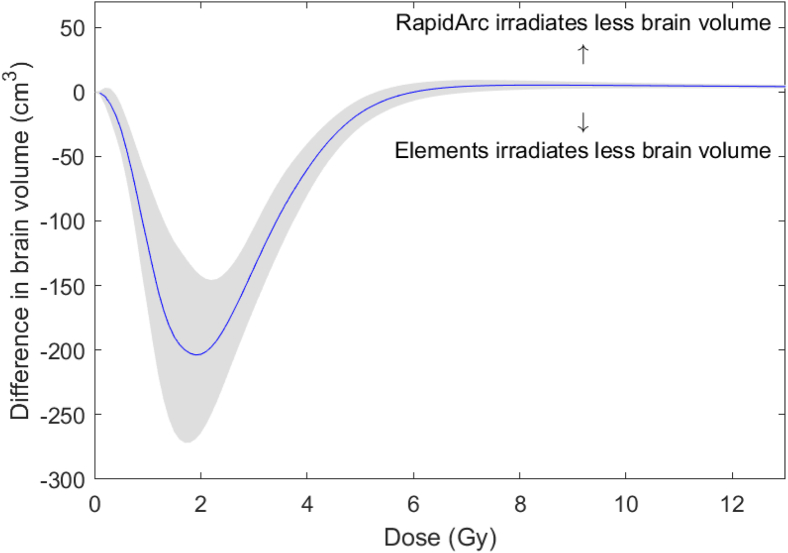
Figure 6 illustrates this phenomenon by showing a plot of the absolute difference at each point in the brain DVH line for the 2 techniques averaged across all 30 cases. The dose level at which the plotted difference in brain volume crosses the x-axis and becomes >0 represents the dose level (~ 6 Gy) above which RapidArc plans demonstrate superior performance. Below this dose level, MME plans demonstrate superior performance, most notably around the 2 Gy isodose line.
Figure 6.
Mean difference between the normal brain dose-volume histograms of BrainLAB Multiple Metastases Elements plans and Varian RapidArc plans. The gray bands indicate the 95% confidence intervals.
Because this was a treatment planning study involving replanning, treatment times could not be compared, but the authors recognize the importance of treatment time in plan consideration. Treatment time is equal to the beam-on time, plus the beam-off time required for room entry, table adjustments, patient positioning corrections, and reimaging. All RA plans in the UAB method use one 360° axial arc, and between 1 and 3 nonaxial 180° arc positions. RA plans rotate through each arc position once. All MME plans used between 4 and 6 noncoplanar table angles with 160° gantry arc length. MME plans sometimes generate 2 gantry arc rotations at the same table position. The total number of arcs were 7.3 ± 1.4 (range, 5-10) for MME plans and 3.8 ± 0.6 (range, 2-4) for RA plans. The median number of table positions was 5 (range, 4-6) for MME plans and 4 (range, 2-4) for RA plans.
The total gantry rotation angles were 1168° ± 223° for MME plans and 872° ± 100° for RA plans. The total number of monitor unit (MU) were 6871 ± 1392 for MME plans and 10225 ± 3449 for RA plans. The gantry on the TrueBeam STx platform cannot rotate faster than 359° per minute. For MME plans, average beam-on time is approximately 6 to 9 minutes at 6 MV FFF (1400 MU/min). Only slight gains in beam-on time would be realized in 10 MV FFF (2400 MU/min) as the plans are still gantry rotation rate constrained. For RA plans, average beam-on time is 3 to 4 minutes in 10 MV FFF. If operated in 6 MV FFF mode, this time would be expected to increase to 4.5 to 6.6 minutes.
Approximately 30 seconds to 1 minute is required for each table position adjustment for either type of plan if no additional imaging is performed in between couch positions, and is similar between the institutions. Because RA plans require fewer arcs in fewer couch positions, and despite their higher average number of MUs per plan, they can be expected to be delivered slightly more efficiently.
Discussion
Treatment planning software and techniques continue to advance; thus, capabilities should be benchmarked and systematically evaluated. An increasingly large number of centers have begun using linear accelerators for SRS instead of Gamma Knife23 because of increased efficiency and comparable plan quality. The platforms evaluated in this study, RA and MME, are 2 of the most recently developed treatment planning platforms for single-isocenter multiple metastasis linac SRS.
Small previous studies have compared the 2 treatment planning systems. Gevaert et al.24 compared these 2 techniques for 10 cases (40 metastases) and concluded that both V12Gy (cm3) and V5Gy (cm3) were significantly higher for RA plans compared with MME plans. The Paddick CI scores for MME and RA plans in their study were 0.65 ± 0.08 and 0.67 ± 0.16, respectively. In our study, the scores were 0.70 ± 0.10 and 0.77 ± 0.12, respectively. The difference between the Paddick CI values for RA plans (0.67 vs. 0.77) suggests that neither the RA nor the MME plans generated in their study were fully optimized. The difference in MME plan quality between our and Gevaert’s study is likely due to improvements in the optimization engine between v1.0 and the more recently available v1.5. We realized that MME plans are easier to achieve consistent plan quality compared with RA plans, which can be both technique and planner dependent. The difference in quality of the RapidArc plans is most likely the increased sensitivity of VMAT plans to planner experience, skill, and adherence to previously demonstrated planning methodology. All 30 VMAT plans in our study were generated by an expert VMAT planner who used the UAB method for single-isocenter VMAT multiple metastasis SRS. Comparing with Gevaert's study, this study has more patients (30 vs 10) and more targets (217 vs 40).
Another study by Narayanasamy et al.25 compared 8 patients (40 targets) and concluded that the 2 techniques achieved comparable CI scores, V12Gy (cm3), and mean brain dose. However, limited case numbers likely provided insufficient power to detect detailed differences between the 2 treatment planning systems. The CI values (inverse Paddick definition) in their study have a mean of 1.8 and 1.7 and maximum of 4.5 and 4.6 for MME and VMAT plans, respectively, which likely represent plans not fully optimized.
Although RA plans tended to be more favorable for conformity and moderate-to-high isodose metrics, and MME plans tended to be more favorable for low-dose metrics, both platforms were able to meet clinical planning goals and generate high-quality radiosurgery plans. Our study design empowered detection of subtle differences in the aforementioned dosimetric quantities, but the clinical sequelae of such differences remains uncertain.
The reason why MME plans were favorable at lower isodose levels and RA plans were favorable at higher isodose levels was not immediately evident; however, a likely contributing factor is that low isodose levels (eg, <50% isodose line reflected by the Paddick Gradient Index or other measures of direct high-to-moderate dose falloff) have not been historically penalized. UAB’s RA planning methodology uses a low-dose constraint limiting the volume of the isodose volume below one-sixth of the prescription dose (eg, 3 Gy for an 18 Gy prescription) but does not penalize the dose spill below that level. MME makes a substantial effort, even to the expense of sometimes a very meaningful increase in treatment time, to never allow island blocking by simply adding additional arcs as necessary. In the version of RapidArc (13.6) tested herein, the planner must manually select a collimator angle, and the optimizer makes no special effort to eliminate island blocking if a fluence pattern is identified that meets the dose-volume criteria specified in the optimizer. The works we previously referenced with regard to this phenomenon mitigate this by using software to select the collimator angles for each beam with the least total in-line area between targets across all control points. This feature is not currently native to Eclipse 13.6, but will be in later VMAT solutions.
With regard to the magnified difference in low-isodose spill and better performance of MME in general for cases with low total target volume, we suspect that leakage doses associated with leaf travel may be a contributing factor. In addition, the shell structures used across all plans may simply not penalize aggressively enough the dose falloff for small targets with inherently less integral buildup necessary for prescription coverage.
Multiple works have retrospectively associated moderate isodose spills (V8Gy, V10Gy, V12Gy) with adverse treatment effect (ie, radionecrosis).26, 27, 28 No studies have yet correlated toxicity with low-dose spill. However, as patients with brain metastases live longer and become more likely to receive sequential salvage radiosurgery or whole brain radiation treatment for distant brain failure, the accumulation of a low dose may become an important prognostic feature of cognitive outcome.
We did not compare differences in homogeneity or maximum dose in this study, because neither of the institutions in this study routinely employs constraints on the maximum dose within targets during radiosurgery planning. We recognize that some clinicians do use hotspot constraints in their linac radiosurgery plans, but we do not recommend the practice because of the associated detriment to other aspects of plan quality.
There a few limitations of our study that bear notation. One limitation is that the single-isocenter dynamic conformal arc plans use a 6 MV FFF beam, whereas the single-isocenter VMAT plans employed a 10 MV FFF beam. These were unavoidable consequences of the commissioned beam availability at each respective institution. Comparisons of 6 MV and 10 MV VMAT plans have been made for spine29 and lung30 but not for multiple brain metastases. We suspect that there are very little differences between the beam types for high and moderate isodoses, but differences in the shapes of the lateral edge of the penumbras between the 6 MV and 10 MV FFF beams might cause small differences in the low isodose volumes or the mean dose to the brain, particularly for the treatment of small targets on field edge being or near the penumbra. This may also explain a portion of the magnified difference we observed between the 2 techniques for cases with very low total target volume. Future work will seek to understand these differences specifically for multiple metastases plans in both VMAT and single-isocenter dynamic conformal arc plans.
As newer and more advanced algorithms are developed (eg, HyperArc option in Eclipse version 15.5), the authors of this study will be using the same data set to evaluate. The existing data in this study provide a good benchmark for future studies. The authors of this study intend make available and share the de-identified data set analyzed herein, so that any interested readers may use the data and develop plans using their own institution's planning software and techniques, and compare with our results.
Conclusions
We systematically compared the plan quality of single-isocenter RapidArc VMAT and Elements MME dynamic conformal arc radiosurgery plans across a large number of patients and determined that both systems reliably generate excellent plans. This study represents the largest and most robust plan quality comparison to date between 2 multiple metastasis radiosurgery planning platforms.
Furthermore, to our knowledge, this is the first multi-institutional study of this kind. Sufficiently large patient and target numbers were available to detect subtle differences in the metrics of plan quality for both platforms. Our data suggest that RA plans are slightly favorable for conformity and high-to-moderate isodose levels, and MME plans are slightly favorable for low isodose spills. Further work will assess how continued updates to each software platform affect these results.
Footnotes
Sources of support: This work had no specific funding.
Disclosures: No financial support was received for the production or publication of this work. Drs. Evan M. Thomas, John B. Fiveash, and Richard A. Popple have existing research support from Varian Medical Systems for unrelated work. Drs. Wenyin Shi and Haisong Liu served as a consultant for Varian Medical Systems for past work. The authors report no other relevant conflicts of interest.
Supplementary material for this article is available at https://doi.org/10.1016/j.adro.2019.10.007.
Supplementary data
References
- 1.Thomas S.S., Dunbar E.M. Modern multidisciplinary management of brain metastases. Curr Oncol Rep. 2010;12:34–40. doi: 10.1007/s11912-009-0073-8. [DOI] [PubMed] [Google Scholar]
- 2.Videtic G.M., Gaspar L.E., Aref A.M. American College of Radiology appropriateness criteria on multiple brain metastases. Int J Radiat Oncol Biol Phys. 2009;75:961–965. doi: 10.1016/j.ijrobp.2009.07.1720. [DOI] [PubMed] [Google Scholar]
- 3.Linskey M.E., Andrews D.W., Asher A.L. The role of stereotactic radiosurgery in the management of patients with newly diagnosed brain metastases: A systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96:45–68. doi: 10.1007/s11060-009-0073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kocher M., Wittig A., Piroth M.D. Stereotactic radiosurgery for treatment of brain metastases: A report of the DEGRO Working Group on Stereotactic Radiotherapy. Strahlenther Onkol. 2014;190:521–532. doi: 10.1007/s00066-014-0648-7. [DOI] [PubMed] [Google Scholar]
- 5.Mehta M.P., Tsao M.N., Whelan T.J. The American Society for Therapeutic Radiology and Oncology (ASTRO) evidence-based review of the role of radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys. 2005;63:37–46. doi: 10.1016/j.ijrobp.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 6.Mayo C.S., Ding L., Addesa A., Kadish S., Fitzgerald T.J., Moser R. Initial experience with volumetric IMRT (RapidArc®) for intracranial stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2010;78:1457–1466. doi: 10.1016/j.ijrobp.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Clark G.M., Popple R.A., Young P.E., Fiveash J.B. Feasibility of single-isocenter volumetric modulated arc radiosurgery for treatment of multiple brain metastases. Int J Radiat Oncol Biol Phys. 2010;76:296–302. doi: 10.1016/j.ijrobp.2009.05.029. [DOI] [PubMed] [Google Scholar]
- 8.Clark G.M., Popple R.A., Prendergast B.M. Plan quality and treatment planning technique for single isocenter cranial radiosurgery with volumetric modulated arc therapy. Practical radiation oncology. 2012;12:306–313. doi: 10.1016/j.prro.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Hardcastle N., Tomé W.A. On a single isocenter volumetric modulated arc therapy SRS planning technique for multiple brain metastases. J Radiosurg SBRT. 2012;2:1–9. [PMC free article] [PubMed] [Google Scholar]
- 10.McDonald D., Schuler J., Takacs I., Peng J., Jenrette J., Vanek K. Comparison of radiation dose spillage from the Gamma Knife Perfexion with that from volumetric modulated arc radiosurgery during treatment of multiple brain metastases in a single fraction. J Neurosurg. 2014;121:51–59. doi: 10.3171/2014.7.GKS141358. [DOI] [PubMed] [Google Scholar]
- 11.Ma L., Nichol A., Hossain S. Variable dose interplay effects across radiosurgical apparatus in treating multiple brain metastases. Int J Comput Assist Radiol Surg. 2014;9:1079–1086. doi: 10.1007/s11548-014-1001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas E.M., Popple R.A., Wu X. Comparison of Plan Quality and Delivery Time between Volumetric Arc Therapy (RapidArc) and Gamma Knife Radiosurgery for Multiple Cranial Metastases. Neurosurgery. 2014;75:409–418. doi: 10.1227/NEU.0000000000000448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu H., Andrews D.W., Evans J.J. Plan quality and treatment efficiency for radiosurgery to multiple brain metastases: Non-coplanar RapidArc vs. Gamma Knife. Front Oncol. 2016;6:26. doi: 10.3389/fonc.2016.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang Y., Chin K., Robbins J.R. Radiosurgery of multiple brain metastases with single-isocenter dynamic conformal arcs (SIDCA) Radiother Oncol. 2014;112:128–132. doi: 10.1016/j.radonc.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 15.Andrews D.W., Scott C.B., Sperduto P.W. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: Phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363:1665–1672. doi: 10.1016/S0140-6736(04)16250-8. [DOI] [PubMed] [Google Scholar]
- 16.Kang J., Ford E.C., Smith K., Wong J., McNutt T.R. A method for optimizing LINAC treatment geometry for volumetric modulated arc therapy of multiple brain metastases. Med Phys. 2010;37:4146–4154. doi: 10.1118/1.3455286. [DOI] [PubMed] [Google Scholar]
- 17.Yuan Y., Thomas E.M., Clark G.A., Markert J.M., Fiveash J.B., Popple R.A. Evaluation of multiple factors affecting normal brain dose in single-isocenter multiple target radiosurgery. J Radiosurg SBRT. 2018;5:131–144. [PMC free article] [PubMed] [Google Scholar]
- 18.Niemierko A., Goitein M. Random sampling for evaluating treatment plans. Med Phys. 1990;17:753–762. doi: 10.1118/1.596473. [DOI] [PubMed] [Google Scholar]
- 19.Ebert M.A., Haworth A., Kearvell R. Comparison of DVH data from multiple radiotherapy treatment planning systems. Phys Med Biol. 2010;55:N337–N346. doi: 10.1088/0031-9155/55/11/N04. [DOI] [PubMed] [Google Scholar]
- 20.Nelms B., Stambaugh C., Hunt D., Tonner B., Zhang G., Feygelman V. Methods, software and datasets to verify DVH calculations against analytical values: Twenty years late. Med Phys. 2015;42:4435–4448. doi: 10.1118/1.4923175. [DOI] [PubMed] [Google Scholar]
- 21.Almond P.R., Biggs P.J., Coursey B.M. AAPM's TG-51 protocol for clinical reference dosimetry of high-energy photon and electron beams. Med Phys. 1999;26:1847–1870. doi: 10.1118/1.598691. [DOI] [PubMed] [Google Scholar]
- 22.Mayo C.S., Pisansky T.M., Petersen I.A. Establishment of practice standards in nomenclature and prescription to enable construction of software and databases for knowledge-based practice review. Pract Radiat Oncol. 2016;6:e117–e126. doi: 10.1016/j.prro.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Park H.S., Wang E.H., Rutter C.E., Corso C.D., Chiang V.L., Yu J.B. Changing practice patterns of Gamma Knife versus linear accelerator-based stereotactic radiosurgery for brain metastases in the US. J Neurosurgery. 2016;124:1018–1024. doi: 10.3171/2015.4.JNS1573. [DOI] [PubMed] [Google Scholar]
- 24.Gevaert T., Steenbeke F., Pellegri L. Evaluation of a dedicated brain metastases treatment planning optimization for radiosurgery: A new treatment paradigm. Radiat Oncol. 2016;11:13. doi: 10.1186/s13014-016-0593-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naryanasamy G., Stathakis S., Gutierrez A.N. A systematic analysis of 2 monoisocentric techniques for the treatment of multiple brain metastases. Technol Cancer Res Treat. 2017;16:639–644. doi: 10.1177/1533034616666998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blonigen B.J., Steinmetz R.D., Levin L., Lamba M.A., Warnick R.E., Breneman J.C. Irradiated volume as a predictor of brain radionecrosis after linear accelerator stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2010;77:996–1001. doi: 10.1016/j.ijrobp.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Flickinger J.C., Kondziolka D., Lunsford L.D. Development of a model to predict permanent symptomatic postradiosurgery injury for arteriovenous malformation patients. Int J Radiat Oncol Biol Phys. 2000;46:1143–1148. doi: 10.1016/s0360-3016(99)00513-1. [DOI] [PubMed] [Google Scholar]
- 28.Minniti G., Clarke E., Lanzetta G. Stereotactic radiosurgery for brain metastases: Analysis of outcome and risk of brain radionecrosis. Radiat Oncol. 2011;6:48. doi: 10.1186/1748-717X-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Balik S., Qi P., Magnelli A., Chao S.T., Suh J.H., Zhuang T. Comparison of Spine SRS VMAT plans with Flattening Filter Free 6 MV or 10 MV beams. Int J Radiat Oncol Biol Phys. 2018;102:e507. [Google Scholar]
- 30.Durmus I.F., Atalay E.D. Dosimetric comparison between 10MV-FFF and 6MV-FFF for lung SBRT. AIP Conf Proc. 2017;1815:150001. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.